Abstract
Background
We developed a heterologous COVID-19 vaccine consisting of two components, a recombinant adenovirus type 26 (rAd26) vector and a recombinant adenovirus type 5 (rAd5) vector, both carrying the gene for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike glycoprotein (rAd26-S and rAd5-S). We aimed to assess the safety and immunogenicity of two formulations (frozen and lyophilised) of this vaccine.
Methods
We did two open, non-randomised phase 1/2 studies at two hospitals in Russia. We enrolled healthy adult volunteers (men and women) aged 18–60 years to both studies. In phase 1 of each study, we administered intramuscularly on day 0 either one dose of rAd26-S or one dose of rAd5-S and assessed the safety of the two components for 28 days. In phase 2 of the study, which began no earlier than 5 days after phase 1 vaccination, we administered intramuscularly a prime-boost vaccination, with rAd26-S given on day 0 and rAd5-S on day 21. Primary outcome measures were antigen-specific humoral immunity (SARS-CoV-2-specific antibodies measured by ELISA on days 0, 14, 21, 28, and 42) and safety (number of participants with adverse events monitored throughout the study). Secondary outcome measures were antigen-specific cellular immunity (T-cell responses and interferon-γ concentration) and change in neutralising antibodies (detected with a SARS-CoV-2 neutralisation assay). These trials are registered with ClinicalTrials.gov, NCT04436471 and NCT04437875.
Findings
Between June 18 and Aug 3, 2020, we enrolled 76 participants to the two studies (38 in each study). In each study, nine volunteers received rAd26-S in phase 1, nine received rAd5-S in phase 1, and 20 received rAd26-S and rAd5-S in phase 2. Both vaccine formulations were safe and well tolerated. The most common adverse events were pain at injection site (44 [58%]), hyperthermia (38 [50%]), headache (32 [42%]), asthenia (21 [28%]), and muscle and joint pain (18 [24%]). Most adverse events were mild and no serious adverse events were detected. All participants produced antibodies to SARS-CoV-2 glycoprotein. At day 42, receptor binding domain-specific IgG titres were 14 703 with the frozen formulation and 11 143 with the lyophilised formulation, and neutralising antibodies were 49·25 with the frozen formulation and 45·95 with the lyophilised formulation, with a seroconversion rate of 100%. Cell-mediated responses were detected in all participants at day 28, with median cell proliferation of 2·5% CD4+ and 1·3% CD8+ with the frozen formulation, and a median cell proliferation of 1·3% CD4+ and 1·1% CD8+ with the lyophilised formulation.
Interpretation
The heterologous rAd26 and rAd5 vector-based COVID-19 vaccine has a good safety profile and induced strong humoral and cellular immune responses in participants. Further investigation is needed of the effectiveness of this vaccine for prevention of COVID-19.
Funding
Ministry of Health of the Russian Federation.
Introduction
COVID-19 was first reported in Wuhan, China, at the end of December, 2019.1 The disease is an acute respiratory illness ranging in severity from mild to severe, with death in some cases; many infected people are asymptomatic. Since the end of January, 2020, cases of COVID-19 have been reported in more than 200 countries around the world. On March 11, 2020, WHO described the spread of COVID-19 as a pandemic.2
The causative agent of COVID-19 is the betacoronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 can be transmitted in many ways, with the main route of transmission via contact with infected people (eg, by secretions, particularly droplets).3 As of Aug 15, 2020, there have been more than 21 million laboratory-confirmed cases of SARS-CoV-2 infection, and more than 750 000 deaths.1
Research in context.
Evidence before this study
We searched ClinicalTrials.gov and PubMed up to Aug 13, 2020, with the terms “COVID-19” OR “SARS-CoV-2” AND “vaccine” AND “clinical trial”, with no date or language restrictions, to find information about adenovirus-based COVID-19 vaccine candidates in active clinical trials. According to WHO, on Aug 13, 2020, 29 candidate vaccines based on different platforms (vectored, DNA, mRNA, inactivated, etc) were being tested in clinical trials against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) proteins. Recombinant viral vector-based vaccines are promising candidates for COVID-19 prevention because they induce humoral and cellular immune responses and can provide protective immunity after one or two doses. Several candidate COVID-19 vaccines have been tested in clinical trials, including an adenovirus type 5 (Ad5) vector-based vaccine (CanSino Biological/Beijing Institute of Biotechnology, China), an Ad26 vector-based vaccine (Johnson & Johnson, USA), and a vaccine containing a simian adenoviral vector (AstraZeneca/University of Oxford, UK). Since boosting vaccination is necessary for formation of a more powerful immune response, the effectiveness of such vaccination can be reduced when using a homologous vector (because of formation of an immune response not only to the target antigen but also to the vector components after priming vaccination).
Added value of this study
We designed a COVID-19 vaccine with two different adenoviral vectors (recombinant Ad26 [rAd26] and recombinant Ad5 [rAd5]), both carrying the gene for SARS-CoV-2 spike glycoprotein (rAd26-S and rAd5-S), and we implemented a prime-boost regimen. We did two open, phase 1/2 non-randomised trials of two formulations (frozen and lyophilised) of the vaccine in healthy adult volunteers. Safety of the two individual vaccine components (rAd26-S and rAd5-S) was confirmed in phase 1. Both components were then administered as a prime-boost vaccination in phase 2, with testing for safety and immunogenicity. The vaccine was well tolerated and produced humoral and cellular immune responses in healthy adults. IgG responses were elicited in all participants, with geometric mean titres significantly higher than those reported in people who have recovered from COVID-19. Antibodies to SARS-CoV-2 glycoprotein and neutralising antibodies increased significantly at day 14 and continued to increase throughout the observation period. Specific T-cell responses peaked at day 28 after vaccination.
Implications of all the available evidence
Our findings indicate that a heterologous rAd26 and rAd5 vector-based COVID-19 vaccine is safe and immunogenic in healthy adults. Further investigation is needed of the effectiveness of this vaccine for prevention of COVID-19.
