Abstract
Purpose
To summarize current understanding of the efficacy, role, and cost-effectiveness of the available epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), and to evaluate sequencing strategies based on the available evidence.
Summary. EGFR TKIs are the current standard of care for patients with EGFR mutation–positive non–small cell lung cancer (NSCLC). Five EGFR TKIs are currently approved in the United States for use in a first-line setting; these TKIs differ in mechanism of action, efficacy, safety, and cost. Most patients develop resistance to first-line EGFR TKIs and require subsequent therapy with additional EGFR TKIs, chemotherapy, and/or other targeted agents. A major consideration when selecting EGFR TKIs, both as first-line or subsequent treatment options, is cost-effectiveness. Although clinical trials have shown that the second- and third-generation EGFR TKIs are superior in efficacy to the first-generation agents, pharmacoeconomic studies suggest that the first-generation agents are the most cost-effective, with the second-generation TKI afatinib also considered cost-effective in some studies. Despite its impressive efficacy, osimertinib appears to be less cost-effective due to substantially higher acquisition costs.
Conclusion
Preliminary data suggest that first-line afatinib followed by osimertinib may offer promising survival outcomes and, on the basis of efficacy alone, may represent an optimal sequencing strategy in the majority of patients with EGFR mutation–positive NSCLC, in particular Asian patients and those with Del19-positive tumors. However, considerably more research into outcomes and costs associated with consecutive sequencing of EGFR TKIs is needed before any conclusions can be reached.
Keywords: cost-effectiveness, EGFR mutation, NSCLC, treatment sequencing, tyrosine kinase inhibitor
KEY POINTS.
Although epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are the standard of care in EGFR mutation–positive non–small cell lung cancer, most patients will develop resistance to first-line therapy with use of these agents.
Clinical efficacy, safety, and cost-effectiveness are important considerations when selecting a first- or subsequent-line EGFR TKI treatment.
Available data suggest that afatinib followed by osimertinib may be a promising treatment sequence; further research to evaluate optimal sequencing strategies is needed.
In the United States, lung cancer is the second most common malignancy and the leading cause of cancer-related mortality, accounting for around 26% of all deaths.1 Non–small cell lung cancer (NSCLC) comprises around 85% of all lung cancer cases in the United States.2 Numerous genetic aberrations are associated with the development of NSCLC; one of the most common sites is the gene encoding epidermal growth factor receptor (EGFR),3 a receptor tyrosine kinase normally found on the cell surface.4 Somatic mutations in the EGFR gene are relatively common, occurring in 10% to 15% of Caucasian patients and approximately 50% of Asian patients with lung adenocarcinoma.5 As a result, it is recommended that all patients with advanced-stage lung adenocarcinoma or a tumor with an adenocarcinoma component undergo EGFR mutational analysis.6 For patients with advanced or metastatic nonsquamous NSCLC in the United States, the National Comprehensive Cancer Network (NCCN) recommends EGFR mutation testing (category 1), ALK gene testing (category 1), ROS1 gene testing, BRAF gene testing, and PD-L1 testing (category 1) and strongly suggests broader molecular profiling (NCCN guidelines accessed in November 2019).7
Tyrosine kinase inhibitors (TKIs) targeting EGFR have been shown to produce high response rates and prolong survival in EGFR mutation–positive NSCLC8-16 and are the current standard of care for patients with sensitizing EGFR mutations, regardless of performance status.17,18 Currently, 5 EGFR TKIs are approved in the United States for the treatment of EGFR mutation–positive NSCLC: the first-generation reversible EGFR TKIs, erlotinib and gefitinib; the second-generation irreversible blockers of the ErbB protein family, afatinib and dacomitinib; and the third-generation irreversible, EGFR wild type–sparing EGFR TKI, osimertinib.19-23 Although an impressive response to these agents is usually seen, resistance almost inevitably develops, resulting in disease progression and necessitating the use of one or more subsequent lines of therapy.17 The most common mechanism of resistance to first- and second-generation EGFR TKIs is the T790M mutation.24
When determining the most appropriate therapy for each individual patient, physicians must consider multiple factors, including the efficacy, safety, and quality of life associated with each agent or combination of agents. Additional considerations include the options available for subsequent lines of treatment and, for many patients and payors, the cost of treatment, in particular the cost of prescribed pharmaceuticals. While substantial data on the clinical benefits of each EGFR TKI are available, less is known about the best order in which to use these agents and which are the most cost-effective in each setting. In this article, we summarize current understanding of the efficacy, role, and cost-effectiveness of the available EGFR TKIs, then discuss current sequencing strategies utilizing the available evidence. Data sources used in this narrative review were derived by searching relevant medical literature on the clinical efficacy, safety, and cost-effectiveness of treatments for EGFR mutation–positive NSCLC. Where available, large (at least 100 patients) and well-designed (eg, randomized controlled) studies were preferred.
Use of EGFR TKIs in the first-line setting: clinical efficacy and cost considerations
Clinical outcomes with first-line EGFR TKIs.
Most clinical trials of first-line EGFR TKIs have focused on patients with NSCLC involving common EGFR mutations (Del19 and L858R mutations).25 Findings from pivotal and additional clinical trials have clearly demonstrated the superior efficacy of all EGFR TKIs relative to platinum-based chemotherapy in the treatment of patients with sensitive EGFR mutation–positive NSCLC (Table 1), with median progression-free survival (PFS) of 9 to 19 months with the EGFR TKIs vs 4 to 7 months with platinum-based chemotherapy.8,10,11,13,15,16,26-30 A discussion of safety and tolerability considerations in use of EGFR TKIs is presented in a separate section of this article.
Table 1.
