Abstract
Commodity polymers are produced in large volumes, providing robust mechanical properties at relatively low costs. The products made from these commodity polymers typically offer only static functionalities. Over the past decade, however, in the scientific literature, stimuli-responsive additives and/or polymer coatings have been introduced to commodity polymers, yielding composites and bilayers that change shape in response to light, temperature, and/or humidity. These stimuli responsive commodity polymers allow the marketing and sales of these otherwise bulk products as “high-end” smart materials for applications spanning from soft actuators to adaptive textiles. This Spotlight on Applications presents an overview of recent intriguing works on how shape changing commodity polymer composite and bilayer actuators based on polyamide 6, poly(ethylene terephthalate), polyethylene, and polypropylene have been fabricated that respond to environmental stimuli and discusses their potential applications.
Keywords: commodity polymers, stimuli-responsive materials, shape changing polymers, soft actuators, smart textile
Introduction
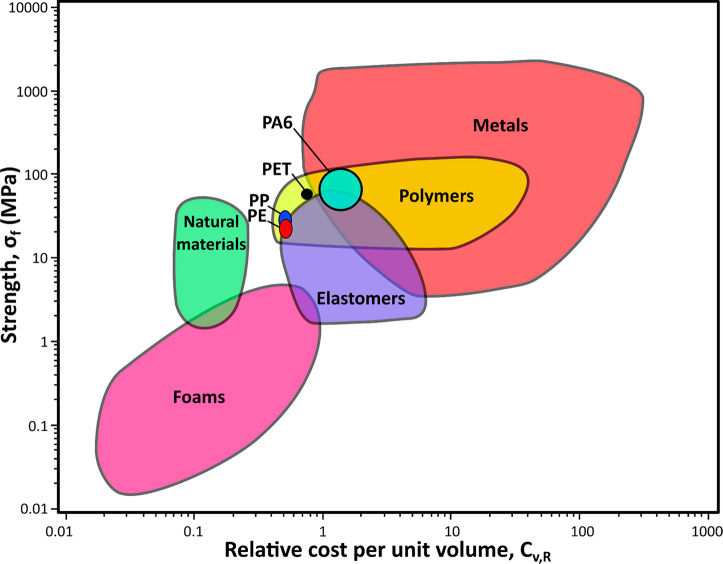
The use of commodity polymers, including polyamide 6 (PA6, Nylon-6), polyethylene (PE), poly(ethylene terephthalate) (PET), and polypropylene (PP), has been well-established in our daily lives with production exceeding millions of tons per year.1 Scale-up of production has resulted in tremendous cost reductions; the materials are mature to the point that it often appears of little financial benefit to continue research on them, given the restricted margins of profitability in these massive-scale productions, see Figure 1.
Figure 1.
Material property chart illustrating strength as a function of the relative cost per unit volume of several materials, including commodity polymers such as PET, PA6, PE, and PP. Chart created using CES EduPack 2018, Granta Design Ltd.2
The research, development, and manufacturing of stimuli-responsive commodity polymers could contribute a great deal to the catalog of industrial production materials, allowing the marketing and sales of these otherwise bulk products as “high-end” “smart” materials. Recently, stimuli- responsive polymers with functional properties that can be changed in response to external environmental stimuli have received considerable attention. Innovations over the past few years have demonstrated the great promise stimuli-responsive commodity polymers hold for high-end, high-value applications ranging from adaptive textiles to soft actuators. It is foreseen that these materials will play a decisive role in meeting societal challenges in the fields of sustainable energy, health care, personal comfort, and food safety.
Road toward Stimuli-Responsive Commodity Polymers
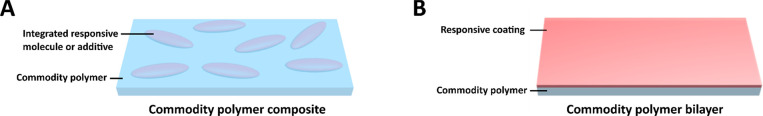
Shape changing commodity polymers can be prepared by blending in low molecular weight stimuli-responsive additives, such as graphene or carbon nanotubes (CNTs). The commodity polymer serves as a host for the responsive additives and thus performs as the actuator itself (see Figure 2). However, in the field of shape changing actuators the commodity polymer is typically employed as a (robust) passive substrate, predominantly focusing on otherwise fragile hydrogels, liquid crystals (LCs) and shape memory polymers (SMPs) to act as the actual actuating initiator.3−5 In the following paragraphs, stimuli-responsive additives, molecules and polymers will be discussed, covering the mechanisms that drive the shape changes in commodity polymer composites and bilayers in response to an environmental stimulus. The shape alterations imposed by changes in the surrounding temperature, humidity and light, are expressed through the actuator design6−8 or via gradients in molecular alignment, temperature or light penetration.9−12 Essentially, macroscopic motion is governed by the combination of material and geometrical parameters, such as mismatches in expansion coefficients, elastic moduli ratios and relative thickness ratios.13 In this Spotlight on Applications, we demonstrate that shape changing hydrogels, LCs and SMPs also can be used to make the commodity polymer stimuli-responsive.
Figure 2.
Two methods of fabricating stimuli-responsive shape changing commodity polymers. (A) In a composite, the responsive additives are embedded in the commodity polymer. (B) A bilayer, shape changing material is typically constructed from a commodity polymer substrate with a responsive coating.
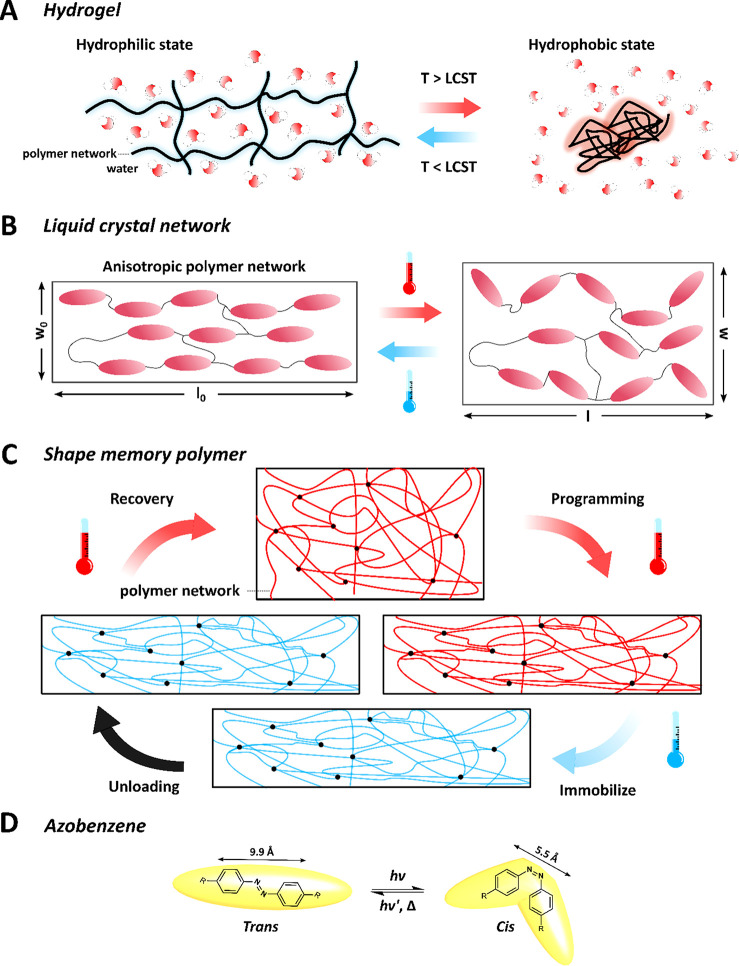
Hydrogels are 3D polymer networks capable of absorbing and retaining up to 90 wt % of water, depending on the cross-link density and polymer–solvent interactions, and swelling close to 10 times its initial volume with large deformations (ε ∼ 90%).14 Thermoresponsive hydrogels, such as poly(N-isopropylacrylamide) (pNIPAM), exhibit a lower critical solution temperature (LCST) and undergo a hydrophilic-to-hydrophobic transition close to the human body temperature, resulting in shrinkage of the polymer.15 At temperatures below the LCST, the polymer network undergoes enthalpy-driven swelling with water via supramolecular interactions including hydrogen bonds.16 In contrast, the heated network becomes hydrophobic as the supramolecular bonds are disrupted and the entropic term stimulates the release of water leading to deswelling (Figure 3A). Shape deformations can be engineered by applying an inhomogeneous stimulus or through gradients in cross-link density through the hydrogel.17
Figure 3.
Schematic illustrations describing the temperature-responsive shape changing properties of hydrogels, liquid crystal networks, shape memory polymers, and the working principle of a light responsive molecular switch. (A) An example of an insoluble polymer network which below the LCST retains significant amounts of water. In transitioning through this LCST the network becomes hydrophobic as the intermolecular bonds are disrupted and the network shrinks considerably. (B) A uniaxially aligned liquid crystal network manifests anisotropic thermal expansion by axial contraction and transverse expansion at elevated temperature. (C) A shape memory polymer can be deformed manually into a “programmed” shape. The polymer chains are then immobilized through cooling, and the deformed shape is “memorized” after unloading and can be recovered by heating the polymer. (D) The light-sensitive azobenzene absorbed electromagnetic radiation to isomerize from the extended trans isomer to the unstable cis isomer. The length of the anisotropically shaped molecules is significantly reduced in this isomerization. The reverse isomerization occurs when exposed to a different (longer) wavelength of light or by heating.
Liquid crystal networks (LCNs) are anisotropic, which make them appealing for fabricating stimuli-responsive polymers.9,18,19 The anisotropic properties arise from alignment and order of the reactive LC mesogens that is preserved by polymerizing them into aligned, cross-linked networks.20,21 More complex alignment profiles than simple uniaxial are possible, including 90° out-of-plane (splay), 90° in-plane (twist) or continuous in-plane (helical).22−24 As with oriented semicrystalline polymers, aligned LCNs demonstrate anisotropic thermal expansion: in their strive to increase entropy the polymer chains take on a more random alignment (order–disorder) evoking an axial contraction and transverse expansion (see Figure 3B).25−27
SMPs are deformable into temporary, “memorized” or “programmed” shapes, from which the original shape can be recovered upon application of an external stimulus (Figure 3C). These semicrystalline polymers28 and chemically or physically cross-linked networks can be programmed to strain fix a higher energy temporary conformation, relying on a sharp transition (a glass (Tg) and/or melting (Tm) transition) to immobilize polymer chains and prevent recovery.29,30 The programmed SMPs can be expanded from one-way shape memory materials, consisting of irreversible recovery of the initial state from a deformed geometry through an applied (thermal) stimulus, into materials with reversible shape changes to attain a tertiary shape.31−34
To create light-sensitive, shape changing hydrogels, LCs and SMPs, light-responsive trigger molecules and additives can be introduced.27 Light-responsive molecules, include azobenzenes, spiropyrans and spirooxazines, while humidity-responsive triggers have polar or ionic moieties.35,36 Photoresponsive dyes absorb electromagnetic radiation and undergo reversible transformations in shape and color.37,38 Azobenzenes, for example, photoisomerize from the extended trans configuration to the unstable cis isomer when exposed to specific wavelengths of light, usually UV (see Figure 3D). The reverse cis-to-trans isomerization occurs thermally or by exposure to visible light. The shape of the molecule is considerably altered when exposed to light: the molecular length of the trans-isomer is nearly halved during isomerization to the cis-isomer. Such molecular changes can be amplified in a polymer matrix leading to macroscopic shape changes of the polymer film. Shape changes in the polymer can also be induced by using photothermal dyes and other additives, including graphene, CNTs, nanoparticles or UV-absorbers, which transduce incident light into heat.39−42
Humidity-responsive shape changing films are generated by embedding moisture sensitive trigger molecules that have polar or ionic moieties in the polymer. In case of hydrogels, the dried hygroscopic polymer is often responding to humidity changes with deformations dependent on the cross-link density, number of ions and polar groups within the polymer network.
