Abstract
In this study, a new type of silver nanoparticles capped with metronidazolium based ionic liquid is synthesized. By this aim, metronidazole is altered to ionic-liquid type structure with citrate counter ion as reducing agent. The produced reducing agent was characterized using 1HNMR and 13CNMR and FT-IR. The capability of metronidazolium-based reducing agent in formation and capping silver nanoparticles was examined in a chemical reaction. More specifically, synthesized silver nanoparticles were synthesized and capped with metronidazolium-citrate based ionic liquid, while the formation of particles in 48 h was monitored by UV-Vis spectroscopy. Fourier transform infrared spectroscopy showed the presence of capping agents around silver nanoparticles. The amount of metronidazolium and citrate as capping agents was determined by thermal gravimetric analysis. The prepared crystalline structure of silver nanoparticles was proved by X-ray diffraction spectroscopy. PSA analysis and TEM was performed to determine the size of particles. The synthesized silver nanoparticle has the potential to be used as an antibacterial agent in preparation of wound dressing with extra capability and efficacy in aerobic and anaerobic bacterium. In this regard, the antibacterial efficacy of discs from different concentration of silver nanoparticles in calcium alginate medium were evaluated in Gram-negative and Gram-positive bacterium.
Keywords: Materials chemistry, Materials science, Metronidazole, Silver nanoparticle, Antibacterial agent
Materials chemistry, Materials science, Metronidazole; Silver nanoparticle; Antibacterial agent.
1. Introduction
Nanotechnology is known as an emerging science with great impact in development of new technologies in the field of medicine [1]. Nanomedicine has revolutionized traditional methods of treatment, especially through the expansion of different types of nanopharmaceuticals, several of which are currently on the market [2]. Progress in the formation of nanoparticles (i.e. metallic and non-metallic) have rendered significant innovations in the methods of drug and gene delivery, diagnosis, antimicrobial agents and health care tools [3, 4, 5, 6, 7]. Among metallic nanoparticles, silver nanoparticles (AgNPs) are of utmost importance and utility for biomedical applications [8]. AgNPs are mostly known for antibacterial, antiviral and antifungal activities, but their applications are not limited to these fields [9, 10]. Interesting scientific advancements of AgNPs' utilization was found in drug delivery, anti-angiogenic and anti-inflammatory activities [11].
Synthesis of AgNPs could be performed through chemical, physical or biological techniques [12, 13]. Chemical technique is one of the most efficient and rapid approaches, which is based on the presence of three components: silver salt, reducing agent and capping or stabilizing agent. The chemical methods of AgNPs could be regarded as “green” protocol, while mostly performed in aqueous solution [14]. The presence of capping agent is mandatory to inhibit naked Ag(0) particles from agglomeration, control sizes and to provide a platform for further functionalization [15]. Citrate capped AgNPs which are mostly synthesized through utilization of sodium citrate as reducing agents are proven to have high stability and be an effective antibacterial agent on Gram-positive and Gram-negative bacteria [16].
Ionic liquids (IL) have also been applied for synthesis of AgNPs as stabilizing agents and even as reaction medium. ILs could provide tri-dimensional hydrophilic networks around nanoparticles and interact with aqueous medium and finely support size and shapes [17]. There is evidence that combining AgNPs and silver complexes with antimicrobial agents can result in enhancement of antibacterial efficacy. Silver sulfadiazine, a widely applied medication in burn treatment, is one of this examples [18]. AgNPs capped with antibiotics (i.e. streptomycine, ampiciline and tetracycline) have rendered increased antibacterial activities in regards of routine types of AgNPs capped with citrate, polyvinylpyrrolidone and sodium dodecylsulfate [19]. Recently, water soluble complexes of metronidazole-silver had been synthesized, and the presence of metronidazole proved to enhance the antibacterial efficiency of silver salts in comparison between silver sulfadiazine and free metronidazole against Gram-positive bacteria [20].
