Abstract
Double-expressor diffuse large B-cell lymphoma (DLBCL) with 17p deletion is an aggressive and refractory disease. Immune checkpoint blockade and epigenetic drugs have been widely used, but the efficacy of different combined applications varied. We report a case with “double-expressor” DLBCL treated with a combined regimen which consisted of programmed cell death protein 1 (PD-1) inhibitor, DNA methyltransferase inhibitor (DNMTi), and histone deacetylase inhibitor (HDACi). A 50-year-old man presented with a 6-month history of hoarseness, and 10 days of progressive shortness of breath was diagnosed of DLBCL, stage IV. The patient failed to respond to the 1st line (R-EPOCH: rituximab, etoposide, vincristine, cyclophosphamide, doxorubicin, and dexamethasone), 2nd line (R-EPOCH + lenalidomide + ibrutinib), and a 3rd line chemotherapy combined with PD-1 inhibitor (sintilimab), decitabine, and GDP (gemcitabine, DDP, and dexamethasone). Surprisingly, patient's condition was improved after treatment with PD-1 inhibitor in combination with DNMTi/HDACi. Restaging PET revealed dramatically radiological response.
1. Background
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma (NHL) [1, 2]. Approximately 60% of DLBCL patients achieve complete remission (CR) with standard R‐CHOP (rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisolone) chemotherapy [3]. However, 30–40% are refractory to therapy or later relapse (R/R cases), especially those with 17p deletion and/or TP53 mutation and high-grade B-cell lymphoma with MYC and BCL2 or BCL6 translocation [2, 4, 5]. Programmed cell death protein 1 (PD-1) is a key immune checkpoint receptor that is frequently expressed on tumor-infiltrating T cells in B-cell lymphomas [6]. In recent years, although therapeutic blockade of PD-1/PD-L1 has shown potential clinical activity in several types of NHL, the efficiency in DLBCL was not as satisfactory as in Classical Hodgkin lymphoma (cHL) [2, 4, 7–10]. The diversity of clinical responses to monotherapy with PD-1/PD-L1 inhibitors is in part explained by genetic heterogeneity and the diversity of signal pathways involved in the development of B-NHL [8, 11]. Therefore, a combinational regimen of PD-1/PD-L1 inhibitors with other synergistic drugs is needed. Here, we report a case in which PD-1 inhibitor in combination with DNA methyltransferase inhibitor (DNMTi) and histone deacetylase inhibitor (HDACi) was administered to a patient diagnosed of refractory “double-expressor” DLBCL with 17p deletion.
2. Case Presentation
A 50-year-old man presented with a 6-month history of hoarseness and 10 days of progressive shortness of breath. Fiberoptic bronchoscopy revealed intratracheal mass in right main bronchi with complete right mainstem bronchus occlusion (Figure 1(a)). Transbronchial biopsy showed a diffuse proliferation of atypical medium- to large-sized lymphoid cells which were positive for CD20, BCL6 (70%), BCL2 (95%), MUM1 (partial), CD79a, PD-L1 (22C3, 25%), PD-L1 (28–8, 25%), TP53, and c-Myc (40%). They are negative for CD3, CD5, and CD10. The Ki67 proliferative fraction