Abstract
The neonatal fragment crystallizable (Fc) receptor (FcRn) functions as a recycling mechanism to prevent degradation and extend the half-life of IgG and albumin in the circulation. Several FcRn inhibitors selectively targeting IgG recycling are now moving rapidly toward clinical practice in neurology and hematology. These molecules accelerate the destruction of IgG, reducing pathogenic IgG and IgG immune complexes, with no anticipated effects on IgA, IgM, IgE, complement, plasma cells, B cells, or other cells of the innate or adaptive immune systems. FcRn inhibitors have potential for future use in a much wider variety of antibody-mediated autoimmune diseases. Given the imminent clinical use, potential for broader utility, and novel mechanism of action of FcRn inhibitors, here we review data from 4 main sources: (a) currently available activity, safety, and mechanism-of-action data from clinical trials of FcRn inhibitors; (b) other procedures and treatments that also remove IgG (plasma donation, plasma exchange, immunoadsorption); (c) diseases resulting in loss of IgG; and (d) primary immunodeficiencies with potential mechanistic similarities to those induced by FcRn inhibitors. These data have been evaluated to provide practical considerations for the assessment, monitoring, and reduction of any potential infection risk associated with FcRn inhibition, in addition to highlighting areas for future research.
Key words: FcRn, neonatal Fc receptor, immunoglobulin, IgG, albumin, hypogammaglobulinemia, autoantibody, antibody-mediated autoimmunity, FcRn inhibitors, infection risk
Abbreviations used: B2M, β2 microglobulin; COVID-19, Coronavirus disease 2019; Fc, Fragment crystallizable; FcRn, Neonatal Fc receptor; FcγR, Fc-gamma receptor; FIH, First-in-human; GI, Gastrointestinal; Gm, Gamma marker; HBV, Hepatitis B virus; IA, Immunoadsorption; IC, Immune complex; IgRT, IgG replacement therapy; ITP, Immune thrombocytopenia; IV, Intravenous; IVIg, Intravenous immunoglobulin; MG, Myasthenia gravis; PLE, Protein-losing enteropathy; PLEX, Plasma exchange; QMG, Quantitative MG; QW, Once-weekly; SARS-CoV-2, Severe acute respiratory syndrome coronavirus-2; SC, Subcutaneous; SID, Secondary immunodeficiency; TEAE, Treatment-emergent adverse event; VL, Viral load; WBC, White blood cell
Autoantibody-mediated autoimmune diseases can result in tissue damage through IgG immune complex (IC) deposition1 , 2 or via monomeric pathogenic autoantibodies, which bind directly to target structures and induce damage through complement activation and/or recruitment of inflammatory phagocytes.2, 3, 4, 5, 6, 7 Current therapeutic strategies for the treatment of chronic autoantibody-mediated diseases consist of reducing antibody production by immunosuppression or B-cell–targeting drugs, and removal of autoantibodies and IC via plasma exchange (PLEX) or immunoadsorption (IA).8, 9, 10 Alternatively, for monomeric IgG-mediated diseases, immunomodulatory high-dose intravenous immunoglobulin (IVIg) therapy acts via a number of mechanisms including increased catabolism of autoantibodies via the neonatal fragment crystallizable (Fc) receptor (FcRn), cytokine neutralization, blockade of activating Fc-gamma receptors (FcγRs), or inhibition of autoantibodies by binding of natural anti-idiotypes11 , 12 (see review by Shock et al13 in this issue).
In many regions, supply and cost of IVIg are important factors. It can be challenging for health care systems to meet the demand for IVIg, which is increasing due to growth in the number of patients requiring IgG replacement therapy (IgRT) for treatment of antibody deficiencies, and the expanding use of immunomodulatory high-dose IVIg (on- and off-label) in the context of a worldwide plasma shortage.14, 15, 16
IgG has one of the longest half-lives of serum proteins, sustained by a specialized recycling pathway involving FcRn (see Patel and Bussel17 review in this issue). FcRn is predominantly expressed on the endothelium, but has also been detected in a diverse range of tissues and cell types.2 Because recycling is the principal mechanism for maintaining a high serum level of IgG (and albumin), modulation of this pathway by blocking FcRn is an attractive mechanism for the reduction of pathogenic IgG autoantibodies. Inhibition of FcRn accelerates destruction of IgG via lysosomal degradation. Using this targeted mechanism to reduce tissue and serum concentrations of IgG has the potential to provide significant therapeutic benefit for patients with both monomeric and IC IgG autoantibody-mediated diseases.18, 19, 20, 21 Should these therapies prove successful, they will provide an alternative to high-dose IVIg and PLEX, reducing treatment burden on health care systems and patients. FcRn inhibitors currently in clinical trials include efgartigimod,20 , 22 , 23 rozanolixizumab,19 , 21 , 24 nipocalimab (M281),25 and orilanolimab (SYNT001)18; additional FcRn inhibitors in development include IMVT-1401/RVT-1401, CSL730/M230, and ABY-039 (see Patel and Bussel17 for further details).
In the absence of large data sets or long-term exposure to FcRn inhibitors, this review aimed to define the immunologic impact of decreasing levels of IgG through FcRn inhibitors. Furthermore, we will review procedures and treatments that also remove IgG (plasma donation, PLEX, and IA), diseases resulting in loss of IgG, and genetic disorders with potential mechanistic similarities to those of FcRn inhibitors, to better understand the risk of infection associated with this novel class of drugs currently in development. Finally, we aim to provide practical considerations on assessment, monitoring, and precautions necessary to ensure adequate humoral immunity in patients receiving an FcRn inhibitor (postapproval) in various clinical scenarios.
Activity and safety of FcRn inhibitors in disease settings
Efgartigimod, currently in phase 3 trials for generalized myasthenia gravis (MG) and immune thrombocytopenia (ITP), is a monoclonal IgG1 Fc fragment that has been mutated at 5 residues to increase its affinity for FcRn at both physiologic and acidic pH.20 In a phase 2 randomized, placebo-controlled study in 24 patients with MG (see Table E1 in this article’s Online Repository at www.jacionline.org),20 following 4 once-weekly (QW) intravenous (IV) administrations of 10 mg/kg efgartigimod, mean maximum reductions in serum IgG of 70.7% from baseline were observed 1 week after the final infusion. Reductions in anti–acetylcholine receptor autoantibodies mirrored the observed reductions in total IgG. Efficacy assessments using the Quantitative MG (QMG), MG Activities of Daily Living, MG Composite, and the revised MG Quality of Life 15-Item scales consistently demonstrated that 75% of patients showed a rapid disease improvement; improvements in QMG, MG Activities of Daily Living, and MG Composite scores were sustained for the duration of the study (through day 78).
Most frequently reported treatment-emergent adverse events (TEAEs) in patients receiving efgartigimod were headache and reduced white blood cell (WBC) counts, all of which were mild in severity. Single TEAE reports considered possibly related to efgartigimod and temporally associated with its administration included decreases in total lymphocyte counts, T lymphocytes and B lymphocytes, and monocytes, and an increase in neutrophil counts (Table I ).18, 19, 20, 21, 22, 23, 24, 25 Hematologic changes (ie, abnormal differential WBC counts) observed in 3 patients following treatment with efgartigimod were mild and asymptomatic, and most likely explained by concomitant use of immunosuppressants. However, the first-in-human (FIH) study of efgartigimod23 also reported abnormal differential WBC counts (mild decrease in CD8, CD3, CD56, CD4, and CD19 lymphocyte counts) after receipt of the drug (3 of 4 healthy volunteers who received 25 mg/kg and 4 of 4 who received 50 mg/kg).
Table I.
Summary of IgG, albumin, adverse event, and infection data for reported clinical trials of FcRn inhibitors
| Indication | N | Intervention and comparator | Duration of follow-up | Mean maximum reduction in IgG (%) | Impact on albumin | TEAEs of interest (%) | Reported infections | |
|---|---|---|---|---|---|---|---|---|
| Efgartigimod (IV) | ||||||||
| MG20 (NCT02965573) | 24 |
|
8 wk | 70.7 week 4 | Not reported |
|
33.3 8.3 8.3 8.3 16.7 16.7 16.7 16.7 |
Herpes zoster: 1 efgartigimod-treated patient (also seen in SoC treatment with prednisone and mycophenolate mofetil) |
| ITP22 (NCT03102593) | 38 |
|
21 wk | 60.4 D25 63.7 D25 |
Similar between groups and within ±10%-15% baseline |
|
15.4 7.7 |
1 pneumonia (deemed unrelated to efgartigimod treatment); no apparent increased risk of infection |
| FIH23 (NCT03457649) | MAD: 32 |
|
58-59 d | 78.5 D24 Emax 73.0 D24 Emax 77.7 D24 Emax |
No significant decrease |
|
n = 4 n = 1 |
None |
| Rozanolixizumab (SC) | ||||||||
| MG19 (NCT03052751) | 43 | Period 1 (D1-29):
|
55 d | 68 D50 in patients receiving 7 mg/kg throughout the study | Not reported |
|
57.1 | Not reported |
| ITP21 (NCT02718716) | 66 |
|
8 wk | 60 D8 for single-dose 20 mg/kg |
Not reported |
|
39.4 12.1 9.1 |
No serious infections |
| FIH24 (NCT02220153) | SAD SC: 24 |
|
79 d | 43.4 for 7 mg/kg D10 | Modest decrease, not significantly different from placebo |
|
27.8 0 16.7 11.1 5.6 |
Incidence of treatment-related infections was lower in the rozanolixizumab total group (13.9%) than in the placebo group (23.1%) |
| Nipocalimab (IV) | ||||||||
| FIH25 (NCT02828046) | MAD: 16 |
|
Up to 14 wk | D20∗: 83 Emax D24∗: 84 Emax |
Asymptomatic and transient reduction in total serum protein and albumin |
|
8.3 8.3 8.3 |
Incidence of treatment-emergent infections and infestations was similar between nipocalimab (41.7%) and placebo (50%) groups |
| Orilanolimab (IV) | ||||||||
| FIH18 (NCT03643627) | SAD: 31 |
|
27 d | 46.21 median for 30 mg/kg dose group within 5 d | No significant changes |
|
34.8 4.3 4.3 4.3 4.3 |
Not reported |
D, Day; Emax, maximum percentage reduction value; MAD, multiple ascending dose; Q4D, every 4 d; SAD, single ascending dose; SoC, standard of care.
For FIH studies, data are presented for multiple doses if available.
Median time to Emax.
Data from a phase 2 trial of efgartigimod in adults with primary ITP reported clinically relevant increases in platelet counts with associated decreases in IgG levels.22 Patients were randomized to receive 4 QW doses of placebo (n = 12), or IV efgartigimod 5 mg/kg (n = 13), or 10 mg/kg (n = 13), with a total of 21 weeks’ follow-up. Patients who experienced a relapse during the follow-up period (platelet count, <30 × 109) had the option to enroll in a 1-year open-label extension (Table E1) during which they received efgartigimod 10 mg/kg QW for 4 weeks. In patients treated with IV efgartigimod 10 mg/kg, a maximum reduction in total serum IgG of 60% to 64% was achieved by day 25 (Table I), with levels returning to baseline over the first part of the study (80 days). Every patient achieved a decrease in all IgG subclasses; antiplatelet autoantibodies were identified in all patients and were reduced by treatment with efgartigimod. In patients receiving IV efgartigimod 10 mg/kg, platelet counts rose from a mean of less than 20 × 109/L at baseline to approximately 40 × 109/L at the end of the treatment phase. During the main part of the study, a platelet count of greater than or equal to 50 × 109/L was achieved by a similar number of patients in the placebo group as in the combined efgartigimod 5 mg/kg and 10 mg/kg groups (54% and 50%, respectively). However, 46% of patients receiving efgartigimod reached this threshold on at least 2 occasions (25% placebo) and 39% maintained counts at or over this threshold for more than 10 cumulative days (0% placebo); 42% achieved a platelet count of greater than or equal to 100 × 109/L (8% placebo).
Efgartigimod was well tolerated over the full duration of the study, with no dose-related safety observations, no increased risk of infection versus placebo, and a safety profile consistent with previous FIH and MG studies. Changes in serum albumin were similar between placebo and efgartigimod groups, mostly within ±10% to 15% of baseline, and changes were not considered clinically relevant, suggesting that efgartigimod does not interfere with albumin binding22 (Table I).
A recent study explored the consequences of engineering the Fc region of IgG1, thus manipulating the FcRn-IgG1 interaction on Fc effector function.26 All 4 recombinant human IgG1 variants, one of which (MST/HN) carried the same 5 point mutations as efgartigimod (M252Y, S254T, T256E, H433K, N434F23), markedly reduced binding to classical FcγRs. Three of the 4 variants (including MST/HN) demonstrated significantly reduced binding to complement factor C1q. Reductions in FcγRs and C1q binding limited the ability of these human IgG1 variants to activate antibody-dependent mechanisms such as cell-mediated cytotoxicity, cellular phagocytosis, and complement-mediated cell lysis. Interestingly, previous successful attempts to treat acute childhood ITP with infusions of unmutated Fcγ fragments suggested a predominant blocking action on classical FcγRs on mononuclear phagocytes.27 To our knowledge, the effects of efgartigimod on classical FcγRs- and C1q-mediated functions have not been published and more information is needed to determine whether efgartigimod, besides inhibiting FcRn, has a significant effect on FcγRs and C1q binding.
