Abstract
A relative haplotype dosage (RHDO)–based method was developed and implemented into routine clinical practice for noninvasive prenatal diagnosis (NIPD) of multiple single-gene disorders: spinal muscular atrophy, Duchenne and Becker muscular dystrophies, and cystic fibrosis. This article describes the experiences of the first 152 pregnancies to have NIPD by RHDO as part of a routine clinical service. Provision of results within a clinically useful time frame (mean, 11 calendar days) was shown to be possible, with a very low failure rate (4%), none being due to a technical failure. Where follow-up confirmatory testing was performed for audit purposes, 100% concordance was seen with the NIPD result, and no discrepancies have been reported. The robust performance of the assay, together with high sensitivity and specificity, demonstrates that NIPD by RHDO is feasible for use in a clinical setting.
The presence of placentally derived fetal cell-free DNA (cfDNA) in the maternal plasma during pregnancy was first described in 1997.1 This discovery allowed significant advances to be made in the field of prenatal diagnoses, and methods, such as fetal sexing,2, 3, 4 RhD blood group genotyping,5, 6, 7 and noninvasive prenatal testing (NIPT) for aneuploidy screening,8, 9, 10, 11, 12 have become well established in clinical practice.
However, the development of noninvasive prenatal diagnosis (NIPD) for single-gene disorders (SGDs) has been hampered because of the complexity of testing required and lack of case studies due to small numbers of patients with individual disorders. Current routine prenatal practice for pregnancies at risk of many SGDs is to analyze fetal DNA obtained by invasive procedures, such as chorionic villus sampling or amniocentesis, to assess the mutational profile of the relevant gene (male pregnancies only for X-linked disorders). These procedures are associated with a risk of miscarriage, with some literature quoting up to a 1% risk,13 although recent studies have shown that it may be as low as 0.2%.14 The introduction of NIPD for SGDs allows an alternative method for prenatal diagnosis, which not only does not carry this associated risk, but also has the added benefits of easier access to sampling, better patient experience, and testing being available from as early as 8 weeks of gestation.15,16 There have been reports in the literature showing case studies and proof-of-principle NIPD assays for several SGDs17; however, these studies have included relatively small numbers of patients, with limited follow-up information. In addition, many of these assays that are designed to directly test for familial variants are not available for maternally inherited variants and because of the fragmented nature of cfDNA cannot be used to test for large deletions or duplications. These assays also cannot test for paternally inherited variants if both parents are carriers of the same mutation, as is often the case for pregnancies at risk of cystic fibrosis (CF) (Supplemental Table S1).
Through the noninvasive prenatal diagnosis for single-gene disorders project, conducted at the Birmingham Women's NHS Foundation Trust, methods were developed to use capture-based targeted enrichment, followed by massively parallel sequencing and analysis by relative haplotype dosage (RHDO),18 to provide NIPD for pregnancies at risk of both X-linked15 and autosomal recessive disorders.16 The analysis of the thousands of single-nucleotide polymorphisms (SNPs) used in RHDO provides the statistical significance required for a robust prenatal test and, unlike other noninvasive assays in current clinical use, allows both maternal and paternal inheritance to be determined. Multiple SGDs can be analyzed in the same sequencing run, thereby allowing maintenance of prenatal turnaround times while keeping costs to an acceptable level to allow implementation into routine clinical practice. Furthermore, as an RHDO assay tracks the inheritance of haplotypes rather than testing for a familial mutation directly, the same assay can be used for all families at risk of a particular disorder, irrespective of the familial variant, thus removing the need for workup of an assay before pregnancy, which reduces both cost and testing time.
Clinical services for NIPD of Duchenne/Becker muscular dystrophies (DMD/BMD) and spinal muscular atrophy (SMA) commenced at the West Midlands Regional Genetics Laboratory (WMRGL) in September 2016, with testing accessible to both UK and international referrals.
This article reports the experiences of the first 152 pregnancies to have NIPD by RHDO as part of a routine clinical service at WMRGL.
Materials and Methods
Patient Samples
This study is a retrospective analysis of patients referred by their local Clinical Genetics Department to the WMRGL for NIPD analysis by RHDO. To be eligible for testing, families needed to have a known family history of the disorder in question, with a confirmed molecular diagnosis and appropriate reference samples available. Samples from consanguineous families cannot currently be accepted, as the NIPD assay requires sufficient informative SNPs to be able to generate a statistically significant result, and it may be expected that in pregnancies of consanguineous couples, the quantity of informative SNPs available for RHDO analysis would be reduced.
Samples were accepted for analysis from pregnant patients with singleton pregnancies at >8 weeks of gestation, confirmed by scan. In pregnancies at risk of DMD/BMD, samples were only accepted from women, following confirmation of male pregnancy on free fetal DNA testing. For every patient, cfDNA was extracted from maternal plasma, which had been isolated from blood samples received in Streck BCT tubes (Streck, Omaha, NE). In addition, maternal, paternal, and reference genomic DNA extracted from leukocytes was required for each analysis. For autosomal recessive disorders, the reference sample required was a child of that couple: an affected child, an unaffected noncarrier child, or, if the parents are carriers of different mutations, a carrier child. For X-linked disorders, the reference sample could be a previous affected male child, a previous unaffected male child, other affected male relative, or unaffected maternal grandfather (if maternal grandmother was known to be a carrier of the disorder).
Targeted Massively Parallel Sequencing
Massively parallel sequencing was performed as previously described.15 In brief, DNA libraries for massively parallel sequencing on the MiSeq sequencing platform (Illumina Inc., San Diego, CA) were prepared from 20 to 100 ng input DNA. Capture enrichment was designed to target highly heterozygous SNPs across the dystrophin (chromosome X: 31,037,731 to 33,457,670), SMN1/SMN2 (chromosome 5: 67,000,530 to 72,999,964), and CFTR (chromosome 7: 117,105,838 to 117,356,025) gene regions. A combined DMD/BMD/SMA/CF probe library was designed for the targeted capture of SNPs covering approximately 4.5 to 6 Mb regions around each gene of interest. Genomic coordinates of SNPs in the DMD, SMN1, and CFTR loci were downloaded from the University of California, Santa Cruz, genome browser (https://genome.ucsc.edu, last accessed July 28, 2020). The SNPs were then filtered to isolate those with a high likelihood of heterozygosity (40% to 50%), increasing suitability for RHDO analysis. The selected SNP coordinates were then uploaded to Roche NimbleDesign software (Roche Molecular Systems, Pleasanton, CA). NimbleDesign software has recently been discontinued and replaced with the Roche KAPA Target Enrichment Portfolio (Roche Molecular Systems) using the highest stringency parameters to minimize the possibility of non-specific capture.
