Abstract
Purpose
Biomarkers serve a number of purposes during drug development including defining the natural history of injury/disease, serving as a secondary endpoint or trigger for intervention, and/or aiding in the selection of an effective dose in humans. BIO 300 is a patent-protected pharmaceutical formulation of nanoparticles of synthetic genistein being developed by Humanetics Corporation. The primary goal of this metabolomic discovery experiment was to identify biomarkers that correlate with radiation-induced lung injury and BIO 300 efficacy for mitigating tissue damage based upon the primary endpoint of survival.
Methods
High-throughput targeted metabolomics of lung tissue from male C57L/J mice exposed to 12.5 Gy whole thorax lung irradiation, treated daily with 400 mg/kg BIO 300 for either 2 weeks or 6 weeks starting 24 h post radiation exposure, were assayed at 180 d post-radiation to identify potential biomarkers.
Results
A panel of lung metabolites that are responsive to radiation and able to distinguish an efficacious treatment schedule of BIO 300 from a non-efficacious treatment schedule in terms of 180 d survival were identified.
Conclusions
These metabolites represent potential biomarkers that could be further validated for use in drug development of BIO 300 and in the translation of dose from animal to human.
Keywords: biomarkers, genistein, lung injury, metabolomics, radiation
INTRODUCTION
Accidental or intentional exposure of individuals to high dose radiation results in acute radiation syndrome (ARS) of the hematopoietic and gastrointestinal systems (1,2). Delayed effects of acute radiation exposure (DEARE) are also significant including pulmonary pneumonitis and fibrosis (3,4). Pneumonitis is an inflammatory reaction that occurs 2 to 6 months post-exposure causing debilitating or life-threatening respiratory complications. Chronic fibrosis of the lung develops months to years post-exposure resulting in diminished quality of life and loss of function (1,3). Accordingly, there is a need to develop therapeutics with the purpose of mitigating radiation-induced lung injury to preserve lung function of individuals exposed to high-dose radiation.
Genistein, a natural component of soy, has been shown to significantly improve survival and reduce lung damage following total body irradiation or organ specific irradiation in rodents (5–7). BIO 300 is a nanosuspension pharmaceutical formulation of synthetic genistein being developed by Humanetics Corporation as a medical countermeasure (MCM) to mitigate radiation-induced pulmonary damage and dysfunction. BIO 300 is wet-nanomilled to reduce mean particle size and improve bioavailability over non-nano formulations which suffer from poor solubility and limited bioavailability (7). BIO 300 has demonstrated efficacy for preventing radiation-induced lung injury when given prophylactically or for mitigating radiation-induced lung injury when given as a post-exposure therapeutic (8,9). A recent study shows that 4 or 6 week daily treatment with 400 mg/kg BIO 300 reduces morbidity/mortality associated with radiation-induced lung injury in a mouse model by improving lung function and reducing morphological damage and airway loss due to edema, congestion, and fibrotic scarring (9,10).
As radiation injuries represent a condition for which clinical trials cannot be ethically conducted in humans, drug development must be conducted under the Federal Drug Administration (FDA) Animal Rule (11). Drug development under the FDA Animal Rule relies upon well-defined animal models and biomarkers where the potential uses of biomarkers include defining the natural history of the injury/disease, serving as a secondary endpoint or trigger for intervention, and/or aiding in the selection of an effective dose in humans (11–13). Whereas there have been numerous reports of molecules that are changed in response to various radiation exposures (14–17), as of yet, there are no FDA qualified biomarkers for any radiation-induced injury and/or for the response to therapeutics used to mitigate radiation-induced injury (18). FDA qualified biomarkers can be used as drug development tools to produce analytically valid measurements that can be relied upon to have a specific use and interpretable meaning during drug development (12).
Ionizing radiation induces DNA damage, oxidative stress, hypoxia, and inflammation which causes the dysregulation of cellular molecules including proteins, metabolites, and lipids (19,20). Endogenous metabolism can serve as an effective readout of altered biochemical pathways that contribute to radiation-induced lung injury (17). Metabolites are small molecules that are intermediates and end products of cellular processes; metabolomic responses are more immediately related to the phenotype as compared to proteomic or genomic readouts. Consequently, metabolomics provides a powerful platform for identifying molecular signatures resulting from biological response to radiation insult. Quantification of individual species can then be used systematically to assess the ability of metabolites to inform on the incidence, severity, latency and progression of radiation-induced lung injury as well as the extent of mitigation of injury by candidate MCMs. Here we used high-throughput targeted metabolomics to identify potential biomarkers for radiation-induced lung injury that could also have utility in the further development of BIO 300.
MATERIALS AND METHODS
Whole Thorax Lung Irradiation (WTLI) Mouse Model
The animal model used in this study has been described in detail elsewhere (9,10). Briefly, age matched male C57L/J mice that were 10–14 weeks old on irradiation day were irradiated with WTLI at 12.5 Gy (~LD90/180) and then administered 400 mg/kg BIO 300 (Humanetics Corporation) by daily oral gavage starting at 24 h post-irradiation for either 2 weeks (2 w) or 6 weeks (6 w). For WTLI, animals were anesthetized with 80–100 mg/kg ketamine and 10–15 mg/kg xylazine and placed in the prone position. Animals were exposed to 0 Gy (sham radiation) or 12.5 Gy of 320 kV x-rays (1.25 Gy min−1; HVL = 1 mm Cu; XRAD320, Precision X-Ray, North Branford, CT). Sham irradiated mice are noted as “sham” and mice irradiated with 12.5 Gy WTLI but untreated with BIO 300 are notated as “0 w” (both are vehicle only). Vehicle, BIO 300 nanosuspension minus the active pharmaceutical ingredient, was provided by Humanetics. Animals were monitored daily and euthanized according to predefined criteria (9). The primary end point of the study from which these samples were derived was defined as a significant improvement in survival time in the BIO 300 treated animals as compared to the control group. The secondary end points for that study were defined as reductions in major morbidity including differences in latency, incidence, severity, and duration of radiation-induced lung injury after WTLI (9). All animal work adhered to the “Principles of Laboratory Animal Care” (NIH publication #85–23, revised in 1985). All experiments were performed in compliance with the animal use protocol approved by the University of Maryland Institutional Animal Care and Use Committee.
Histology
At the time of euthanasia, a bilateral thoracotomy was performed. Gross morphology of the heart and lungs was assessed and documented. Pleural effusions were measured after which the lungs and heart were removed. Lungs were separated into left and right lobes and individually weighed. Heart weights were documented. The left lung was rinsed in PBS and inflated with 10% neutral buffered formalin and placed in 10% formalin for fixation. The right lung was snap frozen in liquid nitrogen and stored at −80°C for future analysis. Five-micron thick tissue sections were stained with hematoxylin and eosin (H&E) for assessment of tissue damage and Masson’s Trichrome for assessment of fibrosis. Brightfield acquisition of lung tissue was obtained using the tile function of Zeiss software ZEN 2011 and a Zeiss Imager.M2 AXIO microscope. Pictures were taken using a 10x Zeiss Plan-Apochromat objective. The entire region of interest was scanned with numerous focus points. Multiple images were then processed using the fuse function in Zen 2011. A typical image of a lung section would be a fusion of up to 180 high resolution images corresponding to a .czi file of approximately 1.5 GB.
High Throughput, Targeted Metabolomics
Targeted, quantitative metabolomics was performed using Biocrates AbsoluteIDQ p180 kit (Biocrates, Life Science AG, Innsbruck, Austria). The AbsoluteIDQp180 kit was prepared as described by the manufacturer. The kit is a combined flow injection analysis (FIA) and liquid chromatography (LC) tandem mass spectrometry assay. The assay quantifies up to 188 metabolites from five metabolite classes: acylcarnitines, amino acids, biogenic amines, glycerophospholipids, sphingolipids, and hexose. Internal standards, analyte derivatization and metabolite extraction are integrated into a 96-well plate kit. Metabolite detection is done via pre-selected multiple reaction monitoring (MRM) transitions.
