Abstract
Currently, there are no approved specific antiviral agents for novel coronavirus disease 2019 (COVID-19). Convalescent plasma has not yet been approved for use in patients with COVID-19 infection; however, it is regulated as an investigational product. This is a case report of a 55-year-old male, with COVID-19 pneumonia who has received convalescent plasma as part of a treatment plan which showed significant radiological and clinical improvement post-treatment.
Key Words: COVID-19, RT-PCR, Convalescent plasma transfusion, Groung-glass opacities, Crazy paving appearance
Abbreviations: CT, computed tomography; CECT, COVID-19, coronavirus disease 2019; RT-PCR, Reverse transcription polymerase chain reaction; ED, emergency department; NC, nasal cannula; CRP, C-reactive protein; ICU, intensive care unit; HRCT, high-resolution computed tomography; CXR, chest x-ray; AJR, American Journal of Roentgenology; ACR, American College of Radiology
Introduction
The pandemic caused by the novel coronavirus, called COVID-19, was first reported in China in December 2019 followed by widespread into all other countries in the world. It is affected by more than 6 million people around the world [1].
COVID-19 clinical spectrum varies from asymptomatic or mild symptoms in most of the cases to severe acute respiratory syndrome, which may lead to death.
There is no vaccine yet available to protect from the infection as well as no specific effective treatment has been found. That's why all therapeutic options for COVID-19 infection need to be discussed scientifically.
Case report
This is a case report of a 55-year-old male, previously healthy and nonsmoker.
He presented with a history of 5 days’ fever and cough associated with shortness of breath and chest pain. The patient is living with 20 persons in the same room and some of them had a fever.
On ED O2 sat was 77% on room air, improved to 91%-97% on 5L NC however still tachypneic.
Labs showed a positive RT-PCR for COVID-19, high inflammatory markers CRP 229 mg/L, and a high ferritin level of 749 ug/L.
Chest x-ray (CXR) (Fig. 1) shows bilateral opacities with ARDS pattern, initial chest CT scan (Fig. 2) confirmed the findings and revealed widespread central and peripheral patchy mixed bilateral ground-glass opacities with consolidations a crazy-paving pattern which are classical findings in COVID-19 pneumonia, graded as severe according to the British Society of Thoracic Imaging. Favipiravir and Hydroxychloroquine were initiated.
Fig. 1.
On ER CXR shows bilateral multifocal confluent consolidations with peripheral predominance. No pleural effusion or pneumothorax.
Fig. 2.
Chest x-ray at the day of ICU admission just before intubation and plasma transfusion (A) shows significant worsening of diffuse airspace opacities bilaterally with retrocardiac consolidation. Day 2 after intubation and plasma transfusion (B) shows improvement. Day 6 after plasma transfusion (C) shows further improvement and patient was extubated.
The patient was transferred to ICU after 4 days with acute hypoxic respiratory failure secondary to severe COVID-19 pneumonia required intubation, mechanical ventilation, and placed on Enoxaparin 40 mg SC BID.
The patient has received convalescent plasma effusion after being consented to participate in a clinical trial as fulfilling the eligibility criteria.
About 300 mL convalescent plasma from recovered COVID-19 patient was transfused over 1 hour, premedications with 1-g paracetamol IV, and 50 mg IV diphenhydramine were administered.
The patient was monitored for any reaction post-transfusion. No significant adverse effects were observed.
There was a significant radiological and clinical improvement in a few days’ postconvalescent plasma transfusion.
Within less than 48 hours, the patients’ lab parameters have also improved, COVID RT-PCR was negative, and Ferritin reduced to 515; however, CRP still high 315 mg/L.
After 2 weeks of plasma transfusion, CRP level 1.99 mg/L, Ferritin level improved as well (385 mcg/L).
Postplasma transfusion, CXRs showed gradual improvement starting from day 2, patient was extubated on day 6 (Fig. 3). Chest CT scan was done on day 12 which showed significant improvement (Fig. 4) and patient was discharged at the same day.
Fig. 3.
Chest HRCT axial (A) and coronal (B) views of COVID-19 patient (preconvalescent plasma transfusion) revealed extensive bilateral mixed ground-glass patchy opacities and consolidations with crazy paving appearance involved both lung lobes with mid and lower zonal predominance.