Up to 12 samples, equivalent to three patients, were multiplexed per sequencing run using 2 × 80 cycles paired-end settings. Bioinformatic analysis included quality trimming of reads, alignment to genome build hg19, removal of duplicates, and variant calling to obtain SNP counts.
RHDO Analysis
RHDO measures the allelic imbalance between two haplotypes in plasma cfDNA to determine which haplotype has been inherited by the fetus, with haplotype phasing conducted through sequencing of SNPs.18, 19, 20
For diagnostic referrals to the WMRGL, RHDO analysis was performed, as described previously.15,16 In brief, RHDO analysis for autosomal recessive disorders was achieved by identifying both the maternal and paternal haplotypes linked with the mutant alleles by DNA sequencing of highly heterozygous bi-allelic SNPs within the gene region of interest in the previous affected child. The genotypes of the same SNPs were determined in maternal and paternal DNA samples to conduct haplotype phasing and identify the haplotypes linked with the normal alleles. SNP counts obtained from cfDNA sequencing were used to determine if the fetus had inherited the mutated or normal maternal allele and the mutated or normal paternal allele. The paternally inherited haplotype was identified using SNPs that were homozygous in the mother and heterozygous in the father. The maternally inherited haplotype was determined using SNPs that were heterozygous in the mother and homozygous in the father. Informative SNPs used to determine maternal and paternal inheritance were separately grouped into haplotype blocks of ≥25 SNPs to form maternal and paternal haplotype blocks, with each block representing a statistically independent result.
RHDO analysis for X-linked disorders requires only maternal haplotyping. The male reference sample provides the haplotype linked with either the mutated copy of the dystrophin gene (if affected male reference used) or the normal copy of the dystrophin gene (if unaffected maternal grandfather used as reference sample). The maternal DNA is sequenced to identify heterozygous informative SNPs. As with analysis for autosomal recessive disorders, SNP counts obtained from cfDNA sequencing are used to determine if the fetus has inherited the reference or alternative haplotype by grouping informative SNPs into statistically significant haplotype blocks of ≥25 SNPs. As there is up to a 12% chance of a recombination event within the dystrophin gene,21 RHDO analysis for pregnancies at risk of DMD/BMD was performed in both directions (ie, 5′ to 3′ and 3′ to 5′) to determine the position of any recombination events with high accuracy.
Fetal fraction for each plasma sample was determined using SNPs that are homozygous in both parents but for different SNP alleles, as described previously.15,16
Follow-Up Studies
Analysis of any follow-up samples received was performed using routine diagnostic procedures. Molecular testing for the familial variant was performed on post-natal cord blood, placenta, or products of conception (POC).
DMD/BMD follow-up studies were performed using multiplex ligation-dependent probe amplification analysis of the dystrophin gene (MRC-Holland kits P034-A2 and P035-A2; MRC-Holland, Amsterdam, the Netherlands) to detect exon deletions/duplications or Sanger sequencing to detect pathogenic single-nucleotide variants.
SMA follow-up studies were performed using multiplex ligation-dependent probe amplification analysis to detect copy number of exons 7 and 8 in the SMN1 and SMN2 genes (MRC-Holland kit P021).
CF follow-up studies were performed using the Luminex xTAG CF 39 assay (Luminex Corp., Austin, TX), the Elucigene CF-EU2v1 assay (Promega, Madison, WI), or by Sanger sequencing of appropriate exons.
Results
Between September 2016 and October 2019, samples from 152 pregnancies have had NIPD by RHDO performed at the West Midlands Regional Genetics Laboratory for CF, SMA, or DMD/BMD (Table 1). Referrals were received from UK Clinical Genetics Departments and from international centers.
Table 1.
Summary of First 152 Pregnancies to Have NIPD by RHDO Performed at the West Midlands Regional Genetics Laboratory
| Disorder | Pregnancies | Second sample requested | Unaffected | Carrier | Affected | Partial result | Suboptimal result | Failed analysis |
|---|---|---|---|---|---|---|---|---|
| SMA | 81 | 6 | 23 | 34 | 15 | 7∗ | 2† | 0 |
| CF | 36 | 3 | 8 | 12 | 11 | 3‡ | 0 | 2§,¶ |
| DMD | 30 | 0 | 11 | NA | 13ǁ | 0 | 2†,∗∗ | 4¶,†† |
| BMD | 5 | 0 | 4 | NA | 0 | 0 | 1‡‡ | 0 |
| Total | 152 | 9 | 46 | 46 | 39 | 10 (6.5) | 5 (3) | 6 (4) |
Data are given as number or number (percentage).
BMD, Becker muscular dystrophy; CF, cystic fibrosis; DMD, Duchenne muscular dystrophy; NA, not applicable; NIPD, noninvasive prenatal diagnosis; RHDO, relative haplotype dosage; SMA, spinal muscular atrophy.
Partial results are cases in which only the inheritance of the paternal allele could be determined. This was due to low fetal fraction, recombination on one allele, or insufficient informative maternal single-nucleotide polymorphisms (three consecutive pregnancies from the same patient).
Suboptimal results are cases in which a full diagnostic result could not be generated, but some information could be reported. This was due to low fetal fraction.
Partial results are cases in which only the inheritance of the paternal allele could be determined. This was due to low fetal fraction.
Failed analysis indicates that no result could be provided, and an invasive test was required. This was due to undisclosed consanguinity.
Failed analysis indicates that no result could be provided, and an invasive test was required. This was due to persistent low fetal fraction.
For DMD cases, an affected result indicates that the fetus has inherited the high-risk haplotype. In five of these cases, the mother had not been shown to be a carrier of DMD, but had a previous affected child. The current fetus was at risk of being affected with DMD because of possible germline mosaicism and follow-up invasive testing was required to determine the presence or absence of the mutation.
Suboptimal results are cases in which a full diagnostic result could not be generated, but some information could be reported. This was due to complex consanguinity.
Failed analysis indicates that no result could be provided, and an invasive test was required. This was due to recombination.
Suboptimal results are cases in which a full diagnostic result could not be generated, but some information could be reported. This was due to recombination.
Referrals
Autosomal Recessive RHDO
A total of 77% of the referrals received (117 pregnancies) were for pregnancies at risk of an autosomal recessive condition (SMA or CF), with most cases (81; 53% of total referrals) being at risk of SMA (Table 1). For all referrals, both parents were confirmed carriers of a pathogenic variant (Supplemental Table S1 provides a full list of variants).
In 31 of the pregnancies (30% of reportable cases), NIPD by RHDO showed that the fetus had inherited both the maternal and paternal low-risk haplotypes. Therefore, the fetus was reported as being predicted to be unaffected (noncarrier) with the condition for which the fetus was at risk (Table 1).
