Abstract
Purpose
Disentangling the effects of endogenous estrogens and inflammation on obesity-related diseases requires a clearer understanding of how the two biological mechanisms relate to each other.
Methods
We studied 155 healthy postmenopausal women not taking menopausal hormone therapy enrolled in the Prostate Lung Colorectal and Ovarian (PLCO) screening cancer trial. From a baseline blood draw, we measured endogenous estradiol and 69 inflammation biomarkers: cytokines, chemokines, adipokines, angiogenic factors, growth factors, acute phase proteins, and soluble receptors. We evaluated the estradiol–inflammation relationship by assessing associations across different models (linear, ordinal logistic, and binary logistic) using a variety of estradiol classifications. We additionally investigated the estradiol–inflammation relationship stratified by baseline obesity status (BMI < 30 stratum and BMI > 30 stratum).
Results
Associations of estradiol with 7 inflammation biomarkers met p < 0.05 statistical significance in linear and ordinal models: C-reactive protein (CRP), adiponectin, chemokine (C-X-C motif) ligand-6, thymus activation-regulated chemokine, eosinophil chemotactic protein, plasminogen activator inhibitor-1, and serum amyloid A. The positive association between estradiol and CRP was robust to model changes. Each standard deviation increase in endogenous estradiol doubled a woman’s odds of having CRP levels higher than the study median (odds ratio 2.29; 95% confidence interval 1.28, 4.09). Estradiol was consistently inversely associated with adiponectin. Other estradiol–inflammation biomarker associations were not robust to model changes.
Conclusions
Endogenous estradiol appears to be associated with CRP and adiponectin; the evidence is limited for other inflammation biomarkers.
Keywords: Endogenous estradiol, Inflammation, Obesity, Serum biomarkers, Cytokines
Introduction
Obesity is a known cause of many diseases including heart disease and stroke, type 2 diabetes, immunologic and metabolic disorders, and many cancers [1–5]. Obesity is a particularly strong risk factor for endometrial cancer—a female cancer that primarily affects those who are postmenopausal—in which it accounts for approximately 40% of all incident cases [6].
Obesity induces many biological changes but its persistently higher levels of estrogens and inflammation are suspected to contribute to carcinogenesis [6, 7]. For instance, excess adipose tissue results in elevated aromatase activity increasing systemic estrogen levels, particularly in postmenopausal women [8]. When estrogen is unopposed by progesterone, it has proliferative effects on the endometrial lining, and when this happens outside of the normal menstrual cycle, the proliferative effects can lead to endometrial hyperplasia and carcinogenesis [9]. Excess adipose tissue also creates a consistent and systemic pro-inflammatory environment conducive to the initiation and growth of tumors [10]. Inflammatory cytokines such as C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-alpha), and interleukin-6 (IL-6) have been implicated in many different cancers [10]. However, inflammation is a complex biological phenomenon unlikely to be fully understood by a few individual biomarkers. Thus, it may require a more comprehensive evaluation [11, 12].
Estrogen and inflammation may interact in a dual hormonal-inflammatory environment to cause obesity-related diseases [13, 14]. However, there is a paucity of evidence regarding circulating estradiol and inflammatory factors. Past research typically has assessed estrogen–disease and inflammation–disease relationships separately, a strategy that misses the broader biological complexity. Indeed, this lack of data limits our ability to study these important carcinogenic pathways in combination [15–19]. The lack of clarity on the relationship between estrogen and inflammation may also partly explain conflicting observational evidence in which endogenous estrogen may be viewed as a causal or preventive factor in other diseases (e.g., cardiovascular disease, hypertension, breast cancer) [20–23].
We sought to investigate the association between endogenous circulating levels of unconjugated estradiol—the most biologically active of three endogenous estrogen metabolites (i.e., estradiol, estriol, and estrone) in postmenopausal women—and 69 inflammation biomarkers spanning pro-and anti-inflammatory cytokines, chemokines, adipokines, angiogenic factors, growth factors, acute phase proteins, and soluble receptors. We focus on the relationship between inflammation and unconjugated estradiol since total estradiol includes conjugated estradiol, such as estradiol glucuronide, which are less biologically active. Additionally, unconjugated estradiol is the estrogen most strongly associated with BMI [24]. We evaluated these relationships in a subset of postmenopausal women within the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial.
Methods
Study population
PLCO was a randomized screening trial of approximately 155,000 men and women aged 55–74 years at multiple centers across the US from 1992 to 2001 [25]. Screening arm participants (n = 77,444) underwent blood draws and were followed for all incident cancers allowing for cancer-specific nested case–control studies. For our analysis, we used the 284 postmenopausal healthy female controls without a hysterectomy from the endometrial cancer-matched case–control study population [26]. We excluded endometrial cancer cases because of concerns about pre-clinical disease influencing the estradiol–inflammation association. Demographic, behavioral, and dietary information were collected, along with non-fasting blood samples at or near baseline; 90.1% of samples were at baseline, 6.7% of samples were within 1 year of baseline, and 3.2% were within 2 years of baseline. An eligible subject had to have consented to biochemical studies, have a previously unthawed serum sample, a completed questionnaire, and no history of cancer other than non-melanoma skin cancer. Since oral hormone therapy may have other effects on inflammatory proteins due to first pass hepatic metabolism [27, 28], we excluded women who reported taking menopausal hormone therapy at baseline (n = 125) and women who reported no hormone use but whose circulating estradiol levels were > 40 pg/ml, indicating possible unreported hormone use at blood draw (n = 3). One subject was missing information on body mass index leaving us with a study population of 155 postmenopausal women. PLCO was approved by the Institutional Review Boards at each participating center and the National Cancer Institute.
Laboratory analysis
Serum specimens were used to measure circulating endogenous levels of unconjugated estradiol in addition to 69 inflammation biomarkers spanning multiple aspects of inflammation [29] (Supplemental Table 1). Unconjugated estradiol was measured via gas chromatography tandem mass spectrometry [26]. In a single laboratory, 68 inflammation markers were measured using five Luminex bead-based commercial assay panels (Millipore, Billerica, MA). The 69th marker, transforming growth factor beta-1 (TGF-beta-1), was measured using a quantitative sandwich enzyme immunoassay (R&D Systems, Minneapolis, MN). Marker concentrations were calculated using a four- or five-parameter logistic curve (Bioplex Manager 6.1, BioRad, Hercules, CA). The high-sensitivity panel used the same manufacturer lot, but the remaining panels were purchased across two lots. We evaluated assay performance by including a blinded duplicate sample from a quality control pool in each batch; information on the quality control is previously published [26]. For the parent study, the estradiol assay coefficient of variation (CV) was 17.1% with an intraclass correlation coefficient (ICC) of 0.97. For the multiplex inflammatory assay, in the parent study, the CVs were on average 9.1% (range 2.7–32.2), with an ICC average of 0.89 (range 0.67–0.99). The study sample CVs are detailed in Supplementary Table 1.