Briefly, lung tissue was snap frozen at the time of euthanasia and stored at −80°C until analysis. Tissue was homogenized in 85:15 (methanol:ethanol, v/v) with 5 mM PBS at a ratio of 5 mg/mL. After centrifugation, 20 μL was loaded onto the 96 well kit plate and dried under a stream of nitrogen. A 5% solution of phenylisothiocyanate in ethanol:water:pyridine (1:1:1, v/v/v) was added for derivatization of biogenic amines and amino acids. Metabolite extraction was then achieved with 5 mM ammonium acetate in methanol. The FIA and LC tandem mass spectrometry platform consisted of a Shimadzu Prominence UFLC XR high-performance liquid chromatograph (HPLC) (Shimadzu, Columbia, MD) coupled to an AB Sciex QTRAP® 5500 hybrid tandem quadrupole/linear ion trap mass spectrometer (AB Sciex, Framingham, MA). The MetIQ software (Biocrates) controlled the assay workflow including sample registration, calculation of metabolite concentrations, and assay validation.
Lipid Structure Confirmation
Diacyl and ether glycerophosphatidylcholine (PCa and PCe, respectively) structures were confirmed via ultra performance liquid chromatography (UPLC) tandem mass spectrometry. Total lipid extracts from fresh mouse lung samples were prepared using a modified Bligh/Dyer protocol (21). The extracted lipids were re-suspended in methanol/chloroform (1:1, v/v) and further diluted with isopropanol/acetonitrile/water (2:1:1, v/v/v) for analysis. UPLC was performed on a Waters ACQUITY UPLC system (Milford, MA). The separation was achieved using a C18 CSH (1.7 μm; 2.1 x 100 mm) column (Waters, Milford, MA). Mobile phase A was 10 mM ammonium formate with 0.1% formic acid in water/acetonitrile (40:60, v/v) and mobile phase B was 10 mM ammonium formate with 0.1% formic acid in acetonitrile/isopropanol (10:90, v/v). The gradient was ramped from 40% to 43% B in 2 min, ramped to 50% in 0.1 min, ramped to 54% B in 9.9 min, ramped to 70% in 0.1 min, and ramped to 99% B in 5.9 min. The gradient was returned to initial conditions in 0.5 min and held for 1.9 min for column equilibration. The flow rate was 0.4 mL/min. The column was maintained at 55°C and the auto-sampler was kept at 5°C. A 2 μL injection was used for all samples. The tandem mass spectrometry experiments were performed on a Waters SYNAPT G2-S traveling wave ion-mobility enabled hybrid quadrupole time-of-flight mass spectrometer (Milford, MA). The instrument was operated in positive and negative ion mode electrospray. The capillary voltage was 2.0 kV and sampling cone voltage was 30 V. Nitrogen at a flow of 650 L/h was used as the desolvation gas with a constant desolvation temperature of 400°C. The source temperature was set at 125°C. Data were acquired over the m/z range of 100–1200. The mass spectrometer was operated in ion-mobility data independent mode (HDMSE) with alternating low- and high-collision energies. The first scan was set at low-collision energy (4 eV) and used to collect precursor ion spectra. The second scan was set at high-collision energy and ramped from 30–55 eV which was used for generation of product ion spectra. Argon gas was used for collision-induced dissociation (CID). Leucine Enkephalin was used as the lock-mass to ensure high mass accuracy data acquisition. Data were acquired and analyzed with Waters MassLynx v4.1 and MSE Data Viewer v1.2.
Statistical Analysis
Statistical analysis of the targeted metabolomics data was performed with the MetaboAnalyst web-based statistical package (22) and GraphPad Prism (v 6.03; LaJolla, CA). Mouse lungs were assigned to one of four cohorts based on irradiation and length of BIO 300 administration. The cohorts were sham (n = 4), 0 w (n = 3), 2 w (n = 3), and 6 w (n = 5).
RESULTS
The primary goal of this metabolomic discovery experiment was to identify biomarkers that correlate with radiation-induced lung injury and BIO 300 efficacy for mitigating radiation-induced lung injury based upon the primary endpoint of survival. In the study from which these samples were derived, mice were irradiated with 12.5 Gy WTLI and then treated with 400 mg/kg BIO 300 daily, starting 24 h post irradiation, for varying durations: 0 w (vehicle only), 2 w, or 6 w (9). Figure 1 shows the Kaplan Meier survival curves for 180 day survival for each treatment group. In this study, the 0 w and 2 w BIO 300 treatment groups both yielded 13% survival while the 6 w BIO 300 treatment group yielded a statistically significant, 34% increase in survival (9). Fig. S1 shows the histopathological lung tissue damage in treated and untreated mice at 180 days post-12.5 Gy WTLI, where the 6 w BIO 300 treatment displayed less overall damage than either the 2 w or 0 w cohort. Lung tissue from surviving animals at 180 d from each of the cohorts was subjected to high throughput, targeted metabolomics followed by statistical processing (multivariate, univariate, and hierarchical clustering data analysis) to identify metabolites that were responsive to MCM efficacy.
Fig. 1.
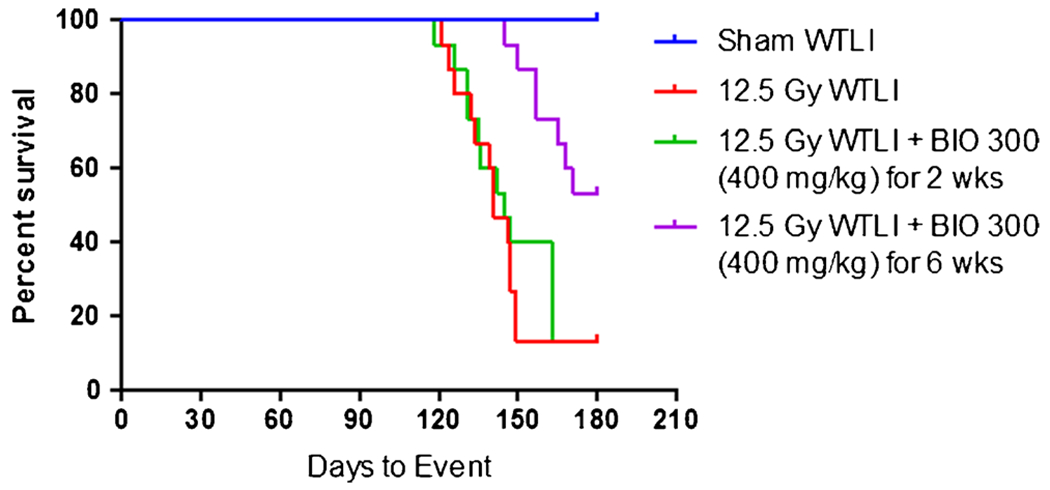
Kaplan Meier mouse survival curves for 180 day survival following 12.5 Gy WTLI with and without administration of BIO 300. Four survival curves are shown: Sham WTLI (blue), 100% survival; mice exposed to 12.5 Gy WTLI with no BIO 300 treatment (red), 13% survival; mice exposed to 12.5 Gy WTLI with 2 week daily administration of 400 mg/kg BIO 300 (green), 13% survival; mice exposed to 12.5 Gy WTLI with 6 week daily administration of 400 mg/kg BIO 300 (purple), 47% survival. Daily BIO 300 administration was initiated starting 24 h post-WTLI.
The metabolites quantified are listed in Supporting Information (SI) Table S1. A total of 188 metabolites are included in the kit. Metabolites (18 in total) designated with an * in Table S1 were removed from the data analysis for quality control purposes (e.g., internal standard was not detected, calibration curve was unacceptable). In addition to the listed metabolites, a series of ratios and sums of individual metabolite concentrations representing additional clinical or pathophysiological information were included. In some cases, using ratios and sums of metabolite concentrations offers the potential advantage for reduced biological and/or analytical variability and improved specificity. In total, there were 208 individual components which included 169 metabolites and 39 metabolite combinations that were used for data processing.
