Abstract
Background/Aim: Aneurysmal bone cyst is a benign bone lesion with a strong tendency to recur. The rearrangement of chromosome band 17p13/USP6 gene is now considered a characteristic genetic feature of aneurysmal bone cyst, with t(16;17)(q22;p13)/CDH11-USP6 as the most frequent chromosomal aberration/fusion gene. We report a novel variant translocation leading to a new fusion gene in an aneurysmal bone cyst. Materials and Methods: Genetic analyses were performed on an aneurysmal bone cyst found in the tibia of a child. Results: G-banding chromosome analysis yielded the karyotype 46,XX,t(12;17)(q21;p13)[5]/46,XX[2]. FISH analysis with a USP6 break-apart probe showed rearrangement of USP6. RNA sequencing detected LUM-USP6 and USP6-LUM fusion transcripts which were subsequently verified by RT-PCR/Sanger sequencing. The two genes exchanged 5’- non-coding exons. Thus, promoter swapping between USP6 and LUM had taken place. Conclusion: We report a novel t(12;17)(q21;p13) chromosome translocation which gave rise to a LUM-USP6 fusion in an aneurysmal bone cyst.
Keywords: Aneurysmal bone cyst, chromosome translocation, LUM, USP6, LUM-USP6 fusion gene
Aneurysmal bone cyst is a rapidly expanding, benign bone lesion with a strong tendency to recur (1-3). It is found in all age groups but most commonly during the first two decades of life (1-3). Aneurysmal bone cyst was originally considered a non-neoplastic lesion of unknown etiology (4). In 1999, however, Panoutsakopoulos et al. (5) reported three cases with clonal acquired chromosomal aberrations, two with t(16;17)(q22;p13) and one with del(16)(q22), providing evidence for a neoplastic origin of these lesions. In 2004, Oliveira et al. showed that the t(16;17)(q21;p13) translocation generated a fusion gene in which the strong promoter of the cadherin 11 gene (CDH11) at 16q21 was fused to the entire ubiquitin-specific protease 6 (USP6; alias Tre2) coding sequence at 17p13 (6,7). The result of the CDH11-USP6 chimeric gene is that USP6 becomes transcriptionally up-regulated. Subsequently, fusion genes corresponding to the variant translocations t(1;17)(p34;p13), t(3;17)(q21;p13), t(9;17)(q22;p13), and t(17;17)(q21;p13) were reported (8). In each translocation, the entire USP6 coding sequence was fused downstream with the promoter region of the partner gene: thyroid hormone receptor associated protein 3 (THRAP3 at 1p34), CCHC-type zinc finger nucleic acid binding protein (CNBP at 3q21), osteomodulin (OMD at 9q22), and collagen type I alpha 1 chain (COL1A1 at 17q21) (8). Additional studies have detected fusion of USP6 with the genes FOS like 2, AP-1 transcription factor subunit (t(2;17)(p23;p13)/FOSL2-USP6), catenin beta 1 (t(3;17)(p22;p13)/CTNNB1-USP6), SEC31 homolog A, COPII coat complex component (t(4;17)(q21;p13)/ SEC31A-USP6), FAT atypical cadherin 1 (t(4;17)(q35;p13)/ FAT1-USP6), secreted protein acidic and cysteine rich (t(5;17)(q33;p13)/SPARC-USP6), RUNX family transcription factor 2 (t(6;17)(p21;p13)/RUNX2-USP6), ArfGAP with SH3 domain, ankyrin repeat and PH domain 1 (t(8;17)(q24;p13)/ ASAP1-USP6), tenascin C (t(9;17)(q33;p13)/TNC-USP6), secretion associated Ras related GTPase 1A (t(10;17)(q22;p13)/ SAR1A-USP6), eukaryotic translation initiation factor 1 (t(17;17)(p13;q21)/EIF1-USP6), platelet activating factor acetylhydrolase 1b regulatory subunit 1 (t(17;17)(p13;p13)/ PAFAH1B1-USP6), signal transducer and activator of transcription 3 (t(17;17)(p13;q21)/STAT3-USP6), and ubiquitin specific peptidase 9 X-linked (t(X;17)(p11;p13)/USP9X-USP6) in other aneurysmal bone cysts (9-13). Thus, rearrangement of chromosome band 17p13 and the USP6 gene is now considered a characteristic genetic feature of aneurysmal bone cyst, with t(16;17)(q22;p13) as the most frequent chromosomal aberration found in 21% (9 out of 43) reported aneurysmal bone cysts with an abnormal karyotype (5,6,8,12,14-25).
Herein, we report an aneurysmal bone cyst in which a novel t(12;17)(q21;p13) translocation was found resulting in fusion of the lumican (LUM at 12q21) gene with USP6.
