Summary
Background
The development of an effective vaccine against Zika virus remains a public health priority. A Zika purified inactivated virus (ZPIV) vaccine candidate has been shown to protect animals against Zika virus challenge and has proven well tolerated and immunogenic in humans through 8 weeks of follow up. Here we assessed the safety and immunogenicity of ZPIV in humans through 52 weeks of follow up when given via standard or accelerated vaccination schedules.
Methods
We conducted a single-center, double-blind, sequential-group, randomized controlled trial at a research clinic in Boston (MA, USA) to evaluate ZPIV given intramuscularly at weeks 0 and 4 (Schedule 1), weeks 0 and 2 (Schedule 2), or week 0 alone (Schedule 3). Participants were healthy adults aged 18–50 years with no known history of flavivirus vaccination or infection, and were enrolled in Schedule 1 first (N=12), followed by Schedule 2 (N=12) and then Schedule 3 (N=12). Within each Schedule, participants were randomized to a sub-group (vaccine or placebo) based on a computer-generated randomization schedule prepared before the study. The 12 participants in each group were randomized in a ratio of five vaccine to one placebo recipient. The primary objectives were safety through 4 weeks after final dose, and immunogenicity at observed peak (the highest level observed for each participant across all time-points) and week 28. We recorded adverse events (AEs) and Zika-specific neutralizing antibody titers for 52 weeks; titers ≥ 100 were considered a positive response. All participants who received ZPIV or placebo, and for whom any post-dose AE or immunogenicity data was available, were included in the safety and observed peak immunogenicity analysis populations, respectively. The week 28 immunogenicity analysis population consisted of all participants who received ZPIV or placebo and had immunogenicity data available at week 28. The trial was registered at ClinicalTrials.gov, number NCT02937233.
Findings
We enrolled 36 participants between Dec 8, 2016 and May 17, 2017. Twelve participants were assigned to each Schedule. There were no serious or grade 3 related AEs. The most common reactions among participants who received vaccine were injection site pain (n=24 [80%]), fatigue (n=16 [53%]), and headache (n=14 [46%]). In the Schedule 1 group, 10/10 participants (100%) who received the vaccine had a positive response, with observed peak geometric mean titers (GMT) of 1154 (95% confidence interval (CI) 455–2925). At week 28 in the Schedule 1 group, 1/8 participants (12·5%) who received the vaccine had a positive response, and GMT declined to 14 (95% CI 4–55). In the Schedule 2 group, 8/10 participants (80%) who received the vaccine had a positive response, with observed peak GMT of 518 (95% CI 143–1876). At week 28 in the Schedule 2 group, 0/7 participants (0%) who received the vaccine had a positive response, and GMT declined to 7 (95% CI 4–12). In the Schedule 3 group, 0/10 participants (0%) who received the vaccine had a positive response, with observed peak GMT of 6 (95% CI 4–11); at week 28, no titers were detectable in any Schedule 3 participant. For all vaccine schedules, GMT peaked two weeks following final vaccination and declined below 100 by study week 16. There was no difference in observed peak GMT between the standard four-week and the accelerated two-week boosting regimens (GMT 1154 vs. GMT 518, Wilcoxon p=0·4494).
Interpretation
ZPIV was safe and well tolerated in humans through 52 weeks of follow up. ZPIV immunogenicity required two doses and had limited durability.
Introduction
Mosquito-borne flaviviruses such as Zika virus (ZIKV), dengue virus (DENV) and yellow fever virus (YFV) cause repeated outbreaks with significant morbidity and mortality(1–3). The 2015–2016 Zika virus epidemic in the Americas was particularly notable for congenital Zika syndrome, in which thousands of children were born with severe developmental problems as a result of prenatal Zika infection(4–6). The development of a safe and effective Zika vaccine is therefore a global public health priority(7, 8). Several vaccine candidates were rapidly moved into clinical trials, including a Zika purified inactivated virus (ZPIV) vaccine, a formalin-inactivated whole virus vaccine produced by Walter Reed Army Institute of Research (WRAIR), Silver Spring, MD. Three ZPIV trials were conducted addressing unique questions, but all three trials included a two-dose prime-boost regimen at a schedule of week 0 and week 4. Interim results of this two-dose regimen from all three studies were published in February 2018 showing ZPIV to be safe and immunogenic through 8 weeks of follow up(9).
Here we present the final results of the ZPIV clinical trial conducted at Beth Israel Deaconess Medical Center (BIDMC), Boston, MA. The focus of this study was to explore the ZPIV vaccination schedule. In addition to testing a two-dose ZPIV schedule at week 0 and week 4, which is a standard regimen for purified inactivated flavivirus vaccines(10–12), we also examined the safety and immunogenicity of a one-dose vaccine and a two-dose schedule at week 0 and week 2. The goal of our study was to determine if an accelerated ZPIV schedule could induce Zika-specific immune responses more rapidly than the standard vaccine schedule, without loss of magnitude or durability. In an outbreak setting, it is critical to elicit protective immunity as quickly as possible. An accelerated vaccine schedule could be beneficial to the general population living in a Zika outbreak, as well as first responders who are deployed to those areas. A one-dose regimen would have the added benefit of not requiring any follow up visits.
Methods
Study design and participants
We performed a single-center, randomized, double-blind, sequential-group, placebo-controlled trial at BIDMC in Boston, Massachusetts. The protocol was approved by the BIDMC Institutional Review Board and the Human Research Protection Office of the US Army Medical Research and Materiel Command. The study was registered at ClinicalTrials.gov, number NCT02937233. Eligible participants were healthy, 18–50 years old, and without a history of known flavivirus infection or previous receipt of flavivirus vaccine. All participants gave written informed consent and successfully completed a test of understanding before the initiation of study procedures. Full eligibility criteria are described in the appendix (pp 220–22).
The study evaluated the safety and immunogenicity of a formalin-inactivated, alum-adjuvanted ZPIV vaccine administered by a standard or accelerated prime-boost regimen to healthy adults. Three vaccination schedules were evaluated. Each participant received either placebo or ZPIV according to either a one-dose schedule (schedule 3) or two-dose schedules at 0/4 weeks (schedule 1) or 0/2 weeks (schedule 2).
