Abstract
Objective
The objective of this study was to describe the lung sonographic findings of COVID-19 patients prospectively and investigate its association with disease severity.
Methods
This study was conducted in an emergency department and included consecutively enrolled laboratory confirmed COVID-19 patients. Lung sonography findings were described in all the included patients and analysed with respect to the clinical severity of the patients.
Results
106 patients were included in the study. Common sonographic findings in COVID-19 patients were pleural line irregularity or shredding (70% of patients), followed by B – profile (59%), pleural line thickening (33%), occasional B – lines (26%), sub-pleural consolidations (35%), deep consolidations (6%), spared areas (13%), confluent B – lines or waterfall sign (14%) and pleural effusion (9%). These findings tended to be present more bilaterally and in lower lung zones. Sonographic characteristics like bilateral lung involvement, B – profile, spared areas and confluent B – lines or waterfall sign were significantly associated (p < 0.01) with clinical severity (more frequent with increasing disease severity).
Conclusion
The lung sonographic findings of COVID-19 were found more bilaterally and in lower lung zones, and specific findings like B – profile, pleural thickening, spared areas and confluent B – lines or waterfall sign were associated with severe COVID-19.
Keywords: COVID-19, Lung sonography, Ultrasound, Characteristics
1. Introduction
Lung ultrasound (LUS) is a powerful bedside tool which helps in clinical decision making in various conditions [1]. In the COVID-19 pandemic, LUS has shown its major utility in triaging and management of patient due to its point of care use, safety and repeatability [2]. The clinical spectrum of COVID-19 patients range from asymptomatic to critical illness, which can include severe acute respiratory distress (ARDS) requiring ventilatory support [[3], [4], [5], [6]]. LUS can help in early detection, triaging the patients and monitoring the progression the disease [7,8].
Various studies have documented the different lung sonographic findings of COVID-19, which include pleural line abnormalities; focal, multifocal, confluent B-lines and varied patterns of consolidation [7,9]. LUS is highly sensitive and specific in detecting findings of pneumonia and are useful alternative to chest radiograph and computed tomography [10]. Switching to ultrasound for clinical evaluation will reduce physicians need to use the stethoscope as it is difficult to use it while wearing personal protective equipment(PPE) [11]. Ultrasound also gives an advantage of limiting the movement of the patient and thus, preventing unnecessary exposure to healthcare workers and other patients.
In our emergency department (ED), we have incorporated LUS in the initial screening of patients with severe acute respiratory infection. Through this study we investigated the various LUS findings of COVID-19 patients.
2. Materials and methods
2.1. Study setting, design and population
This study was conducted in the ED of a tertiary care hospital of India having an emergency medicine residency program, with an annual ED volume of nearly 200,000 patients. The study period was from April 18 to May 30, 2020. All patients (14 year or older) with suspected COVID-19 were screened prospectively and recruited consecutively. ‘Suspect case’ was defined as the patients with acute respiratory infection i.e. “fever with at least one of the respiratory signs and symptoms like cough or dyspnoea”, after exclusion of any alternative diagnosis, with a history of travel to or residence in country or territory which had reported local transmission of COVID-19 during last 2 weeks prior to symptoms, requiring hospitalisation [12,13]. All patients with respiratory illness and a history of contact (providing health care, sharing same environment, traveling together, etc) with a confirmed COVID-19 case in last 2 weeks were also called as ‘suspect case’ [12,13]. Among them, only ‘laboratory confirmed’ cases (positive nucleic acid of SARS-CoV-2 detected by RT-PCR) were included in the study, and their clinical and lung sonographic findings were documented in a pre-designed data collection form [12]. Confirmed COVID-19 patients were categorised by the treating physician (also performed the ultrasound) according to their severity of illness according to Chinese CDC definitions (mild disease: patients with respiratory tract infection, not fulfilling criteria for severe and critical disease, severe disease: any of the following signs or symptoms like shortness of breath, respiratory rate ≥ 30/min or oxygen saturation ≤ 93%, and critical disease: patients requiring intensive care for organ failure or invasive ventilation) [[14]]. Approval from the Institute Ethics Committee was taken prior to the initiation of this study (IEC-262/17.04.2020).
