Abstract
Purpose:
Adjuvant therapy decisions may in part be based on results of Oncotype DX Breast Recurrence Score® (RS) testing of primary tumors. When necessary, lymph node metastases may be considered as a surrogate. Here we evaluate the concordance in gene expression between primary breast cancers and synchronous lymph node metastases, based on results from quantitative RT-PCR-based RS testing between matched primary tumors and synchronous nodal metastases.
Methods:
This retrospective, exploratory study included patients (≥18 years old) treated at our center (2005–2009) who had ER+, HER2-negative invasive breast cancer and synchronous nodal metastases with available tumor blocks from both sites. Paired tissue blocks underwent RS testing, and RS and single-gene results for ER, PR, and HER2 were explored between paired samples.
Results:
A wide distribution of RS results in tumors and in synchronous nodal metastases were modestly correlated between 84 paired samples analyzed (Pearson correlation 0.69 [95% CI 0.55–0.78]). Overall concordance in RS group classification between samples was 63%. ER, PR, and HER2 by RT-PCR between the primary tumor and lymph node were also modestly correlated (Pearson correlation [95% CI]: 0.64 [0.50–0.75], 0.64 [0.49–0.75], and 0.51 [0.33–0.65], respectively). Categorical concordance (positive or negative) was 100% for ER, 77% for PR, and 100% for HER2.
Conclusions:
There is modest correlation in continuous gene expression, as measured by the RS and single-gene results for ER, PR, and HER2 between paired primary tumors and synchronous nodal metastases. RS testing for ER+ breast cancer should continue to be based on analysis of primary tumors.
Keywords: breast cancer, genomics, lymph node, metastases, Oncotype DX®, Recurrence Score®
BACKGROUND
Breast cancer is the leading cause of death in women aged 35 to 55 and the second leading cause of death among women of all ages. In 2017, there were an estimated 255,180 new cases of invasive breast cancer in the United States[1]. About one third of new diagnoses have metastasized to the axillary lymph nodes[2], and a small proportion of new diagnoses (<1%) present as regional nodal disease without clinical evidence of tumor in the breast [3,4]. At least 20% of women with early-stage breast cancer will later develop metastatic disease [5,6]. Many patients with nodal involvement will be recommended adjuvant chemotherapy treatment [7].
Estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) are critical biomarkers used to subgroup and subsequently guide adjuvant treatment decisions in the management of breast cancer [8]. Additionally, the Oncotype DX Breast Recurrence Score® (RS) assay, which uses quantitative RT-PCR to measure RNA expression from archival breast carcinoma tissue, provides prognostic and predictive information regarding chemotherapy benefit in early stage, hormone-receptor-positive (HR+), node-negative, or node-positive breast cancer [9–12]. Quantitative single-gene results for ER, PR, and HER2 are also assessed and reported as part of the Recurrence Score assay. These results have been shown to be highly concordant with immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH) assays (ER concordance: 96%; PR concordance: 90%; HER2 concordance: 95% [IHC] and 97% [FISH]) [13,14].
Comparisons of primary breast carcinomas and their metastases may be performed with either synchronous metastases, most commonly axillary lymph nodes, or asynchronous metastases. In asynchronous metastases, standard IHC markers are frequently discordant between the primary and the asynchronous metastasis [15]. This variability is multifactorial, and includes primary tumor molecular diversity and differential escape of clones [16,17], biological evolution caused by clonal selection following treatment [18], and pre-analytic differences such as delay to fixation, duration of fixation, or fixative differences. The most common difference between primary tumors and metastases is the loss of receptor expression, with the loss of positivity in either or both ER and PR in luminal breast cancer following adjuvant endocrine therapy alone or in combination with chemotherapy [18], and loss of HER2-positivity following trastuzumab therapy [19]. Loss of hormone or HER2 receptors is associated with a worse prognosis [18,19]. Because of such discordances between the primary tumors and the metastases, the American Society of Clinical Oncology (ASCO) recommends biopsy of all metastatic sites after any disease-free interval in order to assure appropriate management based on the biology of the metastasis [20]. Conversely, synchronous metastases show less receptor-discordance between the primary and synchronous distant metastasis [15]. Using more sensitive molecular techniques, a high degree of concordance has been noted in patients with synchronous metastases, although detailed computational analysis reveal that a small number of genes are consistently differentially expressed between tumors and metastases [21,15,22,23].