Randomization and masking
Participants were enrolled in Schedule 1 first (N=12), followed by Schedule 2 (N=12) and then Schedule 3 (N=12). Within each Schedule, participants were randomized to a sub-group (vaccine or placebo) based on a computer-generated randomization schedule prepared before the study. Participants were assigned a unique code that dictated the sub-group assignments. The sponsor, clinical staff, investigators, participants, and laboratory personnel were blinded to sub-group assignment until study completion. There was no blinding regarding Schedule assignment. The pharmacist with primary responsibility for study product preparation and dispensing was not blinded to the Schedule or sub-group assignment. To preserve blinding, he/she placed an overlay on the syringes. The 12 participants in each group were randomized in a ratio of five vaccine to one placebo recipient.
Procedures
Participants received one or two doses of vaccine or placebo at weeks 0/4, weeks 0/2, or week 0 alone. ZPIV contains a chromatographic-column-purified, formalin-inactivated Zika virus strain (PRVABC59) that includes all Zika structural proteins and genomic RNA. The dose was 5 mcg ZPIV and 500 mcg aluminum hydroxide adjuvant (Alhydrogel) per 0·5 mL injection. Placebo consisted of 0·9% saline per 0·5 mL injection. All study products were administered as intramuscular injections into the deltoid (left or right). No local or topical anesthetic was used prior to the injections.
Local and systemic reactogenicity safety data were collected for seven days after each vaccine or placebo administration (see Schedule of Procedures, appendix p. 262–264). Data on unsolicited adverse events (AEs) were collected following the first vaccination until 28 days following last vaccination. Neurologic and neuroinflammatory disorders (e.g., Guillain-Barré syndrome) were considered Adverse Events of Special Interest (AESI). Data on AESI and serious adverse events (SAEs) were collected through Study Day 364. Blood samples for serum chemistry and hematology were collected at 9–12 timepoints throughout the study (depending on Schedule assignment), as were urine samples for pregnancy testing and urinalysis (appendix pp 262–64). Medical monitoring was provided by a Protocol Safety Review Team, an independent Safety Monitoring Committee (SMC), and an independent research monitor appointed by the U.S. Department of Defense. Local and systemic adverse events were graded by the NIAID Division of Microbiology and Infectious Diseases Adult Toxicity Table (www.niaid.nih.gov/sites/default/files/dmidadulttox.pdf). Peripheral blood was collected to determine Zika-specific immune responses through Study Day 364.
Serum neutralizing antibody titers against ZIKV were measured by ZIKV microneutralization (MN) assay using the vaccine-matched ZIKV-PR (PRVABC59) strain, as previously described(9, 13, 14). Neutralization titer was expressed as MN50 titer, e.g. the reciprocal of the serum dilution capable of neutralizing 50 percent of the test virus dose. A positive response was defined as an MN50 titer ≥ 100, as this titer has been determined to be the threshold for complete protection in previous animal studies, where adoptive transfer of vaccine-elicited antibodies at MN50 60 protected against Zika viremia in mice and non-human primates(8, 13, 14). Baseline serum was retrospectively screened for ZIKV, DENV, YFV, Japanese encephalitis virus (JEV) and West Nile virus (WNV). Serum IgG antibodies to ZIKV Env and NS1 were measured by enzyme-linked immunosorbent assays (ELISAs) using commercially available kits (Alpha Diagnostics) as described(15), as well as in-house at BIDMC using Env and NS1 proteins from the SPH2015 strain. ZIKV-specific T cell responses were measured by interferon-gamma (IFNγ) enzyme-linked immunospot (ELISpot) assays, as previously described(16), using pools of ZIKV Cap, ENV, NS1 and prM peptides (JPT, Berlin, Germany). The threshold for a positive ELISpot response was the mean plus 2 standard deviations of a Zika negative sample set. All immunogenicity assays were conducted in a blinded fashion (see Immunogenicity Assays, appendix p. 239–240).
Endpoints
The primary endpoints were regarding safety and tolerability: (a) incidence, intensity, and relationship to vaccination of solicited local and systemic AEs during the seven-day follow-up period (days one to seven) after each ZPIV dose, (b) incidence, intensity, and relationship to vaccination of unsolicited AEs during the 28-day follow-up period (days one to 29) after each ZPIV dose, (c) Grade 2 and Grade 3 laboratory abnormalities at day eight after each ZPIV dose, and (d) incidence of SAEs (including AESIs) and related AEs from day one to day 364. The secondary endpoints were regarding humoral immune responses: proportion (95% CI) of participants per dose group with positive responses and mean response (e.g. GMT) per group with 95% CI for (a) ZIKV MN50 titers at observed peak immunogenicity (e.g., the highest NAb level observed across all time-points) and at six months from day one, and (b) Zika Env-specific endpoint ELISA titers at observed peak immunogenicity and at six months from day one. At study initiation, the six month time-point took place on day 180 (week 26), but this was later amended to day 196 (week 28) to harmonize timepoints with other concurrent ZPIV trials. Additional endpoints included plaque reduction neutralization test titers, durability and kinetics of humoral immune response (through day 364), and cellular immunogenicity. Endpoints for placebo recipients were pooled into a single “Placebo” group.
Statistical analysis
The sample size was not determined by formal hypothesis testing; rather it was selected to provide an assessment of the safety and tolerability of the different vaccine or placebo regimens. The statistical analysis of safety data followed the intention-to-treat principle, including all participants that were randomized and received at least one vaccine or placebo dose. For each vaccine or placebo regimen, the number and proportion of participants experiencing AEs, SAEs and laboratory abnormalities were tabulated, with the denominator including only participants with data available. Immunogenicity outcomes were log transformed and described using geometric mean titer (GMT) and 95% confidence intervals (CIs). Group assignment was known, however, investigators were blinded to active or placebo vaccination status. Two pre-specified blinded interim analyses of safety and immunogenicity (including assay feasibility) was performed for each group once data was collected 28 days and six months post-final vaccination. Interim analyses were reported on Apr 25, 2017 (Group 1 immunogenicity), May 5, 2017 (Group 1 safety), Sep 14, 2017 (Groups 2 and 3 safety) and May 8, 2018 (safety and immunogenicity, all groups). A final analysis was performed when day 364 data were collected (including some delayed six month immunogenicity data for Group 3) and reported on Sep 28, 2018. Peak post-vaccination responses were calculated as the maximum at any time point post vaccination and compared between three active groups and the collapsed placebo using Kruskal Wallis tests (significance level 0·05) followed by pairwise group comparisons using Wilcoxon rank sum tests without multiplicity adjustments. Missing data was not imputed. SAS v9.4 was used for final statistical analysis. Group 1 data through week 8 of follow up was included in a preliminary report published in February, 2018 (9).