Materials and Methods
Ethics statement. The study was approved by the Regional Ethics Committee (Regional komité for medisinsk forskningsetikk Sør-Øst, Norge, http://helseforskning.etikkom.no; 2010/1389/REK sør-øst A). Written informed consent was obtained from the patient’s parents. The Ethics Committee’s approval included a review of the consent procedure. All patient information has been de-identified.
Patient. The patient was a nine-year-old girl with post activity pain and edema in the left ankle the last couple of months. On X-ray there was an osteolytic, benign looking lesion in the metaphysis region of the distal tibia which on MRI appeared multi-locular and confined to the bone. Radiologically and on core needle biopsy aneurysmal bone cyst was the most likely diagnosis and a curettage was performed.
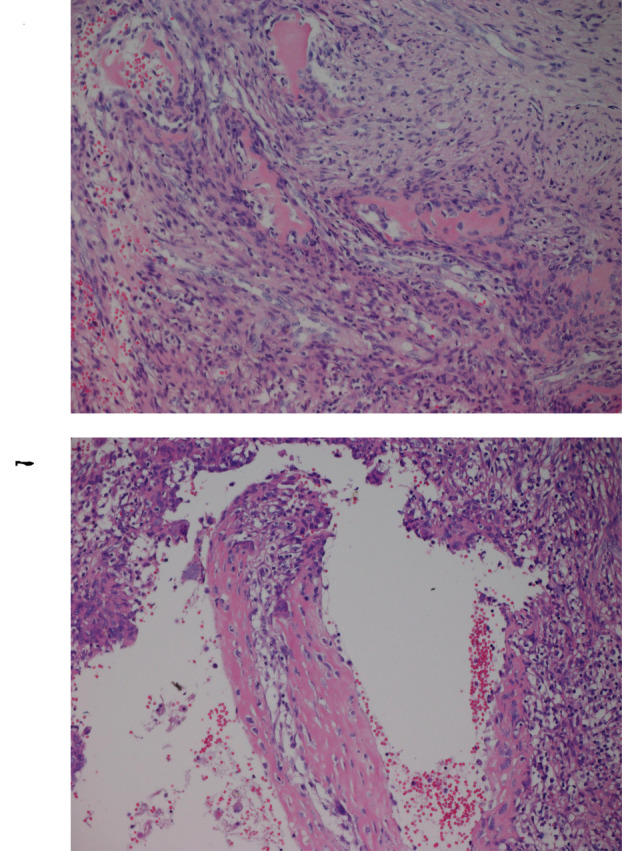
Histologically, the lesion was shown to be fibro-osseous (Figure 1). There was compact growth of fibrous tissue with spindled cells and focal areas with scattered osteoclast-like giant cells (Figure 1A). In some areas, there were islands of osteoid and throughout the biopsy there was osteoid production with focal calcification (Figure 1B). Some of the fibrous areas appeared to be fragments of thin walls. No atypical cells or mitotic activity were seen. The diagnosis was aneurysmal bone cyst.
Figure 1. Microscopic examination of the aneurysmal bone cyst. A) Hematoxylin and eosin (H&E) stained section showing compact growth of fibrous tissue with spindled cells and focal areas with scattered osteoclast-like giant cells, magnification 20×. B) H&E stained section showing cystic space and osteoid production with focal calcification, magnification 20×.
G-banding, karyotyping, and fluorescence in situ hybridization (FISH). Fresh tissue from a representative area of the tumor was analyzed cytogenetically as part of our diagnostic routine. The methodology for G-banding and karyotyping was described elsewhere (26). The samples were disaggregated mechanically and enzymatically with collagenase II (Worthington, Freehold, NJ, USA). The resulting cells were cultured and harvested using standard techniques. Chromosome preparations were G-banded with Wright’s stain (Sigma-Aldrich; St Louis, MO, USA) and examined. Metaphases were analyzed and karyograms prepared using the CytoVision computer assisted karyotyping system (Leica Biosystems, Newcastle, UK). The karyotypes were reported according to the International System for Human Cytogenomic Nomenclature (27). FISH was performed on metaphase spreads using the ZytoLight SPEC USP6 Dual Color Break Apart Probe (ZytoVision, Bremerhaven, Germany). The Probe is a mixture of an orange-labeled-probe and a green-labeled-probe which hybridize proximal and distal to the USP6 gene, respectively. Chromosome preparations were counterstained with 0.2 μg/ml DAPI and overlaid with a 24×50 mm2 coverslip. Fluorescent signals were captured and analyzed using the CytoVision system (Leica Biosystems).