In cases of untreated patients presenting with occult breast carcinoma and axillary lymph node metastases, a high degree of concordance between these tumor tissues might be clinically valuable, if validated, in order to employ molecular assays that have been developed previously and validated using only primary breast cancer tissue [4]. Using the standardized, quantitative RT-PCR-based RS assay, this exploratory study was undertaken with the objective of comparing the RS result and quantitative single-gene results for ER, PR, and HER2 between primary breast carcinomas and their matched synchronous lymph node metastases.
STUDY DESIGN
This was a retrospective study of 100 patients who underwent axillary lymph node dissection based on a positive sentinel lymph node biopsy or presurgical biopsy for breast cancer at Beth Israel Medical Center between January 1, 2005 and January 1, 2009. Patients were identified chronologically through review of pathology reports. Study candidates were deemed eligible if they were 18 years of age or older with ER+ invasive breast cancer, had macrometastatic disease (>2.0 mm) in an axillary lymph node, and had available tumor blocks from both the primary tumor and the positive axillary lymph node(s). Patients with a prior history of breast cancer or who received neoadjuvant chemotherapy or hormonal therapy were excluded from the study.
Tissue blocks from matched primary breast cancer tumor and synchronous lymph node metastases were sent to the Genomic Health Clinical Laboratory for analysis using the Oncotype DX Breast Recurrence Score assay, as previously described [9]. Single-gene ER, PR, and HER2 expression were assessed by RT-PCR for the paired samples. RS groups were predefined as low (RS <18), intermediate (RS 18–30), and high (RS ≥31). ER and PR expression were predefined as negative if <6.5 and <5.5, respectively; HER2 expression was predefined as negative if <10.7, positive if ≥11.5, and equivocal otherwise.
STATISTICAL METHODS
Analyses were performed on all patients with reportable results from the Oncotype DX Breast Recurrence Score assay for both the primary tumor and the metastatic node. Patient characteristics were summarized using descriptive statistics. Scatterplots of RS results, and ER, PR, and HER2 expression from the primary and metastatic tumors were constructed. Pearson’s correlation coefficient and 95% confidence intervals (CI) were calculated to compare RS results and single gene expression between primary tumors and lymph node metastases, and Lin’s concordance statistic was evaluated for agreement. Discordance in RS groups was also measured, with one-level discordance defined as low ↔ intermediate or intermediate ↔ high, and two-level discordance as low ↔ high. General linear models were fit to the difference between the RS result from primary tumor and lymph node metastases to explore potential associations between the difference and pathology measurements. All analyses were performed in SAS 9.2 (SAS Institute, Inc.; Cary, NC).
RESULTS
Tumor specimens from 84 patients with median age 58 years (range 33 to 90 years) were included in the analysis. Most patients had 1–3 positive nodes (36%, 21%, and 14% with 1, 2, and 3 positive nodes, respectively), 24% had 4–9 positive nodes, and 5% had ≥10 positive nodes. Most tumors were grade 2 (61%), and 22% were known to be multicentric. Median tumor size was 2.0 cm (range 0.7 to 7.0 cm) (Table 1).
Table 1.
Baseline patient demographics and disease characteristics.