Rozanolixizumab, currently in phase 3 trials for MG and ITP and a phase 2 trial for chronic inflammatory demyelinating polyneuropathy (CIDP), is a subcutaneously (SC) infused humanized IgG4 mAb that binds to human FcRn, selectively inhibiting IgG binding without affecting albumin.24 In a phase 2 placebo-controlled clinical trial, 43 patients with generalized MG were randomized equally to 3 QW SC infusions of rozanolixizumab 7 mg/kg or placebo in period 1; in period 2, patients were rerandomized to 3 additional QW SC infusions of rozanolixizumab 7 mg/kg or 4 mg/kg (Table E1).19 Treatment with rozanolixizumab 7 mg/kg resulted in rapid reductions in both total IgG and anti–acetylcholine receptor autoantibody concentrations, with a maximum decrease of approximately 68% observed in patients receiving SC rozanolixizumab 7 mg/kg/week throughout the study. Least-squares mean differences between rozanolixizumab and placebo groups at day 29 were −0.7 (P = .221) for the QMG score, −1.4 (P = .036) for the MG Activities of Daily Living score, and −1.8 (P = .089) for the MG Composite score. The most frequently reported TEAE in patients receiving rozanolixizumab was headache. The investigators concluded that despite not meeting the primary end point of change from baseline in the QMG score at day 29, proof of concept was achieved on the basis of clinically meaningful improvements in MG outcomes and reductions in autoantibody titers.19
A phase 2 open-label, multiple-dose clinical trial in 66 patients with persistent/chronic primary ITP21 assessed the effect of different dose schedules of SC rozanolixizumab for a similar total exposure of 15 to 21 mg/kg (Table E1). The primary objectives were safety and tolerability; secondary objectives were efficacy (change in platelet count) and pharmacodynamics (change in total IgG). The most common TEAEs were headache, diarrhea, and vomiting (all mild to moderate in intensity); no serious infections were observed. There were dose-dependent increases in platelet counts: by day 8, a platelet count of greater than or equal to 50 × 109/L was achieved by 54% and 58% of patients in the 20 mg/kg and 15 mg/kg single-dose groups, respectively, with median peak counts exceeding 100 × 109/L in both groups. Dose-dependent decreases in serum IgG concentration were observed by day 8, with the 20 mg/kg (n = 12) single-dose group achieving its nadir of a 60% mean change from baseline in total serum IgG at this time point, whereas the 5 × 4 mg/kg (n = 15) group achieved its nadir of 43.6% change from baseline on day 29.
Nipocalimab (M281), a high-affinity, fully human monoclonal IgG1 anti-FcRn antibody engineered to have no Fc effector potential (no C1q binding, and no binding to activating FcγR), and orilanolimab (SYNT001), a humanized IgG4κ mAb, are 2 additional FcRn inhibitors with published FIH data18 , 25 (Table I). Nipocalimab is currently in phase 2 trials for autoimmune hemolytic anemia, hemolytic disease of the fetus and newborn, and MG. The orilanolimab FIH study assessed the impact on C1q-associated circulating IgG ICs measured by the MicroVue CIC-C1q EIA Kit, in addition to demonstrating reductions in serum IgG. The investigators report that administration of orilanolimab to 31 healthy male volunteers resulted in significant dose-dependent reductions in circulating ICs, and ex vivo, orilanolimab inhibited the ability of IgG ICs to induce secretion of innate inflammatory cytokines by human peripheral blood leukocytes.18 Whether these promising initial findings translate into clinically meaningful therapeutic effects in patients with IgG IC-mediated disease remains to be seen.
Areas where further data are required to contribute to our understanding of the impact of FcRn inhibitors on immune function remain, in addition to data sets anticipated from unpublished phase 2 studies and forthcoming phase 3 trials. It would be of interest to understand details of specific antibody titers before and after inhibition of FcRn, booster responses during treatment, and responses to neoantigens (eg, hepatitis A or rabies vaccine). The impact of dose and route of administration on safety, efficacy, and patient satisfaction with treatment also needs to be evaluated. As patient numbers increase, analyses of bacterial, viral, and fungal infections relative to placebo groups should become possible, as should understanding the impact of previous and concomitant therapies on changes in serum IgG and risks of infection. Further studies should also shed light on the impact of FcRn inhibitors on antigen presentation and follicular dendritic cells, which will be relevant to the understanding of potential FcRn inhibitor–associated infection risk. Finally, more information will emerge on interindividual or age-dependent variability in FcRn expression and, in turn, how this affects response to FcRn inhibitors.
The mechanistic basis of FcRn inhibition: Potential impact on immune function
Current evidence shows that inhibition of FcRn selectively reduces serum IgG, with no relevant reductions in IgM, IgA, IgE, or albumin18 , 23, 24, 25 (Table I). Reduction in IgG is transient and reversible, with mean maximum reductions in serum IgG of 45% to 85% from baseline, returning to near-baseline levels 50 to 57 days (28 days for orilanolimab) after single doses,18 , 24 , 25 and 50 to 80 days after multiple doses,20 , 22 , 23 , 25 indicating that function of the memory B- and plasma-cell compartments remains unaffected by inhibition of FcRn (Table I). During clinical trials of FcRn inhibitors, no rebound effect on levels of total IgG has been observed; for specific antibodies and autoantibodies, rebound is unknown and considered unlikely.
Any differential impact of FcRn inhibitors on reductions in IgG subclasses is of interest due to their potential utility for the treatment of IgG4-related disease.28 An FIH study of efgartigimod reported that following administration of both single and multiple doses of efgartigimod, reductions in IgG1-3 followed a similar pattern, with slightly smaller reductions observed for IgG4.23 This pattern of lower reductions for IgG4 in comparison with IgG1-3 was also observed in the phase 2 studies of efgartigimod,20 , 22 suggesting perhaps less efficient FcRn blockade by efgartigimod (a mutated Fc portion of IgG1) for IgG4. In contrast, FIH studies of rozanolixizumab and orilanolimab both reported that administration of single doses resulted in dose-dependent reductions in IgG1-4, with the most pronounced changes seen for IgG3.18 , 24 A fourth phase 1 study found that each IgG subclass exhibited similar decreases in serum levels after the administration of single doses of nipocalimab; this observation was highly consistent across subjects.25 Although a detailed structure-function analysis is beyond the scope of this review, factors such as half-life of subclasses, gamma marker (Gm) allotypes, and binding characteristics to FcRn deserve some mention. Histidine at position 435 (H435) provides for optimal FcRn-IgG binding and a long half-life (21 days). IgG1, IgG2, and IgG4 carry the H435 variant, whereas an arginine at position 435 (R435) is more common in the IgG3 subclass, leading to reduced binding affinity for FcRn, a shortened half-life (8 days), and reduced transplacental IgG3 transfer.29 , 30 Interestingly, the H435 variant of IgG3 has a different prevalence among Europeans (1%), Asians (10%-25%) and Africans (30%-60%), an observation that has been found to be important in the transplacental transfer of IgG3 antibodies in antimalarial immunity.29 , 31 Similarly, the high number of codominantly inherited Gm allotypes in the IGHG3 locus (13 of all 20 Gm allotypes reside here) suggests a strong functional selection pressure in this part of the genome.32 Whether differences in IgG3 H435/R435 and Gm3 allotype markers (such as G3m5, G3m6, G3m15, G3m16, G3m21 or G3m24, all encoded by 1 or several IGHG3 alleles) could influence its blockade through FcRn inhibitors and the clinical relevance of such a potential effect requires monitoring.
The main TEAEs reported to date appear to be gastrointestinal (GI) disturbances and headache—predominantly mild to moderate in severity, and, in the case of headache, manageable with standard therapies. More data are needed to clarify the effect of FcRn inhibitors on lymphocyte, monocyte, and neutrophil counts given the effects reported in the phase 1 FIH23 and phase 2 trial of efgartigimod in MG24 and ITP.20 , 22 As with all immunomodulatory therapies, there is a concern of increased risk of infection, although there is no evidence of increased infections for FcRn inhibitors reported so far. However, data are still limited, and increased rate of infection is perhaps the greatest theoretical risk associated with these new therapies. By inducing transient reduction in IgG, inhibition of FcRn has interesting parallels with several primary and secondary immunodeficiencies as well as procedures that reduce serum IgG. Assessing data from these examples may aid our understanding of the immunologic impact of FcRn inhibition and thus the potential risk of infection.
Hypogammaglobulinemia and infection risk
Increasing the rate of IgG removal via therapeutic inhibition of FcRn does not distinguish between pathogenic and nonpathogenic IgG and could be considered a type of novel secondary immunodeficiency (SID) (for overview, see Fig E1 in this article’s Online Repository at www.jacionline.org).33 Although SID can result in increased risk of infection, potential severity varies widely, ranging from a modest increase in mild infections to a more serious profile, including opportunistic pathogens.33 In patients with autoimmune diseases, antibody deficiencies can be multifactorial, from underlying disease processes and subsequent treatments. Patients who experience loss of IgG while antibody production and quality remain unaltered (eg, renal or GI losses, or antibody removal by PLEX) retain specific antibody production, and have a lower infection risk and less severe symptoms compared with patients with genetic disorders that result in global deficiency of antibody production.33 Patients with genetic disorders resulting in low IgG who retain normal IgA and/or IgM levels may experience a more benign infection and complication profile.34, 35, 36, 37, 38 SID with a high risk of infections may occur, particularly when antibody production is impaired such as in lymphoid malignancies33 , 39 , 40 or prolonged treatment with B-cell–depleting therapies (eg, rituximab).41, 42, 43, 44, 45, 46
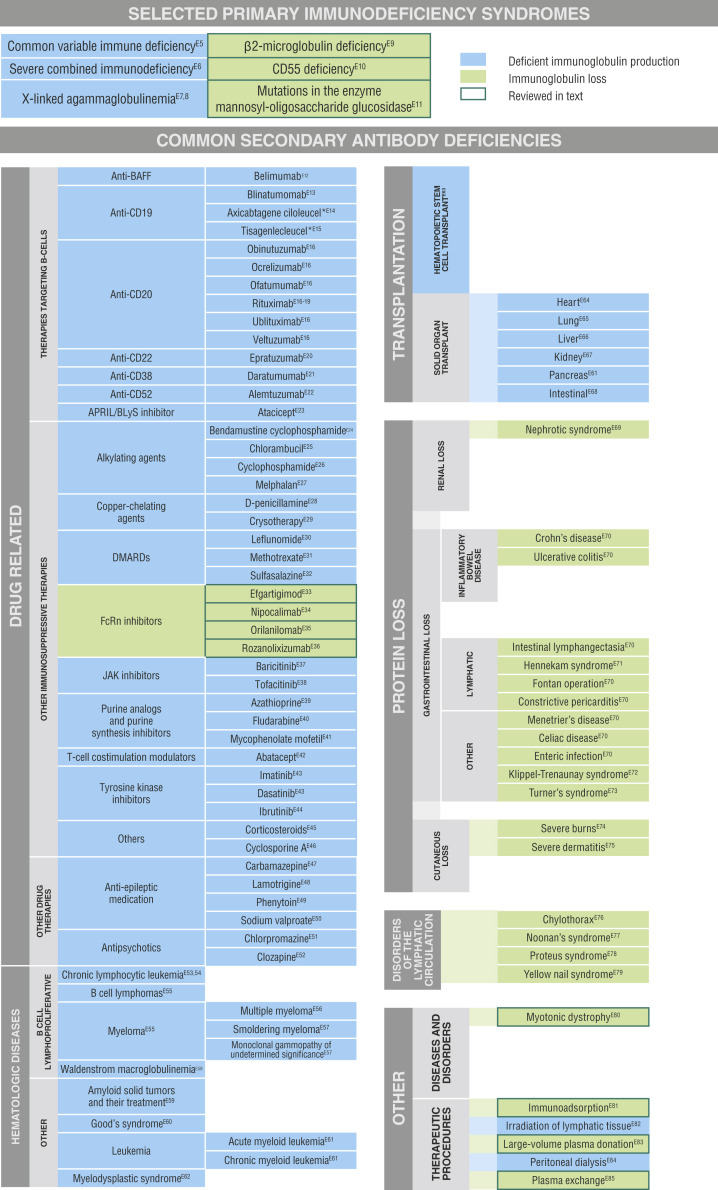
Fig E1.
Impact of selected primary and secondary antibody deficiencies on IgG.E5, E6, E7, E8, E9, E10, E11, E12, E13, E14, E15, E16, E17, E18, E19, E20, E21, E22, E23, E24, E25, E26, E27, E28, E29, E30, E31, E32, E33, E34, E35, E36, E37, E38, E39, E40, E41, E42, E43, E44, E45, E46, E47, E48, E49, E50, E51, E52, E53, E54, E55, E56, E57, E58, E59, E60, E61, E62, E63, E64, E65, E66, E67, E68, E69, E70, E71, E72, E73, E74, E75, E76, E77, E78, E79, E80, E81, E82, E83, E84, E85APRIL, A proliferation-inducing ligand; BAFF, B-cell activating factor; BLyS, B-lymphocyte stimulator; DMARD, disease-modifying antirheumatic drug; JAK, Janus kinase.