RNA sequencing. Total RNA was extracted from frozen (–80˚C) tumor tissue adjacent to that used for cytogenetic analysis and histological examination using miRNeasy Mini Kit and Qiacube (Qiagen, Hilden, Germany). The RNA quality was evaluated using a 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA) according to the manufacturer’s instructions. One μg of total RNA was sent to the Genomics Core Facility at the Norwegian Radium Hospital, Oslo University Hospital (http://genomics.no/oslo/) for high-throughput paired-end RNA-sequencing. For library preparation from total RNA, the Illumina TruSeq RNA Access Library Prep kit was used according to Illuminaʼs protocol (Illumina, San Diego, CA, USA). Sequencing was performed on NextSeq 550 System (Illumina) and 25 million reads were generated. The FASTQC software was used for quality control of the raw sequence data (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The software FusionCatcher was used for detection of possible fusion transcripts (28,29).
Confirmation of the fusion transcripts. The presence of the fusion transcripts was confirmed by reverse transcription (RT) polymerase chain reaction (PCR) and Sanger sequencing analyses. One μg of total RNA was reverse-transcribed in a 20 μl reaction volume using the iScript Advanced cDNA Synthesis Kit for RT-qPCR according to the manufacturer’s instructions (Bio-Rad, Hercules, CA, USA). cDNA corresponding to 20 ng total RNA was used as template in subsequent PCR assays. The BigDye Direct Cycle Sequencing Kit was used to perform both PCR and cycle (Sanger) sequencing following the companyʼs recommendations (ThermoFisher Scientific, Waltham, MA, USA). The primer combinations were M13For-LUM-4F1/ M13Rev-USP6-2600R1 and M13For-USP6-2192F1/ M13Rev-LUM-152-R1. The primers used for RT-PCR/cycle (Sanger) are listed in Table I.
Table I. Primers used for reverse transcription polymerase chain reaction and cycle (Sanger) sequencing. M13 forward primer (TGTAAAACGACGGCCAGT) and M13 reverse primer (CAGGAAACAGCTATGACC) sequences are in italics.
Results
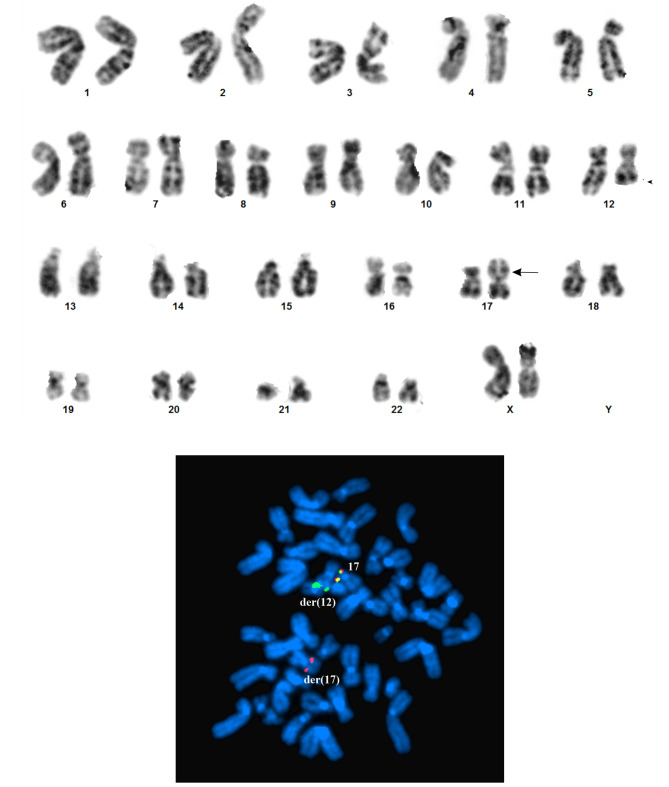
The G-banding analysis yielded a karyotype with a single chromosome abnormality: 46,XX,t(12;17)(q21;p13)[5]/ 46,XX[2] (Figure 2A). FISH analysis using the USP6 break-apart probe showed that the distal part of the probe (green signal) hybridized to the der(12) t(12;17)(q21;p13), whereas the proximal part of the probe (red signal) hybridized to der(17) t(12;17)(q21;p13) (Figure 2B).
Figure 2. G-banding and FISH analyses. A) Karyogram of the aneurysmal bone cyst cells showing the der(12)t(12;17)(q21;p13) and der(17)t(12;17)(q21;p13). Breakpoint positions are indicated by arrows. B) FISH with the USP6 break-apart probe on metaphase spread showing that the distal part of the probe (green signal) hybridized to the der(12)t(12;17)(q21;p13) whereas the proximal part of the probe (red signal) hybridized to der(17)t(12;17)(q21;p13). Both distal and proximal parts of the USP6 probe hybridized to the normal chromosome 17.