Role of the funding source
The funding source was the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. in affiliation with WRAIR. The funders were involved in the study design, study operations, data collection, data analysis, data interpretations as well as reviewing and editing the manuscript. The ZPIV program leads (KM, NLM), BIDMC study leads (KES, DHB), and Emmes data and statistical leads (JT, PD) had access to all the data. The BIDMC study leads (KES, DHB) had final responsibility for the decision to submit for publication.
Results
Seventy-three (73) participants were screened, and 32 were determined to be ineligible. The first participant was enrolled on Dec 8, 2016, the last participant was enrolled on May 17, 2017, and the last study visit was completed on Jun 4, 2018. The most common reason for screen failure was lack of availability for the duration of the trial (Supplemental Table 1, appendix p. 2). Thirty-six (36) participants were randomized and received at least one dose of vaccine or placebo (Figure 1). Twelve participants received only one dose of vaccine and 24 participants received two doses of vaccine (20 ZPIV and four placebo) per protocol. One placebo participant voluntarily withdrew on day 7 and did not contribute any post-vaccination immunogenicity data. Pre-vaccination results for that participant are reported but they are excluded from post-vaccination time points and peak calculations. Demographics of the study population are described in Table 1. Age, ethnicity, and race were comparable across groups. Four participants were seropositive for flavivirus infection on retrospective serologic testing. One participant in Schedule 1 was positive for all four serotypes of DENV, as well as WNV. Two participants in Schedule 2 were positive for DENV serotype 2 (N=1) and YFV (N=1). One placebo recipient was positive for JEV.
Figure 1: Trial profile.
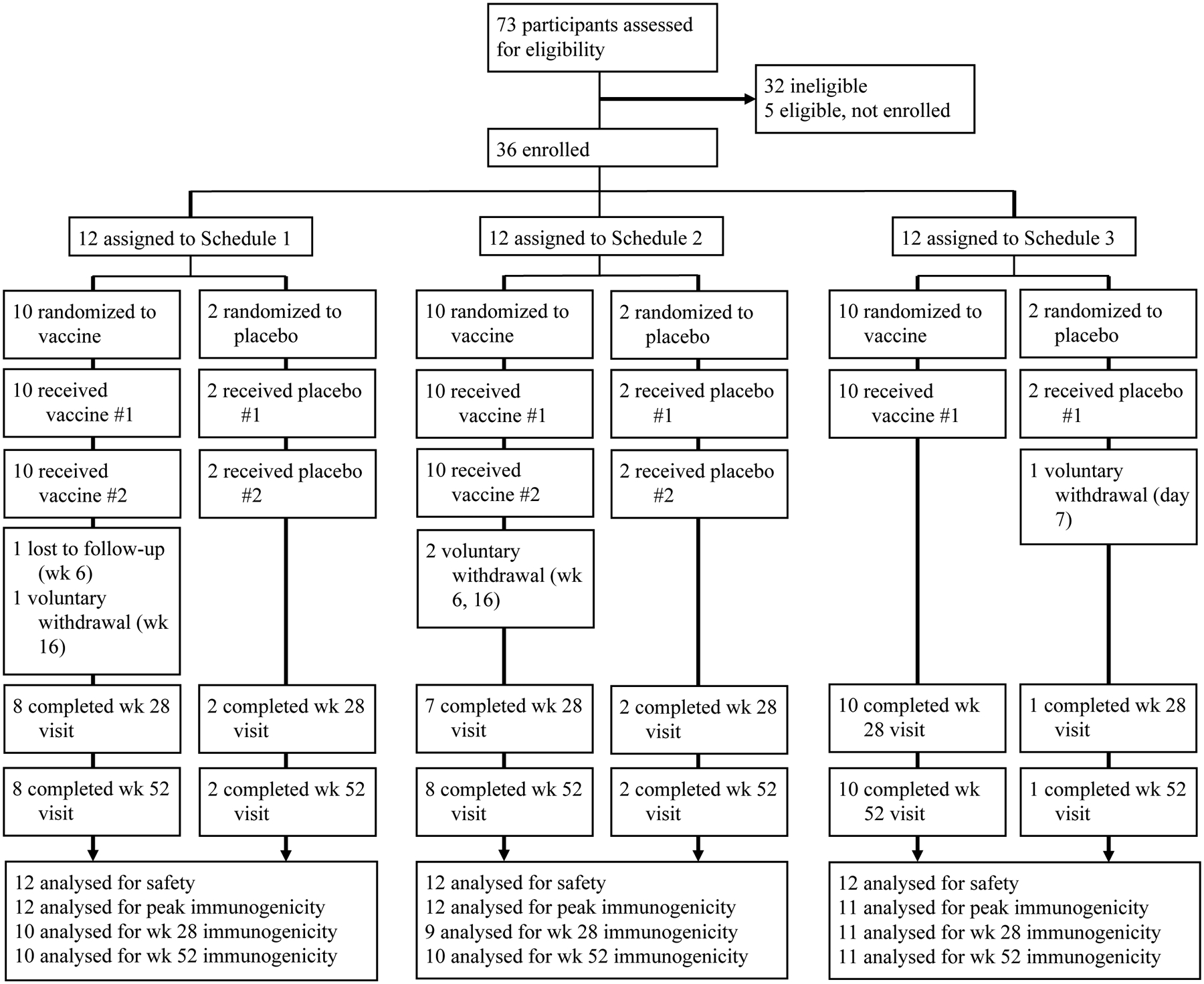
Z001 clinical study.