Summary of Pivotal Studies of Approved EGFR Tyrosine Kinase Inhibitors as First-line Therapies in EGFR Mutation–Positive Non–Small Cell Lung Cancera
| Erlotinib | Gefitinib | Afatinib | Dacomitinib | Osimertinib45 | |||
|---|---|---|---|---|---|---|---|
| Study | EURTAC13 (phase 3) | NEJ00229,75 (phase 3) | LUX-Lung 314,76 (phase 3) | LUX-Lung 661,76 (phase 3) | LUX-Lung 711,12 (phase 2b) | ARCHER 105016,31 (phase 3) | FLAURA15 (phase 3) |
| Treatment | Erlotinib vs cisplatin + docetaxel or gemcitabine | Gefitinib vs carboplatin + paclitaxel | Afatinib vs cisplatin + pemetrexed | Afatinib vs cisplatin + gemcitabine | Afatinib vs gefitinib | Dacomitinib vs gefitinib | Osimertinib vs gefitinib or erlotinib |
| Disease and stage | Stage 3B/4 NSCLC | Stage 3B/4 lung ADC | Stage 3B/4 lung ADC | Stage 3B/4 ADC | Stage 3B/4 ADC | Stage 3B/4 NSCLCa | Stage 3B/4 NSCLC |
| No. of patients | 173 | 230 | 345 | 364 | 319 | 452 | 556 |
| Predominant ethnicities | Not reported (European) | 100% Japanese | 72% East Asian 26% white | 90% Chinese 7% Southeast Asian 4% South Korean | ~57% Asian ~32% white | 51% Chinese 18% Japanese 23% white | 62% Asian 36% white |
| EGFR mutations | Del19, L858R | Del19, L858R, other (6%) | Del19, L858R, other (~11%) | Del19, L858R, other (~11%) | Del19, L858R | Del19, L858R | Del19, L858R |
| ORR | 58% vs 15% P not reported | 74% vs 31% P < 0.001 | 56% vs 23% P = 0.001 | 67% vs 23% P < 0.0001 | 70% vs 56% P = 0.0083 | 75% vs 72% P = 0.42 | 80% vs 76% P = 0.24 |
| Median PFS, mob | 9.7 vs 5.2; HR, 0.37; P < 0.0001 | 10.8 vs 5.4; HR, 0.32; P < 0.001 | 11.1 vs 6.9; HR, 0.58; P = 0.001 | 11.0 vs 5.6; HR, 0.28; P < 0.0001 | 11.0 vs 10.9; HR, 0.73; P = 0.017 | 14.7 vs 9.2; HR, 0.59; P < 0.0001 | 17.7 vs 9.7; HR, 0.45; P < 0.001 |
| Median OS in ITT population, mo | 19.3 vs 19.5; HR, 1.04; P = 0.87 | 27.7 vs 26.6; HR, 0.89; P not reported | 28.2 vs 28.2; HR, 0.88; P = 0.39 | 23.1 vs 23.5; HR, 0.93; P = 0.61 | 27.9 vs 24.5; HR, 0.86; P = 0.26 | 34.1 vs 26.8; HR, 0.76; P = 0.044 | 38.6 vs 31.8; HR, 0.80; P = 0.046 |
| Median OS in Del19-positive patients, mo | Not reported | Not reported | 33.3 vs 21.1; HR, 0.54; P = 0.0015 | 31.4 vs 18.4; HR, 0.64; P = 0.023 | 30.7 vs 26.4; HR, 0.83; P = 0.28 | 34.1 vs NR; HR, 0.88; P = 0.49 | Not reported |
| Median OS in L858R-positive patients, mo | Not reported | Not reported | 27.6 vs 40.3; HR, 1.30; P = 0.29 | 19.6 vs 24.3; HR, 1.22; P = 0.34 | 25.0 vs 21.2; HR, 0.91; P = 0.66 | 32.5 vs 23.2; HR, 0.71; P = 0.081 | Not reported |
| Most common grade 3 or higher AEs | Erlotinib Rash: 13 Aminotransferase elevation: 2% | Gefitinib Aminotransferase elevation: 26% Rash/acne: 5%Appetite loss: 5% | Afatinib Rash/acne: 16%Diarrhea: 14%Paronychia: 11%Stomatitis/mucositis: 9% | Afatinib Rash/acne: 15%Diarrhea: 5%Stomatitis/mucositis: 5% | Gefitinib Liver enzyme elevations: 9% Diarrhea: 1% Rash/acne: 3% Afatinib Diarrhea: 13%Rash/acne: 9%Fatigue: 6% |
Dacomitinib Dermatitis acneiform: 14%Diarrhea: 8%Paronychia: 7%Hypokalemia: 5% Gefitinib ALT elevation: 8%Disease progression: 5%AST elevation: 4%Dyspnea: 3% |
Osimertinib Decreased appetite: 3%Prolonged QT interval: 2%Diarrhea: 2%Rash/acne: 1% |
Abbreviations: ADC, adenocarcinoma; ALT, alanine transaminase; AST, aspartate transaminase; Del19, exon 19 deletion; EGFR, epidermal growth factor receptor; HR, hazard ratio; ITT, intent-to-treat; L858R, leucine to arginine substitution in codon 858; NR, not reached; NSCLC, non–small cell lung cancer; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; TKIs, tyrosine kinase inhibitors.
aExcluded patients with history of brain or leptomeningeal metastases.
bIndependent assessment.
Results of head-to-head studies of different EGFR TKIs suggest that afatinib, dacomitinib, and osimertinib are more efficacious than first-generation TKIs, with significant improvements in PFS achieved with these drugs compared with first-generation agents (Table 1).11,12,15,16,31 To date, no head-to-head studies have compared the second- and third-generation TKIs in the first-line setting, although one network meta-analyses has been conducted.32 Despite the lack of head-to-head comparative data, osimertinib is now listed as the preferred first-line agent (regardless of T790M mutation status) in the NCCN Clinical Practice Guidelines In Oncology (accessed in November 2019),7 presumably due to the impressive PFS seen with first-line osimertinib therapy in the phase 3 FLAURA study.15 Also noteworthy is that afatinib is approved in the United States for treatment of not only patients with common EGFR mutations but also those harboring other nonresistant EGFR mutations.21
Cost considerations.
Early cost-effectiveness analyses comparing first-generation TKIs with the previous standard of care, platinum-based chemotherapy, concluded that the first-line use of EGFR TKIs was cost-effective in China,33 Germany,34 Spain, Italy, and France.35 In contrast, afatinib was not considered cost-effective relative to combination therapy with pemetrexed plus cisplatin in Singapore.36 These were all modeled analyses conducted from the perspectives of healthcare payors in the respective countries and therefore included only direct medical costs.
More recently, several modeled analyses have directly compared the EGFR TKIs (Table 2), and these provided further insights into the relative value of individual agents. However, since the studies were conducted using country-specific pricing and healthcare costs, it is difficult to make any conclusive comparisons. For analyses reporting results in non-US currencies, conversion to US dollars has been provided using the mid-year (July 1) exchange rate (https://www.xe.com/currencytables/) for the year of costing (or for the year prior to publication if no year of costing was provided in the analysis). All cost-effectiveness analyses that were conducted in the United States, as well as most other analyses, included costs related to adverse events (AEs) and, for drug acquisition costs, used publicly available and/or published prices (eg, wholesale acquisition costs); this also applies to analyses discussed in the subsequent section of this article on cost considerations in second-line therapy. In some US analyses,37,38 drug acquisition costs were discounted (eg, by 17%) to account for contract prices.