Because of the rapid global spread of SARS-CoV-2 infection and the high mortality rate, development of a vaccine is an urgent task. Vaccination will restrict the spread of COVID-19 and reduce mortality. Intensive research and development of vaccines is currently underway in China, Russia, the UK, the USA, and other countries.4 According to WHO, on Aug 13, 2020, 29 candidate COVID-19 vaccines based on different platforms (vectored, DNA, mRNA, inactivated, etc) were being tested in clinical trials.4
Prevention of SARS-CoV-2 infection might be achieved by targeting the spike protein (glycoprotein S), which interacts with the ACE2 receptor and enables entry of SARS-CoV-2 into the cell. Blocking this interaction decreases viral internalisation and replication.5, 6, 7 Most vaccines that are currently in development target glycoprotein S as the main antigen. The structure and function of the SARS-CoV-2 glycoprotein S is similar to that of other highly pathogenic betacoronaviruses, such as Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus (SARS-CoV).8 Glycoprotein S consists of two subunits: S1 contains a receptor-binding domain (RBD), which interacts with the ACE2 receptor on the cell surface; S2 mediates the fusion of viral and cell membranes via formation of a six-helix bundle fusion core.9, 10 To protect against SARS-CoV-2 infection, it is important to form neutralising antibodies targeting S1 RBD, S1 N-terminal domain, or the S2 region; these antibodies block binding of the RBD to the ACE2 receptor and prevent S2-mediated membrane fusion or entry into the host cell, thus inhibiting viral infection.11, 12
When developing a vaccine (particularly during a pandemic), it is important to consider that a protective response must develop in a short time (eg, up to 1 month). Moreover, previous work on vaccines for MERS-CoV13 and SARS-CoV14 showed that both humoral and cellular (cytotoxic) immune responses are important to induce a protective immune response. To achieve these goals, one of the most attractive options is for vaccines to be based on recombinant viral vectors, which can induce humoral and cellular immune responses and form protective immunity after one or two doses.15, 16 Recombinant adenovirus vectors have been used for a long time, with safety confirmed in many clinical studies of various preventive and therapeutic drugs.17, 18, 19, 20, 21, 22, 23 Moreover, the long-term effects of vectors based on adenoviruses have been investigated,23 by contrast with newer methods that remain to be studied long term. For formation of a robust long-lasting immune response, a prime-boost vaccination is advisable, which is widely used with registered vaccines for diseases including hepatitis B24 and Ebola virus disease.25 When using vector-based vaccines, immune responses are formed not only to the target antigen but also to the vector component. As a result, the best vaccination scheme is heterologous vaccination, when different viral vectors are used to overcome any negative effects of immune response to vector components.25, 26, 27 Such an approach was successfully used with an Ebola virus disease vaccine developed in Russia and licensed in 2015.25
We designed a novel, heterologous adenoviral vector-based vaccine against SARS-CoV-2 suitable for prime-boost vaccination. The vaccine was designed with two recombinant adenovirus vectors and was developed as two formulations (frozen [Gam-COVID-Vac] and lyophilised [Gam-COVID-Vac-Lyo]). We aimed to assess safety and immunogenicity of both vaccine formulations and to compare the humoral immune response with that recorded in people who have recovered from COVID-19.
Methods
Study design and participants
We did two open, phase 1/2 non-randomised studies at hospitals in Russia (Burdenko Hospital and Sechenov University, Moscow, Russia). For each study, 120 healthy adult volunteers (aged 18–60 years) were preselected to be included in the volunteer register; all adults provided signed informed consent to be included in this database for study participation. Volunteers were screened by demographic data, had a physical examination and bodyweight measured, were assessed for vital functions (eg, blood pressure, pulse, and temperature), had a blood test for clinical and biochemical testing, were screened for infections such as HIV, hepatitis, and syphilis, underwent PCR for SARS-CoV-2 and had a test for antibodies to SARS-CoV-2, and had a urine test for drugs, alcohol, and pregnancy (in women). We included adult volunteers of both sexes with a body-mass index of 18·5–30·0 kg/m2, who had a negative PCR and negative IgG and IgM to SARS-CoV-2, and who had no history of COVID-19 or contact with patients with COVID-19. Volunteers had no infectious diseases at the time of vaccination and for 14 days before vaccination, and they did not receive any other vaccinations within 30 days of participation in the study. Based on the results of the preliminary screening, 100 volunteers were selected (50 for each clinical trial) for inclusion in the register of volunteers planning to take part in the study of vaccines against COVID-19. As soon as the volunteers were included in the register they began self-isolation.
All participants provided written informed consent. The two studies were reviewed and approved by appropriate national and local competent authorities, including the regulator (Department of State Regulation for Medicine Distribution, approval nos 241 and 242) and the ethics committee of the Ministry of Health of the Russian Federation.
Procedures
The vaccine comprises two vector components, recombinant adenovirus type 26 (rAd26) and recombinant adenovirus type 5 (rAd5), both of which carry the gene for SARS-CoV-2 full-length glycoprotein S (rAd26-S and rAd5-S). Both components were developed, manufactured, and stored by N F Gamaleya National Research Centre for Epidemiology and Microbiology (Moscow, Russia) according to Good Manufacturing Practices. A full dose of the vaccine was 1011 viral particles per dose for both recombinant adenoviruses and all participants received full doses. The dose was set based on findings of preclinical studies (unpublished data). The vaccine was manufactured as two formulations, frozen (Gam-COVID-Vac) and lyophilised (Gam-COVID-Vac-Lyo). The frozen vaccine has a volume of 0·5 mL (per dose) and the lyophilised vaccine needs to be reconstituted in 1·0 mL of sterile water for injection (per dose).
The study of Gam-COVID-Vac was done at a branch of Burdenko Hospital, an agency of the Ministry of Defence. Both civilian and military volunteers took part in that study. Military personnel were contract employees (who received a salary for their work) and not individuals conscripted for compulsory military service. The study of Gam-COVID-Vac-Lyo took place at Sechenov University and all volunteers in that study were civilians.
In all cases, vaccines were administered intramuscularly into the deltoid muscle. During phase 1 of both studies, participants received one dose intramuscularly of either rAd26-S or rAd5-S and were assessed for safety over 28 days. Phase 2 of both studies began no earlier than 5 days after phase 1 vaccination, after an interim safety assessment had been done. During phase 2, participants received prime-boost vaccination, with one dose of rAd26-S administered intramuscularly on day 0 and one dose of rAd5-S administered intramuscularly on day 21. Injection-site reactions, systemic reactogenicity, and medication use to alleviate such symptoms were monitored for 28 days after the first injection (in phases 1 and 2) and at day 42 (phase 2 only).
