Summary
There are emerging data that patients <50 years are diagnosed with esophageal adenocarcinoma (EAC) more frequently, suggesting that the age threshold for screening should be revisited. This study aimed to determine the age distribution, outcomes, and clinical features of EAC over time. The pathology database at the Hospital of the University of Pennsylvania was reviewed from 1991 to 2018. The electronic health records and pathology were reviewed for age of diagnosis, pathology grade, race, and gender for a cohort of 630 patients with biopsy proven EAC. For the patients diagnosed from 2009 to 2018, the Penn Abramson Cancer Center Registry was reviewed for survival and TNM stage. Of the 630 patients, 10.3% (65 patients) were <50 years old [median 43 years, range 16–49]. There was no increase in the number of patients <50 years diagnosed with EAC (R = 0.133, P = 0.05). Characteristics of those <50 years versus >50 years showed no difference in tumor grade. Among the 179 eligible patients in the cancer registry, there was no significant difference in clinical or pathological stage for patients <50 years (P value = 0.18). There was no association between diagnosis age and survival (P = 0.24). A substantial subset of patients with EAC is diagnosed at <50 years. There was no increasing trend of EAC in younger cohorts from 1991 to 2018. We could not identify more advanced stage tumors in the younger cohort. There was no significant association between diagnosis age and survival.
Keywords: adenocarcinoma of the esophagus, age distribution, Barrett’s esophagus
INTRODUCTION
Esophageal adenocarcinoma (EAC) has traditionally been thought to be a disease of older Caucasian males. Risk factors for EAC include obesity, chronic gastroesophageal reflux, male gender, Caucasian race, and smoking. Barrett’s esophagus is a condition where chronic inflammation of the esophageal mucosa leads to intestinal metaplasia, which is thought to be a precursor lesion to EAC. The prognosis of EAC remains poor with the estimated 5-year survival of 19%.1 Current professional society guidelines suggest screening for Barrett’s esophagus in patients ≥50 years old with multiple risk factors with the goal of increasing early diagnosis of EAC in a higher risk population.2,3
The incidence of EAC increased dramatically in the beginning of 1970s. However, it appears that the incidence rate of EAC has plateaued in recent years for unclear reasons.4 At the same time, there are emerging data that patients <50 years old are being diagnosed with EAC more frequently, which suggests that the age threshold for screening may need to be revisited. A recent review of the SEER database found that the incidence increased most in the youngest patients.5 In addition, work from the Mayo Clinic found that younger patients presented with later stage disease and had shorter survival.6 Given this information, we hypothesized that the age of diagnosis of EAC decreased over time at our center. The aim of this study was to determine the age distribution, outcomes, and clinical features of EAC over time at a tertiary care academic medical center.
MATERIALS AND METHODS
Setting
This is a single-center study at the Hospital of the University of Pennsylvania. This research was approved by the University of Pennsylvania Institutional Review Board and was given a letter of exemption from the Penn Abramson Cancer Center’s Clinical Trials Scientific Review and Monitoring Committee.
Patients
The pathology database (TIES) at the Hospital of the University of Pennsylvania was reviewed using search terms ‘EAC’ or ‘esophageal squamous cell carcinoma (ESCC)’ or ‘malignant neoplasm of esophagus’ or ‘GE junction tumor’ from January 1, 1991 to September 1, 2018. This identified all patients with EAC. The electronic health records and pathology reports were reviewed for the following variables: age of diagnosis, pathology grade, race, and gender. Our study cohort consisted of patients for whom data for all these variables could be ascertained. Presenting stage information was available for a subcohort of study patients who were diagnosed with EAC after 2009 when the Penn Medicine Cancer Registry was established. The cancer registry conducts long-term follow-up for recurrence and survival for all ‘analytic cases’ (those for which Penn participated in the initial cancer diagnosis or first course of treatment) in addition to capturing cancer stage and other prognostic details. For these patients, survival can be calculated from date of diagnosis to either last follow up or death date. Review of the cancer database for survival and TNM stage created a subgroup of patients for which all variables were available.
Statistical analysis
Linear regression was used to study the relationship between the year of diagnosis and the age of diagnosis first without and then with adjustment for other covariates. Logistic regression was used to compare the relationship between the year of diagnosis and the age of diagnosis in patients under age 50 with those patients diagnosed over age 50. Logistic regression, multinomial regression, and linear regression were performed to study the relationship between the age of diagnosis and grade, age of diagnosis with clinical stage, and age of diagnosis with summary stage. Clinical stage refers to the stage made with information prior to treatment. Pathological staging requires resection of the tumor and regional lymph nodes. Summary stage integrates information from both clinical and pathologic documentation of the extent of disease. For survival analysis, Cox proportional hazards regression was conducted to study the association between age at diagnosis and overall survival.