Statistical analysis
We estimated associations between endogenous estradiol levels and 69 different inflammation biomarkers using a variety of models and variable classifications for both the exposure (estradiol) and the outcomes (inflammation biomarkers). We chose, a priori, to adjust all models for age (continuous), smoking history (categorical), regular aspirin use (binary), regular ibuprofen use (binary), oral contraceptive use (binary), and body mass index (continuous).
Linear models
We fitted linear models after natural log transforming the inflammation biomarkers to normalize them, then standardizing each (mean = 0, standard deviation = 1) so that the results would be comparable across each biomarker. We performed the same transformation to estradiol; thus, each reported regression coefficient represents the standard deviation (SD) change in the geometric mean of the respective inflammation biomarker per SD change in the geometric mean of estradiol [30]. Some inflammation biomarker measurements fell below the limit of detection and thus were considered missing for the linear models; in a sensitivity analysis of the linear models, we imputed those measurements that fell below the limit of detection as one-half of the lowest measured value in the sample for that biomarker. Fifty out of the 69 inflammation biomarkers, however, were missing in fewer than 15% of the samples; another 10 were missing in less than 30% of the samples (see Supplementary Table 1). The nine biomarkers missing in more than 30% are IL21 (36.1% missing), IL16 (37.4%), MCP4 (47.1%), FGF2 (48.4%), IL1 receptor antagonist (52.9%), SCF (58.7%), TNF-beta (65.8%), IL33 (72.9%), and IL29 (74.8%).
Ordinal logistic models
We ran ordinal logistic models that included biomarker measurements that fell below the limit of detection. Biomarkers with < 25% missing were classified into four ordinal groups: biomarker quartiles; biomarkers with 25–49% missing classified into four groups: the undetectable as the lowest group followed by biomarker tertiles; biomarkers with 50–75% missing were classified into three groups: undetectable and median dichotomized biomarkers. Estradiol was natural log transformed and standardized. Thus, the results are the odds of increasing to the next inflammation tertile or quartile category per SD change in the geometric mean of estradiol [30]. We report the results for the inflammation biomarkers that met nominal statistical significance at a p < 0.05 level in either the linear or the ordinal models; we noted any results that met Bonferroni-corrected statistical significance at a p < 0.0007 level (0.05 ÷ 69).
Logistic models
We investigated the robustness of the associations for the 7 nominally significant inflammation biomarkers in both the linear and ordinal models by median dichotomizing the biomarkers and fitting logistic regressions using different classifications of estradiol: (i) keeping estradiol continuous and standardized on the natural log scale; (ii) median dichotomizing estradiol; (iii) estradiol tertiles; (iv) estradiol quartiles in which we also tested a dose–response with a Wald p value by creating a single estradiol variable coded 1 through 4 representing the 4 estradiol quartiles. By examining different classifications, we highlight any conflicting results that may be driven by a specific classification scheme.
Additionally in exploratory analyses using the Logistic models, we stratified the population by BMI to examine the differences between the resulting associations of the 7 nominally significant biomarkers among obese (BMI ≥ 30) and non-obese (BMI < 30) women; we retained a continuous BMI covariate in the model, but we did not perform statistical tests for interaction because of small sample sizes in stratified groups. We analyzed and reported the inflammation biomarker associations with a continuous estradiol variable (standardized on the natural log scale) and for a binary estradiol variable. All statistical tests were performed in SAS version 9.4 [31].
Results
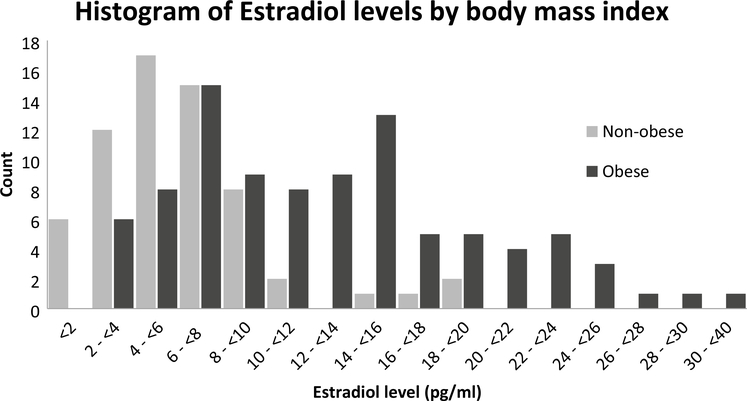
As shown in Table 1, the mean age in the study was 63 years and the population was mostly white with a majority having some college or a college degree. More than half of the women were considered obese (BMI of 30 or more), and among those women, two-thirds had circulating estradiol levels above the study population median of 7.85 pg/ml. Figure 1 is a histogram of circulating estradiol level among obese and non-obese women (BMI < 30). The distribution in obese women appears to be bi-modal and is shifted to higher levels of estradiol compared to the distribution in non-obese women. This led to a very strong unadjusted association between BMI and circulating estradiol with the odds of having a circulating endogenous estradiol measurement above the study population median for obese women being 19 times that of normal weight women (BMI < 25) (OR 19.1, 95% CI 4.14, 88.4). Overweight women had 3 times the odds of having high endogenous estradiol compared to normal weight women (OR 3.1, 95% CI 0.62, 15.4).
Table 1.
Descriptive characteristics of the menopausal hormone therapy-free healthy controls in the endometrial nested study within the Prostate, Lung, Colorectal, and Ovarian cancer trial
| Estradiol levels |
||||
|---|---|---|---|---|
| < 7.85 pg/ml (n = 78) | ≥ 7.85 pg/ml (n = 77) | |||
| Age (mean/IQR) | 62.9 | 58–67 | 63.1 | 60–67 |
| Race | n | % | n | % |
| White | 70 | 89.7 | 73 | 94.8 |
| Non-white | 8 | 10.3 | 4 | 5.2 |
| Education | ||||
| HS grad or less | 24 | 30.8 | 19 | 24.7 |
| More than HS | 31 | 39.7 | 33 | 42.8 |
| College graduate | 23 | 29.5 | 25 | 32.5 |
| BMI | ||||
| Normal (< 25 kg/m2) | 17 | 21.8 | 2 | 2.6 |
| Overweight (25–30) | 33 | 42.3 | 12 | 15.6 |
| Obese (30+) | 28 | 35.9 | 63 | 81.8 |
| Smoking | ||||
| Never | 49 | 62.8 | 48 | 62.3 |
| Former | 24 | 30.8 | 24 | 31.2 |
| Current | 5 | 6.4 | 5 | 6.5 |
| Regular aspirin use | ||||
| No | 37 | 48.0 | 48 | 62.3 |
| Yes | 40 | 52.0 | 29 | 37.7 |
| Regular ibuprofen use | ||||
| No | 50 | 64.1 | 52 | 67.5 |
| Yes | 28 | 35.9 | 25 | 32.5 |
| Hormonal birth control use | ||||
| Never | 56 | 71.8 | 45 | 58.4 |
| Ever | 22 | 28.2 | 32 | 41.6 |
| Parity | ||||
| No | 7 | 9.0 | 10 | 13.0 |
| Yes | 71 | 91.0 | 67 | 87.0 |
| Age at menopause | ||||
| < 50 | 25 | 32.1 | 23 | 29.9 |
| 50–54 | 38 | 48.7 | 40 | 51.9 |
| 55+ | 15 | 19.2 | 14 | 18.2 |
| Diabetes | ||||
| No | 71 | 91.0 | 63 | 82.9 |
| Yes | 7 | 9.0 | 13 | 17.1 |
HS high school, IQR interquartile range, BMI body mass index
Fig. 1.