No randomisation or special selection was done for phases 1 and 2. Participants were included as soon as informed consent was signed. Participants underwent clinical and laboratory assessments on days 0, 2, and 14 in phase 1 and on days 0, 14, 28, and 42 in phase 2. Laboratory analyses included complete blood and urine counts, alanine aminotransferase, aspartate aminotransferase, protein, bilirubin, total cholesterol, lactate dehydrogenase, alkaline phosphatase, prothrombin index, glucose, urea, and creatinine. Immune status was analysed on days 0 and 28 in phase 1 and on days 0, 28, and 42 in phase 2. Volunteers were in hospital for 28 days from the start of vaccination. Information on adverse events was recorded daily.
Determination of immunogenicity is described in detail in the appendix (pp 1–2). In brief, antigen-specific humoral immune responses were analysed on days 0, 14, 21, and 28 in phase 1 and on days 0, 14, 21, 28, and 42 in phase 2. The titre of glycoprotein-specific antibodies in serum was ascertained by ELISA. To test anti-SARS-CoV-2 IgG, we used an ELISA that was developed at N F Gamaleya National Research Centre for Epidemiology and Microbiology and registered for clinical use in Russia (P3H 2020/10393 2020-05-18). The ELISA measures IgGs specific to the RBD of SARS-CoV-2 glycoprotein S. The titre of neutralising antibodies was measured on days 0, 14, and 28 in phase 1 and on days 0, 14, 28, and 42 in phase 2 and was ascertained by microneutralisation assay using SARS-CoV-2 (hCoV-19/Russia/Moscow_PMVL-1/2020) in a 96-well plate and a 50% tissue culture infective dose (TCID50) of 100. Cell-mediated immune responses were measured on days 0, 14, and 28 after the first injection by determination of antigen-specific proliferating CD4+ and CD8+ cells by flow cytometry and by quantification of interferon-γ release.
To compare post-vaccination immunity with natural immunity that forms during infection with SARS-CoV-2, we obtained convalescent plasma from blood samples of 4817 people from Moscow who had recovered after COVID-19 (between March 29 and Aug 11, 2020). Convalescent plasma was obtained from people who had had a laboratory-confirmed COVID-19 diagnosis, who had been recovered for at least 2 weeks, and who had tested negative by PCR twice. The average time from recovery to convalescent plasma collection was about 1 month. Convalescent plasma was collected from people who had had mild (fever ≤39°C without pneumonia) and moderate (fever >39°C with pneumonia) disease severity. Humoral immune responses were ascertained as mentioned above.
Outcomes
Primary outcome measures were safety and immunogenicity of the COVID-19 vaccine. The primary outcome measure for safety was the number of participants with adverse events from day 0 to day 28 after vaccination in phase 1 and from day 0 to day 42 after vaccination in phase 2. The primary outcome measure for immunogenicity was change from baseline in antigen-specific antibody levels at 42 days (from day 0 to day 42), measured by ELISA. Secondary immunogenicity outcome measures were virus neutralising antibody titres (on days 0, 14, and 28 after vaccination in phase 1 and on days 0, 14, 28, and 42 after vaccination in phase 2) and determination of antigen-specific cellular immunity (specific T-cell immunity and interferon-γ production or lymphoproliferation) on days 0, 14, and 28 after vaccination.
Statistical analysis
The sample size for both studies was calculated from previous clinical trials of a MERS vaccine27 based on the same recombinant viral vectors as used in our vaccine but carrying the MERS-CoV glycoprotein S gene. Preliminary results of a study of a MERS vaccine in which more than 100 people participated showed a seroconversion rate of 100%.27 When calculating the sample size for our study, we expected 99% efficiency, which required inclusion of 16 participants in each study. Considering the possibility of early dropout of volunteers, we decided that 20 volunteers should be recruited into the immunogenicity assessment group in phase 2 of each study. A total sample size of 76 (38 in each study) was expected to produce reliable data on adverse events.
All statistical calculations were done in GraphPad Prism 8. Normality of the data distribution was assessed with the d’Agostino-Pearson test. Paired samples were compared with the Wilcoxon test and unpaired samples with the Mann-Whitney U test. Correlation analysis was done with Spearman's test; the correlation coefficient r shows interactions between two datasets and takes values either from 0 to 1 (in the case of a positive correlation) or from –1 to 0 (in the case of a negative correlation). We used the Mann-Whitney U test to compare at various timepoints antibody titres, the level of proliferating CD4 and CD8 cells, and increases in concentrations of interferon-γ between volunteers receiving the two vaccines, and when comparing antibody titres in volunteers on days 28 and 42 after vaccination with antibody titres in convalescent plasma. We used the Wilcoxon test to compare data within the same group of volunteers at different timepoints (eg, when comparing day 0 to day 14).
These trials are registered with ClinicalTrials.gov, NCT04436471 and NCT04437875.
Role of the funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all data in the studies and had final responsibility for the decision to submit for publication.
Results
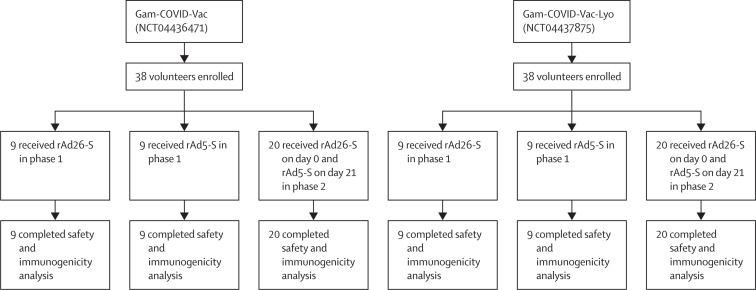
Between June 18 and Aug 3, 2020, 76 healthy adults were enrolled to the two studies from the volunteer register (figure 1 ). 43 adults were selected at the beginning of each study from the volunteer registry; 38 participants were included in each study and five people were kept as backup volunteers in case of dropouts (two for phase 1 and three for phase 2). Nine participants in each study received rAd26-S in phase 1, nine received rAd5-S in phase 1, and 20 received sequential injections of rAd26-S (on day 0) and rAd5-S (on day 21) in phase 2. All volunteers in the main group were analysed and additional volunteers from the backup groups were not needed. Thus, in each study, 38 volunteers were vaccinated. More men than women took part in the study (table 1 ).
Figure 1.
Trial profile
Gam-COVID-Vac=frozen vaccine formulation. Gam-COVID-Vac-Lyo=lyophilised vaccine formulation. rAd26-S=recombinant adenovirus type 26 carrying the gene for SARS-CoV-2 full-length glycoprotein S. rAd5-S=recombinant adenovirus type 5 carrying the gene for SARS-CoV-2 full-length glycoprotein S. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Table 1.
