Abstract
A complex of begomoviruses (Geminiviridae) can cause severe tomato yield losses in the neotropics. Here, next-generation sequencing was employed for large-scale assessment of single-stranded (ss)DNA virus diversity in tomatoes either harboring or lacking the large-spectrum begomovirus tolerance Ty-1 gene. Individual leaf samples exhibiting begomovirus-like symptoms (n = 107) were field-collected, circular DNA-enriched, subdivided into pools (with and without Ty-1), and Illumina-sequenced. Virus-specific PCR and Sanger dideoxy sequencing validations confirmed 15 distinct ssDNA virus/subviral agents (occurring mainly in mixed infections), which highlight the potential drawbacks of employing virus-specific resistance in tomato breeding. More viruses (14 versus 6 species) were observed in tomatoes without the Ty-1 gene. A gemycircularvirus (Genomoviridae), a new alpha-satellite, and two novel Begomovirus species were identified exclusively in samples without the Ty-1 gene. A novel begomovirus was found only in the Ty-1 pool, being the only species associated with severe symptoms in Ty-1 plants in our survey. Our work is the first step towards the elucidation of the potential begomovirus adaptation to Ty-1 and its specific filtering effects on a subset of ssDNA viral/subviral agents.
Keywords: begomoviruses, tomato, resistance gene, NGS, virome
1. Introduction
Geminiviridae is the largest family of plant-infecting viruses, and is currently organized into nine genera: Becurtovirus, Begomovirus, Capulavirus, Curtovirus, Eragrovirus, Grablovirus, Mastrevirus, Topocuvirus, and Turncurtovirus [1]. The classification at the genus level is based upon host range, the associated insect vector(s), genomic organization, and phylogenetic relationships [1,2,3]. In 2016, two novel viral families with non-enveloped, circular, single-stranded DNA (ssDNA) genomes with sizes ranging from 2.0 to 2.4 kb were created and named as Pleolipoviridae and Genomoviridae. The Genomoviridae family also comprises nine genera: Gemycircularvirus, Gemyduguivirus, Gemygorvirus, Gemykibivirus, Gemykolovirus, Gemykrogvirus, Gemykroznavirus, Gemytondvirus, and Gemyvongvirus [1].
The genus Begomovirus is composed of whitefly-transmitted species with one (=monopartite) or two (=bipartite) circular, ssDNA genomic component(s) with ≈2.6 kb that are encapsidated separately into twinned particles formed by two incomplete icosahedrons [2,4]. The begomovirus transmission is characterized as being non-propagative and circulative, and is carried out by members of the Bemisia tabaci (Hemiptera: Aleyrodidae) cryptic species complex [5]. The begomoviruses display a set of mechanisms for generating genetic variability such as mutation, recombination, and pseudo-recombination, which have a direct influence in the continuous emergence of new species that are often reported in this genus [6,7,8].
The tomato (Solanum lycopersicum L.) crop is grown year-round across major tropical and subtropical regions [9]. In Brazil, outbreaks of Begomovirus species in tomatoes became more intensively reported after the invasion of B. tabaci Middle East–Asia Minor 1 (MEAM 1 = biotype B) in the early 1990s [10]. The well-known biological attributes of B. tabaci MEAM 1 (viz. large host range, ability to transmit a wide range of viral species, and adaptation to distinct environmental conditions) facilitated the rapid dispersal of tomato-infecting begomoviruses across all major producing areas of the country [6]. Field surveys conducted afterward have revealed an extremely diverse complex of Begomovirus species (composed mainly by bipartite viruses), occurring in all Brazilian biomes. Currently, over 21 tomato-infecting Begomovirus species have been characterized in Brazil (Table S1), and most of them are already accepted by the International Virus Taxonomy Committee (ICTV) [11]. In addition, begomoviruses initially reported in alternative weed hosts are also occasionally reported asinfecting tomatoes such as Sida mottle virus (SiMoV) and Sida micrantha mosaic virus (SiMMV) [12,13]. Currently, Tomato severe rugose virus (ToSRV; a bipartite species) and Tomato mottle leaf curl virus (ToMoLCV; a monopartite species) are the most widespread and economically important begomoviruses, with occurrence reported across all major tomato-producing regions, including central Brazil. The remaining viral species have an overall more restricted (sometimes endemic) geographic distribution [14].
The preferential strategy for begomovirus management in tomatoes is the employment of cultivars with genetic resistance/tolerance, since the use of insecticides for controlling viruliferous vector populations is neither efficient nor economically and environmentally sustainable [15,16]. Currently, eight resistance/tolerance genes/alleles to begomovirus have been characterized in Solanum (section Lycopersicon) germplasm: Ty-1 [17], Ty-2 [18], Ty-3 [19], Ty-4 [20], ty-5 [21], Ty-6 [22], tcm-1 [23], and tgr-1 [24]. The Ty-1 gene/locus introgressed from Solanum chilense LA 1969 (Zamir et al. 1994) is by far the most employed genetic factor in tomato breeding programs across the globe. In Brazil, cultivars carrying the Ty-1 gene have been widely used, mainly across producing regions in central Brazil [25,26]. The Ty-1 gene is located on chromosome 6 in a genomic region in repulsion phase linkage with resistance genes against other pathogens, including the Mi-1.2 gene that confers resistance in tomato to the three most important root-knot nematode species: Meloidogyne incognita, Meloidogyne javanica, and Meloidogyne arenaria [27,28]. Molecular markers for monitoring the presence of the Ty-1 gene/locus in tomato cultivars are now available [29,30,31].
The phenotypic expression of the Ty-1 gene is best described as a tolerance response [32], since plants harboring this factor allow for a mild manifestation of symptoms, mainly in the apical meristematic regions, which is followed by a progressive recovery as the plant growth/development advances [33]. This tolerant reaction is expressed against a relatively large number of monopartite and bipartite begomoviruses and it is related to the inhibition of viral movement, being more efficient under low inoculum conditions [17,33]. Genetic studies conducted by Verlaan et al., 2011 showed that the Ty-1 gene encodes an RNA-dependent RNA polymerase. Therefore, the Ty-1 gene is representing an entirely new class of disease resistance/tolerance genes that operates by intensifying the levels of transcriptional silencing of viral genes. More recent studies have shown that the Ty-1 gene can also confer resistance to Beet curly top virus (a viral species of the genus Curtovirus) in genetically transformed Nicotiana benthamiana plants [34]. However, no information is yet available about the effects of the Ty-1 gene on ssDNA viruses and subviral agents described in association with tomatoes in neotropical areas.
Next-generation sequencing (NGS) technologies have intensified the advances in elucidating many aspects of plant–microbe interactions by enabling the generation of a huge amount of low-cost sequence data of both hosts and pathogens [35]. Currently, metagenomic analyses with NGS are the best tools available for large-scale assessment of viral diversity under distinct environmental conditions [36,37,38]. NGS has contributed significantly to the sequencing of complete genomes as well as in detecting novel plant-associated viral species [36,37,38]. NGS technologies have also contributed to revealing the viral diversity associated with the tomato crop [39,40,41,42].
Due to the extreme variability of the neotropical tomato-infecting begomoviruses, it is possible that species and strains not yet identified can be emerging in this region. The increase in the crop acreage with tomato varieties and hybrids harboring the Ty-1 gene may represent a relevant selection factor on viral populations that could make them either more adapted or even capable of entirely overcoming this tolerance factor [25,26]. However, the viral diversity associated with the Ty-1 gene and other tomato resistance/tolerance factors have not yet been extensively studied. The complete sequence information of the DNA-A and DNA-B genomic segments generated by NGS provides large-scale assessment tools to study viral population diversity in the tomato–begomovirus pathosystem. In this context, the objective of the present work was to carry out metagenomic analyses aiming to reveal the diversity of Begomovirus species as well as other ssDNA viruses and subviral agents in tomato cultivars either lacking or harboring the Ty-1 gene in central Brazil.
2. Materials and Methods
2.1. Tomato Leaf Samples and Confirmation of the Presence/Absence of the Ty-1 Gene/Locus in the Genome of the Tomato Samples by Employing a Cleaved Amplified Polymorphic Sequence (CAPS) Marker System
Foliar samples (n = 107) of field-grown tomato cultivars/hybrids (with and without the Ty-1 tolerance gene) showing distinct degrees of begomovirus-like symptoms (viz. apical and interveinal chlorosis, yellow spots, golden mosaic, severe rugose mosaic, apical leaf deformation, and stunting) were collected from 2001 to 2016 across three geographic regions (Goiás State—GO, the Federal District—DF, and Minas Gerais State—MG). In our survey, the majority (over 95%) of the samples collected from plants displaying begomovirus-like symptoms (in both pools) were confirmed to be infected by one or more begomovirus species (see description in Section 2.2).
In order to confirm the presence of the Ty-1 gene/locus, we performed PCR assays with the DNA of these 107 tomato leaf samples with positive begomovirus detection. We employed the primer pair UWTyF/UWTyR, which is capable of generating a CAPS marker linked to this tomato genomic region [29]. This codominant marker system is able to discriminate the dominant resistance allele (Ty-1) from the susceptible recessive allele (ty-1) after cleavage with the restriction enzyme Taq I [29]. In order to reveal these alternative alleles for the Ty-1 gene/locus, PCR products (amplicons) were cleaved with the enzyme Taq I for 2 h at a constant temperature of 65 °C. The products obtained after cleavage were analyzed in 1% agarose gels, stained in ethidium bromide, and visualized under ultraviolet light.
2.2. Viral Isolates and Preliminary Confirmation of the Presence of Begomoviruses in the Tomato Leaf Samples
Each individual sample was subjected to total DNA extraction using a modified (high pH buffer) 2X CTAB + organic solvent protocol [43]. These samples/isolates were stored at −20 °C and they currently comprise a section of the begomovirus collection of the Plant Breeding Laboratory at CNPH (Brasília, DF, Brazil). The purified total DNA was subjected to polymerase chain reaction (PCR) assays aiming to confirm the presence of begomovirus(es) in these tomato leaf samples. Amplicons derived from a segment of the DNA-A component were obtained using the “universal” primer pairs PAL1v1978/ PAR1c496 [44] and BegomoAFor1′/‘BegomoARev1 [45], which produce two large and non-overlapping segments (≈1120 bp and ≈1205 bp, respectively). Amplicons derived from a segment of the DNA-B component (≈690 bp) were obtained using the “universal” primer pair PBL1v2040′/‘PCRc1 [44]. The obtained amplicons were analyzed in 1% agarose gels, stained in ethidium bromide, and visualized under ultraviolet light. Only samples displaying begomovirus-derived amplicons were selected for a subsequent enrichment of circular DNAs via rolling circle amplification and for next-generation sequencing (NGS; see sections below).
