Abstract
Purpose of the Meeting
Bladder pain syndrome/interstitial cystitis is a prevalent but underserved disease. At the Global Interstitial Cystitis/Bladder Pain Syndrome Society (GIBS) meeting, the organization and participants were committed to delivering word-class expertise and collaboration in research and patient care. Under the umbrella of GIBS, leading research scholars from different backgrounds and specialties, as well as clinicians, from across the globe interested in the science and art of practice of Bladder Pain Syndrome (BPS)/Interstitial Cystitis (IC) were invited to deliberate on various dimensions of this disease. The meeting aimed to have global guidelines to establish firm directions to practicing clinicians and patients alike on the diagnosis and treatment of this disease entity. Chronic Pelvic Pain Syndrome (CPPS) is defined by pain in the pelvic area that can have different etiologies. This can be due to urologic, gynecologic, musculoskeletal, gastrointestinal, neurologic, and autoimmune or rheumatologic diseases. At the GIBS meeting held in Mumbai, India, in August 2019, a multidisciplinary expert panel of international urologists, gynecologists, pain specialists, and dietitians took part in a think tank to discuss the development of evidence-based diagnostic and treatment algorithms for BPS/IC.
Summary of Presented Findings
The diagnosis of BPS/IC is difficult in daily clinical practice. Patients with BPS/IC present with a variety of signs and symptoms and clinical test results. Hence, they might be misdiagnosed or underdiagnosed, and the correct diagnosis might take a long time. A good history and physical examination, along with cystoscopy, is a must for the diagnosis of IC/BPS. For the treatment, besides lifestyle management and dietary advice, oral medication and bladder instillation therapy, botulinum toxin, and sacral neuromodulation were discussed. The innovation in bladder instillation applicators, as well as battery-free neuromodulation through the tibial nerve, was discussed, as well.
Recommendation for Future Research
As BPS/IC is complex, for many patients, several treatments are necessary at the same time. This was presented at GIBS 2019 as the piano model. In this way, a combination of treatments is tailored to an individual patient depending on the symptoms, age, and patients’ characteristics. In the art of medicine, especially when dealing with BPS/IC patients, pressing the right key at the right time makes the difference.
Keywords: Bladder Pain Syndrome, Interstitial Cystitis Beyond Horizon, Global Interstitial Cystitis, Bladder Pain Society
1. Purpose of Meeting
Chronic Pelvic Pain Syndrome (CPPS) is defined by pain in the pelvic area that can have different etiologies. This can be due to urologic, gynecologic, musculoskeletal, gastrointestinal, neurologic, and autoimmune or rheumatologic diseases with dramatic psychosocial impacts (1, 2). Patients with predominant bladder pain symptoms without any other diagnosis are often referred to as IC/BPS-patients. The clinical diagnosis is made relying on symptoms such as urinary frequency, urgency and bladder pain, and feeling of bladder pressure with discomfort. In addition, all these symptoms must exist after ruling out any other pathological findings.
According to the definition of the International Society for the Study of Interstitial Cystitis/Bladder Pain Syndrome (ESSIC), BPS/IC is defined by the chronic pelvic pain that a patient feels it is related to the bladder, with urgency and/or frequency (3). Several treatments are recommended by various guidelines, including conservative behavioral treatment and oral medication followed by bladder instillation. In the case of the failure of these first-line treatments, second-line invasive treatments are suggested, such as bladder injection with the botulinum toxin, sacral neuromodulation, and surgery (4).
It is estimated that over eight million women around the world are suffering from BPS/IC symptoms, and many believe that the real prevalence is even higher (5, 6). Women are five times more affected than men (2), and various etiological hypotheses have been suggested, including changes in C-fiber neuroplasticity, urothelial dysfunction, mast cell activation, neurogenic inflammation, and central nervous system pain perception (7). Currently, disease management aims to ameliorate the most bothersome symptoms, such as bladder pain or other lower urinary tract symptoms (8).
At the GIBS meeting held in Mumbai, India, in August 2019, a multidisciplinary expert panel of international urologists, gynecologists, pain specialists, and dietitians took part in a think tank to discuss the development of evidence-based diagnostic and treatment algorithms for BPS/IC. Through presentations and discussions, the panelists went through what is known about the disease and its management and exchanged experiences and ideas for future research. Furthermore, the panel discussed the literature and the existing guidelines and put forward proposals for conducting further research and developing a diagnostic and treatment algorithm. This manuscript was finalized following the interactions of the international panel. However, this work does not represent a systematic review of the literature.