RESULTS
The initial search for EAC based on the above search terms produced 2,366 pathology reports as demonstrated in Figure 1. Of this cohort, 895 unique patients with biopsy proven EAC were identified with the remainder of the 2,366 reports being duplicate patients, squamous cell carcinoma, or unspecified pathology. Two hundred and sixty-five patients were excluded because of missing data, which created a cohort of 630 patients for whom all variables could be ascertained. Patient demographics are shown in Table 1. The majority (559) of the patients in the cohort of 630 were Caucasian males. Of the 630 patients, 65 patients (10.3%) were <50 years old [median 43 years, range 16–49 years] as shown in Table 2. Over the study time period, there was no decrease in the age of diagnosis with EAC by simple linear regression (R = 0.133, P = 0.05) as demonstrated in Figure 2. Similarly, there was no decrease in the age of diagnosis by multiple linear regression (R = 0.127, P = 0.07) using gender, race, and grade as covariates. There was also no increase in the probability of being diagnosed at less than 50 years of age over time by logistic regression (P = 0.07). Age of diagnosis, analyzed either dichotomously (i.e. <50 years vs. ≥50 years) or as a continuous variable, was not associated with tumor grade (P = 0.17). Among the 179 eligible patients captured in the cancer registry, there was no significant difference in initial clinical or pathological stage between patients diagnosed <50 years and patients diagnosed ≥50 years (P = 0.16, P = 0.21). There was also no association found between age of diagnosis and overall survival with hazard ratio of 0.53, (95% CI 0.166–1.69, P = 0.24) (Table 3).
Fig. 1.
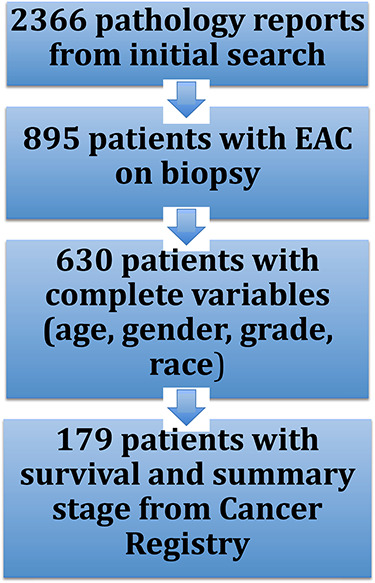
Patient flow.
Table 1.
Patient demographics
| Number of patients (n = 630) | Percentage of total | |
|---|---|---|
| Gender | ||
| Male | 550 | 87.3% |
| Female | 80 | 12.7% |
| Race | ||
| White | 559 | 88.7% |
| African American | 27 | 4.3% |
| Hispanic | 1 | 0.16% |
| Asian | 2 | 0.32% |
| East Indian | 1 | 0.16% |
| American Indian | 16 | 2.5% |
| Other | 24 | 3.8% |
Table 2.
Distribution of patients diagnosed with EAC 1991–2018
| Years | Total number of patients | Number (%) of patients ≥50 | Number (%) of patients <50 | Number (%) of patients <40 | Number (%) of patients <30 | Mean age | Standard deviation |
|---|---|---|---|---|---|---|---|
| 1991–1995 | 20 | 18 (90%) | 2 (10%) | 0 (0%) | 0 (0%) | 58.9 | 12.7 |
| 1996–2000 | 57 | 47 (82.4%) | 10 (17.5%) | 4 (7.0%) | 0 (0%) | 60.3 | 11.8 |
| 2001–2006 | 116 | 104 (89.7%) | 12 (10.3%) | 4 (3.4%) | 0 (0%) | 63.6 | 10.6 |
| 2007–2011 | 140 | 123 (87.8%) | 17 (12.1%) | 3 (2.1%) | 2 (1.4%) | 62.4 | 10.3 |
| 2012–2018 | 291 | 267 (91.7%) | 24 (8.24%) | 7 (2.4%) | 4 (1.3%) | 64.2 | 11.2 |
Percentages are of the patients as part of the total number of patients for each time period.
Fig. 2.