Table 1:
Baseline characteristics of the intention-to-treat population
| Schedule 1 (n=10) | Schedule 2 (n=10) | Schedule 3 (n=10) | Placebo (n=6) | |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (SD) | 28·8 (6·6) | 25·7 (8·3) | 25·1 (7·2) | 23·2 (5·6) |
| Range | 18–38 | 19–46 | 19–42 | 19–33 |
| Sex | ||||
| Female | 5 (50%) | 7 (70%) | 8 (80%) | 5 (83·3) |
| Male | 5 (50%) | 3 (30%) | 2 (20%) | 1 (16·7%) |
| Race | ||||
| White | 7 (70%) | 5 (50%) | 7 (70%) | 3 (50%) |
| Black or African American | 1 (10%) | 1 (10%) | 0 | 1 (16·7%) |
| Asian | 0 | 2 (20%) | 2 (20%) | 2 (33·3%) |
| Other | 0 | 2 (20%) | 1 (10%) | 0 |
| Multiple | 2 (20%) | 0 | 0 | 0 |
| Ethnicity | ||||
| Hispanic or Latino | 1 (10%) | 0 | 1 (10%) | 0 |
| Not Hispanic or Latino | 9 (90%) | 10 (100%) | 9 (90%) | 6 (100%) |
| Flavivirus Positive | ||||
| Yes | 1 (10%) | 2 (20%) | 0 | 1 (16·7%) |
| No | 9 (90%) | 8 (80%) | 10 (100%) | 5 (83.3%) |
One participant from Schedule 3 was unblinded early due to public health concerns. The participant donated platelets six hours following ZPIV administration, and the American Red Cross detected Zika virus RNA in her donated sample using the Hologic Procleix Zika Virus Assay (an investigational ultrasensitive nucleic acid test)(17). The study team was made aware of the donation by the Blood Safety Taskforce at the U.S. Centers for Disease Control after an investigation by the Massachusetts Department of Public Health (DPH) into the donor’s potential Zika exposures. The participant had provided written informed consent to not donate blood (or other body tissues) during the study. Unblinding was approved by the study PI and collaborators at WRAIR. After approval from the study PI, the unblinded treatment assignment was provided in a memo dated Jun 12, 2017, confirming that the participant received ZPIV. The study team and collaborators at WRAIR concurred that ZPIV administration was the most likely cause of the positive ZIKV nucleic acid test, presumably due to detection of ZIKV genomic RNA released from damaged inactivated virions. A similar phenomenon of transient antigenemia detectable in blood donors has been well-described following hepatitis B virus vaccination(18, 19). Further investigation was curtailed by the DPH. The incident was reported to the SMC and the FDA. The data for this participant was included in the analysis.
No SAEs or AESIs were reported during the study (Supplemental Table 2, appendix p. 3). No participants withdrew due to AEs. No pregnancies were reported during study follow-up. There were no related unsolicited AEs. There was one unrelated severe event reported for a participant who suffered a concussion following a sledding accident. No severe laboratory events were reported. No severe vital sign measurements were reported.
Local solicited symptoms were reported by 26 (87%) active ZPIV participants and three (50%) placebo participants (Table 2). Systemic solicited symptoms were reported by 24 (80%) active ZPIV participants and four (67%) placebo participants. The most common vaccine reactions among active ZPIV participants were injection site pain (n=24 [80%]), fatigue (n=16 [53%]), and headache (n=14 [46%]). All vaccine reactions were either mild or moderate. See Supplemental Tables 3–6 (appendix p. 4, 7, 9, and 12, respectively) for number and percentage of participants experiencing solicited events by dose, symptom, maximum severity, and treatment group.
Table 2:
Maximum reactogenicity within 7 days of vaccination
| All ZPIV (N=30) | Placebo (N=6) | |
|---|---|---|
| Local symptoms | ||
| Any local symptom | 26 (87%) | 3 (50%) |
| Mild | 23 (77%) | 3 (50%) |
| Moderate | 3 (10%) | 0 |
| Erythema | 26 (87%) | 3 (50%) |
| Mild | 23 (77%) | 3 (50% |
| Moderate | 3 (10%) | 0 |
| Induration (severity) | 4 (13%) | 1 (17%) |
| Mild | 4 (13%) | 1 (17%) |
| Moderate | 0 | 0 |
| Induration (size) | 4 (13%) | 1 (17%) |
| Mild | 4 (13%) | 1 (17%) |
| Moderate | 0 | 0 |
| Pruritus | 1 (3%) | 0 |
| Mild | 1 (3%) | 0 |
| Moderate | 0 | 0 |
| Pain | 24 (80%) | 2 (33%) |
| Mild | 21 (70%) | 2 (33%) |
| Moderate | 3 (10%) | 0 |
| Systemic symptoms | ||
| Any systemic symptom | 24 (80%) | 4 (67%) |
| Mild | 18 (60%) | 3 (50%) |
| Moderate | 6 (20%) | 1 (17%) |
| Abdominal Pain | 3 (10%) | 1 (17%) |
| Mild | 2 (7%) | 0 |
| Moderate | 1 (3%) | 1 (17%) |
| Arthralgia | 2 (7%) | 0 |
| Mild | 2 (7%) | 0 |
| Moderate | 0 | 0 |
| Diarrhea | 5 (17%) | 0 |
| Mild | 5 (17%) | 0 |
| Moderate | 0 | 0 |
| Fatigue | 16 (53%) | 1 (17%) |
| Mild | 12 (40%) | 1 (17%) |
| Moderate | 4 (13%) | 0 |
| Feverishness | 4 (13%) | 1 (17%) |
| Mild | 3 (10%) | 1 (17%) |
| Moderate | 1 (3%) | 0 |
| Headache | 14 (46%) | 2 (33%) |
| Mild | 10 (33%) | 2 (33%) |
| Moderate | 4 (13%) | 0 |
| Malaise | 10 (34%) | 1 (17%) |
| Mild | 8 (27%) | 1 (17%) |
| Moderate | 2 (7%) | 0 |
| Myalgia | 13 (44%) | 1 (17%) |
| Mild | 11 (37%) | 1 (17%) |
| Moderate | 2 (7%) | 0 |
| Nausea | 7 (23%) | 1 (17%) |
| Mild | 6 (20%) | 1 (17%) |
| Moderate | 1 (3%) | 0 |
| Rash | 1 (3%) | 0 |
| Mild | 0 | 0 |
| Moderate | 1 (3%) | 0 |
| Fever | 1 (3%) | 0 |
| Mild | 0 | 0 |
| Moderate | 1 (3%) | 0 |
| Vomiting | 2 (7%) | 0 |
| Mild | 2 (7%) | 0 |
| Moderate | 0 | 0 |
N = Number of participants in the Safety Population who received the specified dose. ZPIV=Alum-adjuvanted Zika purified inactivated vaccine.