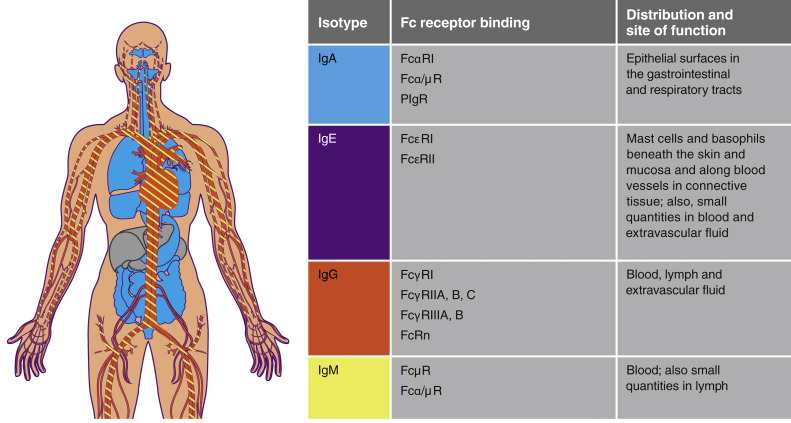
To assess the infection risk in SID or after therapeutic removal of immunoglobulin, it is important to consider which immunoglobulin class(es) has been reduced, where and how they function, and the mechanism and degree of reduction achieved.33 Although FcRn is key to homeostatic regulation of both IgG and albumin, it does not bind to the other immunoglobulin classes (Fig 1 ). As observed in the FcRn inhibitors trials to date, inhibition of FcRn would be expected to induce a transient reduction in IgG with no relevant loss of IgM, IgA, or IgE. FcRn binds to albumin and IgG at 2 distinct sites in its α1 and α2 domain, respectively,47 FcRn inhibitors bind only to the α2 domain; therefore, specific FcRn inhibitors should not impact serum albumin concentrations.
Fig 1.
Distribution and function of immunoglobulin isotypes. PIgR, Polymeric immunoglobulin receptor.
Inhibition of FcRn is not expected to affect plasma cells, B-cell repertoire, or memory B cells, or to interfere with other cells of the innate and adaptive immune systems, or complement. Because of its discrete mechanism of action, FcRn inhibition is less likely to be associated with increased risk of infection compared with other immunomodulators such as glucocorticoids or rituximab, and unlikely to lead to opportunistic infections with intracellular pathogens, particularly those requiring granulomatous inflammation for control (eg, mycobacteria, fungi). However, as a result of the selective loss of IgG, some recipients may be at increased risk for certain sinopulmonary infections, including those caused by respiratory viruses, Hemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. Inhibition of FcRn results in a selective and reversible reduction of IgG that would be predicted to have a lower risk of infection than the impaired production of multiple immunoglobulin classes that results from some genetic disorders. We will therefore also consider infection data associated with procedures that remove IgG, diseases that result in loss of IgG, and genetic disorders with mechanistic similarities to inhibition of FcRn.
What can we learn from other conditions and therapies?
Procedures that remove IgG
Plasma donation, PLEX, and IA all involve removal of blood components with greater or lesser degrees of selectivity for IgG (Table II ).18 , 23, 24, 25 , 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64 These procedures result in the reduction of existing antibodies, while leaving production of immunoglobulins by plasma cells intact (Table II). Levels of serum proteins, including IgG, are subsequently restored by new production and redistribution from the extracellular space.
Table II.
Procedures and conditions causing excessive loss of antibodies
| Procedure/condition |
T cells |
B cells |
NK cells |
IgA |
IgM |
IgE |
IgG |
Albumin |
Infection risk |
References |
|---|---|---|---|---|---|---|---|---|---|---|
| Procedures that remove IgG | ||||||||||
| Plasma donation | Normal humoral and cellular immunity | Decreased (Ab loss) | Normal or reduced | No risk | 48, 49, 50 | |||||
| PLEX | Possible alterations in lymphocyte function due to mechanical damage | Decreased (with rapid recovery) | Decreased (with rapid recovery) | Decreased (Ab loss), recovery may be delayed by weeks | NR | 51,52 | ||||
| IA | Decreased (Ab loss). Dependent on the ligands used to bind different Ig classes | NR | 51 | |||||||
| FcRn inhibitors | Normal | Normal | Normal | Normal | Normal | Normal | Decreased (Ab loss) | Normal or modest decrease | No increased risk of infection reported | 18,23, 24, 25 |
| Conditions that result in loss of IgG | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
Protein loss
|
T-cell abnormalities | B-cell abnormalities | NR | NR | Normal to elevated levels | NR | Decreased (Ab loss) | Decreased; synthesis may be increased | Increased susceptibility, particularly to bacterial infections | 53, 54, 55 |
|
Decreased | Decreased | NR | Decreased | Normal or decreased | Normal | Normal or decreased (Ab loss) | Reduced, synthesis normal or increased | NR | 56,57 |
|
Normal or decreased | Normal | Normal | Variable | Variable | NR | Decreased (Ab loss) | Normal or increased | Delayed clearance of cutaneous viral infections | 58 |
| CD55 deficiency | Normal | Normal | Normal | Decreased (Ab loss) | Decreased (Ab loss) | Decreased (Ab loss) | Decreased (Ab loss) | Decreased | Recurrent respiratory infections | 59 |
| Genetic disorders resulting in the increased catabolism of IgG | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Normal; altered differentiation of CD8 T cells | Decreased | Decreased; but functionally inactive | Normal to elevated | Variable | NR | Decreased (Ab loss) | Decreased | Infections include RTIs; granulomatous dermatitis; recurrent otitis media |
60,61 62 |
|
Normal | Normal or increased | Normal | Decreased (Ab loss) Shortened half-life of Igs due to a glycosylation defect |
Decreased because of reduced half-life of Igs | Normal | Paradoxical increased resistance to some viral infections, due to glycosylation defects in viral receptors and viral envelope (n = 3 patients) | 63,64 | ||
The literature was also assessed for changes to antigen-presenting cells, but no data were reported for this cell type in the articles cited.
Ab, Antibody; CDG, congenital disorders of glycosylation; MOGS, mannosyl-oligosaccharide glucosidase; NK, natural killer; NR, not reported; RTI, respiratory tract infection.
Early onset inflammatory bowel disease caused by single gene defects associated with immune dysregulation.
Plasma donation is perhaps the most common and least documented in terms of understanding the impact on the immune system and the subsequent infection risk. Plasma derivatives, including IVIg and albumin, are derived by processing a large number of pooled plasma donations.65 , 66 Apheresis plasma donations are generally restricted to 400 to 900 mL/donation (anticoagulant excluded) at a frequency of 13 to 104 donations per individual per year,66 with data suggesting a 13% reduction from baseline in donor serum IgG levels over the course of an 800 mL donation.48 A limited number of studies have prospectively assessed the safety of long-term intensive plasmapheresis on donors.49 , 67 , 68 Even with intensive plasmapheresis, there is no evidence of reduced immune responses; donors have normal IgG production and respond normally to antigenic stimulation (Table II).
PLEX involves the removal of 1 to 1.5 plasma volumes (30 to 40 mL/kg) per exchange using centrifugation or filtration-based devices, and replacing volume with isotonic albumin or a mixture of saline and albumin to avoid hypotension.51 The procedure requires large-caliber venous access and may be associated with cardiovascular disturbance; close monitoring of electrolytes is also needed.51 Therapeutic effect is based on the removal of circulating pathogenic immune factors, including autoantibodies and ICs; however, nonpathogenic IgG, IgA, and IgM are also removed, as well as complement components (Table II). PLEX is predominantly used in the acute treatment of several antibody-mediated disorders such as MG, lupus crisis, Guillain-Barré syndrome, and pulmonary-renal syndrome.51 PLEX does not always provide clinical benefit in chronic diseases,69 and may not be the most effective or targeted approach to the treatment of autoantibody-mediated diseases, because it removes all circulating proteins. In addition, as IgG is distributed throughout the extravascular space, its removal from serum by PLEX results in rapid redistribution back into the intravascular space.69
IA is a therapeutic procedure for the specific removal of proteins by passing separated plasma through an adsorption column. Immunoglobulins can be targeted for removal through binding to selected ligands on the backing matrix surface (membranes or beads) of the adsorption column (Table II). Although IA is effective for the treatment of pemphigus vulgaris70 , 71 and CIDP,72 , 73 IA systems are used less frequently than PLEX for the treatment of IgG autoantibody-mediated diseases, due to the availability and complexity of the procedure, and regulatory and economic differences between health care systems.51
The American Society for Apheresis, the American Academy of Neurology, and other organizations have published guidelines and commentaries for PLEX and IA,51 , 74 , 75 noting that side effects and complications of PLEX and IA mostly relate to the procedure itself.75 , 76 Both treatments are used in patients with a range of heterogeneous disorders alongside varied previous therapies and differing numbers of exchanges. Thus, teasing out the specific impact on infection risk of using these procedures to remove serum proteins is challenging, with few studies assessing the risk of infection for these procedures in patients with antibody-mediated autoimmune diseases. Schmaldienst et al77 evaluated the infection risk of IA with and without IVIg substitution. Overall, rates of infection were low and similar between groups (IA + IVIg, 1.3 per patient-year, interquartile range, 0-2; IA alone, 0.9 per patient-year, interquartile range, 0-2).77 In 2012, Som et al78 reported data for PLEX-related complications from the Oklahoma thrombocytopenic purpura-hemolytic uremic syndrome Registry, which provides data from a population-based inception cohort of consecutive patients with a diagnosis of thrombocytopenic purpura or hemolytic uremic syndrome. Over 15 years, the percentage of patients with major complications attributed to PLEX decreased—because of reduced frequency of catheter-related complications, rather than complications related to plasma removal. The researchers make no mention of increased infection risk as a consequence of hypogammaglobulinemia.78
Overall, PLEX and IA appear to be associated with a low risk of infection, and infections that do occur are mainly associated with venous access. In the case of PLEX, which removes IgA, IgM, complement, and other blood components in addition to IgG, the low level of reported infections offers some reassurance regarding the impact of procedures that leave antibody production intact. In clinical practice, the use of these therapies and the duration and degree of IgG reduction that can be realistically achieved may be limited by their other side effects and the burden of treatment on both patient and health care system. The targeted ability of FcRn inhibitors to reduce serum IgG while leaving all other serum constituents intact, and without the associated risks with extended venous access/central venous catheters, anticoagulants, and donor blood products, would suggest a similar or lower infection risk than with PLEX or IA.
Diseases that result in loss of IgG
Further insights into the potential impact of FcRn inhibition on risk of infection can be obtained from conditions associated with loss of fully functional IgG (Table II). Nephrotic syndrome is typically associated with proteinuria, resulting in peripheral edema and hypoalbuminemia due to increased glomerular permeability caused by a number of primary and secondary glomerular diseases.53 Because of its large molecular weight (150 kDa) compared with albumin (70 kDa), only a subset of patients with nephrotic syndrome experience loss of IgG. Nephrotic syndromes with nonselective protein loss that includes IgG have less favorable prognoses than those with selective loss of small proteins, because they frequently lead to tubulointerstitial damage and decreased likelihood of remission.79 Patients with nephrotic syndrome have increased susceptibility to infection due to hypogammaglobulinemia, reduced complement activity, and treatment-related (corticosteroids, immunosuppressive agents) decrease in T-cell function,80 as well as increased risk of clotting disorders and cellulitis due to peripheral edema (Table II). Infections have been reported in up to 20% of adult patients, with bacterial infections such as pneumonia and cellulitis being the most common.53
Protein-losing enteropathy (PLE) can result in loss of all serum proteins, including immunoglobulins, and sometimes WBC into the gut lumen (Table II). PLE has been observed in a range of GI and non-GI conditions including cardiac disease, liver disease, and systemic lupus erythematosus, and, rarely, arises after the Fontan procedure for single-ventricle congenital heart disease.56 Because albumin and IgG contribute to most of the total osmotic effect of human serum, peripheral edema, ascites, and pleural effusions are the most common clinical complications of PLE. The loss of immunoglobulins and coagulation factors increases the risk of bacterial infections and clotting disorders.81 The Fontan procedure leads to increased central venous pressure, hypogammaglobulinemia, and lymphopenia secondary to chronic GI loss of lymph. However, severe bacterial infections are infrequent and only a minority require IgRT.58
CD55 deficiency is an autosomal-recessive syndrome that results in hyperactivation of complement, angiopathic thrombosis, and PLE. All patients described to date have normal lymphocyte subsets and antibody production. The PLE-induced decrease in IgA, IgM, IgG, IgE, and albumin is directly related to primary intestinal lymphangiectasia, intestinal inflammation, and thromboses (Table II).59 Five of 11 patients experienced recurrent respiratory infections associated with hypogammaglobulinemia; infections were reduced in 2 patients after treatment with IVIg.59
Although patients with nephrotic syndrome, PLE, and CD55 deficiency have extensive protein loss in common, their antibody production is normal, suggesting that most symptoms are associated with protein loss. The infection susceptibility profiles observed in these conditions are typically mild and limited to sinopulmonary infections and cutaneous cellulitis, and are less frequent than in patients with abnormal antibody production. In contrast to FcRn inhibitors, the protein loss in these conditions is not limited to IgG but, depending on disease severity, can include low- and high-molecular-weight proteins such as albumin, IgA, IgM, IgG, IgE, and complement, as well as cellular elements. Loss of albumin and associated peripheral edema is the dominant risk factor for cellulitis, and therefore unlikely to be a concern for treatment with FcRn inhibitors.