Baseline characteristics
|
Gam-COVID-Vac |
Gam-COVID-Vac-Lyo |
||||||
|---|---|---|---|---|---|---|---|
| rAd26-S (n=9) | rAd5-S (n=9) | rAd26-S plus rAd5-S (n=20) | rAd26-S (n=9) | rAd5-S (n=9) | rAd26-S plus rAd5-S (n=20) | ||
| Sex | |||||||
| Male | 9 (100%) | 9 (100%) | 14 (70%) | 5 (56%) | 2 (22%) | 14 (70%) | |
| Female | 0 | 0 | 6 (30%) | 4 (44%) | 7 (78%) | 6 (30%) | |
| Height, m | 1·8 (0·1) | 1·8 (0·1) | 1·7 (0·1) | 1·7 (0·1) | 1·7 (0·1) | 1·8 (0·1) | |
| Bodyweight, kg | 80·6 (6·0) | 83·4 (13·8) | 74·6 (12·5) | 72·1 (13·1) | 65·8 (9·4) | 72·0 (12·6) | |
| Age, years | 27·8 (5·1) | 25·3 (6·1) | 26·4 (4·4) | 31·4 (8·2) | 27·0 (7·7) | 26·7 (5·8) | |
| Ethnicity | |||||||
| White | 9 (100%) | 9 (100%) | 20 (100%) | 8 (89%) | 9 (100%) | 19 (95%) | |
| Asian | 0 | 0 | 0 | 1 (11%) | 0 | 1 (5%) | |
| SARS-CoV-2 IgM and IgG negative | 9 (100%) | 9 (100%) | 20 (100%) | 9 (100%) | 9 (100%) | 20 (100%) | |
Data are n (%) or mean (SD). Gam-COVID-Vac=frozen vaccine formulation. Gam-COVID-Vac-Lyo=lyophilised vaccine formulation. rAd26-S=recombinant adenovirus type 26 carrying the gene for SARS-CoV-2 full-length glycoprotein S. rAd5-S=recombinant adenovirus type 5 carrying the gene for SARS-CoV-2 full-length glycoprotein S. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
In both studies, systemic and local reactions (table 2 ) and changes in laboratory variables (appendix pp 3–7) were among the adverse events reported. The most common systemic and local reactions were pain at injection site (44 [58%]), hyperthermia (38 [50%]), headache (32 [42%]), asthenia (21 [28%]), and muscle and joint pain (18 [24%]). Most systemic and local reactions were mild. Changes in laboratory variables were mild and transient. In volunteers who received both vaccine components (rAd26-S and rAd5-S), most adverse events occurred after the second vaccination (appendix pp 5–7). No adverse events, either during phase 1 or phase 2, led to withdrawal of a participant from the study or withdrawal of study drug. In general, adverse events identified during phase 1 and phase 2 of both studies were characteristic of other vaccines (particularly those based on recombinant viral vectors). No serious adverse events were reported and all participants were clinically well throughout the study.
Table 2.
Systemic and local adverse events
|
Gam-COVID-Vac |
Gam-COVID-Vac-Lyo |
||||||
|---|---|---|---|---|---|---|---|
| rAd26-S (n=9) | rAd5-S (n=9) | rAd26-S plus rAd5-S (n=20) | rAd26-S (n=9) | rAd5-S (n=9) | rAd26-S plus rAd5-S (n=20) | ||
| Systemic reactions | |||||||
| Hyperthermia | |||||||
| Mild (37·0–38·4°C; grade 1) | 8 (89%) | 2 (22%) | 19 (95%) | 1 (11%) | 1 (11%) | 6 (30%) | |
| Moderate (38·5–38·9°C; grade 2) | 0 | 1 (11%) | 1 (5%) | 0 | 0 | 1 (5%) | |
| Headache | |||||||
| Mild (grade 1) | 6 (67%) | 3 (33%) | 9 (45%) | 3 (33%) | 4 (44%) | 5 (25%) | |
| Moderate (grade 2) | 0 | 0 | 2 (10%) | 0 | 0 | 0 | |
| Asthenia | |||||||
| Mild (grade 1) | 3 (33%) | 3 (33%) | 11 (55%) | 0 | 0 | 4 (20%) | |
| Muscle and joint pain | |||||||
| Mild (grade 1) | 3 (33%) | 2 (22%) | 4 (20%) | 1 (11%) | 2 (22%) | 4 (20%) | |
| Moderate (grade 2) | 0 | 0 | 1 (5%) | 0 | 0 | 2 (10%) | |
| Heartbeat (subjective palpitation) | |||||||
| Mild (grade 1) | 3 (33%) | 1 (11%) | 0 | 0 | 0 | 0 | |
| Diarrhoea | |||||||
| Mild (grade 1) | 1 (11%) | 0 | 3 (15%) | 0 | 0 | 0 | |
| Rhinorrhoea | |||||||
| Mild (grade 1) | 0 | 0 | 4 (20%) | 0 | 0 | 0 | |
| Loss of appetite | |||||||
| Mild (grade 1) | 2 (22%) | 0 | 1 (5%) | 0 | 0 | 0 | |
| Pain in the oropharynx (pharyngalgia) | |||||||
| Mild (grade 1) | 0 | 1 (11%) | 1 (5%) | 0 | 0 | 0 | |
| Malaise | |||||||
| Mild (grade 1) | 0 | 0 | 2 (10%) | 0 | 0 | 0 | |
| Sore throat (throat irritation) | |||||||
| Mild (grade 1) | 0 | 0 | 2 (10%) | 0 | 0 | 0 | |
| Hives | |||||||
| Mild (grade 1) | 1 (11%) | 0 | 0 | 0 | 0 | 0 | |
| Nasal congestion | |||||||
| Mild (grade 1) | 0 | 0 | 1 (5%) | 0 | 0 | 0 | |
| Cough | |||||||
| Mild (grade 1) | 0 | 0 | 1 (5%) | 0 | 0 | 0 | |
| Sneezing | |||||||
| Mild (grade 1) | 0 | 0 | 1 (5%) | 0 | 0 | 0 | |
| Changes in laboratory variables | |||||||
| Mild (grade 1) | 9 (100%) | 8 (89%) | 20 (100%) | 7 (78%) | 6 (67%) | 18 (90%) | |
| Moderate (grade 2) | 0 | 1 (11%) | 0 | 0 | 0 | 0 | |
| Local reactions | |||||||
| Pain | |||||||
| Mild (grade 1) | 7 (78%) | 5 (56%) | 8 (40%) | 5 (56%) | 7 (78%) | 12 (60%) | |
| Oedema | |||||||
| Mild (grade 1) | 0 | 0 | 0 | 2 (22%) | 1 (11%) | 0 | |
| Hyperthermia | |||||||
| Mild (grade 1) | 0 | 0 | 2 (10%) | 0 | 1 (11%) | 0 | |
| Itch | |||||||