| Variable | N=84 |
|---|---|
| Median age, years (range) | 58 (33 to 90) |
| Median size,a cm (range) | 2.0 (0.7 to 7.0) |
| Histology, n (%)b | |
| Invasive ductal carcinoma | 56 (67%) |
| Invasive lobular carcinoma | 17 (20%) |
| Mixed | 10 (12%) |
| Invasive ductal carcinoma, papillary | 1 (1%) |
| Grade, n (%)b | |
| 1 | 8 (11%) |
| 2 | 46 (61%) |
| 3 | 22 (29%) |
| Unknown | 8 |
| Extracapsular extension,c n (%)b | |
| No | 58 (71%) |
| Yes | 24 (29%) |
| Unknown | 2 |
| Multicentric, n (%)b | |
| No | 63 (78%) |
| Yes | 18 (22%) |
| Unknown | 3 |
| Lymphovascular invasion, n (%)b | |
| No | 44 (54%) |
| Yes | 38 (46%) |
| Unknown | 2 |
| No. of positive nodes, n (%)b | |
| 1 | 30 (36%) |
| 2 | 18 (21%) |
| 3 | 12 (14%) |
| 4 to 9 | 20 (24%) |
| ≥10 | 4 (5%) |
| ER by IHC, n (%)b | |
| 1–2+ | 1 (1%) |
| 2+ | 17 (20%) |
| 2–3+ | 2 (2%) |
| 3+ | 64 (76%) |
| PR by IHC, n (%)b | |
| 0 | 5 (6%) |
| 1+ | 7 (9%) |
| 1–2+ | 3 (4%) |
| 2+ | 16 (20%) |
| 2–3+ | 3 (4%) |
| 3+ | 47 (58%) |
| Unknown | 3 |
| HER2 by IHC, n (%)b | |
| 0 | 38 (45%) |
| 1+ | 22 (26%) |
| 2+ | 24 (29%) |
Tumor size information was available for 82 patients only.
Percentages are reported in non-missing data only.
Extracapsular extension, as detected by lymph node pathology.
There was a wide distribution of RS results for both the primary tumors and the lymph node metastases, and a modest correlation between the paired samples (Pearson correlation [95% CI] 0.69 [0.55–0.78]) (Figure 1). Differences between primary tumor and lymph node RS results varied in magnitude, but with no obvious predictive pattern.
Fig. 1.
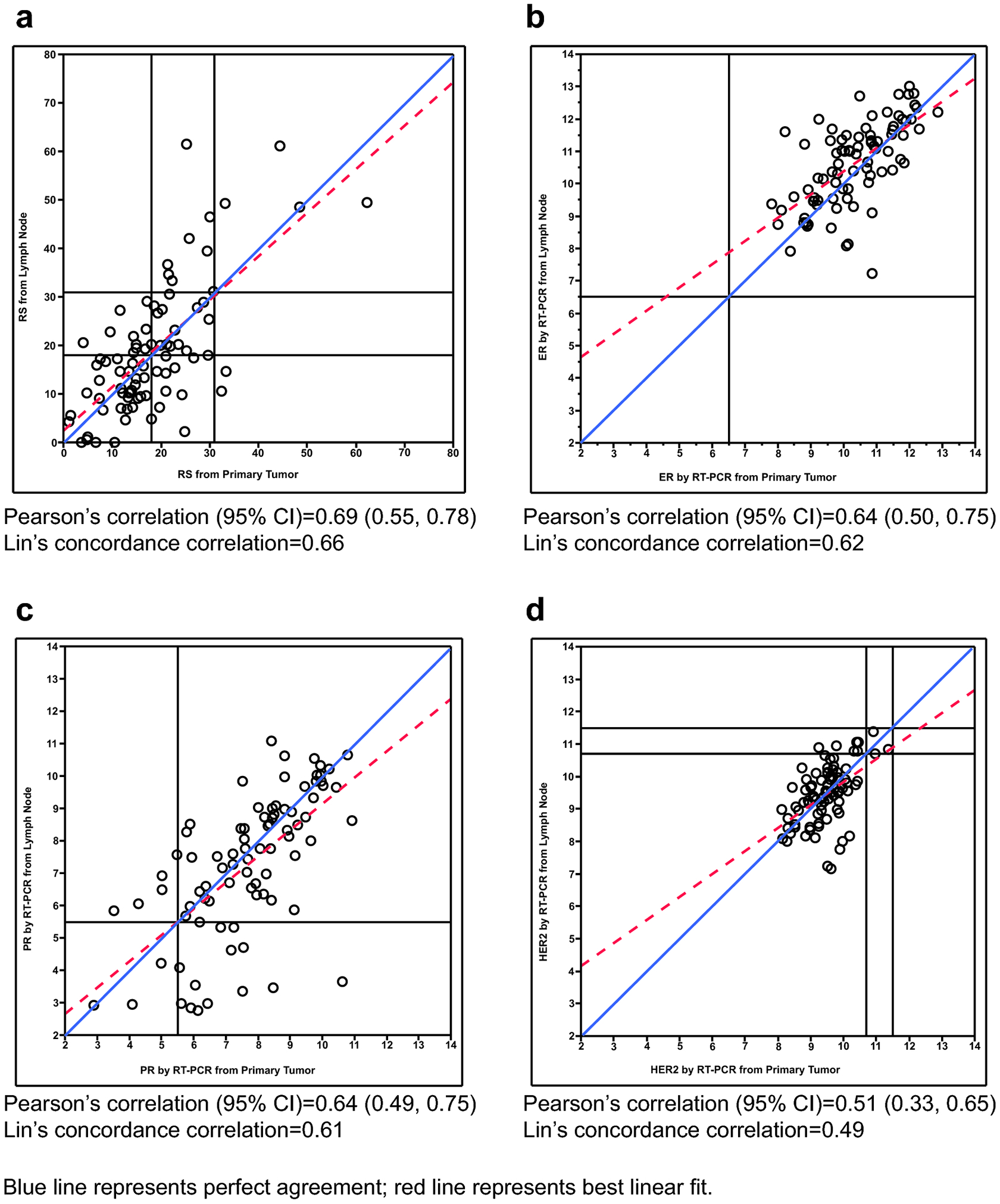
Comparison of Gene Expression Results Measured in the Primary Tumor and the Lymph Node. Scatterplots of (a) Recurrence Score result; (b) ER by RT-PCR; (c) PR by RT-PCR; (d) HER2 by RT-PCR measured in the primary tumor and the lymph node
RS grouping differed between primary tumor and lymph node for 37% of the patients, with 35% displaying one-level discordance, and 2% two-level discordance. Overall concordance in RS classification between samples was 63% (Table 2). The differences in RS groups between primary tumor and lymph node metastases were not associated with histologic subtypes, tumor grade, presence of extracapsular extension, multicentricity, lymphovascular invasion, or the number of positive lymph nodes (Table 3). RS results were not always higher in the lymph node metastases.
Table 2.
