Abstract
Purpose
To report the association of pembrolizumab, an immune checkpoint inhibitor (ICI), with giant cell arteritis (GCA) presenting as paracentral acute middle maculopathy (PAMM) secondary to retinal arterial occlusion.
Observations
86-year old male with history of treated choroidal melanoma now with metastatic uveal melanoma to the liver on pembrolizumab, an ICI, who presented with acute vision loss in the uninvolved left eye. Spectral domain optical coherence tomography showed band-like increased hyperreflectivity in the middle retinal layers at the level of the inner nuclear layer consistent with PAMM. Intravenous fluorescein angiogram demonstrated significant delay in filling of the superotemporal and inferotemporal arteries with nonperfusion of the temporal retina consistent with multiple branch retinal arterial occlusions. Work-up for GCA was performed and temporal artery biopsy showed healed arteritis.
Conclusions and Importance
Pembrolizumab can cause ocular and life-threatening systemic adverse effects and as use of ICIs has increased, it is important to be aware of these associations. There should be a low threshold for GCA work up in patients on ICI therapy who present with acute vision loss and evidence of retinal occlusive disease with or without classic GCA systemic symptoms.
Keywords: Pembrolizumab, Immune checkpoint inhibitor, Giant cell arteritis, Retinal arterial occlusion, Paracentral acute middle maculopathy
1. Introduction
Immune checkpoint inhibitor (ICI) therapy is a class of Food and Drug Administration (FDA) approved therapies used in the treatment of metastatic cutaneous melanoma.1, 2, 3 Pembrolizumab (Merck & Co, Boston, MA) is an ICI that targets programmed cell death protein-1 (PD-1) resulting in T cell upregulation directed against the systemic malignancy.1, 2, 3 Pembrolizumab has been used off-label to treat metastatic uveal melanoma (UM) with early promise.4 Secondary to this amplification of the immune system, however, various ocular and systemic side effects have been reported.5, 6, 7, 8, 9, 10, 11, 12, 13, 14 In this study, we present a case of a patient with metastatic UM to the liver treated with pembrolizumab who developed giant cell arteritis (GCA) presenting as paracentral acute middle maculopathy (PAMM) from retinal arterial occlusion.
2. Case report
An 86-year old Caucasian male with history of previously treated stage T3aN0M015 class 2 (Decision Dx-UM, Castle Biosciences, Inc., Dallas, TX) UM in the right eye was diagnosed with metastatic UM to the liver. He was not a candidate for local liver resection and was treated with 6 cycles of off-label intravenous pembrolizumab (207.6 mg in 50 mL of 0.9% normal saline) with infusions every three weeks.
After 3 cycles of pembrolizumab therapy (84 days following initiation of treatment), the patient developed anterior uveitis that was successfully controlled with topical prednisolone acetate 1% ophthalmic solution. After completion of cycle 6 of treatment (111 days following initiation of treatment), he presented with a 3 day history of acute central and nasal visual field loss in his previously uninvolved and asymptomatic left eye. His visual acuity was count fingers and 20/60 (previously 20/30) in the right and left eye, respectively. Intraocular pressure was 16 and 11 mmHg in the right and left eye, respectively.