2.3. Enrichment via Rolling Circle Amplification of Circular DNA Molecules on Each Individual Sample
The virus-derived circular DNA molecules in the samples were selectively enriched by rolling circle amplification (RCA) assays [46]. After gel electrophoresis, the concentrations were adjusted via NanoVue Plus to 1 microgram per sample and then used to make up the two pools. The CAPS-characterized samples were then subdivided into two pools: one composed of DNAs of tomato plants without the Ty-1 (Table 1) gene (n = 64) and one composed of DNAs of tomato samples with the Ty-1 (Table 2) gene (n = 43).
Table 1.
Identification of 64 samples (=isolates) exhibiting begomovirus-like symptoms that were obtained from tomato plants without the Ty-1 gene/locus in central Brazil. Information is provided about the region where the isolate was collected, year of collection, and the respective isolate code.
| Geographic Region | Year of Collection | Isolate Code |
|---|---|---|
| Goiás State—GO | 2003 | GO-023, GO-046, GO-109, GO-111, GO-118, GO-120, GO-130, GO-134, GO-136, GO-137, GO-142, GO-143, GO-144, GO-168, GO-169, GO-191, GO-192, GO-221, GO-244, GO-245, GO-248, GO-249, GO-250, GO-251 |
| 2004 | GO-298, GO-299, GO-301, GO-322, GO-336 |
|
| 2006 | GO-384, GO-390 | |
| 2011 | GO-493 | |
| 2012 | GO-505, GO-511 | |
| 2015 | GO-594 | |
| Federal District—DF | 2003 | DF-018, DF-023, DF-028, DF-043, DF-045, DF-046, DF-050, DF-062 |
| 2005 | DF-166, DF-167, DF-211 | |
| 2010 | DF-330 | |
| 2011 | DF-447, DF-453 | |
| 2013 | DF-544 | |
| 2014 | DF-566 | |
| 2016 | DF-667 | |
| Minas Gerais State—MG | 2001 | MG-046 |
| 2002 | MG-012, MG-015, MG-016, MG-018, MG-029 |
|
| 2010 | MG-073, MG-113, MG-150 | |
| 2012 | MG-325 | |
| 2015 | MG-378, MG-388 |
Table 2.
Identification of 43 samples (=isolates) exhibiting begomovirus-like symptoms that were obtained from tomato plants harboring the Ty-1 gene/locus in central Brazil. Information is provided about the region where the isolate was collected, year of collection, and the respective isolate code.
| Geographic Region | Year of Collection | Isolate Code |
|---|---|---|
| Goiás State—GO | 2003 | GO-145, GO-148, GO-149, GO-151, GO-157, GO-161, GO-164 |
| 2004 | GO-247, GO-305, GO-307, GO-308, GO-320, GO-326, GO-330 |
|
| 2007 | GO-371 | |
| 2010 | GO-479, GO-487, GO-490 | |
| 2013 | GO-550, GO-582, GO-583 | |
| Federal District—DF | 2007 | DF-227, DF-236, DF-238 |
| 2008 | DF-252 | |
| 2010 | DF-339 | |
| 2011 | DF-438 | |
| 2013 | DF-529, DF-550, DF-556 | |
| 2016 | DF-640 | |
| Minas Gerais State—MG | 2010 | MG-092, MG-122, MG-169, MG-282, MG-283, MG-284, MG-285, MG-286, MG-287 |
| 2012 | MG-326 | |
| 2015 | MG-383, MG-387 |
2.4. Next-Generation Sequencing (NGS) of the Two Tomato DNA Pools and Analysis of the NGS-Derived Sequences
The sample pools (with and without Ty-1 gene) were subjected to high-performance sequencing in an Illumina platform with the HiSeq 2500 system (Macrogen Inc., Seoul, South Korea). The analyses were performed in paired ends reads of 100 nucleotides in length. The number of raw reads (filtered reads in parentheses) obtained for each pool were 16,227.547 (32,455.094) and 16,345.987 (32,691.974) for resistant and susceptible pools, respectively. The NGS-derived sequences were analyzed according to the following workflow: (1) elimination of low-quality reads, (2) assembly of the sequences using the program CLC Genomics Workbench 10, and (3) validation of the contigs via BLASTx and BLASTn algorithms by comparing with the ssDNA virus database of GenBank (https://www.ncbi.nlm.nih.gov/). The viral contigs were annotated and the trimmed reads were mapped back to the annotated genome using the tool “Map to reference” available in the Geneious 11.0 program [47]. The conserved regions/motifs present in the begomovirus genomes such as nonanucleotide, TATA box, stem loop, and iterons were also selectively analyzed [48]. Additionally, individual identification of the viruses was obtained in the NGS-derived dataset by using the SeqMan NGene Metagenomic sequence analysis software (DNAStar, Madison, WI, USA). Viral contigs were analyzed against the RefSeq viral database (NCBI) at a very high stringency conditions (minimum match percentage = 99%). The contig sequences were assembled by CLC Genomics Workbench and subsequently submitted to GenBank (see Section 3.1 below).
2.5. Design of a Collection of Viral Species-Specific PCR Primers for Detection in Individual Samples
For the confirmation of the viral species detected in each individual sample, specific PCR primers (for both DNA-A and DNA-B genomic segments) were designed in opposite and overlapping directions. Primer design was carried out on the basis of the consensus contigs obtained with the Geneious 11.0 program (Table 3). Virus specificity of the primers was double-checked in silico by using the Primer-Blast tool and in preliminary PCR assays using template DNA samples from a reference collection of the NGS-identified viral isolates.
Table 3.
PCR primer pairs designed on the basis of next-generation sequencing (NGS)-derived viral consensus sequences for validation of the Begomovirus species, as well as single-stranded DNA viruses and subviral agents identified in the tomato DNA sample pools (with the Ty-1 gene versus without the Ty-1 gene). For = forward and Rev = reverse direction.
| Viral Species | Primer Name | Sequence 5′–3′ | Annealing Temperature (T °C) |
|---|---|---|---|
| Bean golden mosaic virus (BGMV) DNA-A | BGMV-For | GTGCGTGAATCCATGACCGT | 55 |
| BGMV-Rev | ATTCACGCACAGGGGAACG | ||
| Cleome leaf crumple virus (CILCrV) DNA-A | CILCrV-A-For | GACTCGACGTTCTGTGGT | 51 |
| CILCrV-A-Rev | TCCTAGTCGGGGCTCACT | ||
| Cleome leaf crumple virus (CILCrV) DNA-B | CILCrV-B-For | TAGGAAAGCAAAACGAGAATGGAA | 58 |
| CILCrV-B-Rev | GCTTTCCTAAATCGCAATTGATC | ||
| Tomato severe rugose virus (ToSRV) DNA-A | ToSRV-For5.1 | AGCGTCGTTAGCTGTCTGGCA | 58 |
| ToSRV-Rev5 | TGCCGCAGAAGCCTTGAACGCACCT | ||
| Tomato severe rugose virus (ToSRV) DNA-B | ToSRV-B-For | AAACCCACACGAAAGCAGAGTTT | 55 |
| ToSRV-B-Rev | CACCACGTCTATACATATTGTCCAGG | ||
| Euphorbia yellow mosaic virus (EuYMV) DNA-A | EuYMV-A-R-For | GGGGTTCCAAGTCCAATAAAGATGA | 52 |
| EuYMV-A-R-Rev | CAGACACCTTATATTTGCCGGATTC | ||
| Euphorbia yellow mosaic virus (EuYMV) DNA-B | EuYMV-B-R-For | GCCGAGGATAGAGGACACCAA | 60 |
| EuYMV-B-R-Rev | CCAGGCCCAAACGCATTATATTTTATC | ||
| Tomato chlorotic mottle virus (ToCMoV) DNA-A | ToCMoV-A-For | TTTGGGCCGCTCTTTTGGG | 47 |
| ToCMoV-A-Rev | CAAACTGAATGGGCCTTAAA | ||
| Tomato chlorotic mottle virus (ToCMoV) DNA-B | ToCMoV-B-For | GTATTTGTTCTGGGTGCAATCATAAAAC | 55 |
| ToCMoV-B-Rev | TTGTACTAATGACACATTATTCAATCACGA | ||
| Tomato golden vein virus (TGVV) DNA-A | TGVV-A-For1 | AAAGGAAGATAATTCAAATATAGGGA | 51 |
| TGVV-A-Rev1 | ATCTTCCTTTACTCACGTTCCTGAT | ||
| Tomato golden vein virus (TGVV) DNA-B | TGVV-B-S-For | CCCACTTTCCATAACCTACATGAGA | 55 |
| TGVV-B-S-For | GGAGAGAAAATTGATAAGATCGGCATC | ||
| Tomato mottle leaf curl virus (ToMLCV) DNA-A | ToMoLCV-For | TGTGGTCCAGTCAATAAATG | 47 |
| ToMoLCV-Rev | TGACTGGACCACATAGTAAA | ||
| Tomato common mosaic virus (ToCmMV) DNA-A | ToCmMV-For1 | ATTGCTCTCAACTTCTGTGC | 54 |
| ToCmMV-Rev2 | GCAATCCCTGGTGTCCTCAC | ||
| Tomato rugose mosaic virus (ToRMV) DNA-A | ToRMV-A-For | TGAAAGTAATTTTGACCCAATC | 52 |
| ToRMV-A-Rev | CAATTCATATGAGTTTTAGAGCAGC | ||
| Sida micrantha mosaic virus (SiMMV) DNA-A | SiMMV-For | GATCTCGCTCCCCCCTCT | 58 |
| SiMMV-Rev | AGATCGCACGACAACCAG | ||
| Plant-associated genomovirus 2 | Gemy-For | GCTCTGAATCAAATCTCGCTTACTTG | 54 |
| Gemy-Rev | CGATGTTGATTGGTTGGAAGCAAA | ||
| New Begomovirus Species #1 DNA-A | DF-640-A-For | GTTGACTGACATTTGCCTT | 47 |
| DF-640-A-Rev | TGTCAGTCAACAATCTATACACA | ||
| New Begomovirus Species #1 DNA-B | DF-640-B-For | GTTGTTTCAAGGGCGTCGAC | 55 |
| DF-640-B-Rev | CAACATCAGACATCCAGCAATAATAAACT | ||
| New Begomovirus Species #2 DNA-A | 1ToBYMV-A-For | ATCCATGTCCTCGGCAGTCT | 55 |
| 1ToBYMV-A-Rev | TCACGCACAGAGGAACGC | ||
| New Begomovirus Species #3 DNA-A | Abuti-A-For | GGACTCCAGGGGGCAAAA | 55 |
| Abuti-A-Rev | AGTCCCGTCCGTACCACTTG | ||
| Alpha-satellite | Alfa-For | TGGTGTCCTGGCTTATAT | 46 |
| Alfa-Rev | GGCGGAGTCCTTTTTTTT |
2.6. Validation of NGS-Derived Information via PCR Assays with Virus-Specific Primers
PCR assays with the previously selected virus-specific primers (Table 3) were carried with in all 107 individual DNA samples. These assays were performed in order to validate the NGS results. PCR assays were carried with a total volume of 12.5 µL, containing 1.25 µL of Taq polymerase 10× buffer, 50 mM of MgCl2, 2.5 mM of dNTPs, 10 µM of each primer (forward and reverse), 100 ng of DNA, 8.0 µL of Milli-Q water, and 0.5 U Taq DNA polymerase. The reactions were amplified in a thermal cycler (Bio-Rad Laboratories, Hercules, CA, USA) programmed for 35 cycles with the following conditions: initial denaturation at 94 °C for 3 min, denaturation at 94 °C for 30 s, annealing (ranging from 46 to 60 °C, according to the primer pair employed; Table 3) for 45 s, extension at 72 °C for 3 min, and final extension at 72 °C for 10 min. The begomovirus-derived amplicons were observed to 1.5% agarose gel electrophoresis stained with ethidium bromide and visualized under UV light.