2.2. Ultrasonographic examination
The SonoSite MicroMaxx Ultrasound device (Bothell, WA 98021, USA), equipped with curvilinear (3–5 MHz) and linear (6–13 MHz) transducers, was used. All patients underwent LUS scanning in a standardized way. Eight lung zones (4 in each hemithorax) were scanned (lung zone #1 - extended from 2nd rib to 6th rib in the mid-clavicular line, lung zone #2 - extended from 6th rib to 10th rib in the mid-clavicular line, lung zone #3 - extended from 4th rib to 12th rib in the mid-axillary line and lung zone #4 - extended from 4th rib to 12th rib in the scapular line). The images of different areas of lungs were examined one after another. The above examinations were performed by emergency physicians with formal training (didactic lectures with hands-on training by EM faculty) in ‘Emergency Ultrasonography’ [15] and minimum of 2-years' experience in performing point-of-care ultrasound (POCUS). Separate ultrasound machines and probes were used for imaging to prevent cross infection. Probe covers and low-level disinfectants were utilised for infection control [16].
2.3. Lung sonographic findings
LUS was performed to look for the following features – unilateral or bilateral lung involvement; B – lines (comet-tail or laser-beam like artifacts arising from pleural line, obliterating the normal A – lines and reaching the base of the US screen), B – profile (≥ 3 B – lines in a single lung sonographic field); confluent B – lines or ‘waterfall sign’ (coalescent thick B – lines like white band), pleural line irregularity or shredding (loss of smoothness of pleural line), pleural thickening (≥ 2 mm), consolidation (subpleural and non-subpleural), spared areas (B – lines with patchy areas of normal lung), and pleural effusion [17,18]. Findings like B – profile and B – lines were exclusive to each other. Above-mentioned findings of patients were recorded in a predesigned data collection form during patient evaluation and images were retrieved and stored in a secured hard drive maintaining patient's confidentiality. Later, these records were retrieved for lab confirmed COVID-19 patients only. These findings were categorised according to the individual lung zones and clinical severity at presentation.
2.4. Statistical analysis
All the observations were entered in database for further statistical analysis. Categorical variables were presented in numbers and percentages (%), and continuous variables were presented as median and interquartile range (IQR). Association of sonographic findings with clinical severity (pair-wise and overall) was assessed by Chi-square test. A p-value of <0.05 was considered statistically significant. Statistical analysis was performed with SPSS (version 25; SPSS, Inc., Chicago, IL)
3. Results
3.1. Clinical characteristics of the study population
A total of 408 suspected COVID-19 patients were screened and 106 laboratory confirmed COVID-19 patients were finally included in the study (Supplementary Fig. 1). Detailed study participants characteristics (demographic data, clinical symptoms, vitals and comorbidities) are depicted in Table – 1. Patients were of older age (median age – 47, IQR: 39–57) and majority of them were male (62%). Common presenting symptoms were shortness of breath (72%), cough (61%) and fever (59%). The median duration of shortness of breath was 3 days (IQR: 3–6) and fever was 4 days (IQR: 3–6). More than two-thirds of patients had comorbid illnesses (63%). At presentation, a total of 29 patients had ‘mild’ disease, 41 patients had ‘severe’ disease, and 36 patients had ‘critical’ disease category.
The following are the supplementary data related to this article.Supplementary Fig. 1.
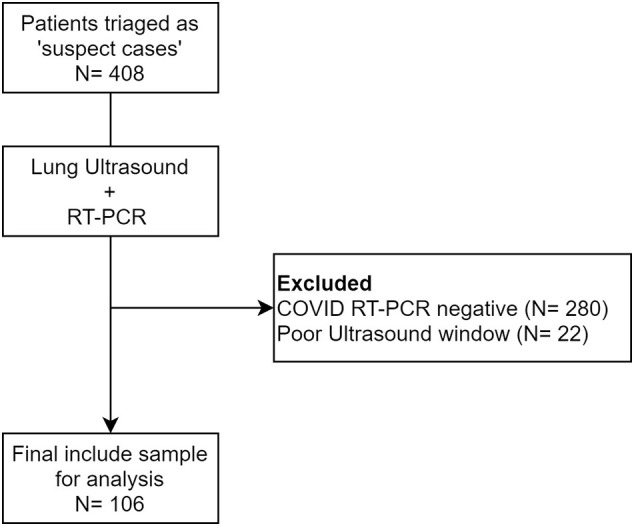
Study flow showing patient inclusion.