Concordance in classification by Recurrence Score group between primary tumor and lymph node.
| Status from primary tumor | Status from lymph node | ||||
| Recurrence Score group | RS <18 | RS 18–30 | RS ≥31 | Concordance | |
| RS <18 | 35 (42%) | 10 (12%) | 0 | 63% | |
| RS 18–30 | 11 (13%) | 14 (17%) | 8 (10%) | ||
| RS ≥31 | 2 (2%) | 0 | 4 (5%) | ||
| ER status by RT-PCR | Negative | Positive | Concordance | ||
| Negative | 0 | 0 | 100% | ||
| Positive | 0 | 84 (100%) | |||
| PR status by RT-PCR | Negative | Positive | Concordance | ||
| Negative | 3 (4%) | 5 (6%) | 77% | ||
| Positive | 14 (17%) | 62 (74%) | |||
| HER2 status by RT-PCR | Negative | Equivocala | Positive | Concordance | |
| Negative | 75 (89%) | 6 (7%) | 0 | 100% | |
| Equivocala | 1 (1%) | 2 (2%) | 0 | ||
| Positive | 0 | 0 | 0 | ||
HER2 equivocal (italics) excluded per ASCO/CAP guidelines [28].
Table 3.
Univariable analysis of difference between Recurrence Score results by primary tumor and lymph node.
| Pathology variable | na | p-value |
|---|---|---|
| Histologic subtype | 84 | 0.57 |
| Tumor grade | 76 | 0.56 |
| Extracapsular extension | 82 | 0.60 |
| Multicentric | 81 | 0.37 |
| Lymphovascular invasion | 82 | 0.62 |
| No. positive lymph nodes | 84 | 0.85 |
Includes only patients with available data for a given variable.
Modest correlations were also observed in ER and PR by RT-PCR between the primary tumor and lymph node metastasis (Pearson correlation [95% CI] 0.64 [0.50–0.75] and 0.64 [0.49–0.75], respectively). Like RS results, differences in ER and PR between primary tumor and lymph node varied in magnitude without any obvious patterns (Figure 2). There was no discordance in categorization of ER-positivity within the samples; all samples were classified as ER-positive (Table 4). By contrast, PR status of the primary tumor differed from that of the lymph node in 23% of cases; 14 cases converted from PR-positive in the primary breast carcinoma to PR-negative in the lymph node metastases, and 5 cases converted from PR-negative in the primary tumor to PR-positive in the metastases. HER2 expression by RT-PCR in the primary tumor was moderately correlated with that in the lymph node metastases (Pearson correlation [95% CI] 0.51 [0.33–0.65]). As there were no HER2-positive primary breast cancer samples included in the study, concordance in HER2 status cannot be fully assessed. (Figure 2, Table 4). Gene expression for ER, PR, and HER2 were not consistently lower in the lymph node metastases.