2.7. Sanger Dideoxy Sequencing Validation of Virus-Specific PCR Amplicons
Direct Sanger dideoxy sequencing reactions of positive virus-derived amplicons were carried out to double-check the viral diversity observed in a subset of individual samples. Sequencing reactions were performed at the Genomic Analysis Laboratory (at CNPH), employing the same virus-specific primer pairs (Table 3) in one ABI PRISM 3130 sequencer using the BigDye Terminator Cycle Sequencing Ready Reaction Kit version 3.1 protocol (Applied Biosystems, São Paulo–SP, Brazil). After contig assembling and quality evaluation, the obtained sequences were analyzed using the BLASTn algorithm. This tool was used to compare our sequences with the ones retrieved from the GenBank–NCBI public database (https://www.ncbi.nlm.nih.gov/), aiming to verify the sample-associated viral species. We adopted the current pairwise identities of 91% and 94% as the demarcation thresholds to identify Begomovirus species and strains, respectively [2].
3. Results
3.1. NGS Detection of Previously Reported Begomovirus Species in the Two Pools of Samples (with and without the Ty-1 Gene)
The assembled contig sequences of both pools that were characterized here and their corresponding GenBank accessions are presented in Table 4 and Table 5. The total number of reads per viral species/genomic component obtained in the pool without the Ty-1 gene is presented in Table 4, whereas the viral sequences obtained in pool from plants with the Ty-1 gene is presented in Table 5. After assembly, 19,487 contigs were obtained in the pool without the Ty-1 gene and 7045 contigs in the sample pool with the Ty-1 gene. ToSRV and Tomato rugose mosaic virus (ToRMV) displayed the two highest numbers of reads, indicating their relative predominance in the tomato samples with the Ty-1 gene. Ten begomoviruses were found in the pool without the Ty-1 gene. Interestingly, only the DNA-A component was recovered from well-characterized bipartite species, including Bean golden mosaic virus (BGMV), Tomato common mosaic virus (ToCmMV), and SiMMV. In the pool harboring the Ty-1 gene, the complete genomes of four previously described bipartite Begomovirus species were recovered viz. ToSRV, Tomato chlorotic mottle virus (ToCMoV), Tomato golden vein virus (TGVV), and ToRMV. The monopartite species ToMoLCV was also recovered in both pools. Some of the neotropical tomato-infecting Begomovirus species (included on the RefSeq database) displayed overall high identity levels (e.g., >97% identity in the case of the DNA-B component that is shared by the species ToSRV and ToRMV). The NGS sequences were then realigned against the viral genomes found using SeqMan NGene (99% stringency parameter) in order to minimize assembling artifacts. This allowed a more precise evaluation of the representation of each virus and subviral agents in the sequenced pools. The validation of the NGS results via PCR assays with virus-specific primers coupled with Sanger dideoxy sequencing was also a very important tool to verify the presence of each individual virus species described here.
Table 4.
Viral circular, single-stranded DNA species (and their corresponding GenBank accessions) detected after Illumina Hiseq sequencing in the pool of tomato DNA samples lacking the Ty-1 gene.
| Viral Species | No. of Reads | Size (nts) | GenBank Accession Numbers |
|---|---|---|---|
| Bean golden mosaic virus (BGMV) DNA-A | 63,525 | 2626 | MT214083 |
| Cleome leaf crumple virus (CILCrV) DNA-A | 566 | 2560 | MN337873 |
| Cleome leaf crumple virus (CILCrV) DNA-B | 702 | 2664 | MN337872 |
| Tomato severe rugose virus (ToSRV) DNA-A | 3,225,120 | 2593 | MT214084 |
| Tomato severe rugose virus (ToSRV) DNA-B | 4,018,351 | 2572 | MT214085 |
| Euphorbia yellow mosaic virus (EuYMV) DNA-A | 1122 | 2609 | MN746971 |
| Euphorbia yellow mosaic virus (EuYMV) DNA-B | 1822 | 2579 | MN746970 |
| Tomato chlorotic mottle virus (ToCMoV) DNA-A | 5,971,019 | 2620 | MT214086 |
| Tomato chlorotic mottle virus (ToCMoV) DNA-B | 1,111,227 | 2600 | MT214087 |
| Tomato golden vein virus (TGVV) DNA-A | 2,639,961 | 2562 | MN928610 |
| Tomato golden vein virus (TGVV) DNA-B | 977,027 | 2512 | MN928611 |
| Tomato mottle leaf curl virus (ToMLCV) DNA-A | 1,784,881 | 2632 | MT214088 |
| Tomato common mosaic virus (ToCmMV) DNA-A | 1,070,674 | 2560 | MT214089 |
| Tomato rugose mosaic virus (ToRMV) DNA-A | 3,267,808 | 2619 | MT214090 |
| Tomato rugose mosaic virus (ToRMV) DNA-B | 4,742,730 | 2571 | MT214091 |
| Sida micrantha mosaic virus (SiMMV) DNA-A | 1,221,062 | 2688 | MT214092 |
| Plant-associated genomovirus 2 | 119 | 2189 | MT214094 |
| New Begomovirus Species #2 DNA-A | 427,646 | 2649 | MT214095 |
| New Begomovirus Species #3 DNA-A | 2839 | 2636 | MT214096 |
| New alpha-satellite | 155,793 | 1321 | MT214093 |
Table 5.
Viral circular, single-stranded DNA species (and their corresponding GenBank accessions) detected after Illumina Hiseq sequencing in the pool of tomato DNA samples harboring the Ty-1 gene.
| Viral Species | N° of Reads | Size (nts) | GenBank Accession Numbers |
|---|---|---|---|
| Tomato severe rugose virus (ToSRV) DNA-A | 7,181,771 | 2592 | MT215001 |
| Tomato severe rugose virus (ToSRV) DNA-B | 5,782,296 | 2570 | MT215002 |
| Tomato golden vein virus (TGVV) DNA-A | 2,358,838 | 2561 | MN928612 |
| Tomato golden vein virus (TGVV) DNA-B | 1,401,684 | 2590 | MN928613 |
| Tomato chlorotic mottle virus (ToCMoV) DNA-A | 4,519,040 | 2623 | MT215003 |
| Tomato chlorotic mottle virus (ToCMoV) DNA-B | 811,733 | 2565 | MT215004 |
| Tomato mottle leaf curl virus (ToMLCV) DNA-A | 2,644,606 | 2631 | MT215005 |
| Tomato rugose mosaic virus (ToRMV) DNA-A | 7,964,942 | 2618 | MT215006 |
| Tomato rugose mosaic virus (ToRMV) DNA-B | 5,780,864 | 2649 | MT215007 |
| New Begomovirus Species #1 DNA-A | 1,270,494 | 2605 | MN147863 |
| New Begomovirus Species #1 DNA-B | 84,022 | 2625 | MN147864 |
3.2. NGS Detection of Putative Three Novel Begomovirus Species as Well as a New Alpha-Satellite Species and a Gemycircularvirus (Genomoviridae) in the Tomato Samples
We were also able to identify three putative new Begomovirus species (one in the pool with the Ty-1 gene and two species in the pool without the Ty-1 gene). In the pool of samples with the Ty-1 gene, a putative new virus (named here as species #1 = isolate DF-640) displayed a bipartite genome organization, having a DNA-A component with 2605 nts and a DNA-B component with 2625 nts. The putative new species #1 displayed the highest level of identity (85%) with Tomato rugose yellow leaf curl virus (TRYLCV) isolates. The isolate DF-640 was recovered from a field-grown tomato plant in the vicinities of Gama city (in the Federal District), with severe symptoms, indicating a putative increase in virulence in relation to the Ty-1 gene. However, we cannot exclude that these severe symptoms might have been induced by synergisms involving DF-640 and RNA viruses (since they were not investigated in the present study). Two putative new species were detected in the pool lacking the Ty-1 gene. The first one was tentatively named here as new species #2 (=isolate MG-378) and displayed only the DNA-A component with 2649 nts. Tomato bright yellow mottle virus (ToBYMoV) was the begomovirus with the highest identity level (84%) to the new species #2. Additional PCR assays were carried out using the isolate MG-378 as a template, but no amplicon for the putative cognate DNA-B component was recovered (data not shown), indicating that it is more likely a monopartite virus. The new species #3 is also more likely a monopartite begomovirus, having a DNA-A genome with 2636 nts. The new species #3 displayed the highest identity level (84%) to Abutilon mosaic Brazil virus (AbMV). In addition, a four genetically identical isolates of a novel alpha-satellite species and two isolates of a gemycircularvirus (Family: Genomoviridae) species were also detected exclusively in the pool of tomato samples without the Ty-1 gene (Table 4).