Table 2.
Summary of Cost-effectiveness Analyses of EGFR Tyrosine Kinase Inhibitors
| Reference and Year Published (Study Country) | Comparators | Inputs | ICER(s)a |
|---|---|---|---|
| Ting et al,37 2015 (United States) |
Erlotinib vs afatinib or cisplatin + pemetrexed | Efficacy/safety data: EURTAC trial (erlotinib), LUX-Lung 3 trial (afatinib, cisplatin + pemetrexed) | Erlotinib vs afatinib: $61,809/QALY Erlotinib vs cisplatin + pemetrexed: $40,106/QALY |
| Graham et al,39 2016 (United States) |
Afatinib vs erlotinib | Efficacy/safety data: available literature | Afatinib vs erlotinib: $77,504/QALY |
| Aguiar et al,41 2018 (United States) |
Osimertinib vs erlotinib, gefitinib, or afatinib | Efficacy/safety data: FLAURA trial (osimertinib), prior meta-analysis (erlotinib, gefitinib, afatinib)77 | Osimertinib vs erlotinib: $226,527/QALY Osimertinib vs gefitinib: $231,123/QALY Osimertinib vs afatinib: $219,874/QALY |
| Ezeife et al,42 2018 (Canada) |
Osimertinib vs gefitinib or afatinib | Efficacy/safety data: available literature | Osimertinib vs gefitinib or afatinib: CaD $223,133 (≈US $169,826) per QALY |
| Chouaid et al,43 2017 (France) |
Afatinib vs gefitinib | Efficacy/safety data: LUX-Lung 7 trial | Afatinib vs gefitinib: €45,211 (≈US $50,361) per QALY |
| Kimura et al,44 2018 (Japan) |
Gefitinib vs afatinib or erlotinib | Efficacy data: literature search/RCTs Safety data: patient records |
Gefitinib vs afatinib: ¥122,071 (≈US $1,086)/MST (per month) Gefitinib vs erlotinib: ¥-69,606 (≈US $-619)/MST (per month) |
CaD = Canadian dollar; EGFR, epidermal growth factor receptor; ICER, incremental cost-effectiveness ratio; MST, median survival time; QALY, quality-adjusted life-year; RCT, randomized controlled trial.
aFor values reported in non-US currencies, conversion to US dollars, as derived from midyear (July 1) exchange rate (https://www.xe.com/currencytables/) for the year of costing (or for the year prior to publication if no year of costing was reported), is provided.
A cost-effectiveness analysis conducted in the United States and comparing combination therapy with cisplatin plus pemetrexed and monotherapy with either erlotinib or afatinib found that both EGFR TKIs were more cost-effective than chemotherapy.37 Based on survival and AE data from the EURTAC and LUX-Lung 3 studies, the investigators concluded that erlotinib was the most cost-effective first-line option. The mean incremental cost-effectiveness ratio (ICER) for erlotinib vs afatinib (year of costing, 2013) was $61,809 per quality-adjusted life-year (QALY), with much of the cost differential due to the additional costs of AE management in afatinib-treated patients. The researchers noted that if the monthly cost of afatinib was decreased by 21%, the drug would be as cost-effective as erlotinib. However, a cost-effectiveness analysis of afatinib and erlotinib in patients with Del19-positive NSCLC in the United States, based on PFS and overall survival (OS), showed that afatinib was cost-effective vs erlotinib at a threshold of $150,000, with an ICER of $77,504/QALY (year of costing, 2016).39 A budget impact analysis of first-line afatinib therapy for patients with Del19 or L858R mutations estimated that increasing the treatment share of afatinib within a United States health plan would increase the proportion of treated patients remaining progression-free while having only a small budget impact on the health plan.40
Although osimertinib is associated with impressive first-line efficacy and less toxicity than first- and second-generation EGFR TKIs, the substantially increased acquisition cost relative to costs of other EGFR TKIs means that osimertinib may be less cost-effective than the alternatives. High ICERs for osimertinib vs first- and second-generation TKIs were reported in a recent cost-effectiveness analysis conducted from the perspectives of US and Brazilian payors.41 In the United States, osimertinib ICERs were $226,527/QALY vs erlotinib, $231,123/QALY vs gefitinib, and $219,874/QALY vs afatinib. In Brazil, the osimertinib ICERs were slightly different from those in the United States: $162,329/QALY vs erlotinib, $180,804/QALY vs gefitinib, and $175,432 vs afatinib (year of costing, 2017). In one-way deterministic sensitivity analyses, hypothetical reductions in the price of osimertinib improved cost-effectiveness substantially. For example, in the United States, a 20% reduction in the acquisition cost of osimertinib could potentially make osimertinib cost-effective, although the study investigators concluded that based on the osimertinib cost at the time of analysis, osimertinib was not cost-effective as a first-line therapy for treatment of EGFR-mutated NSCLC.
In Canada, the use of osimertinib vs the standard of care (gefitinib plus afatinib) was associated with a gain of 0.79 QALY, with an ICER of CaD $223,133 (≈US $169,826) per QALY (year of costing, 2018); osimertinib had a 0% probability of being cost-effective at a willingness to pay threshold of CaD $100,000/QALY.42
A French cost-effectiveness analysis, based primarily on data from the LUX-Lung 7 study, concluded that first-line afatinib appeared to be cost-effective compared with gefitinib, with an ICER of €45,211 (≈US $50,361) per QALY (year of costing not reported).43 ICERs for patient populations with EGFR Del19 and L858R mutations were €38,970 (≈US $43,409) and €52,518 (≈US $58,500) per QALY, respectively. At a willingness-to-pay threshold of €70,000/QALY, afatinib had 100% probability of being cost-effective in patients with common EGFR mutations. In a retrospective Japanese analysis using hospital electronic medical records, gefitinib was considered to be the most cost-effective of the first- and second-generation EGFR TKIs.44 The ICER for gefitinib vs afatinib was ¥122,071 (≈US $1,086) per month of median survival time (MST); and for gefitinib vs erlotinib, ¥-69,606 (≈US $-619)/MST. The cost differential was primarily due to considerably higher drug acquisition costs for afatinib and erlotinib in Japan.