Multivariate analysis of the metabolite concentrations for the four cohorts as individual groups (sham, 0 w, 2 w, and 6 w) did not result in clear discrimination between the groups. Fig. S2 shows the principal component analysis (PCA) and partial least squares-discriminate analysis (PLS-DA) plots with these groups as individual cohorts. However, hierarchical clustering analysis via the use of a dendrogram demonstrated that samples from 6 w and sham clustered similarly and likewise 0 w and 2 w samples clustered together (Fig. S3). This clearly indicated that the metabolomic profile for lung tissue from the 6 w treatment schedule was statistically similar to sham lung tissue and both 6 w and sham metabolomics profiles were statistically different from the other two treatment schedules (0 w and 2 w) which were statistically similar to each other. With this knowledge and the goal of identifying metabolites that distinguished the difference in BIO 300 efficacy towards survival according to treatment schedule, samples from the 6 w BIO 300 treatment group with improved survival and the sham group were combined and samples from the 0 w and the 2 w BIO 300 treatment group that had no effect on survival were combined for data analysis. The PLS-DA plot of these groupings robustly discriminated the 6 w / sham cohort from the 0 w / 2 w cohort (Q2 = 0.70), with the tight clustering of the 6 w / sham group further emphasizing the similarity of the metabolomic profiles of the 6 w and sham samples (Fig. 2a). Additional statistical analysis in the form of hierarchical clustering (heatmap; Fig. 2b) and univariate analysis (volcano plot; Fig. 2c) highlighted individual metabolites that were differentially expressed between the two cohorts. There were a total of 117 metabolites and metabolite combinations that reached statistical significance when comparing the two groupings (p < 0.05; false discovery rate (FDR) < 5%; Table S2).
Fig. 2.
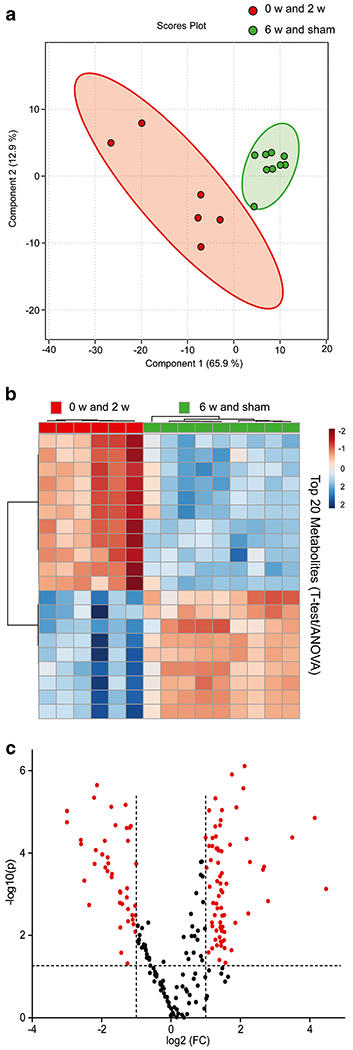
Multivariate analysis, hierarchical clustering and univariate analysis displaying statistical metabolite differences between 6 w / sham and 0 w / 2 w. (a) PLS-DA plot comparing 6 w / sham grouping (green) to 2 w / 0 w grouping (red); R2 = 0.88, Q2 = 0.70. The 95% confidence interval is indicated by the elliptical pattern per group. (b) Heatmap displaying the top 20 metabolites based on t-test/ANOVA, Pearson distancing and Ward clustering. (c) Volcano plot highlighting metabolites (red)that had a p-value <0.05 and a fold change greater than 2 when comparing the groupings of 6 w/sham to 2 w / 0 w.
A number of amino acid, glycerophosphatidylcholine (PC), and sphingomyelin (SM) metabolites and metabolite combinations according to treatment schedule are represented in Figs. 3, 4 and 5. These particular metabolites and metabolite combinations were selected according to statistical significance when comparing 6 w / sham grouping to 0 w / 2 w grouping. Bars representing the individual treatment groups are shown in each figure to highlight the individual effects of the 2 w and 6 w BIO 300 treatment as compared to the untreated and sham-irradiated; however, statistical significance is notated for differences between the 6 w / sham grouping to 0 w / 2 w grouping (Figs. 3, 4 and 5). The lung metabolites shown in Figs. 3, 4 and 5 were responsive to radiation as well as differentially responsive to BIO 300 according to the efficacy of treatment for improvement in 180 day survival. The metabolite changes also correlate with the histopathologic damage (Fig. S1), where the 6 w BIO 300 treatment effected a greater degree of repair as compared to the 2 w treatment. As such, these metabolites may be useful for reflecting the mitigation of radiation-induced lung injury by BIO 300 treatment.
Fig. 3.
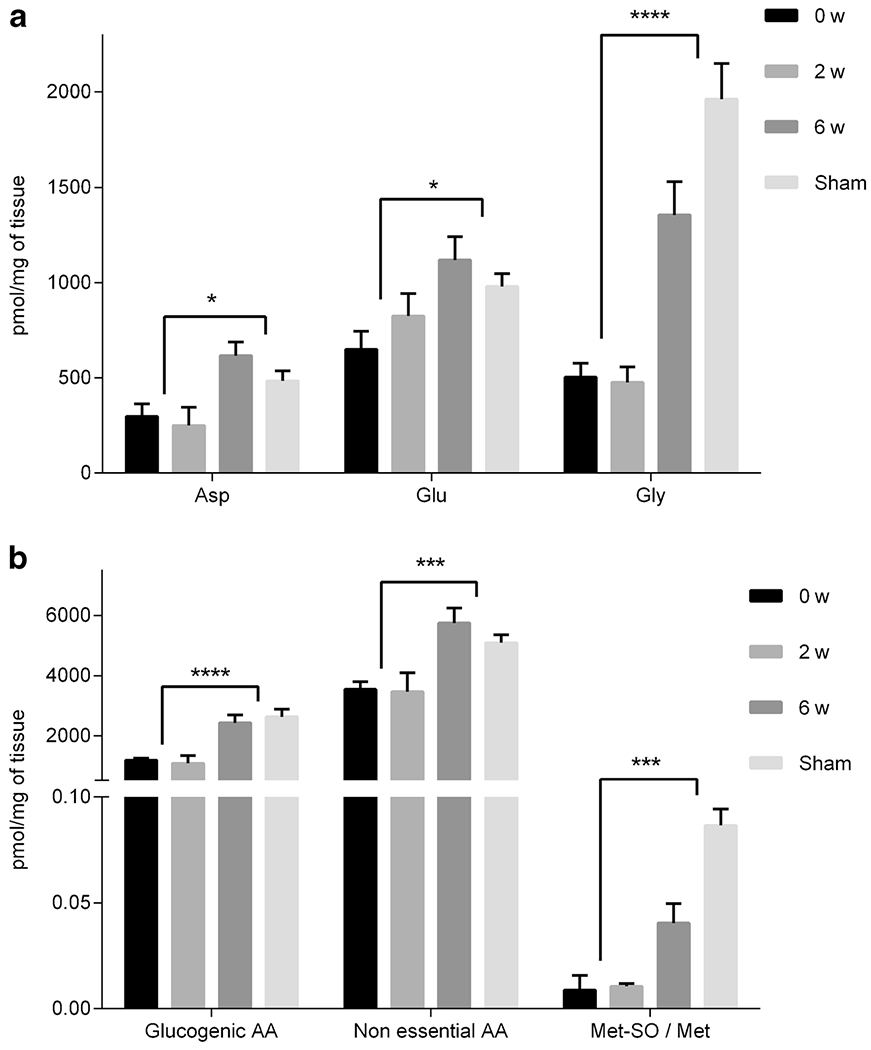
Concentrations of selected amino acids (a) and amino acid combinations (b) for lung tissue from 0 w, 2 w, 6 w, and sham groups. Concentrations are in pmol/mg of tissue and presented as mean ± standard error of mean (sem). Significance (t-test with FDR < 5%) was calculated by grouping 2 w and 0 w together and comparing those values to the grouping of 6 w and sham. * p < 0.05, ** p < 0.01, *** p < 0.005, **** p < 0.0001.
Fig. 4.