Table 1.
Baseline characteristics of the COVID-19 patients.
| All patients (n = 106) | |
|---|---|
| Age (in years), median (IQR) | 47 (39–57) |
| Gender, n (%) | |
| Male | 66 (62.3) |
| Female | 40 (37.7) |
| Presenting symptoms, n (%) | |
| Shortness of breath | 76 (71.7) |
| Cough | 65 (61.3) |
| Fever | 62 (58.5) |
| Muscle aches or joint pain | 19 (17.9) |
| Expectoration | 13 (12.3) |
| Altered sensorium | 13 (12.3) |
| Nausea or vomiting | 12 (11.3) |
| Chills | 11 (10.4) |
| Diarrhea | 10 (9.4) |
| Chest pain | 9 (8.5) |
| Anorexia | 6 (5.7) |
| Abdominal pain | 6 (5.7) |
| Sore throat | 4 (3.8) |
| New onset alteration of taste or smell | 3 (2.8) |
| Running nose | 2 (1.9) |
| Headache | 2 (1.9) |
| Duration of symptoms, median days (IQR) | |
| Fever | 4 (3–6) |
| Shortness of breath | 3 (3–6) |
| Comorbidities, n (%) | |
| Any comorbidity | 67 (63.2) |
| Hypertension | 33 (31.1) |
| Diabetes mellitus | 29 (27.4) |
| Chronic kidney disease | 17 (16) |
| Malignancy | 9 (8.5) |
| Chronic liver disease | 8 (7.5) |
| Coronary artery disease | 8 (7.5) |
| Asthma | 5 (4.7) |
| Cerebrovascular accident | 4 (3.8) |
| Chronic obstructive pulmonary disease | 3 (2.8) |
| Presenting vitals, median (IQR) | |
| Respiratory rate (per minute) | 28 (22−32) |
| Heart rate (per minute) | 111 (88–120) |
| Oxygen saturation (%) | 88 (74–96) |
| Systolic blood pressure (mmHg) | 125 (110–140) |
3.2. Lung sonographic findings
Details of lung sonographic findings are depicted in Table – 2. Most common findings were pleural line irregularity or shredding (70%, Fig. 1A and Video – 1 & 2), followed by B – profile (59%, Fig. 1B and Video 3), pleural line thickening (33%, Fig. 1C) and occasional B – lines (26%, Fig. 1D). Sub-pleural consolidations (35%, Fig. 2A and Video 4) were more frequently found than that of deep consolidations (6%, Fig. 2B). Spared areas and confluent B – lines (Fig. 2C) were found in 13% and 14% of the study population respectively. The pleural effusion was seen in 10 patients (Fig. 2D). These findings tend to be present more bilaterally and in lower lung zones (lung zone – 3 and 4), as shown in Table – 2.
Table 2.
Lung sonographic findings in COVID-19 patients.