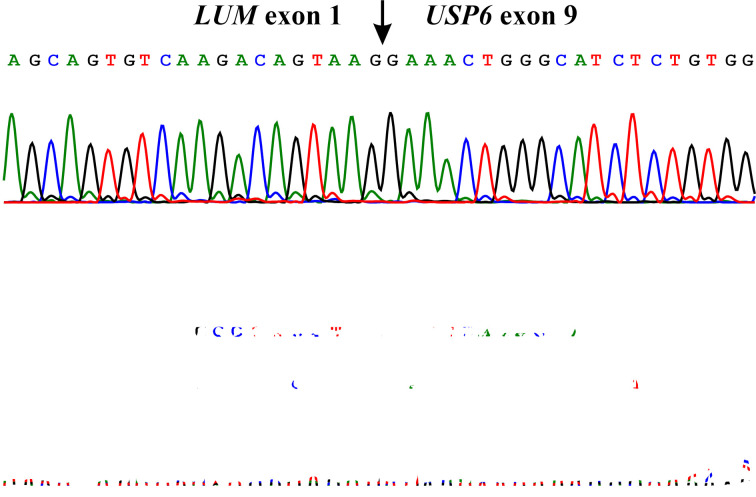
Using the FusionCatcher software with the fastq files from the RNA sequencing, both LUM-USP6 and USP6-LUM fusion transcript sequences were detected. In the LUM-USP6 fusion transcript, exon 1 of LUM (nt 97 of sequence with accession number NM_002345.4) fused to exon 9 of USP6 (nt 2482 of sequence with accession number NM_001304284.2): CATCTGCTTTAAGAATTAACGAAAGCAGTGTCAAGACAGTAAG/GAAACTGGGCATCTCTGTGGCCCTGAACATCCCAGGAGGCCGA. In the USP6-LUM fusion transcript, exon 7 of USP6 (nt 2299 of sequence with accession number NM_001304284.2) fused to exon 2 of LUM (nt 98 of sequence with accession number NM_002345.4): GTGTCCTGAAC TGGGCCCTTCCTCCAGTGAGAAGCCTTCCTGA/GATTCAAACCATTTGCCAAAAATGAGTCTAAGTGCATTTACTC. RT-PCR/cycle (Sanger) sequencing verified the presence of the above-mentioned fusion transcripts (Figure 3).
Figure 3. Results of Sanger sequencing. Partial sequence chromatograms of the cDNA amplified fragment showing the junction position of the LUM and USP6 genes (arrow). The exon numbers were based on the sequences with accession numbers NM_002345.4 for LUM and NM_001304284.2 for USP6.
Discussion
We herein report the LUM gene as a new fusion partner of USP6 in an aneurysmal bone cyst carrying a novel t(12;17)(q21;p13) chromosome translocation as the only cytogenetic aberration. The chromosome translocation resulted in fusion of the 5’-non-coding region of USP6 with the 5’-non-coding region of LUM exchanging the two genes’ regulatory elements. Promoter swapping between USP6 and LUM, thus, took place with the expression of USP6 coming under the control of the LUM promoter leading to overexpression or ectopic activation of USP6. The pattern in the LUM-USP6 fusion gene was thus similar to that seen in previously reported USP6 fusion genes (6,8-13).
USP6 is a hominoid-specific gene derived in the recent evolutionary past from fusion between TBC1D3 and USP32, located on 17q12 and 17q23, respectively (30). The TBC (Tre-2/Bub2/Cdc16) domain of TBC1D3 and the ubiquitin binding domain of USP32 comprise the amino and carboxyl terminal parts of USP6 protein, respectively (30). Expression of USP6 in normal tissues is predominantly found in the testis, but overexpression of USP6 can transform mesenchymal cells, and indeed USP6 was first identified as a potential oncogene based on its transforming properties in transfection studies of NIH-3T3 cells (31).
Overexpression or ectopic activation of USP6 leads to deregulation of USP6-target genes and tumor formation (31-36). Recently, USP6 was found to have Frizzleds, JAK1, and JUN as substrates and consequently to promote Wnt, JAK1-STAT3, and JUN signaling pathways (35-37).
Apart from aneurysmal bone cyst, USP6 activation by promoter-swapping gene fusion has also been found in nodular fasciitis, cranial fasciitis, and myositis ossificans (38-45). Furthermore, USP6 rearrangements were detected in a subset of cellular fibromas of tendon sheath which share similar histological features with nodular fasciitis (46).
The LUM gene codes for lumican which is a member of the small leucine-rich proteoglycan family that also includes decorin, biglycan, fibromodulin, keratocan, epiphycan, and osteoglycin (47-50). Lumican is the major keratan sulfate proteoglycan of the cornea but is also distributed in interstitial collagenous matrices throughout the body (47-50). Lumican may regulate collagen fibril organization and circumferential growth, corneal transparency, and epithelial cell migration and tissue repair (48-53). In cancer, lumican was found to be involved in tumor progression, angiogenesis, and metastasis (49,51,52). Although most of studies showed lumican to have an anti-tumor effect, its role in cancer is dependent on its abundance, distribution, and tumor type and stage (49,51,52).