Histogram of circulating unconjugated estradiol by obesity status (Obese: BMI ≥ 30; Non-obese: BMI < 30), where the number of subjects is the Y-axis and estradiol level in pg/ml is the x-axis. Among the obese the mean and standard deviation are 12.8 pg/ml and 6.9, with a median of 11.9 pg/ml and an interquartile range of 7.1–16.0 pg/ml. Among the non-obese, the mean and standard deviation are 6.2 pg/ml and 3.9, with a median of 5.5 pg/ml and an interquartile range of 3.5–7.7 pg/ml. The unadjusted OR of higher than study median estradiol levels for obese vs normal weight women is OR 19.1 (95% CI 4.14, 88.4). The same odds ratio for overweight vs normal weight women is 3.1 (95% CI 0.62, 15.4)
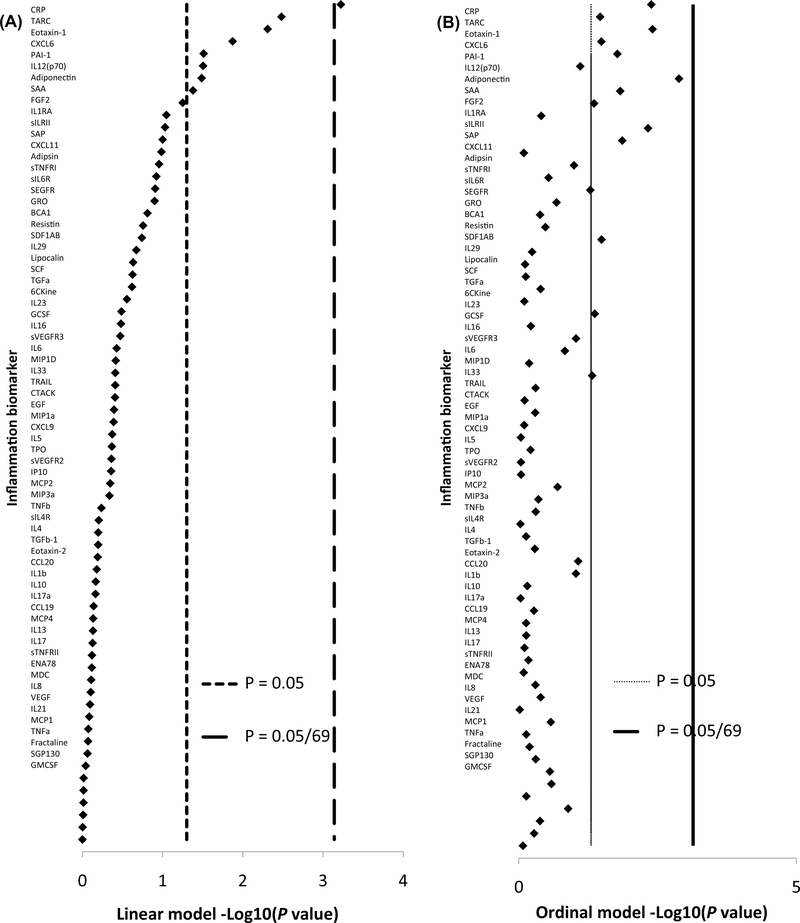
Under the linear models, 8 of the 69 inflammation biomarkers were nominally statistically significantly associated with circulating estradiol at the p < 0.05 level. Four associations suggest that estradiol has pro-inflammatory actions: being positively associated with C-reactive protein (CRP), plasminogen activator protein-1 (PAI-1), and serum amyloid A (SAA), whereas inversely associated with adiponectin. Four associations suggest anti-inflammatory actions of estradiol: being inversely associated with thymus activation-regulated chemokine (TARC), chemokine (C-X-C motif) ligand-6 (CXCL6), eosinophil chemotactic protein (Eotaxin-1), and interleukin-12(p70) (Table 2). The estradiol–CRP association was the only one to meet the Bonferroni-corrected threshold of p < 0.0007 (Fig. 2). Under the ordinal models, 13 of the 69 inflammation biomarkers were nominally associated with estradiol, but none met the Bonferroni threshold. Seven of the 13 were repeat findings from the linear model: CRP, adiponectin, CXCL6, TARC, Eotaxin-1, PAI-1, and SAA. The 6 new findings suggest pro-inflammatory action with all being positively associated with circulating estradiol: serum amyloid P (SAP), soluble IL-1 receptor 2 (sIL-1RII), resistin, chemokine (C–C motif) ligand-21 (CCL21), basic fibroblast growth factor (FGF2), and IL-6 (Table 2).
Table 2.
Associations between continuous estradiol (unit of analysis is per SD on the natural log scale) and separate inflammation biomarkers that were statistically significant (p < 0.05) in models where the inflammation biomarker was natural log transformed and standardized (linear model) or ordered categories (ordinal model)
| Linear model results |
Ordinal model results |
|||
|---|---|---|---|---|
| SD changea | 95% CI | ORb | 95% CI | |
| Biomarkers associated with estradiol in both the linear and ordinal models | ||||
| CRP | 0.314 | (0.140, 0.487) | 1.90 | (1.23, 2.96) |
| Adiponectin | −0.216 | (−0.412, −0.020) | 0.49 | (0.32, 0.76) |
| CXCL6 | −0.255 | (−0.455, −0.055) | 0.66 | (0.45, 0.97) |
| TARC | −0.301 | (−0.499, −0.104) | 0.66 | (0.45, 0.97) |
| Eotaxin-1 | −0.285 | (−0.480, −0.089) | 0.56 | (0.36, 0.83) |
| PAI-1 | 0.239 | (0.024, 0.453) | 1.64 | (1.09, 2.46) |
| SAA | 0.201 | (0.098, 0.393) | 1.63 | (1.10, 2.42) |
| Biomarkers nominally associated with estradiol in only the linear model | ||||
| IL12(p70) | −0.196 | (−0.387, −0.006) | ||
| Biomarkers nominally associated with estradiol in only the ordinal model | ||||
| SAP | 1.66 | (1.11, 2.47) | ||
| sIL-1RII | 1.83 | (1.20, 2.77) | ||
| Resistin | 1.53 | (1.04, 2.26) | ||
| CCL21 | 1.49 | (1.01, 2.18) | ||
| FGF-2 | 1.52 | (1.01, 2.28) | ||
| IL6 | 1.50 | (1.00, 2.24) | ||
SD standard deviation, OR odds ratio, CI confidence interval, CRP C-reactive protein, CXCL6 chemokine (C-X-C motif) ligand-6, TARC thymus activation-regulated chemokine, Eotaxin-1 eosinophil chemotactic protein, PAI-1 plasminogen activator protein-1, SAA serum amyloid A, IL12(p70) interleukin-12(p70), SAP serum amyloid P, sIL-1RII soluble IL-1 receptor 2, CCL21 chemokine (C–C motif) ligand-21, FGF2 basic fibroblast growth factor, IL-6 interleukin-6
The SD change is the standard deviation (SD) change in the geometric mean of the respective inflammation biomarker per SD change in the geometric mean of estradiol; the corresponding study levels are: −2 SD = 1.86 pg/ml; −1 SD = 3.85 pg/ml; study mea n = 7.96 pg/ml; +1 SD = 16.45 pg/ml; +2 SD = 34.01 pg/ml
The OR is interpreted as the odds of increasing to the next inflammation ordered quartile category per SD change in the geometric mean of estradiol
Fig. 2.