Overall, while use of osimertinib may be associated with longer PFS than use of first- and second-generation TKIs, it does not currently appear to be the most cost-effective option for the first-line treatment of EGFR mutation–positive advanced and/or metastatic NSCLC. Variable results regarding the cost-effectiveness of afatinib vs first-generation EGFR TKIs have been reported (Table 2). Some of the variability in results of the cost-effectiveness analyses may be related to heterogeneity in the modeling methodology and lack of adjustments for effect modifiers. In most analyses, efficacy determinations were primarily based on indirect comparisons using network meta-analyses that assumed equal efficacy for control arms and did not adjust for common and uncommon mutations or location of metastases (eg, central nervous system, bone). In addition, further cost-effectiveness analyses incorporating mature OS data from the FLAURA study, which are now available,45 are needed to enable firmer conclusions. Among the inherent uncertainties of economic modeling, the lack of mature OS data may be a particularly important one in terms of its impact on ICER results.
Second-line therapy after failure of first-line EGFR TKI therapy.
Despite the efficacy of EGFR TKIs in the first-line treatment of EGFR mutation–positive NSCLC, acquired resistance to therapy is inevitable. The NCCN guidelines recommend that all patients with progressive disease after first-line afatinib, erlotinib, gefitinib, or dacomitinib therapy should be tested for the presence of the “gatekeeper” T790M resistance mutation,7 which is the most common mechanism of resistance to these agents and is detected in up to two-thirds of patients treated with erlotinib, gefitinib, or afatinib24 (although only rarely in TKI-naive patients46).
T790M-positive patients.
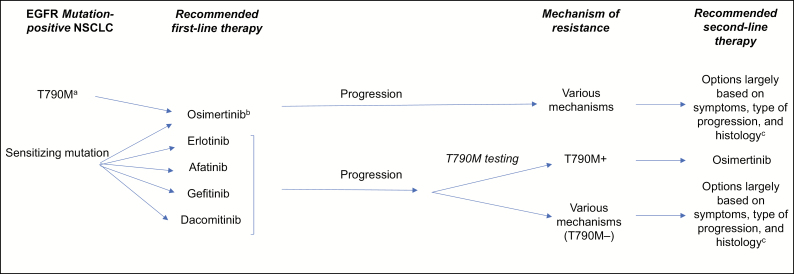
NCCN guidelines recommend that patients with T790M-positive disease following treatment with a first-line EGFR TKI (ie, erlotinib, gefitinib, afatinib, or dacomitinib) are treated with osimertinib (Figure 1).7 Recent clinical trial and real-world data indicate that osimertinib is highly effective in this setting and is associated with PFS ranging from 10 to 12 months.9,24,47,48 Among 279 T790M-positive patients whose disease progressed following erlotinib, gefitinib, and/or afatinib therapy and who subsequently received osimertinib in the AURA3 study, the objective response rate (ORR) was 71%, with a median duration of response of 9.7 months and a median PFS of 10.1 months; PFS was also improved with use of osimertinib vs platinum therapy plus pemetrexed among patients with brain metastases.9
Figure 1.
Current treatment sequencing options in EGFR mutation–positive non–small cell lung cancer (NSCLC).7 EGFR indicates epidermal growth factor receptor; NCCN; National Comprehensive Cancer Network; T790M, threonine to methionine substitution in codon 790 of EGFR; TKI, tyrosine kinase inhibitor.
aRarely seen prior to EGFR TKI therapy,46 and NCCN Guidelines (accessed in November 2019)7 do not explicitly state that testing specifically for the T790M mutation should be done prior to first-line EGFR TKI therapy.
bPreferred treatment, regardless of T790M mutation status, according to NCCN Guidelines (accessed in November 2019).7
cWith no targeted therapy currently available, the recommended treatment depends largely on the extent of disease (eg, symptoms, type of progression, histology). Systemic treatment is recommended for patients with multiple lesions. For more localized disease, patients may continue the initially prescribed EGFR TKI or undergo definitive local therapy, although various other options may be applicable.
Real-world studies support the findings from these clinical trials. In the global ASTRIS real-world study, which enrolled 3,014 T790M-positive patients who had received prior EGFR TKI treatment and were subsequently treated with osimertinib, median PFS at the second interim analysis was 11.0 months, with the endpoint of median OS not yet reached (1-year OS, 76%).48 In an analysis of a French early-access program involving 205 patients who received osimertinib after treatment with a first- or second-generation EGFR TKI (a median of 2.8 prior treatment lines), median PFS was 12.4 months and median OS since osimertinib initiation was 20.5 months.47 Favorable survival results were also reported in the real-world GioTag study to investigate the use of afatinib followed by osimertinib69,70 (see discussion of optimal sequencing paradigms later in this article). Taken together, clinical and real-world data suggest that in patients with acquired T790M mutations, sequential use of EGFR TKIs can achieve a combined disease-free period of at least 2 years, potentially longer.
T790M-negative patients.
In patients who have disease progression despite use of first-line EGFR TKIs but test negative for the T790M mutation, a variety of resistance mechanisms have been identified.49,50 As a result, second-line treatment strategies for patients with T790M-negative tumors are largely based on symptoms, type of progression, and histology (Figure 1).7 Experts generally recommend (1) continuing treatment with the initially prescribed EGFR TKI, since continued benefit may be obtained even after disease progression, (2) considering local therapy, and/or (3) if multiple symptomatic lesions are present, commencing systemic chemotherapy, although other treatment strategies may apply, depending on patient characteristics noted above.7 These recommendations reflect the paucity of clinical data and options available for T790M-negative patients with acquired resistance to EGFR TKIs; this is clearly an area of urgent unmet clinical need. Outcomes in patients who have tumor progression while receiving a first-generation EGFR TKI but not receiving second-line osimertinib therapy are poor, with median PFS values of 4 to 5 months and 4 to 8 months reported with second-line chemotherapy and targeted therapy, respectively, and corresponding median OS values of 12 to 19 months and 17 to 26 months.51-53 Slight improvements have been seen with the use of bevacizumab plus platinum-based chemotherapy vs platinum-based chemotherapy alone (median PFS, 8.2 vs 5.1 months).52 Furthermore, in a subgroup of TKI-pretreated patients with EGFR-sensitizing mutations in the randomized phase 3 IMpower 150 trial, the median PFS was 10.2 months in patients who received atezolizumab and bevacizumab plus chemotherapy, compared with 6.9 months in the bevacizumab plus chemotherapy treatment group.54 Results did not appear to be driven by high PDL1 expression. TKI-pretreated, T790M-negative patients with EGFR-mutant NSCLC (with acquired resistance to EGFR TKIs) treated with afatinib plus bevacizumab had a median PFS of 7.1 months in a multicenter phase 2 trial conducted in Japan (the ABC study).55
By contrast, treatment with gefitinib in combination with cisplatin plus pemetrexed following disease progression on gefitinib had no impact on PFS compared with chemotherapy alone (median PFS, 5.4 months with or without gefitinib).56 An ORR of 25% was observed with use of the combination of afatinib and cetuximab in heavily pretreated patients with acquired T790M-negative resistance following combination therapy with erlotinib and gefitinib; the median response duration was 9.5 months, and median PFS was 4.6 months.57 Additional studies are needed in order to identify more efficacious approaches.