DISCUSSION
In this study of ER+, HER2-negative primary breast carcinomas and paired synchronous lymph node metastases, we found a modest correlation in continuous gene expression, as measured by the RS result and single-gene results for ER, PR, and HER2, between primary carcinoma and synchronous nodal metastasis. These exploratory findings add to the body of literature that has shown concordance of receptor status using IHC between primary breast tumors and paired synchronous nodal metastases. These findings suggest that gene expression results are different between the two sites and that use of lymph node metastases for clinical molecular testing would need validation before implementation. Thus, primary tumor tissue remains the preferred input material for Recurrence Score testing.
Although there is a wide body of literature showing concordance between breast primary and asynchronous metastases (nodal or distant), the literature is quite limited with respect to studies that examine specifically the concordance between breast primary and synchronous lymph nodes metastases. Ba et al evaluated the concordance of ER, PR, and HER2 expression, as measured by IHC, in 209 patients with paired breast primary tumors and synchronous lymph node metastases [24]. They found the rate of discordance in ER status to be 25.0%, PR status 28.9%, and HER2 status 14.0%. A similar study by Falck et al found the concordance rate of ER expression to be 93% (n=262 paired samples), PR expression 84% (n=257 paired samples), and HER2 expression 97% (n=104 paired samples) [25]. A study by Aitken et al involving 211 patients with paired specimens demonstrated the overall risk of discordance to be as high as 46.9% in at least one receptor [26].
This present study found a modest correlation in continuous gene expression, as measured by the RS result and single gene scores for ER, PR, and HER2, between primary carcinoma and synchronous nodal metastasis. Concordance by RS group (<18, 18–30, ≥31) was 63%. Discordance in RS group was not associated with histologic subtype, tumor grade, multicentricity, presence of extracapsular extension, lymphovascular invasion, or the number of positive nodes. With respect to quantitative gene expression for ER, PR and HER2, there were no differences in ER or HER2 status when they were assessed categorically as positive or negative between the primary tumor and the nodal metastasis, despite differences in continuous gene expression. As in prior studies, PR status of the primary tumor differed from that of the metastatic lymph nodes in 23% of cases. Notably, there were no matched patient samples that converted from ER+ primary breast carcinoma to ER-negative lymph node metastasis or from HER2-negative primary breast carcinoma to HER2+ lymph node metastasis, changes that might prompt reconsideration of patient treatment. Also noteworthy is that RS results were not always higher nor single-gene expression levels lower in the lymph node metastases, suggesting that associated lymphocytes do not dilute gene expression as assessed in the lymph node compared to the primary breast carcinoma [27].
The strengths of our study include the use of a rigorously, analytically validated quantitative RT-PCR assay rather than IHC, allowing exploration of the wide dynamic range of quantitative evaluation of gene expression. Inclusion criteria prespecified macrometastatic disease that could be microdissected to enrich for tumor tissue in a central laboratory with expertise in such dissection. Also, synchronous tumors without intervening treatment effect were intentionally chosen. The limitations of this study were that clinical endpoints were not examined, e.g., time to distant recurrence, precluding assessments of which tumor would be most informative regarding the risk of recurrence. Additionally, ER and HER2 expression discordance cannot be fully assessed as patients with ER-negative or HER2+ primary breast cancer were excluded from the study.
CONCLUSION
This exploratory study adds meaningful information to the limited body of knowledge regarding the concordance in biomarkers between a primary breast carcinoma and synchronous lymph node metastases. In most cases, the gene expression profiles of the primary tumor and lymph node were similar; in cases where there was variability, there may be actual biological differences. Confirmation of these data in larger, controlled studies would be necessary before gene expression from lymph node metastases could be used instead of results derived from breast primaries to make adjuvant treatment decisions. We recommend that Recurrence Score testing should continue to be based on analysis of the primary tumor, as is the current standard practice.