Multidrug resistant (MDR) bacteria stand as one of the challenging problems in universal health care. Due to antibiotic resistance of pathogens, new formulations are needed to combat. NPs themselves and in combination with antibiotics offer promising solutions to develop new and prospective nanomedicines to fight bacteria MDR. AgNPs have shown to act as an effective agent against MDR bacteria [5]. A synergistic antibacterial affect was observed while AgNPs were combined with two antibiotics; gentamicin and chloramphenicol in MDR of Enterococcus faecalis [21]. In another researches, a combinatorial system of AgNPs and tetracycline demonstrated to be effective against tetracycline resistant bacteria [22].
Among biodegradable and natural-based polymers [4], sodium alginate fibers have applications in preparation of wound care dressings [23, 24]. Combination of alginate with AgNPs could result in an enhancement in healing properties while broadening the antimicrobial spectrum of wound dresses [25, 26].
Metronidazole is a common type of antibiotic and antiprotozoal that is prescribed for treatment of a wide range of infections. It is categorized under the group of nitro-imidazole as an effective treatment of diseases caused by anaerobic bacteria, such as bacterial vaginosis, endocarditis and pelvic inflammatory diseases [21].
To consider wide arrays of AgNPs applications, and their unique manifestation as antibacterial agents, development of synthetic techniques that resulted in production of particles with enhanced efficiency is of great interest. Herein, a novel synthetic strategy was expended to prepare metronidazole-based ionic liquid with citrate counter ion. This new precursor was applied in synthesis of AgNPs. Finally, to verify the antibacterial efficacy of as-prepared samples, mixture of AgNPs in calcium alginate was prepared.
2. Materials
Alginic acid sodium salt (sodium alginate) with MW = 10–600 kDa, sodium citrate tribasic dehydrate were purchased from Sigma Company. Silver nitrate (AgNO3) was purchased from Daejung Chemical Company. Metronidazole was available from Samen Pharmaceutical Company/Iran (5 mg/ml) and was extracted from injectable solution by recrystallization and purification with deionized water. Calcium chloride, ethanol and acetone was available from Kimia Mavad (Iran). The standard antibiogram discs of several antibiotics such as imipenem (IPM: 10 μg), amikacin (AN: 30 μg), ceftazidime (CAZ: 30 μg), oxacillin (OXA: 5 μg) and clindamycin (CC: 10 μg) for S. Aureus, while for Pseudomonas, antibiotics included gentamicin (GM: 30 μg), azithromycin (AZM: 15 μg), lomefloxacin (LOM: 30 μg), ciprofloxacin (CP: 5 μg) and norfloxacin (NOR: 5 μg) were prepared from Pars Teb company.
3. Instruments
1HNMR and 13CNMR was performed by Brucker Avance DPX (250 MHz) in D2O solvent. Fourier transform infrared (FTIR) spectra was operated by Bruker (VERTEX70) instrument, and the spectrums were recorded by OPUS software. X-ray diffraction (D8 Advance Bruker AXS) with X-ray diffractometer (Cu Kα radiation with λ = 1.540 A°, energy 3 kV, and 15 mA 2θ scan range = 10–90°, 25 °C) was applied to record the crystallinity of synthesized AgNPs. Morphological observation was performed by transmission electron microscope/TEM with a Philips instrument (model CM10). To determine the amount of organic coating, thermal gravimetric analysis (TGA) was performed with Mettler Toledo instrument.