For 46 of the referred pregnancies (45% of reportable cases), the fetus was shown to have inherited one low-risk and one high-risk haplotype and so was reported as being predicted to be a carrier of the condition for which the fetus was at risk (Table 1).
A total of 26 fetuses (25% of reportable cases) were shown to have inherited both the maternal and paternal high-risk haplotypes and so were reported as being predicted to be affected (Table 1). For a further 10 cases, a partial result was issued, whereby only the inheritance from one parent could be reported (Table 1) (see below). For three of these cases, it was conclusively demonstrated that the fetus had inherited one low-risk haplotype and was therefore predicted to be unaffected, although no comment could be made regarding carrier status. For the remaining seven pregnancies, NIPD showed that the fetus had inherited the high-risk paternal haplotype and so invasive testing was recommended to determine maternal inheritance. For one of the pregnancies, invasive testing confirmed that the fetus was a carrier of the condition for which the fetus had been referred. In the remaining six cases, invasive testing confirmed that the fetus was affected.
X-Linked RHDO
Of the 35 cases referred for RHDO analysis of an X-linked disorder, 5 (3% total referrals) were at risk of BMD (Table 1) and all had multi-exon deletions within the dystrophin gene (Supplemental Table S2). For four of these pregnancies, it was shown that the fetus had inherited the low-risk maternal haplotype and so was predicted not to be affected with BMD. For the remaining case, a recombination was detected in the dystrophin gene in close proximity to the familial variant, and so a conclusive report could not be issued (see Partial Results and Unsuccessful Analysis).
Most cases referred for RHDO analysis of an X-linked disorder were pregnancies at risk of DMD (30 cases; 20% of total referrals). These referrals included families with point mutations, deletions (single and multi-exon), and multi-exon duplications within the dystrophin gene (Supplemental Table S2). Of these, in 11 cases (46% of reportable DMD cases), NIPD by RHDO showed that the fetus had inherited the low-risk haplotype and so was predicted not to be affected (Table 1). This group included one pregnancy for which the mother had not been shown to be a carrier of DMD, but had a previous affected child; therefore, the current fetus was at risk of being affected with DMD because of possible germline mosaicism. As it was possible to show that the current fetus had inherited the opposite haplotype to the affected child, the risk could be negated for this pregnancy.
In 13 cases (54% of reportable DMD cases), the fetus was shown to have inherited the high-risk haplotype (Table 1). For eight of these pregnancies, the pregnant woman was known to be a carrier of DMD and so it could be predicted that the fetus would be affected. For the remaining five pregnancies, the current fetus was at risk of being affected with DMD because of possible germline mosaicism. In these cases, it was reported that the fetus had inherited the high-risk maternal haplotype and should be followed up by invasive testing. Invasive testing was performed for two of the pregnancies; one fetus was confirmed to be affected, thus confirming the presence of germline mosaicism, whereas the other fetus was shown not to have inherited the familial variant. Two couples chose not to have invasive testing, and the pregnancies are still ongoing, with diagnostic testing planned postnatally. One pregnancy sadly miscarried shortly after NIPD analysis was performed.
Reporting Times
The mean reporting time for clinical RHDO analyses was 11 calendar days from sample receipt (range, 7 to 17 days).
Partial Results and Unsuccessful Analysis
For this study, a partial result was classified as one for which it was only possible to determine inheritance of the haplotype from one of the parents. This was the case for 10 pregnancies at risk of either SMA or CF (6% of total referrals) and was due to a persistent low fetal fraction, recombination on the opposite allele (Figure 1A), or insufficient informative maternal SNPs (three consecutive pregnancies from the same patient) (Table 1). For three of these pregnancies, it was demonstrated that the fetuses had inherited at least one low-risk haplotype, and so it was possible to conclusively report that the fetuses were predicted not to be affected and no further prenatal analysis was required. In the remaining seven cases, it was demonstrated that the fetuses had inherited the high-risk paternal haplotype, and so it was recommended that invasive testing be performed to determine maternal inheritance. In six cases, the patients went on to have invasive testing, which confirmed that the fetuses was affected. In one case, follow-up analysis of a chorionic villus sample showed the fetus to be a carrier of SMA.
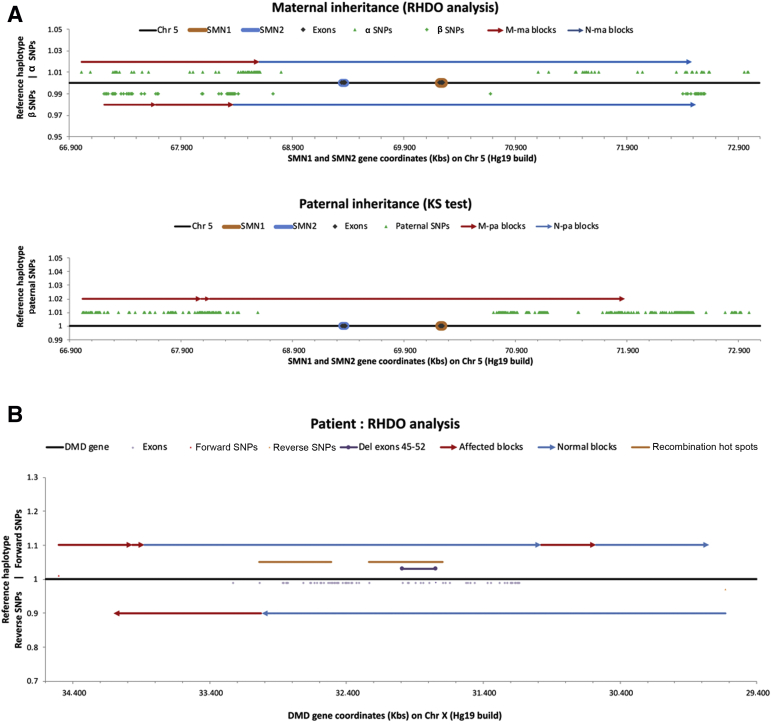
Figure 1.
Recombination events detected by relative haplotype dosage (RHDO) analysis. A: Example of a recombination event in a pregnancy at risk of spinal muscular atrophy. The recombination event on the maternal allele is too close to the site of the familial variant for a conclusive result to be issued. B: Example of a recombination event in a pregnancy at risk of Duchenne muscular dystrophy (DMD). The recombination event is sufficiently distant from the site of the familial variant for a conclusive result to be issued. Chr, chromosome; Del, deleted; KS, Kolmogorov-Smirnov test; M-ma, mutated maternal allele; M-pa, mutated paternal allele; N-ma, normal maternal allele; N-pa, normal paternal allele; SMN, survival motor neuron; SNP, single-nucleotide polymorphism.