Acknowledgement:
The authors thank Anna Lau, PhD for editorial support.
Funding: Genomic Health provided a research grant for the analysis of the specimens and provided editorial support of manuscript development. The preparation of this study was supported in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center.
LIST OF ABBREVIATIONS
- ASCO
American Society of Clinical Oncology
- Cl
confidence interval
- ER
estrogen receptor
- FISH
fluorescent in situ hybridization
- HER2
human epidermal growth factor receptor 2
- IHC
immunohistochemistry
- PR
progesterone receptor
- RS
Recurrence Score®
- RT-PCR
reverse transcriptase-polymerase chain reaction
- transATAC
translational study of the Arimidex, Tamoxifen, Alone or in Combination trial
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Ethical Standards: The study was reviewed and approved by an Institutional Review Board/Ethics Committee and appropriate Scientific Review Committees prior to initiation.
Disclosures of Potential Conflicts of Interest: SKB has served on the speaker’s bureau for Genomic Health. JA was employed at Genomic Health at the time the study occurred. DD, DMJ, and FLB are currently employed at and own stock in Genomic Health. SM has served on the speaker’s bureau for and owns stock in Genomic Health. MH, MC, LK, JMC, and PK declare that they have no conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67 (1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.NCI SEER cancer statistics factsheets: breast cancer. http://seer.cancer.gov/statfacts/html/breast.html. Accessed September 21 2017.
- 3.Fayanju OM, Jeffe DB, Margenthaler JA (2013) Occult primary breast cancer at a comprehensive cancer center. J Surg Res 185 (2):684–689. doi: 10.1016/j.jss.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker GV, Smith GL, Perkins GH, Oh JL, Woodward W, Yu TK, Hunt KK, Hoffman K, Strom EA, Buchholz TA (2010) Population-based analysis of occult primary breast cancer with axillary lymph node metastasis. Cancer 116 (17):4000–4006. doi: 10.1002/cncr.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke M, Collins R, Darby S, Davies C, Evans V, Godwin J, Gray R, McGale P, Peto R, Wang Y, Early Breast Cancer Trialists’ Collaborative G (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717. [DOI] [PubMed] [Google Scholar]
- 6.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD, Feuer EJ, Cancer I, Surveillance Modeling Network C (2005) Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 353 (17):1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network (2017) Clinical Practice Guidelines in Oncology: Breast Cancer. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed February 1 2018.
- 8.[No authors listed (2000) Adjuvant therapy for breast cancer. NIH Consens Statement 17 (4):1–35. [PubMed] [Google Scholar]
- 9.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351 (27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 10.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE, Wickerham DL, Wolmark N (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24 (23):3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 11.Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE, Sledge GW, Winer EP, Hudis C, Ingle JN, Perez EA, Pritchard KI, Shepherd L, Gralow JR, Yoshizawa C, Allred DC, Osborne CK, Hayes DF, Breast Cancer Intergroup of North A (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11 (1):55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, Quinn E, Dunbier A, Baum M, Buzdar A, Howell A, Bugarini R, Baehner FL, Shak S (2010) Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 28 (11):1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 13.Badve SS, Baehner FL, Gray RP, Childs BH, Maddala T, Liu ML, Rowley SC, Shak S, Perez EA, Shulman LJ, Martino S, Davidson NE, Sledge GW, Goldstein LJ, Sparano JA (2008) Estrogen- and progesterone-receptor status in ECOG 2197: comparison of immunohistochemistry by local and central laboratories and quantitative reverse transcription polymerase chain reaction by central laboratory. J Clin Oncol 26 (15):2473–2481. doi: 10.1200/JCO.2007.13.6424. [DOI] [PubMed] [Google Scholar]