4. Experimental section
4.1. Synthesis of metronidazole-based ionic liquid (IL-Met-cit)
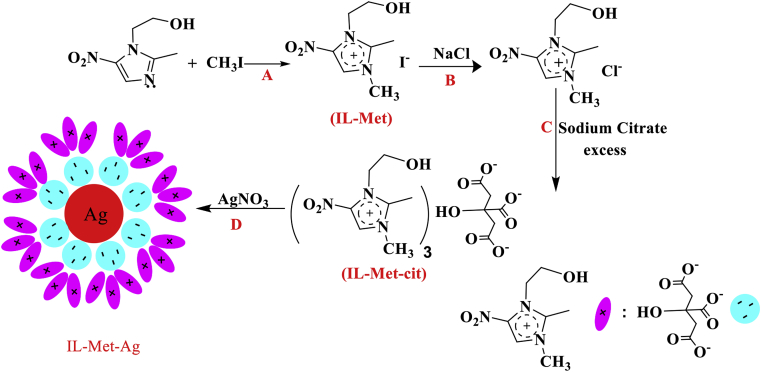
To synthesize metronidazole-based ionic liquid, metronidazole (5.8 mmol) was mixed with ethanol (30 ml). Then, methyl iodide (5.8 mmol) was added, and the reaction flask refluxed at 50 °C for one day. A yellow solution was formed, and the solvent was removed by rotary-evaporator. After that, the precipitated product was mixed with 20 ml of sodium chloride (1% W/W) to replace iodide ion with chloride ion. The product was obtained by evaporation, then re-crystalized in deionized water. Finally, to insert citrate ion to the structure, the product was admixed with 100 ml of sodium citrate solution (2% W/W) overnight at 50 °C. The product was obtained by evaporation and re-crystalized in deionized water (Scheme 1).
Scheme 1.
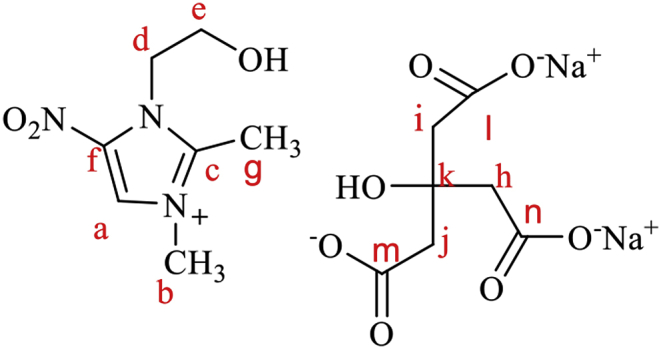
IL-Met-cit structure.
1HNMR and 13CNMR characteristic chemical shifts bands in D2O solvent;
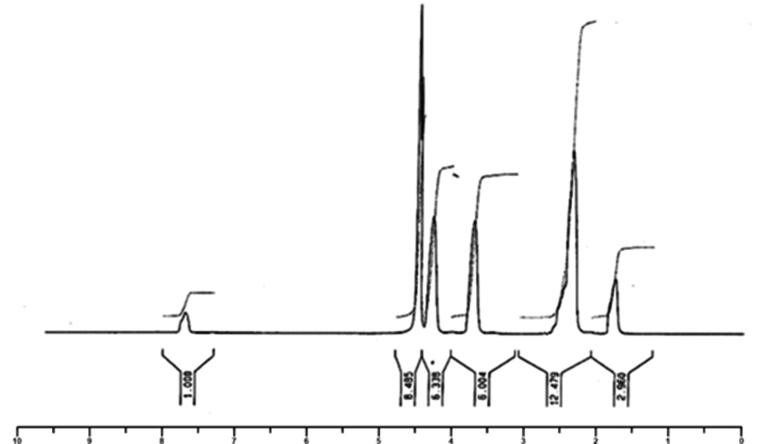
1HNMR: δ7.98 (singlet,1H, Ha), δ4.63 (triplet, 2H, He), δ4.45 (triplet, 2H, Hd), δ3.89 (singlet, 3H, Hb), δ2.43 (singlet, 6H, Hh,j,i), δ1.8 (singlet, 3H, Hg).