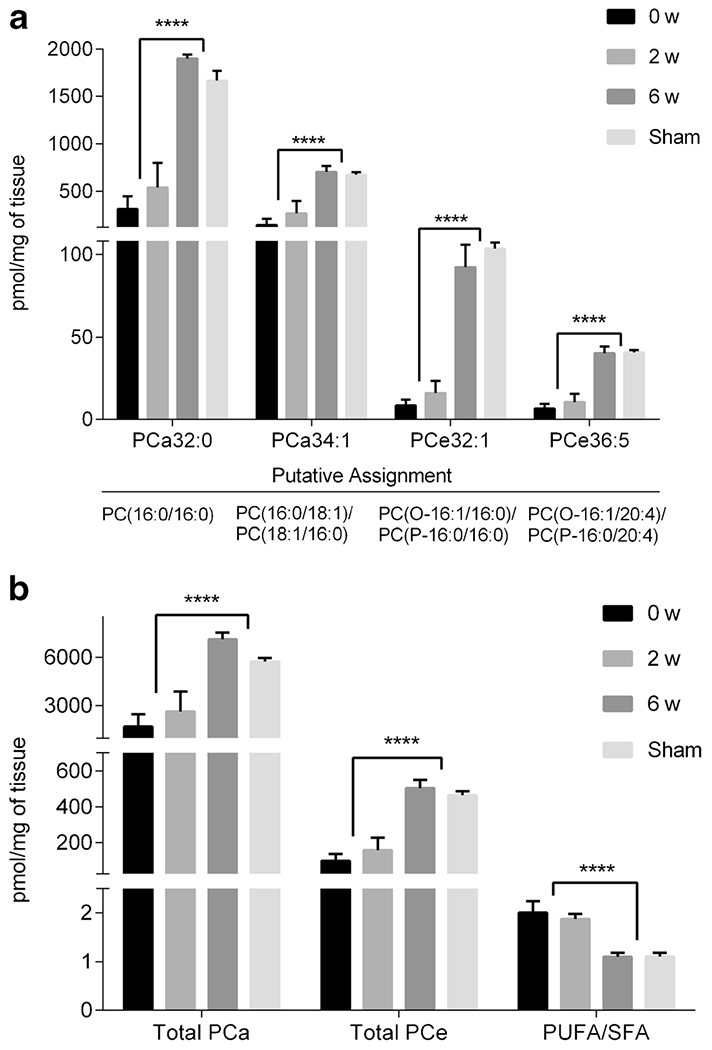
Concentrations of selected PC (a) and PC combinations (b) for lung tissue from 0 w, 2 w, 6 w, and sham groups. Concentrations are in pmol/mg of tissue and presented as mean ± sem. Significance (t-test with FDR < 5%) was calculated by grouping 2 w and 0 w together and comparing those values to the grouping of 6 w and sham. *** p < 0.005, **** p < 0.0001.
Fig. 5.
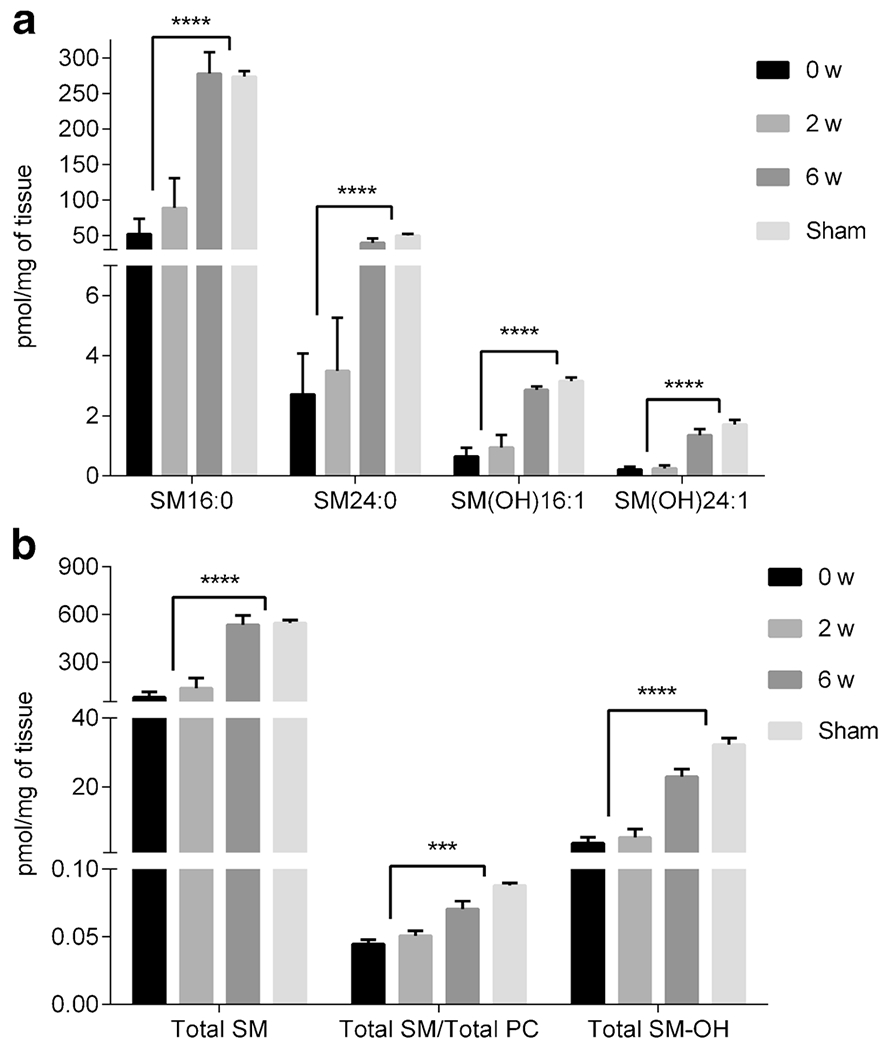
Concentrations of selected SM (a) and SM combinations (b) for lung tissue from 0 w, 2 w, 6 w, and sham groups. Concentrations are in pmol/mg of tissue and presented as mean ± sem. Significance (t-test with FDR < 5%) was calculated by grouping 2 w and 0 w together and comparing those values to the grouping of 6 w and sham. *** p < 0.005, **** p < 0.0001.
Amino acids shown in Fig. 3 included Asp, Glu, and Gly with metabolite combinations including sum of glucogenic amino acids (Ala, Gly, and Ser), sum of non-essential amino acids (Ala, Arg, Asn, Asp, Gln, Glu, Gly, Pro, Ser, and Tyr), and the ratio of methionine sulfoxide to methionine. The individual amino acids and the metabolite combinations for 6 w displayed similar levels to sham (p > 0.05 except for Met-SO/Met) and were systematically elevated when compared to 2 w and 0 w.
Graphical representation of individual PC, SM species, and combinations thereof are displayed in Figs. 4 and 5. Figure 4a shows four individual PC species including diacyl (PCa32:0, PCa34:1) and ether PC (PCe32:1, PCe36:5). Figure 4b shows the trends for total diacyl PC (Total PCa) and total ether PC (Total PCe) as well as the ratio of polyunsaturated fatty acid PC to saturated fatty acid PC (PUFA/SFA). Figure 5a highlights four individual sphingolipid species including two SM (SM16:0, SM24:0) and two hydroxylated SM (SM(OH)16:1, SM(OH)24:1). Figure 5b shows the trends for total sphingomyelins (Total SM), the ratio of total SM to total PC (Total SM/Total PC), and for total hydroxylated SM (Total SM(OH)). These metabolites or metabolite combinations showed statistically similar levels for 6 w and sham (p > 0.05 for comparison of 6 w to sham). All individual concentrations and combinations, excluding the combination of polyunsaturated PCs to fully saturated PCs (PUFA/SFA), for 6 w and sham samples had greater levels when compared to 2 w and 0 w samples.