In conclusion, our finding of a novel variant t(12;17)(q21;p13) chromosome translocation with a LUM-USP6 fusion expands the spectrum of known fusion partner genes of USP6 and emphasizes further its central role in the pathogenesis of aneurysmal bone cyst.
Conflicts of Interest
The Authors declare that they have no conflicts of interest in regard to this study.
Authors’ Contributions
IP designed and supervised the research, performed molecular genetic experiments and bioinformatics analysis, and wrote the article. LG performed cytogenetic analysis and evaluated the FISH data. KA performed molecular genetic experiments, FISH analyses, and evaluated the data. IL performed pathological examination. ML-I performed pathological examination. FM evaluated cytogenetic and FISH data. SH assisted with experimental design and writing of the article. All Authors read and approved the final manuscript.
Acknowledgements
This work was supported by grants from Radiumhospitalets Legater.
References
- 1.Hakim DN, Pelly T, Kulendran M, Caris JA. Benign tumours of the bone: A review. J Bone Oncol. 2015;4(2):37–41. doi: 10.1016/j.jbo.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mascard E, Gomez-Brouchet A, Lambot K. Bone cysts: Unicameral and aneurysmal bone cyst. Orthop Traumatol Surg Res. 2015;101(1 Suppl):S119–127. doi: 10.1016/j.otsr.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 3.Stevens KJ, Stevens JA. StatPearls Publishing LLC.: Treasure Island (FL) 2020. Aneurysmal bone cysts. In: Statpearls. [PubMed] [Google Scholar]
- 4.Kransdorf MJ, Sweet DE. Aneurysmal bone cyst: Concept, controversy, clinical presentation, and imaging. AJR Am J Roentgenol. 1995;164(3):573–580. doi: 10.2214/ajr.164.3.7863874. [DOI] [PubMed] [Google Scholar]
- 5.Panoutsakopoulos G, Pandis N, Kyriazoglou I, Gustafson P, Mertens F, Mandahl N. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosomes Cancer. 1999;26(3):265–266. doi: 10.1002/(sici)1098-2264(199911)26:3<265::aid-gcc12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira AM, Hsi BL, Weremowicz S, Rosenberg AE, Dal Cin P, Joseph N, Bridge JA, Perez-Atayde AR, Fletcher JA. USP6 (Tre2) fusion oncogenes in aneurysmal bone cyst. Cancer Res. 2004;64(6):1920–1923. doi: 10.1158/0008-5472.can-03-2827. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira AM, Perez-Atayde AR, Inwards CY, Medeiros F, Derr V, Hsi BL, Gebhardt MC, Rosenberg AE, Fletcher JA. USP6 and CDH11 oncogenes identify the neoplastic cell in primary aneurysmal bone cysts and are absent in so-called secondary aneurysmal bone cysts. Am J Pathol. 2004;165(5):1773–1780. doi: 10.1016/s0002-9440(10)63432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliveira AM, Perez-Atayde AR, Dal Cin P, Gebhardt MC, Chen CJ, Neff JR, Demetri GD, Rosenberg AE, Bridge JA, Fletcher JA. Aneurysmal bone cyst variant translocations upregulate USP6 transcription by promoter swapping with the ZNF9, COL1A1, TRAP150, and OMD genes. Oncogene. 2005;24(21):3419–3426. doi: 10.1038/sj.onc.1208506. [DOI] [PubMed] [Google Scholar]
- 9.Guseva NV, Jaber O, Tanas MR, Stence AA, Sompallae R, Schade J, Fillman AN, Miller BJ, Bossler AD, Ma D. Anchored multiplex PCR for targeted next-generation sequencing reveals recurrent and novel USP6 fusions and upregulation of USP6 expression in aneurysmal bone cyst. Genes Chromosomes Cancer. 2017;56(4):266–277. doi: 10.1002/gcc.22432. [DOI] [PubMed] [Google Scholar]
- 10.Warren M, Xu D, Li X. Gene fusions PAFAH1B1-USP6 and RUNX2-USP6 in aneurysmal bone cysts identified by next generation sequencing. Cancer Genet. 2017;212-213:13–18. doi: 10.1016/j.cancergen.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Sekoranja D, Bostjancic E, Salapura V, Mavcic B, Pizem J. Primary aneurysmal bone cyst with a novel SPARC-USP6 translocation identified by next-generation sequencing. Cancer Genet. 2018;228-229:12–16. doi: 10.1016/j.cancergen.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Blackburn PR, Davila JI, Jackson RA, Fadra N, Atiq MA, Pitel BA, Nair AA, VanDeWalker TJ, Hessler MG, Hovel SK, Wehrs RN, Fritchie KJ, Jenkins RB, Halling KC, Geiersbach KB. Rna sequencing identifies a novel USP9X-USP6 promoter swap gene fusion in a primary aneurysmal bone cyst. Genes Chromosomes Cancer. 2019;58(8):589–594. doi: 10.1002/gcc.22742. [DOI] [PubMed] [Google Scholar]