Genetic disorders with mechanistic similarities to inhibition of FcRn
The β2 microglobulin (B2M) gene is required for cell-surface expression of nearly all members of the MHC-I family, including FcRn. B2M deficiency has been described in 2 pairs of siblings.60 , 61 Although the genetic mutations and subsequent levels of B2M expression differed between the pairs of siblings, all 4 patients had normal-to-high IgA levels, variable IgM levels, and low serum albumin and IgG levels due to a lack of functional FcRn (Table II). Because of its role in mediating cell-surface expression of MHC-I molecules, the impact of B2M deficiency was not limited to humoral immunity. The patients had a complex immunodeficiency impacting both the innate and the adaptive immune systems, characterized by altered differentiation of CD8 T cells and a lack of functional natural killer cells.61 Clinically, patients presented with recurrent bacterial respiratory tract infections, bronchiectasis, and inflammatory skin lesions, all of varying severity, resembling transport associated with antigen processing deficiency and other MHC-I deficiencies.60 , 61
Myotonic dystrophy is an autosomal-dominant disorder characterized by muscle weakness, myotonia, cataracts, and cardiac conduction defects. Hypogammaglobulinemia is a lesser-known symptom of myotonic dystrophy type 1, with patients typically having low IgG1 and IgG3, with normal levels of IgM, IgA, IgG2, IgG4, and albumin; patients retain the ability to produce protective specific vaccine-related antibody titers (Table II).62 It has previously been proposed that alterations to the FcRn receptor in myotonic dystrophy type 1 lead to impaired recycling and hypercatabolism of IgG.82 Although patients with myotonic dystrophy type 1 and hypogammaglobulinemia have been reported to experience increased infection risk, the resulting burden of infection in these patients does not appear to correlate directly with serum levels of IgG, and the mechanism by which IgG levels are reduced remains unclear.62
Mannosyl-oligosaccharide glucosidase mutations represent 1 of more than 100 types of congenital disorders of glycosylation identified to date and caused by defects in protein or lipid glycosylation.63 N-glycosylation disorders such as mannosyl-oligosaccharide glucosidase-congenital disorders of glycosylation result in intrinsic defects, affecting immunoglobulin structure and stability, and can alter IgG function and half-life (Table II).83 Case studies of 6 patients have reported variable infection rates and a complex influence on vaccine responses.63 , 64 , 84
B2M deficiency presents with a complex immunologic picture, myotonic dystrophy, and mannosyl-oligosaccharide glucosidase-congenital disorders of glycosylation selectively shorten the half-life of IgG without impacting IgA, IgM, IgE, or other components of the immune system. In addition, in each of these conditions, reductions in IgG do not appear to correlate directly with infection risk.
Practical considerations
Inhibition of FcRn reduces both pathogenic and nonpathogenic IgG (but does not change the production or quality of IgG), without affecting other components of the innate or adaptive immune systems. Based on the multiple lines of evidence reviewed, it would be expected that patients receiving an FcRn inhibitor will have limited risk of increased infection. Here, we provide our considerations for clinicians in terms of diagnostic assessments, monitoring of patients, and vaccination, before and during FcRn inhibition. These principles are based on the limited currently available clinical evidence and are driven by theoretical and scientific deliberations, because FcRn inhibitors are still in clinical development and yet to be licensed for use. We expect these principles to evolve as clinical trial data and real-world evidence emerge, and in accordance with future individual drug labels.
Diagnostic assessments before FcRn inhibition
- Establish any possible preexisting risks for infection from the clinical history and with the following assessments:
-
•WBC count with differential; C-reactive protein, liver, and renal function profiles; serum albumin; and IgG, IgA, and IgM.
-
•
- Establish presence of hepatitis B virus (HBV), hepatitis C virus, and/or HIV in patients at risk for infection:
-
•Serologic and/or PCR screening may need to be performed.
-
•Capacity for seroconversion may be affected by a primary immune defect or by previous therapies; therefore, assessment of the corresponding viral load (VL) may be the preferred approach.
-
○Where possible, storage of serum for future analysis should be considered, because serology testing may be less reliable after treatment.
-
○
-
•
In patients with a family history and/or clinical suspicion of primary immunodeficiency or SID, additional testing beyond immunoglobulin levels could be considered—including (but not limited to) T-, B-, and natural killer–cell enumeration and subset differentiation.
These immunologic tests provide a baseline assessment of the patient to facilitate interpretation of potential changes in these parameters, which is useful because patients with primary immunodeficiency may present with autoimmune complications.
Prophylactic antibiotics before FcRn inhibition
Routine antibiotic prophylaxis for patients before or during FcRn inhibition was not recommended for the clinical trials.
-
•
Specific approaches may be required for some underlying conditions (eg, CD4+ T lymphopenia, splenectomy, or multilobar bronchiectasis) or if there is a burden of infection before initiation of an FcRn inhibitor.
Vaccination
The immunogenicity of vaccines is not currently expected to be compromised by FcRn inhibition. However, the ability to respond to vaccines may be impaired because of other recent immunosuppressant therapy.
- For FcRn inhibition in treatment-naive patients, we would recommend the following:
-
•Recombinant or inactive vaccinations be up-to-date more than or 2 to 4 weeks before commencing treatment, allowing time for adaptive immunity to develop.
-
•Live attenuated vaccines be administered more than or 8 weeks before initiating an FcRn inhibitor, to ensure enough time for clearance of vaccine-associated viremia.
-
○Live attenuated vaccines are generally contraindicated in patients with proven or suspected immunodeficiency, because of the potential for vaccine-related complications.
-
○
-
•
- For patients already receiving an FcRn inhibitor:
-
•There is currently no evidence against administering inactivated and recombinant vaccines.
-
•Kinetics of IgG restoration, following single or multiple dosing of an FcRn inhibitor, suggest that vaccines should be given 2 months or more after the last dose, and more than or 2 to 4 weeks before the next.
-
•Inactivated influenza vaccine should be administered annually, with the timing based on the scenarios described above.
-
•
The safety of live attenuated vaccines in patients receiving an FcRn inhibitor is currently unclear; some clinical trials stipulated that they should be avoided during the trial. Given the current lack of evidence, they should therefore be generally avoided.
Management of infections during long-term FcRn inhibition
Although bacterial respiratory tract infections are thought to be the most likely consequence of FcRn inhibitors, any infection should be thoroughly investigated to establish the anatomical site and microbial etiology, and reported while we gain experience with these agents. Reassessment of the baseline immunologic parameters may also help to determine any changes in immunophenotype that may be associated with the infection.
Novel, rare, or recurring viral infections are always concerning. Measles, influenza, and HBV are vaccination preventable and immunity may be boosted before or during FcRn inhibition, whereas for some other viruses, including the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), at the time of writing there exists no vaccine and no effective antiviral treatment. A case report of nonsevere coronavirus disease 2019 (COVID-19) demonstrated recruitment of immune cell populations and IgM and IgG SARS-CoV-2-binding antibodies before the resolution of symptoms.85 Although the primary antiviral immune response involving IFNs, IgM, IgA, complement, and natural killer and T cells is unlikely to be affected by FcRn inhibition, the presence and the degree of low IgG levels and the potential impact on the primary immune response when exposed to a new virus or the clinical course and severity of COVID-19 is unknown. It is therefore prudent to pause the FcRn inhibitors if a diagnosis of COVID-19 or other severe respiratory virus is confirmed, to mitigate against any consequences of reduced IgG in this scenario.
The risk of FcRn inhibitors in the setting of HBV, hepatitis C virus, and HIV is unknown but would be theorized to be favorable to that of some other immunosuppressive options.
- For patients with active viral infection (eg, detectable VL in blood):
-
•It may be prudent to initiate antiviral treatment before the use of FcRn inhibitors.
-
•Monitor VL while on an FcRn inhibitor.
-
•
- For chronic HBV (characterized by persistence of HBV surface antigen:
-
•Antiviral prophylaxis may be considered before and during FcRn inhibition.
-
•HBV VL monitoring should be considered.
-
•Active monitoring (eg, with liver enzymes and HBV VL), with preemptive initiation of antiviral therapy as required.
-
•Possible referral to an infectious disease specialist.
-
•
Appropriate antimicrobial therapy should be implemented if needed—the choice dependent on severity of infection, anatomical site, microbial etiology, local epidemiological pattern of antimicrobial resistance, and, where possible, microbiological sensitivities. For severe infections (ie, requiring hospitalization or IV therapy), withholding any immunosuppressant treatment should be considered; this decision likely also applies to FcRn inhibition. In nonsevere cases, it is possible that FcRn inhibitors can be continued with concomitant antimicrobial therapy and close monitoring of IgG levels, particularly if the patient demonstrates a favorable evolution. Where an FcRn inhibitor is continued during or reinstituted after treatment of infection, close monitoring for clinical and/or laboratory evidence of disease recurrence is recommended. In cases of severe infection that cannot be controlled with other specific treatments (eg, antibiotics), IgRT may be considered. Any subsequent decision about continued FcRn inhibition would need to be based on individual assessment of the patient, considering also any anticipated further need for IgRT.
As with other novel biologic modulators, the emergence of unanticipated side effects such as opportunistic infections should be monitored in the real-world setting by building registries. Opportunistic infections will need to be reported to the manufacturer and/or regulatory agency for safety purposes and the cases published.
Patients with primary or secondary immune deficiency: Specific considerations
Patients who require IgRT because of primary or secondary immune deficiency may be at risk of serious infections. FcRn inhibition is unlikely to be appropriate for these patients.
Patients who have undergone splenectomy: Specific considerations
Splenectomy remains a treatment option for patients with ITP or common variable immune deficiency with severe autoimmune cytopenias who have not responded to other therapies.86 , 87 In the absence of any FcRn inhibitor–specific recommendations, standard guidelines for patients undergoing splenectomy88 should be followed.
Patients with splenectomy are at risk of overwhelming postsplenectomy infection, a life-threatening sepsis. Where overwhelming postsplenectomy infection develops despite appropriate vaccination, chronic FcRn inhibitor therapy is unlikely to be appropriate and alternative treatments for the underlying condition should be considered.
Monitoring during long-term FcRn inhibition
Postapproval, monitoring of patients receiving repeat cycles of an FcRn inhibitor may include the following:
Summary and conclusions
Based on the evidence available to date, hypogammaglobulinemia caused by FcRn blockade is transient and reversible, and affects only the IgG isotype. Despite the absence of data on specific vaccine and infection-induced antibody titers before, during, and after FcRn blockade, we do not expect that this inhibition impacts plasma cells or B cells, or cells of the innate and adaptive immune systems. By harnessing the IgG salvage and recycling pathway, FcRn inhibitors have the potential to meet an urgent need for a more targeted therapeutic approach to pathogenic IgG reduction and provide a less invasive and time-consuming alternative to PLEX, IA, and immunomodulatory high-dose IVIg therapies.
Glossary.
AUTOANTIBODIES
Antibodies that react with the host’s own tissues.
COMPLEMENT
A cascade of plasma proteins that is activated directly by pathogens or indirectly by pathogen-bound antibody to enhance the ability of antibodies and phagocytic cells to clear microbes and damaged cells.
Fc REGION
The tail region of an antibody that contains the constant regions of the heavy chains and interacts with cell surface Fc receptors or serum complement molecules.
HYPOGAMMAGLOBULINEMIA
The presence of abnormally low IgG levels within the blood.
IMMUNE COMPLEX
A molecule composed of a cluster of interlocked antibodies and bound antigens.
IMMUNOADSORPTION
A procedure wherein separated plasma is passed through an adsorber column to remove specific antibodies and immune complexes. Because other plasma components are unaffected, it has the advantage of removing the need for plasma replacement.
IMMUNOGENICITY
The ability of a foreign substance to elicit an immune response.
LYSOSOMAL DEGRADATION
The digestion of macromolecules, including proteins, nucleic acids, lipids, and oligosaccharides, into their building block molecules within intracellular lysosomes.
OPPORTUNISTIC INFECTIONS
Microbial infections that exhibit increased frequency and severity in patients with compromised immune systems.
PLASMA EXCHANGE
A procedure wherein blood plasma is removed and exchanged with either donor plasma or a plasma substitute, such as albumin and saline.
PRIMARY IMMUNODEFICIENCY
A disorder that results from deficiency in the development and/or function of 1 or more components of the immune system. To be considered primary, the immunodeficiency must not be secondary in nature, such as being a result of other disease, drug treatment, or environmental exposure.
The Editors wish to acknowledge Jared Travers, MD, PhD, for preparing the glossary.
Key concepts and therapeutic implications
-
•
Inhibition of FcRn is a promising therapeutic approach for the treatment of IgG-mediated autoimmune diseases that has the potential to provide an alternative to immunomodulatory high-dose IVIg therapy, PLEX, IA, and other less targeted immunosuppressive therapies.
-
•
Successful treatment with an FcRn inhibitor will induce transient low levels of IgG, including a lowering of IgG autoantibodies and IgG-containing ICs.
-
•
There are no anticipated effects of FcRn inhibitors on IgA, IgM, IgE, complement, plasma cells, B cells, or other cells of the innate or adaptive immune systems.
-
•
Short-term treatment experiences with FcRn inhibitors currently in development do not indicate an increased infection risk.
-
•
Described treatment-related side effects of FcRn inhibitors are headaches and mild GI symptoms (diarrhea, vomiting).