Graph of the −Log10 p values of the associations between estradiol and each inflammation biomarker in the linear model (a) ordered from the most significant (CRP) to the least significant (GMCSF). b is the same order of inflammation biomarker, but the −Log10 p values are those for the ordinal models to demonstrate how the association changes with a different classification. The vertical lines are the cutpoints for statistical significance at the < 0.05 level and the Bonferroni-corrected level < 0.0007 (i.e., 0.05/69)
We investigated the robustness of the associations for the 7 biomarkers that were statistically significant in both the linear and ordinal models (Table 3). We dichotomized each inflammation biomarker at its median and ran logistic regressions with different categorizations of estradiol (continuous, binary, tertiles, and quartiles). For CXCL6, TARC, and Eotaxin-1, no association, under any estradiol classification, met nominal statistical significance. For those three, the different estradiol classifications resulted in relatively weak odds ratios, inconsistent directionality, and/or little suggestion of a dose–response relationship. The biomarkers PAI-1 and SAA were borderline significant with the linear estradiol variable, but the resulting associations from the binary, tertile, or quartile estradiol variable did not meet nominal statistical significance and did not suggest a dose–response (P for trend = 0.09 for PAI-1; P for trend = 0.14 for SAA). The associations for CRP and adiponectin, however, were robust to classification changes in estradiol. For CRP, we observed an OR 2.29 (95% CI 1.28, 4.09) for each SD increase in estradiol on the natural log scale, which corresponds to an estradiol increase from the study mean of 7.96 to 16.45 pg/ml. The quartile estradiol classification suggested an increasing dose–response (P for trend = 0.01) with the highest quartile of estradiol compared to the lowest resulting in an OR 7.48 (95% CI 1.63, 34.4). For adiponectin, the results were slightly more muted though they did suggest a consistent association with a borderline decreasing dose–response (P for trend = 0.06): OR 0.26 (95% CI 0.06, 1.07) when comparing the highest estradiol quartile to the lowest (Table 3).
Table 3.
Associations of dichotomized inflammation biomarkers with different classifications of estradiol
| CRP** |
Adiponectin* |
CXCL6 |
TARC |
Eotaxin-1 |
PAI-1* |
SAA |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Continuousa | 2.29 | (1.28, 4.09) | 0.46 | (0.26, 0.82) | 0.65 | (0.42, 1.02) | 0.81 | (0.53, 1.25) | 0.64 | (0.40, 1.02) | 1.67 | (1.00, 2.79) | 1.77 | (1.10, 2.85) |
| Binaryb | ||||||||||||||
| < 7.85 | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | ref | 1.0 | ref |
| ≥ 7.85 | 2.05 | (0.86, 4.88) | 0.54 | (0.23, 1.30) | 0.79 | (0.36, 1.76) | 1.33 | (0.61, 2.91) | 0.65 | (0.30, 1.42) | 1.79 | (0.75, 4.29) | 1.57 | (0.71, 3.46) |
| Tertilesb | ||||||||||||||
| < 6.3 | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | ref | 1.0 | ref |
| 6.3–11.5 | 2.20 | (0.86, 5.66) | 0.52 | (0.20, 1.34) | 0.98 | (0.42, 2.30) | 1.25 | (0.54, 2.91) | 0.76 | (0.33, 1.77) | 1.45 | (0.58, 3.58) | 1.67 | (0.71, 3.97) |
| ≥ 11.5 | 6.74 | (1.87, 24.2) | 0.23 | (0.07, 0.75) | 0.41 | (0.14, 1.19) | 0.74 | (0.27, 2.04) | 0.48 | (0.17, 1.34) | 2.95 | (0.88, 9.91) | 2.38 | (0.83, 6.83) |
| Quartilesb | ||||||||||||||
| < 5.05 | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | ref | 1.0 | ref |
| 5.05–7.85 | 2.31 | (0.77, 6.94) | 0.53 | (0.18, 1.59) | 0.96 | (0.36, 2.52) | 0.76 | (0.29, 2.01) | 0.55 | (0.20, 1.46) | 1.59 | (0.57, 4.43) | 2.03 | (0.74, 5.53) |
| 7.85–14.3 | 2.54 | (0.77, 8.43) | 0.41 | (0.13, 1.36) | 1.29 | (0.45, 3.71) | 1.43 | (0.50, 4.03) | 0.48 | (0.17, 1.38) | 2.04 | (0.66, 6.35) | 2.41 | (0.82, 7.07) |
| ≥ 14.3 | 7.48 | (1.63, 34.4) | 0.26 | (0.06, 1.07) | 0.30 | (0.08, 1.07) | 0.76 | (0.23, 2.48) | 0.39 | (0.12, 1.30) | 3.41 | (0.83, 14.0) | 2.54 | (0.74, 8.64) |
Median cut points for CPR: > 36.1 mg/l; Adiponectin: > 16.3 mg/l; CXCL6: > 84.5 pg/ml; TARC: > 88.9 pg/ml; Eotaxin-1: > 108.1 pg/ml; PAI-1: > 78.3 ng/ml; SAA: 9.7 mg/l
OR odds ratio, CI confidence interval, CRP C-reactive protein, CXCL6 chemokine (C-X-C motif) ligand-6, TARC thymus activation-regulated chemokine, Eotaxin-1 eosinophil chemotactic protein, PAI-1 lasminogen activator protein-1, SAA serum amyloid A
p value for trend with estradiol quartiles < 0.10
p value for trend with estradiol quartiles < 0.05
Estradiol was natural log transformed and standardized (unit of analysis is per SD); the corresponding study levels are: −2 SD = 1.86 pg/ml; −1 SD = 3.85 pg/ml; study mea n = 7.96 pg/ml; +1 SD = 16.45 pg/ml; +2 SD = 34.01 pg/ml
Estradiol is in pg/ml
Following the Logistic models, we conducted exploratory analyses stratified by obesity status (BMI < 30 vs. BMI ≥ 30). The positive estradiol–CRP association was stronger among the obese relative to the non-obese (OR 3.51, 95% CI 1.33, 9.32; vs. OR 1.95, 95% CI 0.90, 4.23; per SD increase in estradiol), while conversely, the positive associations for PAI-1 and SAA appeared stronger among the non-obese (Table 4). The inverse estradiol–adiponectin association was stronger among the non-obese relative to the obese (OR 0.29, 95% CI 0.10, 0.88; vs. OR 0.65, 95% CI 0.30, 1.38; per SD increase in estradiol).