2. Summary of Presented Findings
2.1. Diagnosis of IC/BPS
In daily clinical practice, the diagnosis of BPS/IC is difficult to make. Patients present with a variety of complaints, signs, and clinical test results. Hence, these patients are often misdiagnosed or underdiagnosed (9). A good history and physical examination along with cystoscopy is a must for the diagnosis of IC/BPS (10).
Many patients have a history of mental trauma and stress, which needs to be dealt with. In addition, pain management needs to focus on the character, intensity, duration, and radiation of the pain. The pain score is important in determining the treatment strategy. On the examination, one can find palpable “Myofascial Bands” in addition to undefined nodules in the perineum, suprapubic region, thigh, and even back, of unknown origin. Assurance is very important at each step and the patient should be educated about the disease, and the goals of management should be discussed. Patients and their families should also be told that it takes time for the pain to be controlled, and there is a stepwise approach.
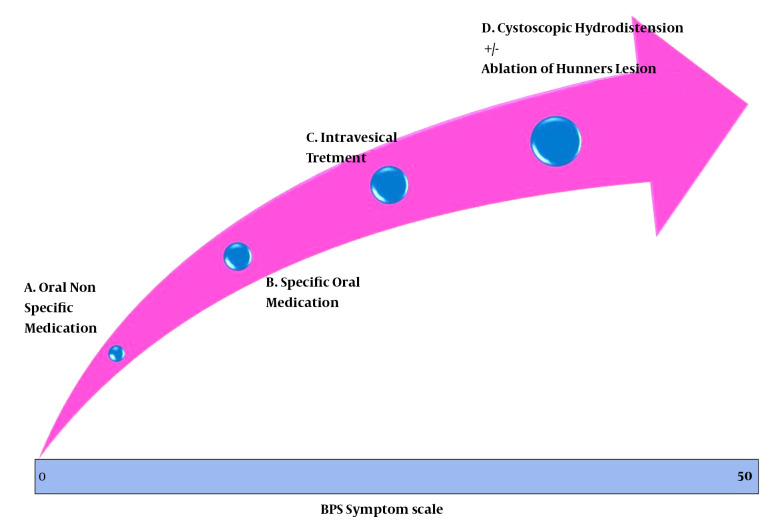
As a corollary, it is essential to clinically grade the patients from the commencement according to the severity and follow the patients on the same scale to objectively assess the response and downgrade or upgrade the already ongoing treatment (11). The symptom-measuring scales, such as the O’Leary-Sant symptom and problem index, help characterize patients, and measure treatment outcomes (10). The mentioned scoring system has four domains with equal weights, i.e., urgency, frequency, nocturia, and pain, and each domain needs to be scored 0 - 5, with a total score of 0 - 20.
Pain severity, as the main and most prominent symptom, has been correlated with frequency and urgency. Similarly, it was discussed in the meeting that nocturia, which may prevent people from attending work, could certainly be more significant when compared to frequency alone. There has been no domain for sexual dysfunction though it may be an indication of more inflammation. Deep dyspareunia may signify a higher intensity of the disease. In addition, no weighting is given to the psychological impact of the disease by this system (12). Therefore, a new scoring system was presented and discussed at the GIBS 2019 meeting, which uses four times amplified score for pain and a double score for nocturia (13). The pain score will then range from 0 to 20 (14) while that of nocturia ranges from 0 to 10. In addition, one domain was added for each sexual dysfunction and psychological impact. Each of these new domains carries a score of 0 - 5. Separate sets of questions have been designed for gender-specific sexual dysfunction evaluation, bringing a total score of 0 - 50. The score can be plotted on a horizontal virtual scale and linked to possible treatment modalities (Figure 1). The initial evaluations have shown that it helps identify patients who may be treated with lifestyle and dietary modifications along with nonspecific oral medications like hydroxyzine and amitriptyline, and at the other hand, this scoring system could help with the choice for more aggressive treatments like ablation of cystoscopically identifiable Hunner’s lesion and hydrodistention. The response to treatment can be quantified using this scale periodically. During the follow-up period, treatment may be modified as per the scale. For example, someone who has initially benefitted from hydrodistension may be managed with medication, but if there is a relapse, as is also documented by the rising score, repeat hydrodistension may be indicated. This New scoring system needs larger multicenter validation studies.
Figure 1. Pictorial Representation of Therapeutic Modalities Linked to the Clinical Scores of BPS/IC.