There are currently only limited published data on patients receiving second-line therapy following first-line osimertinib therapy; this is clearly an area that requires further investigation. It is known, however, that resistance to osimertinib is mediated by a range of mechanisms, with 60% of patients not exhibiting putative resistance mechanisms,58 including additional EGFR mutations (eg, the C797S mutation) or mutations in other genes (eg, KRAS)59; this heterogeneity will make therapeutic targeting of osimertinib resistance mechanisms difficult.
Cost considerations.
The cost- effectiveness of second-line therapy following the failure of first-line treatment with EGFR TKIs in patients with EGFR mutation–positive NSCLC has not been extensively investigated to date. However, the available data suggest that, primarily due to its high acquisition cost, osimertinib may be a less cost-effective option in the second-line setting in the United States.
Wu et al analyzed data from the AURA3 study to investigate the costs associated with testing for T790M mutations in the plasma and tissue of patients who had disease progression despite first-line treatment with EGFR TKIs followed by second-line treatment with osimertinib or chemotherapy with cisplatin plus pemetrexed in the United States and in China.38 The ICERs for osimertinib vs chemotherapy were $232,895 and $48,081 per QALY in the United States and China, respectively (year of costing, 2017). The researchers concluded that osimertinib treatment for T790M mutation–positive NSCLC is unlikely to be cost-effective in either country. As in the first-line setting, the unfavorable ICERs of osimertinib vs chemotherapy were primarily due to the higher acquisition costs of osimertinib, with treatment becoming cost-effective if the price of osimertinib was discounted by 50%.
A cost-effectiveness analysis of osimertinib for the second-line treatment of T790M-positive NSCLC in the United Kingdom, conducted using data from the phase 2, single-arm AURA extension and AURA2 studies and taking into account the cost of T790M testing, reported an ICER for osimertinib vs platinum-doublet chemotherapy of £41,705 (≈US $65,139) per QALY gained (year of costing, 2015).60 The investigators concluded that osimertinib may be considered cost-effective in this setting.
Safety of EGFR TKIs
EGFR TKIs are associated with some serious AEs that require ongoing management (Table 1). While EGFR TKIs result in common class-related AEs, each TKI also has distinct AEs that may ultimately lead to treatment discontinuation. AEs were initially common with afatinib, with an overall incidence of grade 3 or higher AEs of up 50%14,61; however, tolerability-guided dose reductions have been found to reduce the incidence and severity of AEs, permitting patients to remain on therapy for longer and reducing the cost of managing treatment-related AEs.62,63 Afatinib dose reductions in the LUX-Lung 3 and LUX-Lung 6 trials resulted in major reductions in rates of treatment-related grade 3 or higher AEs (from 73.0% to 20.5% and from 80.6% to 11.9%, respectively) without altering efficacy outcomes64; these management strategies have led to low rates of afatinib treatment discontinuation in clinical studies (8.0%, 5.9%, and 6.3% in the LUX-Lung 3, 6, and 7 trials, respectively).11,14,61 In a noninterventional, observational study conducted in a real-world setting, afatinib dose reductions were shown to reduce the frequency and intensity of treatment-related AEs without compromising treatment effectiveness.65 The incidence of grade 3 or higher AEs is lowest with osimertinib,15 probably because osimertinib does not target wild-type (ie, nonmutated) EGFR. The AE profiles of each drug, while important clinically, can also have a significant impact on the cost-effectiveness of each agent. As noted previously, all of the US cost-effectiveness analyses (and most non-US analyses) included costs related to AEs.
The development of optimal sequencing paradigms
Given that all patients inevitably experience disease progression during use of EGFR TKIs, subsequent therapy is an important consideration when choosing first-line treatment. The ultimate goal of treatment is to maximize not just the initial PFS duration (as is often the focus of clinical studies) but the entire length and quality of the patient’s remaining months or years. A critical issue, however, is how to determine which treatment sequence will provide the best outcomes for each individual patient. In addition to treatment efficacy and tolerability, multiple other factors are usually considered, including patient characteristics (eg, age, performance status), patient preferences, and anticipated quality of life.18,66,67
Available evidence indicates that first-line afatinib therapy followed by osimertinib therapy is an appropriate treatment regimen in T790M-positive patients.12,68 The promising results seen in small numbers of patients in the LUX-Lung 7 trial who were treated with afatinib followed by a third-generation EGFR TKI suggest that sequential use of afatinib and osimertinib may offer prolonged disease control.12 In a post hoc analysis of patients who had received osimertinib or olmutinib following discontinuation of afatinib or gefitinib, there was a trend towards longer survival in patients who had received first-line treatment with afatinib vs gefitinib (median OS, not evaluable vs 46.0 months; hazard ratio [HR], 0.51; P = 0.22), suggesting that the benefits of afatinib extend beyond the first treatment line.12 In addition, the results of the global, retrospective, real-world GioTag study to investigate the use of afatinib followed by osimertinib showed a favorable rate of 2-year survival (78.9%) and a median time on treatment of 27.6 months with sequential use of these agents.69 Median time on treatment was particularly long in patients with Del19-positive tumors (30.3 months) and in Asian patients (46.7 months). In a recent updated report on the GioTag study, the 2-year survival rate was 80% for patients treated with sequential afatinib and osimertinib therapy; the updated median time to treatment failure was 28.1 months. In addition, the median OS was 41.3 months for the overall patient population and 45.7 months for patients with Del19-positive tumors.70