3.3. Confirmation via PCR Assays with Virus-Specific Primers and Sanger Dideoxy Sequencing of the Viral and Subviral ssDNA Species Present in each Individual Tomato Sample and Quantification of Mixed Infections
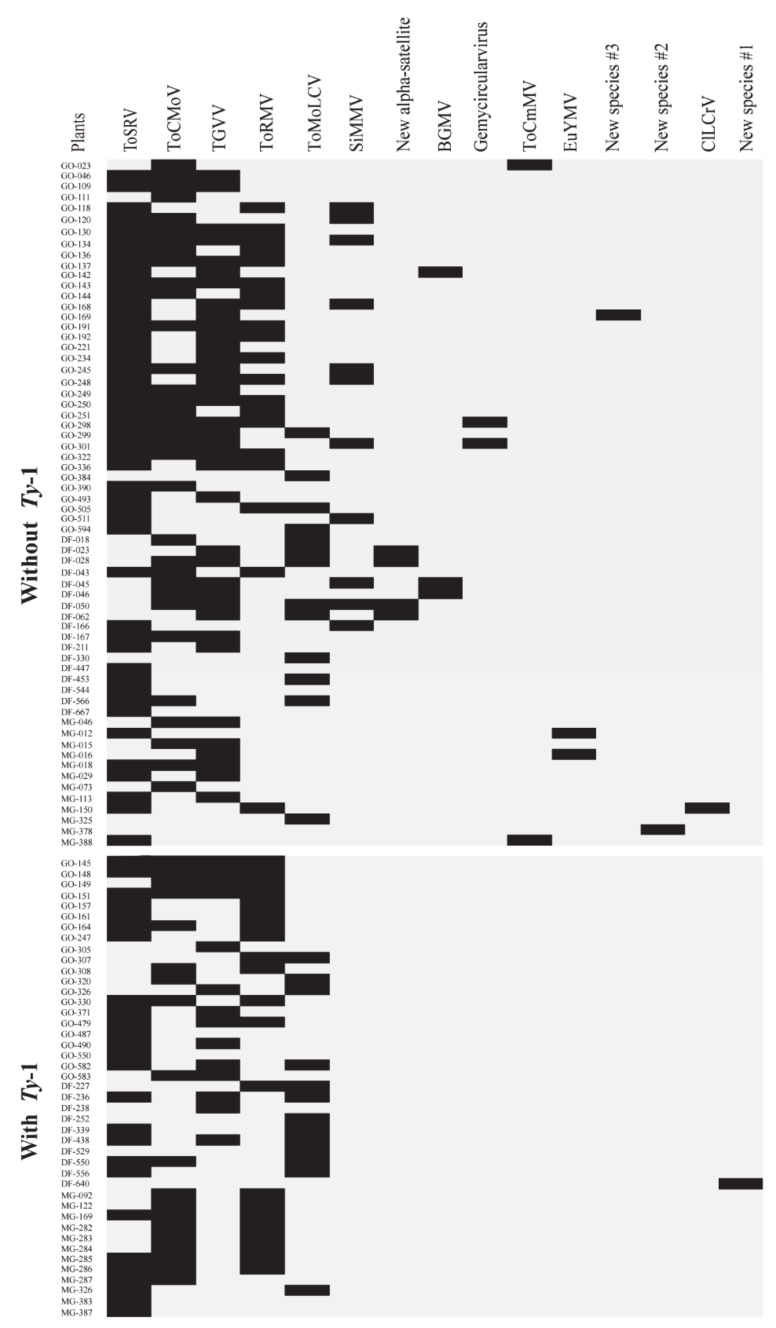
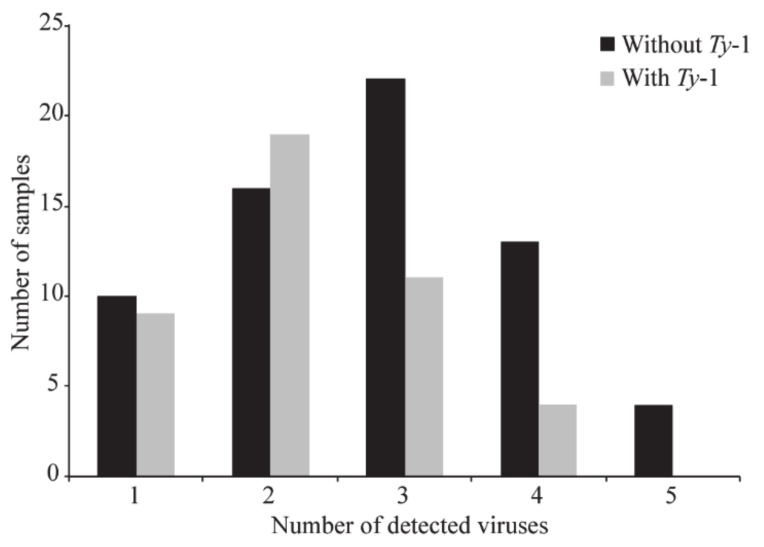
After carrying out PCR assays with virus-specific primers (Table 3) and Sanger sequencing, we were able to catalog all the viral and subviral ssDNA species present in each individual tomato sample comprising the two pools. In the samples of the pool without the Ty-1 gene, it was possible to confirm the presence of all Begomovirus species reported initially by the analyses of the NGS-derived results (Table 6). ToSRV was the most prevalent begomovirus, mainly in samples from Goiás (GO) State. In the samples of the pool with the Ty-1 gene, all species identified after NGS analyses were also confirmed via PCR assays with virus-specific primers (Table 7). In addition, it is important to highlight that the majority of the samples displayed mixed infections, with two to five viral species being simultaneously detected in a single tomato plant (Figure 1 and Figure 2).
Table 6.
Relative frequency of Begomovirus and other circular single-stranded DNA viruses detected after Illumina Hiseq sequencing of 63 tomato DNA samples lacking the Ty-1 gene.
| Viral Species* Followed by the Respective Number of Occurrences in Each Region | Goiás State (GO) | Federal District (DF) | Minas Gerais State (MG) |
|---|---|---|---|
|
ToSRV
(32 + 9 + 5) = 46 |
GO-046, GO-109, GO-118, GO-120, GO-130, GO-134, GO-136, GO-137, GO-142, GO-143, GO-144, GO-168, GO-169, GO-191, GO-192, GO-221, GO-244, GO-245, GO-248, GO-249, GO-250, GO-251, GO-298, GO-299, GO-301, GO-322, GO-336, GO-390, GO-493, GO-505, GO-511, GO-594 | DF-043, DF-166, DF-167, DF-211, DF-447, DF-453, DF-544, DF-566, DF-667 | MG-012, MG-018, MG-029, MG-150, MG-388 |
|
TGVV
(23 + 8 + 5) = 36 |
GO-046, GO-109, GO-130, GO-134, GO-137, GO-142, GO-143, GO-168, GO-169, GO-191, GO-192, GO-221, GO-244, GO-245, GO-248, GO-249, GO-250, GO-298, GO-299, GO-301, GO-322, GO-336, GO-493 | DF-023, DF-028, DF-045, DF-046, DF-050, DF-062, DF-167, DF-211 | MG-015, MG-016, MG-018, MG-029, MG-046 |
|
ToCMoV
(21 + 8+5) = 34 |
GO-023, GO-046, GO-109, GO-111, GO-120, GO-130, GO-134, GO-136, GO-137, GO-143, GO-144, GO-191, GO-245, GO-249, GO-250, GO-251, GO-298, GO-299, GO-301, GO-322, GO-390 | DF-018, DF-028, DF-043, DF-045, DF-046, DF-050, DF-167, DF-566 | MG-015, MG-018, MG-046, MG-073, MG-150 |
|
ToCmMV
(1 + 0 + 1) = 2 |
GO-023 | --- | MG-388 |
|
BGMV
(1 + 2 + 0) = 3 |
GO-142 | DF-045, DF-046 | --- |
|
CILCrV
(0 + 0 + 1) = 1 |
--- | --- | MG-150 |
|
EuYMV
(0 + 0 + 2) = 2 |
--- | --- | MG-012, MG-016 |
|
ToMLCV
(4 + 8 + 1) = 13 |
GO-299, GO-384, GO-505, GO-594 | DF-018, DF-023, DF-028, DF-050, DF-062, DF-330, DF-453, DF-566 | MG-325. |
|
ToRMV
(19 + 1 + 1) = 21 |
GO-109, GO-118, GO-130, GO-134, GO-136, GO-137, GO-143, GO-144, GO-168, GO-191, GO-192, GO-244, GO-248, GO-250, GO-251, GO-298, GO-322, GO-336, GO-505 | DF-043 | MG-150 |
|
SiMMV
(8 + 3 + 0) = 11 |
GO-118, GO-120, GO-134, GO-168, GO-245, GO-248, GO-301, GO-511 | DF-045, DF-050, DF-166 | --- |
|
Plant-associated genomovirus 2
(2 + 0 + 0) = 2 |
GO-298, GO-301 | --- | --- |
|
Alpha-satellite
(0 + 4 + 0) = 4 |
--- | DF-023, DF-028, DF-050, DF-062 | --- |
|
New Begomovirus species #2
(0 + 0 + 1) = 1 |
--- | --- | MG-378 |
|
New Begomovirus species #3
(1 + 0 + 0) = 1 |
GO-169 | --- | --- |
* ToSRV = Tomato severe rugose virus, TGVV = Tomato golden vein virus, ToCMoV = Tomato chlorotic mottle virus, ToCmMV = Tomato common mosaic virus, BGMV = Bean golden mosaic virus, CILCrV = Cleome leaf crumple virus, EuYMV = Euphorbia yellow mosaic virus, ToMLCV = Tomato mottle leaf curl virus, ToRMV = Tomato rugose mosaic virus, and SiMMV = Sida micrantha mosaic virus.
Table 7.
Relative frequency of Begomovirus and other circular single-stranded DNA viruses in association with 43 tomato DNA samples harboring the Ty-1 gene detected after Illumina Hiseq sequencing.
| Viral Species* Followed by the Respective Number of Occurrences in Each Region | Goiás State (GO) | Federal District (DF) | Minas Gerais State (MG) |
|---|---|---|---|
|
ToSRV
(14 + 5 + 7) = 26 |
GO-145, GO-148, GO-151, GO-157, GO-161, GO-164, GO-247, GO-330, GO-371, GO-479, GO-487, GO-490, GO-550, GO-582 | DF-236, DF-339, DF-438, DF-550, DF-556 |
MG-169, MG-285, MG-286, MG-287, MG-326, MG-383, MG-387 |
|
TGVV
(12 + 3 + 0) = 15 |
GO-145, GO-148, GO-149, GO-151, GO-305, GO-320, GO-326, GO-371, GO-479, GO-490, GO-582, GO-583 | DF-236, DF-238, DF-438 |
--- |
|
ToCMoV
(12 + 1 + 9) = 22 |
GO-145, GO-148, GO-149, GO-305, GO-320, GO-326, GO-330, GO-371, GO-479, GO-490, GO-582, GO-583 | DF-550 | MG-092, MG-122, MG-169, MG-282, MG-283, MG-284, MG-285, MG-286, MG-287 |
|
ToMLCV
(4 + 8 + 1) = 13 |
GO-307, GO-320, GO-326, GO-582 | DF-227, DF-236, DF-252, DF-339, DF-438, DF-529, DF-550, DF-556 |
MG-326 |
|
ToRMV
(13 + 1 + 8) = 22 |
GO-145, GO-148, GO-149, GO-151, GO-157, GO-161, GO-164, GO-247, GO-307, GO-308, GO-320, GO-330, GO-479 | DF-227 | MG-092, MG-122, MG-169, MG-282, MG-283, MG-284, MG-285, MG-286 |
|
New Begomovirus species #1
(0 + 1 + 0) = 1 |
--- | DF-640 | --- |
* ToSRV = Tomato severe rugose virus, TGVV = Tomato golden vein virus, ToCMoV = Tomato chlorotic mottle virus, and ToRMV = Tomato rugose mosaic virus.