Hunner Lesion (HL) has been described in 3.5% to 55% of BPS/IC patients. The condition is found mostly in women with an average age of 50 - 60 years. Comorbidities as IBS (irritable bowel syndrome), migraine, vulvodynia, and fibromyalgia were found to be less prevalent. The symptoms are correlated with the extent of the lesion. More pain with penetration and pain distinctly provoked during palpation of the bladder at the site of the lesion have been described. The diagnosis is made on cystoscopy, and the endoscopic features have been well described before, as are the findings of histopathological investigation (15).
Biomarkers of BPS/IC have been the subject of attention in the last decades. It would be very valuable to have such biologic characteristics that can be measured objectively and evaluated as markers for the pathologic state of the patient. The biomarker requirements are not easy to meet, including the measurement in blood or urine specimens, serial measurement to indicate the increases or decreases, and a fast turnaround. For BPS/IC, an additional problem is that neither proper causative definitions nor diagnostic/treatment criteria are well-defined. This can explain why, despite a very long list of biomarkers that have been studied in this syndrome, none has so far been universally accepted (16). In BPS/IC, pathological changes in the bladder wall can be caused by or result in changes in urine components. The Antiproliferative Factor (APF) was found in the urine of 95% of BPS/IC patients, compared to 9% in controls. This may indicate that this factor, if is produced by the urothelium in excessive quantities, can injure the urothelium while also inhibiting the regeneration. In theory, it would make a good biomarker for classic and non-classic BPS/IC. However, no correlation has been found with biopsy findings. After 15 years, the original results have not yet been replicated, and no clinical test is available. Products indicating a deficiency in the GAG layer are still under investigation. Urine inflammatory mediators are not useful, as they are nonspecific. Other factors, such as urine cytokines, substance P, and urine pro-inflammatory genes have been proposed. The macrophage migration inhibitory factor is elevated in HL patients, and an increased level of urinary pro-inflammatory gene expression has also been found in this group. Uromoduline (Tamm-Horsfall protein) may play a role in the pathophysiology. Tryptase, PGE, kallikrein, and many others are probably not specific enough. Nitric oxide appears to be a definitive marker in distinguishing the HL disease from the non-HL disease. Urinary metabolomics could identify a molecular correlate of BPS/IC in a multidisciplinary approach. The MAPP Research Network Cohort has been able with Etiocholan-3α-ol-17-one sulfate (Etio-S) to distinguish female BPS/IC and controls with sensitivity and specificity of > 90% (17) and it’s urinary levels were found to be correlated with elevated symptom scores. This may indicate that underlying biochemical abnormalities contribute to the symptoms of patients with severe IC/BPS.
2.2. Treatment
Lifestyle changes are typically prescribed as the first step of treatment in many ailments. The same applies to IC/BPS. Dietary changes, sometimes minor and sometimes major, go a long way in alleviating the symptoms of IC. Moreover, the majority of patients with BPS/IC have evident food sensitivities and dietary modification is an important part of their treatment plans (18). Because of the various mechanisms by which the bladder can be affected by food components, it is difficult to prescribe a standard IC/BPS-friendly diet that works for all patients effectively. It is not enough to simply identify the offending food. The frequency of intake and portions of the offending food component play significant roles in initiating and triggering bladder flare-ups. This is the reason why the IC/BPS-friendly diet usually provides broad guidelines and a framework to follow but requires customization at the individual level.
The elimination diet is the standard protocol used to identify individual triggers (19). Based on the pathophysiology of IC/BPS, the dietary restrictions include limiting or excluding foods that change the urine to an acidic pH (e.g. coffee, carbonated drinks, some citrus fruits, and chocolates), omitting foods that increase the potassium content of the urine (e.g. tomatoes and sweet lime), excluding any allergy-initiating component present in the food (e.g. gluten, soy, and tofu), and eliminating the components of foods to which the pain receptors in the bladder are sensitive, as these components excite the local nerve endings (e.g. hot spices including chilies, caffeine in tea and other beverages, carbonated beverages, and alcoholic drinks).
Moreover, the increased awareness of these food triggers (coffee, tea, alcoholic beverages, carbonated drinks, citrus fruits, and their juices, tomato-based products, artificial sweeteners, chilies, and spicy foods, and supplements of Vitamin C) remains a central feature of coping with IC/BPS (20). Oral preparations of sodium bicarbonate and calcium glycerophosphate help neutralize the acid in the food and counter the symptoms during a flare-up. Naturally found mucopolysaccharides in oral formulations of Aloe Vera supposedly work by replenishing the defective GAG layer on the bladder lining and preventing irritants from acting on it (21).