ZIKV-specific neutralizing antibody (NAb) titers using the microneutralization MN50 assay in each treatment group are shown in Figure 2 and Supplemental Tables 7 and 8 (appendix p. 14, and 15, respectively). Among participants who received ZPIV at weeks 0 and 4 (Schedule 1), geometric mean NAb titers were highest (for the group) at the six week time-point (GMT = 983·3, 95% CI 425·5–2272·5). All participants (10/10 [100%]) in Schedule 1 reached a threshold MN50 titer of ≥ 100 at their observed peak (i.e., the highest NAb level observed across all time-points), with observed peak GMT of 1153·9 (95% CI 455·2–2925·2). By week 28 in the Schedule 1 group, 1/8 participants (12.5%) maintained a MN50 titer of ≥ 100 and GMT declined to 14 (95% CI 4–55). Among participants who received ZPIV at weeks 0 and 2 (Schedule 2), geometric mean NAb titers were highest for the group at the four week time-point (GMT = 477·4, 95% CI 111·7–2039·8), and the observed peak GMT was 517·7 (95% CI 142·9–1875·6). Compared to Schedule 1, fewer participants in Schedule 2 (8/10 [80%]) reached a threshold MN50 ≥ 100 at their observed peak. By week 28 in the Schedule 2 group, 0/7 participants (0%) maintained a MN50 titer of ≥ 100 and GMT declined to 7 (95% CI 4–12). Among participants who received a single administration of ZPIV at week 0 (Schedule 3), only one participant had detectable NAbs (peaking at 53 at two weeks), and no participant reached a threshold MN50 ≥ 100. No placebo participants reached a threshold titer of ≥ 10. For all vaccine schedules, geometric mean NAb titers peaked two weeks following final vaccination and declined below 100 by study week 16. At the end of study follow up (week 52), one participant in Schedule 1 and one participant in Schedule 2 maintained MN50 titers ≥ 100.
Figure 2: Neutralizing antibody responses against Zika Virus (ZIKV) following vaccination.
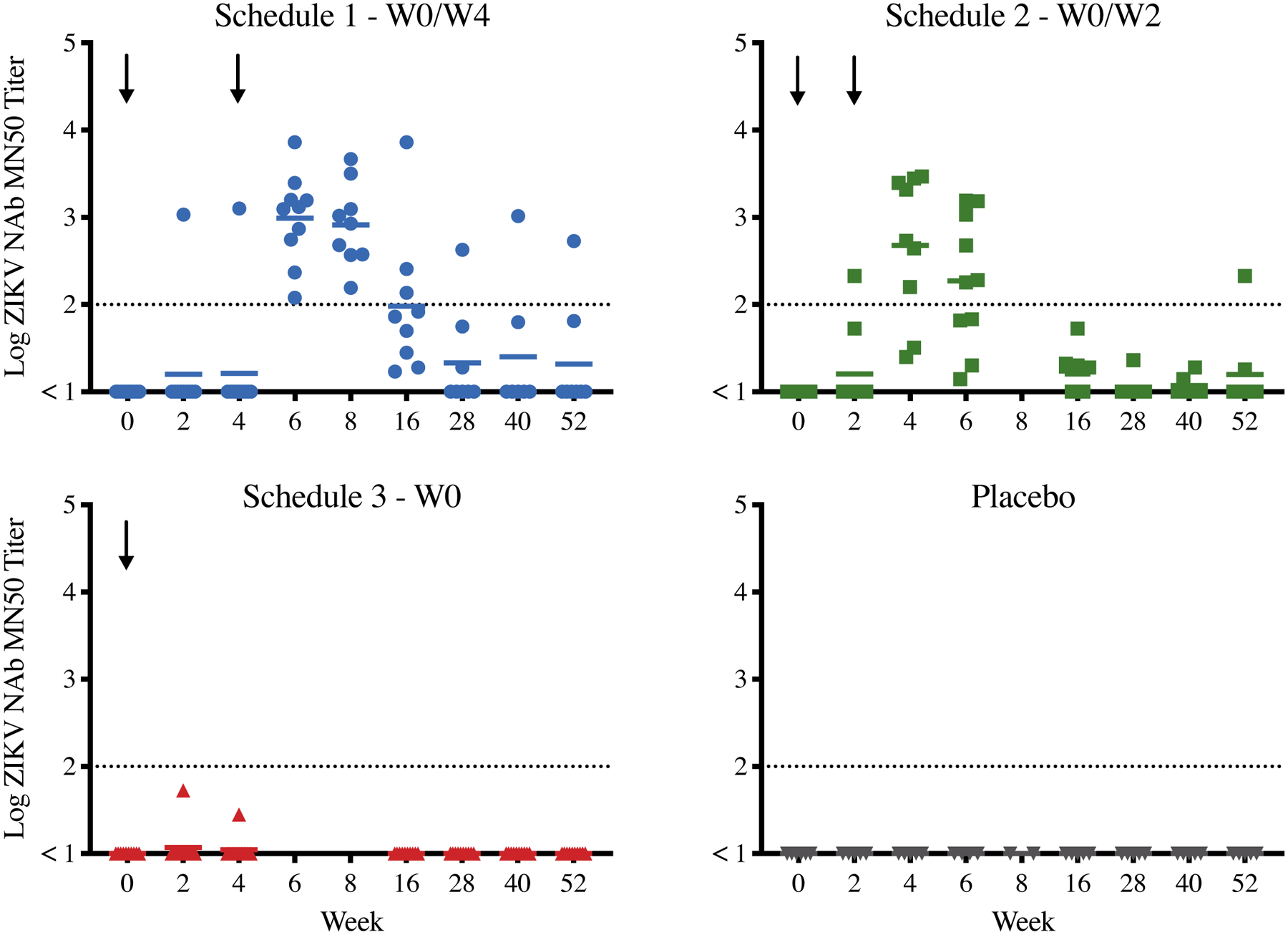
Log ZIKV-specific MN50 titers after Zika purified inactivated vaccine (ZPIV) administration by treatment group and study week. Dotted line is threshold of MN50 ≥ 100. Solid lines reflect log-transformed geometric mean titers. Black arrows indicate time of ZPIV injection.
Table 3 shows the results of the pre-specified hypothesis tests for the MN50 immunogenicity data. Initial testing showed significant differences between the 3 active groups and placebo for the observed peak (Kruskal Wallis p<0·0001) but no significant difference at the final study visit (p=0·2456). Pairwise group comparisons were performed for the observed peak timepoint and are displayed in Supplemental Table 9 (appendix p. 16). ZPIV Schedules 1 and 2 were significantly different from Schedule 3 (Wilcoxon p<0·0001 and p=0·0001, respectively) and placebo (both Wilcoxon p=0·0018). Significant differences were not observed between Schedules 1 and 2 (Wilcoxon p=0·4494) or Schedule 3 and placebo (Wilcoxon p=0·4795). These data suggest that the shorter two-week regimen in Schedule 2 was comparably immunogenic to the four-week regimen in Schedule 1, although the study was not powered to make this conclusion definitively. Schedule 2 titers were also noted to decline more quickly than Schedule 1 titers.