Lipid structure impacts biological function. Structure confirmation for the diacyl and ether PC presented in Fig. 4 was pursued in order to gain insight into what acyl chains were present including the presence of alkyl and/or vinyl ethers. Structure confirmation was a result of the following criteria: 1.) chromatographic retention time on a C18 column, 2.) accurate mass (<6.5 ppm) of precursor ion, 3.) HDMSE spectra, and 4.) corresponding negative ion mode accurate mass and HDMSE spectra. Of note, HDMSE denotes that the product ion spectrum are not only time-aligned with the precursor ion spectrum by chromatographic separation but also by ion mobility drift time (23). All species in Fig. 4a have putative structure identification from a secondary lipid analysis, including PCa32:0, PCa34:1, PCe32:1, and PCe36:5 as indicated in the Fig. 4.
Figure 6 highlights an example of the use of UPLC combined with HDMSE for structure confirmation of PCe36:5. The use of a C18 chromatographic separation allowed for PCs in the total lipid extract to be separated by acyl chain hydrophobicity. This resulted in characteristic retention times based on equivalent carbon number (24) and reduced sample complexity at the point of ionization and subsequent detection. The ion at m/z 766.5786 which corresponded to PCe36:5 had a retention time of 7.62 min (Fig. 6a and 6b). A LIPID MAPS database search using a 6.5 ppm (5.0 mmu) mass tolerance for the precursor ion at m/z 766.5786 resulted in 6 possible isomeric ether PC lipid structures (Fig. 6b inset). The specific location and stereochemistry of the points of unsaturation within a particular acyl chain were not considered due to the complexity in resolving these types of positional/stereo-isomers (e.g., PC(O-16:1(9Z)/20:4(8Z,11Z,14Z,17Z) from LIPID MAPS was referred to as PC(O-16:0/20:5) with no designation of double bond location or stereochemistry). Further confirmation of the lipid structure was accomplished via the use of combined mass spectral interpretation of both the positive and negative ion mode HDMSE. The positive ion mode HDMSE mass spectrum (Fig. 6c) revealed an abundant product ion at m/z 184.1 ([PCho]+) indicating the presence of a phosphocholine head group. Less abundant product ions at m/z 480.3 and 526.3 corresponded to a neutral 20:4 acyl chain loss as a ketene and a 16:1 acyl chain as a free fatty acid. These product ions are consistent with an ether PC (PCe36:5) with the following configuration: PC(O-16:1/20:4) and PC(P-16:0/20:4). Other product ions were also observed and were associated with other PC lipids that co-eluted and co-drifted with m/z 766.5786.
Fig. 6.
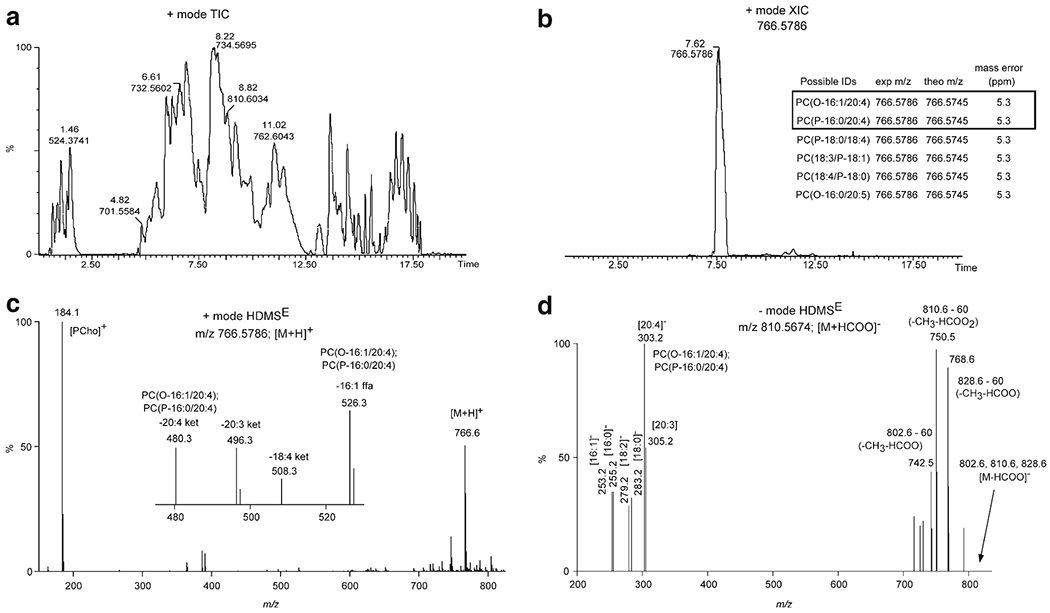
UPLC-HDMSE structure confirmation for PCe36:5. (a) Positive ion mode total ion chromatogram (TIC) of mouse lung total lipid extract. Retention time (min) and m/z value denoted for several of the most abundant chromatographic peaks. (b) Extracted ion chromatogram (XIC) for the m/z value at 766.5786. The inset table lists the possible lipid structures for accurate mass (<6.5 ppm) database searching from LIPID MAPS. PC(O-16:1/22:4) and PC(P-16:0/22:4) were the structures confirmed based on UPLC-HDMSE. (c) Positive ion mode HDMSE high-collision energy scan for m/z 766.5786 ([M + H]+ ion for PCe36:5). The inset mass spectrum is the zoomed in region for m/z 470–530. The m/z value and associated product ion or neutral loss is listed next to the peak. (d) Negative ion mode HDMSE high-collision energy scan for m/z 810.5674 ([M + HCOO]− ion for PCe36:5). The m/z value and associated product ion or neutral loss is listed next to the peak.
The putative ether PC structures noted above were confirmed by analysis of the corresponding negative ion HDMSE mass spectrum (Fig. 6d). Note, the negative ion mode precursor ion was the formate anion ([M + HCOO]− = 810.5674; tR = 7.59). PC formate anions both of diacyl and ether structures characteristically fragment upon low-energy (LE) collision-induced dissociation (CID) via the neutral loss of 60 (loss of CH3 and HCOO) and charge retention on fatty acyls (i.e., fatty acid anions) (25). Diacyl PCs are susceptible to producing fatty acid product ions at either the sn-1 or sn-2 position. In contrast, ether PCs preferentially dissociate at only the sn-1 or sn-2 position depending on the location of the ether linkage. Congruently, the product ion at m/z 750.5 corresponded to the neutral loss of 60 from m/z 810.6 and the product ion at m/z 303.2 corresponded to the fatty acid anion of [20:4]−. This was consistent with an ether PC (PCe36:5) with the following configuration: PC(O-16:1/20:4) and PC(P-16:0/20:4). The other product ions observed were associated with other PC lipids that co-eluted and co-drifted. Absolute distinction between the alkyl and vinyl ether structures was not obtained at this level of structural analysis.
Although the metabolomic profiles between 6 w and sham samples were similar when juxtaposed to 0 w and 2 w samples, Fig. 1 shows that the 6 w BIO 300 treatment does not fully mitigate the radiation-induced damage. Accordingly, we performed an isolated comparison of 6 w and sham samples to interrogate residual damage and this analysis yielded differential expression for a number of metabolites indicating that there is still some residual dysfunction in the 6 w group as compared to the sham (Fig. 7). The PLS-DA plot discriminated between 6 w and sham samples (Fig. 7a; Q2 = 0.94) and hierarchical clustering clearly delineated samples between the groups (Fig. 7b). A number of amino acids and amino acid combinations were identified to be up-regulated in the 6 w cohort (Fig. 7c and 7d). In total there were 55 metabolites and metabolite combinations that reached statistical significance when comparing 6 w samples to sham samples (p < 0.05; FDR < 5%; Table S3). These results agree with the lung histopathology in Fig. S1 which shows remaining focal areas of injury amongst large areas of normal aerated lung.
Fig. 7.