| Lung sonography findings | Among all 106 patients in any lung zone, n (%) |
Lung zones-wise findings, n (% among all 106 patients) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Right lung |
Left lung |
||||||||
| Zone-1 | Zone-2 | Zone-3 | Zone-4 | Zone-1 | Zone-2 | Zone-3 | Zone-4 | ||
| Pleural shredding or irregularity | 72 (67.9) | 25 (23.6) | 41 (38.7) | 54 (50.9) | 43 (40.6) | 23 (21.7) | 34 (32.1) | 47 (44.3) | 40 (37.7) |
| B-profile (≥3 per field) | 63 (59.4) | 40 (37.7) | 46 (43.4) | 56 (52.8) | 50 (47.2) | 35 (33.0) | 42 (39.6) | 49 (46.2) | 48 (45.3) |
| Subpleural consolidation | 37 (34.9) | 10 (9.4) | 16 (15.1) | 29 (17.4) | 25 (23.6) | 9 (8.5) | 15 (14.2) | 25 (23.6) | 22 (20.8) |
| Pleural thickening | 35 (33.0) | 14 (13.2) | 15 (14.2) | 25 (23.6) | 13 (12.3) | 12 (11.3) | 18 (17.0) | 23 (21.7) | 20 (18.9) |
| B-lines (<3 per field) | 27 (25.5) | 8 (7.5) | 21 (19.8) | 25 (23.6) | 15 (14.2) | 7 (6.6) | 16 (15.1) | 20 (18.9) | 15 (14.2) |
| Confluent B-lines (waterfall sign) | 15 (14.2) | 9 (8.5) | 12 (11.3) | 14 (13.2) | 14 (13.2) | 7 (6.6) | 8 (7.5) | 14 (13.2) | 15 (14.2) |
| Spared areas | 14 (13.2) | 7 (6.6) | 12 (11.3) | 13 (12.3) | 13 (12.3) | 8 (7.5) | 9 (8.5) | 13 (12.3) | 13 (12.3) |
| Pleural effusion | 10 (9.4) | 0 (0) | 0 (0) | 4 (3.8) | 4 (3.8) | 1 (0.9) | 2 (1.9) | 8 (7.5) | 8 (7.5) |
| Deeper consolidation | 6 (5.7) | 3 (2.8) | 3 (2.8) | 1 (0.9) | 1 (0.9) | 0 (0) | 0 (0) | 1 (0.9) | 1 (0.9) |
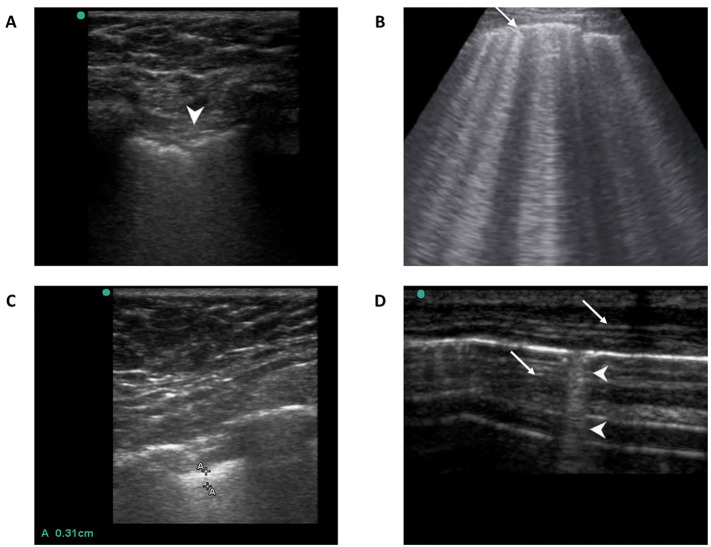
Fig. 1.
Lung sonographic findings; A. Pleural line irregularity or shredding (arrowhead), B. Pleural line thickening (≥ 2 mm), C. B – lines or lung comets (arrowheads), D. B – profile (≥ 3 B – lines in a view shown by thin arrows).
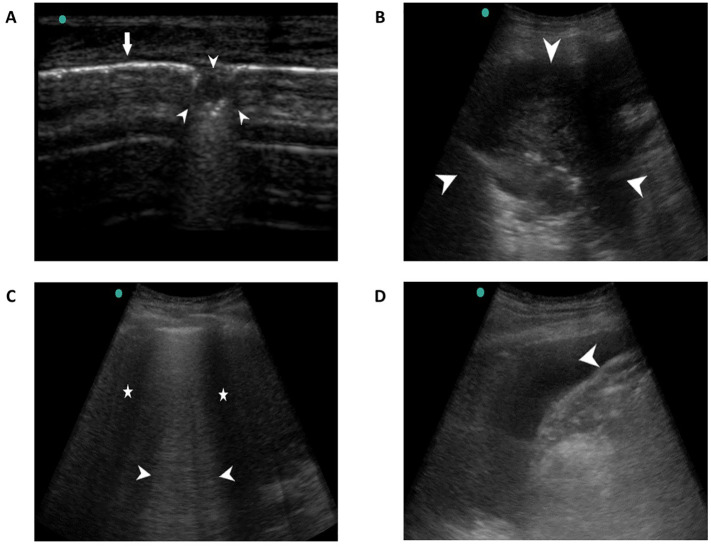
Fig. 2.