| Mild (grade 1) | 1 (11%) | 0 | 0 | 0 | 0 | 0 | |
| Swelling | |||||||
| Mild (grade 1) | 0 | 0 | 1 (5%) | 0 | 0 | 0 | |
The table shows the total number (%) of volunteers who developed adverse events, according to severity (mild [grade 1], moderate [grade 2], and severe [grade 3]). No grade 3 adverse events were reported. Some volunteers had adverse events of two degrees of severity. Gam-COVID-Vac=frozen vaccine formulation. Gam-COVID-Vac-Lyo=lyophilised vaccine formulation. rAd26-S=recombinant adenovirus type 26 carrying the gene for SARS-CoV-2 full-length glycoprotein S. rAd5-S=recombinant adenovirus type 5 carrying the gene for SARS-CoV-2 full-length glycoprotein S. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
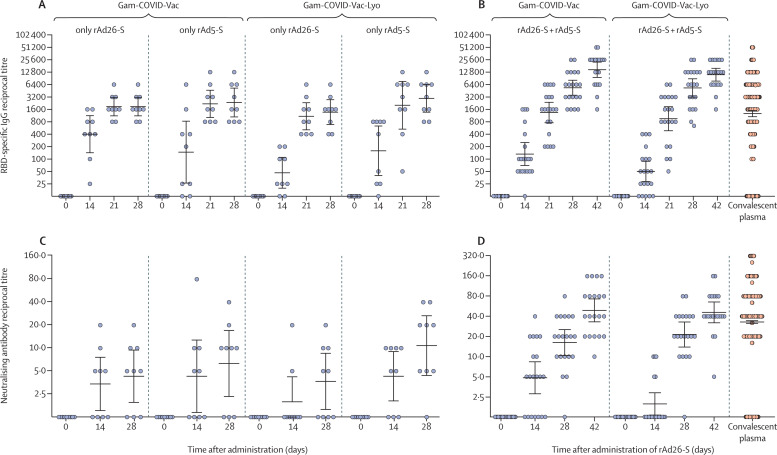
During phase 1 of both studies (administration of either rAd26-S or rAd5-S alone), SARS-CoV-2 RBD-specific IgGs were detected on day 14 in 88·9% of participants after administration of rAd26-S and in 84·2% of participants after administration of rAd5-S (combined data for both the lyophilised and frozen vaccine formulations); beginning from day 21, SARS-CoV-2 RBD-specific IgGs were detected in 100% of vaccinated participants. During phase 2, SARS-CoV-2 RBD-specific IgGs were detected in 85·0% of participants on day 14 (after priming with rAd26-S) and in 100% of participants from day 21 (geometric mean titre [GMT] 1345 with the frozen formulation [Gam-COVID-Vac] and 951 with the lyophilised formulation [Gam-COVID-Vac-Lyo]; figure 2 ). Boosting with rAd5-S led to an increase in SARS-CoV-2 RBD-specific IgG titres; 7 days after boost, GMTs had increased to 5382 with Gam-COVID-Vac (p<0·0001 at day 28 vs day 21) and 5322 with Gam-COVID-Vac-Lyo (p<0·0001 at day 28 vs day 21). On day 42, GMTs of SARS-CoV-2 RBD-specific IgGs were 14 703 with Gam-COVID-Vac and 11 143 with Gam-COVID-Vac-Lyo (figure 2). On day 28 after vaccination with rAd26-S only (in phase 1), SARS-CoV-2 RBD-specific GMTs were significantly lower than in volunteers who had prime-boost vaccination (in phase 2): 1866 after rAd26-S of Gam-COVID-Vac (p=0·0047) and 1372 after rAd26-S of Gam-COVID-Vac-Lyo (p=0·0042). SARS-CoV-2 S1 subunit-specific IgGs were also assessed on days 0 and 42 in volunteers who received combined rAd26-S and rAd5-S (in phase 2). GMTs were 53 006 with Gam-COVID-Vac and 51 200 with Gam-COVID-Vac-Lyo (p=0·78; appendix p 12). Analysis of neutralising antibodies to SARS-CoV-2 showed that only administration of both rAd26-S and rAd5-S led to production of neutralising antibodies in 100% of participants (GMT 49·25 with Gam-COVID-Vac and 45·95 with Gam-COVID-Vac-Lyo at day 42), whereas administration of only rAd26-S led to a seroconversion rate of 61·1% (combined data for both the lyophilised and frozen vaccine formulations). Comparing data for antibody responses to SARS-CoV-2 at days 28 and 42 with data for antibody responses in convalescent plasma showed that post-vaccination ELISA titres were significantly higher than were titres after COVID-19 (for both days 28 and 42, p<0·0001), whereas significant differences in neutralising antibodies were not seen (p=0·55; figure 2). We also analysed the correlation between SARS-CoV-2 RBD ELISA titres and neutralising antibody titres and noted a strong correlation between these variables (r=0·82, 95% CI 0·77–0·86; p<0·0001; appendix p 13).
Figure 2.
Humoral immune response
Data are geometric mean titres and 95% CIs. (A) RBD-specific antibodies on days 0, 14, 21, and 28, as measured by ELISA, in participants vaccinated with rAd26-S or rAd5-S only. (B) RBD-specific antibodies on days 0, 14, 21, 28, and 42, as measured by ELISA, in participants vaccinated with rAd26-S on day 0 and rAd5-S on day 21. (C) Neutralising antibodies on days 0, 14, and 28, as measured by neutralisation assay with 100 TCID50, in participants vaccinated with rAd26-S or rAd5-S only. (D) Neutralising antibodies on days 0, 14, 28, and 42, as measured by microneutralisation assay with 100 TCID50, in participants vaccinated with rAd26-S on day 0 and rAd5-S on day 21. RBD-specific IgGs and neutralising antibodies of in convalescent plasma are also shown in (B) and (D). Gam-COVID-Vac=frozen vaccine formulation. Gam-COVID-Vac-Lyo=lyophilised vaccine formulation. rAd26-S=recombinant adenovirus type 26 carrying the gene for SARS-CoV-2 full-length glycoprotein S. rAd5-S=recombinant adenovirus type 5 carrying the gene for SARS-CoV-2 full-length glycoprotein S. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. RBD=receptor-binding domain. TCID50=50% tissue culture infective dose.