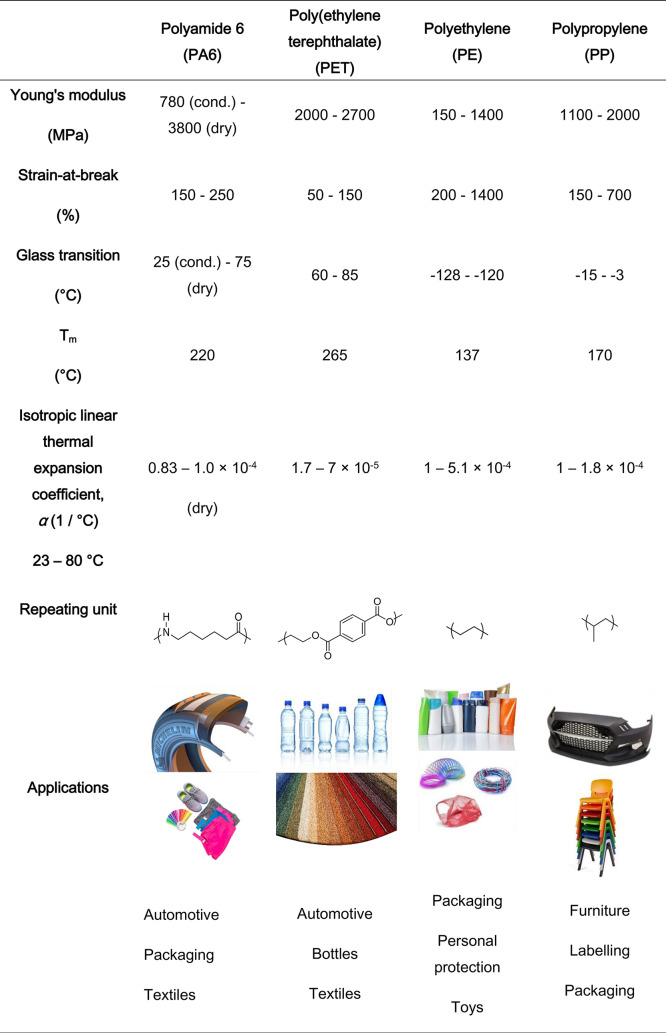
In this Spotlight on Applications, stimuli-responsive commodity polymer composites and bilayers that change shape will be discussed. We compare and contrast scientific findings and offer our projection of future applications utilizing heat-, humidity- and light-responsive commodity polymers in soft actuators and adaptive textiles. Other compelling stimuli-responsive functional properties, such as switching of permeability or optical properties, will not be considered in this work.43−45 We restrict our focus to the fabrication and (future) application of emerging materials composed of common textile polymers PA6 and PET, and the large volume polymers PE and PP, each of which shall be discussed individually (Figure 1). The approaches presented in this Spotlight on Applications can be equally applied to other commodity and noncommodity or specialty polymers not mentioned explicitly here.46−49 A comparison of some of the basic properties of these commodity polymers are summarized in Table 1, including temperatures relevant for processing and use, Young’s moduli, strains-at-break, (isotropic) linear thermal expansion coefficients, and several product examples. The (thermo)mechanical properties are important parameters to develop shape changing commodity polymers as deformations are determined by a mismatch in for example elastic moduli and thermal expansion coefficients.13,50 In these shape changing composites and bilayer actuators, the benefits of using commodity polymers will be highlighted.
Table 1. An Overview of Commodity Polymers, Including (Thermo)mechanical Properties, Chemical Structures, and Diverse Applicationsa.
In Table 2, an overview is given of the different shape changing commodity polymer composites and bilayer actuators discussed in this Spotlight on Applications. How the actuator benefits from the commodity polymer is highlighted: either actively performing macroscopic shape changes or providing mechanical stability as a passive bulk polymer.
Table 2. Summary of Different Shape Changing Stimuli-Responsive Commodity Polymer Composites and Bilayers.
| commodity polymer | shape changing composite, bilayer or multilayer | active layer | stimulus | refs |
|---|---|---|---|---|
| PA6 | bilayer | conductive paint, graphene | light, thermal | (11) |
| PA6 | bilayer | LCN | humidity | (22) |
| PA6 | bilayer | hydrogel | humidity | (60) |
| PA6 | bilayer | PA6 | humidity | (61) |
| PA6 | bilayer | PA6 | humidity, thermal | (63) |
| PA6 | n/a | PA6 | thermal | (64) |
| PET | bilayer | azobenzene | light | (67) |
| PET | bilayer | CNF | humidity, thermal | (68) |
| PET | bilayer | Ag-plated | (electro)thermal | (69) |
| PE | n/a | PE | thermal | (64) |
| PE | composite | azobenzene | light, thermal | (71, 72) |
| PE | bilayer | azobenzene | light, thermal | (73) |
| PE | bilayer | hydrogel, CNT | humidity, light, thermal | (74) |
| PE | multilayer | azobenzene | light, thermal | (75−77) |
| PE | bilayer | azobenzene | light, thermal | (78, 79) |
| PP | composite | LCN | humidity | (83) |
| PP | bilayer | hydrogel | humidity, light, thermal | (84) |
| PP | bilayer | azobenzene | light, thermal | (86) |
Polyamide 6 (PA6)
PA6, commonly known as nylon-6, is a semicrystalline polymer with repeating units linked through amide groups that provide high tensile strength by forming intermolecular hydrogen bonds.55 These flexible thermoplastics can be dyed, mechanically oriented and exhibit excellent wear resistance, all features of significant interest to many industries (including automotive, packaging, textile, and many others).56−59 The sensitivity of the hydrophilic amide groups in PA6 (see Table 1) to humidity is often exploited in (hygro)thermal actuators, where changes in temperature are coupled to changes in humidity. Anisotropy is frequently introduced by solid-state stretching during manufacturing to provide a strong thermal contraction and linear swelling along the polymer backbone.
A bilayer actuator was fabricated by drop casting a hydrogel solution (made of a poly(acrylic acid)/polyacrylamide copolymer grafted onto a carboxymethyl cellulose (poly(AA-co-AAm)-g-CMC)) onto a PA6 film.60 The hydrogel was photo cross-linked, the combination yielding an actuator which readily swelled in water, causing the bilayer to bend. In this actuator, PA6 provided mechanical robustness as the hydrogels absorbed significantly more moisture. When submerged in water, the shape changing bilayer took over 5 min to fully bend, with the PA6 substrate along the inner radius. The bilayer unbent in 3.5 min after adding ethanol; the absorbed water molecules migrated into the ethanol owing to good miscibility of the solvent. The addition of a polysaccharide-based hydrogel increased biocompatibility, which is considered appealing for potential drug delivery or implantable device applications.
Humidity-sensitive bilayer actuators have also been prepared by spraying LCs from solvent onto oriented, anisotropic PA6 substrates; spraying is compatible with high-throughput industrial standards.22 The rod-like LCs self-organize, with the stretching direction of the PA6 substrate acting as an alignment layer. The LC alignment at the PA6 interface is planar, but the LC director gradually rotates through the film depth to stand perpendicular to the substrate at the air interface to minimize the surface free energy. This “splay” alignment is fixed during photo cross-linking to form an LCN composed of carboxylic acids. A subsequent alkaline treatment generates a carboxylate sodium salt gradient in the LCN coating, which becomes humidity-sensitive. Thus, both the LCN and PA6 absorb moisture, though the hygroscopic polymer salt expands more than the PA6. At lower humidity, the bilayer is bent with the LCN on the inner radius. With increasing humidity, the swelling splay aligned polymer salt pushes away the PA6 underlayer.
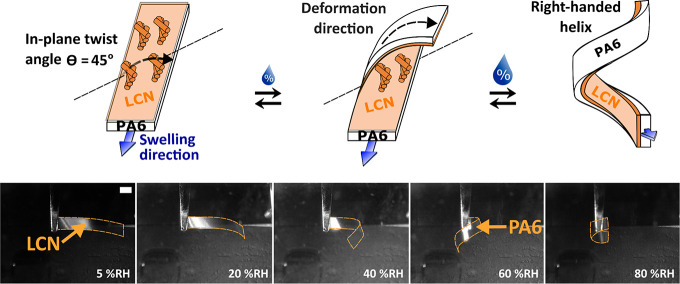
A more complex response was programmed in the LCN/PA6 bilayers; the in-plane twist rotation of the LCs was controlled by the addition of chiral molecules, and this LCN twisting was mirrored in its humidity response.61 In this case, the 3D response of the bilayer was fully determined by the swelling of the anisotropic PA6 substrate, as the LCN did not contain humidity-sensitive carboxylate salt units but did act as an in situ template to direct the response. At lower humidity, the bilayer is nearly straight. As PA6 absorbs moisture, the in-plane twist angles 0 < θ < 90° of the LCN layer directed the formation of a helix. Adjusting the concentration of chiral dopant defines the in-plane rotation of the LCs through the film depth, and the greatest macroscopic deformation of the bilayer was found when the LC twist is θ = 45° (see Figure 4). The formation of helical structures using chiral molecules in the LCN coating eliminated the need to cut oriented (bi)layers at oblique angles to the alignment direction to achieve curling motion.19,23,62 It is notable that a coating of only 4 μm is capable of guiding the macroscopic response of a 15 μm PA6 substrate.
Figure 4.
Actuation behavior of a PA6-based bilayer actuator. The spray-applied coating consists of a self-organized LCN with a 45° in-plane twist angle. At increasing humidity, the oriented PA6 substrate absorbs significant amounts of moisture, deforming the bilayer actuator. The in-plane twist angle within the LCN coating controls the humidity-driven three-dimensional deformation of the actuators. Reproduced from 61 with permission from The Royal Society of Chemistry.
As temperature changes are coupled with variations in environmental humidity, the response of PA6 could be tailored to fabricate dual-stimuli responsive polymers. A layer of continuous carbon fiber reinforced copolyamide (cCF:PA6-I) was 3D printed onto PA6.63 The bilayer laminates feature PA6’s humidity/thermal-sensitivity coupled with the mechanically resistant copolyamide in which the embedded carbon fibers act as an electrical circuit for Joule heating. In conditions of high humidity (∼98%), the bilayer was nearly straight, bending toward the PA6 side after the desorption of moisture by (electro)heating. The versatile composite actuators demonstrated significantly improved actuation speeds compared to other hydrogel systems, aided by the tethered Joule heating.
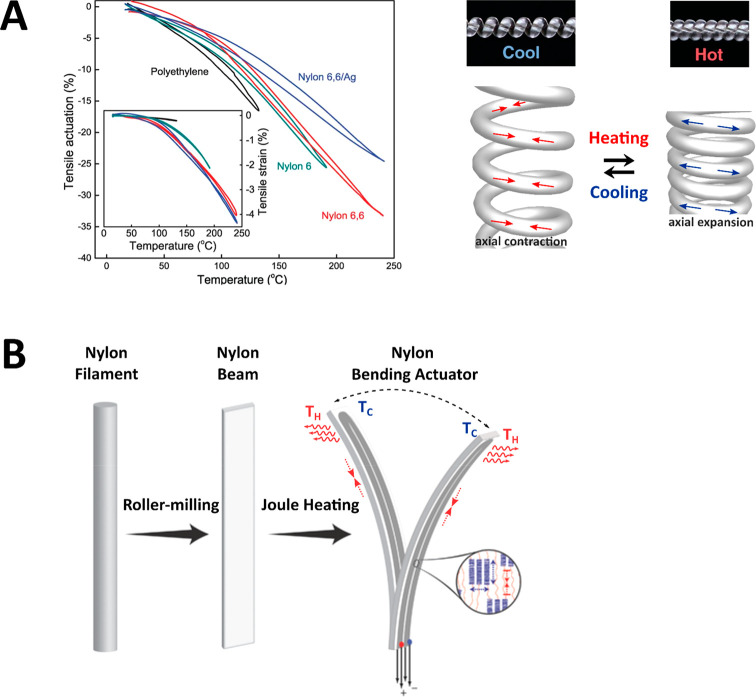
Compelling work was conducted on PA6 fibers which were wound with a predefined number of twists per meter to provide highly anisotropic actuators (see Figure 5A).64 The high strength, twist-inserted PA6 fibers attained large, reversible dimensional changes when heated, able to reach contractions up to 49% originating from the large negative thermal expansion coefficient amplified by the twist of the fiber. Instead of using radiative heating, multiwalled CNTs or metal wires could also be incorporated enabling conductivity driven Joule heating. A heat-activated muscle-like actuator was built from twisted Ø ∼ 860 μm PA6 fibers which could lift a 1 kg weight 7 mm, corresponding to a specific actuation stress of 2100 J/kg. A textile was prepared by weaving the coiled, conductive PA6 monofilaments (warp direction) together with polyester and cotton fibers (weft direction). The porosity of the weave was dynamically altered by Joule heating; the controlled opening and closing intended to enhance the wearer’s comfort. Similarly, window shutters were designed which opened or closed through thermal stimulus, anticipated for use in self-regulating indoor temperatures.