Footnotes
Medical writing support was provided by Sarah Feaver, PhD, on behalf of iMed Comms, an Ashfield Company, part of UDG Healthcare plc, and funded by UCB Pharma in accordance with Good Publications Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). The authors acknowledge Linda Feighery, PhD, CMPP, and Veronica Porkess, PhD, CMPP, of UCB Pharma for publication and editorial support. The article was reviewed and approved for publication by all authors and the sponsor.
Disclosure of potential conflict of interest: H.-H. Peter is receiving consulting fees from and has acted as a member of data monitoring committees for UCB; is an elected member of the Working Group “Blood” of the Robert Koch Institute, Berlin; and is a member of the Drug Safety Commission of the German Medical Association (no fees). H.D. Ochs has received consulting fees from and has acted as a member of a Data Monitoring Committee for UCB. C. Cunningham-Rundles is on advisory committees for UCB Pharma and Momenta, is a consultant for Pharming Group and Atara Biotherapeutics, and has received clinical trials funding from CSL Behring. D.C. Vinh is supported by the Fonds de la Recherche en Santé du Quebec (FRQS) Clinician-scientist scholar Junior 2 program; has also received consultancy fees, clinical trials funding, and honoraria and taken part in speaker bureaus for Avir Pharma, CSL Behring, Cidara Therapeutics, Janssen, and UCB Pharma; and has received research funding from the Canadian Institutes of Health Research. P. Kiessling and B. Greve are employees of UCB Pharma and may hold stock and/or stock options. UCB Pharma is the manufacturer and sponsor of rozanolixizumab, an FcRn inhibitor, which is currently being investigated in clinical trials in a range of autoimmune disorders. S. Jolles has received consultancy fees from and has acted as a member of a Drug Safety Committee for UCB Pharma; has also received consultancy fees, research funding, meeting support, and honoraria, and taken part in speaker bureaus and clinical trials, for CSL Behring, Shire/Takeda, Octapharma, Biotest, SOBI, Grifols, Sanofi, GlaxoSmithKline, The Binding Site, Weatherden, Zarodex, LFB, and Pharming.
Terms in boldface and italics are detailed in the glossary on page 480.
Appendix
Table E1.
Summary of methodology for reported phase 2 clinical trials of FcRn inhibitors
| Indication | No. of patients randomized | Intervention and comparator | Primary end point | Duration of follow-up | Geographical region of recruited patients | Methodology for infection screening before enrollment | Reported infections∗ | Reference |
|---|---|---|---|---|---|---|---|---|
| Efgartigimod: IgG1 Fc fragment modified by 5 point mutations | ||||||||
| MG (NCT02965573) | 24 | IV efgartigimod 10 mg/kg QW vs placebo | Safety and tolerability | 11 wk | Canada, Europe, US | Exclusion criteria
|
Herpes zoster: 1 efgartigimod- treated patient (also on SoC treatment with prednisone and mycophenolate mofetil) | E1 |
| ITP (NCT03102593) | 38 | IV efgartigimod 5 mg/kg or 10 mg/kg, QW (4 doses) vs placebo | Safety and tolerability | Main study: 21 wk OLE: 1 y |
Europe, Ukraine | Not provided | Pneumonia: 1 efgartigimod- treated patient who was deemed unrelated to treatment. No increased risk of infection was apparent |
E2 |
| Rozanolixizumab: IgG4 mAb with a S241P stabilizing hinge mutation | ||||||||
| MG (NCT03052751) | 43 | Period 1: 3 doses of SC rozanolixizumab 7 mg/kg QW Period 2: 3 doses of SC rozanolixizumab 4 mg/kg QW or 7 mg/kg QW vs placebo |
Clinically meaningful improvements in MG outcomes and reductions in autoantibody titers Safety and tolerability |
99 d | Canada, Europe, US | Not provided | Not reported | E3 |
| ITP (NCT02718716) | 66 | Single (15 or 20 mg/kg) or multiple (5 × 4 mg/kg, 3 × 7 mg/kg, 2 × 10 mg/kg weekly) doses of SC rozanolixizumab; total dose in each group ranged from 15 to 21 mg/kg No comparator |
Safety and tolerability | 12 wk | Australia, Europe | Exclusion criteria:
|
No serious infections were seen | E4 |
HCV, Hepatitis C virus; Ig, immunoglobulin; OLE, open-label extension; SoC, standard of care; TB, tuberculosis.
Although neither FcRn inhibitor appears to increase in risk of infection, limitations of these data include small sample sizes and trial duration.
References
- 1.Elkon K., Casali P. Nature and functions of autoantibodies. Nat Clin Pract Rheumatol. 2008;4:491–498. doi: 10.1038/ncprheum0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pyzik M., Sand K.M.K., Hubbard J.J., Andersen J.T., Sandlie I., Blumberg R.S. The neonatal Fc receptor (FcRn): a misnomer? Front Immunol. 2019;10:1540. doi: 10.3389/fimmu.2019.01540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodsky R.A. Warm autoimmune hemolytic anemia. N Engl J Med. 2019;381:647–654. doi: 10.1056/NEJMcp1900554. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y., Chernyavsky A., Webber R.J., Grando S.A., Wang P.H. Critical role of the neonatal Fc receptor (FcRn) in the pathogenic action of antimitochondrial autoantibodies synergizing with anti-desmoglein autoantibodies in pemphigus vulgaris. J Biol Chem. 2015;290:23826–23837. doi: 10.1074/jbc.M115.668061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cines D.B., Bussel J.B., Liebman H.A., Luning Prak ET The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113:6511–6521. doi: 10.1182/blood-2009-01-129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilhus N.E., Tzartos S., Evoli A., Palace J., Burns T.M., Verschuuren J. Myasthenia gravis. Nat Rev Dis Primers. 2019;5:30. doi: 10.1038/s41572-019-0079-y. [DOI] [PubMed] [Google Scholar]
- 7.Roggenbuck J.J., Boucraut J., Delmont E., Conrad K., Roggenbuck D. Diagnostic insights into chronic-inflammatory demyelinating polyneuropathies. Ann Transl Med. 2018;6:337. doi: 10.21037/atm.2018.07.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cacoub P., Comarmond C., Domont F., Savey L., Saadoun D. Cryoglobulinemia vasculitis. Am J Med. 2015;128:950–955. doi: 10.1016/j.amjmed.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Greco A., Rizzo M.I., De Virgilio A., Gallo A., Fusconi M., Pagliuca G., et al. Goodpasture’s syndrome: a clinical update. Autoimmun Rev. 2015;14:246–253. doi: 10.1016/j.autrev.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Kronbichler A., Brezina B., Gauckler P., Quintana L.F., Jayne D.R.W. Refractory lupus nephritis: when, why and how to treat. Autoimmun Rev. 2019;18:510–518. doi: 10.1016/j.autrev.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Nagelkerke S.Q., Kuijpers T.W. Immunomodulation by IVIg and the role of Fc-gamma receptors: classic mechanisms of action after all? Front Immunol. 2014;5:674. doi: 10.3389/fimmu.2014.00674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez E.E., Orange J.S., Bonilla F., Chinen J., Chinn I.K., Dorsey M., et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139:S1–S46. doi: 10.1016/j.jaci.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 13.Shock A., Humphreys D., Nimmerjahn F. Dissecting the mechanism of action of intravenous immunoglobulin in human autoimmune disease: lessons from therapeutic modalities targeting Fcγ receptors. J Allery Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.06.036. [DOI] [PubMed] [Google Scholar]
- 14.FDA Center for Biologics Research . FDA; 2019. Information about immune globulin (human) product shortage. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/information-about-immune-globulin-human-product-shortage. Accessed August 10, 2020. [Google Scholar]
- 15.NHS medical data solutions services. The 8th National Immunoglobulin Database Report 2017 – 2018 version 1: NHS2018 2018/12//. Available at: http://igd.mdsas.com/wp-content/uploads/ImmunoglobulinDatabaseReport201718_v1.pdf. Accessed August 10, 2020.
- 16.Strengers P.F., Klein H.G. Plasma is a strategic resource. Transfusion. 2016;56:3133–3137. doi: 10.1111/trf.13913. [DOI] [PubMed] [Google Scholar]
- 17.Patel D.D., Bussel J.B. Neonatal Fc receptor in human immunity: function and role in therapeutic intervention. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Blumberg L.J., Humphries J.E., Jones S.D., Pearce L.B., Holgate R., Hearn A., et al. Blocking FcRn in humans reduces circulating IgG levels and inhibits IgG immune complex-mediated immune responses. Sci Adv. 2019;5 doi: 10.1126/sciadv.aax9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bril V., Benatar M., Brock M., Greve B., Kiessling P., Woltering F., et al. Proof-of-concept and safety of the anti-FcRn antibody rozanolixizumab in patients with moderate-to-severe generalized myasthenia gravis (GMG): a phase 2a study. Neurology. 2019;92 S43.001. [Google Scholar]
- 20.Howard J.F., Jr., Bril V., Burns T.M., Mantegazza R., Bilinska M., Szczudlik A., et al. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology. 2019;92:e2661–e2673. doi: 10.1212/WNL.0000000000007600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robak T, Kazmierczak M, Jarque I, Musteata V, Trelinski J, Cooper N, et al. Phase 2 multiple dose study of an FcRn inhibitor, rozanolixizumab, in patients with primary immune thrombocytopenia (ITP). Blood Adv. In press. [DOI] [PMC free article] [PubMed]
- 22.Newland A.C., Sánchez-González B., Rejtő L., Egyed M., Romanyuk N., Godar M., et al. Phase 2 study of efgartigimod, a novel FcRn antagonist, in adult patients with primary immune thrombocytopenia. Am J Hematol. 2020;95:178–187. doi: 10.1002/ajh.25680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulrichts P., Guglietta A., Dreier T., van Bragt T., Hanssens V., Hofman E., et al. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J Clin Invest. 2018;128:4372–4386. doi: 10.1172/JCI97911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiessling P., Lledo-Garcia R., Watanabe S., Langdon G., Tran D., Bari M., et al. The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: a randomized phase 1 study. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aan1208. [DOI] [PubMed] [Google Scholar]
- 25.Ling L.E., Hillson J.L., Tiessen R.G., Bosje T., van Iersel M.P., Nix D.J., et al. M281, an anti-FcRn antibody: pharmacodynamics, pharmacokinetics, and safety across the full range of IgG reduction in a first-in-human study. Clin Pharmacol Ther. 2019;105:1031–1039. doi: 10.1002/cpt.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grevys A., Bern M., Foss S., Bratlie D.B., Moen A., Gunnarsen K.S., et al. Fc engineering of human IgG1 for altered binding to the neonatal Fc receptor affects Fc effector functions. J Immunol. 2015;194:5497–5508. doi: 10.4049/jimmunol.1401218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Debre M., Bonnet M.C., Fridman W.H., Carosella E., Philippe N., Reinert P., et al. Infusion of Fc gamma fragments for treatment of children with acute immune thrombocytopenic purpura. Lancet (London, England) 1993;342:945–949. doi: 10.1016/0140-6736(93)92000-j. [DOI] [PubMed] [Google Scholar]
- 28.Kamisawa T., Zen Y., Pillai S., Stone J.H. IgG4-related disease. Lancet (London, England) 2015;385:1460–1471. doi: 10.1016/S0140-6736(14)60720-0. [DOI] [PubMed] [Google Scholar]
- 29.Dechavanne C., Dechavanne S., Sadissou I., Lokossou A.G., Alvarado F., Dambrun M., et al. Associations between an IgG3 polymorphism in the binding domain for FcRn, transplacental transfer of malaria-specific IgG3, and protection against Plasmodium falciparum malaria during infancy: a birth cohort study in Benin. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stapleton N.M., Andersen J.T., Stemerding A.M., Bjarnarson S.P., Verheul R.C., Gerritsen J., et al. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat Commun. 2011;2:599. doi: 10.1038/ncomms1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lefranc M.-P., Lefranc G. Human Gm, Km, and Am allotypes and their molecular characterization: a remarkable demonstration of polymorphism. Methods Mol Biol. 2012;882:635–680. doi: 10.1007/978-1-61779-842-9_34. [DOI] [PubMed] [Google Scholar]
- 32.Dechavanne C., Guillonneau F., Chiappetta G., Sago L., Lévy P., Salnot V., et al. Mass spectrometry detection of G3m and IGHG3 alleles and follow-up of differential mother and neonate IgG3. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel S.Y., Carbone J., Jolles S. The expanding field of secondary antibody deficiency: causes, diagnosis, and management. Front Immunol. 2019;10:33. doi: 10.3389/fimmu.2019.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durandy A., Peron S., Fischer A. Hyper-IgM syndromes. Curr Opin Rheumatol. 2006;18:369–376. doi: 10.1097/01.bor.0000231905.12172.b5. [DOI] [PubMed] [Google Scholar]