Suboptimal results were classified as those that were not diagnostic, but for which some information could be provided to guide any further testing required. In three samples, the fetal fraction was persistently too low to conclusively determine the inheritance of either the maternal or the paternal haplotype. However, despite suboptimal data, some information was provided for each of the pregnancies. Two pregnancies were reported as being highly unlikely to be affected with SMA; one patient declined invasive testing, and an unaffected result was confirmed on cord blood after delivery, and the second pregnancy miscarried at approximately 12 weeks of gestation and the unaffected result was confirmed on POC. The third persistently low fetal fraction sample was from a pregnancy at risk of DMD. The data were consistent with the fetus being affected and invasive prenatal testing later confirmed this result.
For one pregnancy at risk of DMD, a result of sufficient quality to allow a diagnostic result could not be generated; however, it was determined that the fetus was unlikely to be affected. It was later disclosed that there was complex consanguinity within the family, and it is likely that this affected the level of informative SNPs. The patient went on to have chorionic villus sampling, which confirmed that the fetus was unaffected.
For one pregnancy at risk of BMD, a recombination event was detected upstream of the familial mutation (deletion of exons 45 to 48 of the dystrophin gene); however, several statistically significant haplotype blocks were generated between the site of the recombination and the familial mutation. The result was reported as the fetus being highly likely to be affected, and the pregnancy was terminated without any further confirmatory testing.
In 4% of all referrals (four pregnancies at risk of DMD; two pregnancies at risk of CF), reportable results could not be generated (Table 1). In two of the pregnancies at risk of DMD, a recombination event was detected close to the familial variant, which meant that the haplotype in this region of the gene could not be conclusively determined. For one pregnancy at risk of CF, sufficient informative SNPs in either parent could not be identified. It was later disclosed that this family was consanguineous. For the remaining three pregnancies (two pregnancies at risk of DMD; one pregnancy at risk of CF), a persistently low fetal fraction meant that no result could be issued.
Confirmation of Results
To allow ongoing validation of this method, when possible, follow-up testing was performed on cord blood, placenta, or POC.
Of the 146 pregnancies to date for which a diagnostic result could be issued, follow-up genetic testing was performed for 70. In all cases, the follow-up testing confirmed the result generated by RHDO (Table 2), and no discrepancies were reported. For a further six pregnancies for which NIPD had predicted that the fetuses were not affected with the condition for which they were at risk, it was reported that a healthy baby had been born, but no follow-up molecular testing had been performed (Table 2). For 16 pregnancies that were predicted by NIPD to be affected, the pregnancy was terminated, with no further testing performed (Table 2).
Table 2.
Follow-Up of Pregnancies Referred for NIPD by RHDO
| Disorder | Follow-up |
||||
|---|---|---|---|---|---|
| Confirmed by molecular testing | Discordant by molecular testing | Healthy baby following neg NIPD (no testing) | TOP following positive NIPD (no testing) | No follow-up information available | |
| SMA | 44 | 0 | 5 | 6 | 26 (7 ongoing) |
| CF | 14 | 0 | NA | 4 | 18 (7 ongoing) |
| DMD | 9 | 0 | NA | 5 | 16 (7 ongoing) |
| BMD | 3 | 0 | 1 | 1 | 0 |
| Total | 70/70 (100) | 0/70 (0) | 6 | 16 | 54 (21 ongoing) |
Data are given as number or number/total (percentage).
BMD, Becker muscular dystrophy; CF, cystic fibrosis; DMD, Duchenne muscular dystrophy; NA, not applicable; NIPD, noninvasive prenatal diagnosis; RHDO, relative haplotype dosage; SMA, spinal muscular atrophy; TOP, termination of pregnancy.
For 54 of the pregnancies in the cohort, no follow-up data were available. At the time of writing, 21 pregnancies are still ongoing and no further information was available. For the remaining 33 pregnancies, no discordant results have been reported postnatally.
Fetal Fraction
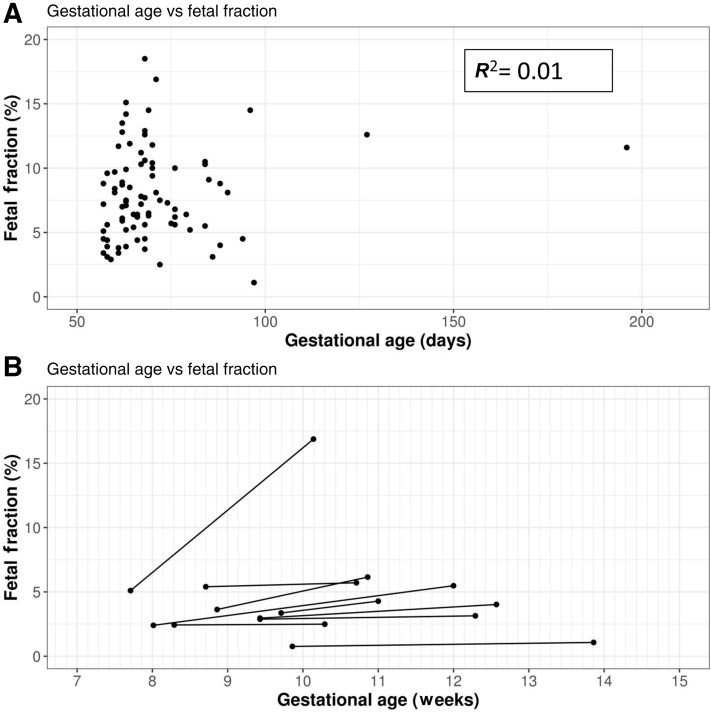
For 86 of the tested pregnancies, an accurate gestational age of the fetus at the time of sampling was provided. For these pregnancies, fetal fraction could be plotted, calculated as previously described,15,16 against gestational age (Figure 2A). Linear regression analysis of data did not show a correlation between gestational age and fetal fraction (R 2 = 0.01).
Figure 2.
Fetal fraction variation with gestational age. A: Fetal fraction of 86 pregnancies at different gestational ages. B: Change in fetal fraction between samples for nine pregnancies. Linear regression analysis was performed using Prism software version 8 (GraphPad Software Inc., San Diego, CA).
In nine cases in which a low fetal fraction at initial sampling complicated analysis, a second sample was requested at a later gestation. Figure 2B shows the change in fetal fraction over time for each of these pregnancies. For one pregnancy, the fetal fraction more than tripled over the space of 17 days (5.1% to 16.88%) (Figure 2B). For the remaining eight pregnancies, little or no increase was seen in fetal fraction between samples (Figure 2B).