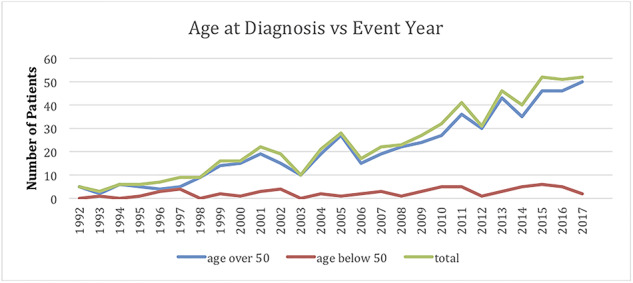
Age at diagnosis versus event year.
Table 3.
Race, grade, and stage for patients diagnosed at age <50 and ≥50 years old
| Number of patients <50 years |
Number of patients ≥50 years |
|
|---|---|---|
| Race | (Total = 65) | (Total = 565) |
| White | 54 | 505 |
| African American | 2 | 25 |
| Hispanic | 0 | 1 |
| Asian | 1 | 1 |
| East Indian | 0 | 1 |
| American Indian | 4 | 12 |
| Other | 4 | 20 |
| Grade | ||
| Well | 2 | 15 |
| Well to moderate | 0 | 10 |
| Moderate | 31 | 227 |
| Moderate to poor | 13 | 122 |
| Poor | 19 | 191 |
| Summary stage | (Total = 15) | (Total = 164) |
| 1 | 2 | 27 |
| 2 | 5 | 56 |
| 3 | 7 | 72 |
| 4 | 1 | 9 |
DISCUSSION
Over the study time period from 1991 to 2018, we found no increase in the number of patients <50 years diagnosed with EAC in our center. Characteristics of those <50 years versus ≥50 years showed no difference with respect to tumor grade. Furthermore, in the subset of 179 patients captured in the cancer registry, there was no significant difference in initial clinical or pathological stage between patients <50 years compared with patients ≥50 years. There was also no association between diagnosis age and survival.
EAC has until recently been considered a disease of middle aged or older obese, Caucasian men who smoke and have chronic gastroesophageal reflux disease. However, just like in colon cancer, emerging data suggest that patients <50 years are being diagnosed with EAC more frequently.7 The increasing rates of colon cancer in younger patients are possibly secondary to increasing rates of obesity, environmental exposures, or microbiome risk factors.8 The changing trends of EAC have been addressed in recent years in larger populations, but none in a single health system. Work from the Cleveland Clinic evaluated their registry of 837 patients with Barrett’s esophagus from 1979 to 2002. They found that 638 (76%) patients were 50 or older. Of the patients with EAC, five patients in the registry (8.5%) were younger than 50 years with an age range of 30–49 years9 Notably, that study was focused on those patients who had Barrett’s esophagus, but not all of the patients within that health system with EAC. In terms of large datasets, El-Serag et al. examined the SEER database from 1977 to 1996 and found that the incidence of EAC continued to rise and noted an increase in the diagnosis of younger patients (defined as ages 45–65).10 More recently, Islami et al. revisited the SEER database and found that the incidence of EAC increased in patients <50 years over time with the largest increase being in non-Hispanic white patients age <39 years from 1997 to 2014. Overall rates had showed an initial increase from 1996 to 2006 but then rates decreased in Hispanics and stabilized in non-Hispanic whites.5 Petrick et al. reviewed the Cerner Health Facts Database from 2001 to 2010 of 35 million patients with esophageal cancer by ICD codes and found that the rate of esophageal cancer more than doubled during the time period but noted no changes in the rates of patients diagnosed <50 years11 However, they were not able to separate out EAC and ESCC cases. Our data also did not find a decrease in the average age of diagnosis of EAC. While SEER is a much larger database that is thought to be representative of the US population, the limitation of SEER is that the ages listed are not necessarily the age the patient was first diagnosed with EAC.