is 80%+ (Figure 2). Fluorescence in situ hybridization (FISH) results demonstrated the presence of 17p deletion and BCL-2 rearrangement, while c-Myc translocation was negative. Initial fluorodeoxyglucose positron emission tomography (FDG-PET) revealed left hilum and mediastinal mass (41 × 87 × 76 mm, FDG uptake 20.6) with compression of the pulmonary artery, lower tracheal segment, bilateral main bronchi, and superior vena cava (Figure 1(b)). Vocal cords were involved, and there was a small pericardial effusion. Bone marrow and cerebrospinal fluid are negative for lymphoma. Gene mutation by “Next-generation” sequencing showed STAT6, EZH2, TP53, KMT2D, BCL6, and CREBBP mutation. These findings were consistent with DLBCL, stage IV. The patient was started urgently on dose-adjusted R-EPOCH therapy (rituximab, etoposide, vincristine, cyclophosphamide, doxorubicin, and dexamethasone) since DLBCL patients with poor prognosis treated with R-CHOP usually had shorter remission according to our clinical observations. It is also reported that DA-EPOCH-R produced durable remission in patients with aggressive B-cell lymphomas [12, 13], so we did not risk choosing R-CHOP. A chest computed tomography (CT) scan showed no sign of improvement. In a phase II study from MD Anderson Cancer Center of using rituximab, lenalidomide, and ibrutinib lead in prior to combination with chemotherapy for patients with newly diagnosed DLBCL, the CR rate was 96%. The regimen also has promising activity in R/R Non-Germinal Center B-cell-like DLBCL [14]. Therefore, R2-CHOP-I therapy (rituximab, lenalidomide, ibrutinib, vincristine, cyclophosphamide, doxorubicin, and dexamethasone) was administered as 2nd line. However, the patient developed respiratory distress soon, and a tracheal stent was implanted into the completely occluded bronchus (Figure 1(c)). A repeat PET showed progressive disease. In view of the patient's clinical status with rapidly progressing respiratory distress, a combination regimen with PD-1 inhibitor (Sintilimab, 10 ml: 100 mg), DNMTi (Decitabine, 10 mg), and GDP (gemcitabine, DDP and dexamethasone) was given as 3rd line. Follow-up CT scan showed only a slight improvement (much less than 50%) of bronchus occlusion without the shrinkage of the hilum and mediastinal mass.
Figure 1.
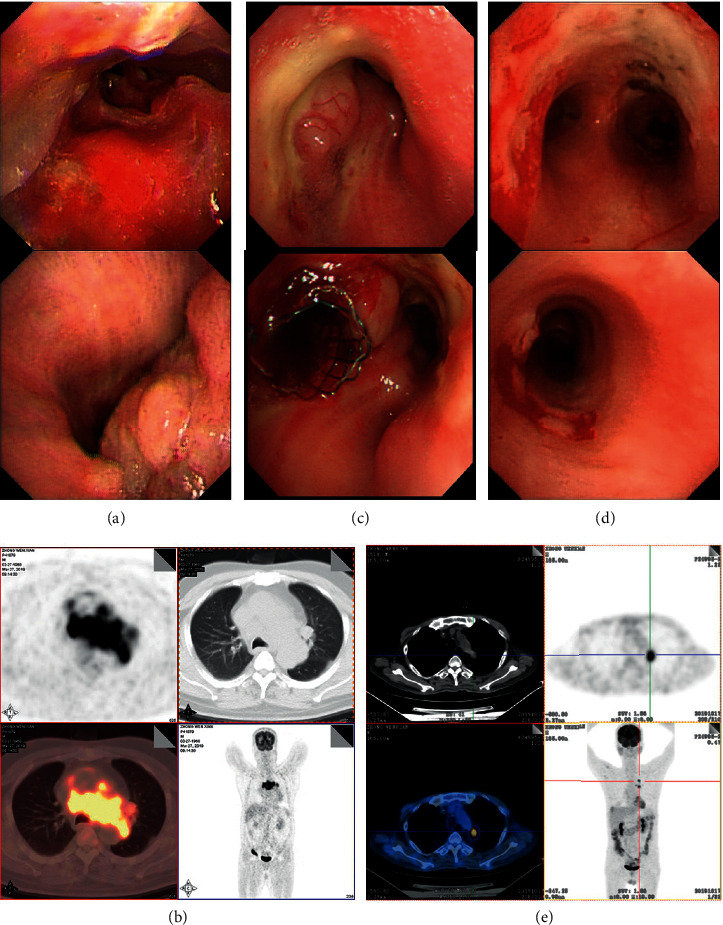
Fiberoptic bronchoscopy and PET/CT images. (a) Fiberoptic bronchoscopy revealed intratracheal mass in right main bronchi with complete right mainstem bronchus occlusion before treatment. (b) PET/CT image showing left hilum and mediastinal mass (41 × 87 × 76 mm, FDG uptake 20.6) with compression of the pulmonary artery, lower tracheal segment, bilateral main bronchi, and superior vena cava. (c) Right mainstem bronchus was completely occluded, and a tracheal stent was implanted. (d) Bronchus occlusion was significantly improved after treatment with PD-1 inhibitor and DNMTi/HDACi. (e) Restaging PET image showing dramatically shrinkage of hilum and mediastinal mass (16 × 15 mm, FDG uptake 8.0) after a triple combination treatment of DNMTi/HDACi plus PD-1 inhibitor.