- 35.Filion C.A., Taylor-Black S., Maglione P.J., Radigan L., Cunningham-Rundles C. Differentiation of common variable immunodeficiency from IgG deficiency. J Allergy Clin Immunol Pract. 2019;7:1277–1284. doi: 10.1016/j.jaip.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta S., Gupta A. Selective IgM deficiency—an underestimated primary immunodeficiency. Front Immunol. 2017;8:1056. doi: 10.3389/fimmu.2017.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodkinson J.P., Bangs C., Wartenberg-Demand A., Bauhofer A., Langohr P., Buckland M.S., et al. Low IgA and IgM is associated with a higher prevalence of bronchiectasis in primary antibody deficiency. J Clin Immunol. 2017;37:329–331. doi: 10.1007/s10875-017-0381-y. [DOI] [PubMed] [Google Scholar]
- 38.Yel L. Selective IgA deficiency. J Clin Immunol. 2010;30:10–16. doi: 10.1007/s10875-009-9357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamblin A.D., Hamblin T.J. The immunodeficiency of chronic lymphocytic leukaemia. Br Med Bull. 2008;87:49–62. doi: 10.1093/bmb/ldn034. [DOI] [PubMed] [Google Scholar]
- 40.Morrison V.A. Infectious complications in patients with chronic lymphocytic leukemia: pathogenesis, spectrum of infection, and approaches to prophylaxis. Clin Lymphoma Myeloma. 2009;9:365–370. doi: 10.3816/CLM.2009.n.071. [DOI] [PubMed] [Google Scholar]
- 41.Barmettler S., Ong M.S., Farmer J.R., Choi H., Walter J. Association of immunoglobulin levels, infectious risk, and mortality with rituximab and hypogammaglobulinemia. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mikulska M., Lanini S., Gudiol C., Drgona L., Ippolito G., Fernandez-Ruiz M., et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52) Clin Microbiol Infect. 2018;24:S71–S82. doi: 10.1016/j.cmi.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Thiel J., Rizzi M., Engesser M., Dufner A.-K., Troilo A., Lorenzetti R., et al. B cell repopulation kinetics after rituximab treatment in ANCA-associated vasculitides compared to rheumatoid arthritis, and connective tissue diseases: a longitudinal observational study on 120 patients. Arthritis Res Ther. 2017;19:101. doi: 10.1186/s13075-017-1306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thiel J., Troilo A., Salzer U., Schleyer T., Halmschlag K., Rizzi M., et al. Rituximab as induction therapy in eosinophilic granulomatosis with polyangiitis refractory to conventional immunosuppressive treatment: a 36-month follow-up analysis. J Allergy Clin Immunol Pract. 2017;5:1556–1563. doi: 10.1016/j.jaip.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 45.Venhoff N., Effelsberg N.M., Salzer U., Warnatz K., Peter H.H., Lebrecht D., et al. Impact of rituximab on immunoglobulin concentrations and B cell numbers after cyclophosphamide treatment in patients with ANCA-associated vasculitides. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zugmaier G., Topp M.S., Alekar S., Viardot A., Horst H.A., Neumann S., et al. Long-term follow-up of serum immunoglobulin levels in blinatumomab-treated patients with minimal residual disease-positive B-precursor acute lymphoblastic leukemia. Blood Cancer J. 2014;4:244. doi: 10.1038/bcj.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sand K.M., Bern M., Nilsen J., Noordzij H.T., Sandlie I., Andersen J.T. Unraveling the interaction between FcRn and albumin: opportunities for design of albumin-based therapeutics. Front Immunol. 2015;5:682. doi: 10.3389/fimmu.2014.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burkhardt T., Rothe R., Moog R. Immunoglobulin G levels during collection of large volume plasma for fractionation. Transfus Apher Sci. 2017;56:417–420. doi: 10.1016/j.transci.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Tran-Mi B., Storch H., Seidel K., Schulzki T., Haubelt H., Anders C., et al. The impact of different intensities of regular donor plasmapheresis on humoral and cellular immunity, red cell and iron metabolism, and cardiovascular risk markers. Vox Sanguinis. 2004;86:189–197. doi: 10.1111/j.0042-9007.2004.00408.x. [DOI] [PubMed] [Google Scholar]
- 50.Flesland O., Halvorsen R., Solheim B.G., Orjasaeter H. The effect of plasmapheresis on IgG and albumin [in Norwegian] Tidsskr Nor Laegeforen. 1990;110:1936–1937. [PubMed] [Google Scholar]
- 51.Padmanabhan A., Connelly-Smith L., Aqui N., Balogun R.A., Klingel R., Meyer E., et al. Guidelines on the use of therapeutic apheresis in clinical practice—evidence-based approach from the Writing Committee of the American Society for Apheresis: the eighth special issue. J Clin Apher. 2019;34:171–354. doi: 10.1002/jca.21705. [DOI] [PubMed] [Google Scholar]
- 52.Guptill J.T., Juel V.C., Massey J.M., Anderson A.C., Chopra M., Yi J.S., et al. Effect of therapeutic plasma exchange on immunoglobulins in myasthenia gravis. Autoimmunity. 2016;49:472–479. doi: 10.1080/08916934.2016.1214823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hull R.P., Goldsmith D.J.A. Nephrotic syndrome in adults. BMJ. 2008;336:1185–1189. doi: 10.1136/bmj.39576.709711.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaysen G.A., al Bander H. Metabolism of albumin and immunoglobulins in the nephrotic syndrome. Am J Nephrol. 1990;10:36–42. doi: 10.1159/000168192. [DOI] [PubMed] [Google Scholar]
- 55.Kemper M.J., Meyer-Jark T., Lilova M., Müller-Wiefel D.E. Combined T- and B-cell activation in childhood steroid-sensitive nephrotic syndrome. Clin Nephrol. 2003;60:242–247. doi: 10.5414/cnp60242. [DOI] [PubMed] [Google Scholar]
- 56.Levitt D.G., Levitt M.D. Protein losing enteropathy: comprehensive review of the mechanistic association with clinical and subclinical disease states. Clin Exp Gastroenterol. 2017;10:147–168. doi: 10.2147/CEG.S136803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Braamskamp M.J.A.M., Dolman K.M., Tabbers M.M. Clinical practice. Protein-losing enteropathy in children. Eur J Pediatr. 2010;169:1179–1185. doi: 10.1007/s00431-010-1235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morsheimer M.M., Rychik J., Forbes L., Dodds K., Goldberg D.J., Sullivan K., et al. Risk factors and clinical significance of lymphopenia in survivors of the fontan procedure for single-ventricle congenital cardiac disease. J Allergy Clin Immunol Pract. 2016;4:491–496. doi: 10.1016/j.jaip.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 59.Ozen A., Comrie W.A., Ardy R.C., Dominguez Conde C., Dalgic B., Beser O.F., et al. CD55 deficiency, early-onset protein-losing enteropathy, and thrombosis. N Engl J Med. 2017;377:52–61. doi: 10.1056/NEJMoa1615887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waldmann T.A., Terry W.D. Familial hypercatabolic hypoproteinemia: a disorder of endogenous catabolism of albumin and immunoglobulin. J Clin Invest. 1990;86:2093–2098. doi: 10.1172/JCI114947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ardeniz Ö., Unger S., Onay H., Ammann S., Keck C., Cianga C., et al. β2-Microglobulin deficiency causes a complex immunodeficiency of the innate and adaptive immune system. J Allergy Clin Immunol. 2015;136:392–401. doi: 10.1016/j.jaci.2014.12.1937. [DOI] [PubMed] [Google Scholar]
- 62.Sasson S.C., Corbett A., McLachlan A.J., Chen R., Adelstein S.A., Riminton S., et al. Enhanced serum immunoglobulin G clearance in myotonic dystrophy-associated hypogammaglobulinemia: a case series and review of the literature. J Med Case Rep. 2019;13:338. doi: 10.1186/s13256-019-2285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim Y.M., Seo G.H., Jung E., Jang J.H., Kim S.Z., Lee B.H. Characteristic dysmorphic features in congenital disorders of glycosylation type IIb. J Hum Genet. 2018;63:383–386. doi: 10.1038/s10038-017-0386-7. [DOI] [PubMed] [Google Scholar]
- 64.Sadat M.A., Moir S., Chun T.-W., Lusso P., Kaplan G., Wolfe L., et al. Glycosylation, hypogammaglobulinemia, and resistance to viral infections. N Engl J Med. 2014;370:1615–1625. doi: 10.1056/NEJMoa1302846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barahona Afonso A.F., Joao C.M.P. The production processes and biological effects of intravenous immunoglobulin. Biomolecules. 2016;6:15. doi: 10.3390/biom6010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laub R., Baurin S., Timmerman D., Branckaert T., Strengers P. Specific protein content of pools of plasma for fractionation from different sources: impact of frequency of donations. Vox Sanguinis. 2010;99:220–231. doi: 10.1111/j.1423-0410.2010.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bechtloff S., Tran-My B., Haubelt H., Stelzer G., Anders C., Hellstern P. A prospective trial on the safety of long-term intensive plasmapheresis in donors. Vox Sanguinis. 2005;88:189–195. doi: 10.1111/j.1423-0410.2005.00632.x. [DOI] [PubMed] [Google Scholar]
- 68.Schulzki T., Seidel K., Storch H., Karges H., Kiessig S., Schneider S., et al. A prospective multicentre study on the safety of long-term intensive plasmapheresis in donors (SIPLA) Vox Sanguinis. 2006;91:162–173. doi: 10.1111/j.1423-0410.2006.00794.x. [DOI] [PubMed] [Google Scholar]
- 69.Lehmann H.C., Hartung H.P., Hetzel G.R., Stuve O., Kieseier B.C. Plasma exchange in neuroimmunological disorders: part 1: rationale and treatment of inflammatory central nervous system disorders. Arch Neurol. 2006;63:930–935. doi: 10.1001/archneur.63.7.930. [DOI] [PubMed] [Google Scholar]
- 70.Behzad M., Mobs C., Kneisel A., Moller M., Hoyer J., Hertl M., et al. Combined treatment with immunoadsorption and rituximab leads to fast and prolonged clinical remission in difficult-to-treat pemphigus vulgaris. Br J Dermatol. 2012;166:844–852. doi: 10.1111/j.1365-2133.2011.10732.x. [DOI] [PubMed] [Google Scholar]
- 71.Kasperkiewicz M., Shimanovich I., Meier M., Schumacher N., Westermann L., Kramer J., et al. Treatment of severe pemphigus with a combination of immunoadsorption, rituximab, pulsed dexamethasone and azathioprine/mycophenolate mofetil: a pilot study of 23 patients. Br J Dermatol. 2012;166:154–160. doi: 10.1111/j.1365-2133.2011.10585.x. [DOI] [PubMed] [Google Scholar]
- 72.Galldiks N., Burghaus L., Dohmen C., Teschner S., Pollok M., Leebmann J., et al. Immunoadsorption in patients with chronic inflammatory demyelinating polyradiculoneuropathy with unsatisfactory response to first-line treatment. Eur Neurol. 2011;66:183–189. doi: 10.1159/000331011. [DOI] [PubMed] [Google Scholar]
- 73.Lieker I., Slowinski T., Harms L., Hahn K., Klehmet J. A prospective study comparing tryptophan immunoadsorption with therapeutic plasma exchange for the treatment of chronic inflammatory demyelinating polyneuropathy. J Clin Apher. 2017;32:486–493. doi: 10.1002/jca.21546. [DOI] [PubMed] [Google Scholar]
- 74.Cortese I., Chaudhry V., So Y.T., Cantor F., Cornblath D.R., Rae-Grant A. Evidence-based guideline update: plasmapheresis in neurologic disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011;76:294–300. doi: 10.1212/WNL.0b013e318207b1f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Winters J.L. Plasma exchange: concepts, mechanisms, and an overview of the American Society for Apheresis guidelines. Hematology Am Soc Hematol Educ Program. 2012;2012:7–12. doi: 10.1182/asheducation-2012.1.7. [DOI] [PubMed] [Google Scholar]
- 76.Kronbichler A., Brezina B., Quintana L.F., Jayne D.R.W. Efficacy of plasma exchange and immunoadsorption in systemic lupus erythematosus and antiphospholipid syndrome: a systematic review. Autoimmun Rev. 2016;15:38–49. doi: 10.1016/j.autrev.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 77.Schmaldienst S., Mullner M., Goldammer A., Spitzauer S., Banyai S., Horl W.H., et al. Intravenous immunoglobulin application following immunoadsorption: benefit or risk in patients with autoimmune diseases? Rheumatology (Oxford, England) 2001;40:513–521. doi: 10.1093/rheumatology/40.5.513. [DOI] [PubMed] [Google Scholar]
- 78.Som S., Deford C.C., Kaiser M.L., Terrell D.R., Kremer Hovinga J.A., Lammle B., et al. Decreasing frequency of plasma exchange complications in patients treated for thrombotic thrombocytopenic purpura-hemolytic uremic syndrome, 1996 to 2011. Transfusion. 2012;52:2525–2532. doi: 10.1111/j.1537-2995.2012.03646.x. quiz 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bazzi C., Petrini C., Rizza V., Arrigo G., D’Amico G. A modern approach to selectivity of proteinuria and tubulointerstitial damage in nephrotic syndrome. Kidney Int. 2000;58:1732–1741. doi: 10.1046/j.1523-1755.2000.00334.x. [DOI] [PubMed] [Google Scholar]
- 80.KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl. 2012;2:139. [Google Scholar]
- 81.Parrish C.R., Dibaise J.K., Copland A.P. Protein losing enteropathy: diagnosis and management. Pract Gastroenterol. 2017;162:22–37. [Google Scholar]