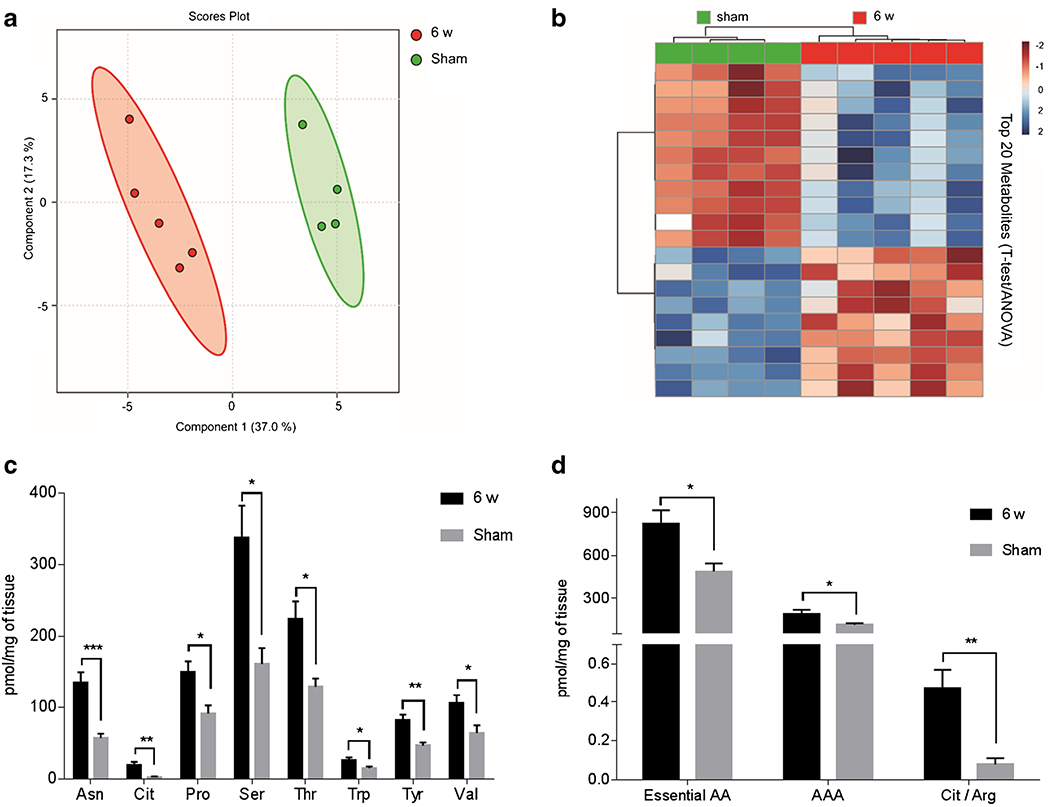
Multivariate analysis, hierarchical clustering and selected amino acid and amino acid combinations displaying statistical metabolite differences between 6 w and sham. (a) PLS-DA plot comparing 6 w (red) to sham (green); R2 = 0.98, Q2 = 0.94. The 95% confidence interval is indicated by the elliptical pattern per group. (b) Heatmap displaying the top 20 metabolites based on t-test/ANOVA, Pearson distancing and Ward clustering. (c) Concentrations of selected amino acids from 6 wand sham. (d) Concentrations of selected amino acid combinations from 6 wand sham. Concentrations are in pmol/mg of tissue and presented as mean ± sem. Significance was calculated via a t-test with FDR < 5%. * p < 0.05, ** p <0.01, *** p < 0.005.
DISCUSSION
Metabolite biomarkers are of great interest due to their relative ease to detect in biological samples and their correlation to phenotypic expression. Metabolites are a product of the preceding biological cascade of genomic and proteomic processes and, as such, their presence and abundance are intrinsically linked to phenotype making them ideal markers for disease/injury progression and drug efficacy. The investigation of metabolite biomarkers in animal models has predominantly been via the use of mass spectrometry-based techniques (14,15,17,26). Among the various approaches applied to lung, untargeted techniques are discovery-based, yield relative abundance, and require extensive identification of identified metabolites (14). In contrast, targeted techniques, although much narrower in their scope, yield metabolite quantification from a set panel of previously characterized metabolites. A common and efficient targeted approach that has been applied to various models, including lung, is the use of a kit-based assay that has a predetermined list of metabolites from a variety of metabolite classes (27–30). Such kits incorporate sample preparation into the kit and include the use of isotopically labeled internal standards. The use of a targeted, kit-based platform is advantageous for several reasons including built-in quality control for both intra- and inter-experimental consistency, streamlined sample preparation in a 96-well plate format, inclusion of internal standards that provide quantitative results, and known assignment of metabolites to biochemical pathways.
Both targeted and untargeted metabolomic approaches have been carried out previously on models of radiation-induced lung injury (14,17,31). The radiation models have consisted of various species and radiation exposures covering a range of radiological doses and times after exposure. The use of untargeted lipidomics (lipidomics is a subset of metabolomics) has recently been reported for identification of lung lipids deferentially expressed in the mouse WTLI model (14). A number of ceramide, PC, and glycerophosphoethanolamine (PE) species were identified that were differentially expressed in lung tissue 24-h following WTLI exposure. The extent to which these early lipid markers are diagnostic for the latency, incidence, severity and progression of radiation-induced lung injury is still under investigation. The lack of information regarding metabolites as biomarkers of radiation that could also be responsive to indicating MCM efficacy for mitigating radiation-induced lung injury was a motivation for this study. Towards these ends, we utilized a high throughput, targeted metabolomics approach to identify metabolites that are responsive to a specific MCM, BIO 300, in the WTLI mouse model.
We identified a number of amino acids as reflective of BIO 300 efficacy (Fig. 3). Amino acids are directly involved in a number of key biochemical processes including protein synthesis, osmoregulation, hormone secretion, gene expression, and cell signaling (32). In general, abnormal metabolism of amino acids disrupts whole body homeostasis, impairs growth, development, and immune response, and contributes to mortality (33,34). Accordingly, there is a greater appreciation that amino acids are not only the building blocks of proteins and polypeptides but amino acids are dynamic regulators of key metabolic pathways that are essential for maintenance, growth, reproduction, and immunity for general overall health (34). As such, disruption of amino acid metabolism within the lung as a result of radiation likely has profound effects on lung function. The identification of Asp, Glu, and Gly as potential markers of radiation-induced lung injury is a novel finding that may have implications for N-methyl-D-aspartate (NMDA) receptor function. The NMDA receptor is a non-specific cation channel that allows influx of Ca2+ and Na+ and efflux of K+. NMDA receptor requires the binding of glutamate and aspartate as well as the binding of co-agonist glycine for efficient opening of the ion channel portion of the receptor (35). NMDA receptors are most notably linked to neurological disorders, but have also been identified in lung tissue and associated with lung pathologies (36). These pathologies include acute lung injury induced by bleomycin (37), lipopolysaccharide (38), cecal ligation and perforation (39), and hyperoxia (40). A number of other amino acids remained dysregulated after the 6 w treatment schedule (Fig. 7) indicating that amino acid homeostasis in the lung may play a role in the mechanisms of injury and repair in the lung after exposure to high-dose radiation.
We also identified a number of lipids including diacyl PC, ether PC and SM species (Fig. 4, Fig. 5). PC are a major component of cellular membranes and perform important biological functions such as regulation of the physiochemical properties of the cell membrane and lipoproteins, involvement in cell signaling, and are essential in buffering lipid peroxidation (41). In regards to lung physiology, PC account for approximately 80% of total pulmonary surfactant phospholipids and two-thirds of whole surfactant (42). Pulmonary surfactant is vital for maintaining the proper surface tension dynamics during respiratory function by increasing lung compliance (the ability of lung tissue to stretch and expand) while preventing alveolar collapse (43). Furthermore, lung surfactant contributes to pulmonary defense mechanisms from environmental toxins, microbes and local immunomodulation (43). Radiation pneumonitis and fibrosis are consequences of radiation-induced lung injury and disruption of pulmonary surfactant is intimately involved in these sequelae. Radiation to the lungs leads to alveolar accumulation of surfactant inhibitors by disruption of the alveolar wall. This disruption hinders the alveolar epithelial type II cell function that is important for secretion of pulmonary surfactant and potentially initiates a switch from surfactant phospholipid synthesis to cell membrane repair (44). Membrane phospholipids are also targets of reactive oxygen species resulting from ionizing radiation (45). The damage incurred to the cellular membrane as a byproduct of lipid peroxidation results in disruption of both the structural and functional integrity of the membrane leading to further cell injury. Although all phospholipids are susceptible to lipid peroxidation, polyunsaturated diacyl PC and ether PC are more prone to oxidative reactions due to their abundance and lipid structure (i.e., multiple methylene groups or the enol-ether functional site) (46). Ether PC in particular has been suggested to play a role as a sacrificial oxidant, sparing lipid peroxidation of other membrane-bound lipids, in effect becoming endogenous antioxidants (47). In this work, we were able to positively confirm the presence of ether-linked lipids via additional lipid structural analyses.