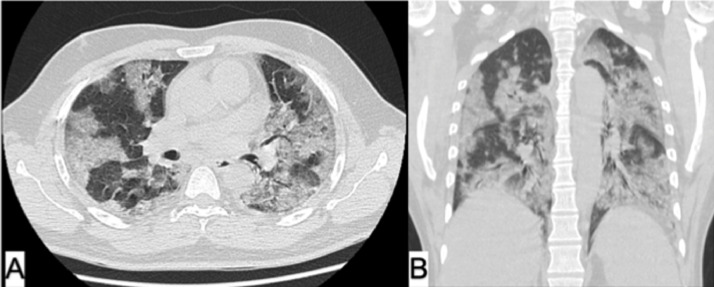
Fig. 4.
Chest HRCT axial (A) and coronal (B) views of COVID-19 patient (12 days postconvalescent plasma transfusion) revealed significant improvement in lung condition with regression of previously noted diffuse bilateral airspace consolidations; however, residual ground-glass opacities and septal thickening are still noted.
Discussion
Convalescent plasma transfusion has been used as a possible passive immunotherapy treatment option for moderate to severe cases [1,2].
The administration of convalescent plasma has been shown to reduce morbidity and mortality rates as well as shorten the hospital stay in patients with severe acute respiratory syndrome in uncontrolled nonrandomized clinical trials [3,4].
A prospective, double-blind, randomized, controlled clinical trial using H1N1 convalescent plasma showed a significant reduction in viral load with reduced mortality rate within 5 days of the symptom onset [5].
During the outbreaks of the Ebola virus in 2014, the WHO recommended the use of Ebola convalescent plasma transfusion as empirical treatment [5].
In the diagnosis and management of COVID-19 patients, imaging has played a controversial role [6].
In February of 2020, AJR found that the rate of missed COVID-19 on HRCT chest is very low; which means negative CT may help physicians in managing patients within the incubation window [7].
In March of 2020, the ACR urges caution in utilizing chest CT to take the decisions in testing the patients for COVID-19 or not [8].
Common chest CT findings are noted in COVID-19 patients include bronchovascular thickening, peripheral and bilateral distributions of patchy subsegmental ground-glass opacity, and patchy consolidations in later phases of the infection. Intralobular septal thickening along with ground-glass opacity forming crazy-paving appearance may be noted as well. For follow-up scans, better to assess the severity score which depends on scoring the percentages of each of the 5 lobes range from 0 (no involvement) to 25 (maximum involvement) when all the 5 lobes show more than 75% involvement [9,10].
Conclusion
The COVID-19 infection may cause severe inflammatory response followed by serious lung damage that can be detected, scored, and followed up by chest HRCT.
In the absence of definitive curative treatment, the use of plasma collected from recovered patients shows an initial promise; however, no enough published data are there to support this passive antibody therapy [11].
Footnotes
Consent waved on IRB applied in our institute.
References
- 1.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunnigham A.C., Goh H.P., Koh D. Treatment of COVID-19: old tricks for new challenges. Crit Care. 2020;24:91. doi: 10.1186/s13054-020-2818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Y., Wong R., Soo Y.O., Wong W.S., Lee C.K., Ng M.H. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hung I.F., To K.K., Lee C.K., Lee K.L., Chan K., Yan W.W. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52:447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. 2014. Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion, as an empirical treatment during outbreaks, Interim Guidance for National Health Authorities and Blood Transfusion Services Version 1.0 September 2014. P8-9.
- 6.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y., Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020 doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 8.American College of Radiology ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection. Am Coll Radiol. 2020 [Google Scholar]
- 9.Kong W., Agarwal P. Chest imaging appearance of COVID-19 infection. Radiol Cardiothorac Imaging. 2020 doi: 10.1148/ryct.2020200028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou S. et al. CT Features of Coronavirus Disease 2019 (COVID-19) Pneumonia in 62 Patients in Wuhan, China, AJR 2020; 214:18. https://radiologyassistant.nl/chest/lk-jg-1. [DOI] [PubMed]
- 11.Casadevall A., Pirofski L.A. The convalescent sera option for containing COVID19. J Clin Invest. 2020;130:1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]