Figure 5.
Actuation behavior of oriented polyamide 6 (PA6) fibers. (A) Thermal contraction of diverse oriented polymer fibers, before (inset) and after twisting. A polymer fiber is wound to form a twist-coil actuator that strongly contracts at elevated temperature, further winding the actuator. Reproduced with permission from 64. Copyright 2014 AAAS. (B) An oriented PA6 fiber is roller-pressed and coated with conductive paint to yield multidirectional bending by side-selective Joule heating. Reproduced with permission from 11. Copyright 2017 Wiley-VCH.
Bending deformations were made multidirectional by generating asymmetry through heat conductivity differences across the thickness of roller-pressed PA6 fibers.11 Conductive coatings are applied to otherwise poorly conducting PA6 fibers to obtain a temperature gradient generated bending motion through heat convection (see Figure 5B). The PA6 modulus decreased as heat was transferred across the thickness of the roller-pressed fiber and, combined with strong thermal contraction, resulted in bending of the oriented film. Essentially, the temperature mismatch across the thickness of the oriented film subdivides the oriented PA6 filament into “passive” and “active” regions, accounting for directional actuation. Two different conductive coatings were explored: a paint consisting of silver flakes and metallic nanowires embedded in an adhesive resin (Ag/Nb paint) and dip coating the films into a solution containing graphene flakes. The Ag/Nb paint was developed to electrothermally actuate, whereas the graphene flakes could be controlled photothermally. Interestingly, by painting Ag/Nb traces along the longitudinal planes of a roller-pressed square fiber, selective Joule heating could bend the beam in the ±X, ±Y direction or contract the beam (in the −Z direction) upon simultaneous laser excitation; the actuator went through over 100 000 bending cycles with less than 5% amplitude loss. In contrast, a highly focused laser source could only initiate modest bending, as only a small area was exposed compared to Joule heating.
Poly(ethylene terephthalate) (PET)
The semiaromatic thermoplastic PET is produced via condensation reactions and widely used in textile and packaging applications as it forms an excellent moisture barrier (also see Table 1).65 Spun PET fibers may be dyed, are significantly oriented, and offer high crystallinity, tensile strength and thermostability. In contrast to PA6, it is considered less sensitive to humidity, while still providing anisotropic thermal expansion after solid-state stretching. Among all commodity polymers, PET is regarded as the most sustainable polymer, as it is frequently recycled to serve a second life.66 It is not limited to just lower grade products: a virgin grade is typically blended with recycled PET to fabricate products appearing in garments, home textiles (duvets, pillows, or carpeting) and automotive components (sound insulation, seat covers).
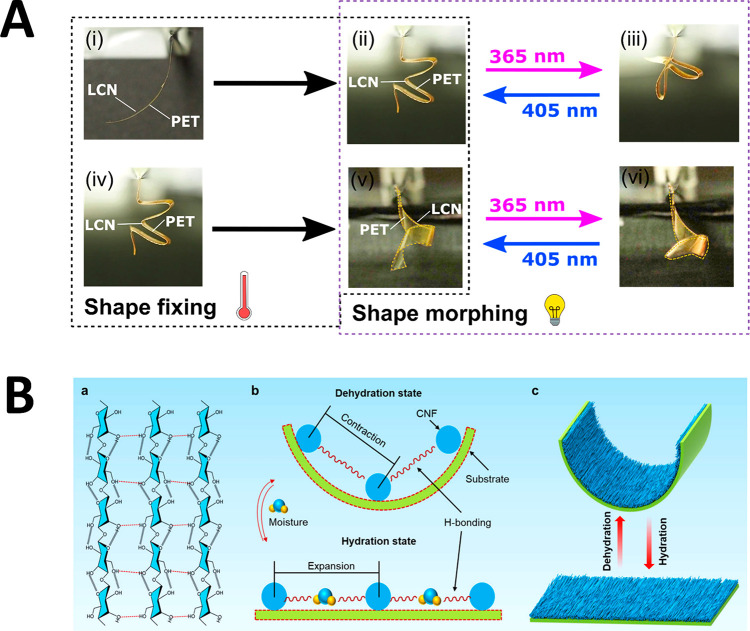
Thin, anisotropic PET films are attractive for their mechanical robustness and ductility, but also for their thermoplastic moldability. PET can be thermally shaped into any complex, even origami-like, shape when deformed around its Tg.67 In combining PET with photoactuators, intricate architectures can be designed with arbitrary initial shapes that reversibly actuate upon illumination. An azobenzene-containing LC mixture has been spray-applied onto oriented PET films, and self-aligned in the nematic phase prior to photo cross-linking. The photoinert PET support layer was bent with the LCN on the inner radius of the bilayer upon illumination with UV light, which anisotropically contracts the LCN undergoing an order-to-disorder transition (Figure 3D). When the light is turned off, the bilayer retained its bent shape for hours in the dark; the device quickly returned to its initial shape by exposing to visible light. The bilayer was wrapped in aluminum foil, manually deformed into any arbitrary shape (via shape fixing), and when heated to 100 °C for 15 min, the segmental polymer chain mobility allows adoption of the new shape, with subsequent cooling freezing in the new configuration. The bilayer undergoes shape morphing upon exposure to light, even allowing area-selective reversible actuation (Figure 6A). The structure can be erased, and a new structure imposed by again reheating and forming the device, establishing easy fabrication of mechanically robust, reprogrammable photoactuators.
Figure 6.
Actuation of stimuli-responsive PET-based actuators. (A) A ductile PET substrate is spray-applied with a light-responsive, brittle LCN. Arbitrary shapes can be accessed through thermal programming: the bilayers can be reversibly actuated using UV (365 nm) and visible (405 nm) light before being thermally shape fixed again. Reproduced with permission from ref (67). Copyright 2020 Wiley-VCH. (B) A bilayer actuator is fabricated by depositing CNFs onto a PET substrate. The CNFs self-assembled perpendicular to the long axis of the substrate. The ab- and desorption of moisture by the aligned CNFs reversibly deforms the bilayer. Reproduced from ref (68). Copyright 2019 Elsevier.
A humidity-sensitive PET-based actuator is prepared by depositing a suspension of cellulose nanofibers (CNFs) onto a substrate.68 The bilayer actuator is prepared by evaporation-assisted uniaxial self-assembly of the CNFs. The physically cross-linked CNF gel network aligned perpendicular to the long axis of the PET support layer (see Figure 6B). The humidity-sensitive gel rapidly ab- and desorbs moisture, causing reversible bending of the bilayer strip, even capable of lifting heavy weights owing to strong intermolecular hydrogen bonds between individual CNFs.
In a twisted-coil actuator (TCA), the negative thermal expansion coefficient of PET was exploited by twist-inserting silver-plated fibers, followed by Joule heating to obtain actuation.69 The PET fibers underwent metallization via activation of hydroxyl and carboxylic acid end groups at the surface which were deposited with silver, also known as an electroless plating technique. At an initial length of 50 cm, the fiber bundle was fixed to a rotating motor while a mass hung at the end to prepare ∼10 cm long TCA. The highly oriented PET fibers thus serve as a robust base layer, which strongly contracts by the efficient transduction of heat from the metal coating. Large stresses of 368 and 357 MPa, respectively, are reported at 160 °C either through electroheating or with a heat gun. It was disclosed that the rapid tensile actuation of ∼12% was facilitated at 6 V.
Polyethylene (PE)
PEs account for the largest share of the annual production of commodity polymers.70 These thermoplastic polyolefins can be subdivided by density, degree of branching and molecular weight (such as long-branched low-density (LD), short-branched linear low-density (LLD), high-density (HD), and ultrahigh molecular weight (UHMW), as examples). These macromolecular differences are reflected in their mechanical properties, affecting strength, impact resistance, and flexibility, providing a great diversity in applications ranging from packaging to toys, and even personal protection. Additionally, the PEs are humidity-insensitive and photoinert, and the (thermo)mechanical properties become highly anisotropic upon solid-state stretching (Figure 5A), which is greatly benefitted from in constructing shape changing commodity polymers.
Uniaxially stretched PE films with a high specific surface area of ∼40 m2/g and porosity of ∼40–50% have been doped with an azobenzene-containing LC mixture.71 Thermal polymerization of the LCs resulted in a yellow colored, relatively transparent composite with the oriented PE and anisotropic pores serving as a good alignment material for the LC mesogens. The photoisomers aligned along the PE’s stretching direction and the films reversibly bent toward the UV light source due to an increasing cis azobenzene content when examined above the Tg (∼95 °C) of the LC polymer. The composite unbent through thermal relaxation due to segmental mobility of the LCN, which was accelerated by exposure to visible light, inducing cis-to-trans isomerization and bending away from the light source.
In a similar way, azobenzene was embedded in ultrahigh molecular-weight polyethylene (UHMWPE), this time using direct casting.72 Compatibility of the azobenzene with the apolar UHMWPE matrix was increased by attaching PE (C78) side chains to the azobenzene core. The as-cast composites were solid-state drawn to draw ratio 60, yielding high Young’s modulus (∼100 GPa) films with a high degree of chromophore alignment. The films were periodically exposed to either UV or visible light at increasing intensities. As the films were illuminated a photomechanical stress was generated, attributed to the strong thermal contraction of the ultradrawn UHMPWE matrix surrounding the heat-producing azobenzene chromophores. The photomechanical stress rapidly disappeared when the light source was turned off. Remarkably, photoinduced mechanical stresses exceeding 65 MPa at only 0.06% strain were generated. In longer-term experiments, periodic light exposure of the composite films showed excellent photomechanical stability over at least ∼2600 cycles. The high-modulus, lightweight composites remotely attained specific photomechanical stress values >6 × 104 Pa·(kg·m–3)−1, which are comparable to values found for conventional hard metallic or ceramic actuators.
Other photothermal dyes, such as C20-azobenzene or 2-(2H-benzotriazol-2-yl)-4,6-ditertpentylphenol (BZT), are also reported to introduce responsivity in PE (see Figure 7A).37,73 The azobenzene photoswitch is adsorbed onto the surface of oriented UHMWPE serving as an alignment layer to fabricate light-responsive actuators, enabling stress generation (up to ∼3 MPa), although ultimately lacking the performance demanded for putting UHMWPE to practical use. Additionally, blending BZT with UHMWPE also allowed direct laser writing, inducing surface deformations through local heating.
Figure 7.