Pregnancy Outcomes
In total, 45 at-risk pregnancies were demonstrated to either be affected with the disorder for which they had been referred for prenatal diagnosis or be at risk of being affected with DMD due to possible germline mosaicism (Table 1). Of the five pregnancies at risk of DMD due to germline mosaicism, follow-up results were available for two; one pregnancy was shown to be affected, and one pregnancy was shown to be unaffected. Therefore, of the 152 pregnancies tested to date, 41 were predicted to be affected with the disorder for which they had been referred.
Of these 41 affected pregnancies, 29 were terminated following the result (Table 3), and follow-up testing on material from 13 of these terminations confirmed that the fetus was affected. No follow-up testing was performed on the other 16 cases.
Table 3.
Pregnancy Outcomes following a Positive NIPD Result
| Disorder | TOP following positive NIPD | Pregnancy continued following positive NIPD | Unknown outcome |
|---|---|---|---|
| SMA | 16 | 2 | 1 |
| CF | 8 | 2 | 4 |
| DMD | 5 | 2 | 1 |
| BMD | NA | NA | NA |
Data are given as number.
BMD, Becker muscular dystrophy; CF, cystic fibrosis; DMD, Duchenne muscular dystrophy; NA, not applicable; NIPD, noninvasive prenatal diagnosis; SMA, spinal muscular atrophy; TOP, termination of pregnancy.
In six pregnancies predicted to be affected with the disease for which they had been referred, the family decided to continue with the pregnancy. Postnatal testing for each of these cases confirmed the child's affected status. No outcome information is available for the remaining six pregnancies.
Following negative NIPD analysis, two local patients were also offered NIPT for common trisomies due to an increased nuchal translucency and/or a high screening risk for trisomy 21. NIPT analysis showed both pregnancies to be highly likely to be affected with Down syndrome. One patient had an amniocentesis, which confirmed the diagnosis, and the pregnancy was terminated. The second patient terminated the pregnancy on the basis of the NIPT result.
Discussion
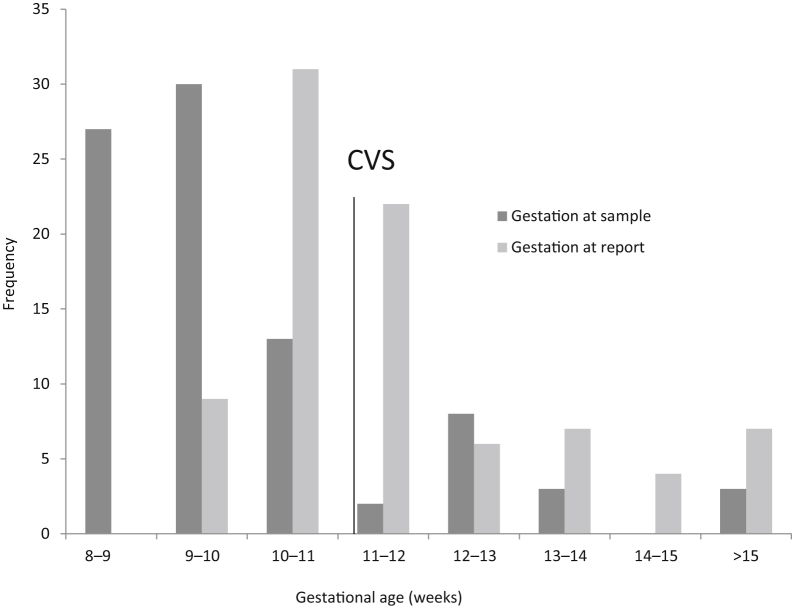
Through the noninvasive prenatal diagnosis for single-gene disorders project, assays suitable for NIPD for pregnancies at risk of both X-linked15 and autosomal recessive disorders16 as part of routine clinical practice were developed. These assays were implemented into clinical service in September 2016 and, since this date, referrals from throughout the United Kingdom and further afield have been received. These referrals include multiple referrals from some women for subsequent pregnancies, and as far as is known, no patients with a conclusive NIPD result had further testing unless later screening indicated it was required for a different condition. This suggests that patients are confident in the results, and anecdotal patient feedback has been positive. On average, results were issued 11 days after sample receipt at a mean gestational age of 11 + 4 weeks, which meant that the information was available to the patients within a clinically useful time frame and in general before the results of invasive testing would have been available (Figure 3). The main drawback to this technique is that analysis is only available in families for which an appropriate reference sample is available. However, proof-of-principle studies have demonstrated that microfluidics-based linked-read sequencing technology can be used to deduce haplotypes directly without the need for a reference sample.22,23 Although the cost of this analysis is currently prohibitive for routine clinical use, it may in the future allow further development of this service to at-risk couples identified through carrier screening programs.
Figure 3.
Gestational age of 86 pregnancies at time of sampling and time of reporting. Gestational age at sampling ranged from 8 + 1 to ≥15 weeks (mean, 10 + 1 weeks). Gestational age at reporting ranged from 9 + 2 to 29 weeks (mean, 11 + 4 weeks). The minimum gestational age at which chorionic villus sampling (CVS) is performed is 11 weeks.
Confirmation of Results
Although NIPD does not require confirmatory testing, to allow ongoing validation of service, it was requested that follow-up testing be performed on cord blood, placenta, or POC where possible. This analysis was performed at either WMRGL or the local referring laboratory. NIPD results were confirmed in all 63 pregnancies in which follow-up testing was performed, demonstrating 100% concordance (Table 2). For a further 39 cases in the cohort, no postnatal discrepancies have been reported. It can be inferred that these cases were also true results. As this assay tracks the inheritance of haplotypes, it does not account for the acquisition of de novo pathogenic variants in the fetus, and it is important that families accessing NIPD are counseled regarding this. However, this is also the case for targeted mutation analysis, whether using NIPD or invasive techniques, which would only test for variants previously identified in the family. Therefore, the utility of this method as a diagnostic prenatal assay has been demonstrated.
NIPD for Germline Mosaicism
There is a high incidence of germline mosaicism for DMD, with a recurrence risk of 9% associated with a high-risk haplotype.24 Of referrals for pregnancy at risk of DMD, 6 (20%) were at risk because of possible germline mosaicism. Although an invasive test was recommended if the fetus was shown to have inherited the high-risk haplotype, demonstration that the fetus has inherited the low-risk haplotype means that the risk of germline mosaicism is negated. Therefore, NIPD is still able to significantly reduce the number of invasive tests required for pregnancies at risk of DMD attributable to germline mosaicism. In addition, of the five pregnancies that were shown to have inherited the high-risk haplotype, only two went on to have invasive testing, with another two opting to have postnatal testing. In one case, the pregnancy miscarried shortly after NIPD was performed, and it is not known whether this family was planning on accessing invasive testing. It can be assumed that those families opting for postnatal testing would not have pursued prenatal diagnosis of any form were NIPD not available to them. Therefore, NIPD by RHDO is increasing the available options for the prenatal management of these families.