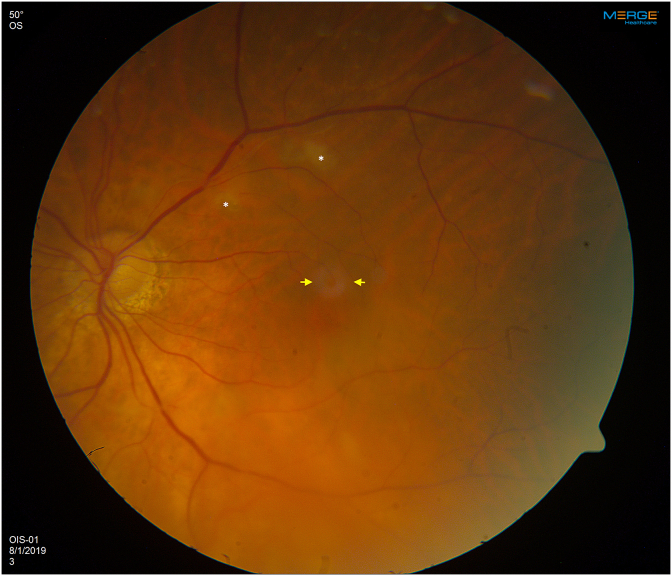
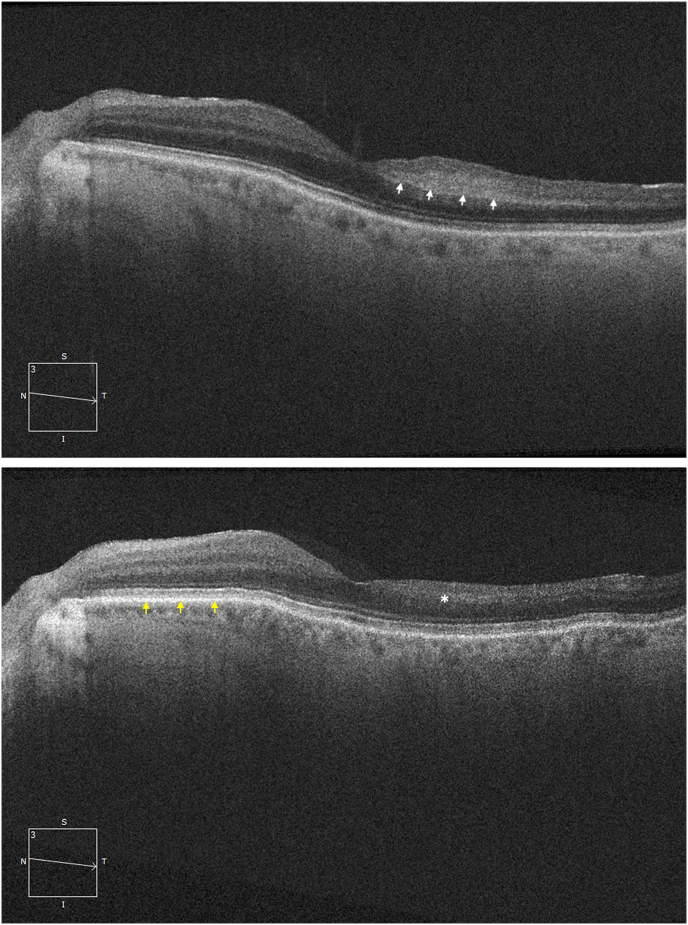
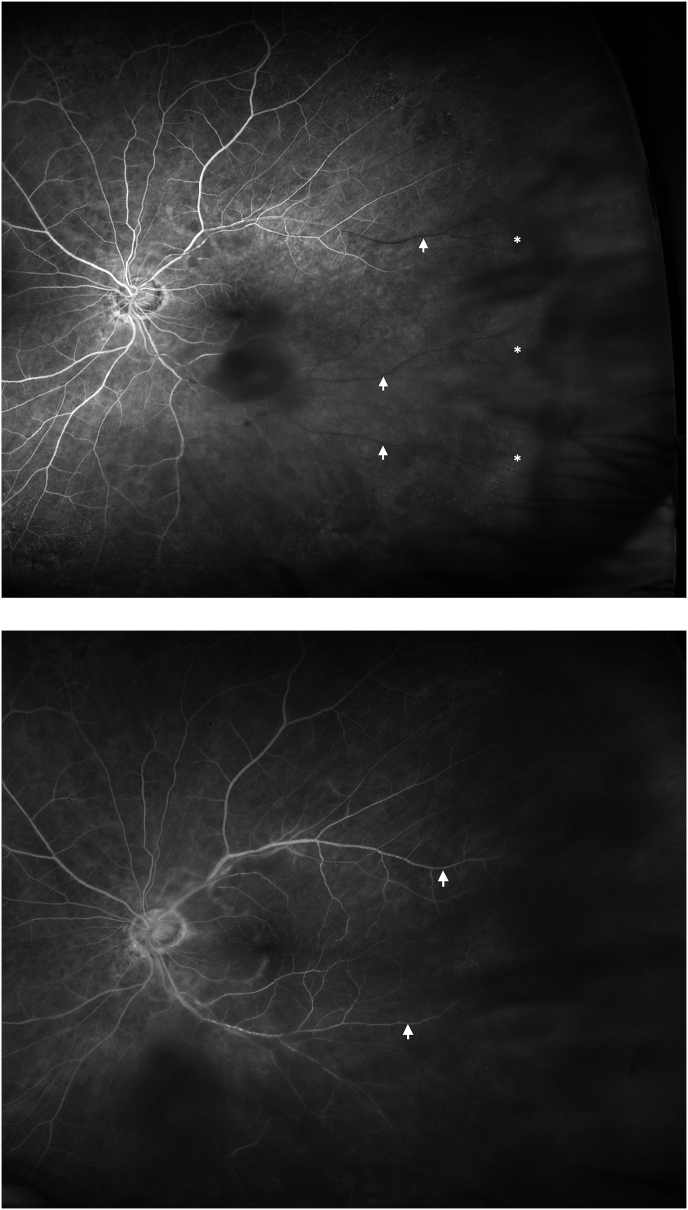
On examination, the right eye had evidence of treated UM with extensive radiation retinopathy but no neovascularization. In the left eye, there was no anterior chamber or vitreous inflammation. On dilated fundus examination, there was no sign of optic disc swelling or pallor. There were scattered cotton wool spots and a localized area of perifoveal retinal whitening (Fig. 1) with arterial attenuation and dropout of the vasculature in the temporal peripheral retina. Spectral domain optical coherence tomography (SD-OCT) of the left eye (Fig. 2A) demonstrated band-like increased hyperreflectivity in the middle retinal layers at the level of the inner nuclear layer temporal to the fovea consistent with PAMM. Intravenous fluorescein angiogram (IVFA) showed significant delay in filling of the superotemporal and inferotemporal arteries with nonperfusion of the temporal raphe and temporal peripheral retina with filling finally occurring at around 5 minutes (Fig. 3A and B). There was no evidence of choroidal ischemia and retinal vasculitis or leakage on IVFA.
Fig. 1.
Color fundus photograph (Zeiss, Jena, Germany) of the left eye demonstrating cotton wool spots (white asterisks) in the superotemporal macula. There is some mild retinal whitening superior to the fovea (yellow arrows). . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
(A) Spectral domain optical coherence tomography (SD-OCT) of the left eye at presentation showing a localized area of band-like hyperreflectivity (white arrows) temporal to the fovea in the middle retinal layers at the level of the inner nuclear layer consistent with paracentral acute middle maculopathy (PAMM). (B) SD-OCT of the left eye 1 month after presentation and initiation of oral prednisone for giant cell arteritis. In contrast to the intact nasal retina (yellow arrows), there is retinal thinning temporal to the fovea with loss of the inner retinal layers in the area of prior PAMM lesion (white asterisk). . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
(A) Intravenous fluorescein angiogram (IVFA) of the left eye at 1:00 minute showing hypofluorescence of the peripheral temporal retina and temporal raphe corresponding to nonperfusion (white asterisks). There is absence of filling of the distal inferotemporal and superotemporal retinal arteries (white arrows) as well as the distal arteries in the superior and temporal macula. (B) In very late frames (5:00 minutes), there is filling of the inferotemporal and superotemporal arteries that were previously nonperfused (white arrows).