Several studies have looked at stage at presentation and survival times in younger patients with EAC in larger populations. Zeng et al. reviewed SEER for esophageal carcinoma (both squamous and adenocarcinoma) from 2004 to 2013 and found that 8.37% of patients with both ESCC and EAC were <50 years. They found that younger patients with ESCC or EAC were more likely to present with stage III/IV disease but had better survival than patients >50 years12 A review of the Netherlands Cancer Registry similarly found that patients <50 years presented with more advanced stages and that 5 year survival was higher in the younger population (18.2%) compared with 16.4% in the older population.13 A review of the SEER database from 1998 to 2011 looked at 2601 patients who underwent primary tumor resection without pre-operative radiotherapy. They found that patients <45 years were more likely to have lymph node metastasis and had lower survival rate after radical esophagectomy.14 A review of literature in pediatrics from 1950 to 2015 showed 19 cases of EAC in pediatric literature with median age of 16 years with 68.4% of these patients having metastatic disease at presentation.15
Several studies have looked at the stage of presentation in younger EAC patients within single health systems. Recent work from the Mayo Clinic found that 15.4% of 662 patients were less than 50 years from 2009 to 2012. They found higher proportions of patients <50 years old with stage III or IV disease compared with patients 51–70 years of age.6 Other studies have found similar findings at single centers.16–18 Overall, other studies done at single centers have suggested that younger patients present with more advanced cancers, which we did not find in our study.
Several studies have looked at the survival of younger EAC patients within health systems with varying results. In the study at the Mayo Clinic, the mean survival time was lower in the younger patients compared with the middle-aged patients. Notably, they had separated patients >70 years from this comparison given that their comorbidities might decrease their survival time. The University of Southern California in Los Angeles from 1990 to 2013 found that younger patients had shorter median survival time compared with those >40 years.16 However, a retrospective study done in a specialized esophagogastric center in London from 2000 to 2007 did not find a difference in survival time.17 Similarly, work at Jefferson Medical College with data from 1994 to 2004 did not show a difference in survival.19 The data about survival time appear mixed, and this might depend on the varying definitions of younger patients and regional variation. Compared with other work, to date, our study covered a longer time period, introducing more heterogeneity about treatment modalities and changes in treatment paradigms, which might affect survival times.
Although overall rates of EAC have plateaued in recent years, there appears to be a substantial subset of patients under the age of 50. Screening for Barrett’s esophagus with endoscopy is encouraged to detect patients at increased risk for EAC. However, endoscopy is not a cost-effective screening process for the general population given the low prevalence of Barrett’s, and while considerable research into less expensive and invasive screening modalities is ongoing, none are yet ready for routine clinical practice. The diagnosis of EAC is clearly not just a disease of patients >50 years, and further research should focus on creating mechanisms to diagnose these patients earlier and increase awareness of this phenomenon.
There were several limitations to this study. Since the time period studied included the transition from paper records to an electronic health record, there were inconsistencies in uniform documentation. As such, a substantial number of patients from the original cohort were excluded due to incomplete data, with more missing data in the older records.
The number of total cancers in the earlier time periods was lower, so the proportion of cancers during that time had higher confidence intervals. This was also a representation of one urban, tertiary care health system with a relatively small affected population that may not be representative of the larger population and has selection bias given the referral nature of the setting. The size of the cohort could lead to a Type I error when looking at differences among age subgroups over the period of the study. The subgroup of the patients in the Cancer Registry was small in comparison to the rest of the cohort, making the interpretation of survival and stage limited. Strengths of this study include the duration of the cohort from 1991 to 2018, which is much longer than similar studies from tertiary care centers. Furthermore, all of the patients had pathology proven EAC compared with studies where ICD codes or other diagnosis codes were used. The sample size may also not be large enough to have adequate power for detecting the association and trends of interest.
In summary, we found no increase in the percentage of patients <50 years diagnosed with EAC in our setting. Characteristics of those <50 years versus ≥50 years showed no difference with respect to tumor grade, race, and gender. Among the 179 eligible patients captured in the cancer registry, there was no significant difference in initial clinical or pathological stage between patients <50 years compared with patients ≥50 years There was also no association between diagnosis age and survival. However, 10.3% of the cohort was diagnosed at an age less than 50 years. These and other findings suggest that age-based screening recommendations merit additional examination.
ACKNOWLEDGEMENTS
We would like to thank Michael Feldman MD, PhD for his assistance with the TIES pathology database. We would also like to thank Abigail Doucette for her assistance with the Cancer Center Database. We would also like to acknowledge our grant support: P30CA016520, NIH P30-DK050306, and NCI U54-CA163004.
†Specific author contributions: Study planned by Alexandra Strauss, Gary W. Falk. Data collected by Alexandra Strauss, Peter Gabriel. Data interpreted by Alexandra Strauss, Gary W. Falk, Eun Jeong Min, Qi Long, Yu-Xiao Yang. Manuscript prepared by Alexandra Strauss, Gary W. Falk. Manuscript reviewed by Alexandra Strauss, Gary W. Falk, Eun Jeong Min, Qi Long, Yu-Xiao Yang, Peter Gabriel.