Stimuli-responsive shape changing PE. (A) Epitaxially grown crystals of light-sensitive azobenzene dopants bearing C20 aliphatic side chains adsorbed onto oriented PE as seen with optical microscopy. In stress relaxation experiments, the adsorbed chromophores allowed for repeated stress generation in response to UV-light exposure. Adapted from ref (73) with permission from The Royal Society of Chemistry. (B) A flexible LDPE substrate is locally coated with carbon nanotube doped poly(N-isopropylacrylamide) to form bilayer, hinged actuators. The hinges change shape when being heated above the lower critical solution temperature enabling bending. Reprinted with permission from ref (74). Copyright 2011 American Chemical Society. (C) A light-driven plastic motor is prepared by connecting the ends of a photoinert LDPE substrate coated with an azobenzene-containing liquid crystal elastomer. The bilayer actuator continuously rotates using simultaneous UV and visible light illumination driving cooperative azobenzene isomerization. Reproduced with permission from ref (76). Copyright 2008 Wiley-VCH.
Thermoresponsive, self-folding bilayer actuators were fabricated employing a flexible isotropic LDPE substrate locally coated with stimuli-responsive hinges operating at temperatures below 60 °C.74 In designing complex architectures, the temperature response of predefined hinge actuators was programmed by solution deposition of single-walled carbon nanotube (SWCNT)–pNIPAM followed by UV-light polymerization, forming thin thermosensitive coatings on the LDPE layer acting as the support layer. As the SWCNT–pNIPAM hydrogel was heated above the lower critical solution temperature (around ∼33 °C), water was expelled due to the hydrophilic-to-hydrophobic transition, resulting in macroscopic film bending. The addition of uniformly dispersed SWCNT into the hydrogel was shown to significantly enhance response time; bending at predefined sites formed a cube within only 35 s compared to 150 s for the hydrogel alone (Figure 7B). This rapid actuation is explained by two possible mechanisms: either the SWCNT improved convection through the hydrogel, or optimized water diffusion within SWCNT channels. Additionally, laser irradiation of the near-IR absorbing nanotubes resulted in ultrafast (<1 s), thermal actuation, desirable in biocompatible architectures for drug delivery, for example. Near-IR radiation is of particular interest as it has a lower photon energy than UV-light and therefore less harmful to living organisms.
Photopolymerized light-responsive azobenzene liquid crystal elastomers (LCEs) aligned in a higher order smectic phase were attached to an unstretched passive LDPE utilizing an adhesion layer.75 The low-modulus LDPE support permits sufficient flexibility to undergo large, macroscopic deformations. Cooperative dimensional changes of the azobenzenes in the LCE drove macroscopic unidirectional movement of the multilayer film, the directional motion resulted from the asymmetric leg shapes; by alternating UV and visible light exposures of the sharp tip and the flat edge, “walking” motion of a film was demonstrated. This untethered movement was expanded by using selective local exposures with different light intensities to prepare a flexible robotic arm.
In contrast to conventional bending, Ikeda and coworkers demonstrated how azobenzene-functionalized LCEs applied to sheets of otherwise photoinert PE could be applied as a light-driven motor, see Figure 7C.76 The alignment of the LCE was preserved as a cross-linked elastomer with Tg around room temperature. The ends of the LCE/PE bilayer strip were attached after threading around two pulleys to form a belt-like actuator. Simultaneous UV and visible light exposure at different film positions resulted in a slow rolling toward the light sources; UV exposure forced a contraction along the long axis of the mesogens, whereas visible light reversed this length change. The demonstrator beautifully displayed how light can induce motor-like motion through simultaneous UV and visible light illumination at two different positions.
A lightweight robot was built using a responsive LCN coating applied onto PE substrates to form shape changing bilayer in an effort to replace heavy, rigid, tethered metal robots.77 The LCN was composed of thermally polymerized azobenzene chromophores. Two distinct LCN/PE actuators were prepared. The first was a flat, multilayer composite using an adhesive and a ring-shaped laminate in which the substrate was strained 2%, the coating applied and then allowed to elastically recover. The resulting untethered robot had a ring-shaped “arm” and flat bilayer films as “wrist” and “hand” connected by passive joints to independently control movement. The hand with inactive “fingers” was capable of gripping a 10 mg load by trans-to-cis isomerization, the cargo held firmly by cis-to-trans isomerization and transferred by bending the arm, displacing over a total distance of 20 mm. Finally, the cargo was released into a container by reopening the hand, demonstrating the ability to manipulate material positions with light. Repeatedly moving objects in the horizontal and vertical directions was estimated to attain photoinduced stresses similar to human muscles (∼320 kPa).
A layer of linear polymer with side-chain azobenzene mesogens was bar coated onto unstretched, photoinert LDPE.78 The azobenzene chromophores were aligned by using polarized light and adhered via electron beam (EB) irradiation in an inert atmosphere to prepare large (14 × 14 cm2) samples without using an adhesive. The formation of intra- and interlayer chemical bonds via one step EB polymerization is considered industrially relevant, potentially improving processing speeds. The direct cross-linking was also anticipated to provide sufficient mechanical strength with efficient translation of anisotropic expansion to the flexible substrate. The dose of absorbed ionizing radiation was found to be key in preparing reversible bilayer actuators; at low dose, no bending was observed during UV exposure, with bending becoming evident only in a certain EB dose window. The bilayers bent toward the light source when illuminated with UV-light and reverted upon visible light exposure with excellent bond durability and reversible bending. The adhesive-free bilayer withstood prolonged UV exposure (for 5 h), showing no signs of fatigue cracking or delamination, while the multilayer actuator with adhesive layer failed after only 10 min.
Azobenzene derivatives can be adsorbed and aligned onto the surface of isotropic LDPE.79 Flexible PE substrates were initially rubbed with silicon carbide sandpaper to induce microgrooves as alignment layers for the chromophores.80 Then, the passive LDPE films were immersed in azobenzene/ethanol solutions. When vertically removed from the solution, the cast composites had azobenzene derivatives adsorbed to its surface due to capillary action in the microgrooves. Unsubstituted and mono- and disubstituted hydroxy azobenzene had good alignments, defined as order parameters (S) exceeding 0.46. The order parameter was calculated from dichroism values derived from polarized absorption spectra, with a random alignment corresponding to S = 0 and perfect order to S = 1, respectively.81 However, only small deformations took place for the unsubstituted azobenzene, contrasting with the large and fast photoresponses of hydroxy substituted analogues. It was reported that an UV intensity >100 mW/cm2 led to permanent damage to the surface of the photoactuators. Well below this intensity (at 20 mW/cm2), large photoinduced stresses of ∼0.26 MPa were repeatedly generated indicating good fatigue resistance.
Finally, a twisted PE fiber bundle actuator (similar to the PA6 and PET fiber actuators described earlier, see Figure 5A) was able to generate a specific actuation stress of 2630 J/kg at only 4.5% contraction.64 The twisted fibers were found to generate a high torque due to heat-driven reversible untwisting attributed to the negative axial and positive radial expansion of oriented polymer fibers.
Polypropylene (PP)
The second-most produced commodity polymer after PE is PP. This thermoplastic polyolefin is humidity-insensitive, photoinert and provides anisotropic (themo)mechanical properties through solid-state stretching. This semicrystalline polymer is well-known to be exceptionally tough, durable, both chemically and heat resistant and hence finding application in furniture, labeling, packaging, and ropes.82
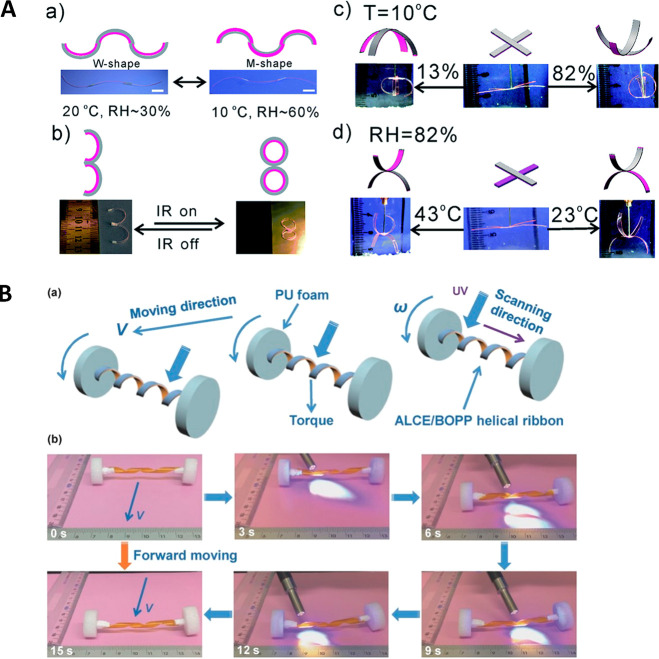
As with PE, oriented porous PP polymers may serve as flexible substrates, which serve to align LCs infiltrated into the pores. A nematic LC mixture consisting of carboxylic acid moieties22 was used to fill the porous PP (∼55%) via capillary forces; the LC was subsequently photopolymerized.83 Tensile tests revealed the composite became significantly tougher than the naked LCN film: the elongation-at-break (εb) improved from εb,LCN∼ 2% to εb,composite ∼225%, which in terms of energy required for material failure was ∼200 times that of the LCN alone. The ductile composite films were made humidity-responsive by immersing them in a 0.1 M aqueous potassium hydroxide solution for 20 min, forming a humidity-sensitive LCN with carboxylate salt moieties. Cutting the film at an angle with respect to the nematic director altered the mode of deformation: as the initially flat strip absorbed moisture, swelling occurred predominantly in the perpendicular direction, directing the composite to bend, curl or twist. Double composite layers were prepared by filling two orthogonally oriented substrates: it is noteworthy that these double-layered actuators demonstrated a handedness inversion in helicity at ∼40% RH. Without further details, the authors contemplate how these composite salts potentially could be integrated in soft piezo-electric materials making use of humidity fluctuations for energy harvesting.
A hydrogel actuator solution cast onto biaxially oriented PP displayed humidity, temperature and infrared (IR) light responses.84 The commercially available PP substrate contained a poly(acrylic acid) (PAA) coating, facilitating anchoring of a cast borax cross-linked N1,N1-diethylethane-1,2-diamine-modified poly(vinyl alcohol) (PVA-DEEDA-borax) to the substrate. Sensitivity to fluctuating humidity and temperature levels influenced swelling of the hydrogel and hence bent the passive PP substrate. Similarly, IR light was used to photothermally initiate deswelling when going through the lower critical solution temperature (LCST). It was suggested that tetra-coordinated boron enhanced the swelling and deswelling kinetics via charge repulsion which increased the free volume within the film network. In diverse experimental conditions (10–60 °C and 13–82% RH, respectively) the bilayer actuator demonstrated large, reversible deformations ranging from −360 to +360°. Several 3D shapes were constructed by connecting multiple bilayer actuators, resulting in shape changing numbers, grippers and artificial muscles to lift objects (Figure 8A).
Figure 8.
Actuation of PP-based actuators. (A) A multiresponsive bilayer actuator was fabricated by applying a hydrogel coating onto PP. Several shape changing demonstrators were prepared, displaying its responsiveness to heat, changing humidity, and IR light. Reproduced from ref (84) with permission from The Royal Society of Chemistry. (B) A schematic of a spring-like motor composed of a light-responsive liquid crystal network attached to a PP substrate which is end-capped by PU foam wheels. The photographs demonstrate isomerization-driven forward motion through reversible winding and unwinding of the bilayer. Reproduced with permission from ref (85). Copyright 2017 Wiley-VCH.
A spring-like motor composed of a light-responsive LCN attached to a PP substrate has also been reported (Figure 8B).85 Oriented azobenzene-doped LCNs were attached to the photoinert, biaxially oriented PP with a conventional tape adhesive, and a wheel made by connecting the ends of the bilayer strip above Tg. Shining UV-light leads to asymmetry: exposing only one side evokes a contractive force which shifts the center of mass. Rolling directionality of the multilayer actuators was determined by the position of the LCN; when located on the inner side of the wheel, it rolled toward the LED, whereas placing it on the outer side caused rolling away from the light. This simple concept demonstrated that by preparing a helical ribbon end-linked to polyurethane foam wheels, a continuous, light-driven motion could be obtained akin to a spring-like motor. A bilayer film was wrapped around a metal rod and shape-fixed upon cooling into a helical ribbon. The ribbon showed not only fast photocontrolled motion but also the ability to reprocess the LCN using exchangeable chemical bonds.