To adopt a more holistic approach to the diet, several plant-based foods containing bioactive compounds, which have anti-inflammatory and anti-oxidant properties, should be included in the plan. Bioactive compounds such as flavonoids and Omega 3 fats can be incorporated into the eating plan through some easily available foods. The seeds of mustard, fenugreek, pumpkin, cucumber, flaxseed, sesame, fatty fish, nuts, leafy vegetables, and legumes are the sources of such compounds. Promoting a healthy gut microbiota through the diet or food supplements is also part of an IC diet treatment plan (22). A healthy population of gut microbes flourishes when there are adequate non-digestible portions of food (prebiotics) and probiotics available in the diet. A combination of vegetables, fruits, legumes, nuts, seeds, psyllium, and food products containing probiotics can provide the right medium/substrate to promote healthy gut bacteria. At the same time, avoiding processed and ultra-processed foods that contain synthetic ingredients, preservatives, and artificial sweeteners is crucial in the IC diet.
Today, the BPS/IC diet is no longer a restrictive one, but a modified, personalized version of a diet composed of natural and minimally processed foods from all essential food groups.
In addition to lifestyle management and dietary advice, oral treatment with agents such as Pentosane Polysulfate Sodium (PPS), hydroxyzine hydrochloride, tricyclic antidepressants (including amitriptyline HCL and imipramine), and anticholinergics are recommended for alleviating the lower urinary tract symptoms (23). Moreover, intravesical therapy is frequently used as a second-line treatment for patients who are not benefitting sufficiently from oral treatment (24). The growing prevalence of BPS/IC and the high costs of long-lasting, maintenance therapy make a change of paradigm inevitable. Regularly performed self-instillations significantly reduce expenses, but conventionally used catheterization and the proper identification of the urethral orifice still raise difficulties (25).
To overcome these drawbacks, an easy-to-use device for aiding the procedure of self-instillation and performing the treatment by using the syringe-adapter of the catheter-free instillation was presented and discussed (26). To evaluate the usefulness and applicability of this device, a two-center, prospective clinical trial authorized and governed by the relevant Hungarian regulatory agency (OGYEI) was initiated. Patients were asked to compare their impressions and experiences by employing the new aiding device with the earlier used conventional treatment method. Their experiences were assessed by completing questionnaires at the origin and the conclusion of an eight-week test period. they also collected incidental complications, patients’ feedback, comments, and propositions to improve the product. Pursuing a teaching course, all patients of both groups were capable of using the aiding device alone, without external assistance. Patients performed the self-instillation biweekly (four times during the study period). All of them could instill the drug solution by reducing the leakage by the end of the study. Based on the patients’ feedback, the adequate handling of the device was relatively easy. The advantages of the device got more pronounced in motility-disabled patients and those living far from medical centers.
The self-treatment of BPS/IC by using the newly developed aiding device and the catheter-free instillation with the non-invasive syringe-adapter could represent the optimal treatment solution of the future. Its main advantages included the significant reduction of costs and time, the precise targeting of the orifice, and lower complication rates, which carry the promise of the improved quality of life. All patients would like to keep on using the aiding device and would recommend it to other patients.
Furthermore, a separate discussion was made on pain treatment in patients with IC/BPS. To begin the treatment, the World Health Organization (WHO) stepladder analgesic pattern is followed, which states to use non-opioids, paracetamol, or non-steroidal anti-inflammatory drugs such as diclofenac 50 mg twice to three times a day or Etoricoxib twice a day. However, these medications cannot be given for a long time because of their side effects. If the patient does not respond, we should go to the next step and use weak opioids like tramadol/tapentadol 50 mg twice a day and raise if needed to as high as four times a day. Also, an adjuvant can be added as by the time the patient is diagnosed with chronic pain, and with neuropathic o central sensitization. Adjuvants may include gabapentin starting from 100 mg once to three times a day and can be given up to 900 mg at divided doses. Pregabalin can be used instead of gabapentin at starting doses of 75 mg up to 300 mg at divided doses. Amitryptiline can be given along with all these drugs starting at the dose of 10 mg at night time and the gradual increase to 75 mg at divided doses (27). Moreover, physical therapy may be indicated. It can be started along with pharmacological management in the form of TENS, the release of myofascial bands, and relaxation exercises for pelvic floor (28-30). Pelvic floor strengthening is contraindicated in patients with IC/BPS (29). Along with physical therapy, underlying stress is dealt with by involving psychological counselors for starting cognitive-behavioral therapy. If pharmacological pain management does not work, minimally invasive interventions can be done. Trigger point injections can be given at myofascial bands, which can relieve pain significantly (30, 31). The continuation of physical therapy and cognitive-behavioral therapy is advised.