Lung sonographic findings; A. Small sub-pleural consolidation shown by arrowheads and pleural line by thin arrow, B. Large consolidation (not sub-pleural, arrowheads), C. Pleural effusion (arrowhead), D. Confluent B – lines (waterfall sign, arrowhead) with spared areas (asterisk).
3.3. Association of lung sonographic findings with clinical severity
All the sonographic findings were analysed according the patient's presenting disease severity (Table – 3). All these findings were more common in ‘severe’ and ‘critically’ ill patients, than that of ‘mild’ ill patients, except occasional B – lines (common in mild disease). Overall comparison of prevalence of bilateral lung involvement was not significant across the disease severity categories (p = 0.095), but pairwise comparison showed more bilateral lung involvement in severe/critical disease, as compared to that of mild disease (p = 0.034). B – profile, pleural thickening, spared areas and confluent B – lines or waterfall sign were significantly correlated with clinical severity (p < 0.05).
Table 3.
Association of lung sonographic findings with disease severity.
| Disease Severity, n (% among the severity category) |
||||
|---|---|---|---|---|
| Lung sonographic findings | Mild disease, 29 (100) |
Severe disease, 41 (100) |
Critical disease, 36 (100) |
p-value |
| Bilateral lung involvement | 20 (60.9) | 36 (87.8) | 31 (86.1) | 0.095# |
| Pleural shredding or irregularity | 18 (62.1) | 30 (73.2) | 24 (66.7) | 0.606 |
| B – profile (≥3 B-lines per field) | 9 (31) | 27 (65.9) | 27 (75) | 0.001* |
| Sub-pleural consolidation | 8 (27.6) | 13 (31.7) | 16 (44.4) | 0.315 |
| Pleural thickening (≥ 2 mm) | 4 (13.8) | 16 (39) | 15 (41.7) | 0.035* |
| B – lines (<3 per field) | 10 (34.5) | 9 (22.0) | 8 (22.2) | 0.426 |
| Confluent B-lines (waterfall sign) | 0 (0) | 9 (22) | 6 (16.7) | 0.030* |
| Spared areas | 0 (0) | 9 (22) | 5 (13.9) | 0.028* |
| Pleural effusion | 3 (10.3) | 4 (9.8) | 3 (8.3) | 0.959 |
| Deeper consolidation | 3 (10.3) | 1 (2.4) | 2 (5.6) | 0.370 |
4. Discussion
The utility of LUS in diagnosis and management of patients with respiratory illness is well documented [1,19]. During this COVID-19 pandemic, our emergency department incorporated the ultrasound in screening patients of acute respiratory illness at the separate triage desk for COVID-19 ‘suspects’ [20,21]. Use of POCUS in clinical evaluation of the patients has many advantages. First, it acts as a visual stethoscope aiding the EP with real time images of the lung, improving their decision power. Second, it removes the need of using an actual stethoscope for auscultation, which becomes difficult to use with the personal protective equipment. Third, this helped us in preventing the movement of patients to a radiology suite, reducing unnecessary exposure to healthcare workers. Studies have shown the LUS findings in COVID-19 correlate strongly with CT findings, so replacing LUS with CT scan reduces radiation exposures to the patient [22,23].
Our study demonstrated COVID-19 LUS findings like pleural line abnormalities (pleural line irregularity or shredding and thickening), B-profile and sub pleural consolidations. In two case series of 20 patients each with confirmed COVID-19 by Peng et al [9] and Huang et al [24], demonstrated similar LUS findings which were consistent with CT findings. Pleural line abnormalities were the most common finding seen in our study. Sub pleural consolidations were more frequently seen when compared to deeper consolidation. This correlated with the more peripheral involvement of the lung in the disease process [17]. Pleural effusion was rarely seen in patients.