Table 3:
Comparison of MN Geometric Mean Titer (GMT) by Treatment Group –Comparison Across Groups
| Time Point | Statistic | Schedule 1 (N=10) | Schedule 2 (N=10) | Schedule 3 (N=10) | Placebo (N=6) | P-value** |
|---|---|---|---|---|---|---|
| Observed Peak* | N | 10 | 10 | 10 | 5 | <0·0001 |
| GMT | 1153·9 | 517·7 | 6·3 | 5·0 | ||
| 95% CI | [455·2, 2925·2] | [142·9, 1875·6] | [3·7, 10·8] | -- | ||
| 363 Days Post-First Administration | N | 8 | 8 | 10 | 5 | 0·2456 |
| GMT | 12·4 | 9·4 | 5·0 | 5·0 | ||
| 95% CI | [2·8, 54·2] | [3·1, 28·7] | -- | -- |
N=Number of participants in immunogenicity population.
Peak result of a participant observed across all visits
p-value is calculated via Kruskal-Wallis test
Figure 3A displays the binding antibody titers against Env and NS1 ZIKV antigens at observed peak levels. Binding Env-specific antibody titers were detectable at low levels at baseline (particularly among participants with pre-existing flavivirus immunity by MN assay), indicating significant cross-reactivity of pre-existing binding antibodies to ZIKV antigens (Supplemental Figure 1, appendix p. 17). At observed peak levels, Schedule 1 and 2 antibody titers against both Env and NS1 were approximately one log greater than Schedule 3 and placebo titers. We did not perform binding ELISA assays for post-peak timepoints due to the significant cross-reactivity of the assay. Plaque reduction neutralization tests were also not performed as the study team determined these assays would be redundant with the microneutralization assays.
Figure 3: Binding antibody titers and cellular immune responses against Zika Virus (ZIKV) following vaccination.
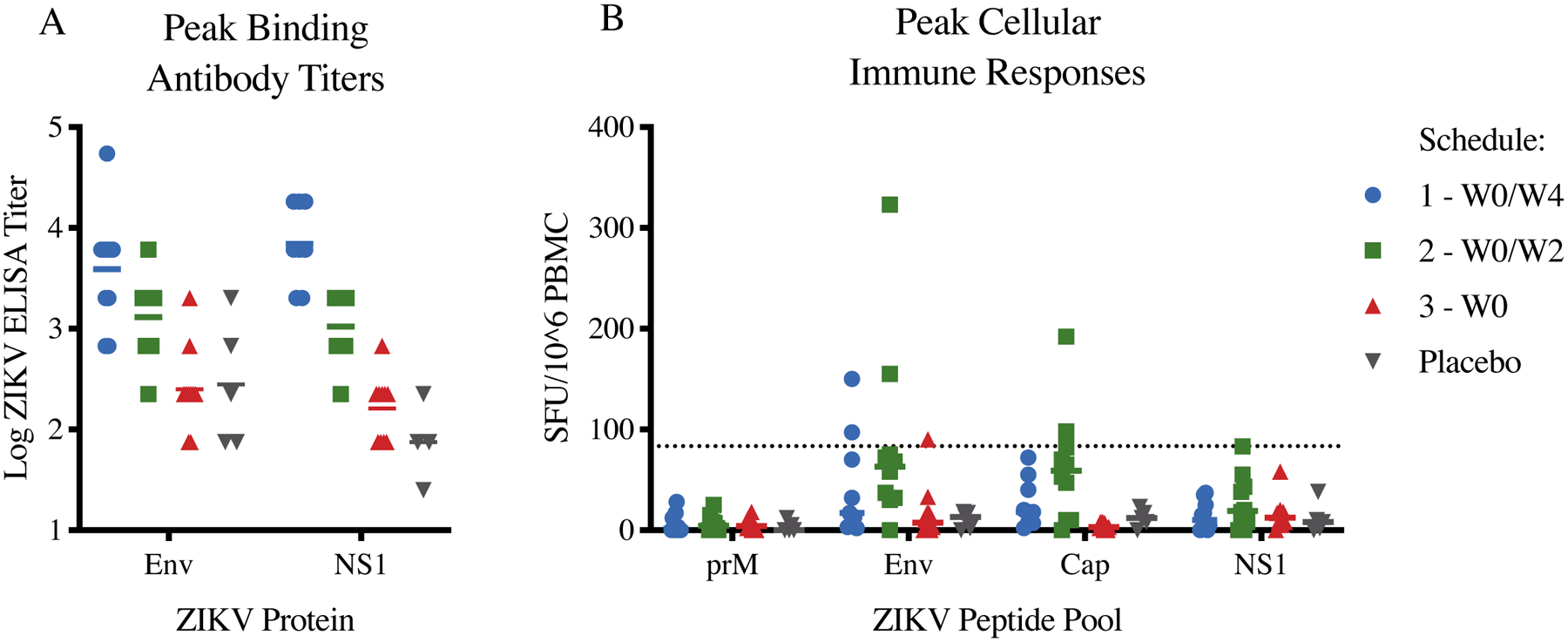
(A) Log ELISA titers by treatment group against ZIKV Envelope and NS1 (SPH2015 strain) antigens at observed peak. (B) Cellular immune responses by treatment group against ZIKV Env, NS1, Cap, and prM peptide pools at observed peak. Dotted line is threshold for a positive response. CI=confidence interval. SFU=spot forming units. PBMC=peripheral blood mononuclear cells.
Figure 3B displays cellular immune responses against prM, Env, Cap, and NS1 ZIKV antigens at observed peak levels. ZIKV-specific cellular immune responses were low across all groups, as expected for an inactivated virus vaccine. Exploratory studies including but not limited to epitope-specific antibody binding patterns, system serology, and the development of Zika-specific monoclonal antibodies are ongoing and will be published at a future date as appropriate.