Table 4.
Associations of dichotomized inflammation biomarkers with different classifications of estradiol stratified by obesity
| CRP |
Adiponectin |
CXCL6 |
TARC |
Eotaxin-1 |
PAI-1 |
SAA |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Among BMI <30 | ||||||||||||||
| Continuousa | 1.95 | (0.90, 4.23) | 0.29 | (0.10, 0.88) | 0.70 | (0.36, 1.35) | 0.84 | (0.44, 1.58) | 0.73 | (0.38, 1.40) | 2.24 | (1.03, 4.88) | 2.80 | (1.22, 6.44) |
| Binaryb | ||||||||||||||
| <8 pg/ml | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref |
| ≥8 pg/ml | 0.83 | (0.21, 3.33) | 0.32 | (0.07, 1.53) | 0.86 | (0.22, 3.35) | 1.03 | (0.28, 3.75) | 1.03 | (0.28, 3.79) | 3.47 | (0.70, 17.3) | 1.99 | (0.53, 7.44) |
| Among BMI ≥ 30 | ||||||||||||||
| Continuousa | 3.51 | (1.33, 9.32) | 0.65 | (0.30, 1.38) | 0.58 | (0.30, 1.13) | 0.70 | (0.36, 1.36) | 0.55 | (0.28, 1.07) | 1.47 | (0.67, 3.22) | 1.47 | (0.75, 2.87) |
| Binaryb | ||||||||||||||
| < 8 pg/ml | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref |
| ≥ 8 pg/ml | 6.37 | (1.54, 26.3) | 0.82 | (0.26, 2.56) | 0.79 | (0.28, 2.22) | 1.56 | (0.54, 4.47) | 0.44 | (0.16, 1.27) | 1.62 | (0.48, 5.48) | 1.57 | (0.55, 4.52) |
Median cut points for CPR: > 36.1 mg/l; Adiponectin: > 16.3 mg/l; CXCL6: > 84.5 pg/ml; TARC: > 88.9 pg/ml; Eotaxin-1: > 108.1 pg/ml; PAI-1: > 78.3 ng/ml; SAA: 9.7 mg/l;
OR odds ratio, CI confidence interval, BMI body mass index, CRP C-reactive protein, CXCL6 chemokine (C-X-C motif) ligand-6, TARC thymus activation-regulated chemokine, Eotaxin-1 eosinophil chemotactic protein, PAI-1 plasminogen activator protein-1, SAA serum amyloid A
Estradiol was natural log transformed and standardized (unit of analysis is per SD); the corresponding study levels are: −2 SD = 1.86 pg/ml; −1 SD = 3.85 pg/ml; study mean = 7.96 pg/ml; +1 SD = 16.45 pg/ml; +2 SD = 34.01 pg/ml
Estradiol is in pg/ml
Discussion
In this study of 155 healthy postmenopausal women not taking menopausal hormones, we found that circulating levels of endogenous estradiol was associated with 7 of 69 inflammation biomarkers measured in multiplex panels (CRP, adiponectin, CXCL6, TARC, Eotaxin-1, PAI-1, and SAA). The estradiol association with CRP was robust to false discovery considerations, and the association with adiponectin was consistently inversely associated by alternative classifications of estradiol. No prior study has examined the estradiol–inflammation link with such an expansive list of inflammation biomarkers.
Past research typically has assessed estrogen–disease and inflammation–disease relationships separately, a strategy that misses the broader biological complexity. It has been theorized that the two mechanisms are interrelated [13, 14], quite possibly through obesity. Adipose tissue is known to be biologically active, affecting many biological processes including sex hormones [5] and inflammation [32]. Indeed, high levels of estrogen and inflammation are two mechanisms by which obesity increases a person’s risk of cancer and other disease [5, 10]. Gaining a better understanding of these disease pathways, as many researchers are trying to do [15–19], means studying the two mechanisms together. This requires disentangling any effect that estrogen and inflammation may have on each other. The current analysis is a step in that direction. We analyzed the association of endogenous estradiol and 69 inflammation biomarkers in a menopausal hormone therapy-free female population—a population whose circulating estradiol is believed to result mostly from adipose tissue [33, 34]. To the extent that they are linked, the current analysis helps future studies be cognizant of, and account for, the complex relationships among obesity, estrogen, and inflammation.
To put our results in the proper context, it is important to discuss what is currently known about estradiol and individual inflammation biomarkers. CRP is a well-studied marker that is related to many acute or chronic inflammatory conditions. Despite its lack of specificity, it is an important marker because it is used clinically as a first test to evaluate various inflammatory conditions. Furthermore, CRP has been linked to cancer [35], specifically endometrial cancer in this dataset [26] and in others [15,16, 36]. In this analysis, CRP was the biomarker most consistently associated with circulating estradiol; we found estradiol positively associated with CRP, but interestingly, only borderline significant with IL-6, a promoter of CRP [37]. Our findings add to the evidence regarding endogenous estrogens and CRP in postmenopausal women. Our findings generally agree with those of Störk [38], Folsom [39], and Maggio [40], while they disagree with Crandall [41], Karim [42], Joffe [43], and Güdücü [44]. One possible reason for the conflicting findings is population age. The studies that found an association, including ours, were generally older (~64.5 years), while the studies that did not find an association were younger (~58.7 years) and thus more likely to include recently postmenopausal/perimenopause women whose estrogen levels may be influenced by other factors in addition to adipose tissue. Additionally, our study population was more obese (mean BMI = 32.7) than previous studies (mean BMI range 26–29), and we observed a stronger estradiol–CRP association among obese women than non-obese women suggesting the need for future studies to conduct stratified analyses. Given the conflicting evidence, CRP and estradiol may not be directly causally linked, but may have a more complex relationship—one that is influenced by obesity and which may be influenced by androgens, which are still produced after menopause, or estrogen binding proteins (sex hormone binding globulin).