Furthermore, if an invasive test following NIPD demonstrates the presence of the familial mutation on the high-risk haplotype, mosaicism in the mother can be confirmed, and this allows a more accurate recurrence risk to be calculated for the family. Conversely, if the familial mutation is not detected on the high-risk haplotype, the risk of germline mosaicism and hence recurrence risk is reduced. This is an advantage of NIPD over invasive testing methods, which do not establish which haplotype the fetus has inherited.
Although such a scenario has not been encountered in the cases to date, NIPD for SMA can be similarly informative for families in which one parent is suspected to be a 2 + 0 carrier. It is estimated that approximately 4% of the northern European population have two copies of SMN1 on one allele,25 and in such individuals, a deletion on the opposite allele would not be detected by traditional carrier testing methods, such as multiplex ligation-dependent probe amplification, that rely on copy number analysis. If the fetus of a suspected 2 + 0 carrier was shown to have inherited the associated high-risk haplotype, invasive testing would be able to confirm or rule out carrier status.
Partial, Suboptimal, and Failed Analysis
Our failure rate for this service has been low, with only 4% of referrals not receiving a result and 3% receiving a suboptimal result, none being due to a technical failure. A common reason for an inconclusive/suboptimal result was a recombination within the dystrophin gene (Table 2). Recombination events within dystrophin are not unexpected as the recombination rate across the whole gene can be as high as 12%.21 To reduce this risk, reference samples for X-linked RHDO analyses are selected to ensure the fewest possible meiosis between phasing sample and current pregnancy. RHDO analysis for pregnancies at risk of DMD/BMD is also performed in both directions (ie, 5′ to 3′ and 3′ to 5′) to allow the recombination site to be positioned with high accuracy.15 If the recombination event is sufficiently distant from the familial mutation, then an accurate diagnosis can still be achieved (Figure 1B). However, for four cases within the cohort, a recombination event was detected too close to the familial mutation to be reportable. As discussed below, recombination events have also been detected in pregnancies at risk of an autosomal recessive inherited disorder (Figure 1A). However, recombination events in the CFTR and SMN gene regions are much rarer, and in these cases, it is expected that the inheritance of the opposite haplotype would still be reportable, meaning that a partial result would be generated.
A second common reason for failure to generate a diagnostic result was an unresolvable low fetal fraction. Although a minimum fetal fraction threshold is not set for the assay, other suboptimal conditions, such as low numbers of informative SNPs, can be exacerbated by a low fetal fraction. For five cases in the cohort, a level high enough to generate a reportable result could not be reached, even with a repeated sample (discussed further below). NIPD is a diagnostic assay that does not require confirmatory testing as standard; therefore, it is important that suboptimal results are treated with caution. In each of these cases, the pregnancy was reported to be highly likely/unlikely to be affected, but it was recommended that if the patient wanted a definitive diagnosis, the patient could consider an invasive test. One patient whose pregnancy was reported to be highly likely to be affected with DMD went on to have invasive testing that confirmed the NIPD result and the pregnancy was later terminated. Two pregnancies were reported as highly unlikely to be affected with SMA; one result was confirmed post-natally, and one pregnancy later miscarried and the NIPD result was confirmed on POC. No follow-up information is yet available on the other two pregnancies.
Our NIPD assay requires sufficient informative SNPs to be able to generate a statistically significant result, and it may be expected that for pregnancies in consanguineous couples, the quantity of informative SNPs available for RHDO analysis would be reduced. Within this cohort, referrals from two cases with undisclosed consanguinity were received. In one case, there were sufficient informative SNPs for a suboptimal result to be generated, but in the second, no analysis could be performed. The level of available SNPs is likely to be related to the degree of consanguinity between the couple, but as this cannot be accurately predicted, NIPD by RHDO cannot currently be offered to consanguineous couples. In the future, it may be possible to offer NIPD for these families using SNPs that are heterozygous in both parents,19 although further testing would be required to validate this method for clinical use.
For 10 pregnancies at risk of an autosomal recessive disorder, only a partial result was generated (ie, only inheritance of one of the parental haplotypes could be determined). This was due to either unavoidable biological factors, such as insufficient informative maternal SNPs, or a recombination event on the opposite allele or due to a fetal fraction that was sufficient to determine paternally inherited haplotypes, but not sufficient to determine the maternally inherited haplotype above the background maternal material. Seven cases within this data set were shown to have inherited the high-risk paternal haplotype and so invasive testing was required to determine maternal inheritance. However, for three of these cases, it was shown that the fetus had inherited a low-risk allele from one of his/her parents. This meant that no further testing was required to determine whether the fetus was affected, and carrier testing could be offered at an appropriate stage later in life.
Fetal Fraction
There is a general consensus in the literature that fetal fraction increases with gestational age.26, 27, 28, 29 However, for the cohort of patients in the current study, a statistical relationship between gestational age and fetal fraction was not demonstrated (Figure 2A), in keeping with a recent study that showed no correlation between fetal fraction and gestational age during the first trimester.30 Furthermore, of the nine pregnancies with a low fetal fraction for which a repeated sample was received, in eight cases little or no increase was seen in the repeated sample taken ≥2 weeks later (Figure 2B). In the one case in which a significant increase was seen in fetal fraction between samples (Case 1) (Figure 2B), the first sample was taken earlier than the recommended threshold for testing of 8 weeks. These data support the requirement of a minimal gestational age for testing, but suggest that beyond this, fetal fraction can remain relatively stable in the first trimester. This finding is consistent with a study by Wang et al26 that showed, between 10 and 21 weeks of gestation, fetal fraction increases by 0.1% per week.
To determine whether a low fetal fraction could be overcome by sequencing to a greater depth, average read depth was assessed for all samples affected by a low fetal fraction. A total of 90% of the cases had an average sequencing depth of >200 for the SNPs used in haplotype generation (range, 105 to 518). Where repeated samples were tested, improved results were only obtained if there was an increased fetal fraction, even if an average read depth was reduced. An increase in read depth without an increase in fetal fraction did not yield improved results. Therefore, it appears that a low fetal fraction cannot be overcome by sequencing to a greater depth.
Pregnancy Outcomes
For the referrals received for pregnancies at risk of an autosomal recessively inherited SGD, outcomes fitted the expected mendelian ratios (25% affected, 45% carrier, and 30% noncarrier unaffected). Similarly, it was possible to demonstrate an expected ratio of affected and unaffected pregnancies at risk of an X-linked disorder.