A recent retrospective analysis of 111 T790M mutation-positive patients who acquired resistance to afatinib or first-generation TKIs during any line of therapy showed significantly higher rates of objective response and disease control with use of afatinib followed by osimertinib vs use of first-generation TKIs followed by osimertinib (82.9% vs 53.9% [P = 0.0065] and 91.4% vs 71.1% [P = 0.032], respectively).71 Preliminary data suggest that median PFS was also longer with use of afatinib followed by osimertinib compared with use of first-generation TKIs followed by osimertinib (15.7 vs 8.9 months, P = 0.195).71 Finally, a post hoc analysis of the LUX-Lung 3, 6, and 7 studies demonstrated encouraging OS in a small number of patients who received osimertinib following afatinib therapy, with a 3-year OS rate of over 90%.12,68
As mentioned above, osimertinib was recently recommended as the preferred first-line option for EGFR mutation–positive NSCLC.7,72 A strong argument for this approach is that approximately one-third of patients will never develop T790M mutations24 and, consequently, never benefit from osimertinib in any line of therapy if it is not given as a front-line treatment. Further, initial analysis of extended outcomes from the FLAURA study suggest that the benefits of first-line osimertinib therapy may extend beyond the time osimertinib treatment is stopped due to initial disease progression.73 In the study arms evaluating osimertinib vs erlotinib or gefitinib, 26% vs 38% of all patients experienced a second progression or died (HR for second progression or death after initiation of second-line treatment, 0.58; P = 0.0004). Median time to second subsequent therapy or death was not calculable for patients in the osimertinib arm, compared with 25.9 months in the erlotinib or gefitinib arm (HR, 0.6; P = 0.0005). In addition, final OS data now available from the FLAURA study show that median OS was prolonged with use of osimertinib vs the comparator agents: 38.6 months (95% confidence interval [CI], 34.5–41.8) vs 31.8 months (95% CI, 26.6–36.0); HR, 0.8 (95.05% CI, 0.64–1.00; P = 0.046.45 However, a number of unanswered questions regarding the first-line use of osimertinib remain, including questions as to the comparative effects of using osimertinib vs afatinib and/or dacomitinib. In addition, as a result of the apparently heterogeneous mechanisms of resistance to first-line osimertinib treatment,59 defined second-line therapeutic options targeting these mechanisms are currently lacking.
Clearly, data on long-term outcomes following sequential EGFR TKI therapy are urgently needed. Two investigator-initiated trials are underway, in Germany and Japan, to assess the benefits of sequencing afatinib and osimertinib. The ongoing APPLE trial comparing first-line osimertinib therapy until disease progression with use of gefitinib followed by osimertinib will provide further insight into the optimal role for osimertinib (ie, first- or second-line therapy), albeit in comparison to a first- rather than a second-generation EGFR TKI.74
In addition to long-term outcomes and tolerability, cost considerations are relevant when determining the most appropriate sequencing strategies. Cost-effectiveness analyses have suggested that, at current US pricing, osimertinib is not particularly cost-effective in any treatment line.38,41 By contrast, first- and second-generation agents have varying degrees of cost-effectiveness in the first-line setting in the United States.37 These pharmacoeconomic benefits, coupled with the known mechanism of acquired resistance in a majority of patients, provide an advantage for selecting a first- or second-generation TKI as a first-line option when defining a cost-effective treatment strategy. Given the absence of robust data clearly showing that first-line osimertinib is cost-effectiveness, it may be preferable to reserve osimertinib for use as a second-line therapy in a smaller number of patients who may potentially derive greater benefit from its use.
Conclusion
Available data suggest that the efficacy of the second- and third-generation EGFR TKIs is superior to that of the first-generation agents, although a higher frequency of grade 3 or higher AEs has been reported with use of the second-generation agents. However, pharmacoeconomic analyses suggest that the first-generation EGFR TKIs are the more cost-effective first-line options, with afatinib also considered cost-effective in some studies. Based on recent data, it appears that first-line osimertinib confers longer PFS than the first-generation agents; however, due to its high acquisition cost, osimertinib may not be the most cost-effective option in this setting.
First-line treatment with afatinib followed by osimertinib has been demonstrated to produce promising survival outcomes in a post hoc analysis and in a preliminary analysis of real-world data and, on the basis of efficacy alone, may represent an optimal sequencing strategy in the majority of patients with EGFR mutation–positive NSCLC, in particular Asians and patients with Del19-positive tumors. However, considerably more research into outcomes and costs associated with consecutive sequencing of EGFR TKIs is needed before any conclusions can be reached. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors received no direct compensation related to the development of the manuscript.
Supplementary Material
Acknowledgments
Writing and editorial support was provided by Hashem Dbouk, PhD.