Figure 2.
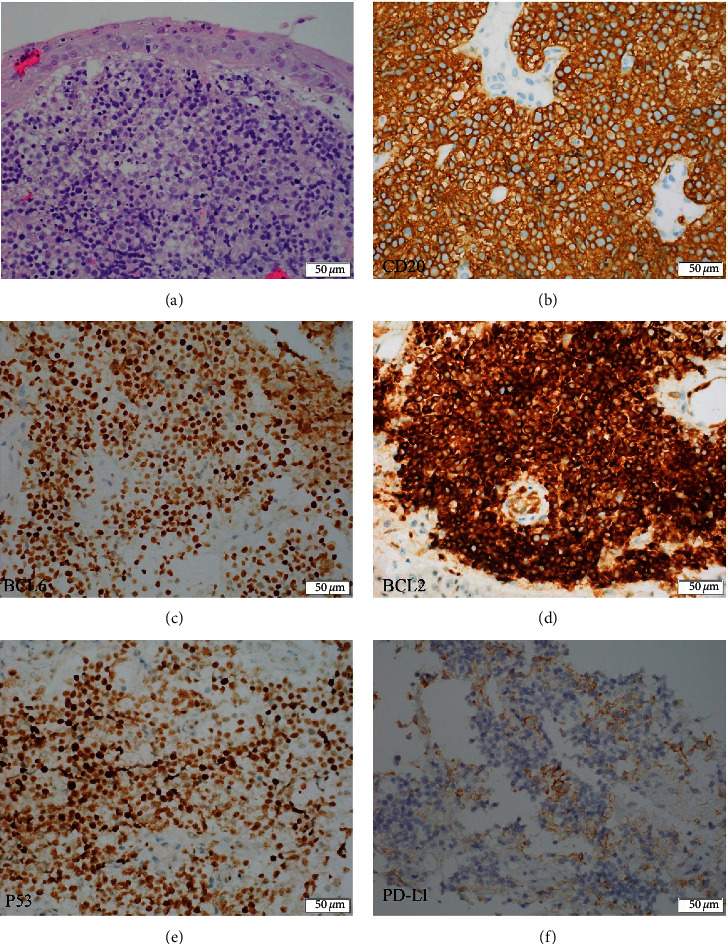
Immunohistochemical characteristics of tumor cells. (a) Transbronchial biopsy of the mediastinal mass (H&E staining) showing a diffuse proliferation of atypical medium- to large-sized lymphoid cells. (b) CD20-positive neoplastic cells from mediastinal biopsy aspirate. (c) BCL6-positive neoplastic cells (70%) from mediastinal biopsy aspirate. (d) BCL2-positive neoplastic cells (95%) from mediastinal biopsy aspirate. (e) P53-positive neoplastic cells from mediastinal biopsy aspirate. (f) PD-L1-positive neoplastic cells from mediastinal biopsy aspirate.