Adiponectin, a cell signaling protein released from adipose tissue, regulates glucose levels and is inversely related to obesity and central adiposity [45]. The adipocy tokines (adiponectin, leptin, resistin, etc.) are recognized as potential mediators in the inflammatory and immune response [46]. Although the inverse association between estradiol and adiponectin observed in this study did not meet Bonferroni statistical significance, it was the most consistent estradiol–biomarker association after CRP. Unlike CRP for which there are conflicting prior studies, adiponectin shows a consistent inverse association with estradiol [47–51], with only two smaller studies suggesting no association [52, 53]. Furthermore, conditions that increase estrogen levels in women such as pregnancy [54], polycystic ovary syndrome [55], and taking unopposed estrogen hormone therapy [56, 57] tend to lower a woman’s adiponectin levels; though the effect from exogenous hormones appears to be restricted to oral rather than transdermal administration.
The other inflammation biomarkers that were nominally associated with estradiol in the linear and ordinal models were PAI-1, SAA, and the chemokines CXCL6, TARC, and Eotaxin-1. All have limited observational evidence in humans and require further study. SAA is an acute phase inflammatory protein, like CRP, that increases in response to unopposed oral estrogen hormone therapy [58]. PAI-1 is an inhibitory protein that impedes the degradation of blood clots. Interestingly, unlike SAA, PAI-1 decreases in response to unopposed estrogen hormone therapy [59]. Eotaxin-1 recruits eosinophils, a white blood cell associated with the allergic response. Laboratory evidence suggests that estradiol levels may inhibit eosinophil recruitment [60]. TARC is a chemokine expressed in the thymus by dendritic cells and is related to some autoimmune skin diseases [61]. Estradiol may not alter TARC directly, but may alter levels indirectly via lipopolysaccharide-stimulated dendritic cells [62]. CXCL6 is a chemokine that recruits neutrophils, a phagocytic white blood cell. Like TARC, estradiol may alter levels of CXCL6 indirectly via IL-8 [63].
IL-6 showed a positive association with estradiol, of borderline significance, in the ordinal model. This contrasts with reports of an inverse association [64–66] or no association with estradiol [40, 41]. For other commonly studied inflammation biomarkers such as tumor necrosis factor-alpha (TNF-alpha), transforming growth factor beta-1 (TGF-beta-1), IL-1, IL-8, and vascular endothelial growth factor (VEGF), we found no association with circulating estradiol. For TNF-alpha, the literature reports inverse or no association with estradiol [40, 53, 67]. The other biomarkers are more commonly studied in laboratory settings, or with unopposed or combined estrogen hormone therapy, but there is limited evidence on their associations with endogenous estradiol in humans.
Perhaps the most important limitation of our study is the small sample size coupled with the number of statistical tests performed. By performing many statistical tests, we increase the probability that some of our findings are false positives. We addressed this by the Bonferroni-corrected P value for our initial findings. We also altered our variable classification schemes to investigate the robustness of the associations. In theory, the tertile and quartile classification schemes can detect a curvilinear relationship, however, the smaller sample size in our study limits our ability to detect such relationships between estradiol and inflammation. The large number of inflammatory markers we examined with estradiol—more than any previous study—is also a strength of our analysis. Inflammation is a complex process not easily measured by a few individual biomarkers [11, 12]. Since the goal of the analysis was discovery based, we report nominally significant results knowing that replication of our findings is needed. For that reason, we are careful not to ascribe any strong conclusions from our results alone, but rather to place them in the context of previous literature. Some of our findings had prior literature for comparisons, but many of them were novel. Though the multiplex panel has good reproducibility [68], we relied on a single baseline blood draw for all our biomarker measurements. We excluded women who were currently taking menopausal hormone therapy, but did so by relying on self-report. It is possible that some women may have been misclassified. Thus, we also excluded women who had very high levels of circulating estradiol (> 40 pg/ml) suggesting that they may have been using exogenous hormones at blood draw.
We examined an extensive list of inflammatory biomarkers with endogenous estradiol in menopausal hormone therapy-free women. Our results combined with prior studies suggest that CRP, a marker of non-specific inflammation, is possibly linked with endogenous estradiol and should continue to be studied. Additionally, adiponectin, a protein involved in regulating glucose and fatty acids, may be inversely linked with estradiol. The complex relationship between inflammation and estradiol may necessitate researchers to measure both mechanisms when studying disease as one may act as an important confounder, mediator, or moderator of the other. Putting these interrelated obesity mechanisms into a larger framework will clarify their effects on cancer and other disease.
Supplementary Material
Acknowledgments
Funding This work was supported in part by the Cancer Prevention Fellowship Program and the Intramural Research Program of the National Cancer Institute, National Institutes of Health, as well as the Georgia CTSA (UL 1TR002378) and (KL2TR002381).
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10552-020-01280-6) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX et al. (2006) Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease From the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 113(6):898–918 [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444:840. [DOI] [PubMed] [Google Scholar]
- 3.Martí A, Marcos A, Martínez J (2001) Obesity and immune function relationships. Obes Rev 2(2):131–140 [DOI] [PubMed] [Google Scholar]
- 4.Ritchie SA, Connell JMC (2007) The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis 17(4):319–326 [DOI] [PubMed] [Google Scholar]
- 5.Calle EE, Kaaks R (2004) Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4(8):579–591 [DOI] [PubMed] [Google Scholar]
- 6.Kaaks R, Lukanova A, Kurzer MS (2002) Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev 11(12):1531–1543 [PubMed] [Google Scholar]
- 7.Wallace AE, Gibson DA, Saunders PTK, Jabbour HN (2010) Inflammatory events in endometrial adenocarcinoma. J Endocrinol 206(2):141–157 [DOI] [PubMed] [Google Scholar]