Disclosures
Dr. Hirsh has received honoraria for advisory board service from AstraZeneca, Roche, Pfizer, Bristol-Myers Squibb, Merck, and Boehringer Ingelheim. Dr. Singh has received honoraria from Medtronic Global Lung Health Solutions and is on the physician advisory board of Somnoware, Inc. Writing and editorial support for the development of the manuscript of this article was funded by Boehringer Ingelheim, which was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. [DOI] [PubMed] [Google Scholar]
- 2. Molina JR, Yang P, Cassivi SD et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell. 2014;25(3):282-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res. 2015;4(1):36-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leighl NB, Rekhtman N, Biermann WA et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathology guideline. J Clin Oncol. 2014;32(32):3673-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Comprehensive Cancer Network. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer V1. 2020. ©National Comprehensive Cancer Network, Inc 2019. All rights reserved. Accessed November 6, 2019. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. [Google Scholar]
- 8. Mitsudomi T, Morita S, Yatabe Y et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121-128. [DOI] [PubMed] [Google Scholar]
- 9. Mok TS, Wu YL, Ahn MJ et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mok TS, Wu YL, Thongprasert S et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. [DOI] [PubMed] [Google Scholar]
- 11. Park K, Tan EH, O’Byrne K et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577-589. [DOI] [PubMed] [Google Scholar]
- 12. Paz-Ares L, Tan EH, O’Byrne K et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol. 2017;28(2):270-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosell R, Carcereny E, Gervais R et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. [DOI] [PubMed] [Google Scholar]
- 14. Sequist LV, Yang JC, Yamamoto N et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327-3334. [DOI] [PubMed] [Google Scholar]
- 15. Soria JC, Ohe Y, Vansteenkiste J et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. [DOI] [PubMed] [Google Scholar]
- 16. Wu YL, Cheng Y, Zhou X et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454-1466. [DOI] [PubMed] [Google Scholar]
- 17. Ettinger DS, Wood DE, Aisner DL et al. Non-small cell lung cancer, version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(4):504-535. [DOI] [PubMed] [Google Scholar]
- 18. Novello S, Barlesi F, Califano R et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v1-v27. [DOI] [PubMed] [Google Scholar]
- 19. Tarceva [prescribing information]. South San Francisco, CA: Genentech USA; revised October 2016. [Google Scholar]
- 20. Iressa [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals; revised August 2018. [Google Scholar]
- 21. Gilotrif [prescribing information]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; revised January 2018. [Google Scholar]
- 22. Tagrisso [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals; revised April 2018. [Google Scholar]
- 23. Vizimpro [prescribing information]. New York, NY: Pfizer Inc.; revised September 2018. [Google Scholar]
- 24. Yang JC, Ahn MJ, Kim DW et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase ii extension component. J Clin Oncol. 2017;35(12):1288-1296. [DOI] [PubMed] [Google Scholar]
- 25. Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Sci. 2016;107(9):1179-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Douillard JY, Ostoros G, Cobo M et al. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. Br J Cancer. 2014;110(1):55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fukuoka M, Wu YL, Thongprasert S et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866-2874. [DOI] [PubMed] [Google Scholar]
- 28. Zhou C, Wu YL, Chen G et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. [DOI] [PubMed] [Google Scholar]
- 29. Maemondo M, Inoue A, Kobayashi K et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380-2388. [DOI] [PubMed] [Google Scholar]
- 30. Wu YL, Zhou C, Liam CK et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883-1889. [DOI] [PubMed] [Google Scholar]
- 31. Mok TS, Cheng Y, Zhou X et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018:JCO2018787994. [DOI] [PubMed] [Google Scholar]
- 32. Lin JZ, Ma SK, Wu SX et al. A network meta-analysis of nonsmall-cell lung cancer patients with an activating EGFR mutation: should osimertinib be the first-line treatment? Medicine (Baltimore). 2018;97(30):e11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang S, Peng L, Li J et al. A trial-based cost-effectiveness analysis of erlotinib alone versus platinum-based doublet chemotherapy as first-line therapy for Eastern Asian nonsquamous non-small-cell lung cancer. PLoS One. 2013;8(3):e55917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schremser K, Rogowski WH, Adler-Reichel S et al. Cost-effectiveness of an individualized first-line treatment strategy offering erlotinib based on EGFR mutation testing in advanced lung adenocarcinoma patients in Germany. Pharmacoeconomics. 2015;33(11):1215-1228. [DOI] [PubMed] [Google Scholar]
- 35. Vergnenegre A, Massuti B, de Marinis F et al. Economic analysis of first-line treatment with erlotinib in an EGFR-mutated population with advanced NSCLC. J Thorac Oncol. 2016;11(6):801-807. [DOI] [PubMed] [Google Scholar]
- 36. Tan PT, Aziz MIA, Pearce F et al. Cost effectiveness analysis of afatinib versus pemetrexed-cisplatin for first-line treatment of locally advanced or metastatic EGFR mutation positive non-small-cell lung cancer from the Singapore healthcare payer’s perspective. BMC Cancer. 2018;18(1):352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ting J, Tien Ho P, Xiang P et al. Cost-effectiveness and value of information of erlotinib, afatinib, and cisplatin-pemetrexed for first-line treatment of advanced EGFR mutation-positive non-small-cell lung cancer in the United States. Value Health. 2015;18(6):774-782. [DOI] [PubMed] [Google Scholar]
- 38. Wu B, Gu X, Zhang Q. Cost-effectiveness of osimertinib for EGFR mutation-positive non-small cell lung cancer after progression following first-line EGFR TKI therapy. J Thorac Oncol. 2018;13(2):184-193. [DOI] [PubMed] [Google Scholar]
- 39. Graham J, Earnshaw S, Lim J et al. Cost-effectiveness of afatinib versus erlotinib in the first-line treatment of patients with metastatic non-small cell lung cancer with EGFR exon 19 deletion mutations. J Clin Path. 2016;2(4):31-39. [Google Scholar]
- 40. Graham J, Earnshaw S, Burslem K et al. Budget impact analysis of afatinib for first-line treatment of patients with metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 substitution mutations in a U.S. health plan. J Manag Care Spec Pharm. 2018;24(6):544-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aguiar PN Jr., Haaland B, Park W et al. Cost-effectiveness of osimertinib in the first-line treatment of patients with EGFR-mutated advanced non-small cell lung cancer. JAMA Oncol. 2018;4(8):1080-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ezeife DA, Kirk V, Chew DS et al. Economic analysis of osimertinib in previously untreated EGFR-mutant advanced non-small cell lung cancer in Canada. Lung Cancer. 2018;125:1-7. [DOI] [PubMed] [Google Scholar]
- 43. Chouaid C, Luciani L, LeLay K et al. Cost-effectiveness analysis of afatinib versus gefitinib for first-line treatment of advanced EGFR-mutated advanced non-small cell lung cancers. J Thorac Oncol. 2017;12(10):1496-1502. [DOI] [PubMed] [Google Scholar]
- 44. Kimura M, Yasue F, Usami E et al. Cost-effectiveness and safety of the molecular targeted drugs afatinib, gefitinib and erlotinib as first-line treatments for patients with advanced EGFR mutation-positive non-small-cell lung cancer. Mol Clin Oncol. 2018;9(2):201-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ramalingam SS, Vansteenkiste J, Planchard D et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41-50. [DOI] [PubMed] [Google Scholar]
- 46. Lavdovskaia ED, Iyevleva AG, Sokolenko AP et al. EGFR T790M mutation in TKI-naive clinical samples: frequency, tissue mosaicism, predictive value and awareness on artifacts. Oncol Res Treat. 2018;41(10):634-642. [DOI] [PubMed] [Google Scholar]
- 47. Auliac JB, Perol M, Planchard D et al. Efficacy and tolerance of osimertinib in real world setting: results of the French Early Access Program (EXPLORE T790M GFPC study). Paper presented at 19th World Congress on Lung Cancer; September 23-26, 2018; Toronto, Canada.
- 48. Wu YL, Cho BC, Zhou Q et al. A real-world treatment study of osimertinib in patients with EGFR T790M-positive NSCLC. Paper presented at 19th World Congress on Lung Cancer; September 23-26, 2018; Toronto, Canada.