Of the 152 pregnancies in the cohort, 41 were predicted to be affected with the disorder for which they were referred (Table 1). Following this result, most affected pregnancies for which outcomes are known were terminated. However, at least six couples opted to continue with the pregnancy following a positive NIPD result, and all had their result confirmed postnatally. With the increase in effective therapies becoming available for SGDs, it may be that more families choose to continue with affected pregnancies and that prenatal diagnosis is used to enable access to early treatment. This is the case for at least one of the pregnancies that was reported to be affected with SMA.
Sadly, for two of the patients who received a negative NIPD result, later screening indicated that the pregnancy was at a high risk of trisomy 21. Both patients went on to have NIPT for common trisomies, which showed that the pregnancies were highly likely to be affected with Down syndrome. Both pregnancies were terminated: one on the basis of the NIPT result, and one following invasive testing to confirm the diagnosis of Down syndrome. This highlights the importance of patients receiving comprehensive prenatal counseling when being referred with a pregnancy at risk of an SGD and either going through the routine screening pathway or having access to NIPT once the pregnancy reaches 10 weeks of gestation.
Conclusion
This article demonstrated that NIPD by RHDO can be performed efficiently in a clinical setting for both autosomal recessive and X-linked disorders, with both high sensitivity and specificity. Anecdotal data from families have shown the availability of this service to have a significant impact, with the testing being perceived as much less traumatic and the option to have samples taken near to home rather than having to travel to a specialist center, improving their overall experience. This has been reflected in an increase in referrals to this service over time, with several couples going on to have NIPD for multiple, subsequent pregnancies. Of interest, a small but significant proportion of the families have chosen to continue with their pregnancies following a positive result, with the NIPD outcome being used for information only. It is not clear how many of these families would have pursued invasive prenatal testing if NIPD were not available, but with the advent of treatments, such as nusinersen (Spinraza; Biogen, Cambridge, MA) for SMA, early diagnosis can prove critical to ensure optimal outcomes. Looking forward, it is possible to envision that NIPD could be used to inform in utero treatment options in the future.
These assays can now be extended to increase the availability for additional monogenic disorders, thus providing accessibility for many more couples with a pregnancy at risk of an SGD.
Acknowledgments
We thank all patients involved in this service and our collaborators, both nationally and internationally, for referring patients to us and providing follow-up information on the pregnancies; and the Health Innovation Challenge Fund (HICF-R6-381), a parallel funding partnership between the Wellcome Trust and the Department of Health, for funding the research that allowed this service to be developed. The views expressed in this publication are those of the authors and do not necessarily represent those of Wellcome Trust or the Department of Health.
Footnotes
Supported by the Health Innovation Challenge Fund HICF-R6-381.
Disclosures: None declared.
Current address of M.P., Nonacus Ltd, Birmingham, United Kingdom.
Supplemental material for this article can be found at http://doi.org/10.1016/j.jmoldx.2020.06.001.
Supplemental Data
References
- 1.Lo Y.M., Corbetta N., Chamberlain P.F., Rai V., Sargent I.L., Redman C.W., Wainscoat J.S. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–487. doi: 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- 2.Rijnders R.J., van der Schoot C.E., Bossers B., de Vroede M.A., Christiaens G.C. Fetal sex determination from maternal plasma in pregnancies at risk for congenital adrenal hyperplasia. Obstet Gynecol. 2001;98:374–378. doi: 10.1016/s0029-7844(01)01480-6. [DOI] [PubMed] [Google Scholar]
- 3.Devaney S.A., Palomaki G.E., Scott J.A., Bianchi D.W. Noninvasive fetal sex determination using cell-free fetal DNA: a systematic review and meta-analysis. JAMA. 2011;306:627–636. doi: 10.1001/jama.2011.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill M., Lewis C., Jenkins L., Allen S., Elles R.G., Chitty L.S. Implementing noninvasive prenatal fetal sex determination using cell-free fetal DNA in the United Kingdom. Expert Opin Biol Ther. 2012;12(Suppl 1):S119–S126. doi: 10.1517/14712598.2012.666522. [DOI] [PubMed] [Google Scholar]
- 5.Lo Y.M., Hjelm N.M., Fidler C., Sargent I.L., Murphy M.F., Chamberlain P.F., Poon P.M., Redman C.W., Wainscoat J.S. Prenatal diagnosis of fetal RhD status by molecular analysis of maternal plasma. N Engl J Med. 1998;339:1734–1738. doi: 10.1056/NEJM199812103392402. [DOI] [PubMed] [Google Scholar]
- 6.van der Schoot C.E., Hahn S., Chitty L.S. Non-invasive prenatal diagnosis and determination of fetal Rh status. Semin Fetal Neonatal Med. 2008;13:63–68. doi: 10.1016/j.siny.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Bombard A.T., Akolekar R., Farkas D.H., VanAgtmael A.L., Aquino F., Oeth P., Nicolaides K.H. Fetal RHD genotype detection from circulating cell-free fetal DNA in maternal plasma in non-sensitized RhD negative women. Prenat Diagn. 2011;31:802–808. doi: 10.1002/pd.2770. [DOI] [PubMed] [Google Scholar]
- 8.Hill M., Wright D., Daley R., Lewis C., McKay F., Mason S., Lench N., Howarth A., Boustred C., Lo K., Plagnol V., Spencer K., Fisher J., Kroese M., Morris S., Chitty L.S. Evaluation of non-invasive prenatal testing (NIPT) for aneuploidy in an NHS setting: a reliable accurate prenatal non-invasive diagnosis (RAPID) protocol. BMC Pregnancy Childbirth. 2014;14:229. doi: 10.1186/1471-2393-14-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu R.W.K., Chan K.C.A., Gao Y., Virginia Y., Lau M., Zheng W., Leung T.Y., Foo C.H.F., Xie B., Nancy B., Tsui Y., Lun F.M.F., Zee B.C.Y., Lau T.K., Cantor C.R., Dennis Lo Y.M. Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma. Proc Natl Acad Sci U S A. 2008;105:20458–20463. doi: 10.1073/pnas.0810641105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianchi D.W., Platt L.D., Goldberg J.D., Abuhamad A.Z., Sehnert A.J., Rava R.P. Maternal blood is source to accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119:890–901. doi: 10.1097/AOG.0b013e31824fb482. [DOI] [PubMed] [Google Scholar]