For 4th line, a triple combination of decitabine, sintilimab plus HDACi (Chidamide, 5 mg) were administered (decitabine 10 mg i. v. d1-5, Sintilimab 200 mg, i. v., d1, Chidamide 20 mg p. o. twice a week). Chidamide, which inhibits class I HDACs 1, 2, 3, as well as class II HDAC 10, is the first oral subtype-selective histone deacetylase inhibitor approved in China. It was rapidly absorbed after oral administration and exhibited an elimination half-life in plasma of 17–18 hrs. The recommended dose for lymphomas was 10 mg twice per week for 4 consecutive weeks in a 6-week cycle [15, 16]. Right after the 4th line treatment, a significant clinical improvement was noted. Bilateral bronchus was clear, and restaging PET 2 weeks later revealed over 90% shrinkage of hilum and mediastinal mass (16 × 15 mm, FDG uptake 8.0, PET-CT score 5) and significant improvement of bronchus occlusion (Figures 1(d) and 1(e)). The patient achieved partial remission (PR), and a total of 3 cycles of this combination therapy were given. The patient had only myelosuppression presented with absolute neutrophil count <1.5 × 109/L and platelets <80 × 109/L which lasted for less than a week. The PR duration was about 40 days until a repeat PET-CT showed PD (41 × 33 mm, FDG uptake 18.4, PET-CT score 5). Then, venetoclax was used due to BCL-2 rearrangement, in combination with Chidamide, Sintilimab, and radiotherapy (50 Gy). Decrease in lesion size and FDG uptake were observed (15 × 13 mm, FDG uptake 1.9, PET-CT score 4). However, PET-CT revealed new metastatic foci (10 × 10 mm, FDG uptake 3.4, PET-CT score 5) on the right cardiodiaphragmatic angle 1 month after radiotherapy. Fortunately, the patient had a twin brother as transplant donor and syngeneic stem cell transplantation was performed about 6 months after diagnosis and CR was confirmed by a repeat PET-CT +35 d posttransplant. The patient is alive without lymphoma, and thymosin alpha-1 and recombinant human interleukin-2 are used to induce GVL. Treatment timescales are shown in Table 1, and response assessment was made according to the Lugano classification [17].
Table 1.
Treatment timescales.
| Start time | Therapy | Number of cycles | Time for repeat CT/PET | Effect | |
|---|---|---|---|---|---|
| 1st | 2019/7/19 | R-EPOCH: rituximab, etoposide, vincristine, cyclophosphamide, doxorubicin, and dexamethasone | 1 | 2019/8/8 (CT) | Stable disease |
| 2nd | 2019/8/15 | R-EPOCH + lenalidomide (25 mg d1-7) + ibrutinib (420 mg once a day) | 1 | 2019/9/4 (PET) | Progressive metabolic disease |
| 3rd | 2019/9/5 | GDP (gemcitabine, DDP, and dexamethasone) + Sintilimab (200 mg d1) + Decitabine (10 mg d1-5) | 1 | 2019/9/17 (CT) | Stable disease |
| 4th | 2019/9/29 | Sintilimab (200 mg, iv. d1) + Decitabine (10 mg, iv, d1-5) + Chidamide (20 mg, po, twice a week) | 3. | 2019/10/17 (PET) | Partial metabolic response and >90% shrinkage of mass |
| 2019/10/22 | |||||
| 2019/11/12 | 2019/11/26 (PET) | Progressive metabolic disease | |||
| 5th | 2019/11/29 | Radiotherapy (50 Gy) + Sintilimab (200 mg d1) + Chidamide (20 mg twice a week) + venetoclax | 1 | 2020-2-5 (PET) | Progressive metabolic disease |
| 2020/2/10 | Syngeneic stem cell transplantation | 2020-3-25 (PET) | Complete metabolic response |
CT, computed tomography; PET, positron emission tomography. Response assessment was made according to the Lugano classification [17]: Stable disease: <50% decrease from baseline in SPD of up to 6 dominant, measurable nodes, and extranodal sites; no criteria for progressive disease are met. Progressive metabolic disease: PET-CT Score 4 or 5 with an increase in intensity of uptake from baseline and/or new foci consistent with lymphoma at interim or end-of-treatment assessment. Partial metabolic response: PET-CT score 4 or 5 with reduced uptake compared with baseline and residual mass(es) of any size. Complete metabolic response: score 1, 2, or 3 with or without a residual mass on 5 PET-CT score.