- 49. Corallo S, D’Argento E, Strippoli A et al. Treatment options for EGFR T790M-negative EGFR tyrosine kinase inhibitor-resistant non-small cell lung cancer. Target Oncol. 2017;12(2):153-161. [DOI] [PubMed] [Google Scholar]
- 50. van der Wekken AJ, Saber A, Hiltermann TJ et al. Resistance mechanisms after tyrosine kinase inhibitors afatinib and crizotinib in non-small cell lung cancer, a review of the literature. Crit Rev Oncol Hematol. 2016;100:107-116. [DOI] [PubMed] [Google Scholar]
- 51. Vavala T, Follador A, Tiseo M et al. BE-POSITIVE: Beyond progression after tyrosine kinase inhibitor in EGFR-positive non small cell lung cancer patients: results from a multicenter Italian observational study. Lung Cancer. 2016;95:73-81. [DOI] [PubMed] [Google Scholar]
- 52. Jiang Z, Zhang Y, Yang Y et al. Efficacy of pemetrexed and carboplatin with or without bevacizumab in lung adenocarcinoma patients with EGFR non-T790M mutations after progression on first-line EGFR-tyrosine kinase inhibitors. Thorac Cancer. 2018;9(9):1151-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liam CK, Ho GF, Chai CS et al. Real-world experience with afatinib after failure of first-generation epidermal growth factor receptor-tyrosine kinase inhibitor. Paper presented at 19th World Congress on Lung Cancer; September 23-26, 2018; Toronto, Canada.
- 54. Reck M, Mok TSK, Nishio M et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7(5):387-401. [DOI] [PubMed] [Google Scholar]
- 55. Hata A, Katakami N, Kaji R et al. Afatinib plus bevacizumab combination after acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant non–small cell lung cancer: Multicenter, single-arm, phase 2 trial (ABC Study). Cancer. 2018;124(19):3830-3838. [DOI] [PubMed] [Google Scholar]
- 56. Soria JC, Wu YL, Nakagawa K et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16(8):990-998. [DOI] [PubMed] [Google Scholar]
- 57. Janjigian YY, Smit EF, Groen HJ et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov. 2014;4(9):1036-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ramalingam SS, Cheng Y, Zhou C et al. Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the phase III FLAURA study. Ann Oncol. 2018;29(suppl 8):abstract LBA50. [Google Scholar]
- 59. Oxnard GR, Hu Y, Mileham KF et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M-positive lung cancer and acquired resistance to osimertinib. JAMA Oncol. 2018;4(11):1527-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bertranou E, Bodnar C, Dansk V et al. Cost-effectiveness of osimertinib in the UK for advanced EGFR-T790M non-small cell lung cancer. J Med Econ. 2018;21(2):113-121. [DOI] [PubMed] [Google Scholar]
- 61. Wu YL, Zhou C, Hu CP et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213-222. [DOI] [PubMed] [Google Scholar]
- 62. Califano R, Tariq N, Compton S et al. Expert consensus on the management of adverse events from EGFR tyrosine kinase inhibitors in the UK. Drugs. 2015;75(12):1335-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Melosky B, Hirsh V. Management of common toxicities in metastatic NSCLC related to anti-lung cancer therapies with EGFR-TKIs. Front Oncol. 2014;4:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yang JC, Sequist LV, Zhou C et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann Oncol. 2016;27(11):2103-2110. [DOI] [PubMed] [Google Scholar]
- 65. Halmos B, Tan EH, Soo RA et al. Impact of afatinib dose modification on safety and effectiveness in patients with EGFR mutation-positive advanced NSCLC: results from a global real-world study (RealGiDo). Lung Cancer. 2019;127:103-111. [DOI] [PubMed] [Google Scholar]
- 66. Hirsh V. Is the evaluation of quality of life in NSCLC trials important? Are the results to be trusted? Front Oncol. 2014;4:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Girard N. Optimizing outcomes in EGFR mutation-positive NSCLC: which tyrosine kinase inhibitor and when? Future Oncol. 2018;14(11):1117-1132. [DOI] [PubMed] [Google Scholar]
- 68. Sequist LV, Wu YL, Schuler M et al. Subsequent therapies post-afatinib among patients with EGFRmutation-positive NSCLC in LUX-Lung (LL) 3, 6 and 7 [poster presentation]. Abstract 1349P. Paper presented at ESMO 2017 Congress; 2017; Madrid, Spain.
- 69. Hochmair MJ, Morabito A, Hao D et al. Sequential treatment with afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: an observational study. Future Oncol. 2018;14:2861-2874. [DOI] [PubMed] [Google Scholar]
- 70. Hochmair MJ, Morabito A, Hao D et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: updated analysis of the observational GioTag study. Future Oncol. 2019;15(25):2905-2914. [DOI] [PubMed] [Google Scholar]
- 71. Tamiya M, Tamiya A, Suzuki H et al. Which is better EGFR-TKI followed by osimertinib between afatinib and gefitinib/erlotinib? Ann Oncol. 2018;29(Suppl_8):viii493-viii547. [Google Scholar]
- 72. Tan CS, Kumarakulasinghe NB, Huang YQ et al. Third generation EGFR TKIs: current data and future directions. Mol Cancer. 2018;17(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Planchard D, Boyer M, Lee JS et al. Osimertinib vs standard of care (SoC) EGFR-TKI as first-line therapy in patients (pts) with untreated EGFRm advanced NSCLC: FLAURA post-progression outcomes. J Thorac Oncol. 2018;13(4):S72-S73. [Google Scholar]
- 74. Remon J, Menis J, Hasan B et al. The APPLE trial: feasibility and activity of AZD9291 (osimertinib) treatment on positive plasma T790M in EGFR-mutant NSCLC patients. EORTC 1613. Clin Lung Cancer. 2017;18(5):583-588. [DOI] [PubMed] [Google Scholar]
- 75. Inoue A, Kobayashi K, Maemondo M et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol. 2013;24(1):54-59. [DOI] [PubMed] [Google Scholar]
- 76. Yang JC, Wu YL, Schuler M et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141-151. [DOI] [PubMed] [Google Scholar]
- 77. Haaland B, Tan PS, de Castro G Jr et al. Meta-analysis of first-line therapies in advanced non-small-cell lung cancer harboring EGFR-activating mutations. J Thorac Oncol. 2014;9(6):805-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.