Figure 1.
Frequency and relative predominance of Begomovirus species and single-stranded DNA viruses detected with Illumina Hiseq sequencing of tomato samples with (n = 43) and without (n = 64) the Ty-1 gene. Results were validated by PCR assays with virus-specific primers and by Sanger dideoxy sequencing. Viruses found were Tomato severe rugose virus (ToSRV); Tomato golden vein virus (TGVV); Tomato chlorotic mottle virus (ToCMoV); Tomato rugose mosaic virus (ToRMV); Tomato mottle leaf curl virus (ToMoLCV); Sida micrantha mosaic virus (SiMMV); Bean golden mosaic virus (BGMV); Tomato common mosaic virus (ToCmMV); Euphorbia yellow mosaic virus (EuYMV); and Cleome leaf crumple virus (CILCrV). A new alpha-satellite species and three putative novel begomovirus species (=new species #1, new species #2, and new species #3) were also detected. Black bars in each line indicate the presence of a given virus in a given individual sample = isolates (left column). Isolate with GO abbreviation = isolates collected in Goiás State; DF abbreviation = isolates collected in the Federal District; and MG abbreviation = isolates collected in Minas Gerais State, in central Brazil.
Figure 2.
Number of samples displaying single and mixed (ranging from two to five viruses per sample) infections with Begomovirus species and single-stranded DNA viruses detected with Illumina Hiseq sequencing of tomato samples with (n = 43) and without (n = 64) the Ty-1 gene. Results were validated by PCR assays with virus-specific primers and by Sanger dideoxy sequencing.
4. Discussion
Over 286 viral species have been reported to infect tomatoes in Brazil and worldwide [5,49,50,51]. The emergence per se of a large number of novel species is somewhat expected since the begomoviruses display a well-known set of mechanisms for generating genetic variability such as mutation, recombination, and pseudo-recombination [6,7,52]. The scenario of immense begomovirus variability in the neotropics favors the emergence of new species, which can be intensified by the frequent occurrence of mixed infections. However, there is a surprisingly scarce amount of information quantifying the frequency of mixed infections of tomato plants by members of the neotropical Begomovirus species complex under natural conditions. Our NGS-derived results displayed a substantial number of the tomato samples with events of co-infection in both pools (with and without the Ty-1 gene). The simultaneous presence of distinct virus species detected in single plants ranged from two to up to five (Figure 1 and Figure 2). However, it is interesting to highlight that the Ty-1 gene did not have a significant impact on reducing the overall number of multiple viral infections, since samples with this genetic factor displayed non-significant differences when compared to samples without this gene (chi-square test = 6.5193; p-value = 0.1635, which was found to be not significant at p < 0.05).
In our study, in addition to the detection of Begomovirus species already reported in the neotropics, we were able to detect two putative new Begomovirus species in the samples without the Ty-1 and one novel Begomovirus species in a sample with the Ty-1 gene. The putative new species #1 (DF-640) displays all typical features of the New World bipartite begomoviruses, having a DNA-A with a size of 2605 nts and a DNA-B component with 2625 nts. The new species #1 was the only begomovirus found to be associated with severe (dwarf) symptoms in plants harboring the Ty-1 gene in our survey. The new species #1 displayed the highest identity level (85%) with Tomato rugose yellow leaf curl virus (TRYLCV). Only the DNA-A components were found in the putative new species #2 (=isolate MG-378) and in the new species #3 (=isolate GO-169), suggesting that both might be novel monopartite viruses. The new species #2 (2649 nts) displayed the highest identity (84%) with the Tomato bright yellow mottle virus (ToBYMoV), and the new species #3 (2636 nts) displayed the highest identity level (84%) with Abutilon mosaic Brazil virus (AbMBV). According to the current criteria for species demarcation in the genus Begomovirus, nucleotide identities of the DNA-A component that are less than 91% with the complete DNA-A genome of any other known begomovirus sequence will correspond to a new species [2]. The overall low number of samples detected with these putative new begomoviruses indicates that they may represent extremely rare emergence events of novel viral variants. Therefore, it is most likely that we were able to identify these emerging viruses here due to the enhanced analytical power provided by the NGS technology.
A single alpha-satellite isolate with 1321 nts was detected in four individual samples collected in distinct areas of the Federal District in plants lacking the Ty-1 gene. Alpha-satellite DNA molecules are subviral agents classified in the family Alphasatellitidae that have been found in association with Begomovirus [1,53]. The genera of alpha-satellites associated with the geminiviruses are found in the subfamily Geminialphasatellitinae, viz. Ageyesisatellite, Clecrusatellite, Colecusatellite, and Gosmusatellite. Nucleotide identity less than 88% (in comparison with complete sequences of the known alpha-satellites) is the criterion currently used for the classification of a new species within the family Geminialphasatellitinae [1,53]. The alpha-satellite isolates found in the present study showed the highest level of identity (81%) with other New World species that were found in association with bipartite begomoviruses in Brazil, Cuba, and Venezuela [54,55]. Thus, according to the demarcation within the subfamily, the alpha-satellite is more likely a new species, probably of the genus Clecrusatellite, which is composed of species found in association with bipartite Begomovirus from the New World [1,54,55]. This putative new alpha-satellite species was detected in constant association with two begomoviruses (TGVV and ToMLCV). Therefore, additional bioassays will be necessary to identify which associated Begomovirus species is able to transreplicate this novel alpha-satellite.
A plant-associated genomovirus 12, classified into the genus Gemycircularvirus (family Genomoviridae), was also detected in two tomato samples from the pool without the Ty-1 gene. Both isolates were collected in Leopoldo de Bulhões, Goiás (GO) State in 2004. These isolates displayed 98% identity to Capybara genomovirus 9 isolate cap1_561 (MK483081.1) from Brazil [56]. The gemycircularviruses have ssDNA, and some species of this genus have been reported in association with plants [57,58,59]. In Brazil, two gemycircularviruses were previously described in samples of Momorcadia charantia and Euphorbia heterophylla [60]. However, according to our knowledge, this is the first report of a gemycircularvirus associated with tomatoes in Brazil and worldwide.
In the present study, the complete DNA-A sequences of the begomoviruses BGMV, SiMMV, and ToCmMV were detected and subsequently confirmed in the individual samples via PCR assays and Sanger sequencing. BGMV was found in two samples collected in the Gama (DF) region in 2003 (isolates DF-045 and DF-046) and in one sample collected in Leopoldo de Bulhões (GO) (isolate GO-142). In fact, BGMV has been previously found in association with tomatoes in the Submédio São Francisco River valley in northeast Brazil [61]. However, this initial detection was carried out by using only DNA-A-specific probes without additional molecular characterization of the putative BGMV isolates [61]. Therefore, our work is the first to characterize tomato-infecting BGMV isolates. Interestingly, the DNA-B components of these BGMV isolates were not recovered from the samples, indicating that they might be using one alternative DNA-B component from another co-infecting species. Additional bioassays will be necessary to confirm this hypothesis.
SiMMV was detected in association with tomatoes in Goiás State (nine samples) and in the Federal District (three samples). SiMMV was already reported infecting tomatoes in Brazil [12]. All SiMMV isolates were found only in the pool without the Ty-1 gene (Table 5), suggesting virus-specific filtering effects by this genetic factor. It will be of interest to challenge plants harboring the Ty-1 gene with infectious SiMMV clones to confirm this potential high level of resistance to this pathogen. This work is now underway.
Somewhat surprising, only the DNA-A component of the bipartite species ToCmMV was detected in two samples of the pool without the Ty-1 gene (GO-023 and MG-388) collected in Luziânia (GO) and Viçosa (MG), respectively. The isolate GO-023 is a mixed infection with ToCMoV, and the isolate MG-388 is a mixed infection with ToSRV. The absence of the DNA-B component of ToCmMV also suggests that these isolates might be using this component of these co-infecting species. This hypothesis remains to be investigated. ToCmMV was initially reported infecting tomato plants in southeast Brazil [62,63]. However, ToCmMV was not yet reported in Goiás State (GO-023). Even though both ToCmMV isolates were found in the pool without the Ty-1 gene, there are reports indicating that this virus can replicate and cause mild symptoms in tomato plants carrying this tolerance factor (manuscript in preparation).
The DNA-A and DNA-B genome sequences of Euphorbia yellow mosaic virus (EuYMV) and Cleome leaf crumple virus (CILCrV) were also recovered in our NGS analyses only from samples without the Ty-1 gene. EuYMV and CILCrV were detected in samples from Minas Gerais State. EuYMV was first characterized as infecting the weed E. heterophylla [64], and CILCrV was first reported infecting the weed Cleome affinis [54]. However, according to our knowledge, this is the first report of these two viral species naturally associated with tomatoes. The detection of these two species reinforces the hypothesis that weeds can serve as a natural reservoir for begomoviruses that may be able to move and be able to infect cultivated plants such as tomatoes.
We found that the NGS analyses in combination with PCR assays with virus-specific primers and Sanger sequencing to be powerful tools that allow us to assess the relative prevalence of the predominant Begomovirus species in distinct geographic areas across central Brazil. ToSRV has been described as the predominant begomovirus species, as indicated by independent surveys carried out across all tomato-producing regions in Brazil [13,65,66]. In our study, ToSRV was also the predominant begomovirus, being found in 46 samples of the pool without the Ty-1 gene and in 26 samples in the pool harboring the Ty-1 gene. This ability of ToSRV isolates replicate in plants with the Ty-1 gene could also be considered as an additional factor that explains the overall predominance of this virus under Brazilian conditions.
ToSRV is the prevalent begomovirus in the central and meridional regions of Brazil [66,67], whereas ToMoLCV is predominant in the northeast region [66]. However, ToMoLCV is also often found in central Brazil [68], which was confirmed by our results. Tomato chlorotic mottle virus (ToCMoV) has already been reported across the northeast, southeast, and central Brazil [6,69]. However, our results indicated that besides the Federal District, a large number of ToCMoV-infected tomato samples were also identified in Goiás State. Tomato golden vein virus (TGVV) is commonly found in central Brazil [68], and our results are in agreement with this observation. Tomato rugose mosaic virus (ToRMV) is a recombinant viral species with genomic contributions of ToSRV and ToCMoV [69]. In accordance with our results, ToRMV was found to be one of the predominant viral species in the central region of Brazil, especially in the Goiás State [6,70].