3. Discussion
Immune checkpoint blockade has been considered an important breakthrough in cancer treatment. The field of clinical trials in DLBCL associated with immune checkpoint blockade such as PD-1/PD-L1 inhibitor is being actively studied. Unlike cHL, the clinical response to monotherapy with PD-1/PD-L1 blockade in R/R DLBCL was not confirmed [2, 4, 7–9]. Results in a phase I trial of nivolumab monotherapy were promising with a response rate of 36% in R/R DLBCL. Clinical trials of different PD-1/PD-L1 inhibitors are ongoing, and a subset of patients experienced progressive disease after a short response [18]. Therefore, there is an urgent need to designate an effective PD-1/PD-L1 inhibitor-based combination regimen for R/R DLBCL.
This “double-expressor” DLBCL with 17p deletion failed the 1st line chemotherapy and presented with increase in overall tumor burden both in lesion size and FDG uptake after 2nd line chemotherapy. It is difficult to identify whether these responses were “tumor flare” or true disease progression since lenalidomide and ibrutinib were used in the 2nd line treatment. “Tumor flare” has been observed in patients treated with immunomodulatory drugs [19], but aggressive lymphoma is different from solid tumor or chronic lymphocytic leukemia possibly presented with self-limited lymphocytosis or increase in the size of lymph nodes. Increased FDG uptake with a concomitant increase in lesion size was consistent with what was found by fiberoptic bronchoscopy in this case. Thus, the Lugano classification was used to assess lymphoma response rather than the lymphoma response to immunomodulatory therapy criteria (LYRIC) [19]. The most important reason to change the regimen was that the rapid progression of mass could result in suffocating and death. The PD-1 inhibitor was chosen since PD-L1 was positive in 25% of tumor cells. Rapid progression of disease did not allow us to choose monotherapy of PD-1 inhibitor because of the low response rates and lack of durability. On one hand, it is reported that PD-L1 expression on tumor cells in DLBCL was shown to be associated with poor prognosis in DLBCL in most clinical data [7, 20]. One the other hand, resistant DLBCL has been identified to simultaneously utilize multiple pathways that could compensate for the one provided by PD1 blockade. The PD-1/PD-L1 signal pathway turns down the activation of the immune response, and PD-1 blockade only modestly improved T cells proliferation and cytokine production but was insufficient to restore the antitumor activity [21]. By targeting multiple pathways, which in addition to PD1, combination regimen may overcome the resistance to anti-PD1 therapy.
Multiple components of DLBCL microenvironment are affected by epigenetic regulators such as HDACis or DNMTi [22]. In this report, STAT6, EZH2, TP53, KMT2D, BCL6, and CREBBP mutations are mostly associated with epigenetic dysregulation. After decitabine was added to the treatment, the patient showed a slight improvement rather than progression for the first time. Moreover, mutation of CREBBP encoding proteins with established roles in histone acetylation often results in impaired p53 activation while also promoting the oncogenic effects of BCL-6 [23]. HDACis as epigenetic modulators could reduce PD-L1 and PD-L2 expression rapidly on tumor cells, upregulate the immune response, and alter the drug resistance [22, 24]. Therefore, a triple combination of DNMTi/HDACi plus PD-1 inhibitor was performed, and the therapy led to a significant radiological response. EZH2 mutation was also found in this case. It is reported that the EZH2 inhibitor demonstrated an enhanced clinical activity in DLBCL, so EZH2 inhibition could be a promising strategy [25].
Despite this triplet therapy provided very good partial metabolic response which lasted for 40 days, the patient had progressive disease inevitably even after radiotherapy was performed. However, over 50% shrinkage of lesion size and decreased FDG uptake were observed after radiotherapy. Though the presence of bulky disease remains a significant predictor of disease recurrence, radiotherapy as bridging strategy for stem cell transplantation played an important role in reducing tumor burden.