When analysing antigen-specific IgGs, the seroconversion rate was 100% for both vaccine formulations on days 28 and 42 of the study, and when analysing neutralising antibody responses, seroconversion was 100% on day 42 of the study for both vaccine formulations. Seroconversion rates on days 0, 14, 28, and 42 (in phase 2) are presented in the appendix (pp 8–11). Descriptive statistics for humoral immune responses are presented in the appendix (pp 8–11).
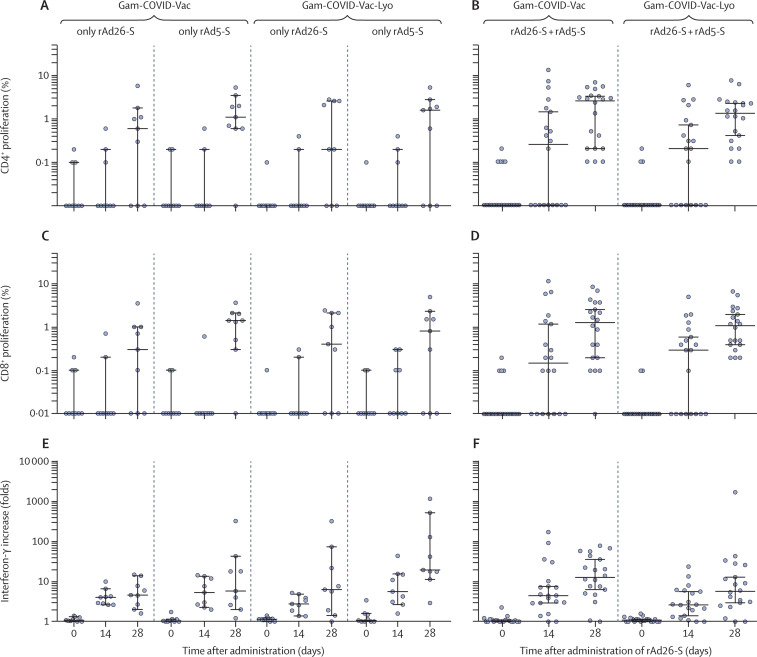
Cellular immune responses showed formation of antigen-specific cells of both T-helper (CD4+) and T-killer (CD8+) cells, and an increase in the concentration of interferon-γ secretion in peripheral blood mononuclear cells, in 100% of volunteers (figure 3 ). Cells from vaccinated participants proliferated significantly in response to glycoprotein S, particularly on day 28. The number of participants with CD4+ and CD8+ proliferative responses to antigen are shown in the appendix (p 14). Cell-mediated responses were detected in all participants at day 28, with median cell proliferation of 2·5% CD4+ and 1·3% CD8+ with the frozen formulation (Gam-COVID-Vac), and a median cell proliferation of 1·3% CD4+ and 1·1% CD8+ with the lyophilised formulation (Gam-COVID-Vac-Lyo). The mononuclear cell response was evaluated on days 0, 14, and 28 by interferon-γ secretion and reported as fold increase in secretion on exposure to glycoprotein S of SARS-CoV-2 (figure 3). The number of participants with interferon-γ response to antigen are shown in the appendix (p 15). Descriptive statistics for cellular immune responses are presented in the appendix (pp 16–21).
Figure 3.
Cell-mediated immune response to SARS-CoV-2 glycoprotein
Data are median and 95% CI. Antigen-specific proliferation of CD4+ and CD8+ T cells and increase in interferon-γ secretion in peripheral blood mononuclear cells in participants vaccinated with rAd26-S or rAd5-S only (A, C, E) and in participants vaccinated with rAd26-S on day 0 and rAd5-S on day 21 (B, D, F). Gam-COVID-Vac=frozen vaccine formulation. Gam-COVID-Vac-Lyo=lyophilised vaccine formulation. rAd26-S=recombinant adenovirus type 26 carrying the gene for SARS-CoV-2 full-length glycoprotein S. rAd5-S=recombinant adenovirus type 5 carrying the gene for SARS-CoV-2 full-length glycoprotein S. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
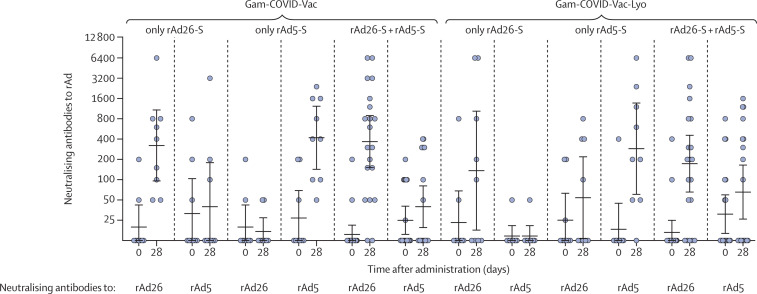
To investigate the effect of the pre-existing immune response to adenoviral vectors, neutralising antibodies to recombinant vectors were measured in all participants on days 0 and 28 in both studies (figure 4 ). After one injection of vaccine components, not only is an immune response to target antigen formed but also an immune response is seen to components of the vaccine vector. Further, a correlation analysis was done to compare the level of neutralising antibodies to recombinant vectors with the level of antigen-specific antibodies (appendix p 22). No significant correlation was noted between the titre of neutralising antibodies to recombinant viral vectors on day 0 and the titre of RBD-specific IgGs in serum samples of participants on days 14, 21, and 28 from the start of vaccination in participants in phase 1 of each study and on days 14, 21, 28, and 42 from the start of vaccination in participants in phase 2 of each study. Moreover, formation of cross-reactive neutralising antibodies to vectors rAd26 and rAd5 was analysed. Administration of rAd26 did not increase the titre of neutralising antibodies to rAd5 on day 28, and vice versa, which indicates the absence of cross-reactivity with respect to vaccine components (figure 4). Thus, the presence of a pre-existing immune response to the components of vaccine vectors rAd26 and rAd5 does not affect the titre of RBD-specific antibodies in the serum of participants.
Figure 4.