There are some roles for the Pudendal nerve block in patients whose pain area is in the perineum and anal region. It can be given as a guided block by ultrasound (30, 32). If the pain is widespread in pubic and suprapubic area and is perveived as neuropathic pain, the superior hypogastric plexus block can be regarded as an option for managing the pain (33). Assessment and reassessment is the key to managing the pain. The discussed pain management algorithm is presented in Figure 2.
Figure 2. Algorithm for pain management in patients with BPS/IC.

As the third-line therapy of patients with BPS/IC, both intravesical botulinum toxin A treatment and Sacral Neuromodulation (SNM) have been shown to have positive effects (7).
Percutaneous Tibial Nerve Stimulation (PTNS) has shown good success rates accompanying with almost no side effects when compared to SNM (34, 35). More recently, some clinical trials have reported positive outcomes for battery-free implantable devices that can stimulate the tibial nerve transcutaneously in patients with overactive bladder syndrome (36). It needs to be studied if it is the case in BPS/IC patients. At GIBS 2019, a case series was presented concerning BPS/IC-patients implanted with a battery-free implantable tined lead device to stimulate the tibial nerve (StimRouter™). It resulted in a reduction of urgency incontinence and pain scores. However, these data have not been published and therefore, the implantable tibial nerve stimulation for BPS/IC should only be applied in research settings.
3. Recommendation for Future Research
The presented diagnostic and treatment pathways for BPS/IC at GIBS 2019 once again underlined the variety of non-evidence-based approaches that are currently carried out by physicians throughout the world. However, there was a consensus on the urgent need for new definitions and terminology changes to distinguish between different groups. It was announced and suggested that in the next ESSIC meeting, the subgroup of HL patients should be separated from the rest of the bladder pain syndrome patients who do not have visible changes in their cystoscopy. The underlying reason is that in this subgroup, the therapeutic approach and the prognosis are different. In addition, there was a consensus on performing cystoscopy in patients who fulfill the BPS/IC criteria and have symptoms for more than six months. Cystoscopy in these patients is carried out to rule out conditions that mimic BPS/IC symptoms such as bladder cancer, carcinoma in situ, and bladder tuberculosis.
In the therapeutic approach, there was an agreement on the use of oral agents such as pentosan polysulfate and amitriptyline, as well as intravesical instillation therapy. A novel syringe adapter converts bladder instillations into a pain-free procedure. An assistive device for self-instillation was presented that proved to be an easy to use solution, significantly reducing the treatment expenses. Intravesical botulinum toxin injection, PTNS, and SNM were presented as second-line therapy for BPS/IC that have documented positive outcomes.
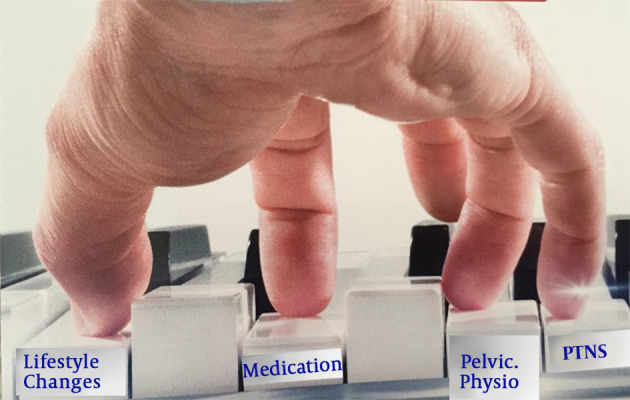
A stepwise approach was presented in a staircase model (Figure 3). In this approach, the least invasive therapy is tried initially, followed by more invasive treatments if needed. Many of the treatments need further research. However, it should be recognized that clinical research in this relatively rare condition with homogenous groups of patients with unclear pathology and phenotype is very difficult. Therefore, the participants of GIBS agreed on the collaboration in research so that resources and patients could be bundled to achieve the maximum output and desired answers from trials.
Figure 3. A stepwise approach to BPS/IC patients.