- 82.Kim J., Hayton W.L., Robinson J.M., Anderson C.L. Kinetics of FcRn-mediated recycling of IgG and albumin in human: pathophysiology and therapeutic implications using a simplified mechanism-based model. Clin Immunol (Orlando, Fla) 2007;122:146–155. doi: 10.1016/j.clim.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gudelj I., Lauc G., Pezer M. Immunoglobulin G glycosylation in aging and diseases. Cell Immunol. 2018;333:65–79. doi: 10.1016/j.cellimm.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 84.Li M., Xu Y., Wang Y., Yang X.-A., Jin D. Compound heterozygous variants in MOGS inducing congenital disorders of glycosylation (CDG) IIb. J Hum Genet. 2019;64:265–268. doi: 10.1038/s10038-018-0552-6. [DOI] [PubMed] [Google Scholar]
- 85.Thevarajan I., Nguyen T.H.O., Koutsakos M., Druce J., Caly L., van de Sandt C.E., et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med. 2020;26:453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chaturvedi S., Arnold D.M., McCrae K.R. Splenectomy for immune thrombocytopenia: down but not out. Blood. 2018;131:1172–1182. doi: 10.1182/blood-2017-09-742353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wong G.K., Goldacker S., Winterhalter C., Grimbacher B., Chapel H., Lucas M., et al. Outcomes of splenectomy in patients with common variable immunodeficiency (CVID): a survey of 45 patients. Clin Exp Immunol. 2013;172:63–72. doi: 10.1111/cei.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonanni P., Grazzini M., Niccolai G., Paolini D., Varone O., Bartoloni A., et al. Recommended vaccinations for asplenic and hyposplenic adult patients. Hum Vaccin Immunother. 2017;13:359–368. doi: 10.1080/21645515.2017.1264797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maccioni L., Weber S., Elgizouli M., Stoehlker A.-S., Geist I., Peter H.-H., et al. Obesity and risk of respiratory tract infections: results of an infection-diary based cohort study. BMC Public Health. 2018;18:271. doi: 10.1186/s12889-018-5172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nieters A., Blagitko-Dorfs N., Peter H.-H., Weber S. Psychophysiological insomnia and respiratory tract infections: results of an infection-diary-based cohort study. Sleep. 2019;42:zsz098. doi: 10.1093/sleep/zsz098. [DOI] [PubMed] [Google Scholar]
- 91.Nieters A., Weber S., Elgizouli M., Maccioni L., Wolfrum S., Tshiang J.T., et al. Screening score to identify people prone to respiratory tract infections in the community. Int J Respir Med. 2017;2:6–13. [Google Scholar]
- 92.Wilod Versprille L.J.F., van de Loo A.J.A.E., Mackus M., Arnoldy L., Sulzer T.A.L., Vermeulen S.A., et al. Development and validation of the Immune Status Questionnaire (ISQ) Int J Environ Res Public Health. 2019;16:4743. doi: 10.3390/ijerph16234743. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- Howard J.F., Jr., Bril V., Burns T.M., Mantegazza R., Bilinska M., Szczudlik A., et al. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology. 2019;92:e2661–e2673. doi: 10.1212/WNL.0000000000007600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland A.C., Sánchez-González B., Rejtő L., Egyed M., Romanyuk N., Godar M., et al. Phase 2 study of efgartigimod, a novel FcRn antagonist, in adult patients with primary immune thrombocytopenia. Am J Hematol. 2020;95:178–187. doi: 10.1002/ajh.25680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bril V., Benatar M., Brock M., Greve B., Kiessling P., Woltering F., et al. Proof-of-concept and safety of the anti-FcRn antibody rozanolixizumab in patients with moderate-to-severe generalized myasthenia gravis (GMG): a phase 2a study. Neurology. 2019;92 S43.001. [Google Scholar]
- Robak T., Kazmierczak M., Jarque I., Musteata V., Trelinski J., Cooper N., et al. Rozanolixizumab, an anti-FcRn antibody: final results from a phase II, multiple-dose study in patients with primary immune thrombocytopenia. Blood. 2019;134:897. doi: 10.1182/bloodadvances.2020002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameratunga R., Woon S.T., Gillis D., Koopmans W., Steele R. New diagnostic criteria for common variable immune deficiency (CVID), which may assist with decisions to treat with intravenous or subcutaneous immunoglobulin. Clin Exp Immunol. 2013;174:203–211. doi: 10.1111/cei.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcwilliams L.M., Dell Railey M., Buckley R.H. Positive family history, infection, low absolute lymphocyte count (ALC), and absent thymic shadow: diagnostic clues for all molecular forms of severe combined immunodeficiency (SCID) J Allergy Clin Immunol Pract. 2015;3:585–591. doi: 10.1016/j.jaip.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.-F., Wang W.-F., Zhang Y.-D., Zhao W., Wu J., Chen T.-X. Clinical characteristics and genetic profiles of 174 patients with X-linked agammaglobulinemia: report from Shanghai, China (2000-2015) Medicine. 2016;95:e4544. doi: 10.1097/MD.0000000000004544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein J.A., Marino M.C., Lederman H.M., Jones S.M., Sullivan K., Burks A.W., et al. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine. 2006;85:193–202. doi: 10.1097/01.md.0000229482.27398.ad. [DOI] [PubMed] [Google Scholar]
- Ardeniz Ö, Unger S., Onay H., Ammann S., Keck C., Cianga C., et al. β2-Microglobulin deficiency causes a complex immunodeficiency of the innate and adaptive immune system. J Allergy Clin Immunol. 2015;136:392–401. doi: 10.1016/j.jaci.2014.12.1937. [DOI] [PubMed] [Google Scholar]
- Ozen A., Comrie W.A., Ardy R.C., Dominguez Conde C., Dalgic B., Beser O.F., et al. CD55 deficiency, early-onset protein-losing enteropathy, and thrombosis. N Engl J Med. 2017;377:52–61. doi: 10.1056/NEJMoa1615887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadat M.A., Moir S., Chun T.-W., Lusso P., Kaplan G., Wolfe L., et al. Glycosylation, hypogammaglobulinemia, and resistance to viral infections. N Engl J Med. 2014;370:1615–1625. doi: 10.1056/NEJMoa1302846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl W., Hiepe F., Latinis K.M., Thomas M., Scheinberg M.A., Clarke A.N.N., et al. Belimumab reduces autoantibodies, normalizes low complement, and reduces select B-cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. 2012;64:2328–2337. doi: 10.1002/art.34400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugmaier G., Topp M.S., Alekar S., Viardot A., Horst H.A., Neumann S., et al. Long-term follow-up of serum immunoglobulin levels in blinatumomab-treated patients with minimal residual disease-positive B-precursor acute lymphoblastic leukemia. Blood Cancer J. 2014;4:244. doi: 10.1038/bcj.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez L., Sánchez-Escamilla M., Perales M.-A. CAR T cell toxicity: current management and future directions. HemaSphere. 2019;3:e186. doi: 10.1097/HS9.0000000000000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger U., Tam C., Borchmann P., Mcguirk J., Holte H., Waller E., et al. Intravenous immunoglobulin therapy use in patients with relapsed/refractory diffuse large B-cell lymphoma treated with tisagenlecleucel in the Juliet trial. Hematol Oncol. 2019;37:505–507. [Google Scholar]
- Mikulska M., Lanini S., Gudiol C., Drgona L., Ippolito G., Fernandez-Ruiz M., et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52) Clin Microbiol Infect. 2018;24:S71–S82. doi: 10.1016/j.cmi.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Thiel J., Rizzi M., Engesser M., Dufner A.-K., Troilo A., Lorenzetti R., et al. B cell repopulation kinetics after rituximab treatment in ANCA-associated vasculitides compared to rheumatoid arthritis, and connective tissue diseases: a longitudinal observational study on 120 patients. Arthritis Res Ther. 2017;19:101. doi: 10.1186/s13075-017-1306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel J., Troilo A., Salzer U., Schleyer T., Halmschlag K., Rizzi M., et al. Rituximab as induction therapy in eosinophilic granulomatosis with polyangiitis refractory to conventional immunosuppressive treatment: a 36-month follow-up analysis. J Allergy Clin Immunol Pract. 2017;5:1556–1563. doi: 10.1016/j.jaip.2017.07.027. [DOI] [PubMed] [Google Scholar]
- Venhoff N., Effelsberg N.M., Salzer U., Warnatz K., Peter H.H., Lebrecht D., et al. Impact of rituximab on immunoglobulin concentrations and B cell numbers after cyclophosphamide treatment in patients with ANCA-associated vasculitides. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowse M.E.B., Wallace D.J., Furie R.A., Petri M.A., Pike M.C., Leszczyński P., et al. Efficacy and safety of epratuzumab in moderately to severely active systemic lupus erythematosus: results from two phase III randomized, double-blind, placebo-controlled trials. Arthritis Rheum. 2017;69:362–375. doi: 10.1002/art.39856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerichs K.A., Bosman P.W.C., Velzen J.F.V., Fraaij P.L.A., Koopmans M.P.G., Rimmelzwaan G.F., et al. Effect of daratumumab on normal plasma cells, polyclonal immunoglobulin levels, and vaccination responses in extensively pre-treated multiple myeloma patients. Haematologica. 2020;105:e302–e306. doi: 10.3324/haematol.2019.231860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhn N., Pfeuffer S., Ruck T., Gross C.C., Skripuletz T., Klotz L., et al. Alemtuzumab therapy changes immunoglobulin levels in peripheral blood and CSF. Neurol Neuroimmunol Neuroinflamm. 2019;7:e654. doi: 10.1212/NXI.0000000000000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung H.-P., Kieseier B.C. Atacicept: targeting B cells in multiple sclerosis. Ther Adv Neurol Disord. 2010;3:205–216. doi: 10.1177/1756285610371146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafter-Gvili A., Polliack A. Bendamustine associated immune suppression and infections during therapy of hematological malignancies. Leuk Lymphoma. 2016;57:512–519. doi: 10.3109/10428194.2015.1110748. [DOI] [PubMed] [Google Scholar]
- Hoofnagle J.H., Davis G.L., Schafer D.F., Peters M., Avigan M.I., Pappas S.C., et al. Randomized trial of chlorambucil for primary biliary cirrhosis. Gastroenterology. 1986;91:1327–1334. doi: 10.1016/0016-5085(86)90183-6. [DOI] [PubMed] [Google Scholar]
- Fassbinder T., Saunders U., Mickholz E., Jung E., Becker H., Schlüter B., et al. Differential effects of cyclophosphamide and mycophenolate mofetil on cellular and serological parameters in patients with systemic lupus erythematosus. Arthritis Res Ther. 2015;17:92. doi: 10.1186/s13075-015-0603-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin G.D., Owen B.A., Atchley C.E., Novelli G.D., Solomon A. Decreased immunoglobulin production by a human lymphoid cell line following melphalan treatment. Cancer Res. 1982;42:4505–4510. [PubMed] [Google Scholar]
- Bodenheimer H.C., Charland C., Thayer W.R., Schaffner F., Staples P.J. Effects of penicillamine on serum immunoglobulins and immune complex-reactive material in primary biliary cirrhosis. Gastroenterology. 1985;88:412–417. doi: 10.1016/0016-5085(85)90500-1. [DOI] [PubMed] [Google Scholar]
- Lorber A., Simon T., Leeb J., Peter A., Wilcox S., Chrysotherapy Suppression of immunoglobulin synthesis. Arthritis Rheum. 1978;21:785–791. doi: 10.1002/art.1780210708. [DOI] [PubMed] [Google Scholar]
- Mladenovic V., Domljan Z., Rozman B., Jajic I., Mihajlovic D., Dordevic J., et al. Safety and effectiveness of leflunomide in the treatment of patients with active rheumatoid arthritis. Arthritis Rheum. 1995;38:1595–1603. doi: 10.1002/art.1780381111. [DOI] [PubMed] [Google Scholar]
- Glaesener S., Quách T.D., Onken N., Weller-Heinemann F., Dressler F., Huppertz H.-I., et al. Distinct effects of methotrexate and etanercept on the B cell compartment in patients with juvenile idiopathic arthritis. Arthritis Rheum. 2014;66:2590–2600. doi: 10.1002/art.38736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.H., Levinson A.I., Schumacher H.R. Hypogammaglobulinemia and rheumatic disease. Semin Arthritis Rheum. 1993;22:252–264. doi: 10.1016/0049-0172(93)80073-o. [DOI] [PubMed] [Google Scholar]
- Ulrichts P., Guglietta A., Dreier T., Van Bragt T., Hanssens V., Hofman E., et al. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J Clin Invest. 2018;128:4372–4386. doi: 10.1172/JCI97911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L.E., Hillson J.L., Tiessen R.G., Bosje T., Van Iersel M.P., Nix D.J., et al. M281, an anti-FcRn antibody: pharmacodynamics, pharmacokinetics, and safety across the full range of IgG reduction in a first-in-human study. Clin Pharmacol Ther. 2019;105:1031–1039. doi: 10.1002/cpt.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg L.J., Humphries J.E., Jones S.D., Pearce L.B., Holgate R., Hearn A., et al. Blocking FcRn in humans reduces circulating IgG levels and inhibits IgG immune complex-mediated immune responses. Sci Adv. 2019;5 doi: 10.1126/sciadv.aax9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling P., Lledo-Garcia R., Watanabe S., Langdon G., Tran D., Bari M., et al. The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: a randomized phase 1 study. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aan1208. [DOI] [PubMed] [Google Scholar]