Complex pathways are commonly followed to obtain stimuli-responsive garments. Light-insensitive PP fabric was functionalized by physical adsorption of a poly[di(ethyelene glycol) methyl ether methacrylate-copentafluorophenyl acrylate] (P(DEGMA-co-PFPA)) precursor.86 Postpolymerization modification of the precursor took place at the reactive pentafluorophenyl ester, which was replaced with a light-responsive azobenzene. The azobenzene-containing copolymer was spin-coated onto PP fabric from solution and dried in an oven, yielding a bilayer actuator. The 1 × 10 mm2 freestanding fabric rapidly bent over 70° within <2 s when exposed to UV at two different positions. Unfortunately, unbending did not occur on a similar time scale; the bent state did not revert even after exposure with visible light for an hour. However, when the bilayer was stored at 50 °C, the initial shape recovered within 10 min, showing reversible actuation was possible.
Conclusions and Outlook
This Spotlight on Applications has demonstrated how shape changes can be introduced in commodity polymer composites and bilayers in response to light, temperature, and/or humidity changes. In fact, all stimuli-responsive commodity polymers discussed follow similar design guidelines: thin (μm-range), high-aspect ratio (L||/L⊥ ≫ 1) films, or fibers with strongly deviating expansion coefficients (see Figure 4).4,13 In this field, two approaches are used to induce shape changes in commodity polymers: doping stimuli-responsive additives into the commodity polymer or coating stimuli-responsive materials onto the reinforcing commodity polymers (Table 2). In this section, we will reflect on these novel materials in the form of the next challenges, while focusing on how the commodity polymer itself can be made stimuli-responsive.
Scalability and Stability
Adding responsive features to inexpensive, bulk, otherwise static commodity polymers will extend their range to more high-end applications, but this most likely will require more extended product lifetimes of the responsive elements than currently required for polymers used in typical disposable consumables (drinking cups, packaging material, and so forth). The blending of low molecular weight responsive additives or applying responsive coatings must be easy to integrate into industrial processes with only small production modifications and preferable to developing completely new polymers, itself a lengthy, expensive process. Time consuming, expensive techniques such as film transfer and casting are interesting from a scientific point of view but are of limited use in high-throughput processes. Co-extrusion of intermediate adhesive and functional layers,77,78 compression molding of reprocessable networks,85 spraying of LCs as top coats,22,61,67 infiltration in porous polymers,71,83 blending in low molecular weight additives,72 and applying conductive paints11 can all find their way in the commodity polymer industry.
It is desirable that the performance of shape changing actuators does not deteriorate under conditions of continual temperature, humidity, or light changes.11 Among the diverse stimuli exploited as triggers in actuators, we consider illumination as most favorable, as it is area selective, remotely controllable, and capable of initiating fast shape changes with possible additional selectivity based on dependence on light polarization, if desired.72 Furthermore, as stimuli are often interconnected,87 i.e. surrounding humidity is coupled with temperature, illumination can directly impact these conditions photothermally.84 Obviously, this all depends on the intended application; for example, homogeneous heating may be more applicable in adaptive garments than employing (local) photothermal heating.
From an application point-of-view, repeated or long-term exposure to light, especially UV light, is of key interest with respect to product lifetime. Especially for polymers, which tend to discolor due to degradation, and photochromic dyes, prone to photofatigue, it is beneficial to add UV-absorbers or free-radical scavengers which are well-known to extend product lifetimes.88−92 In addition, it is appealing to develop molecular switches that respond to visible or even (near-)IR light.93 Regarding humidity-sensitive materials, hydrogels frequently lack mechanical robustness, and their operation window is typically restricted to aqueous environments, which considerably limits their usefulness.17 Depending on the application, long-term product life can be improved by applying a protective top coat endowing polymers with abrasion-, impact-, and/or scratch-resistance.
Fabrication Guidelines
A more appealing solution to address all of these shortcomings (time-consuming costly techniques, inferior mechanical properties, and susceptible responsive parts) is found in using additive blending to create stimuli-responsive commodity polymer composites. Typically, in high-throughput industrial processes a blend of additives is supplied to the commodity polymer before undergoing elaborate processing conditions (i.e., solid-state stretching) to yield highly oriented, anisotropic composites with extended product lifetime. The addition of the stimuli-responsive molecules to these packages endows the final product with responsive parts shielded by the robust, encapsulating commodity polymer. An example of such a robust, anisotropic UHMWPE actuator is given in ref (72). This composite design contrasts bilayer-type actuators in which hydrogels, azobenzene-containing coatings, or LCN coatings are repeatedly exposed to daily life conditions, requiring top-coats to protect the relatively fragile materials.
Among the four commodity polymers, the polyolefins (PE and PP) may be considered most useful for improving the robustness of current shape changing polymer actuators based on hydrogels and liquid crystals. The widely applied polyolefins offer chemical and photoinertness, are humidity-insensitive and vary in (thermo)mechanical properties depending on the degree of branching, molecular weight and manufacturing processes (e.g., solid-state stretching). PA6 for example, is susceptible to temperature–humidity cross-sensitivity which is anticipated to be unfavorable for most shape changing hydrogel and LC actuators. As an alternative to polyolefins, PET may be used as it provides good optical clarity while being less sensitive to moisture than PA6.
Applications
A particularly attractive application for these emerging responsive bulk polymers is in the field of consumer or medical soft robotics. In particular, there has been an explosion of growth in the consumer robot market, which is expected to further increase 110–175% over the next 5 years.94−96 These consumer robots come in many forms for many functions but hold in common the need to interface with humans, requiring them to provide appropriate responses for physical contact between robot and human, creation of artificial muscles driving natural 3D motion, and manipulating delicate objects with soft grippers.
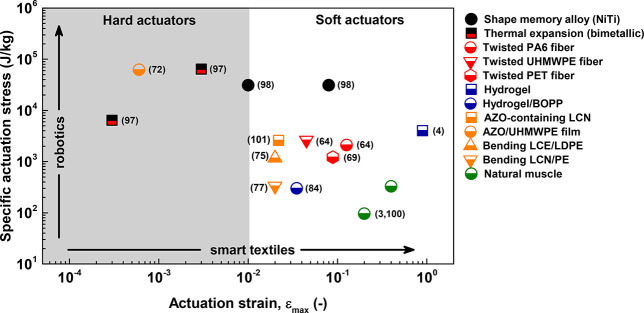
Developing stimuli-responsive commodity polymer contact interfaces within “soft” robots would seem natural, as long as the physical properties of the “soft” material are adequate for the job at hand. Soft shape changing actuators typically require compliant polymers with large reversible strains (ε ≫ 1%), while being low modulus materials (E < 1 GPa), whereas hard robotics commonly demand bulky metal-based actuators (E ≫ 1 GPa) operating at low strain values (ε < 1%) (for a comparison of “hard” and “soft” actuators described in the literature, see Figure 9). In contrast, hard robotic actuators are generally powered by an electric stimulus to provoke immediate, fast stress responses, often coupled to larger strains through hydraulics or pneumatics.102 Lightweight, “soft” alternatives employing highly oriented UHMWPE films doped with azobenzene chromophores generated high stresses at similar strain-values to metallic actuators.72 Large anisotropic expansions of highly oriented, high-strength fibers (E ≫ 1 GPa) were combined by twisting fiber bundles so they exert large, reversible, mechanical strains in response to a stimulus, generating large stresses.103,104 These compelling examples suggest the design of mechanical compressors, artificial muscles,64,105 and even exoskeletons and prosthetic limbs employing high-aspect ratio fibers. While many human-mimetic challenges remain, including the design and integration of skin-like compliant materials for robot-human physical interaction combined with adaptive sensing, control and actuation,106 these intricate devices show promise for robotic applications, although the challenges of remote control and/or lower operating temperatures must be addressed. An increasing demand for these new technologies is anticipated,5,107 reaching a market size of over 100 billion USD in 2025.108,109
Figure 9.
Stimuli-responsive actuator performance defined by the specific actuation stress and the actuation strain. Symbols in black refer to (partially) metal actuators, whereas (partially) organic actuators are found in white, and human muscle actuators are colored green. The metal and organic actuators are colored according the external stimuli applied, that being humidity (swelling, blue), light (orange), and photothermal/electro-heating (red). Commodity polymer-based composite and bilayer actuators are built from PA6, PET, PE, and PP. In accordance with future applications, robotics are considered to demand large specific actuation stresses, whereas smart textiles are anticipated to benefit from a larger actuation strain. Data were taken from refs (3, 4, 64, 69, 72, 75, 77, 84, and 97−101).
Responsive commodity polymers hold promise as adaptive textiles, in which fiber packing density can be engineered to dynamically change upon exposure to external triggers.110 We envision the opening of textile weaves in warm environments, allowing eased perspiration and helping maintain safe body temperatures, and hence increase the wearer’s comfort. Alternately, a denser fiber packing in colder temperatures will preserve body heat, keeping one warm. Similarly, smart window shutters could self-regulate indoor temperature by triggered opening and closing.64 Hence, tunability in, for example, twist fibers is particularly interesting as the coil spring index, correlates the mean coil to fiber diameter, can be modified to attain task-dependent large reversible strain or maximal actuation stress through optimized design. The major challenge to fulfill requirements for both stimuli-responsive soft actuators and adaptive textiles will be found in covering actuation specifications key to device architectures. A combination of high stresses and large actuation strains in polymer actuators is still sought after to execute all possible tasks. Nevertheless, we envision that shape changing commodity polymers shall become accessible by using simple processing techniques (solid-state stretching) to yield high-aspect ratio composite actuators meeting the desired actuation stress–strain performance.
In addition to the above-mentioned stimuli-responsive commodity polymers, other (commodity) polymers such as polycarbonate and poly(methyl methacrylate) are frequently reported as featuring optical responsivity, which is interesting for applications in sensors, anticounterfeit labeling, and aesthetics.37,41,45,111,112 Currently, the combination of polymer fibers and photonic coatings is also explored to prepare thermoregulating textiles relying on the reflection of IR light.113 The application of light, heat, or humidity to change photonic colors is appealing, though alternative stimuli such as mechanical force should also be considered for fabricating strain sensors, for example.114−116 These developments point out that commodity polymers can still expand their range of applications, leaving plenty of room for innovations.
In summary, inexpensive commodity polymers are being utilized to develop new stimuli-responsive soft actuators and adaptive textiles. By using rather simple coating or blending techniques to introduce responsiveness without requiring striking modifications in the existing infrastructure, new applications may be found for these otherwise static polymers. It is foreseen that shape changing commodity polymers will continue to expand at a rapid pace and will play a important role in meeting social challenges in the fields of sustainable energy, health, soft robotics, and “smart” textiles.
Glossary
Abbreviations
- BZT
2-(2H-benzotriazol-2-yl)-4,6-ditertpentylphenol
- cCF PA6-I
continuous carbon fiber reinforced copolyamide
- CNC/PEGDA
cellulose nanocrystal/polyethylene glycol diacrylate
- CNF
cellulose nanofiber
- CNT
carbon nanotube
- EB
electron beam
- HD
high-density
- IR
infrared
- LC
liquid crystal
- LCE
liquid crystal elastomer
- LCN
liquid crystal network
- LCST
lower critical solution temperature
- LD
low-density
- LLD
linear low-density
- LPL
linearly polarized light
- MWCNT
multiwalled carbon nanotube
- PA6
polyamide 6
- PA6.6
polyamide 6.6
- PAA
poly(acrylic acid)
- PE
polyethylene
- PET
poly(ethylene terephthalate)
- pNIPAM
poly(N-isopropylacrylamide)
- poly(AA-co-AAm)-g-CMC
polyacrylamide/poly(acrylic acid) copolymer grafted onto carboxymethyl cellulose
- poly(DEGMA-co-PFPA)
poly[di(ethyelene glycol) methyl ether methacrylate-copentafluorophenyl acrylate]
- PP
polypropylene
- PVA-DEEDA-borax
borax cross-linked N1,N1-diethylethane-1,2-diamine-modified poly(vinyl alcohol)
- RT
room temperature
- SMP
shape memory polymer
- SWCNT
single-walled carbon nanotube
- TCA
twisted-coil actuator
- Tg
glass transition temperature
- UHMW
ultrahigh molecular weight
- UV
ultraviolet
The work of R.C.P.V. forms part of the research program of DPI, project 731.015.502.