Discussion
In this phase 1 randomized clinical trial, we evaluated the safety, tolerability and immunogenicity of the ZPIV vaccine administered according to either a one-dose vaccination regimen or two-dose regimens with a standard week 0 and week 4 schedule or a shortened week 0 and week 2 schedule. We found that ZPIV was safe and well tolerated across regimens. There were no related unsolicited adverse events, serious adverse events (related or unrelated), or adverse events of special interest (e.g., Guillain-Barre syndrome). The most commonly reported symptom post-vaccination was local pain at the injection site, and all solicited symptoms were either mild or moderate. Vaccine immunogenicity required two doses, and the two boosting intervals proved similar, though MN50 titers persisted at high levels for a greater duration with the longer four-week boosting interval. ZPIV elicited NAb titers in nearly all participants who received either two-dose schedule in Schedule 1 and 2 with MN50 titer ≥ 100. This threshold titer protected mice and non-human primates against ZIKV challenge(13, 14, 20). In contrast, no participant who received the one-dose schedule developed MN50 titers ≥ 100 at any point in the study. This data demonstrates that a single ZPIV dose is not sufficient to induce robust MN50 titers and that a boost is required.
There was no statistically significant difference between observed peak NAb titers elicited by the standard four-week regimen versus the accelerated two-week regimen (Wilcoxon p=0·4494), although the study was not formally powered to identify differences between schedules. Observed peak geometric mean MN50 titers of 517·7 were achieved in the accelerated schedule, compared to observed peak GMT of 1153·9 in the standard schedule. In both Schedule 1 and 2, antibody titers sharply declined to MN50 GMT of ≥ 100 by week 16, with a more rapid decline in titers noted with the accelerated regimen compared to the standard regimen. These data suggest that an accelerated schedule with a two-week boost may be a feasible option in an outbreak setting if rapid induction of immune responses is desired, but that these MN50 titers may be less durable.
At the final study visit, there was no significant difference in antibody titers between the three active groups and placebo (Kruskal Wallis p=0·2456). These data are consistent with the reported decline in immunogenicity observed at one year following vaccination with the JEV purified inactivated vaccine (IXIARO)(21–23). It is likely that the short durability of ZPIV humoral immune responses is related to the lack of Zika-specific T cell responses elicited by the inactivated vaccine, indicating a potential absence of CD4 T cell help shown to be important for controlling ZIKV infection in mouse models(24–26). Additional boosts or higher doses of ZPIV may therefore be required for durable titers to be sustained for longer than 16 weeks. For IXIARO, the U.S. Advisory Committee on Immunization Practices recommends a boost at one year(27). Ongoing studies of a late ZPIV boost (NCT02963909) and higher ZPIV doses (NCT02952833) will shed light on whether the ZPIV regimen can be optimized further.
Our results are limited by the small sample size of the study (36 participants), the lack of randomization between groups, the relatively short follow up (52 weeks), and the demographic characteristics of the study population (predominantly female, white, and non-Hispanic). Data from a ZPIV study ongoing in Ponce, Puerto Rico (NCT03008122), where flaviviruses are endemic, will be important for extending the generalizability of our results.
In conclusion, ZPIV was safe, well-tolerated and immunogenic when given as a two-dose regimen at either 0/4 weeks or 0/2 weeks. A single dose of ZPIV was minimally immunogenic. The accelerated two-week boosting regimen elicited equivalent peak NAb titers to a standard four-week boosting regimen, and was able to achieve these peak levels sooner (four vs. six weeks after initial prime). However, the accelerated two-dose regimen appeared to have faster antibody decline than the standard regimen, although durability with both regimens was limited. Additional studies to optimize ZPIV dose and schedule are underway to extend the durability of these responses.
Supplementary Material
Research in context.
Evidence before this study
The Zika virus epidemic in the Americas in 2015–2016 prompted an urgent international effort to develop a safe and effective Zika vaccine. In 2016, the Walter Reed Army Institute of Research, Silver Spring, MD, USA, designed and manufactured an inactivated and purified whole Zika virus vaccine with aluminium hydroxide gel adjuvant (ZPIV). In preclinical studies, ZPIV was shown to protect mice and monkeys from Zika virus challenge, particularly when Zika-specific neutralizing antibody (NAb) titers were greater than 1:100. Three ZPIV clinical trials commenced in late 2016, with all three trials including a two-dose prime-boost regimen at a schedule of week 0 and week 4. Interim results of this two-dose regimen from all three studies were published in February 2018 showing ZPIV to be safe and immunogenic in humans through 8 weeks of follow up. According to a search of PubMed for articles published up to January 3, 2020 using the terms “Zika”, “vaccine”, and “Zika vaccine”, there are two additional published reports of Zika vaccine clinical trial results (also interim) in humans. In these reports, three different DNA vaccines expressing pre-membrane and envelope Zika virus proteins were shown to be safe and immunogenic through 24 weeks of follow up. To date, there are no published reports of any completed Zika vaccine trial in humans (using ZPIV, DNA or other platform), nor any published reports of the safety and immunogenicity of alternative vaccine schedules of ZPIV in humans.
Added value of this study
Here we present the final results of the ZPIV clinical trial conducted at Beth Israel Deaconess Medical Center (BIDMC), Boston, MA. In addition to testing a two-dose ZPIV schedule at week 0 and week 4, we also examined the safety and immunogenicity of a one-dose vaccine and a two-dose schedule at week 0 and week 2. The goal of our study was to determine if an accelerated ZPIV schedule could induce Zika-specific immune responses more rapidly than the standard vaccine schedule, without loss of magnitude or durability. We found that ZPIV was safe and well tolerated across regimens through 52 weeks of follow up. Vaccine immunogenicity required two doses, and the two boosting intervals proved similar, with nearly all participants developing NAb titers >1:100 at peak levels. By week 28, however, all groups had low titers.
Implications of all the available evidence
The evidence suggests that ZPIV is safe and well-tolerated in humans, and when given in a two-dose regimen, can elicit Zika-specific antibody titers that are predicted to be protective based on animal models. An accelerated schedule with a two-week boost may be a feasible option in an outbreak setting if rapid induction of immune responses is desired. A single dose of ZPIV, however, would not be sufficient to elicit protective immunity. Additional boosts or higher doses of ZPIV will likely be required to improve durability.
Acknowledgements
We thank the clinical trial participants and staff at the Center for Virology and Vaccine Research Clinical Trials Unit and the Harvard Catalyst Clinical Research Center. This work was supported by a cooperative agreement W81XWH-07-2-0067 between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense; National Institutes of Health grants AI060354, AI124377, AI126603, AI128751, A129797, OD024917, and TR001102; the Ragon Institute of MGH, MIT, and Harvard; and Harvard Catalyst. The content is solely the responsibility and opinions of the authors. We acknowledge generous advice and assistance from Dr. Richard G. Jarman at Walter Reed Army Institute of Research, the BIDMC research and clinical teams, the International AIDS Vaccine Initiative, and Emmes.
Funding
The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc.