Neutralising antibody response to rAd26 and rAd5 vectors after immunisation
The ordinate axis designates reciprocal neutralising antibody titres to recombinant adenoviral vectors. rAd=recombinant adenovirus. Gam-COVID-Vac=frozen vaccine formulation. Gam-COVID-Vac-Lyo=lyophilised vaccine formulation. rAd26-S=recombinant adenovirus type 26 carrying the gene for SARS-CoV-2 full-length glycoprotein S. rAd5-S=recombinant adenovirus type 5 carrying the gene for SARS-CoV-2 full-length glycoprotein S. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Discussion
These findings of two open, phase 1/2 non-randomised studies of a heterologous prime-boost COVID-19 vaccine based on recombinant adenoviral vectors rAd26-S and rAd5-S show that the vaccine is safe, well tolerated, and induces strong humoral and cellular immune responses in 100% of healthy participants. All reported adverse events were mostly mild. The most common systemic and local reactions were pain at the injection site, hyperthermia (body temperature 37–38°C), headache, asthenia, and muscle and joint pain, which are typical for vaccines based on recombinant viral vectors. No serious adverse events were reported during the study. In general, the adverse event profile did not differ from those reported in published work for other vector-based vaccines.25, 28, 29, 30 The incidence of adverse events in our studies was slightly lower than in other work; a comparative clinical study with other vaccines is needed to confirm these findings.
In preclinical studies of the vaccine (unpublished data), robust humoral and cellular immune responses were elicited in non-human primates, providing protection from SARS-CoV-2 infection. The vaccine showed 100% protectivity in a lethal model of SARS-CoV-2 challenge in immunosuppressed hamsters. No antibody-dependent enhancement of infection was seen in vaccinated and SARS-CoV-2-challenged animals.
In general, titres of neutralising antibodies to SARS-CoV-2 were lower than those reported in studies of vaccines based on mRNA and ChAdOx1.28, 31 In our study, we used a high dose of virus (100 TCID50) and a small amount of serum (50 μL serum and 50 μL of virus), whereas in studies of other vaccines, doses of 58–70 TCID50 and a larger amount of serum were used for analyses.28, 31 Despite the fact that research results cannot be compared with each other in this case, we can make a comparison between titres of neutralising antibodies in vaccinated volunteers and in convalescent plasma. We showed that volunteers who received the heterologous rAd26 and rAd5 vaccine elicited the same titre of SARS-CoV-2 neutralising antibodies as did people who had recovered from COVID-19.
According to our study protocols (NCT04436471 and NCT04437875), the T-cell response in healthy adult volunteers after vaccination was to be assessed using two methods. First, by measuring percentages of proliferating CD4 and CD8 T (CD3+) cells in response to antigenic re-stimulation in culture. Second, by measuring interferon-γ in culture medium produced by peripheral blood mononuclear cells. Interferon-γ is a marker cytokine of T-helper-1 biased cellular response towards vaccination,32 and high rates of antigen-specific CD8+ T cells generally correspond to potentiation of T-helper-1 polarisation.33 We understand that results obtained from both assays could indirectly characterise the T-helper-1 response. In the phase 3 clinical trial, we will supplement our research methods with more focus on T-helper-1 and T-helper-2 polarisation.
The main issue that can limit use of vectors based on recombinant adenoviruses is widespread pre-existing immunity in the human population. After vaccination with an adenoviral vector, immune responses form not only to the target antigen but also to the vector proteins (particularly in case of pre-existing immunity). In our study, despite formation of neutralising antibodies to recombinant adenoviruses after vaccination with rAd26 and rAd5, formation of a humoral immune response to target antigen (SARS-CoV-2 glycoprotein S) in vaccinated volunteers was not affected. Moreover, neutralising antibodies to rAd26 did not neutralise rAd5 when serum samples from vaccinated volunteers were obtained and analysed 28 days after immunisation (and vice versa). Thus, use of a heterologous prime-boost immunisation, when rAd26-S is used for priming and rAd5-S is used for boosting, is an effective approach to elicit a robust immune response and to overcome the immune response that is formed to the components of a viral vector. For more accurate estimation of the effect of pre-existing immunity on vaccination, the number of observations should be increased and analysed during future research.
Limitations of our studies include the short duration of follow-up (42 days), inclusion of only male volunteers in some parts of phase 1, the low number of participants (n=76), and no placebo or control vaccine. Despite planning to recruit healthy volunteers aged 18–60 years, in general, our study included fairly young volunteers. Further research is needed to evaluate the vaccine in different populations, including older age groups, individuals with underlying medical conditions, and people in at-risk groups. Participants in these phase 1/2 trials will be followed up to 180 days after initial immunisation.
We designed the vaccine in two formulations, frozen (storage at –18°C) and lyophilised (storage at 2–8°C). The lyophilised form was developed for vaccine delivery to hard-to-reach regions of Russia, and the frozen form was developed for large-scale use. Production volumes in a pandemic will be strongly biased towards the frozen vaccine, since production of a lyophilised form takes much more time and resources.
In conclusion, these data collectively show that the heterologous vaccine based on rAd26-S and rAd5-S is safe, well tolerated, and does not cause serious adverse events in healthy adult volunteers. The vaccine is highly immunogenic and induces strong humoral and cellular immune responses in 100% of healthy adult volunteers, with antibody titres in vaccinated participants higher than those in convalescent plasma. Unprecedented measures have been taken to develop a COVID-19 vaccine in Russia. Based on our own experience in developing vaccines against Ebola virus disease and MERS, the COVID-19 vaccine has been developed in a short time. Preclinical and clinical studies have been done, which has made it possible to provisionally approve the vaccine under the current Decree of the Government of the Russian Federation of April 3, 2020, no 441 on Aug 11, 2020 (registration no LP-006395 [Gam-COVID-Vac]) and on Aug 26, 2020 (registration no LP-006423 [Gam-COVID-Vac-Lyo]). Provisional licensure requires a large-scale study, allows vaccination in a consented general population in the context of a phase 3 trial, allows the vaccine to be brought into use in a population under strict pharmacovigilance, and to provide vaccination of risk groups. The phase 3 clinical trial was approved by the appropriate national and local competent authorities, including the regulator (Department of State Regulation for Medicine Distribution) and the ethics committee of the Ministry of Health of the Russian Federation, on Aug 26, 2020 (approval 450). The phase 3 clinical trial is planned with involvement of 40 000 volunteers from different age and risk groups. The phase 3 clinical trial will be undertaken with constant monitoring of the condition of volunteers through an online application, and each dose of vaccine will have its own QR code, which will be assigned to the volunteer.