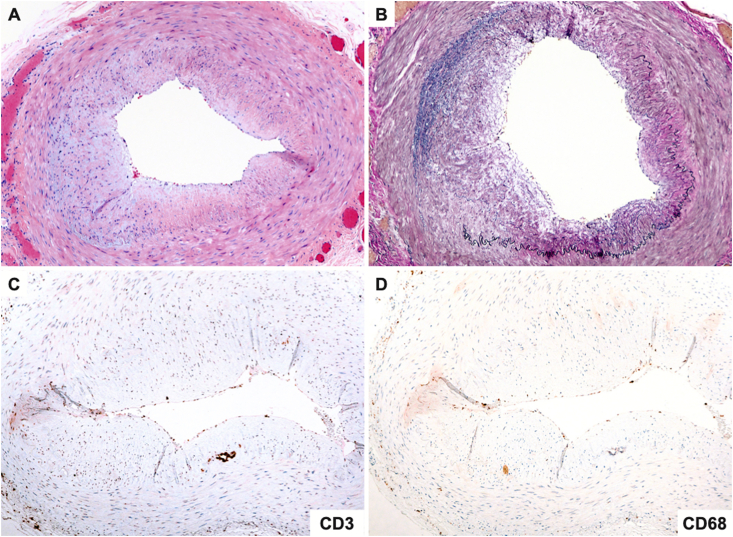
Given the presentation of sudden onset of vision loss with PAMM and retinal arterial occlusions, the patient was sent to the emergency department for expedited imaging for source of retinal occlusive disease and laboratory work up for inflammatory conditions, infectious conditions, and GCA. Blood pressure in the emergency department was 152/76. Carotid ultrasound did not show significant stenosis and echocardiogram was normal. Given prior episode of anterior uveitis, laboratory work up to evaluate for infectious and inflammatory causes was performed: rapid plasma reagin, fluorescent treponemal antibody absorption test, quantiferon tuberculosis gold test, lysozyme, and angiotensin converting enzyme were all negative. Although the patient denied systemic symptoms such as jaw claudication, scalp tenderness, and headache associated with GCA, the incidence of occult GCA with visual loss without systemic symptoms is 21.2%.16 Therefore, GCA work up was performed. Laboratory results revealed erythrocyte sedimentation rate (ESR) of 41 mm/hour (reference range 0–20) and C-reactive protein (CRP) of 3.0 mg/dl (reference < 0.5). Although ESR was not elevated by the Miller Criteria,17 because of elevation of CRP greater than 2.45 mg/dl which is a stronger predictor for GCA than ESR,18 the presence of retinal arterial occlusions, and vision loss in a patient over the age of 50 with normal cardiac and carotid ultrasound and no known cardiac risk factors, a high suspicion for GCA remained. Temporal artery biopsy was performed, and the pathology showed patchy loss of the internal elastic lamina and concentric intimal thickening without mural inflammation corresponding to healed arteritis (Fig. 4A–D). A diagnosis of presumed GCA was given.
Fig. 4.
(A) Cross sectional segment of temporal artery showing concentric intimal thickening without mural inflammation (H&E x100). (B) The elastic stain demonstrating asymmetric loss of the internal elastic membrane indicative of prior vascular injury (Elastic van Gieson x100). (C) The pan-T cell immunohistochemical stain, CD3 showing absence of inflammatory cells within the intimal and medial layers. (D) The macrophage immunostain, CD68 is also negative. The findings are consistent with healed arteritis.
Pembrolizumab was discontinued, given progression of his metastatic UM while on therapy and new onset GCA, and the GCA was treated with high dose oral prednisone (80 mg daily with gradual taper to 10 mg over 3 months) with improvement of visual acuity to 20/30 from 20/60 in the left eye. After 1 month of oral prednisone therapy, follow-up SD-OCT showed inner retinal atrophy temporal to the fovea in the same location as prior PAMM, notable in comparison to the intact normal retina nasal to the fovea (Fig. 2B). The patient ultimately elected to pursue hospice given progression of metastatic disease while on systemic treatment.