4. Conclusions
The combination of epigenetic-modulating agents with immune checkpoint blockade provides exciting avenue for future research. This case suggests that PD-1 blockade in combination with DNMTi/HDACi can be encouraging in refractory DLBCL. Further research is warranted to evaluate this novel therapeutic regimen.
Acknowledgments
The authors would like to acknowledge Juhong Jiang in the Pathology Department for histological interpretation of lymphoid tissue and providing high-resolution images. This work was supported by grant from the Guangdong Province Natural Science Foundation (2018A030313661).
Abbreviations
- CR:
Complete remission
- cHL:
Classical Hodgkin lymphoma
- DLBCL:
Diffuse large B-cell lymphoma
- DNMTi:
DNA methyltransferase inhibitor
- FISH:
Fluorescence in situ hybridization
- GDP:
Gemcitabine, DDP, and dexamethasone
- HDACi:
Histone deacetylase inhibitor
- PD-1:
Programmed cell death protein 1
- PET:
Positron emission tomography
- PMBCL:
Primary mediastinal B-cell lymphoma
- NHL:
Non-Hodgkin lymphoma
- R‐CHOP:
Rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisolone
- R-EPOCH:
Rituximab, etoposide, vincristine, cyclophosphamide, doxorubicin, and dexamethasone
- R/R:
Relapse or refractory.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
Runhui Zheng assembled, analyzed, and interpreted the patient data regarding the hematological disease; Xiaodan Luo assembled and analyzed the patient data and wrote the manuscript; Xiaobo Chen performed fiberoptic bronchoscopy and provided related high-resolution images; Chunyan Wang and Pengfei Qin assisted with data analysis and edited the manuscript; Huo Tan assisted with data analysis and edited the manuscript. All authors read and approved the final manuscript. Xiaodan Luo and Runhui Zheng equally contributed to this work.
References
- 1.Sukswai N., Lyapichev K., Khoury J. D., Medeiros L. J. Diffuse large B-cell lymphoma variants: an update. Pathology. 2019;52(1):53–67. doi: 10.1016/j.pathol.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Crombie J. L., Armand P. Diffuse large B-cell lymphoma and high-grade B-cell lymphoma. Hematology/Oncology Clinics of North America. 2019;33(4):575–585. doi: 10.1016/j.hoc.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Feugier P., Van Hoof A., Sebban C., et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the groupe d’Etude des Lymphomes de l’Adulte. Journal of Clinical Oncology. 2005;23(18):4117–4126. doi: 10.1200/jco.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 4.Pascual M., Mena-Varas M., Robles E. F., et al. PD-1/PD-L1 immune checkpoint and p53 loss facilitate tumor progression in activated B-cell diffuse large B-cell lymphomas. Blood. 2019;133(22):2401–2412. doi: 10.1182/blood.2018889931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crump M., Neelapu S. S., Farooq U., et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808. doi: 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ok C. Y., Young K. H. Checkpoint inhibitors in hematological malignancies. Journal of Hematology & Oncology. 2017;10(1):p. 103. doi: 10.1186/s13045-017-0474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu-Monette Z. Y., Zhou J., Young K. H. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131(1):68–83. doi: 10.1182/blood-2017-07-740993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juárez-Salcedo L. M., Sandoval-Sus J., Sokol L., Chavez J. C., Dalia S. The role of anti-PD-1 and anti-PD-L1 agents in the treatment of diffuse large B-cell lymphoma: the future is now. Critical Reviews in Oncology/Hematology. 2017;113:52–62. doi: 10.1016/j.critrevonc.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 9.Song M. K., Park B. B., Uhm J. Understanding immune evasion and therapeutic targeting associated with PD-1/PD-L1 pathway in diffuse large B-cell lymphoma. International Journal of Molecular Sciences. 