Footnotes
Permissions
Early safety and immunogenicity data are reprinted from The Lancet, Vol. 391, Modjarrad, et al., Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: phase 1, randomised, double-blind, placebo-controlled clinical trials, Pages 563–571, Copyright (2017), with permission from Elsevier.
Data Sharing
We provide the protocol and informed consent form in the appendix, and we can share raw data upon request by contacting the corresponding author.
Declaration of Interests
KHE, SJT and RADLB are co-inventors on the patent for ZPIV. All other authors declare no competing interests.
References
- 1.Espinal MA, Andrus JK, Jauregui B, Waterman SH, Morens DM, Santos JI, et al. Emerging and Reemerging Aedes-Transmitted Arbovirus Infections in the Region of the Americas: Implications for Health Policy. Am J Public Health. 2019;109(3):387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fauci AS, Morens DM. Zika Virus in the Americas--Yet Another Arbovirus Threat. N Engl J Med. 2016;374(7):601–4. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brasil P, Pereira JP, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med. 2016;375(24):2321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, et al. Birth Defects Among Fetuses and Infants of US Women With Evidence of Possible Zika Virus Infection During Pregnancy. JAMA. 2017;317(1):59–68. [DOI] [PubMed] [Google Scholar]
- 6.de Oliveira WK, Carmo EH, Henriques CM, Coelho G, Vazquez E, Cortez-Escalante J, et al. Zika Virus Infection and Associated Neurologic Disorders in Brazil. N Engl J Med. 2017;376(16):1591–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organization WH. Zika Strategic Response Framework and Joint Operations Plan: January-June 2016. 2016. [Google Scholar]
- 8.Abbink P, Stephenson KE, Barouch DH. Zika virus vaccines. Nat Rev Microbiol. 2018;16(10):594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Modjarrad K, Lin L, George SL, Stephenson KE, Eckels KH, De La Barrera RA, et al. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet. 2018;391(10120):563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer M, Lindsey N, Staples JE, Hills S, Centers for Disease C, Prevention. Japanese encephalitis vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-1):1–27. [PubMed] [Google Scholar]
- 11.Schmidt AC, Lin L, Martinez LJ, Ruck RC, Eckels KH, Collard A, et al. Phase 1 Randomized Study of a Tetravalent Dengue Purified Inactivated Vaccine in Healthy Adults in the United States. Am J Trop Med Hyg. 2017;96(6):1325–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez LJ, Lin L, Blaylock JM, Lyons AG, Bauer KM, De La Barrera R, et al. Safety and Immunogenicity of a Dengue Virus Serotype-1 Purified-Inactivated Vaccine: Results of a Phase 1 Clinical Trial. Am J Trop Med Hyg. 2015;93(3):454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353(6304):1129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larocca RA, Abbink P, Peron JPS, Zanotto PMdA, Iampietro MJ, Badamchi-Zadeh A, et al. Vaccine protection against Zika virus from Brazil. Nature. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox F, van der Fits L, Abbink P, Larocca RA, van Huizen E, Saeland E, et al. Adenoviral vector type 26 encoding Zika virus (ZIKV) M-Env antigen induces humoral and cellular immune responses and protects mice and nonhuman primates against ZIKV challenge. PloS one. 2018;13(8):e0202820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barouch DH, Tomaka FL, Wegmann F, Stieh DJ, Alter G, Robb ML, et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13–19). Lancet. 2018;392(10143):232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saá P, Proctor M, Foster G, Krysztof D, Winton C, Linnen JM, et al. Investigational Testing for Zika Virus among U.S. Blood Donors. N Engl J Med. 2018;378(19):1778–88. [DOI] [PubMed] [Google Scholar]
- 18.Rysgaard CD, Morris CS, Drees D, Bebber T, Davis SR, Kulhavy J, et al. Positive hepatitis B surface antigen tests due to recent vaccination: a persistent problem. BMC Clin Pathol. 2012;12:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kloster B, Kramer R, Eastlund T, Grossman B, Zarvan B. Hepatitis B surface antigenemia in blood donors following vaccination. Transfusion. 1995;35(6):475–7. [DOI] [PubMed] [Google Scholar]
- 20.Abbink P, Larocca RA, Visitsunthorn K, Boyd M, De La Barrera RA, Gromowski GD, et al. Durability and correlates of vaccine protection against Zika virus in rhesus monkeys. Sci Transl Med. 2017;9(420):eaao4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuller E, Jilma B, Voicu V, Golor G, Kollaritsch H, Kaltenbock A, et al. Long-term immunogenicity of the new Vero cell-derived, inactivated Japanese encephalitis virus vaccine IC51 Six and 12 month results of a multicenter follow-up phase 3 study. Vaccine. 2008;26(34):4382–6. [DOI] [PubMed] [Google Scholar]
- 22.Dubischar-Kastner K, Eder S, Buerger V, Gartner-Woelfl G, Kaltenboeck A, Schuller E, et al. Long-term immunity and immune response to a booster dose following vaccination with the inactivated Japanese encephalitis vaccine IXIARO, IC51. Vaccine. 2010;28(32):5197–202. [DOI] [PubMed] [Google Scholar]
- 23.Eder S, Dubischar-Kastner K, Firbas C, Jelinek T, Jilma B, Kaltenboeck A, et al. Long term immunity following a booster dose of the inactivated Japanese Encephalitis vaccine IXIARO(R), IC51. Vaccine. 2011;29(14):2607–12. [DOI] [PubMed] [Google Scholar]
- 24.Elong Ngono A, Young MP, Bunz M, Xu Z, Hattakam S, Vizcarra E, et al. CD4+ T cells promote humoral immunity and viral control during Zika virus infection. PLoS Pathog. 2019;15(1):e1007474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucas CGO, Kitoko JZ, Ferreira FM, Suzart VG, Papa MP, Coelho SVA, et al. Critical role of CD4(+) T cells and IFNgamma signaling in antibody-mediated resistance to Zika virus infection. Nat Commun. 2018;9(1):3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassert M, Wolf KJ, Schwetye KE, DiPaolo RJ, Brien JD, Pinto AK. CD4+T cells mediate protection against Zika associated severe disease in a mouse model of infection. PLoS Pathog. 2018;14(9):e1007237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease C, Prevention. Recommendations for use of a booster dose of inactivated vero cell culture-derived Japanese encephalitis vaccine: advisory committee on immunization practices, 2011. MMWR Morbidity and mortality weekly report. 2011;60(20):661–3. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