- 13.Sekoranja D, Zupan A, Mavcic B, Martincic D, Salapura V, Snoj Z, Limpel Novak AK, Pizem J. Novel ASAP1-USP6, FAT1-USP6, SAR1A-USP6, and TNC-USP6 fusions in primary aneurysmal bone cyst. Genes Chromosomes Cancer. 2020;59(6):357–365. doi: 10.1002/gcc.22836. [DOI] [PubMed] [Google Scholar]
- 14.Althof PA, Ohmori K, Zhou M, Bailey JM, Bridge RS, Nelson M, Neff JR, Bridge JA. Cytogenetic and molecular cytogenetic findings in 43 aneurysmal bone cysts: Aberrations of 17p mapped to 17p13.2 by fluorescence in situ hybridization. Mod Pathol. 2004;17(5):518–525. doi: 10.1038/modpathol.3800090. [DOI] [PubMed] [Google Scholar]
- 15.Baruffi MR, Neto JB, Barbieri CH, Casartelli C. Aneurysmal bone cyst with chromosomal changes involving 7q and 16p. Cancer Genet Cytogenet. 2001;129(2):177–180. doi: 10.1016/s0165-4608(01)00453-8. [DOI] [PubMed] [Google Scholar]
- 16.Dal Cin P, Kozakewich HP, Goumnerova L, Mankin HJ, Rosenberg AE, Fletcher JA. Variant translocations involving 16q22 and 17p13 in solid variant and extraosseous forms of aneurysmal bone cyst. Genes Chromosomes Cancer. 2000;28(2):233–234. [PubMed] [Google Scholar]
- 17.Ellison DA, Sawyer JR, Parham DM, Nicholas R Jr. Soft-tissue aneurysmal bone cyst: Report of a case with t(5;17)(q33;p13) Pediatr Dev Pathol. 2007;10(1):46–49. doi: 10.2350/06-03-0070.1. [DOI] [PubMed] [Google Scholar]
- 18.Geiersbach K, Rector LS, Sederberg M, Hooker A, Randall RL, Schiffman JD, South ST. Unknown partner for USP6 and unusual SS18 rearrangement detected by fluorescence in situ hybridization in a solid aneurysmal bone cyst. Cancer Genet. 2011;204(4):195–202. doi: 10.1016/j.cancergen.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Hurd LM, Thacker MM, Okenfuss E, Duker AL, Lou Y, Harty MP, Conard K, Lian JB, Bober MB. Aneurysmal bone cysts and pathologic fracture associated with supernumerary ring chromosome 6 in two unrelated patients. Am J Med Genet A. 2017;173(12):3205–3210. doi: 10.1002/ajmg.a.38498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacquot C, Szymanska J, Nemana LJ, Steinbach LS, Horvai AE. Soft-tissue aneurysmal bone cyst with translocation t(17;17)(p13;q21) corresponding to COL1A1 and USP6 loci. Skeletal Radiol. 2015;44(11):1695–1699. doi: 10.1007/s00256-015-2205-6. [DOI] [PubMed] [Google Scholar]
- 21.Kenney B, Richkind KE, Zambrano E. Solid variant of aneurysmal bone cyst with a novel (X;9) translocation. Cancer Genet Cytogenet. 2007;178(2):155–159. doi: 10.1016/j.cancergencyto.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Pan Z, Sanger WG, Bridge JA, Hunter WJ, Siegal GP, Wei S. A novel t(6;13)(q15;q34) translocation in a giant cell reparative granuloma (solid aneurysmal bone cyst) Hum Pathol. 2012;43(6):952–957. doi: 10.1016/j.humpath.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Sciot R, Dorfman H, Brys P, Dal Cin P, De Wever I, Fletcher CD, Jonson K, Mandahl N, Mertens F, Mitelman F, Rosai J, Rydholm A, Samson I, Tallini G, Van den Berghe H, Vanni R, Willen H. Cytogenetic-morphologic correlations in aneurysmal bone cyst, giant cell tumor of bone and combined lesions. A report from the champ study group. Mod Pathol. 2000;13(11):1206–1210. doi: 10.1038/modpathol.3880224. [DOI] [PubMed] [Google Scholar]
- 24.Winnepenninckx V, Debiec-Rychter M, Jorissen M, Bogaerts S, Sciot R. Aneurysmal bone cyst of the nose with 17p13 involvement. Virchows Arch. 2001;439(5):636–639. doi: 10.1007/s004280100449. [DOI] [PubMed] [Google Scholar]