As BPS/IC is complex, for many patients, several treatments are necessary at the same time. This was presented at GIBS 2019 as the piano model (Figure 4). In this way, a combination of treatments is tailored to an individual patient depending on the symptoms, age, and patients’ characteristics. In the art of medicine, especially when dealing with BPS/IC patients, pressing the right key at the right time makes the difference.
Figure 4. An example of a combination of treatments tailored to BPS/IC patients.
More studies are needed to develop painful bladder syndrome symptom and life quality-measuring scales to apply universally to all treatments and trials of painful bladder syndrome. In addition, large randomized controlled trials are needed for oral and intravesical agents. The clinical benefit of intravesical agents needs to be studied in more detail to determine the best frequency and duration of treatment, the correct dose, and solution composition. In addition, for Sacral nerve modulation, we need an assessment of bilateral stimulation and its role in pain and further evaluation of pudendal nerve stimulation. Furthermore, international common datasets and follow-ups are needed to determine the best selection of surgical methods and outcomes.
Acknowledgments
GIBS is the first global initiative taken by INDIA under the aegis of Swati Spentose Pvt Ltd. This is an independent scientific body conceptualized by a team of physicians lead by Dr. Rajesh Taneja, Dr. Sanjay Pandey and SSPL’ Founder Mr. Vishal Jajodia dedicated exclusive to the IC/BPS.
Footnotes
Authors' Contribution: Drafting of the manuscript: S. R, A. J, N. V, S. L, N. S, M.C, S. P, J. W, and R. T; data collection: S. R, A. J, N. V, S. L, N. S, M.C, S. P, J. W, and R. T; setting tables and graphics: S. R, A. J, N. V, S. L, N. S, M.C, S. P, J. W, and R. T; submission and final revision: A. J; supervision: S. R, A. J, and R. T; organization: S. R, A. J, and R. T.
Conflict of Interests: Dr. Taneja: The organization committee of GIBS; Dr. Rahnama'i: The consultant for Dr. Pfleger, Astellas, Janssen en Biones
Funding/Support: None.
Contributor Information
Mohammad Sajjad Rahnama'i, Email: sajjad_r@yahoo.com.
Aida Javan, Email: aida.javan@hotmail.com.
Navita Vyas, Email: drnavita.nv@gmail.com.
Sandor Lovasz, Email: lovipapa@gmail.com.
Neelanjana Singh, Email: neelanjanasingh@gmail.com.
Mauro Cervigni, Email: mauro.cervigni@libero.it.
Sanjay Pandey, Email: sanjaypdr@gmail.com.
Jean Jacques Wyndaele, Email: wyndaelejj@skynet.be.
Rajesh Taneja, Email: rajeshtanejadr@yahoo.com.
References
- 1.Doggweiler R, Whitmore KE, Meijlink JM, Drake MJ, Frawley H, Nordling J, et al. A standard for terminology in chronic pelvic pain syndromes: A report from the chronic pelvic pain working group of the international continence society. Neurourol Urodyn. 2017;36(4):984–1008. doi: 10.1002/nau.23072. [DOI] [PubMed] [Google Scholar]
- 2.Patnaik SS, Lagana AS, Vitale SG, Buttice S, Noventa M, Gizzo S, et al. Etiology, pathophysiology and biomarkers of interstitial cystitis/painful bladder syndrome. Arch Gynecol Obstet. 2017;295(6):1341–59. doi: 10.1007/s00404-017-4364-2. [DOI] [PubMed] [Google Scholar]
- 3.van de Merwe JP, Nordling J, Bouchelouche P, Bouchelouche K, Cervigni M, Daha LK, et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur Urol. 2008;53(1):60–7. doi: 10.1016/j.eururo.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Khullar V, Digesu GA, Veit-Rubin N, Sahai A, Rahnama'i MS, Tarcan T, et al. How can we improve the diagnosis and management of bladder pain syndrome? Part 2:ICI-RS 2018. Neurourol Urodyn. 2019;38 Suppl 5:S71–s81. doi: 10.1002/nau.24245. [DOI] [PubMed] [Google Scholar]
- 5.Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186(2):540–4. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts RO, Bergstralh EJ, Bass SE, Lightner DJ, Lieber MM, Jacobsen SJ. Incidence of physician-diagnosed interstitial cystitis in Olmsted County: a community-based study. BJU Int. 2003;91(3):181–5. doi: 10.1046/j.1464-410x.2003.04060.x. [DOI] [PubMed] [Google Scholar]