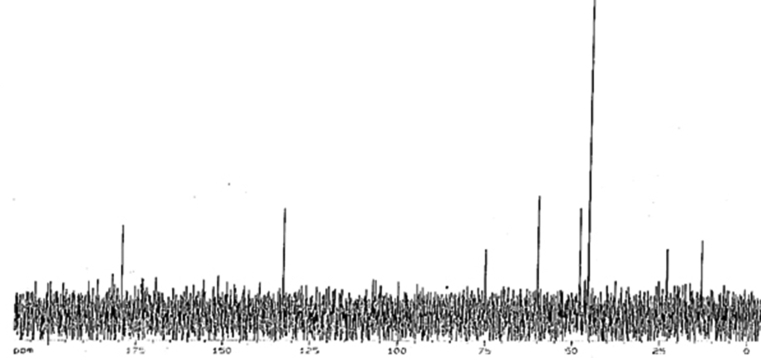
13CNMR: δ 181, 179.2 (Cl,Cn, Cm), δ132.7(Cc), δ 108 (Ca, Cc), δ75.1(Ck), δ60.08 (Cb), δ48 (Ch), 45.6 (Ce, Cd), δ23.203 (Cb), δ 13.3(Cg).
Other characteristics of IL-Met-cit (Scheme 1) containing: melting point: 152–154 °C, white powder without odor and color.
4.2. Synthesis of metronidazole-capped AgNPs (IL-Met-Ag)
To synthesize metronidazole-capped AgNPs, 20 ml of IL-Met-cit (100 mM) was first dissolved in DI water and stirred at 50 °C. Then 20 ml of silver nitrate (AgNO3, 10 mM) was added drop-wise over 15 min. The mixture was stirred and refluxed for 24 h until the creamy mixture obtained. Finally, in order to extract AgNPs, sodium hydroxide solution (10% W/W) was added drop-wise until precipitation began. The precipitate was extracted from supernatant and washed in deionized water (x3). The obtained AgNPs (IL-Met-Ag) were dried at room temperature and stored for further analysis.
4.3. Disc diffusion test
To evaluate antibacterial efficiency, an aqueous solution (5 ml) of IL-Met-Ag with various concentrations (1, 3, 5, 6, 7, 10, 12, 14, 20, 50, 100, 200, 300 and 400 μg/ml) were prepared in small vials. Then sodium alginate (0.03 g) was included in the solution, and the mixture was stirred at room temperature. After 30 min, 0.5 ml of calcium chloride (1% W/W) was added to the mixture. After 2 h, paper discs were inserted into each sample and maintained for 24 h. Then, the discs were dried in an oven at 50 °C. The anti-microbial efficiency of discs from each samples was evaluated against Staphylococcus aureus (S. aureus) and Pseudomonas in comparison to antibiotic discs.
The disc diffusion test by the above mentioned procedures were repeated for IL-Met-cit and metronidazole with different concentration (0.15, 0.3, 0.6, 1, 2.5, 5, 10, 15, 20, 50, 100 μg/ml).
All procedures were performed according to guidelines of Clinical and Laboratory Standard Institute (CLSI) for primary evaluation of the materials. Briefly, all glassware and medium were sterilized in an autoclave for 20 min at 121 °C. A suspension of Gram-negative and Gram-positive bacteria Pseudomonas and S. Aureus, respectively was combined with 0.5 McFarland and cultured overnight. The turbidity was adjusted to 108 CFU. To run the tests, the suspension was diluted 105-fold, and 10 μl of suspension was added to Mueller-Hinton agar plates and dispersed by swab. The paper discs, with a diameter of 6 mm, contained a certain concentration of antibiotics, for example, S. Aureus contained antibiotics IPM, AN, CAZ, OXA and CC, while Pseudomonas contained antibiotics like GM, AZM, LOM, CP and NOR. On the other hand, the discs prepared in the previous section were also placed in the plates, which were incubated at 37 °C for 24 h. After this time frame, the zone of inhibition (ZOI) was measured, and the inhibitory percent was calculated according to the following formula:
| Inhibitory % = ZOI of samples/ZOI of antibiogram discs∗100 |
5. Results
5.1. Synthesis and characterization of IL-Met-cit and IL-Met-Ag
Today, AgNPs have found fascinating applications, especially in the biomedical field, as a masterpiece of nanotechnology progress. In this regard, it is possible to present innovation from synthetic routes to their application. Herein, a novel innovation in chemical synthetic routes of AgNPs is introduced, which leads to preparation of more stable AgNPs with broader antimicrobial properties.