This online publication has been corrected. The corrected version first appeared at thelancet.com on January 7, 2020
Data sharing
Individual participant data will be made available on request, directed to the corresponding author (DYL). After approval of a proposal, data can be shared through a secure online platform.
Acknowledgments
Acknowledgments
This research was supported by the Ministry of Health of the Russian Federation (state assignment no 056-00034-20-02, to ALG and DYL). We thank the study participants, site research staff, and members of the trial management groups, trial steering committee, and independent data monitoring committee.
Contributors
DYL is the principal investigator, did research, and coordinated the study. IVD and DVS wrote the draft report. IVD, NLL, YVS, and EAT coordinated the study. IVD, OVZ, AIT, ASD, DMG, ASE, AVK, AGB, FMI, OP, TAO, IBE, IAF, DIZ, DVV, DNS, and ASS collected data. IVD, OVZ, AIT, YVS, EAT, NLL, DAE, NAN, and MMS contributed to data analysis and data interpretation. DYL, DVS, BSN, and ALG edited the report. LFM, EAS, EVK, VFB, and SVB did research. ALG organised the research and had final responsibility for the decision to submit for publication. All authors critically reviewed the report and approved the final version.
Declaration of interests
ALG and DYL report funding from the Ministry of Health of the Russian Federation. OVZ, TAO, IVD, OP, DVS, DMG, ASD, AIT, DNS, IBE, EAT, AGB, ASE, ASS, SVB, DYL, BSN, and ALG report patent pending (patent for the use of vector constructs for the induction of immunity to SARS-CoV-2). All other authors declare no competing interests.
Supplementary Material
References
- 1.WHO Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2.WHO WHO Director-General's opening remarks at the media briefing on COVID-19. March 11, 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020
- 3.WHO Transmission of SARS-CoV-2: implications for infection prevention precautions: scientific brief. July 9, 2020. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions
- 4.WHO Draft landscape of COVID-19 candidate vaccines. Aug 28, 2020. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 5.Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020;291:135–145. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H, Penninger JM, Li Y. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datta PK, Liu F, Fischer T, Rappaport J, Qin X. SARS-CoV-2 pandemic and research gaps: understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics. 2020;10:7448–7464. doi: 10.7150/thno.48076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Astuti I, Ysrafil Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metab Syndr. 2020;14:407–412. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia S, Liu M, Wang C. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Othman H, Bouslama Z, Brandenburg JT. Interaction of the spike protein RBD from SARS-CoV-2 with ACE2: similarity with SARS-CoV, hot-spot analysis and effect of the receptor polymorphism. Biochem Biophys Res Commun. 2020;527:702–708. doi: 10.1016/j.bbrc.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Mai J, Zhou W. Immunoinformatic analysis of T- and B-cell epitopes for SARS-CoV-2 vaccine design. Vaccines. 2020;8:355. doi: 10.3390/vaccines8030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mubarak A, Alturaiki W, Hemida MG. Middle East respiratory syndrome coronavirus (MERS-CoV): infection, immunological response, and vaccine development. J Immunol Res. 2019 doi: 10.1155/2019/6491738. published online April 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, Gao X. Immunological responses against SARS-coronavirus infection in humans. Cell Mol Immunol. 2004;1:119–122. [PubMed] [Google Scholar]
- 15.Rauch S, Jasny E, Schmidt KE, Petsch B. New vaccine technologies to combat outbreak situations. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolzhikova IV, Tokarskaya EA, Dzharullaeva AS. Virus-vectored Ebola vaccines. Acta Naturae. 2017;9:4–11. [PMC free article] [PubMed] [Google Scholar]
- 17.Wold WS, Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr Gene Ther. 2013;13:421–433. doi: 10.2174/1566523213666131125095046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volpers C, Kochanek S. Adenoviral vectors for gene transfer and therapy. J Gene Med. 2004;6:S164–S171. doi: 10.1002/jgm.496. [DOI] [PubMed] [Google Scholar]
- 19.Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheridan C. Gene therapy finds its niche. Nat Biotechnol. 2011;29:121–128. doi: 10.1038/nbt.1769. [DOI] [PubMed] [Google Scholar]
- 21.Afkhami S, Yao Y, Xing Z. Methods and clinical development of adenovirus-vectored vaccines against mucosal pathogens. Mol Ther Methods Clin Dev. 2016;3 doi: 10.1038/mtm.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Zhou D. Adenoviral vector-based strategies against infectious disease and cancer. Hum Vaccin Immunother. 2016;12:2064–2074. doi: 10.1080/21645515.2016.1165908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang WW, Li L, Li D. The first approved gene therapy product for cancer Ad-p53 (Gendicine): 12 years in the clinic. Hum Gene Ther. 2018;29:160–179. doi: 10.1089/hum.2017.218. [DOI] [PubMed] [Google Scholar]
- 24.US Centers for Disease Control and Prevention Hepatitis B VIS. Aug 15, 2020. https://www.cdc.gov/vaccines/hcp/vis/vis-statements/hep-b.html
- 25.Dolzhikova IV, Zubkova OV, Tukhvatulin AI. Safety and immunogenicity of GamEvac-Combi, a heterologous VSV- and Ad5-vectored Ebola vaccine: an open phase I/II trial in healthy adults in Russia. Hum Vaccin Immunother. 2017;13:613–620. doi: 10.1080/21645515.2016.1238535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu S. Heterologous prime-boost vaccination. Curr Opin Immunol. 2009;21:346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovyrshina AV, Dolzhikova IV, Grousova DM. A heterologous vectored vaccine for prevention of Middle East respiratory syndrome induces long protective immune response against MERS-CoV. Immunologia. 2020;41:135–143. (in Russian). [Google Scholar]
- 28.Jackson LA, Anderson EJ, Rouphael NG. An mRNA vaccine against SARS-CoV-2: preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2022483. published online July 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu FC, Guan XH, Li YH. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu F-C, Li Y-H, Guan X-H. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folegatti PM, Ewer KJ, Aley PK. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans TG, Fitzgerald T, Gibbons DC, Keefer MC, Soucier H. Th1/Th2 cytokine responses following HIV-1 immunization in seronegative volunteers. Clin Exp Immunol. 1998;111:243–250. doi: 10.1046/j.1365-2249.1998.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahlers JD, Belyakov IM. Memories that last forever: strategies for optimizing vaccine T-cell memory. Blood. 2010;115:1678–1689. doi: 10.1182/blood-2009-06-227546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data will be made available on request, directed to the corresponding author (DYL). After approval of a proposal, data can be shared through a secure online platform.