3. Discussion
Pembrolizumab is a FDA approved treatment for metastatic cutaneous melanoma and has been shown to improve progression-free and overall survival in these patients.2,3 In contrast, metastatic UM has no approved treatment. However due to pembrolizumab's efficacy in the management of metastatic cutaneous melanoma, it has been applied to metastatic UM but with limited efficacy.4 Kottschade et al. demonstrated a 50% progression free survival in metastatic UM patients treated with pembrolizumab with 12.5%, 12.5%, and 25% of patients showing stable disease, complete response, and partial response, respectively.4 Although ICIs are effective in the treatment of previously incurable malignancies, they are associated with various ocular and systemic adverse effects1,5, 6, 7, 8, 9, 10, 11, 12, 13, 14 with 64–75% of patients experiencing these immune related adverse effects.5
The most common ocular adverse effects from ICIs are uveitis (1%) and dry eye (1–24%) syndrome.7 Other reported effects include but are not limited to conjunctivitis, myasthenia gravis, choroidopathy, retinal vasculitis, Vogt-Koyanagi-Harada syndrome, inflammatory orbitopathy, and optic neuropathy.1,7 Pembrolizumab associated uveitis has been described to cause variable presentations.8, 9, 10, 11, 12 Aaberg et al. presented a case of a patient with metastatic choroidal melanoma who developed nongranulomatous panuveitis with retinal vasculitis after starting pembrolizumab.9 Samra et al. reported a case of anterior uveitis and papillitis that occurred following initiation of pembrolizumab and resolved after stopping the medication.10
In addition to ocular side effects, ICIs have been described to cause systemic nonrheumatic and rheumatic immune related adverse effects.5,8,12,14 The most common nonrheumatic adverse effects include enterocolitis, dermatitis, endocrinopathies, and hepatitis.8,14 Richter et al. described 61 of 1311 patients who developed rheumatic immune related adverse effects: 2% had inflammatory arthritis, 0.8% had a life-threatening myopathy, and the remainder had other rheumatic syndromes including GCA, Sjogren's syndrome, vasculitis, polymyalgia rheumatica, and others.13
The association between ICIs and GCA has been reported in a few cases in the literature.8,13,14 Goldstein et al. described one case of GCA associated with ipilimumab, another ICI that targets cytotoxic T-lymphocyte associated protein 4, where the patient presented with one episode of transient diplopia and amaurosis fugax.8 This patient also had a history of ipilimumab related colitis and hypophysitis.8 In another case report, an 88-year old female with non-small cell lung carcinoma developed sudden onset blurry vision in one eye along with colitis and abdominal pain after pembrolizumab infusion. A temporal artery biopsy was positive for GCA.14 In both reports, the authors did not elaborate on ophthalmic findings or examination and no ophthalmic imaging was discussed.
Immune checkpoints such as PD-L1 and PD-1 are vital for balancing stimulatory T cell dependent immune responses and preventing autoimmune disease.19 Zhang et al. studied normal, control arterial grafts and compared them to grafts treated with anti PD-1 antibodies.19 In the anti-PD1 grafts, there was marked T cell recruitment, intimal hyperplasia, and vessel remodeling leading to occlusion of the arterial lumina.19 It is plausible that pembrolizumab inhibits PD1 expression on vessels walls and via the same mechanism caused retinal arterial occlusions in our patient.
Ours is the first report of pembrolizumab-associated, presumed GCA manifesting as PAMM secondary to retinal arterial occlusion. In our patient under active treatment for unresectable metastatic UM, we had to balance systemic GCA treatment in an elderly patient with systemic corticosteroid immunosuppression with continuation of ICI therapy. Our patient had a prior history of anterior uveitis while on pembrolizumab which may have placed him at greater risk for additional inflammatory side effects.
4. Conclusion
It is important to recognize the potentially life-threatening systemic implications of pembrolizumab and other ICIs which are being used more frequently to treat metastatic melanoma and other malignancies. There should be a low threshold for systemic work up for GCA, a life-threatening rheumatic disorder, that can occur secondary to ICI therapy. Any patient with sudden vision loss on pembrolizumab should have a thorough dilated fundus examination. If there is concern for retinal, choroidal, or optic nerve ischemia, IVFA should be performed to evaluate for occlusive disease. If imaging demonstrates occlusive disease, it is important to perform a laboratory work up for GCA with possible temporal artery biopsy if there is a high level of suspicion. The mechanism of pembrolizumab induced GCA may be secondary to inhibition of PD-1 in vessels walls with subsequent recruitment of T cells leading to intimal hyperplasia, vessel remodeling, and vascular occlusion.
Patient consent
Written informed consent was obtained from patients for publication of these case reports and any accompanying images.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Financial disclosure
None of the authors has any financial interest in the subject matter and no financial support is involved in the study.
Declaration of competing interest
None of the authors have any financial disclosures.
Acknowledgements
No further acknowledgements.