First, an antibiotic type (metronidazole)-based reducing agent that also act as stabilizer in the form of ionic liquid is introduced and applied in preparation of AgNPs (Figure 1). In this method, metronidazole is reacted chemically with methyl iodide to change the imidazolium ring to quaternary iodide salt and form IL-Met (Figure 1A) [27, 28]. Then, to prepare a chemically stable ionic liquid type compound, IL-Met is reacted with sodium chloride to change the iodide ion, making the molecule stable for long-term storage at room temperature (Figure 1B). In the next step, the product is reacted with sodium citrate to prepare IL-Met-cit, which could have several functions including antibacterial efficacy, ionic liquid type stabilizing agent and reducing capability (Figure 1C). The capacity of IL-Met-cit is tested in preparation of AgNPs while reacted in aqueous medium with silver nitrate for 24 h, which results in the formation of stabilized AgNPs (IL-Met-Ag) (Figure 1D).
Figure 1.
Synthetic processes of AgNPs: first preparation of IL-Met-cit, then reaction with silver nitrate.
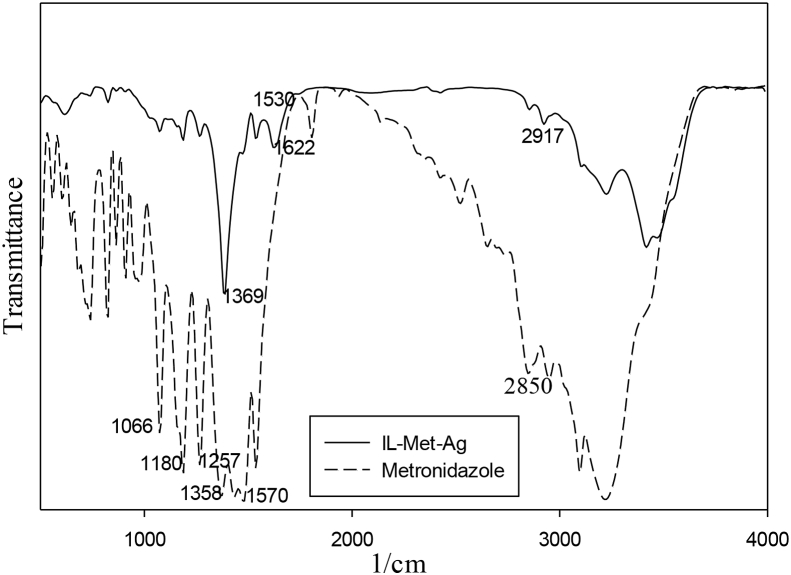
The characterization of products from each steps is determined by FT-IR, 1HNMR and 13CNMR support the correct synthetic strategies of IL-Met-cit preparation (Figures 2 and 3). AgNPs formation is performed in aqueous solution and FT-IR spectrum shows successful formation of metronidazolium- and citrate-capped silver particles (Figure 4). The FTIR spectrum of IL-Met-Ag is compared with metronidazole. The characteristic peaks of pure metronidazole such as imidazolium ring attributed to N–C=N and C=N group appears at 1530 and 1622 cm−1. C–H stretching vibration is evident at 1180 cm−1, the N–O stretching band of NO2 group appears at 1358 cm−1 and C–O stretching vibration is at 1180 cm−1. Regarding IL-Met-Ag spectrum, all attributed peaks of metronidazole appear with lower intensity and slight chemical shift to higher frequency, due to the presence of silver. The peak at 1369 cm−1 could be attributed to NO2 group. However, the C=O group of citrate could appear at 1622 cm−1, which has incidence with N–C=N group.
Figure 2.
1HNMR spectrum of IL-Met-cit in D2O solvent.
Figure 3.
13CNMR spectrum of IL-Met-cit in D2O solvent.
Figure 4.
FTIR spectrum of metronidazole and IL-Met-Ag.