- 8.Simpson ER, Merrill JC, Hollub AJ, Graham-Lorence S, Mendelson CR (1989) Regulation of estrogen biosynthesis by human adipose cells. Endocr Rev 10(2):136–148 [DOI] [PubMed] [Google Scholar]
- 9.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I (2005) Endometrial cancer. Lancet 366(9484):491–505 [DOI] [PubMed] [Google Scholar]
- 10.van Kruijsdijk RCM, van der Wall E, Visseren FLJ (2009) Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomark Prev 18(10):2569–2578 [DOI] [PubMed] [Google Scholar]
- 11.Chaturvedi AK, Moore SC, Hildesheim A (2013) Invited commentary: circulating inflammation markers and cancer risk—implications for epidemiologic studies. Am J Epidemiol 177(1):14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eldridge RC, Flanders WD, Bostick RM, Fedirko V, Gross M, Thyagarajan B et al. (2017) Using multiple biomarkers and determinants to obtain a better measurement of oxidative stress: a latent variable structural equation model approach. Biomarkers 22(6):517–524 [DOI] [PubMed] [Google Scholar]
- 13.Modugno F, Ness RB, Chen C, Weiss NS (2005) Inflammation and endometrial cancer: a hypothesis. Cancer Epidemiol Biomarkers Prev 14(12):2840–2847 [DOI] [PubMed] [Google Scholar]
- 14.Straub RH (2007) The complex role of estrogens in inflammation. Endocr Rev 28(5):521–574 [DOI] [PubMed] [Google Scholar]
- 15.Wang T, Rohan TE, Gunter MJ, Xue X, Wactawski-Wende J, Rajpathak SN et al. (2011) A prospective study of inflammation markers and endometrial cancer risk in postmenopausal hormone nonusers. Cancer Epidemiol Biomarkers Prev 20(5):971–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dossus L, Rinaldi S, Becker S, Lukanova A, Tjonneland A, Olsen A et al. (2010) Obesity, inflammatory markers, and endometrial cancer risk: a prospective case–control study. Endocr Relat Cancer 17(4):1007–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Gaal LF, Mertens IL, De Block CE (2006) Mechanisms linking obesity with cardiovascular disease. Nature 444(7121):875–880 [DOI] [PubMed] [Google Scholar]
- 18.Goodwin PJ, Ennis M, Fantus IG, Pritchard KI, Trudeau ME, Koo J et al. (2005) Is leptin a mediator of adverse prognostic effects of obesity in breast cancer? J Clin Oncol 23(25):6037–6042 [DOI] [PubMed] [Google Scholar]
- 19.Dandona P, Aljada A, Bandyopadhyay A (2004) Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 25(1):4–7 [DOI] [PubMed] [Google Scholar]
- 20.Vegeto E, Benedusi V, Maggi A (2008) Estrogen anti-inflammatory activity in brain: a therapeutic opportunity for menopause and neurodegenerative diseases. Front Neuroendocrinol 29(4):507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue B, Johnson AK, Hay M (2013) Sex differences in angiotensin II- and aldosterone-induced hypertension: the central protective effects of estrogen. Am J Physiol-Regul Integr Comp Physiol 305(5):R459–R463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X-P, Reckelhoff JF (2011) Estrogen, hormonal replacement therapy and cardiovascular disease. Curr Opin Nephrol Hypertens 20(2):133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iyengar NM, Hudis CA, Dannenberg AJ (2013) Obesity and inflammation: new insights into breast cancer development and progression. Am Soc Clin Oncol Educ 33:46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh H, Coburn SB, Matthews CE, Falk RT, LeBlanc ES, Wactawski-Wende J et al. (2017) Anthropometric measures and serum estrogen metabolism in postmenopausal women: the Women’s Health Initiative Observational Study. Breast Cancer Res 19(1):28–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, David Crawford E et al. (2000) Design of the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control Clin Trials 21(6):273S–309S [DOI] [PubMed] [Google Scholar]
- 26.Trabert B, Eldridge RC, Pfeiffer RM, Shiels MS, Kemp TJ, Guillemette C et al. (2017) Prediagnostic circulating inflammation markers and endometrial cancer risk in the prostate, lung, colorectal and ovarian cancer (PLCO) screening trial. Int J Cancer 140(3):600–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vongpatanasin W, Tuncel M, Wang Z, Arbique D, Mehrad B, Jialal I (2003) Differential effects of oral versus transdermal estrogen replacement therapy on C-reactive protein in postmenopausal women. J Am Coll Cardiol 41(8):1358–1363 [DOI] [PubMed] [Google Scholar]
- 28.Cushman M, Legault C, Barrett-Connor E, Stefanick ML, Kessler C, Judd HL et al. (1999) Effect of postmenopausal hormones on inflammation-sensitive proteins, the Postmenopausal Estrogen/Progestin Interventions (PEPI) study. Circulation 100(7):717–722 [DOI] [PubMed] [Google Scholar]
- 29.Chaturvedi AK, Kemp TJ, Pfeiffer RM, Biancotto A, Williams M, Munuo S et al. (2011) Evaluation of multiplexed cytokine and inflammation marker measurements: a methodologic study. Cancer Epidemiol Biomark Prev 20(9):1902–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.FAQ: How do I interpret a regression model when some variables are log transformed? UCLA: statistical consulting group; https://stats.idre.ucla.edu/other/mult-pkg/faq/general/faqhow-do-i-interpret-a-regression-model-when-some-variables-are-log-transformed/ Accessed 28 June 2018. [Google Scholar]
- 31.SAS, Institute, Inc. (2012) Base SAS 9.4 Procedures Guide. 2nd SAS Institute Inc., Cary [Google Scholar]
- 32.Cancello R, Clément K (2006) Review article: is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG 113(10):1141–1147 [DOI] [PubMed] [Google Scholar]
- 33.Simpson ER (2003) Sources of estrogen and their importance. J Steroid Biochem Mol Biol 86(3):225–230 [DOI] [PubMed] [Google Scholar]
- 34.Potischman N, Swanson CA, Siiteri P, Hoover RN (1996) Reversal of relation between body mass and endogenous estrogen concentrations with menopausal status. J Natl Cancer Inst 88(11):756–758 [DOI] [PubMed] [Google Scholar]
- 35.Heikkila K, Harris R, Lowe G, Rumley A, Yarnell J, Gallacher J et al. (2009) Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control 20(1):15–26 [DOI] [PubMed] [Google Scholar]
- 36.Dossus L, Lukanova A, Rinaldi S, Allen N, Cust AE, Becker S et al. (2013) Hormonal, metabolic, and inflammatory profiles and endometrial cancer risk within the EPIC cohort: a factor analysis. Am J Epidemiol 177(8):787–799 [DOI] [PubMed] [Google Scholar]
- 37.Tanaka T, Narazaki M, Kishimoto T (2014) IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspect Biol 6(10):a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Störk S, Bots ML, Grobbee DE, Van Der Schouw YT (2008) Endogenous sex hormones and C-reactive protein in healthy postmenopausal women. J Intern Med 264(3):245–253 [DOI] [PubMed] [Google Scholar]
- 39.Folsom AR, Golden SH, Boland LL, Szklo M (2005) Association of endogenous hormones with C-reactive protein, fibrinogen, and white blood count in post-menopausal women. Eur J Epidemiol 20(12):1015–1022 [DOI] [PubMed] [Google Scholar]