The present work is the first exploratory study on the potential impact of the Ty-1 gene on the diversity of neotropical Begomovirus species. The majority of the samples obtained from tomato plants carrying the Ty-1 gene displayed mild symptoms restricted to the apical leaves. It was possible to observe putative filtering effects as well as gene-specific viral selection in samples with the Ty-1 gene, indicating a potential evolution of viral populations more adapted to this genetic factor. It would be interesting to know if the viruses detected in the apical mild symptoms in plants carrying the Ty-1 gene are indeed able to escape its effects or if the occurrence of multiple infections on these plants makes a more permissive cellular environment. However, an illustrative example is the isolate DF-640 that was the only one found in association with severe disease symptoms in a field-grown Ty-1 tomato plant. This strong susceptible-like reaction associated with the isolate DF-640 may indicate its potential ability to overcome the Ty-1 gene. Another possibility is that the isolate DF-640 may represent a singular “host switch” event that is not necessary associated with viral adaptation to the Ty-1 gene. The production of infectious DF-640 clones is now underway, and they will be used to verify this hypothesis. Nevertheless, it is well documented that the increase in the acreage of cultivars harboring resistance genes such as Ty-1 can result in strong selection forces towards more aggressive viral isolates, accelerating the change in the composition of the viral population and potentially culminating with the loss of effectivity of the source of resistance/tolerance [71,72,73,74,75]. Studies have also reported the “breakdown” of the resistance mediated by the Ty-2 gene caused by a strain of Tomato yellow leaf curl virus (TYLCV) [76] and by a strain of the Tomato leaf curl Bangalore virus (ToLCBV) in India [77].
Our preliminary set of analyses showed no unique (i.e., pool-specific) polymorphisms (data not shown). Several point mutations were found, but none of them were specific to the viruses present in the pool with or without the Ty-1 gene. Thus, another plausible explanation for some of the reported field events of Ty-1-mediated resistance/tolerance “breakdown” under Brazilian conditions could be related to some natural synergistic interactions with distinct group of viruses. In fact, it has been demonstrated that the Ty-1 gene does not confer resistance to major tomato-infecting RNA viruses such as Tomato spotted wilt virus (TSWV) and Cucumber mosaic virus (CMV) [78]. However, it has been demonstrated that RNA viruses can compromise resistance against begomoviruses, as previously shown during TYLCV and CMV co-infection, where there was a significant increase in TYLCV concentration that was due to the inhibition of the transcriptional gene silencing response by CMV 2b RNAi suppressor protein [78,79,80]. In the present work, it was not possible to assess the diversity of RNA viruses associated with the samples because the employed methodological approach did not allow us to analyze this group of viruses.
5. Conclusions
The results reported here provide useful information about the population dynamics of begomoviruses associated with tomato crops across three major tomato-producing regions of central Brazil in the last decade. Our work is in agreement with the notion that NGS is a powerful strategy to identify not only known viruses but also novel viruses and subviral agents. The present work is the first step towards the elucidation of the potential begomovirus adaptation to the tolerance factor Ty-1. However, in order to carry out a more precise study on the selective impact of the Ty-1 locus on begomovirus diversity and evolution, a distinct experimental strategy would probably be more appropriate, since our analysis was conducted on samples collected in different regions of a large country, in different years and from tomato plants grown in different microenvironmental situations. Therefore, it is possible that these variables (geographic area, climate, and year) can generate some biases that may not allow us to estimate the actual effect of the Ty-1 gene. For this purpose, the analysis could be more appropriately conducted on samples collected from experimental plots cultivated with tomato isolines with and without the Ty-1 gene. On the other side, our ecologically oriented approach allowed us to carry out a more ample exploration of an array of environments, which may enhance the opportunity to detect a larger number of yet undescribed viral species associated with the tomato crop. Even though with a slightly different number of evaluated samples in the pools with (n = 43) and without (n = 64) the Ty-1 gene, virus-specific PCR assays and Sanger sequencing validations of NGS-derived data indicated greater diversity (14 versus 6 species) in samples lacking this gene. Moreover, two novel Begomovirus species, one gemycircularvirus (Genomoviridae) and one alpha-satellite were identified exclusively in samples without Ty-1, whereas a novel begomovirus was found exclusively in the Ty-1 gene pool. These results indicated a potential viral adaptation to this tolerance factor, as well as virus-specific filtering effects of the Ty-1 on a subset of single-stranded DNA viruses and subviral agents. However, these hypotheses will be better tested with tomato isolines (with and without the Ty-1 gene) after controlled experiments employing infectious clones.
Acknowledgments
The authors would like to thank Antonio Francisco Costa and Fernando Lucas Melo for their technical support in Sanger dideoxy sequencing.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4915/12/8/819/s1, Table S1. Geographic distribution of 21 species of begomovirus reported naturally infecting tomatoes in Brazil. Sample sequences with Ty-1 NGS with codes.
Author Contributions
Conceptualization, L.d.N.A.d.R., S.G.R., M.E.d.N.F., L.S.B. and R.d.C.P.-C.; methodology, L.d.N.A.d.R., F.Y.B.N., L.S.B., M.E.d.N.F. and R.d.C.P.-C.; software, M.E.d.N.F., L.d.N.A.d.R. and R.d.C.P.-C.; validation, L.d.N.A.d.R. and F.Y.B.N.; formal analysis, L.d.N.A.d.R. and F.Y.B.N.; investigation, L.d.N.A.d.R. and F.Y.B.N., resources, M.E.d.N.F., L.S.B. and R.d.C.P.-C.; data curation, M.E.d.N.F., L.S.B., writing—original draft, L.d.N.A.d.R., L.S.B. and R.d.C.P.-C.; preparation, L.d.N.A.d.R., L.S.B. and R.d.C.P.-C.; writing—review and editing, S.G.R., M.E.d.N.F., L.S.B. and R.d.C.P.-C.; visualization, L.S.B. and R.d.C.P.-C.; supervision, L.S.B. and R.d.C.P.-C.; project administration, L.S.B. and R.d.C.P.-C.; funding acquisition, M.E.d.N.F., L.S.B. and R.d.C.P.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants, scholarships, and post-doc fellowships from CNPq, CAPES, FAP–DF, and the Empresa Brasileira de Pesquisa Agropecuária (Embrapa), project Improvement of the tomato plant to add value and sustainability of the crop in Brazil.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.ICTV International Committee on Taxonomy of Viruses. [(accessed on 6 January 2020)]; Available online: http://www.ictvonline.org.
- 2.Brown J.K., Zerbini F.M., Navas-Castillo J., Moriones E., Ramos-Sobrinho R., Silva J.C., Fiallo-Olivé E., Briddon R.W., Hernández-Zepeda C., Idris A. Revision of Begomovirus taxonomy based on pairwise sequence comparisons. Arch. Virol. 2015;160:1593–1619. doi: 10.1007/s00705-015-2398-y. [DOI] [PubMed] [Google Scholar]
- 3.Varsani A., Roumagnac P., Fuchs M., Navas-Castillo J., Moriones E., Idris A., Briddon R.W., Rivera-Bustamante R., Zerbini F.M., Martin D.P. Capulavirus and Grablovirus: Two new genera in the family Geminiviridae. Arch. Virol. 2017;162:1819–1831. doi: 10.1007/s00705-017-3268-6. [DOI] [PubMed] [Google Scholar]
- 4.Rojas M.R., Macedo M., Maliano M., Soto-Aguilar M., Souza J., Briddon R., Kenyon L., Rivera Bustamante R., Zerbini F., Adkins S., et al. World management of geminiviruses. Annu. Rev. Phytopathol. 2018;56:637–677. doi: 10.1146/annurev-phyto-080615-100327. [DOI] [PubMed] [Google Scholar]
- 5.De Barro P., Liu S.-S., Boykin L., Dinsdale A. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011;56:1–19. doi: 10.1146/annurev-ento-112408-085504. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro S., Ambrozevicius L., Avila A., Bezerra I., Calegario R., Fernandes J., Lima M., De Mello R., Rocha H., Zerbini F., et al. Distribution and genetic diversity of tomato-infecting begomoviruses in Brazil. Arch. Virol. 2003;148:281–295. doi: 10.1007/s00705-002-0917-0. [DOI] [PubMed] [Google Scholar]
- 7.Seal S., VandenBosch F., Jeger M. Factors influencing begomovirus evolution and their increasing global significance: Implications for sustainable control. Crit. Rev. Plant Sci. 2006;25:23–46. doi: 10.1080/07352680500365257. [DOI] [Google Scholar]
- 8.Silva F.N., Lima A., Rocha C., Castillo-Urquiza G., Alves-Júnior M., Zerbini F. Recombination and pseudorecombination driving the evolution of the begomoviruses Tomato severe rugose virus (ToSRV) and Tomato rugose mosaic virus (ToRMV): Two recombinant DNA-A components sharing the same DNA-B. Virol. J. 2014;11:66. doi: 10.1186/1743-422X-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FAO Food and Agriculture Organization of the United Nations (FAO) [(accessed on 8 January 2020)]; Available online: http://www.faostat.fao.org.