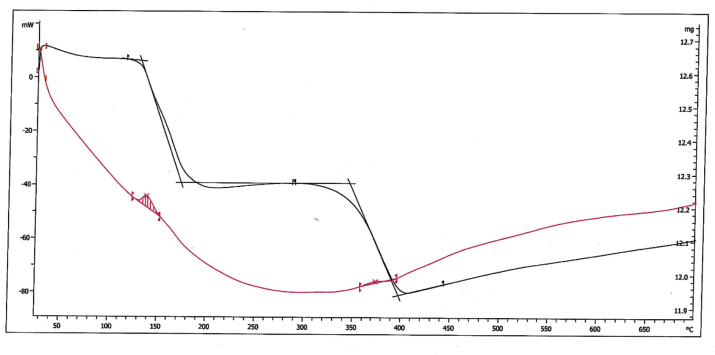
TGA analysis is performed to determine the amount of organic layer around IL-Met-Ag particles (Figure 5). The diagram shows weight losses in two regions. The first region begins at 133–176 °C, which is related to water and humidity, while the second is between 348-399 °C, indicating about 2.65% related to weight loss of IL-Met-cit molecules.
Figure 5.
TGA diagram of IL-Met-Ag.
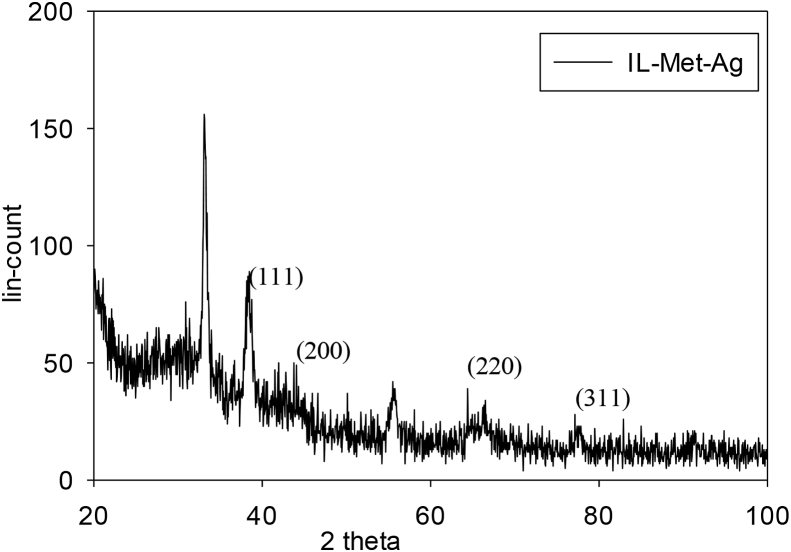
X-ray is a powerful technique for determination of crystalline structure at the atomic scale. XRD analysis is performed to identify the crystalline nature and purity of samples [29]. For IL-Met-Ag, the diffraction peaks appear at 2 angle of 38.3°, 44.0°, 66.0° and 77.6°, which can be attributed to the planes of reflection in xyz directions of (111), (200), (220) and (311) of AgNPs that confirm face centered cubic structure of silver (Figure 6). Two other diffractions recorded in 32.6° and 55.3° directions could be attributed to silver oxide formation [30, 31].
Figure 6.
XRD diagram of IL-Met-Ag.