2019;20(6) doi: 10.3390/ijms20061326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X., Chen L., Zhao Y., Yin H., Ma H., He M. Safety and efficacy in relapsed or refractory classic hodgkin’s lymphoma treated with PD-1 inhibitors: a meta-analysis of 9 prospective clinical trials. BioMed Research International. 2019;2019:13. doi: 10.1155/2019/9283860.9283860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruger S., Ilmer M., Kobold S., et al. Advances in cancer immunotherapy 2019 - latest trends. Journal of Experimental & Clinical Cancer Research. 2019;38(1):p. 268. doi: 10.1186/s13046-019-1266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson W. H., Jung S.-H., Porcu P., et al. A Cancer and Leukemia Group B multi-center study of DA-EPOCH-rituximab in untreated diffuse large B-cell lymphoma with analysis of outcome by molecular subtype. Haematologica. 2012;97(5):758–765. doi: 10.3324/haematol.2011.056531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunleavy K., Fanale M. A., Abramson J. S., et al. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: a prospective, multicentre, single-arm phase 2 study. The Lancet Haematology. 2018;5(12):e609–e617. doi: 10.1016/s2352-3026(18)30177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goy A., Ramchandren R., Ghosh N., et al. Ibrutinib plus lenalidomide and rituximab has promising activity in relapsed/refractory non-germinal center B-cell-like DLBCL. Blood. 2019;134(13):1024–1036. doi: 10.1182/blood.2018891598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong M., Ning Z.-Q., Xing P.-Y., et al. Phase I study of chidamide (CS055/HBI-8000), a new histone deacetylase inhibitor, in patients with advanced solid tumors and lymphomas. Cancer Chemotherapy and Pharmacology. 2012;69(6):1413–1422. doi: 10.1007/s00280-012-1847-5. [DOI] [PubMed] [Google Scholar]
- 16.Shi Y., Jia B., Xu W., et al. Chidamide in relapsed or refractory peripheral T cell lymphoma: a multicenter real-world study in China. Journal of Hematology & Oncology. 2017;10(1):p. 69. doi: 10.1186/s13045-017-0439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheson B. D., Fisher R. I., Barrington S. F., et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. Journal of Clinical Oncology. 2014;32(27):3059–3067. doi: 10.1200/jco.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lesokhin A. M., Ansell S. M., Armand P., et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. Journal of Clinical Oncology. 2016;34(23):2698–2704. doi: 10.1200/jco.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheson B. D., Ansell S., Schwartz L., et al. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. 2016;128(21):2489–2496. doi: 10.1182/blood-2016-05-718528. [DOI] [PubMed] [Google Scholar]
- 20.Kiyasu J., Miyoshi H., Hirata A., et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126(19):2193–2201. doi: 10.1182/blood-2015-02-629600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Donnell J. S., Long G. V., Scolyer R. A., Teng M. W. L., Smyth M. J. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treatment Reviews. 2017;52:71–81. doi: 10.1016/j.ctrv.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Banik D., Moufarrij S., Villagra A. Immunoepigenetics combination therapies: an overview of the role of HDACs in cancer immunotherapy. International Journal of Molecular Sciences. 2019;20(9) doi: 10.3390/ijms20092241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasqualucci L., Dominguez-Sola D., Chiarenza A., et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 2011;471(7337):189–195. doi: 10.1038/nature09730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Waschke B. C., Woolaver R. A., et al. Histone deacetylase inhibition sensitizes PD1 blockade-resistant B-cell lymphomas. Cancer Immunology Research. 2019;7(8):1318–1331. doi: 10.1158/2326-6066.CIR-18-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lue J. K., Amengual J. E. Emerging EZH2 inhibitors and their application in lymphoma. Current Hematologic Malignancy Reports. 2018;13(5):369–382. doi: 10.1007/s11899-018-0466-6. [DOI] [PubMed] [Google Scholar]