The authors declare no competing financial interest.
References
- PlasticsEurope Market Research Group (PEMRG)/Consultic Marketing & Industrieberatung GmbH . Plastics – the Facts 2017, 16.
- Material property charts. Granta Design. https://grantadesign.com/education/students/charts/ (accessed June 9, 2020).
- Miriyev A.; Stack K.; Lipson H. Soft Material for Soft Actuators. Nat. Commun. 2017, 8 (1), 596. 10.1038/s41467-017-00685-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee H.; Suhail M.; Ren H. Hydrogel Actuators and Sensors for Biomedical Soft Robots: Brief Overview with Impending Challenges. Biomimetics 2018, 3 (3), 15. 10.3390/biomimetics3030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majidi C. Soft Robotics: A Perspective - Current Trends and Prospects for the Future. Soft Robot. 2014, 1 (1), 5–11. 10.1089/soro.2013.0001. [DOI] [Google Scholar]
- Agrawal A.; Yun T.; Pesek S. L.; Chapman W. G.; Verduzco R. Shape-Responsive Liquid Crystal Elastomer Bilayers. Soft Matter 2014, 10 (9), 1411–1415. 10.1039/C3SM51654G. [DOI] [PubMed] [Google Scholar]
- Schenning A. P. H. J. Intelligent Stimuli-Responsive Materials. From Well-Defined Nanostructures to Applications. Edited by Q. Li. Angew. Chem., Int. Ed. 2014, 53 (42), 11130–11131. 10.1002/anie.201406937. [DOI] [Google Scholar]
- Sawa Y.; Ye F.; Urayama K.; Takigawa T.; Gimenez-Pinto V.; Selinger R. L. B.; Selinger J. V. Shape Selection of Twist-Nematic-Elastomer Ribbons. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (16), 6364–6368. 10.1073/pnas.1017658108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol G. N.; Harris K. D.; Bastiaansen C. W. M.; Broer D. J. Thermo-Mechanical Responses of Liquid-Crystal Networks with a Splayed Molecular Organization. Adv. Funct. Mater. 2005, 15 (7), 1155–1159. 10.1002/adfm.200400503. [DOI] [Google Scholar]
- De Haan L. T.; Verjans J. M. N.; Broer D. J.; Bastiaansen C. W. M.; Schenning A. P. H. J. Humidity-Responsive Liquid Crystalline Polymer Actuators with an Asymmetry in the Molecular Trigger That Bend, Fold, and Curl. J. Am. Chem. Soc. 2014, 136 (30), 10585–10588. 10.1021/ja505475x. [DOI] [PubMed] [Google Scholar]
- Mirvakili S. M.; Hunter I. W. Multidirectional Artificial Muscles from Nylon. Adv. Mater. 2017, 29 (4), 1604734. 10.1002/adma.201604734. [DOI] [PubMed] [Google Scholar]
- Van Oosten C. L.; Corbett D.; Davies D.; Warner M.; Bastiaansen C. W. M.; Broer D. J. Bending Dynamics and Directionality Reversal in Liquid Crystal Network Photoactuators. Macromolecules 2008, 41 (22), 8592–8596. 10.1021/ma801802d. [DOI] [Google Scholar]
- Timoshenko S. Analysis of Bi-Metal Thermostats. J. Opt. Soc. Am. 1925, 11 (3), 233–255. 10.1364/JOSA.11.000233. [DOI] [Google Scholar]
- Ionov L. Polymeric Actuators. Langmuir 2015, 31 (18), 5015–5024. 10.1021/la503407z. [DOI] [PubMed] [Google Scholar]
- Voncina B.; Zemljič Fras L.; Ristic T.. Active Textile Dressings for Wound Healing. In Advances in Smart Medical Textiles; Elsevier, 2016; pp 73–92. [Google Scholar]
- Kang H.; Suich D. E.; Davies J. F.; Wilson A. D.; Urban J. J.; Kostecki R. Molecular Insight into the Lower Critical Solution Temperature Transition of Aqueous Alkyl Phosphonium Benzene Sulfonates. Commun. Chem. 2019, 2 (1), 1–11. 10.1038/s42004-019-0151-2. [DOI] [Google Scholar]
- Ionov L. Hydrogel-Based Actuators: Possibilities and Limitations. Mater. Today 2014, 17 (10), 494–503. 10.1016/j.mattod.2014.07.002. [DOI] [Google Scholar]
- Hikmet R. A. M.; Broer D. J. Dynamic Mechanical Properties of Anisotropic Networks Formed by Liquid Crystalline Acrylates. Polymer 1991, 32 (9), 1627–1632. 10.1016/0032-3861(91)90398-3. [DOI] [Google Scholar]
- Boothby J. M.; Ware T. H. Dual-Responsive, Shape-Switching Bilayers Enabled by Liquid Crystal Elastomers. Soft Matter 2017, 13 (24), 4349–4356. 10.1039/C7SM00541E. [DOI] [PubMed] [Google Scholar]
- Hamley I. W.Introduction to Soft Matter– Revised ed.; John Wiley & Sons, Ltd: Chichester, UK, 2007. [Google Scholar]
- White T. J.; Broer D. J. Programmable and Adaptive Mechanics with Liquid Crystal Polymer Networks and Elastomers. Nat. Mater. 2015, 14 (11), 1087–1098. 10.1038/nmat4433. [DOI] [PubMed] [Google Scholar]
- Dai M.; Picot O. T.; Verjans J. M. N.; De Haan L. T.; Schenning A. P. H. J.; Peijs T.; Bastiaansen C. W. M. Humidity-Responsive Bilayer Actuators Based on a Liquid-Crystalline Polymer Network. ACS Appl. Mater. Interfaces 2013, 5 (11), 4945–4950. 10.1021/am400681z. [DOI] [PubMed] [Google Scholar]
- Wie J. J.; Lee K. M.; Ware T. H.; White T. J. Twists and Turns in Glassy, Liquid Crystalline Polymer Networks. Macromolecules 2015, 48 (4), 1087–1092. 10.1021/ma502563q. [DOI] [Google Scholar]
- Mitov M. Cholesteric Liquid Crystals with a Broad Light Reflection Band. Adv. Mater. 2012, 24 (47), 6260–6276. 10.1002/adma.201202913. [DOI] [PubMed] [Google Scholar]
- Choy C. L.; Chen F. C.; Young K. Negative Thermal Expansion in Oriented Crystalline Polymers. J. Polym. Sci., Polym. Phys. Ed. 1981, 19 (2), 335–352. 10.1002/pol.1981.180190213. [DOI] [Google Scholar]
- Broer D. J.; Mol G. N. Anisotropic Thermal Expansion of Densely Cross-linked Oriented Polymer Networks. Polym. Eng. Sci. 1991, 31 (9), 625–631. 10.1002/pen.760310902. [DOI] [Google Scholar]
- Bisoyi H. K.; Li Q. Light-Driven Liquid Crystalline Materials: From Photo-Induced Phase Transitions and Property Modulations to Applications. Chem. Rev. 2016, 116 (24), 15089–15166. 10.1021/acs.chemrev.6b00415. [DOI] [PubMed] [Google Scholar]
- Wu X. L.; Yang C. P.; Guo Y. Q.; Wang H. Y. Triple-Shape Memory Effect in Poly (Ethylene Terephthalate) (PET) Film. Pigm. Resin Technol. 2018, 47 (1), 55–62. 10.1108/PRT-03-2017-0031. [DOI] [Google Scholar]
- Boydston A. J.; Cao B.; Nelson A.; Ono R. J.; Saha A.; Schwartz J. J.; Thrasher C. J. Additive Manufacturing with Stimuli-Responsive. J. Mater. Chem. A 2018, 6 (42), 20621–20645. 10.1039/C8TA07716A. [DOI] [Google Scholar]
- Liu C.; Qin H.; Mather P. T. Review of Progress in Shape-Memory Polymers. J. Mater. Chem. 2007, 17 (16), 1543–1558. 10.1039/b615954k. [DOI] [Google Scholar]
- Lendlein A.; Gould O. E. C. Reprogrammable Recovery and Actuation Behaviour of Shape-Memory. Polymers. Nat. Rev. Mater. 2019, 4 (2), 116–133. 10.1038/s41578-018-0078-8. [DOI] [Google Scholar]
- Jin B.; Song H.; Jiang R.; Song J.; Zhao Q.; Xie T. Programming a Crystalline Shape Memory Polymer Network with Thermo- and Photo-Reversible Bonds toward a Single-Component Soft Robot. Sci. Adv. 2018, 4 (1), eaao3865. 10.1126/sciadv.aao3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes M.; Verduzco R. Direct Shape Programming of Liquid Crystal Elastomers. Soft Matter 2019, 15 (5), 870–879. 10.1039/C8SM02174K. [DOI] [PubMed] [Google Scholar]
- McBride M. K.; Martinez A. M.; Cox L.; Alim M.; Childress K.; Beiswinger M.; Podgorski M.; Worrell B. T.; Killgore J.; Bowman C. N. A Readily Programmable, Fully Reversible Shape-Switching Material. Sci. Adv. 2018, 4 (8), eaat4634. 10.1126/sciadv.aat4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese S.; Severn J. R.; Schenning A. P. H. J.. Photoresponsive Polyolefins. In Photoactive Functional Soft Materials: Preparation, Properties, and Applications; Li Q., Ed.; John Wiley & Sons, 2019; pp 319–340. [Google Scholar]
- Bandara H. M. D.; Burdette S. C. Photoisomerization in Different Classes of Azobenzene. Chem. Soc. Rev. 2012, 41 (5), 1809–1825. 10.1039/C1CS15179G. [DOI] [PubMed] [Google Scholar]
- Lafleur S. S. D.; Severn J. R.; Verpaalen R. C. P.; Schenning A. P. H. J.; Bastiaansen C. W. M. Rewritable Optical Patterns in Light-Responsive Ultrahigh Molecular Weight Polyethylene. ACS Appl. Polym. Mater. 2019, 1 (3), 392–396. 10.1021/acsapm.8b00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micciche F.; Ramakrishnan V.; Hoeks T. L. The Effect of Molecular Structure on the Secondary Transitions and Their Influence on the Decoloration Kinetics of Photochromic Dyes in Co-Polycarbonates. J. Polym. Sci., Part B: Polym. Phys. 2016, 54 (16), 1593–1601. 10.1002/polb.24062. [DOI] [Google Scholar]
- Bisoyi H. K.; Urbas A. M.; Li Q. Soft Materials Driven by Photothermal Effect and Their Applications. Adv. Opt. Mater. 2018, 6 (15), 1800458. 10.1002/adom.201800458. [DOI] [Google Scholar]
- Stoychev G.; Kirillova A.; Ionov L. Light-Responsive Shape-Changing Polymers. Adv. Opt. Mater. 2019, 7 (16), 1900067. 10.1002/adom.201900067. [DOI] [Google Scholar]
- Lafleur S. S. D.; Shen L.; Kamphuis E. J. T. W.; Houben S. J. A.; Balzano L.; Severn J. R.; Schenning A. P. H. J.; Bastiaansen C. W. M. Optical Patterns on Drawn Polyethylene by Direct Laser Writing. Macromol. Rapid Commun. 2019, 40 (9), 1800811. 10.1002/marc.201800811. [DOI] [PubMed] [Google Scholar]