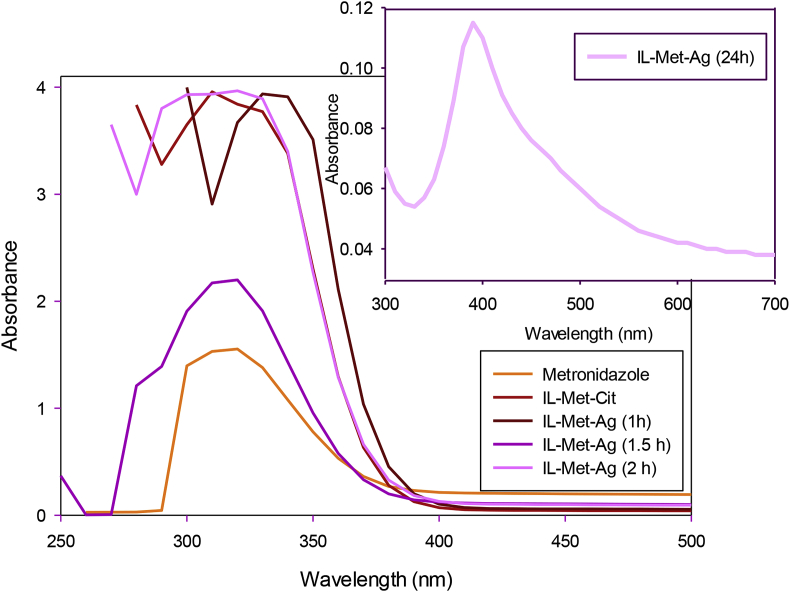
The formation of AgNPs was monitored by UV-Vis spectroscopy (Figure 7). For this purpose, metronidazole and IL-Met-cit are analyzed to determine the maximum absorbance wavelength. The maximum absorbance wavelength of metronidazole is between 310-330 nm, similar to a previous report [32]. The UV-Vis absorbance of IL-Met-cit lies at 310 nm. During silver nitrate reaction with IL-Met-cit, the recorded UV-Vis spectrums in the first two hours did not show changes indicating absorbance of IL-Met-cit. However, after 24 h, a peak appeared at 410 nm as a result of AgNPs formation (Figure 7). The sharpness of this peak is dependent on the concentration of the AgNPs [22].
Figure 7.
UV-Vis spectrum of metronidazole, IL-Met-cit and the reaction medium of synthetic process of AgNO3 with IL-Met-cit during first two hours: IL-Met-Ag (1 h), IL-Met-Ag (1.5 h) and IL-Met-Ag (2 h), and IL-Met-Ag (24 h).
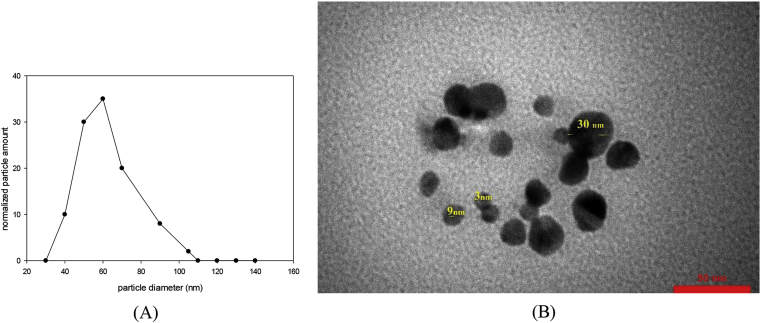
The particle sizes are measured by PSA. The PSA histogram shows symmetrical distribution of particle diameter with average size of 60 nm (Figure 8A). TEM image also confirmed the formation of spherical particles with the sizes of less than 10 nm. Also, larger particles with low availability and maximum sizes of 30 nm could be observed (Figure 8B).
Figure 8.
(A) PSA diagram of IL-Met-Ag dispersed in DI, (B) TEM image of IL-Met-Ag.