- 40.Maggio M, Ceda GP, Lauretani F, Bandinelli S, Corsi AM, Giallauria F et al. (2011) SHBG, sex hormones, and inflammatory markers in older women. J Clin Endocrinol Metab 96(4):1053–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crandall C, Palla S, Reboussin B, Hu P, Barrett-Connor E, Reuben D et al. (2006) Cross-sectional association between markers of inflammation and serum sex steroid levels in the postmenopausal estrogen/progestin interventions trial. J Women’s Health 15(1):14–23 [DOI] [PubMed] [Google Scholar]
- 42.Karim R, Stanczyk FZ, Hodis HN, Cushman M, Lobo RA, Hwang J et al. (2010) Associations between markers of inflammation and physiological and pharmacological levels of circulating sex hormones in postmenopausal women. Menopause (New York, NY) 17(4):785–790 [PMC free article] [PubMed] [Google Scholar]
- 43.Joffe HV, Ridker PM, Manson JE, Cook NR, Buring JE, Rexrode KM (2006) Sex hormone-binding globulin and serum testosterone are inversely associated with C-reactive protein levels in postmenopausal women at high risk for cardiovascular disease. Ann Epidemiol 16(2):105–112 [DOI] [PubMed] [Google Scholar]
- 44.Guducu N, Gormus U, Kutay SS, Kavak ZN, Telatar B (2013) Endogenous sex hormones and their associations with cardiovascular risk factors in post-menopausal women. J Endocrinol Invest 36(8):588–592 [DOI] [PubMed] [Google Scholar]
- 45.Yang W-S, Lee W-J, Funahashi T, Tanaka S, Matsuzawa Y, Chao C-L et al. (2002) Plasma adiponectin levels in overweight and obese asians. Obes Res 10(11):1104–1110 [DOI] [PubMed] [Google Scholar]
- 46.Tilg H, Moschen AR (2006) Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6(10):772–783 [DOI] [PubMed] [Google Scholar]
- 47.Cust AE, Kaaks R, Friedenreich C, Bonnet F, Laville M, Lukanova A et al. (2007) Plasma adiponectin levels and endometrial cancer risk in pre- and postmenopausal women. J Clin Endocrinol Metab 92(1):255–263 [DOI] [PubMed] [Google Scholar]
- 48.Laughlin GA, Barrett-Connor E, May S (2006) Sex-specific determinants of serum adiponectin in older adults: the role of endogenous sex hormones. Int J Obes 31(3):457–465 [DOI] [PubMed] [Google Scholar]
- 49.Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C et al. (2003) Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab 88(10):4823–4831 [DOI] [PubMed] [Google Scholar]
- 50.Tworoger SS, Mantzoros C, Hankinson SE (2007) Relationship of plasma adiponectin with sex hormone and insulin-like growth factor levels. Obesity 15(9):2217–2224 [DOI] [PubMed] [Google Scholar]
- 51.Miyatani Y, Yasui T, Uemura H, Yamada M, Matsuzaki T, Kuwahara A et al. (2008) Associations of circulating adiponectin with estradiol and monocyte chemotactic protein-1 in postmenopausal women. Menopause 15(3):536–541 [DOI] [PubMed] [Google Scholar]
- 52.Sieminska L, Wojciechowska C, Niedziolka D, Marek B, Kos-Kudla B, Kajdaniuk D et al. (2005) Effect of postmenopause and hormone replacement therapy on serum adiponectin levels. Metabolism 54(12):1610–1614 [DOI] [PubMed] [Google Scholar]
- 53.Hong SC, Yoo SW, Cho GJ, Kim T, Hur JY, Park YK et al. (2007) Correlation between estrogens and serum adipocytokines in premenopausal and postmenopausal women. Menopause 14(5):835–840 [DOI] [PubMed] [Google Scholar]
- 54.Mazaki-Tovi S, Kanety H, Sivan E (2005) Adiponectin and human pregnancy. Curr DiabRep 5(4):278–281 [DOI] [PubMed] [Google Scholar]
- 55.Panidis D, Kourtis A, Farmakiotis D, Mouslech T, Rousso D, Koliakos G (2003) Serum adiponectin levels in women with polycystic ovary syndrome. Hum Reprod 18(9):1790–1796 [DOI] [PubMed] [Google Scholar]
- 56.Kunnari A, Santaniemi M, Jokela M, Karjalainen AH, Heikkinen J, Ukkola O et al. (2008) Estrogen replacement therapy decreases plasma adiponectin but not resistin in postmenopausal women. Metabolism 57(11):1509–1515 [DOI] [PubMed] [Google Scholar]
- 57.Chu MC, Cosper P, Nakhuda GS, Lobo RA (2006) A comparison of oral and transdermal short-term estrogen therapy in postmenopausal women with metabolic syndrome. Fertil Steril 86(6):1669–1675 [DOI] [PubMed] [Google Scholar]
- 58.Abbas A, Fadel PJ, Wang Z, Arbique D, Jialal I, Vongpatanasin W (2004) Contrasting effects of oral versus transdermal estrogen on serum amyloid A (SAA) and high-density lipoprotein-SAA in postmenopausal women. Arterioscler Thromb Vasc Biol 24(10):e164–e167 [DOI] [PubMed] [Google Scholar]
- 59.Vehkavaara S, Silveira A, Hakala-Ala-Pietilä T, Virkamäki A, Hovatta O, Hamsten A et al. (2001) Effects of oral and transdermal estrogen replacement therapy on markers of coagulation, fibrinolysis, inflammation and serum lipids and lipoproteins in postmenopausal women. Thromb Haemost 85(4):619–625 [PubMed] [Google Scholar]
- 60.Douin-Echinard V, Calippe B, Billon-Gales A, Fontaine C, Lenfant F, Tremollieres F et al. (2011) Estradiol administration controls eosinophilia through estrogen receptor-alpha activation during acute peritoneal inflammation. J Leukoc Biol 90(1):145–154 [DOI] [PubMed] [Google Scholar]
- 61.Saeki H, Tamaki K (2006) Thymus and activation regulated chemokine (TARC)/CCL17 and skin diseases. J Dermatol Sci 43(2):75–84 [DOI] [PubMed] [Google Scholar]
- 62.Bengtsson ÅK, Ryan EJ, Giordano D, Magaletti DM, Clark EA (2004) 17β-Estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood 104(5):1404–1410 [DOI] [PubMed] [Google Scholar]
- 63.Lockwood CJ, Arcuri F, Toti P, Felice CD, Krikun G, Guller S et al. (2006) Tumor necrosis factor-a and interleukin-1β regulate interleukin-8 expression in third trimester decidual cells. Am J Pathol 169(4):1294–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papadopoulos NG, Georganas K, Skoutellas V, Konstantellos E, Lyritis GP (1997) Correlation of interleukin-6 serum levels with bone density in postmenopausal women. Clin Rheumatol 16(2):162–165 [DOI] [PubMed] [Google Scholar]
- 65.Scheidt-Nave C, Bismar H, Leidig-Bruckner G, Woitge H, Seibel MJ, Ziegler R et al. (2001) Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past menopause. J Clin Endocrinol Metab 86(5):2032–2042 [DOI] [PubMed] [Google Scholar]
- 66.Yasui T, Maegawa M, Tomita J, Miyatani Y, Yamada M, Uemura H et al. (2007) Changes in serum cytokine concentrations during the menopausal transition. Maturitas 56(4):396–403 [DOI] [PubMed] [Google Scholar]
- 67.Punnonen J, Heinonen PK, Teisala K, Kujansuu E, Jansen CT, Punnonen R (1992) Demonstration of tumor necrosis factor-a in preovulatory follicular fluid: its association with serum 17β-estradiol and progesterone. Gynecol Obstet Invest 33(2):80–84 [DOI] [PubMed] [Google Scholar]
- 68.Wong H-L, Pfeiffer RM, Fears TR, Vermeulen R, Ji S, Rabkin CS (2008) Reproducibility and correlations of multiplex cytokine levels in asymptomatic persons. Cancer Epidemiol Biomark Prev 17(12):3450–3456 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.