ABBREVIATIONS
EAC, esophageal adenocarcinoma; ESCC, esophageal squamous cell carcinoma; TIES, text information extraction system pathology database; UPHS, University of Pennsylvania Health System
References
- 1. Siegel R L, Miller K D, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 1: 7–34. [DOI] [PubMed] [Google Scholar]
- 2. Sami S S, Iyer P G. Recent advances in screening for Barrett's esophagus. Curr Treat Options Gastroenterol 2018; 16: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clermont M, Falk G W. Clinical guidelines update on the diagnosis and management of Barrett's esophagus. Dig Dis Sci 2018; 68: 2122–8. [DOI] [PubMed] [Google Scholar]
- 4. Pohl H, Sirovich B, Welch H G. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomarkers Prev 2010; 19: 1468–70. [DOI] [PubMed] [Google Scholar]
- 5. Islami F, DeSantis C E, Jemal A. Incidence trends of esophageal and gastric cancer subtypes by race, ethnicity, and age in the United States, 1997–2014. Clin Gastroenterol Hepatol 2019; 17: 429–39. [DOI] [PubMed] [Google Scholar]
- 6. Sawas T, Manrique G C, Iyer P G, Wang K K, Katka D A. Young adults with esophageal adenocarcinoma present with more advanced stage tumors and have shorter survival times. Clin Gastroenterol Hepatol 2019; 9: 1756–62. [DOI] [PubMed] [Google Scholar]
- 7. Siegel R L, Miller K D, Jemal A. Colorectal cancer mortality rates in adults aged 20 to 54 years in the United States, 1970–2014. JAMA 2017; 318: 572–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dwyer A J, Muphy C C, Boland C R et al. A summary of the fight colorectal cancer working meeting: exploring risk factors and etiology of sporadic early-age onset colorectal cancer. Gastroenterology 2019; 157: 280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guardino J M, Khandwala F, Lopez R, Wachsberger D M, Eichter J E, Falk G W. Barrett's esophagus at a tertiary care center: association of age on incidence and prevalence of dysplasia and adenocarcinoma. Am J Gastroenterol 2006; 101: 2187–93. [DOI] [PubMed] [Google Scholar]
- 10. El-Serag H, Mason A C, Petersen N, Key C R. Epidemiological differences between adenocarcinoma of the oesophagus and adenocarcinoma of the gastric cardia in the USA. Gut 2002; 50: 368–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petrick J L, Nguyen T, Cook M B. Temporal trends of esophageal disorders by age in the Cerner Health Facts database. Ann Epidemiol 2016; 26: 151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeng Y, Ruan W, Liu J et al. Esophageal cancer in patients under 50: a SEER analysis. J Thorac Dis 2018; 10: 2542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Nistelrooij A M J, van Steenbergen L N, Spaander M C W et al. Treatment and outcome of young patients with esophageal cancer in the Netherlands. J Surg Oncol 2014; 109: 561–6. [DOI] [PubMed] [Google Scholar]
- 14. Yang S, Li H, Jia C, Ma X, Guo W, Li H. Clinicopathological features and prognosis of patients <45 years old with esophageal adenocarcinoma comparing to other age groups. Thorac Dis 2016; 8: 2724–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Theilen T M, Chou A J, Klimstra D S, LaQuaglia M P. Esophageal adenocarcinoma and squamous cell carcinoma in children and adolescents: report of 3 cases and comprehensive literature review. J Pediatr Surg Case Rep 2016; 5: 23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boys J A, Oh D S, Lewis J S, DeMeester S R, Hagen J A. Esophageal adenocarcinoma in patients younger than 40 years: a two-decade experience at a public and private hospital. Am Surg 2015; 81: 974–8. [PubMed] [Google Scholar]
- 17. Hamouda A, Forshaw M, Rohatgi A, Mirnesami R, Botha A, Mason R. Presentation and survival of operable esophageal cancer in patients 55 years of age and below. World J Surg 2010; 34: 744–9. [DOI] [PubMed] [Google Scholar]
- 18. Oezcelik A, Ayazi S, DeMeester S R et al. Adenocarcinoma of the esophagus in the young. J Gastrointest Surg 2013; 17: 1032–5. [DOI] [PubMed] [Google Scholar]
- 19. Hashemi N, Loren D, DiMarino A J, Cohen S. Presentation and prognosis of esophageal adenocarcinoma in patients below age 50. Dig Dis Sci 2009; 54: 1708–12. [DOI] [PubMed] [Google Scholar]