- 10.Ribeiro S., Mello L., Boiteux L., Kitajima E., Faria J. Tomato infection by a geminivirus in the Federal District, Brazil. Fitopatol. Bras. 1994;19:330. [Google Scholar]
- 11.Rêgo-Machado C.M., Nakasu E.Y., Blawid R., Nagata T., Inoue-Nagata A.K. Complete genome sequence of a new bipartite begomovirus infecting tomato in Brazil. Arch. Virol. 2019;164:2873–2875. doi: 10.1007/s00705-019-04380-0. [DOI] [PubMed] [Google Scholar]
- 12.Calegario R., Ferreira S., Andrade C., Zerbini F. Caracterização de um isolado do begomovírus Sida micrantha mosaic virus (SiMMV) obtido de tomateiro. Fitopatol. Bras. 2004;29:150. [Google Scholar]
- 13.Cotrim M.A.A., Krause-Sakate R., Narita N., Zerbini F.M., Pavan M.A. Diversidade genética de begomovírus em cultivos de tomateiro no Centro-Oeste Paulista. Summa Phytopathol. 2007;33:300–303. doi: 10.1590/S0100-54052007000300017. [DOI] [Google Scholar]
- 14.Inoue-Nagata A.K., Lima M.F., Gilbertson R.L. A review of geminivirus diseases in vegetables and other crops in Brazil: Current status and approaches for management. Hortic. Bras. 2016;34:8–18. doi: 10.1590/S0102-053620160000100002. [DOI] [Google Scholar]
- 15.Silva L.D., Omoto C., Bleicher E., Dourado P. Monitoring the susceptibility to insecticides in Bemisia tabaci (Gennadius)(Hemiptera: Aleyrodidae) populations from Brazil. Neotrop. Entomol. 2009;38:116–125. doi: 10.1590/s1519-566x2009000100013. [DOI] [PubMed] [Google Scholar]
- 16.Yao F.L., Zheng Y., Huang X.Y., Ding X.L., Zhao J.W., Desneux N., He Y.X., Weng Q.Y. Dynamics of Bemisia tabaci biotypes and insecticide resistance in Fujian province in China during 2005–2014. Sci. Rep. 2017;7:1–12. doi: 10.1038/srep40803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamir D., Ekstein-Michelson I., Zakay Y., Navot N., Zeidan M., Sarfatti M., Eshed Y., Harel E., Pleban T., Van-Oss H., et al. Mapping and introgression of a Tomato yellow leaf curl virus tolerance gene, Ty-1. Theor. Appl. Genet. 1994;88:141–146. doi: 10.1007/BF00225889. [DOI] [PubMed] [Google Scholar]
- 18.Hanson P., Green S., Kuo G. Ty-2, a gene on chromosome 11 conditioning geminivirus resistance in tomato. Tomato Genet. Coop. Rep. 2006;56:17–18. [Google Scholar]
- 19.Ji Y., Scott J. Ty-3, a begomovirus resistance locus linked to Ty-1 on chromosome 6 of tomato. Tomato Genet. Coop. Rep. 2006;56:22–25. [Google Scholar]
- 20.Ji Y., Scott J., Schuster D. Molecular mapping of Ty-4, a new Tomato yellow leaf curl virus resistance locus on chromosome 3 of tomato. J. Am. Soc. Hortic. Sci. 2009;134:281–288. doi: 10.21273/JASHS.134.2.281. [DOI] [Google Scholar]
- 21.Anbinder I., Reuveni M., Azari R., Paran I., Nahon S., Shlomo H., Chen L., Lapidot M., Levin I. Molecular dissection of Tomato leaf curl virus resistance in tomato line TY172 derived from Solanum peruvianum. Theor. Appl. Genet. 2009;119:519–530. doi: 10.1007/s00122-009-1060-z. [DOI] [PubMed] [Google Scholar]
- 22.Hutton S., Scott J. Ty-6, a major begomovirus resistance gene located on chromossome 10. Res. Rep. 2014;64:14–16. [Google Scholar]
- 23.Giordano L., Silva-Lobo V., Santana F., Fonseca M., Boiteux L. Inheritance of resistance to the bipartite Tomato chlorotic mottle begomovirus derived from Lycopersicon esculentum cv.‘Tyking’. Euphytica. 2005;143:27–33. doi: 10.1007/s10681-005-1685-1. [DOI] [Google Scholar]
- 24.Bian X.Y., Thomas M., Rasheed M., Saeed M., Hanson P., De Barro P., Rezaian M. A recessive allele (tgr-1) conditioning tomato resistance to geminivirus infection is associated with impaired viral movement. Phytopathology. 2007;97:930–937. doi: 10.1094/PHYTO-97-8-0930. [DOI] [PubMed] [Google Scholar]
- 25.Boiteux L., Fonseca M., Giordano L., Melo P. Produção de Tomate para Processamento Industrial. Embrapa; Brasília-DF, Brazil: 2012. Melhoramento genético; pp. 31–50. [Google Scholar]
- 26.Boiteux L., Fonseca M., Viera J., Pereira-Carvalho R. Melhoramento de Plantas para Condições de Estresses Bióticos. MG Suprema; Visconde de Rio Branco-MG, Brazil: 2012. Melhoramento para resistência a doenças virais; pp. 89–119. [Google Scholar]
- 27.Pereira-Carvalho R., Boiteux L., Fonseca M., Díaz-Pendón J., Moriones E., Fernández-Muñoz R., Charchar J., Resende R. Multiple resistance to Meloidogyne spp. and to bipartite and monopartite Begomovirus spp. in wild Solanum (Lycopersicon) accessions. Plant Dis. 2010;94:179–185. doi: 10.1094/PDIS-94-2-0179. [DOI] [PubMed] [Google Scholar]
- 28.Verlaan M.G., Szinay D., Hutton S.F., de Jong H., Kormelink R., Visser R.G., Scott J.W., Bai Y. Chromosomal rearrangements between tomato and Solanum chilense hamper mapping and breeding of the TYLCV resistance gene Ty-1. Plant J. 2011;68:1093–1103. doi: 10.1111/j.1365-313X.2011.04762.x. [DOI] [PubMed] [Google Scholar]
- 29.Maxwell D.M.C., Salus M., Montes L., Mejía L. Tagging begomovirus resistance gene. [(accessed on 6 May 2020)]; Available online: www.plantpath.wisc.edu.
- 30.Caro M., Verlaan M.G., Julián O., Finkers R., Wolters A.-M.A., Hutton S.F., Scott J.W., Kormelink R., Visser R.G., Díez M.J. Assessing the genetic variation of Ty-1 and Ty-3 alleles conferring resistance to Tomato yellow leaf curl virus in a broad tomato germplasm. Mol. Breed. 2015;35:132. doi: 10.1007/s11032-015-0329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung J., Kim H.J., Lee J.M., Oh C.S., Lee H.J., Yeam I. Gene-based molecular marker system for multiple disease resistances in tomato against Tomato yellow leaf curl virus, late blight, and verticillium wilt. Euphytica. 2015;205:599–613. doi: 10.1007/s10681-015-1442-z. [DOI] [Google Scholar]
- 32.Cooper J., Jones A. Responses of plants to viruses: Proposals for the use of terms. Phytopathology. 1983;73:127–128. doi: 10.1094/Phyto-73-127. [DOI] [Google Scholar]
- 33.Boiteux L., Oliveira V., Silva C., Makishima N., Inoue-Nagata A., Fonseca M., Giordano L. Reaction of tomato hybrids carrying the Ty-1 locus to Brazilian bipartite Begomovirus species. Hortic. Bras. 2007;25:20–23. doi: 10.1590/S0102-05362007000100005. [DOI] [Google Scholar]
- 34.Voorburg C.M., Yan Z., Bergua-Vidal M., Wolters A.M.A., Bai Y., Kormelink R. Ty-1, a universal resistance gene against geminiviruses that is compromised by co-replication of a betasatellite. Mol. Plant Pathol. 2020;21:160–172. doi: 10.1111/mpp.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knief C. Analysis of plant microbe interactions in the era of next generation sequencing technologies. Front. Plant Sci. 2014;5:216. doi: 10.3389/fpls.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams I., Fox A. Current Research Topics in Plant Virology. Springer; Cham, Switzerland: 2016. Diagnosis of plant viruses using next-generation sequencing and metagenomic analysis; pp. 323–335. [Google Scholar]
- 37.Hadidi A. Next-Generation Sequencing and CRISPR/Cas13 editing in viroid research and molecular diagnostics. Viruses. 2019;11:120. doi: 10.3390/v11020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villamor D., Ho T., Al Rwahnih M., Martin R., Tzanetakis I. High throughput sequencing for plant virus detection and discovery. Phytopathology. 2019;109:716–725. doi: 10.1094/PHYTO-07-18-0257-RVW. [DOI] [PubMed] [Google Scholar]
- 39.Li R., Gao S., Hernandez A., Wechter W., Fei Z., Ling K.S. Deep sequencing of small RNAs in tomato for virus and viroid identification and strain differentiation. PLoS ONE. 2012;7:e37127. doi: 10.1371/journal.pone.0037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu C., Sun X., Taylor A., Jiao C., Xu Y., Cai X., Wang X., Ge C., Pan G., Wang Q. Diversity, distribution, and evolution of tomato viruses in China uncovered by small RNA sequencing. J. Virol. 2017;91:1–14. doi: 10.1128/JVI.00173-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fontenele R.S., Lamas N.S., Lacorte C., Lacerda A.L.M., Varsani A., Ribeiro S.G. A novel geminivirus identified in tomato and cleome plants sampled in Brazil. Virus Res. 2017;240:175–179. doi: 10.1016/j.virusres.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez-Negrete E.A., Morales-Aguilar J.J., Domínguez-Duran G., Torres-Devora G., Camacho-Beltrán E., Leyva-López N.E., Voloudakis A.E., Bejarano E.R., Méndez-Lozano J. High-throughput sequencing reveals differential Begomovirus species diversity in non-cultivated plants in Northern-Pacific Mexico. Viruses. 2019;11:594. doi: 10.3390/v11070594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boiteux L., Fonseca M., Simon P. Effects of plant tissue and DNA purification method on randomly amplified polymorphic DNA-based genetic fingerprinting analysis in carrot. J. Am. Soc. Hortic. Sci. 1999;124:32–38. doi: 10.21273/JASHS.124.1.32. [DOI] [Google Scholar]
- 44.Rojas M.R., Gilbertson R., Russell D., Maxwell D. Use of degenerate primers in the polymerase chain reaction to detect whitefly-transmitted geminiviruses. Plant Dis. 1993;77:340–347. doi: 10.1094/PD-77-0340. [DOI] [Google Scholar]
- 45.Ha C., Coombs S., Revill P., Harding R., Vu M., Dale J. Corchorus yellow vein virus, a New World geminivirus from the Old World. J. Gen. Virol. 2006;87:997–1003. doi: 10.1099/vir.0.81631-0. [DOI] [PubMed] [Google Scholar]
- 46.Inoue-Nagata A.K., Albuquerque L., Rocha W., Nagata T. A simple method for cloning the complete begomovirus genome using the bacteriophage φ29 DNA polymerase. J. Virol. Methods. 2004;116:209–211. doi: 10.1016/j.jviromet.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 47.Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Argüello-Astorga G., Ruiz-Medrano R. An iteron-related domain is associated to Motif 1 in the replication proteins of geminiviruses: Identification of potential interacting amino acid-base pairs by a comparative approach. Arch. Virol. 2001;146:1465–1485. doi: 10.1007/s007050170072. [DOI] [PubMed] [Google Scholar]
- 49.Virus-HostDB Host Index. [(accessed on 10 February 2020)]; Available online: https://www.genome.jp/virushostdb/index/host/all.