Out of the 106 patients included in our study, 36 had ‘critical’ illness who presented to emergency in acute respiratory distress. Acute respiratory distress syndrome (ARDS) is characterized by heterogeneous B lines, with or without lung sliding and subpleural consolidations [25]. Early detection of these finding on a lung ultrasound can help predict the disease severity of the patient. Our study is consistent with Smith et al. [17] that also demonstrated these findings among patients with increased clinical severity. Patients with severe and critical disease had more bilateral lung involvement; there were ‘Confluent B-lines’ known as the waterfall sign with spared areas, which is more specific for a critical illness. As the disease progresses, there is more interstitial thickening and inflammation, leading to an increase in pleural line irregularities and B-line artifacts seen on LUS [9].
It is important to recognize the different characteristic of LUS in COVID-19 patient in different stages of this disease. This will help in initial triage and decision making for such patients. There are certain limitations of using LUS; firstly, it is operator-dependent and requires training for image acquisition and interpretation. Second; an extensive examination of lung using ultrasound can take at least 10 min for the physician [26], which increases the risk of contracting infection from the patient. This could be minimised by following infection control protocols (gowns, masks, gloves and face shields). Third, there is an increased chance of cross-infection if the same probe is used in evaluating COVID and non-COVID patients. This can be prevented by using separate probe covers and low level disinfectants (LLDs; ethyl or isopropyl alcohol, 70%–90%) after each patient [21].
4.1. Limitations
This was a single-centred study, which may not reflect other ED patient population and owing to the small sample size, there is a possibility of missing few LUS characteristics of COVID19. This was a single arm observational design, so further studies comparing the lung ultrasound findings of COVID and non-COVID patient, as well as comparing them with a CT scan of chest, would help in finding specific features unique to COVID-19 patients. Since more patients with severe symptoms presented to our emergency department, the proportion of mild disease was less in our cohort. It is usual to find ‘silent hypoxia’ in COVID-19 patients, so certain early sonographic findings could have been missed as the patients usually presented late. In our study, the same physicians who classified the disease severity were also the ones who performed the POCUS of the patient, which may have introduced bias.
5. Conclusion
Bedside lung sonography has a key role in screening and management of COVID-19 patients. Emergency physicians should be well informed of the sonographic findings of COVID-19 pneumonia and its association of the disease severity. This will aid their decision making process of appropriately triaging the patient and deciding further line of management.
Thickened (> 2 mm thick) and irregular pleural line.
Pleural shredding (irregular pleural line).
B-profile (> 3 B-lines in a lung window) with pleural line irregularity.
Subpleural consolidation.
Author's contributions
Conceptualization of the study: SB, TS.
Data collection: AK, RM.
Data analysis: AK, RM.
Manuscript writing: AK, RM.
Overall conduct of the study: SB.
Data availability statement
The data that supports the findings of this study are available in the supplementary material of this article.
Funding
The authors did not receive any financial support from any source.
Declaration of Competing Interest
The authors did not have any conflicts of interest.
References
- 1.Lichtenstein D.A. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4:1. doi: 10.1186/2110-5820-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith M.J., Hayward S.A., Innes S.M., Miller A.S.C. Point-of-care lung ultrasound in patients with COVID −19 – a narrative review. Anaesthesia. 2020 doi: 10.1111/anae.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottlieb M., Sansom S., Frankenberger C., Ward E., Hota B. Clinical course and factors associated with hospitalization and critical illness among COVID-19 patients in Chicago, Illinois. Academic Emergency Medicine. 2020 doi: 10.1111/acem.14104. n.d.;n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lentz S., Roginski M.A., Montrief T., Ramzy M., Gottlieb M., Long B. Initial emergency department mechanical ventilation strategies for COVID-19 hypoxemic respiratory failure and ARDS. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volpicelli G., Gargani L. Sonographic signs and patterns of COVID-19 pneumonia. The Ultrasound Journal. 2020;12:22. doi: 10.1186/s13089-020-00171-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manivel V., Lesnewski A., Shamim S., Carbonatto G., Govindan T. CLUE: COVID-19 lung ultrasound in emergency department. Emergency Medicine Australasia. 2020 doi: 10.1111/1742-6723.13546. n.d.;n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chinese Critical Care Ultrasound Study Group (CCUSG), Peng Q.-Y., Wang X.-T., Zhang L.-N. Intensive Care Medicine. 2020. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laursen C.B., Sloth E., Lassen A.T., Christensen R dePont, Lambrechtsen J, Madsen PH, et al. Point-of-care ultrasonography in patients admitted with respiratory symptoms: a single-blind, randomised controlled trial. Lancet Respir Med. 2014;2:638–646. doi: 10.1016/S2213-2600(14)70135-3. [DOI] [PubMed] [Google Scholar]
- 11.Buonsenso D., Pata D., Chiaretti A. COVID-19 outbreak: less stethoscope, more ultrasound. Lancet Respir Med. 2020;8 doi: 10.1016/S2213-2600(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Global Surveillance for human infection with coronavirus disease (COVID-19) https://www.who.int/publications-detail/global-surveillance-for-human-infection-with-novel-coronavirus-(2019-ncov) n.d.