References
- 1.Noble C.W., Gangaputra S.S., Thompson I.A. Ocular adverse events following use of immune checkpoint inhibitors for metastatic malignancies. Ocul Immunol Inflamm. 2019:1–6. doi: 10.1080/09273948.2019.1583347. 0(0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C., Schachter J., Long G.V. KEYNOTE-006 investigators. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 3.Ribas A., Puzanov I., Dummer R. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomized, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kottschade L., McWilliams R.R., Markovic S.N. The use of pembrolizumab for the treatment of metastatic uveal melanoma. Melanoma Res. 2016;26(3):300–303. doi: 10.1097/CMR.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 5.Ye C., Jamal S., Hudson M., Fifi-Mah A., Roberts J. Immune checkpoint inhibitor associated adverse events: a review of their presentations and treatments. Curr Treat Options Rheumatol. 2019;5:272–289. [Google Scholar]
- 6.Abdel-Rahman O., Oweira H., Petrausch U. Immune-related ocular toxicities in solid tumor patients treated with immune checkpoint inhibitors: a systematic review. Expet Rev Anticancer Ther. 2017;17(4):387–394. doi: 10.1080/14737140.2017.1296765. [DOI] [PubMed] [Google Scholar]
- 7.Dalvin L.A., Shields C.L., Orloff M., Sato T., Shields J.A. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina. 2018;38(6):1063–1078. doi: 10.1097/IAE.0000000000002181. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein B.L., Gedmintas L., Todd D.J. Drug associated polymyalgia rheumatica/giant cell arteritis occurring in two patients after treatment with ipilimumab, an antagonist of CTLA-4. Arthritis Rheumatol. 2013;66(3):768–769. doi: 10.1002/art.38282. [DOI] [PubMed] [Google Scholar]
- 9.Aaberg M.T., Aaberg T.M., Jr. Pembrolizumab administration associated with posterior uveitis. Retin Cases Brief Rep. 2017;11(4):348–351. doi: 10.1097/ICB.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 10.Samra K.A., Valdes-Navarro M., Lee S., Swan R., Foster C.S., Anesi S.D. A case of bilateral uveitis and papillitis in a patient treated with pembrolizumab. Eur J Ophthalmol. 2016;26(3):46–48. doi: 10.5301/ejo.5000724. [DOI] [PubMed] [Google Scholar]
- 11.Taylor S.C., Hrismalos G., Linette G.P., Rao P.K. A case of recurrent bilateral uveitis independently associated with dabrafenib and pembrolizumab therapy. Am J Ophthalmol Case Rep. 2016;2:23–25. doi: 10.1016/j.ajoc.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basilious A., Lloyd J.C. Posterior subcapsular cataracts and hypotony secondary to severe pembrolizumab induced uveitis: case report. Can J Ophthalmol. 2016;51(1):4–6. doi: 10.1016/j.jcjo.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Richter M.D., Crowson C., Kottschade L.A., Finnes H.D., Markovic S.N., Thanarajasingham U. Rheumatic syndromes associated with immune checkpoint inhibitors: a single-center cohort of sixty-one patients. Arthritis Rheumatol. 2018;71(3):468–475. doi: 10.1002/art.40745. [DOI] [PubMed] [Google Scholar]
- 14.Micaily I., Chernoff M. An unknown reaction to pembrolizumab: giant cell arteritis. Ann Oncol. 2017;28(10):2621–2622. doi: 10.1093/annonc/mdx306. [DOI] [PubMed] [Google Scholar]
- 15.Gershenwald J.E., Scolyer R.A., Hess K.R. Melanoma staging: evidence‐based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA A Cancer J Clin. 2017;67(6):472–492. doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayreh S.S., Podhasjsky P.A., Zimmerman B. Occult giant cell arteritis: ocular manifestations. Am J Ophthalmol. 1998;125(4):521–526. doi: 10.1016/s0002-9394(99)80193-7. [DOI] [PubMed] [Google Scholar]
- 17.Miller A., Green M., Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. B Med J (Clin Res Ed) 1983;286:266. doi: 10.1136/bmj.286.6361.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayreh S.S., Podhasky P.A., Raman R., Zimmerman B. Giant cell arteritis: validity and reliability of various diagnostic criteria. Am J Ophthalmol. 1997;123:285–296. doi: 10.1016/s0002-9394(14)70123-0. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H., Watanabe R., Berry G.J. Immunoinhibitory checkpoint deficiency in medium and large vessel vasculitis. Proc Natl Acad Sci Unit States Am. 2017;114(6):970–979. doi: 10.1073/pnas.1616848114. [DOI] [PMC free article] [PubMed] [Google Scholar]