- Pan X.; Shen L.; Schenning A. P. H. J.; Bastiaansen C. W. M. Transparent, High-Thermal-Conductivity Ultradrawn Polyethylene/Graphene Nanocomposite Films. Adv. Mater. 2019, 31 (40), 1904348. 10.1002/adma.201904348. [DOI] [PubMed] [Google Scholar]
- Van Kuringen H. P. C.; Leijten Z. J. W. A.; Gelebart A. H.; Mulder D. J.; Portale G.; Broer D. J.; Schenning A. P. H. J. Photoresponsive Nanoporous Smectic Liquid Crystalline Polymer Networks: Changing the Number of Binding Sites and Pore Dimensions in Polymer Adsorbents by Light. Macromolecules 2015, 48 (12), 4073–4080. 10.1021/acs.macromol.5b00623. [DOI] [Google Scholar]
- Wang J.; Jakli A.; West J. L. Airbrush Formation of Liquid Crystal/Polymer Fibers. ChemPhysChem 2015, 16 (9), 1839–1841. 10.1002/cphc.201500096. [DOI] [PubMed] [Google Scholar]
- Van Heeswijk E. P. A.; Kloos J. J. H.; Grossiord N.; Schenning A. P. H. J. Humidity-Gated, Temperature-Responsive Photonic Infrared Reflective Broadband Coatings. J. Mater. Chem. A 2019, 7 (11), 6113–6119. 10.1039/C9TA00993K. [DOI] [Google Scholar]
- Liu Y.; Shaw B.; Dickey M. D.; Genzer J. Sequential Self-Folding of Polymer Sheets. Sci. Adv. 2017, 3 (3), e1602417. 10.1126/sciadv.1602417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Boyles J. K.; Genzer J.; Dickey M. D. Self-Folding of Polymer Sheets Using Local Light Absorption. Soft Matter 2012, 8 (6), 1764–1769. 10.1039/C1SM06564E. [DOI] [Google Scholar]
- Stroganov V.; Al-Hussein M.; Sommer J.-U.; Janke A.; Zakharchenko S.; Ionov L. Reversible Thermosensitive Biodegradable Polymeric Actuators Based on Confined Crystallization. Nano Lett. 2015, 15 (3), 1786–1790. 10.1021/nl5045023. [DOI] [PubMed] [Google Scholar]
- Cui J.; Yao S.; Huang Q.; Adams J. G. M.; Zhu Y. Controlling the Self-Folding of a Polymer Sheet Using a Local Heater: The Effect of the Polymer–Heater Interface. Soft Matter 2017, 13 (21), 3863–3870. 10.1039/C7SM00568G. [DOI] [PubMed] [Google Scholar]
- Sol J. A. H. P.; Peeketi A. R.; Vyas N.; Schenning A. P. H. J.; Annabattula R. K.; Debije M. G. Butterfly Proboscis-Inspired Tight Rolling Tapered Soft Actuators. Chem. Commun. 2019, 55 (12), 1726–1729. 10.1039/C8CC09915D. [DOI] [PubMed] [Google Scholar]
- Wypych G.Handbook of Polymers: Second ed.; Elsevier Inc., 2016. [Google Scholar]
- Vegt A. K.; van der Govaert L. E.. Polymeren: Van Keten Tot Kunststof; VVSD, 2003. [Google Scholar]
- Mark J.; Ngai K.; Graessley W.; Mandelkern L.; Samulski E.; Koenig J.; Wignall G.. Physical Properties of Polymers; Cambridge University Press, 2004. [Google Scholar]
- Krevelen D. W.; van Nijenhuis K.. Properties of Polymers : Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier, 2009. [Google Scholar]
- Reuvers N. J. W.; Huinink H. P.; Fischer H. R.; Adan O. C. G. Quantitative Water Uptake Study in Thin Nylon-6 Films with NMR Imaging. Macromolecules 2012, 45 (4), 1937–1945. 10.1021/ma202719x. [DOI] [Google Scholar]
- Inoue K.; Hoshino S. Effect of Molecular Orientation on the Swelling of Nylon 6. J. Polym. Sci., Polym. Phys. Ed. 1977, 15, 1363–1378. 10.1002/pol.1977.180150805. [DOI] [Google Scholar]
- Parodi E.; Peters G. W. M.; Govaert L. E. Prediction of Plasticity-Controlled Failure in Polyamide 6: Influence of Temperature and Relative Humidity. J. Appl. Polym. Sci. 2018, 135 (11), 15–17. 10.1002/app.45942. [DOI] [Google Scholar]
- Baldrian J. Effect of Swelling on the Structure of Oriented Polyamide 6. Polymer 1991, 32 (4), 740–744. 10.1016/0032-3861(91)90489-6. [DOI] [Google Scholar]
- Puffr R.; Bebenda J.; Šebenda J.; Bebenda J.; Šebenda J. On the Structure and Properties of Polyamides. XXVII. The Mechanism of Water Sorption in Polyamides. J. Polym. Sci., Part C: Polym. Symp. 1967, 16 (1), 79–93. 10.1002/polc.5070160109. [DOI] [Google Scholar]
- Haldorai Y.; Shim J. Chemo-Responsive Bilayer Actuator Film: Fabrication, Characterization and Actuator Response. New J. Chem. 2014, 38 (6), 2653–2659. 10.1039/C4NJ00014E. [DOI] [Google Scholar]
- Verpaalen R. C. P.; Debije M. G.; Bastiaansen C. W. M.; Halilović H.; Engels T. A. P.; Schenning A. P. H. J. Programmable Helical Twisting in Oriented Humidity-Responsive Bilayer Films Generated by Spray-Coating of a Chiral Nematic Liquid Crystal. J. Mater. Chem. A 2018, 6 (36), 17724–17729. 10.1039/C8TA06984K. [DOI] [Google Scholar]
- Iamsaard S.; Aßhoff S. J.; Matt B.; Kudernac T.; Cornelissen J. J. L. M.; Fletcher S. P.; Katsonis N. Conversion of Light into Macroscopic Helical Motion. Nat. Chem. 2014, 6 (3), 229–235. 10.1038/nchem.1859. [DOI] [PubMed] [Google Scholar]
- Le Duigou A.; Chabaud G.; Scarpa F.; Castro M. Bioinspired Electro-Thermo-Hygro Reversible Shape-Changing Materials by 4D Printing. Adv. Funct. Mater. 2019, 29 (40), 1903280. 10.1002/adfm.201903280. [DOI] [Google Scholar]
- Haines C. S.; Lima M. D.; Li N.; Spinks G. M.; Foroughi J.; Madden J. D. W.; Kim S. H.; Fang S.; Jung de Andrade M.; Goktepe F.; Goktepe O.; Mirvakili S. M.; Naficy S.; Lepro X.; Oh J.; Kozlov M. E.; Kim S. J.; Xu X.; Swedlove B. J.; Wallace G. G.; Baughman R. H. Artificial Muscles from Fishing Line and Sewing Thread. Science 2014, 343 (6173), 868–872. 10.1126/science.1246906. [DOI] [PubMed] [Google Scholar]
- Lepoittevin B.; Roger P.. Poly(Ethylene Terephthalate). In Handbook of Engineering and Speciality Thermoplastics; John Wiley & Sons, Inc.: Hoboken, NJ, 2011; pp 97–126. [Google Scholar]
- Park S. H.; Kim S. H. Poly (Ethylene Terephthalate) Recycling for High Value Added Textiles. Fash. Text. 2014, 1 (1), 1. 10.1186/s40691-014-0001-x. [DOI] [Google Scholar]
- Verpaalen R. C. P.; Pilz da Cunha M.; Engels T. A. P.; Debije M. G.; Schenning A. P. H. J. Liquid Crystal Networks on Thermoplastics: Reprogrammable Photo-Responsive Actuators. Angew. Chem., Int. Ed. 2020, 59 (11), 4532–4536. 10.1002/anie.201915147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang Y.; Chen C.; Cheng J.; Pastel G.; Li T.; Song J.; Jiang F.; Li Y.; Zhang Y.; Jang S. H.; Chen G.; Li T.; Hu L. Selectively Aligned Cellulose Nanofibers towards High-Performance Soft Actuators. Extrem. Mech. Lett. 2019, 29, 100463. 10.1016/j.eml.2019.100463. [DOI] [Google Scholar]
- Park J.; Yoo J. W.; Seo H. W.; Lee Y.; Suhr J.; Moon H.; Koo J. C.; Choi H. R.; Hunt R.; Kim K. J.; Kim S. H.; Nam J. Do. Electrically Controllable Twisted-Coiled Artificial Muscle Actuators Using Surface-Modified Polyester Fibers. Smart Mater. Struct. 2017, 26 (3), 035048. 10.1088/1361-665X/aa5323. [DOI] [Google Scholar]
- Hutley T. J.; Ouederni M. Polyolefins—The History and Economic Impact. Polyolefin Compounds and Materials 2016, 13–50. 10.1007/978-3-319-25982-6_2. [DOI] [Google Scholar]
- Ryabchun A.; Bobrovsky A.; Stumpe J.; Shibaev V. Novel Generation of Liquid Crystalline Photo-Actuators Based on Stretched Porous Polyethylene Films. Macromol. Rapid Commun. 2012, 33 (11), 991–997. 10.1002/marc.201100837. [DOI] [PubMed] [Google Scholar]
- Verpaalen R. C. P.; Varghese S.; Froyen A.; Pilz da Cunha M.; Pouderoijen M. J.; Severn J. R.; Bhatti M. R.; Peijs T.; Bastiaansen C. W. M.; Debije M. G.; Engels T. A. P.; Schenning A. P. H. J. Fast, light responsive metal-like polymer actuators generating high stresses at low strain. Matter 2020, 2 (6), 1522–1534. 10.1016/j.matt.2020.03.001. [DOI] [Google Scholar]
- Varghese S.; Fredrich S.; Vantomme G.; Prabhu S. R.; Teyssandier J.; De Feyter S.; Severn J.; Bastiaansen C. W. M.; Schenning A. P. H. J. Epitaxial Growth of Light-Responsive Azobenzene Molecular Crystal Actuators on Oriented Polyethylene Films. J. Mater. Chem. C 2020, 8 (2), 694–699. 10.1039/C9TC05407C. [DOI] [Google Scholar]
- Zhang X.; Pint C. L.; Lee M. H.; Schubert B. E.; Jamshidi A.; Takei K.; Ko H.; Gillies A.; Bardhan R.; Urban J. J.; Wu M.; Fearing R.; Javey A. Optically- and Thermally-Responsive Programmable Materials Based on Carbon Nanotube-Hydrogel Polymer Composites. Nano Lett. 2011, 11 (8), 3239–3244. 10.1021/nl201503e. [DOI] [PubMed] [Google Scholar]
- Yamada M.; Kondo M.; Miyasato R.; Naka Y.; Mamiya J. I.; Kinoshita M.; Shishido A.; Yu Y.; Barrett C. J.; Ikeda T. Photomobile Polymer Materials - Various Three-Dimensional Movements. J. Mater. Chem. 2009, 19 (1), 60–62. 10.1039/B815289F. [DOI] [Google Scholar]
- Yamada M.; Kondo M.; Mamiya J. I.; Yu Y.; Kinoshita M.; Barrett C. J.; Ikeda T. Photomobile Polymer Materials: Towards Light-Driven Plastic Motors. Angew. Chem., Int. Ed. 2008, 47 (27), 4986–4988. 10.1002/anie.200800760. [DOI] [PubMed] [Google Scholar]
- Cheng F.; Yin R.; Zhang Y.; Yen C. C.; Yu Y. Fully Plastic Microrobots Which Manipulate Objects Using Only Visible Light. Soft Matter 2010, 6 (15), 3447–3449. 10.1039/c0sm00012d. [DOI] [Google Scholar]
- Naka Y.; Mamiya J. I.; Shishido A.; Washio M.; Ikeda T. Direct Fabrication of Photomobile Polymer Materials with an Adhesive-Free Bilayer Structure by Electron-Beam Irradiation. J. Mater. Chem. 2011, 21 (6), 1681–1683. 10.1039/C0JM03901B. [DOI] [Google Scholar]