- 7.Rahnama'i MS, Marcelissen T, Apostolidis A, Veit-Rubin N, Schurch B, Cardozo L, et al. The efficacy of botulinum toxin A and sacral neuromodulation in the management of interstitial cystitis (IC)/bladder pain syndrome (BPS), what do we know? ICI-RS 2017 think thank, Bristol. Neurourol Urodyn. 2018;37(S4):S99–S107. doi: 10.1002/nau.23493. [DOI] [PubMed] [Google Scholar]
- 8.Offiah I, Didangelos A, Dawes J, Cartwright R, Khullar V, Bradbury EJ, et al. The Expression of Inflammatory Mediators in Bladder Pain Syndrome. Eur Urol. 2016;70(2):283–90. doi: 10.1016/j.eururo.2016.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson JE, Johnson KE. Ambiguous chronic illness in women: community health nursing concern. J Community Health Nurs. 2006;23(3):159–67. doi: 10.1207/s15327655jchn2303_3. [DOI] [PubMed] [Google Scholar]
- 10.Engeler DS, Baranowski AP, Dinis-Oliveira P, Elneil S, Hughes J, Messelink EJ, et al. The 2013 EAU guidelines on chronic pelvic pain: is management of chronic pelvic pain a habit, a philosophy, or a science? 10 years of development. Eur Urol. 2013;64(3):431–9. doi: 10.1016/j.eururo.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 11.Kushner L, Moldwin RM. Efficiency of questionnaires used to screen for interstitial cystitis. J Urol. 2006;176(2):587–92. doi: 10.1016/j.juro.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 12.Nickel JC, Tripp D, Teal V, Propert KJ, Burks D, Foster HE, et al. Sexual function is a determinant of poor quality of life for women with treatment refractory interstitial cystitis. J Urol. 2007;177(5):1832–6. doi: 10.1016/j.juro.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 13.Taneja R, Massand S. A modified clinical scoring system for bladder pain syndrome: Long term experience. Int J Urol. 2019;26 Suppl 1:61–7. doi: 10.1111/iju.13977. [DOI] [PubMed] [Google Scholar]
- 14.O'Leary MP, Sant GR, Fowler FJ, Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49(5A Suppl):58–63. doi: 10.1016/s0090-4295(99)80333-1. [DOI] [PubMed] [Google Scholar]
- 15.Whitmore KE, Fall M, Sengiku A, Tomoe H, Logadottir Y, Kim YH. Hunner lesion versus non-Hunner lesion interstitial cystitis/bladder pain syndrome. Int J Urol. 2019;26 Suppl 1:26–34. doi: 10.1111/iju.13971. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Freeman MR. Antiproliferative factor signaling and interstitial cystitis/painful bladder syndrome. Int Neurourol J. 2011;15(4):184–91. doi: 10.5213/inj.2011.15.4.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker KS, Crowley JR, Stephens-Shields AJ, van Bokhoven A, Lucia MS, Lai HH, et al. Urinary Metabolomics Identifies a Molecular Correlate of Interstitial Cystitis/Bladder Pain Syndrome in a Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network Cohort. EBioMedicine. 2016;7:167–74. doi: 10.1016/j.ebiom.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herati AS, Shorter B, Srinivasan AK, Tai J, Seideman C, Lesser M, et al. Effects of foods and beverages on the symptoms of chronic prostatitis/chronic pelvic pain syndrome. Urology. 2013;82(6):1376–80. doi: 10.1016/j.urology.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Friedlander JI, Shorter B, Moldwin RM. Diet and its role in interstitial cystitis/bladder pain syndrome (IC/BPS) and comorbid conditions. BJU Int. 2012;109(11):1584–91. doi: 10.1111/j.1464-410X.2011.10860.x. [DOI] [PubMed] [Google Scholar]
- 20.Bassaly R, Downes K, Hart S. Dietary consumption triggers in interstitial cystitis/bladder pain syndrome patients. Female Pelvic Med Reconstr Surg. 2011;17(1):36–9. doi: 10.1097/SPV.0b013e3182044b5c. [DOI] [PubMed] [Google Scholar]
- 21.Whitmore KE. Complementary and alternative therapies as treatment approaches for interstitial cystitis. Rev Urol. 2002;4 Suppl 1:S28–35. [PMC free article] [PubMed] [Google Scholar]