Sphingolipids that correlated with BIO 300 efficacy included SM and hydroxylated SM species. SM is a component of lipid rafts, organized domains of the plasma membrane essential for cell signaling and endothelial cell stress response (48). We observed a decreased in SM species in response to radiation consistent with reports that ionizing radiation increases the activity of acid sphingomyelinase, an enzyme that metabolizes SM into ceramide species (49). Increased ceramide species resulting from the breakdown of SM disrupts lipid rafts impairing cellular signaling that leads to increased apoptosis and inflammation (50). Whereas, the pathological role of these identified lipid biomarkers is not fully understood, this repo rt provides the foundation for gaining mechanistic insight by which acute radiation exposure leads to delayed on-set lung injury.
CONCLUSION
These data provide a panel of lung metabolites that are responsive to radiation and indicative of radiation-induced lung injury. Importantly, these select metabolites are able to distinguish an efficacious treatment schedule of BIO 300 that improved 180 day survival after 12.5 Gy WTLI from a non-efficacious treatment schedule that had no impact on survival as compared to an untreated, irradiated control. As such, these metabolites represent potential biomarkers that could be further validated to be used in the continued development of BIO 300 and in the translation of dose from animal to human. The next steps in biomarker development toward FDA Biomarker Qualification would be the validation of biomarker response under various conditions to further define the biomarker-clinical endpoint relationship including the dose-dependency and biokinetics of the metabolite response post-radiation. Qualified biomarkers would be a significant and valuable drug development tool because, in addition to its development as an MCM, BIO 300 is currently in a Phase I/II clinical trial in patients undergoing radiochemotherapy for lung cancer (NCT02567799).
Supplementary Material
Acknowledgments and Disclosures.
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272201000046C. Additional support was provided by the University of Maryland School of Pharmacy Mass Spectrometry Center (SOP1841-IQB2014). The authors would like to thank the members of the Medical Countermeasures Against Radiological Threats (MCART) consortium for their dedication, support, and guidance. Additionally, we acknowledge and thank the members of the Kane laboratory. Preclinical efficacy studies were supported with Federal funds from the Biomedical Advanced Research and Development Authority, Office of the Assistant Secretary for Preparedness and Response, Department of Health and Human Services under Contract #HHSO100201100026C (Humanetics Corporation). BARDA acknowledges the presentation, data analysis, and the interpretation are the choice of the Contractor and do not necessarily represent the only possible analysis and interpretation. Drs. Kane and Jones declare no competing financial interest. Dr. Isabel L. Jackson serves as a scientific advisor for Humanetics Corporation. Dr. Zeljko Vujaskovic serves as medical advisor for Humanetics Corporation. Dr. Michael D. Kaytor is the Vice President of Research and Development for Humanetics Corporation.
ABBREVIATIONS
- ARS
Acute radiation syndrome
- CID
Collision-induced dissociation
- DEARE
Delayed effects of acute radiation exposure
- FDA
Federal Drug Administration
- FDR
False discovery rate
- FIA
Flow injection analysis
- H&E
Hematoxylin and eosin
- HDMSE
High definition mass spectrometry
- HPLC
High-performance liquid chromatography
- LC
Liquid chromatography
- LD
Lethal dose
- MCM
Medical countermeasure
- MRM
Multiple reaction monitoring
- NMDA
N-methyl-D-aspartate
- PC
Glycerophosphatidylcholine
- PCa
Diacyl glycerophosphatidylcholine
- PCA
Principal component analysis
- PCe
Ether glycerophosphatidylcholine
- PCho
Phosphocholine
- PE
Glycerophosphoethanolamine
- PLS-DA
Partial least squares-discriminate analysis
- PUFA
Polyunsaturated fatty acid
- Sem
Standard error of the mean
- SFA
Saturated fatty acid
- SM
Sphingomyelin
- TIC
Total ion chromatogram
- UPLC
Ultra performance liquid chromatography
- XIC
Extracted ion chromatogram
- WTLI
Whole thorax lung irradiation
Footnotes
Electronic supplementary material The online version of this article (doi: 10.1007/s11095-017-2200-9) contains supplementary material, which is available to authorized users.
REFERENCES
- 1.Dorr H, Meineke V. Acute radiation syndrome caused by accidenttal radiation exposure - therapeutic principles. BMC med. 2011;9: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacVittie TJ, Farese AM, Jackson W III. The hematopoietic syndrome of the acute radiation syndrome in rhesus macaques: a systematic review of the lethal dose response relationship. Health Phys. 2015;109(5):342–66. [DOI] [PubMed] [Google Scholar]
- 3.Van Dyk J, Keane TJ, Kan S, Rider WD, Fryer CJ. Radiation pneumonitis following large single dose irradiation: a re-evaluation based on absolute dose to lung. Int J Radiat Oncol Biol Phys. 1981;7(4):461–7. [DOI] [PubMed] [Google Scholar]
- 4.Mah K, Van Dyk J. Quantitative measurement of changes in human lung density following irradiation. Radiother Oncol. 1988;11(2):169–79. [DOI] [PubMed] [Google Scholar]
- 5.Day RM, Barshishat-Kupper M, Mog SR, McCart EA, Prasanna PG, Davis TA, et al. Genistein protects against biomarkers of delayed lung sequelae in mice surviving high-dose total body irradiation. J Radiat res. 2008;49(4):361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calveley VL, Jelveh S, Langan A, Mahmood J, Yeung IW, Van Dyk J, et al. Genistein can mitigate the effect of radiation on rat lung tissue. Radiat res. 2010;173(5):602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Z, Kulkarni K, Zhu W, Hu M. Bioavailability and pharma-cokinetics of genistein: mechanistic studies on its ADME. Anti Cancer Agents med Chem. 2012;12(10):126–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ha CT, Li XH, Fu D, Xiao M, Landauer MR. Genistein nanoparticles protect mouse hematopoietic system and prevent proinflammatory factors after gamma irradiation. Radiat res. 2013;180(3):316–25. [DOI] [PubMed] [Google Scholar]
- 9.Jackson IL, Zodda A, Gurung G, Pavlovic R, Kaytor MD, Kuskowski MA, Vujaskovic Z. BIO 300, a nanosuspension of Genistein, mitigates pneumonitis/fibrosis following high dose radiation exposure in the C57L/J murine model. Br J Pharmacol 2017. (in review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson IL, Xu P, Hadley C, Katz BP, McGurk R, Down JD, et al. A preclinical rodent model of radiation-induced lung injury for medical countermeasure screening in accordance with the FDA animal rule. Health Phys. 2012;103(4):463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Product development under the animal rule: Guidance for industry. U.S. Department of Health and Human Services, Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) 2015. Available from: https://www.fda.gov/downloads/drugs/guidances/ucm399217.pdf. [Google Scholar]
- 12.Guidance for industry and FDA staff: Qualification process for drug development tools. U.S. Department of Health and Human Services, Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) 2014. Available from: https://www.fda.gov/downloads/drugs/guidances/ucm230597.pdf. [Google Scholar]
- 13.Biomarkers Definitions Working G. Biomarkers and surrogate end-points: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. [DOI] [PubMed] [Google Scholar]
- 14.Jones JW, Carter CL, Li F, Yu J, Pierzchalski K, Jackson IL, et al. Ultraperformance convergence chromatography-high resolution tandem mass spectrometry for lipid biomarker profiling and identification. Biomed Chromatogr. 2017;31(3):e3822. doi: 10.1002/bmc.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyburski JB, Patterson AD, Krausz KW, Slavik J, Fornace AJ Jr, Gonzalez FJ, et al. Radiation metabolomics. 1. Identification of minimally invasive urine biomarkers for gamma-radiation exposure in mice. Radiat res. 2008; 170(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ossetrova NI, Sandgren DJ, Blakely WF. Protein biomarkers for enhancement of radiation dose and injury assessment in nonhuman primate total-body irradiation model. Radiat Prot Dosim. 2014;159(1–4):61–76. [DOI] [PubMed] [Google Scholar]
- 17.Jones JW, Scott AJ, Tudor G, Xu PT, Jackson IL, Vujaskovic Z, et al. Identification and quantitation of biomarkers for radiation-induced injury via mass spectrometry. Health Phys. 2014;106(1): 106–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biomarkers Used as Outcomes in Development of FDA-Approved Therapeutics (October 2007-December 2015) [10/18/2016]. Available from: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/ucm483052.htm.