- Liu Z.; Tang R.; Xu D.; Liu J.; Yu H. Precise Actuation of Bilayer Photomechanical Films Coated with Molecular Azobenzene Chromophores. Macromol. Rapid Commun. 2015, 36 (12), 1171–1176. 10.1002/marc.201500177. [DOI] [PubMed] [Google Scholar]
- Hu J.; Li X.; Ni Y.; Ma S.; Yu H. A Programmable and Biomimetic Photo-Actuator: A Composite of a Photo-Liquefiable Azobenzene Derivative and Commercial Plastic Film. J. Mater. Chem. C 2018, 6 (40), 10815–1082. 10.1039/C8TC03693D. [DOI] [Google Scholar]
- Lub J.; Broer D. J.; Wegh R. T.; Peeters E.; Van Der Zande B. M. I. Formation of Optical Films by Photo-Polymerisation of Liquid Crystalline Acrylates and Application of These Films in Liquid Crystal Display Technology. Mol. Cryst. Liq. Cryst. 2005, 429, 77–99. 10.1080/15421400590930773. [DOI] [Google Scholar]
- Lewin M.Handbook of Fiber Chemistry, 3rd ed.; CRC/Taylor & Francis, 2006. [Google Scholar]
- Ryabchun A.; Lancia F.; Nguindjel A.-D.; Katsonis N. Humidity-Responsive Actuators from Integrating Liquid Crystal Networks in an Orienting Scaffold. Soft Matter 2017, 13, 8070–8075. 10.1039/C7SM01505D. [DOI] [PubMed] [Google Scholar]
- Gao L.; Guo G.; Liu M.; Tang Z.; Xie L.; Huo Y. Multi-Responsive, Bidirectional, and Large Deformation Bending Actuators Based on Borax Cross-Linked Polyvinyl Alcohol Derivative Hydrogel. RSC Adv. 2017, 7 (63), 40005–40014. 10.1039/C7RA06617A. [DOI] [Google Scholar]
- Lu X.; Guo S.; Tong X.; Xia H.; Zhao Y. Tunable Photocontrolled Motions Using Stored Strain Energy in Malleable Azobenzene Liquid Crystalline Polymer Actuators. Adv. Mater. 2017, 29 (28), 1606467. 10.1002/adma.201606467. [DOI] [PubMed] [Google Scholar]
- Shang J.; Lin S.; Theato P. UV-Triggered Shape-Controllable PP Fabric. Polym. Chem. 2018, 9 (23), 3232–3237. 10.1039/C8PY00411K. [DOI] [Google Scholar]
- Verpaalen R. C. P.; Souren A. E. J.; Debije M. G.; Engels T. A. P.; Bastiaansen C. W. M.; Schenning A. P. H. J. Unravelling Humidity-Gated, Temperature Responsive Bilayer Actuators. Soft Matter 2020, 16 (11), 2753–2759. 10.1039/D0SM00030B. [DOI] [PubMed] [Google Scholar]
- Gijsman P. New Synergists for Hindered Amine Light Stabilizers. Polymer 2002, 43 (5), 1573–1579. 10.1016/S0032-3861(01)00708-X. [DOI] [Google Scholar]
- Wypych G.Handbook of UV Degradation and Stabilization, 2nd ed.; 2015. [Google Scholar]
- Zayat M.; Garcia-Parejo P.; Levy D. Preventing UV-Light Damage of Light Sensitive Materials Using a Highly Protective UV-Absorbing Coating. Chem. Soc. Rev. 2007, 36 (8), 1270–1281. 10.1039/b608888k. [DOI] [PubMed] [Google Scholar]
- Raab M.; Kotulák L.; Kolařík J.; Pospíšil J. The Effect of Ultraviolet Light on the Mechanical Properties of Polyethylene and Polypropylene Films. J. Appl. Polym. Sci. 1982, 27 (7), 2457–2466. 10.1002/app.1982.070270716. [DOI] [Google Scholar]
- Yousif E.; Haddad R. Photodegradation and Photostabilization of Polymers, Especially Polystyrene: Review. SpringerPlus 2013, 2 (1), 1–32. 10.1186/2193-1801-2-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bléger D.; Schwarz J.; Brouwer A. M.; Hecht S. O -Fluoroazobenzenes as Readily Synthesized Photoswitches Offering Nearly Quantitative Two-Way Isomerization with Visible Light. J. Am. Chem. Soc. 2012, 134 (51), 20597–20600. 10.1021/ja310323y. [DOI] [PubMed] [Google Scholar]
- Global Robotics Revenue to Reach $248.5 Billion by 2025, as the Market for Non-Industrial Robots Maintains Strong Growth. Tractica. https://www.tractica.com/newsroom/press-releases/global-robotics-revenue-to-reach-248-5-billion-by-2025-as-the-market-for-non-industrial-robots-maintains-strong-growth/ (accessed June 9, 2020).
- World Robotics Report: Global Sales of Robots Hit $16.5B in 2018. https://www.roboticsbusinessreview.com/research/world-robotics-report-global-sales-of-robots-hit-16-5b-in-2018/ (accessed June 9, 2020).
- Robotics industry growing faster than expected. The Robot Report. https://www.therobotreport.com/robotics-industry-growing-faster-than-expected/ (accessed June 9, 2020).
- Huber J. E.; Fleck N. A.; Ashby M. F. The Selection of Mechanical Actuators Based on Performance Indices. Proc. R. Soc. London, Ser. A 1997, 453 (1965), 2185–2205. 10.1098/rspa.1997.0117. [DOI] [Google Scholar]
- Madden J. D. W.Dielectric Elastomers as Electromechanical Transducers; Elsevier, 2008. [Google Scholar]
- Lipton J. I.; Angle S.; Banai R. E.; Peretz E.; Lipson H. Electrically Actuated Hydraulic Solids. Adv. Eng. Mater. 2016, 18 (10), 1710–1715. 10.1002/adem.201600271. [DOI] [Google Scholar]
- Mirvakili S. M.; Hunter I. W. Artificial Muscles: Mechanisms, Applications, and Challenges. Adv. Mater. 2018, 30 (6), 1704407. 10.1002/adma.201704407. [DOI] [PubMed] [Google Scholar]
- Kondo M.; Sugimoto M.; Yamada M.; Naka Y.; Mamiya J. I.; Kinoshita M.; Shishido A.; Yu Y.; Ikeda T. Effect of Concentration of Photoactive Chromophores on Photomechanical Properties of Crosslinked Azobenzene Liquid-Crystalline Polymers. J. Mater. Chem. 2010, 20 (1), 117–122. 10.1039/B917342K. [DOI] [Google Scholar]
- Bauer S.; Bauer-Gogonea S.; Graz I.; Kaltenbrunner M.; Keplinger C.; Schwödiauer R. 25th Anniversary Article: A Soft Future: From Robots and Sensor Skin to Energy Harvesters. Adv. Mater. 2014, 26 (1), 149–162. 10.1002/adma.201303349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima M. D.; Li N.; De Andrade M. J.; Fang S.; Oh J.; Spinks G. M.; Kozlov M. E.; Haines C. S.; Suh D.; Foroughi J.; Kim S. J.; Chen Y.; Ware T.; Shin M. K.; Machado L. D.; Fonseca A. F.; Madden J. D. W.; Voit W. E.; Galvão D. S.; Baughman R. H. Electrically, Chemically, and Photonically Powered Torsional and Tensile Actuation of Hybrid Carbon Nanotube Yarn Muscles. Science 2012, 338 (6109), 928–932. 10.1126/science.1226762. [DOI] [PubMed] [Google Scholar]
- Hiraoka M.; Nakamura K.; Arase H.; Asai K.; Kaneko Y.; John S. W.; Tagashira K.; Omote A. Power-Efficient Low-Temperature Woven Coiled Fibre Actuator for Wearable Applications. Sci. Rep. 2016, 6 (1), 36358. 10.1038/srep36358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.; Jung de Andrade M.; Saharan L. K.; Rome R. S.; Baughman R. H.; Tadesse Y. Compact and Low-Cost Humanoid Hand Powered by Nylon Artificial Muscles. Bioinspir. Biomim. 2017, 12 (2), 026004. 10.1088/1748-3190/aa52f8. [DOI] [PubMed] [Google Scholar]
- Alici G. Softer Is Harder: What Differentiates Soft Robotics from Hard Robotics?. MRS Adv. 2018, 3 (28), 1557–1568. 10.1557/adv.2018.159. [DOI] [Google Scholar]
- Cianchetti M.; Laschi C.; Menciassi A.; Dario P. Biomedical Applications of Soft Robotics. Nat. Rev. Mater. 2018, 3 (6), 143–153. 10.1038/s41578-018-0022-y. [DOI] [Google Scholar]
- Prescouter. Disruption in Human Robot Collaboration. https://www.prescouter.com/report/disruption-in-human-robot-collaboration/ (accessed June 9, 2020).
- Advanced Robotics in the Factory of the Future. https://www.bcg.com/publications/2019/advanced-robotics-factory-future.aspx (accessed June 9, 2020).
- Zhang X. A.; Yu S.; Xu B.; Li M.; Peng Z.; Wang Y.; Deng S.; Wu X.; Wu Z.; Ouyang M.; Wang Y. H. Dynamic Gating of Infrared Radiation in a Textile. Science 2019, 363 (6427), 619–623. 10.1126/science.aau1217. [DOI] [PubMed] [Google Scholar]
- Guan Y.; Agra-Kooijman D. M.; Fu S.; Jákli A.; West J. L. Responsive Liquid-Crystal-Clad Fibers for Advanced Textiles and Wearable Sensors. Adv. Mater. 2019, 31 (29), 1902168. 10.1002/adma.201902168. [DOI] [PubMed] [Google Scholar]
- Van Der Werff L.; Kyratzis I. L.; Robinson A.; Cranston R.; Peeters G.; O’Shea M.; Nichols L. Thermochromic Composite Fibres Containing Liquid Crystals Formed via Melt Extrusion. J. Mater. Sci. 2013, 48 (14), 5005–5011. 10.1007/s10853-013-7287-8. [DOI] [Google Scholar]
- Pakdel E.; Naebe M.; Sun L.; Wang X. Advanced Functional Fibrous Materials for Enhanced Thermoregulating Performance. ACS Appl. Mater. Interfaces 2019, 11 (14), 13039–13057. 10.1021/acsami.8b19067. [DOI] [PubMed] [Google Scholar]
- Picot O. T.; Dai M.; Broer D. J.; Peijs T.; Bastiaansen C. W. M. New Approach toward Reflective Films and Fibers Using Cholesteric Liquid-Crystal Coatings. ACS Appl. Mater. Interfaces 2013, 5 (15), 7117–7121. 10.1021/am401438z. [DOI] [PubMed] [Google Scholar]
- Picot O. T.; Dai M.; Billoti E.; Broer D. J.; Peijs T.; Bastiaansen C. W. M. A Real Time Optical Strain Sensor Based on a Cholesteric Liquid Crystal Network. RSC Adv. 2013, 3 (41), 18794–18798. 10.1039/c3ra42986e. [DOI] [Google Scholar]
- Park T. H.; Yu S.; Cho S. H.; Kang H. S.; Kim Y.; Kim M. J.; Eoh H.; Park C.; Jeong B.; Lee S. W.; Ryu D. Y.; Huh J.; Park C. Block Copolymer Structural Color Strain Sensor. NPG Asia Mater. 2018, 10 (4), 328–339. 10.1038/s41427-018-0036-3. [DOI] [Google Scholar]