- 50.Inoue-Nagata A.K., Lopes C., Reis A., Pereira R., Quezao-Durval A., Pinheiro J., Lima M. Doenças do Tomateiro. In: Amorim L.B.F., Rezende J.A.M., Camargo L.E.A., editors. Manual de Fitopatologia. 5th ed. Volume 2. Agronômica Ceres; Sao Paulo-SP, Brazil: 2016. pp. 697–729. [Google Scholar]
- 51.Rosen R., Kanakala S., Kliot A., Pakkianathan B.C., Farich B.A., Santana-Magal N., Elimelech M., Kontsedalov S., Lebedev G., Cilia M. Persistent, circulative transmission of begomoviruses by whitefly vectors. Curr. Opin. Virol. 2015;15:1–8. doi: 10.1016/j.coviro.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Sahu A.K., Verma R.K., Gaur R., Sanan-Mishra N. Complexity and recombination analysis of novel begomovirus associated with spinach yellow vein disease in India. Plant Gene. 2018;13:42–49. doi: 10.1016/j.plgene.2018.01.001. [DOI] [Google Scholar]
- 53.Briddon R.W., Martin D.P., Roumagnac P., Navas-Castillo J., Fiallo-Olivé E., Moriones E., Lett J.M., Zerbini F.M., Varsani A. Alphasatellitidae: A new family with two subfamilies for the classification of geminivirus-and nanovirus-associated alphasatellites. Arch. Virol. 2018;163:2587–2600. doi: 10.1007/s00705-018-3854-2. [DOI] [PubMed] [Google Scholar]
- 54.Paprotka T., Metzler V., Jeske H. The first DNA 1-like α satellites in association with New World begomoviruses in natural infections. Virology. 2010;404:148–157. doi: 10.1016/j.virol.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Romay G., Chirinos D., Geraud-Pouey F., Desbiez C. Association of an atypical alphasatellite with a bipartite New World begomovirus. Arch. Virol. 2010;155:1843–1847. doi: 10.1007/s00705-010-0760-7. [DOI] [PubMed] [Google Scholar]
- 56.Fontenele R.S., Lacorte C., Lamas N.S., Schmidlin K., Varsani A., Ribeiro S.G. Single stranded DNA viruses associated with capybara faeces sampled in Brazil. Viruses. 2019;11:710. doi: 10.3390/v11080710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Male M.F., Kami V., Kraberger S., Varsani A. Genome sequences of Poaceae-associated gemycircularviruses from the Pacific Ocean island of Tonga. Genome Announc. 2015;3:e01144-15. doi: 10.1128/genomeA.01144-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marzano S.Y.L., Domier L.L. Novel mycoviruses discovered from metatranscriptomics survey of soybean phyllosphere phytobiomes. Virus Res. 2016;213:332–342. doi: 10.1016/j.virusres.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 59.Krupovic M., Ghabrial S.A., Jiang D., Varsani A. Genomoviridae: A new family of widespread single-stranded DNA viruses. Arch. Virol. 2016;161:2633–2643. doi: 10.1007/s00705-016-2943-3. [DOI] [PubMed] [Google Scholar]
- 60.De Rezende R.R., Mar T.B., Páez L.M.C., da Silva Xavier A., Xavier C.A.D., Navas-Castillo J., Zerbini F.M., Alfenas-Zerbini P. Complete genome sequences of two gemycircularviruses associated with non-cultivated plants in Brazil. Arch. Virol. 2018;163:3163–3166. doi: 10.1007/s00705-018-3924-5. [DOI] [PubMed] [Google Scholar]
- 61.Lima M.F., Bezerra I., Ribeiro S., Ávila A. Distribuição de geminivírus nas culturas do tomate e pimentão em doze municípios do Submédio do Vale São Francisco. Fitopatol. Bras. 2001;26:81–85. doi: 10.1590/S0100-41582001000100014. [DOI] [Google Scholar]
- 62.Castillo-Urquiza G., Beserra J., Bruckner F., Lima A., Varsani A., Alfenas-Zerbini P., Zerbini F. Six novel begomoviruses infecting tomato and associated weeds in Southeastern Brazil. Arch. Virol. 2008;153:1985–1989. doi: 10.1007/s00705-008-0172-0. [DOI] [PubMed] [Google Scholar]
- 63.Barbosa J.C., Albuquerque L., Rezende J., Inoue-Nagata A., Bergamin Filho A., Costa H. Occurrence and molecular characterization of Tomato common mosaic virus (ToCmMV) in tomato fields in Espírito Santo state, Brazil. Trop. Plant Pathol. 2016;41:62–66. doi: 10.1007/s40858-015-0064-2. [DOI] [Google Scholar]
- 64.Fernandes F.R., Albuquerque L.C., de Oliveira C.L., Cruz A.R., da Rocha W.B., Pereira T.G., Naito F.Y., Dias N.D.M., Nagata T., Faria J.C., et al. Molecular and biological characterization of a new Brazilian begomovirus, Euphorbia yellow mosaic virus (EuYMV), infecting Euphorbia heterophylla plants. Arch. Virol. 2011;156:2063. doi: 10.1007/s00705-011-1070-4. [DOI] [PubMed] [Google Scholar]
- 65.Rezende W., Militão Neto V., Goulart L., GiovaniniI M., Juliatti F., Fernandes J. Mixed infection by geminiviruses in tomato plants detected by LIS-SSCP-PCR. Fitopatol. Bras. 1997;22:338–339. [Google Scholar]
- 66.Fernandes F.R., Albuquerque L., Giordano L., Boiteux L., Avila A., Inoue-Nagata A. Diversity and prevalence of Brazilian bipartite Begomovirus species associated to tomatoes. Virus Genes. 2008;36:251–258. doi: 10.1007/s11262-007-0184-y. [DOI] [PubMed] [Google Scholar]
- 67.Rocha C.S., Castillo-Urquiza G.P., Lima A.T., Silva F.N., Xavier C.A., Hora-Júnior B.T., Beserra-Júnior J.E., Malta A.W., Martin D.P., Varsani A., et al. Brazilian begomovirus populations are highly recombinant, rapidly evolving, and segregated based on geographical location. J. Virol. 2013;87:5784–5799. doi: 10.1128/JVI.00155-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Albuquerque L.C., Varsani A., Fernandes F., Pinheiro B., Martin D., Ferreira P., Lemos T., Inoue-Nagata A. Further characterization of tomato-infecting begomoviruses in Brazil. Arch. Virol. 2012;157:747–752. doi: 10.1007/s00705-011-1213-7. [DOI] [PubMed] [Google Scholar]
- 69.Ribeiro S., Martin D., Lacorte C., Simões I., Orlandini D., Inoue-Nagata A. Molecular and biological characterization of Tomato chlorotic mottle virus suggests that recombination underlies the evolution and diversity of Brazilian tomato begomoviruses. Phytopathology. 2007;97:702–711. doi: 10.1094/PHYTO-97-6-0702. [DOI] [PubMed] [Google Scholar]
- 70.Fernandes J.J., Carvalho M., Andrade E., Brommonschenkel S., Fontes E., Zerbini F. Biological and molecular properties of Tomato rugose mosaic virus (ToRMV), a new tomato-infecting begomovirus from Brazil. Plant Pathol. 2006;55:513–522. doi: 10.1111/j.1365-3059.2006.01395.x. [DOI] [Google Scholar]
- 71.Belabess Z., Dallot S., El-Montaser S., Granier M., Majde M., Tahiri A., Blenzar A., Urbino C., Peterschmitt M. Monitoring the dynamics of emergence of a non-canonical recombinant of Tomato yellow leaf curl virus and displacement of its parental viruses in tomato. Virology. 2015;486:291–306. doi: 10.1016/j.virol.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 72.Granier M., Tomassoli L., Manglli A., Nannini M., Peterschmitt M., Urbino C. First report of TYLCV-IS141, a Tomato yellow leaf curl virus recombinant infecting tomato plants carrying the Ty-1 resistance gene in Sardinia (Italy) Plant Dis. 2019;103:1437. doi: 10.1094/PDIS-09-18-1558-PDN. [DOI] [Google Scholar]
- 73.Panno S., Caruso A.G., Davino S. The nucleotide sequence of a recombinant tomato yellow leaf curl virus strain frequently detected in Sicily isolated from tomato plants carrying the Ty-1 resistance gene. Arch. Virol. 2018;163:795–797. doi: 10.1007/s00705-017-3674-9. [DOI] [PubMed] [Google Scholar]
- 74.Torre C., Agüero J., Aranda M. First evidence of Tomato yellow leaf curl virus-Israel IS76 recombinant isolates associated with severe yellow leaf curl epidemics in resistant tomatoes in Spain. Plant Dis. 2019;103:780. doi: 10.1094/PDIS-09-18-1619-PDN. [DOI] [Google Scholar]
- 75.Rêgo C.D.M. Diversidade Genômica de Begomovírus em Tomateiros Resistente (BRS SENA) e Susceptível (H-9553) Universidade de Brasília; Brasilia-DF, Brazil: 2016. [Google Scholar]
- 76.Ohnishi J., Yamaguchi H., Saito A. Analysis of the mild strain of Tomato yellow leaf curl virus, which overcomes Ty-2 gene–mediated resistance in tomato line H24. Arch. Virol. 2016;161:2207–2217. doi: 10.1007/s00705-016-2898-4. [DOI] [PubMed] [Google Scholar]
- 77.Tiwari N., Padmalatha K., Singh V., Haq Q., Malathi V. Tomato leaf curl Bangalore virus (ToLCBV): Infectivity and enhanced pathogenicity with diverse betasatellites. Arch. Virol. 2010;155:1343–1347. doi: 10.1007/s00705-010-0710-4. [DOI] [PubMed] [Google Scholar]
- 78.Butterbach P., Verlaan M.G., Dullemans A., Lohuis D., Visser R.G., Bai Y., Kormelink R. Tomato yellow leaf curl virus resistance by Ty-1 involves increased cytosine methylation of viral genomes and is compromised by Cucumber mosaic virus infection. Proc. Natl. Acad. Sci. USA. 2014;111:12942–12947. doi: 10.1073/pnas.1400894111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.González I., Martínez L., Rakitina D.V., Lewsey M.G., Atencio F.A., Llave C., Kalinina N.O., Carr J.P., Palukaitis P., Canto T. Cucumber mosaic virus 2b protein subcellular targets and interactions: Their significance to RNA silencing suppressor activity. Mol. Plant Microbe Interact. 2010;23:294–303. doi: 10.1094/MPMI-23-3-0294. [DOI] [PubMed] [Google Scholar]
- 80.Hamera S., Song X., Su L., Chen X., Fang R. Cucumber mosaic virus suppressor 2b binds to AGO4-related small RNAs and impairs AGO4 activities. Plant. J. 2012;69:104–115. doi: 10.1111/j.1365-313X.2011.04774.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.