- 25.Wyatt-Ashmead J, Bao L, Eilert RE, Gibbs P, Glancy G, McGavran L. Primary aneurysmal bone cysts: 16q22 and/or 17p13 chromosome abnormalities. Pediatr Dev Pathol. 2001;4(4):418–419. doi: 10.1007/s10024-001-0035-0. [DOI] [PubMed] [Google Scholar]
- 26.Panagopoulos I, Gorunova L, Andersen HK, Pedersen TD, Lomo J, Lund-Iversen M, Micci F, Heim S. Genetic characterization of myoid hamartoma of the breast. Cancer Genomics Proteomics. 2019;16(6):563–568. doi: 10.21873/cgp.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGowan-Jordan J, Simons A, Schmid M. Karger: Basel. 2016. ISCN 2016: An international system for human cytogenomic nomenclature; p. pp 140. [Google Scholar]
- 28.Kangaspeska S, Hultsch S, Edgren H, Nicorici D, Murumagi A, Kallioniemi O. Reanalysis of rna-sequencing data reveals several additional fusion genes with multiple isoforms. PLoS One. 2012;7(10):e48745. doi: 10.1371/journal.pone.0048745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicorici D, Satalan H, Edgren H, Kangaspeska S, Murumagi A, Kallioniemi O, Virtanen S, Kikku O. Fusioncatcher – a tool for finding somatic fusion genes in paired-end RNA-sequencing data. bioRxiv. 2014 doi: 10.1101/011650. [DOI] [Google Scholar]
- 30.Paulding CA, Ruvolo M, Haber DA. The Tre2 (USP6) oncogene is a hominoid-specific gene. Proc Natl Acad Sci USA. 2003;100(5):2507–2511. doi: 10.1073/pnas.0437015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura T, Hillova J, Mariage-Samson R, Onno M, Huebner K, Cannizzaro LA, Boghosian-Sell L, Croce CM, Hill M. A novel transcriptional unit of the Tre oncogene widely expressed in human cancer cells. Oncogene. 1992;7(4):733–741. [PubMed] [Google Scholar]
- 32.Ye Y, Pringle LM, Lau AW, Riquelme DN, Wang H, Jiang T, Lev D, Welman A, Blobel GA, Oliveira AM, Chou MM. TRE17/USP6 oncogene translocated in aneurysmal bone cyst induces matrix metalloproteinase production via activation of NF-κB. Oncogene. 2010;29(25):3619–3629. doi: 10.1038/onc.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pringle LM, Young R, Quick L, Riquelme DN, Oliveira AM, May MJ, Chou MM. Atypical mechanism of NF-κB activation by TRE17/ubiquitin-specific protease 6 (USP6) oncogene and its requirement in tumorigenesis. Oncogene. 2012;31(30):3525–3535. doi: 10.1038/onc.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliveira AM, Chou MM. USP6-induced neoplasms: The biologic spectrum of aneurysmal bone cyst and nodular fasciitis. Hum Pathol. 2014;45(1):1–11. doi: 10.1016/j.humpath.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Madan B, Walker MP, Young R, Quick L, Orgel KA, Ryan M, Gupta P, Henrich IC, Ferrer M, Marine S, Roberts BS, Arthur WT, Berndt JD, Oliveira AM, Moon RT, Virshup DM, Chou MM, Major MB. USP6 oncogene promotes wnt signaling by deubiquitylating frizzleds. Proc Natl Acad Sci USA. 2016;113(21):E2945–2954. doi: 10.1073/pnas.1605691113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quick L, Young R, Henrich IC, Wang X, Asmann YW, Oliveira AM, Chou MM. JAK1-STAT3 signals are essential effectors of the USP6/TRE17 oncogene in tumorigenesis. Cancer Res. 2016;76(18):5337–5347. doi: 10.1158/0008-5472.CAN-15-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Yang H, He Y, Li T, Feng J, Chen W, Ao L, Shi X, Lin Y, Liu H, Zheng E, Lin Q, Bu J, Zeng Y, Zheng M, Xu Y, Liao Z, Lin J, Lin D. Ubiquitin-specific protease USP6 regulates the stability of the c-JUN protein. Mol Cell Biol. 2018;38(2) doi: 10.1128/MCB.00320-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erickson-Johnson MR, Chou MM, Evers BR, Roth CW, Seys AR, Jin L, Ye Y, Lau AW, Wang X, Oliveira AM. Nodular fasciitis: A novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91(10):1427–1433. doi: 10.1038/labinvest.2011.118. [DOI] [PubMed] [Google Scholar]
- 39.Patel NR, Chrisinger JSA, Demicco EG, Sarabia SF, Reuther J, Kumar E, Oliveira AM, Billings SD, Bovee J, Roy A, Lazar AJ, Lopez-Terrada DH, Wang WL. USP6 activation in nodular fasciitis by promoter-swapping gene fusions. Mod Pathol. 2017;30(11):1577–1588. doi: 10.1038/modpathol.2017.78. [DOI] [PubMed] [Google Scholar]