- 22.Arora HC, Eng C, Shoskes DA. Gut microbiome and chronic prostatitis/chronic pelvic pain syndrome. Ann Transl Med. 2017;5(2):30. doi: 10.21037/atm.2016.12.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng E, Hsu YC, Chuang YC. Advances in intravesical therapy for bladder pain syndrome (BPS)/interstitial cystitis (IC). Low Urin Tract Symptoms. 2018;10(1):3–11. doi: 10.1111/luts.12214. [DOI] [PubMed] [Google Scholar]
- 24.Wyndaele JJJ, Riedl C, Taneja R, Lovasz S, Ueda T, Cervigni M. GAG replenishment therapy for bladder pain syndrome/interstitial cystitis. Neurourol Urodyn. 2019;38(2):535–44. doi: 10.1002/nau.23900. [DOI] [PubMed] [Google Scholar]
- 25.Christison K, Walter M, Wyndaele JJM, Kennelly M, Kessler TM, Noonan VK, et al. Intermittent Catheterization: The Devil Is in the Details. J Neurotrauma. 2018 doi: 10.1089/neu.2017.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovasz S. Minimally invasive device for intravesical instillation by urological syringe adapter (MID-ii U.S.A.) for catheter-free instillation therapy of the bladder in interstitial cystitis/bladder pain syndrome. Int J Urol. 2019;26 Suppl 1:57–60. doi: 10.1111/iju.13976. [DOI] [PubMed] [Google Scholar]
- 27.Foster HJ, Hanno PM, Nickel JC, Payne CK, Mayer RD, Burks DA, et al. Effect of amitriptyline on symptoms in treatment naive patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183(5):1853–8. doi: 10.1016/j.juro.2009.12.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson RU, Wise D, Sawyer T, Chan C. Integration of myofascial trigger point release and paradoxical relaxation training treatment of chronic pelvic pain in men. J Urol. 2005;174(1):155–60. doi: 10.1097/01.ju.0000161609.31185.d5. [DOI] [PubMed] [Google Scholar]
- 29.Peters KM, Carrico DJ, Kalinowski SE, Ibrahim IA, Diokno AC. Prevalence of pelvic floor dysfunction in patients with interstitial cystitis. Urology. 2007;70(1):16–8. doi: 10.1016/j.urology.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 30.Han E, Nguyen L, Sirls L, Peters K. Current best practice management of interstitial cystitis/bladder pain syndrome. Ther Adv Urol. 2018;10(7):197–211. doi: 10.1177/1756287218761574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.FitzGerald MP, Payne CK, Lukacz ES, Yang CC, Peters KM, Chai TC, et al. Randomized multicenter clinical trial of myofascial physical therapy in women with interstitial cystitis/painful bladder syndrome and pelvic floor tenderness. J Urol. 2012;187(6):2113–8. doi: 10.1016/j.juro.2012.01.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labat JJ, Riant T, Robert R, Amarenco G, Lefaucheur JP, Rigaud J. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurourol Urodyn. 2008;27(4):306–10. doi: 10.1002/nau.20505. [DOI] [PubMed] [Google Scholar]
- 33.El-Hefnawy AS, Makharita MY, Abed A, Amr YM, Salah El-Badry M, Shaaban AA. Anesthetic Bladder Hydrodistention Is Superior to Superior Hypogastric Plexus Neurolysis in Treatment of Interstitial Cystitis-bladder Pain Syndrome: A Prospective Randomized Trial. Urology. 2015;85(5):1039–44. doi: 10.1016/j.urology.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Kabay S, Kabay SC, Yucel M, Ozden H. Efficiency of posterior tibial nerve stimulation in category IIIB chronic prostatitis/chronic pelvic pain: a Sham-Controlled Comparative Study. Urol Int. 2009;83(1):33–8. doi: 10.1159/000224865. [DOI] [PubMed] [Google Scholar]
- 35.Gokyildiz S, Kizilkaya Beji N, Yalcin O, Istek A. Effects of percutaneous tibial nerve stimulation therapy on chronic pelvic pain. Gynecol Obstet Invest. 2012;73(2):99–105. doi: 10.1159/000328447. [DOI] [PubMed] [Google Scholar]
- 36.van Breda HMK, Martens FMJ, Tromp J, Heesakkers J. A New Implanted Posterior Tibial Nerve Stimulator for the Treatment of Overactive Bladder Syndrome: 3-Month Results of a Novel Therapy at a Single Center. J Urol. 2017;198(1):205–10. doi: 10.1016/j.juro.2017.01.078. [DOI] [PubMed] [Google Scholar]