- 14.Baehner FL, Achacoso N, Maddala T, Shak S, Quesenberry CP Jr., Goldstein LC, Gown AM, Habel LA (2010) Human epidermal growth factor receptor 2 assessment in a case-control study: comparison of fluorescence in situ hybridization and quantitative reverse transcription polymerase chain reaction performed by central laboratories. J Clin Oncol 28 (28):4300–4306. doi: 10.1200/JCO.2009.24.8211. [DOI] [PubMed] [Google Scholar]
- 15.Kroigard AB, Larsen MJ, Thomassen M, Kruse TA (2016) Molecular concordance between primary breast cancer and matched metastases. Breast J 22 (4):420–430. doi: 10.1111/tbj.12596. [DOI] [PubMed] [Google Scholar]
- 16.Marusyk A, Polyak K (2010) Tumor heterogeneity: causes and consequences. Biochim Biophys Acta 1805 (1):105–117. doi: 10.1016/j.bbcan.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellsworth RE, Blackburn HL, Shriver CD, Soon-Shiong P, Ellsworth DL (2017) Molecular heterogeneity in breast cancer: State of the science and implications for patient care. Semin Cell Dev Biol 64:65–72. doi: 10.1016/j.semcdb.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Lindstrom LS, Karlsson E, Wilking UM, Johansson U, Hartman J, Lidbrink EK, Hatschek T, Skoog L, Bergh J (2012) Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol 30 (21):2601–2608. doi: 10.1200/JCO.2011.37.2482. [DOI] [PubMed] [Google Scholar]
- 19.Amir E, Miller N, Geddie W, Freedman O, Kassam F, Simmons C, Oldfield M, Dranitsaris G, Tomlinson G, Laupacis A, Tannock IF, Clemons M (2012) Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol 30 (6):587–592. doi: 10.1200/JCO.2010.33.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, Fallowfield L, Fowble B, Ingle JN, Jahanzeb M, Johnston SR, Korde LA, Khatcheressian JL, Mehta RS, Muss HB, Burstein HJ (2016) Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol 34 (25):3069–3103. doi: 10.1200/JCO.2016.67.1487. [DOI] [PubMed] [Google Scholar]
- 21.van Agthoven T, Timmermans M, Dorssers LC, Henzen-Logmans SC (1995) Expression of estrogen, progesterone and epidermal growth factor receptors in primary and metastatic breast cancer. Int J Cancer 63 (6):790–793. [DOI] [PubMed] [Google Scholar]
- 22.Ellsworth RE, Seebach J, Field LA, Heckman C, Kane J, Hooke JA, Love B, Shriver CD (2009) A gene expression signature that defines breast cancer metastases. Clin Exp Metastasis 26 (3):205–213. doi: 10.1007/s10585-008-9232-9. [DOI] [PubMed] [Google Scholar]
- 23.Hao X, Sun B, Hu L, Lahdesmaki H, Dunmire V, Feng Y, Zhang SW, Wang H, Wu C, Wang H, Fuller GN, Symmans WF, Shmulevich I, Zhang W (2004) Differential gene and protein expression in primary breast malignancies and their lymph node metastases as revealed by combined cDNA microarray and tissue microarray analysis. Cancer 100 (6):1110–1122. doi: 10.1002/cncr.20095. [DOI] [PubMed] [Google Scholar]
- 24.Ba JL, Liu CG, Jin F (2014) Alterations in hormonal receptor expression and HER2 status between primary breast tumors and paired nodal metastases: discordance rates and prognosis. Asian Pac J Cancer Prev 15 (21):9233–9239. [DOI] [PubMed] [Google Scholar]
- 25.Falck AK, Ferno M, Bendahl PO, Ryden L (2010) Does analysis of biomarkers in tumor cells in lymph node metastases give additional prognostic information in primary breast cancer? World J Surg 34 (7):1434–1441. doi: 10.1007/s00268-010-0499-z. [DOI] [PubMed] [Google Scholar]
- 26.Aitken SJ, Thomas JS, Langdon SP, Harrison DJ, Faratian D (2010) Quantitative analysis of changes in ER, PR and HER2 expression in primary breast cancer and paired nodal metastases. Ann Oncol 21 (6):1254–1261. doi: 10.1093/annonc/mdp427. [DOI] [PubMed] [Google Scholar]
- 27.Dabbs DJ, Klein ME, Mohsin SK, Tubbs RR, Shuai Y, Bhargava R (2011) High false-negative rate of HER2 quantitative reverse transcription polymerase chain reaction of the Oncotype DX test: an independent quality assurance study. J Clin Oncol 29 (32):4279–4285. doi: 10.1200/jco.2011.34.7963. [DOI] [PubMed] [Google Scholar]
- 28.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF, American Society of Clinical O, College of American P (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31 (31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]