- 40.Bekers EM, Eijkelenboom A, Grunberg K, Roverts RC, de Rooy JWJ, van der Geest ICM, van Gorp JM, Creytens D, Flucke U. Myositis ossificans - another condition with USP6 rearrangement, providing evidence of a relationship with nodular fasciitis and aneurysmal bone cyst. Ann Diagn Pathol. 2018;34:56–59. doi: 10.1016/j.anndiagpath.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Flucke U, Bekers EM, Creytens D, van Gorp JM. COL1A1 is a fusionpartner of USP6 in myositis ossificans - fish analysis of six cases. Ann Diagn Pathol. 2018;36:61–62. doi: 10.1016/j.anndiagpath.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Lenz J, Michal M, Svajdler M, Ptakova N, Lenz D, Konecna P, Kavka M. Novel EIF5A-USP6 gene fusion in nodular fasciitis associated with unusual pathologic features: A report of a case and review of the literature. Am J Dermatopathol. 2020;42 (7):539–543. doi: 10.1097/DAD.0000000000001602. [DOI] [PubMed] [Google Scholar]
- 43.Paulson VA, Stojanov IA, Wasman JK, Restrepo T, Cano S, Plunkitt J, Duraisamy S, Harris MH, Chute DJ, Al-Ibraheemi A, Church AJ. Recurrent and novel USP6 fusions in cranial fasciitis identified by targeted RNA sequencing. Mod Pathol. 2019;33(5):775–780. doi: 10.1038/s41379-019-0422-6. [DOI] [PubMed] [Google Scholar]
- 44.Svajdler M, Michal M, Martinek P, Ptakova N, Kinkor Z, Szepe P, Svajdler P, Mezencev R, Michal M. Fibro-osseous pseudotumor of digits and myositis ossificans show consistent COL1A1-USP6 rearrangement: A clinicopathological and genetic study of 27 cases. Hum Pathol. 2019;88:39–47. doi: 10.1016/j.humpath.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Salib C, Edelman M, Lilly J, Fantasia JE, Yancoskie AE. USP6 gene rearrangement by FISH analysis in cranial fasciitis: A report of three cases. Head Neck Pathol. 2020;14(1):257–261. doi: 10.1007/s12105-019-01018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carter JM, Wang X, Dong J, Westendorf J, Chou MM, Oliveira AM. USP6 genetic rearrangements in cellular fibroma of tendon sheath. Mod Pathol. 2016;29(8):865–869. doi: 10.1038/modpathol.2016.83. [DOI] [PubMed] [Google Scholar]
- 47.Nikitovic D, Katonis P, Tsatsakis A, Karamanos NK, Tzanakakis GN. Lumican, a small leucine-rich proteoglycan. IUBMB Life. 2008;60(12):818–823. doi: 10.1002/iub.131. [DOI] [PubMed] [Google Scholar]
- 48.Hultgårdh-Nilsson A, Boren J, Chakravarti S. The small leucine-rich repeat proteoglycans in tissue repair and atherosclerosis. J Intern Med. 2015;278(5):447–461. doi: 10.1111/joim.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Appunni S, Anand V, Khandelwal M, Gupta N, Rubens M, Sharma A. Small leucine rich proteoglycans (decorin, biglycan and lumican) in cancer. Clin Chim Acta. 2019;491:1–7. doi: 10.1016/j.cca.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Matsushima N, Takatsuka S, Miyashita H, Kretsinger RH. Leucine rich repeat proteins: Sequences, mutations, structures and diseases. Protein Pept Lett. 2019;26(2):108–131. doi: 10.2174/0929866526666181208170027. [DOI] [PubMed] [Google Scholar]
- 51.Brézillon S, Pietraszek K, Maquart FX, Wegrowski Y. Lumican effects in the control of tumour progression and their links with metalloproteinases and integrins. FEBS J. 2013;280(10):2369–2381. doi: 10.1111/febs.12210. [DOI] [PubMed] [Google Scholar]
- 52.Nikitovic D, Papoutsidakis A, Karamanos NK, Tzanakakis GN. Lumican affects tumor cell functions, tumor-ecm interactions, angiogenesis and inflammatory response. Matrix Biol. 2014;35:206–214. doi: 10.1016/j.matbio.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Karamanou K, Franchi M, Vynios D, Brezillon S. Epithelial-to-mesenchymal transition and invadopodia markers in breast cancer: Lumican a key regulator. Semin Cancer Biol. 2020;62:125–133. doi: 10.1016/j.semcancer.2019.08.003. [DOI] [PubMed] [Google Scholar]