- 19.Mukherjee D, Coates PJ, Lorimore SA, Wright EG. Responses to ionizing radiation mediated by inflammatory mechanisms. J Pathol. 2014;232(3):289–99. [DOI] [PubMed] [Google Scholar]
- 20.Fleckenstein K, Zgonjanin L, Chen L, Rabbani Z, Jackson IL, Thrasher B, et al. Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Int J Radiat Oncol Biol Phys. 2007;68(1):196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter CL, Jones JW, Farese AM, MacVittie TJ, Kane MA. Inflation-fixation method for Lipidomic mapping of lung biopsies by matrix assisted laser desorption/ionization-mass spectrometry imaging. Anal Chem. 2016;88(9):4788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia J, Wishart DS. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr Protoc Bioinformatics. 2016;55(14.10):1–14. [DOI] [PubMed] [Google Scholar]
- 23.Castro-Perez J, Roddy TP, Nibbering NM, Shah V, McLaren DG, Previs S, et al. Localization of fatty acyl and double bond positions in phosphatidylcholines using a dual stage CID fragmentation coupled with ion mobility mass spectrometry. J am Soc Mass Spectrom. 2011;22(9): 1552–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lisa M, Cifkova E, Holcapek M. Lipidomic profiling of biological tissues using off-line two-dimensional high-performance liquid chromatography-mass spectrometry. J Chromatogr a. 2011;1218(31):5146–56. [DOI] [PubMed] [Google Scholar]
- 25.Houjou T, Yamatani K, Nakanishi H, Imagawa M, Shimizu T, Taguchi R. Rapid and selective identification of molecular species in phosphatidylcholine and sphingomyelin by conditional neutral loss scanning and MS3. Rapid Commun Mass Spectrom. 2004;18(24):3123–30. [DOI] [PubMed] [Google Scholar]
- 26.Pannkuk EL, Fornace AJ Jr, Laiakis EC. Metabolomic applications in radiation biodosimetry: exploring radiation effects through small molecules. Int J Radiat Biol. 2017;12:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunelli L, Caiola E, Marabese M, Broggini M, Pastorelli R. Comparative metabolomics profiling of isogenic KRAS wild type and mutant NSCLC cells in vitro and in vivo. Sci Report. 2016;6: 28398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnackenberg LK, Pence L, Vijay V, Moland CL, George N, Cao Z, et al. Early metabolomics changes in heart and plasma during chronic doxorubicin treatment in B6C3F1 mice. J Appl Toxicol. 2016;36(11): 1486–95. [DOI] [PubMed] [Google Scholar]
- 29.Qiu Y, Zhou B, Su M, Baxter S, Zheng X, Zhao X, et al. Mass spectrometry-based quantitative metabolomics revealed a distinct lipid profile in breast cancer patients. Int J Mol Sci. 2013;14(4): 8047–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conlon TM, Bartel J, Ballweg K, Gunter S, Prehn C, Krumsiek J, et al. Metabolomics screening identifies reduced L-carnitine to be associated with progressive emphysema. Clin Sci (Lond.). 2016;130(4):273–87. [DOI] [PubMed] [Google Scholar]
- 31.Carter CL, Jones JW, Barrow K, Kieta K, Taylor-Howell C, Kearney S, et al. A MALDI-MSI approach to the characterization of radiation-induced lung injury and medical countermeasure development. Health Phys. 2015;109(5):466–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu G Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37(1):1–17. [DOI] [PubMed] [Google Scholar]
- 33.Orlando GF, Wolf G, Engelmann M. Role of neuronal nitric oxide synthase in the regulation of the neuroendocrine stress response in rodents: insights from mutant mice. Amino Acids. 2008;35(1):17–27. [DOI] [PubMed] [Google Scholar]
- 34.Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc Rhoads J, et al. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37(1):153–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen PE, Geballe MT, Stansfeld PJ, Johnston AR, Yuan H, Jacob AL, et al. Structural features of the glutamate binding site in recom-binant NR1/NR2A N-methyl-D-aspartate receptors determined by site-directed mutagenesis and molecular modeling. Mol Pharmacol. 2005;67(5):1470–84. [DOI] [PubMed] [Google Scholar]
- 36.Said SI, Berisha HI, Pakbaz H. Excitotoxicity in the lung: N-methyl-D-aspartate-induced, nitric oxide-dependent, pulmonary edema is attenuated by vasoactive intestinal peptide and by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S a. 1996;93(10):4688–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Liu Y, Peng X, Liu W, Zhao F, Feng D, et al. NMDA receptor antagonist attenuates bleomycin-induced acute lung injury. PLoS One. 2015;10(5):e0125873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.da Cunha AA, Pauli V, Saciura VC, Pires MG, Constantino LC, de Souza B, et al. N-methyl-D-aspartate glutamate receptor blockade attenuates lung injury associated with experimental sepsis. Chest. 2010;137(2):297–302. [DOI] [PubMed] [Google Scholar]
- 39.da Cunha AA, Nunes FB, Lunardelli A, Pauli V, Amaral RH, de Oliveira LM, et al. Treatment with N-methyl-D-aspartate receptor antagonist (MK-801) protects against oxidative stress in lipopolysaccharide-induced acute lung injury in the rat. Int Immunopharmacol. 2011;11(6):706–11. [DOI] [PubMed] [Google Scholar]
- 40.Tang F, Yue S, Luo Z, Feng D, Wang M, Qian C, et al. Role of N-methyl-D-aspartate receptor in hyperoxia-induced lung injury. Pediatr Pulmonol. 2005;40(5):437–44. [DOI] [PubMed] [Google Scholar]
- 41.Engelmann B, Brautigam C, Thiery J. Plasmalogen phospholipids as potential protectors against lipid peroxidation of low density lipoproteins. Biochem Biophys res Commun. 1994;204(3):1235–42. [DOI] [PubMed] [Google Scholar]
- 42.Harwood JL. Lung surfactant. Prog Lipid res. 1987;26(3):211–56. [DOI] [PubMed] [Google Scholar]
- 43.Griese M Pulmonary surfactant in health and human lung diseases: state of the art. Eur Respir J. 1999;13(6): 1455–76. [DOI] [PubMed] [Google Scholar]
- 44.Finkelstein JN. Physiologic and toxicologic responses of alveolar type II cells. Toxicology. 1990;60(1–2):41–52. [DOI] [PubMed] [Google Scholar]
- 45.Agrawal A, Kale RK. Radiation induced peroxidative damage: mechanism and significance. Indian J Exp Biol. 2001;39(4):291–309. [PubMed] [Google Scholar]
- 46.Catala A Lipid peroxidation of membrane phospholipids gen-erates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids. 2009; 157(1):1–11. [DOI] [PubMed] [Google Scholar]
- 47.Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta. 2012;1822(9): 1442–52. [DOI] [PubMed] [Google Scholar]
- 48.Niaudet C, Bonnaud S, Guillonneau M, Gouard S, Gaugler MH, Dutoit S, et al. Plasma membrane reorganization links acid sphingomyelinase/ceramide to p38 MAPKpathways in endothelial cells apoptosis. Cell Signal. 2017;33:10–21. [DOI] [PubMed] [Google Scholar]
- 49.Corre I, Guillonneau M, Paris F. Membrane signaling induced by high doses of ionizing radiation in the endothelial compartment. Relevance in radiation toxicity. Int J Mol Sci. 2013; 14(11):22678–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22(37):5897–906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