- 13.Sahu A.K., Nayer J., Aggarwal P. Novel coronavirus: a capsule review for primary care and acute care physicians. Journal of Family Medicine and Primary Care. 2020;9:1820. doi: 10.4103/jfmpc.jfmpc_217_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 15.AIIMS EMERGENCY SONOGRAPHY (EM SONO) -AIIMS Rishikesh-06 th −07 th May 2019 – The International Council For Critical Emergency Sonography. http://aiimsultrasound.com/courses/aiims-em-sono/ n.d.
- 16.Bhoi S., Sahu A.K., Mathew R., Sinha T.P. Point-of-care ultrasound in COVID-19 pandemic. Postgrad Med J. 2020 doi: 10.1136/postgradmedj-2020-137853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith M.J., Hayward S.A., Innes S.M., Miller A.S.C. Point-of-care lung ultrasound in patients with COVID −19 – a narrative review. Anaesthesia. 2020 doi: 10.1111/anae.15082. anae.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buonsenso D., Piano A., Raffaelli F., Bonadia N., Donati K.D.G., Franceschi F. Point-of-Care Lung Ultrasound findings in novel coronavirus disease-19 pnemoniae: a case report and potential applications during COVID-19 outbreak. Eur Rev Med Pharmacol Sci. 2020;24(5):2776–2780. doi: 10.26355/eurrev_202003_20549. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenstein D.A. Point-of-care ultrasound: infection control in the intensive care unit. Crit Care Med. 2007;35:S262–S267. doi: 10.1097/01.CCM.0000260675.45549.12. [DOI] [PubMed] [Google Scholar]
- 20.Mathew R., Sinha T., Sahu A., Bhoi S. COVID-19 pandemic: a two-step triage protocol for emergency department. J Emerg Trauma Shock. 2020 doi: 10.4103/JETS.JETS_33_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhoi S., Sahu A., Mathew R., Sinha T. Point-of-care ultrasound in COVID-19 pandemic. Postgrad Med J. 2020 doi: 10.1136/postgradmedj-2020-137853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poggiali E., Dacrema A., Bastoni D., Tinelli V., Demichele E., Mateo Ramos P., et al. Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID-19) pneumonia? Radiology. 2020;200847 doi: 10.1148/radiol.2020200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoo J., Murray D., Frenkel O., Barbic D., Francispragasm M., Scheuermeyer F. Emergency department lung ultrasound findings in novel coronavirus. Ann Emerg Med. 2020 doi: 10.1016/j.annemergmed.2020.03.031. [DOI] [Google Scholar]
- 24.Huang Y., Wang S., Liu Y., Zhang Y., Zheng C., Zheng Y., et al. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical novel coronavirus pneumonia (COVID-19) SSRN Electron J. 2020 doi: 10.2139/ssrn.3544750. [DOI] [Google Scholar]
- 25.Baston C., West T.E. Lung ultrasound in acute respiratory distress syndrome and beyond. J Thorac Dis. 2016;8 doi: 10.21037/jtd.2016.12.74. E1763–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rouby J.-J., Arbelot C., Gao Y., Zhang M., Lv J., An Y., et al. Training for lung ultrasound score measurement in critically ill patients. Am J Respir Crit Care Med. 2018;198:398–401. doi: 10.1164/rccm.201802-0227LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Thickened (> 2 mm thick) and irregular pleural line.
Pleural shredding (irregular pleural line).
B-profile (> 3 B-lines in a lung window) with pleural line irregularity.
Subpleural consolidation.
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.