- 11.Futch T., Spinosa J., Bhatt S., de Feo E., Rava R.P., Sehnert A.J. Initial clinical laboratory experience in noninvasive prenatal testing for fetal aneuploidy from maternal plasma DNA samples. Prenat Diagn. 2013;33:569–574. doi: 10.1002/pd.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porreco R.P., Garite T.J., Maurel K., Marusiak B., Obstetrix Collaborative Research Network, Ehrich M., van den Boom D., Deciu C., Bombard A. Noninvasive prenatal screening for fetal trisomies 21, 18, 13 and the common sex chromosome aneuploidies from maternal blood using massively parallel genomic sequencing of DNA. Am J Obstet Gynecol. 2014;211:365. doi: 10.1016/j.ajog.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 13.Chorionic Villus Sampling (CVS) and Amniocentesis: Information for Parents. NHS Fetal Anomaly Screening Programme. PHE publications gateway number: GW-437. Public Health England; London, England: 2017. [Google Scholar]
- 14.Saloman L.J., Sotiriadis A., Wuff C.B., Odibo A., Akolekar R. Risk of miscarriage following amniocentesis or chorionic villus sampling: systematic review of literature and updated meta-analysis. Ultrasound Obstet Gynecol. 2019;54:442–451. doi: 10.1002/uog.20353. [DOI] [PubMed] [Google Scholar]
- 15.Parks M., Court S., Cleary S., Clokie S., Hewitt J., Williams D., Cole T., MacDonald F., Griffiths M., Allen S. Non-invasive prenatal diagnosis of Duchenne and Becker muscular dystrophies by relative haplotype dosage. Prenat Diagn. 2016;36:312–320. doi: 10.1002/pd.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parks M., Court S., Bowns B., Cleary S., Clokie S., Hewitt J., Williams D., Cole T., MacDonald F., Griffiths M., Allen S. Non-invasive prenatal diagnosis of spinal muscular atrophy by relative haplotype dosage. Eur J Hum Genet. 2017;25:416–422. doi: 10.1038/ejhg.2016.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen S., Young E., Bowns B. Noninvasive prenatal diagnosis for single gene disorders. Curr Opin Obstet Gynecol. 2017;29:73–79. doi: 10.1097/GCO.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 18.Lo Y.M., Chan K.C., Sun H., Chen E.Z., Jiang P., Lun F.M., Zheng Y.W., Leung T.Y., Lau T.K., Cantor C.R., Chiu R.W. Maternal plasma DNA sequencing reveals the genome wide genetic and mutational profile of the fetus. Sci Transl Med. 2010;2:61ra91. doi: 10.1126/scitranslmed.3001720. [DOI] [PubMed] [Google Scholar]
- 19.Lam K.W., Jiang P., Liao G.J., Chan K.C., Leung T.Y., Chiu R.W., Lo Y.M. Noninvasive prenatal diagnosis of monogenic diseases by targeted massively parallel sequencing of maternal plasma: application to β-thalassemia. Clin Chem. 2012;58:1467–1475. doi: 10.1373/clinchem.2012.189589. [DOI] [PubMed] [Google Scholar]
- 20.New M.I., Tong Y.K., Yuen T., Jiang P., Pina C., Chan K.C., Khattab A., Liao G.J., Yau M., Kim S.M., Chiu R.W., Sun L., Zaidi M., Lo Y.M. Noninvasive prenatal diagnosis of congenital adrenal hyperplasia using cell-free fetal DNA in maternal plasma. J Clin Endocrinol Metab. 2014;99:E1022-30. doi: 10.1210/jc.2014-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbs S., Roberts R.G., Mathew C.G., Bentley D.R., Bobrow M. Accurate assessment of intragenic recombination frequency within the Duchenne muscular dystrophy gene. Genomics. 1990;7:602–606. doi: 10.1016/0888-7543(90)90205-9. [DOI] [PubMed] [Google Scholar]
- 22.Zheng G.X., Lau B.T., Schnall-Levin M., Jarosz M., Bell J.M., Hindson C.M. Haplotyping germline and cancer genomes with high-throughput linked-read sequencing. Nat Biotechnol. 2016;34:303–311. doi: 10.1038/nbt.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hui W.W., Jiang P., Tong Y.K., Lee W.S., Cheng Y.K., New M.I., Kadir R.A., Chan K.C., Leung T.Y., Lo Y.M., Chiu R.W. Universal haplotype-based noninvasive prenatal testing for single gene disorders. Clin Chem. 2017;63:513–524. doi: 10.1373/clinchem.2016.268375. [DOI] [PubMed] [Google Scholar]
- 24.Helderman-van den Enden A.T., de Jong R., den Dunnen J.T., Houwing-Duistermaat J.J., Kneppers A.L., Ginjaar H.B., Breuning M.H., Bakker E. Recurrence risk due to germ line mosaicism: Duchenne and Becker muscular dystrophy. Clin Genet. 2009;75:465–472. doi: 10.1111/j.1399-0004.2009.01173.x. [DOI] [PubMed] [Google Scholar]
- 25.Sugarman E.A., Nagan N., Zhu H., Akmaev V.R., Zhou Z., Rohlfs E.M., Flynn K., Hendrickson B.C., Scholl T., Sirko-Osadsa D.A., Allitto B.A. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72,400 specimens. Eur J Hum Genet. 2012;20:27–32. doi: 10.1038/ejhg.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang E., Batey A., Struble C., Musci T., Song K., Oliphant A. Gestational age and maternal weight effects on fetal cell-free DNA in maternal plasma. Prenat Diagn. 2013;33:662–666. doi: 10.1002/pd.4119. [DOI] [PubMed] [Google Scholar]
- 27.Hudecova I., Sahota D., Heung M.M., Jin Y., Lee W.S., Leung T.Y., Lo Y.M., Chiu R.W. Maternal plasma fetal DNA fractions in pregnancies with low and high risks for fetal chromosomal aneuploidies. PLoS One. 2014;9:e88484. doi: 10.1371/journal.pone.0088484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y., Zhu Z., Gao Y., Yuan Y., Guo Y., Zhou L., Liao K., Wang J., Du B., Hou Y., Chen Z., Chen F., Zhang H., Yu C., Zhao L., Lau T.K., Jiang F., Wang W. Effects of maternal and fetal characteristics on cell-free fetal DNA fraction in maternal plasma. Reprod Sci. 2015;22:1429–1435. doi: 10.1177/1933719115584445. [DOI] [PubMed] [Google Scholar]
- 29.Lee T.J., Rolnik D.L., Menezes M.A., McLennan A.C., da Silva Costa F. Cell-free fetal DNA testing in singleton IVF conceptions. Hum Reprod. 2018;33:572–578. doi: 10.1093/humrep/dey033. [DOI] [PubMed] [Google Scholar]
- 30.Hestand M.S., Bessem M., van Rijn P., de Menezes R.X., Sie D., Bakker I., Boon E.M.J., Sistermans E.A., Weiss M.M. Fetal fraction evaluation in non-invasive prenatal screening (NIPS) Eur J Hum Genet. 2019;27:198–202. doi: 10.1038/s41431-018-0271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.