- Olumiant EPAR summary of product characteristics. Eli Lilly. https://www.ema.europa.eu/en/documents/product-information/olumiant-epar-product-information_en.pdf Available at:
- Onda M., Ghoreschi K., Steward-Tharp S., Thomas C., O’shea J.J., Pastan I.H., et al. Tofacitinib suppresses antibody responses to protein therapeutics in murine hosts. J Immunol. 2014;193:48–55. doi: 10.4049/jimmunol.1400063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J., Barnett E.V., Macdonald N.S., Klinenberg J.R., Pearson C.M. The effect of azathioprine on gammaglobulin synthesis in man. J Clin Invest. 1972;51:2233–2238. doi: 10.1172/JCI107031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison V.A. Infectious complications in patients with chronic lymphocytic leukemia: pathogenesis, spectrum of infection, and approaches to prophylaxis. Clin Lymphoma Myeloma. 2009;9:365–370. doi: 10.3816/CLM.2009.n.071. [DOI] [PubMed] [Google Scholar]
- Ritter M.L., Pirofski L. Mycophenolate mofetil: effects on cellular immune subsets, infectious complications, and antimicrobial activity. Transpl Infect Dis. 2009;11:290–297. doi: 10.1111/j.1399-3062.2009.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conigliaro P., Triggianese P., Giampa E., Chimenti M.S., Kroegler B., Perricone R. Effects of abatacept on T-lymphocyte sub-populations and ommunoglobulins in patients affected by rheumatoid arthritis. Isr Med Assoc J. 2017;19:406–410. [PubMed] [Google Scholar]
- Rajala H.L.M., Missiry M.E., Ruusila A., Koskenvesa P., Brümmendorf T.H., Gjertsen B.T., et al. Tyrosine kinase inhibitor therapy-induced changes in humoral immunity in patients with chronic myeloid leukemia. J Cancer Res Clin Oncol. 2017;143:1543–1554. doi: 10.1007/s00432-017-2378-6. [DOI] [PubMed] [Google Scholar]
- Sun C., Tian X., Lee Y.S., Gunti S., Lipsky A., Herman S.E.M., et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood. 2015;126:2213–2219. doi: 10.1182/blood-2015-04-639203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirsum C., Glaser C., Gutenberger S., Keller B., Unger S., Voll R.E., et al. Secondary antibody deficiency in glucocorticoid therapy clearly differs from primary antibody deficiency. J Clin Immunol. 2016;36:406–412. doi: 10.1007/s10875-016-0264-7. [DOI] [PubMed] [Google Scholar]
- Lee J., Choi T.G., Ha J., Kim S.S. Cyclosporine A suppresses immunoglobulin G biosynthesis via inhibition of cyclophilin B in murine hybridomas and B cells. Int Immunopharmacol. 2012;12:42–49. doi: 10.1016/j.intimp.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Go T. Carbamazepine-induced IgG1 and IgG2 deficiency associated with B cell maturation defect. Seizure. 2004;13:187–190. doi: 10.1016/S1059-1311(03)00086-4. [DOI] [PubMed] [Google Scholar]
- Svalheim S., Mushtaq U., Mochol M., Luef G., Rauchenzauner M., Frøland S.S., et al. Reduced immunoglobulin levels in epilepsy patients treated with levetiracetam, lamotrigine, or carbamazepine. Acta Neurol Scand Suppl. 2013 doi: 10.1111/ane.12044. 11-5. [DOI] [PubMed] [Google Scholar]
- Aarli J.A. Changes in serum immunoglobulin levels during phenytoin treatment of epilepsy. Acta Neurol Scand. 1976;54:423–430. doi: 10.1111/j.1600-0404.1976.tb04374.x. [DOI] [PubMed] [Google Scholar]
- Ashrafi M.-R., Hosseini S.-A., Biglari M., Abolmaali S., Azizi Malamiri R., Mombeini H., et al. Effect of anti-epileptic drugs on serum level of IgG subclasses. Iran J Pediatr. 2010;20:269–276. [PMC free article] [PubMed] [Google Scholar]
- Abe S., Suzuki T., Hori T., Baba A., Shiraishi H. Hypogammaglobulinemia during antipsychotic therapy. Psychiatry Clin Neurosci. 1998;52:115–117. doi: 10.1111/j.1440-1819.1998.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Ponsford M., Castle D., Tahir T., Robinson R., Wade W., Steven R., et al. Clozapine is associated with secondary antibody deficiency. Br J Psychiatry. 2018;214:1–7. doi: 10.1192/bjp.2018.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin A.D., Hamblin T.J. The immunodeficiency of chronic lymphocytic leukaemia. Br Med Bull. 2008;87:49–62. doi: 10.1093/bmb/ldn034. [DOI] [PubMed] [Google Scholar]
- Tamburello A., Castelnovo L., Faggioli P., Bompane D., Brando B., Gatti A., et al. Good’s syndrome, a rare form of acquired immunodeficiency associated with thymomas. Clin Pract. 2019;9:1112. doi: 10.4081/cp.2019.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M., Berger M., Gale R.P., Lazarus H.M. Immunoglobulin therapy in hematologic neoplasms and after hematopoietic cell transplantation. Blood Rev. 2018;32:106–115. doi: 10.1016/j.blre.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Sørrig R., Klausen T.W., Salomo M., Vangsted A.J., Frølund U.C., Andersen K.T., et al. Immunoparesis in newly diagnosed multiple myeloma patients: effects on overall survival and progression free survival in the Danish population. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Persona E., Vidriales M.-B., Mateo G., García-Sanz R., Mateos M.-V., De Coca A.G., et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110:2586–2592. doi: 10.1182/blood-2007-05-088443. [DOI] [PubMed] [Google Scholar]
- Hunter Z.R., Manning R.J., Hanzis C., Ciccarelli B.T., Ioakimidis L., Patterson C.J., et al. IgA and IgG hypogammaglobulinemia in Waldenström’s macroglobulinemia. Haematologica. 2010;95:470–475. doi: 10.3324/haematol.2009.010348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegmann J.L., Moreno G., Aláez C., Osorio S., Granda A., De La Cámara R., et al. Chronic myeloid leukemia patients resistant to or intolerant of interferon alpha and subsequently treated with imatinib show reduced immunoglobulin levels and hypogammaglobulinemia. Haematologica. 2003;88:762–768. [PubMed] [Google Scholar]
- Kelesidis T., Yang O. Good’s syndrome remains a mystery after 55 years: a systematic review of the scientific evidence. Clin Immunol. 2010;135:347–363. doi: 10.1016/j.clim.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.Y., Carbone J., Jolles S. The expanding field of secondary antibody deficiency: causes, diagnosis, and management. Front Immunol. 2019;10:33. doi: 10.3389/fimmu.2019.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufti G.J., Figes A., Hamblin T.J., Oscier D.G., Copplestoni J.A. Immunological abnormalities in myelodysplastic syndromes, I: serum immunoglobulins and autoantibodies. Br J Haematol. 1986;63:143–147. doi: 10.1111/j.1365-2141.1986.tb07504.x. [DOI] [PubMed] [Google Scholar]
- Frangoul H., Min E., Wang W., Chandrasekhar R., Calder C., Evans M., et al. Incidence and risk factors for hypogammaglobulinemia in pediatric patients following allo-SCT. Bone Marrow Transplant. 2013;48:1456–1459. doi: 10.1038/bmt.2013.76. [DOI] [PubMed] [Google Scholar]
- Sarmiento E., Rodríguez-Molina J., Muñoz P., Fernández-Yánez J., Palomo J., Fogueda M., et al. Decreased levels of serum immunoglobulins as a risk factor for infection after heart transplantation. Transplant Proc. 2005;37:4046–4049. doi: 10.1016/j.transproceed.2005.09.153. [DOI] [PubMed] [Google Scholar]
- Goldfarb N.S., Avery R.K., Goormastic M., Mehta A.C., Schilz R., Smedira N., et al. Hypogammaglobulinemia in lung transplant recipients. Transplantation. 2001;71:242–246. doi: 10.1097/00007890-200101270-00013. [DOI] [PubMed] [Google Scholar]
- Carbone J., Micheloud D., Salcedo M., Rincon D., Bañares R., Clemente G., et al. Humoral and cellular immune monitoring might be useful to identify liver transplant recipients at risk for development of infection. Transpl Infect Dis. 2008;10:396–402. doi: 10.1111/j.1399-3062.2008.00329.x. [DOI] [PubMed] [Google Scholar]
- Ku G., Varghese Z., Fernando O.N., Baillod R., Hopewell J.P., Moorhead J.F. Serum IgG and renal transplantation. Br Med J. 1973;4:702–707. doi: 10.1136/bmj.4.5894.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer D.G., Kattan O.M., Wozniak L.J., Marcus E., Ponthieux S., Hwang V., et al. Incidence, timing, and significance of early hypogammaglobulinemia after intestinal transplantation. Transplantation. 2013;95:1154–1159. doi: 10.1097/TP.0b013e3182869d05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysen G.A., Al Bander H. Metabolism of albumin and immunoglobulins in the nephrotic syndrome. Am J Nephrol. 1990;10:36–42. doi: 10.1159/000168192. [DOI] [PubMed] [Google Scholar]
- Levitt D.G., Levitt M.D. Protein losing enteropathy: comprehensive review of the mechanistic association with clinical and subclinical disease states. Clin Exp Gastroenterol. 2017;10:147–168. doi: 10.2147/CEG.S136803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Sinani S., Rawahi Y.A., Abdoon H. Octreotide in Hennekam syndrome-associated intestinal lymphangiectasia. World J Gastroenterol. 2012;18:6333–6337. doi: 10.3748/wjg.v18.i43.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda A., Soriano H., Espino A. Gastrointestinal tract involvement in Klippel-Trénaunay syndrome. Lancet Gastroenterol Hepatol. 2018;3:518. doi: 10.1016/S2468-1253(18)30140-7. [DOI] [PubMed] [Google Scholar]
- Lorini R., Ugazio A.G., Cammareri V., Larizza D., Castellazzi A.M., Brugo M.A., et al. Immunoglobulin levels, T-cell markers, mitogen responsiveness and thymic hormone activity in Turner’s syndrome. Thymus. 1983;5:61–66. [PubMed] [Google Scholar]
- Hansbrough J.F., Miller L.M., Field T.O., Gadd M.A. High dose intravenous immunoglobulin therapy in burn patients: pharmacokinetics and effects on microbial opsonization and phagocytosis. Pediatr Infect Dis J. 1988;7:S49–S56. [PubMed] [Google Scholar]
- Celiksoy M.H., Topal E., Sancak R., Catal F., Sogut A. Relationship between hypogammaglobulinemia and severity of atopic dermatitis. Ann Allergy Asthma Immunol. 2014;113:467–469. doi: 10.1016/j.anai.2014.06.025. [DOI] [PubMed] [Google Scholar]
- Hoskote A.U., Ramaiah R.N., Cale C.M., Hartley J.C., Brown K.L. Role of immunoglobulin supplementation for secondary immunodeficiency associated with chylothorax after pediatric cardiothoracic surgery. Pediatr Crit Care Med. 2012;13:535–541. doi: 10.1097/PCC.0b013e318241793d. [DOI] [PubMed] [Google Scholar]
- Hostoffer R.W., Macleish S. Noonan’s syndrome associated with hypogammaglobulinemia. J Allergy Clin Immunol. 2005;115:S160. [Google Scholar]
- Hodge D., Misbah S., Mueller R., Glass E., Chetcuti P. Proteus syndrome and immunodeficiency. Arch Dis Child. 2000;82:234–235. doi: 10.1136/adc.82.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Samra D., Yel L., Agrawal S. T and B cell deficiency associated with yellow nail syndrome. Scand J Immunol. 2012;75:329–335. doi: 10.1111/j.1365-3083.2011.02653.x. [DOI] [PubMed] [Google Scholar]
- Sasson S.C., Corbett A., Mclachlan A.J., Chen R., Adelstein S.A., Riminton S., et al. Enhanced serum immunoglobulin G clearance in myotonic dystrophy-associated hypogammaglobulinemia: a case series and review of the literature. J Med Case Rep. 2019;13:338. doi: 10.1186/s13256-019-2285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenger V., Morath C. Immunoadsorption in nephrology and kidney transplantation. Nephrol Dial Transplant. 2010;25:2407–2413. doi: 10.1093/ndt/gfq264. [DOI] [PubMed] [Google Scholar]
- Krüsmann W., Slanina J., Boser F., Löhr G.W. Changes in concentrations of IgM, IgA and IgM immune globulins after megavolt therapy with the large-field technic in patients with Hodgkin’s disease [in German] Strahlenther Onkol. 1988;164:323–329. [PubMed] [Google Scholar]
- Burkhardt T., Rothe R., Moog R. Immunoglobulin G. levels during collection of large volume plasma for fractionation. Transfus Apher Sci. 2017;56:417–420. doi: 10.1016/j.transci.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Lalan S., Dai H., Warady B.A. Hypogammaglobulinemia in infants receiving chronic peritoneal dialysis. Pediat Nephrol. 2017;32:503–509. doi: 10.1007/s00467-016-3487-1. [DOI] [PubMed] [Google Scholar]
- Guptill J.T., Juel V.C., Massey J.M., Anderson A.C., Chopra M., Yi J.S., et al. Effect of therapeutic plasma exchange on immunoglobulins in myasthenia gravis. Autoimmunity. 2016;49:472–479. doi: 10.1080/08916934.2016.1214823. [DOI] [PMC free article] [PubMed] [Google Scholar]