5.2. Evaluation of antibacterial efficiency of IL-Met-Ag nanoparticles
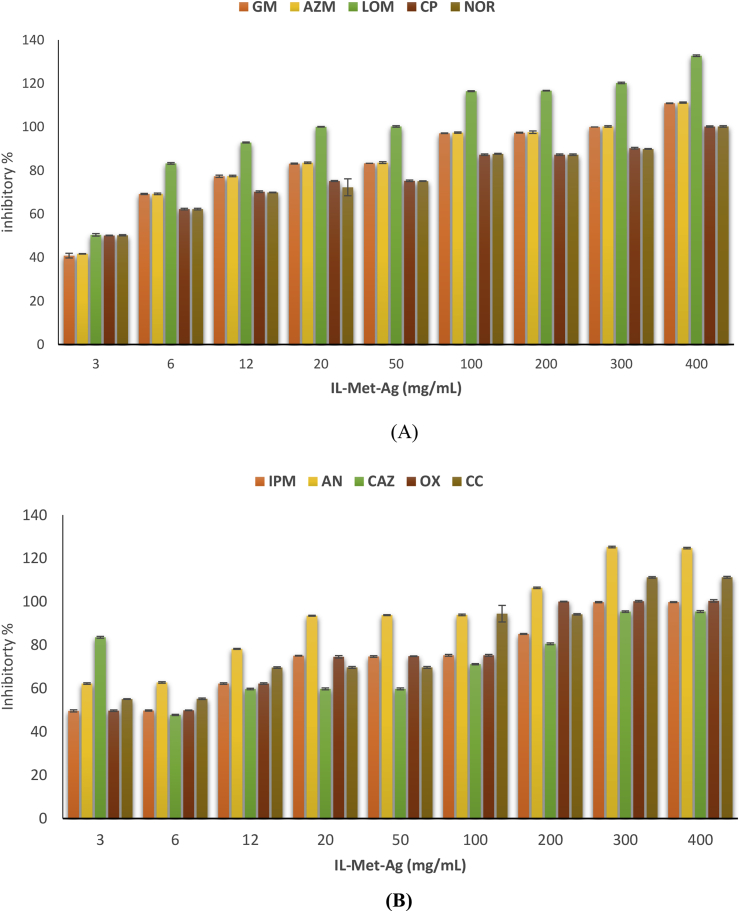
AgNPs have found impactful commercial applications as antimicrobial agents, especially in preparation of wound dresses. By this aim, the antimicrobial efficacy of IL-Met-Ag nanoparticles is evaluated in calcium alginate medium as potent wound care dresses. Two different types of bacteria including Gram S.aureus (as Gram-positive bacteria) and Pseudomonase aeruguinosa (P. aeruguinosa) as (Gram-negative) bacteria are tested. Antimicrobial discs of different concentrations of IL-Met-Ag are prepared, then the antibacterial efficiency is reported while compared with antibiotic discs. The antibiotics applied for efficacy evaluation in Gram-positive bacteria were IPM, AN, CAZ, OX and CC, while for Gram-negative, GM, AZM, LOM, CP and NOR were selected. The percent of inhibition in regards of types of antibiotics for each samples concentrations are presented in two diagrams for Gram-positive and Gram-negative bacteria (Figure 9). As a comparison, the antibacterial efficiency of metronidazole and IL-Met-cit disks in calcium alginate solution in various concentrations were assessed on Gram-positive and Gram-negative bacteria. However, the disk containing IL-Met-cit and metronidazole couldn't inhibit bacterial growth. Of course, it was previously well studied that metronidazole is not effective against S. aureus and P. aeruguinosa in concentration less than 128 mg/L [5].
Figure 9.
Antibacterial efficacy of calcium alginate containing IL-Met-Ag solution with different concentrations (3, 6, 12, 20, 50, 100, 200, 300 and 400 μg/ml) for: A) P. aeruguinosa bacteria in regards to GM, AZM, LOM, CP and NOR antibiotics and B) S. aureus bacteria in regard to IPM, AN, CAZ, OX and CC antibiotics.
6. Discussion
Two novel synthetic strategies are developed in this study. The first one results in the transformation of metronidazole to a potent reducing agent that could stimulate antimicrobial efficiency for anaerobic bacteria and also have ionic liquid type structure. This new introduced low molecular weight compound (IL-Met-cit) that contains citrate ion in the structure was fully characterized by 1HNMR and 13CNMR and FTIR spectroscopy (Figures 2, 3, and 4). This compound was produced with commercially available materials and has the potential to be registered as a new ionic liquid with metronidazolium-based structure and high reducing capability. The efficiency of this compound could be evaluated in nanoparticle formation. By this aim, the synthesis of AgNPs was performed with IL-Met-cit compound.
Synthesis of AgNPs has been efficiently performed with ionic liquids by N-alkylethylenediamine [17]. On the other hand, metronidazole complexes of silver were also synthesized and evaluated for antimicrobial efficacy [20]. Antibiotic type capping agents are proven to have higher antibacterial efficiency rather than AgNPs [19]. In this regard, the synthesis of AgNPs is performed in aqueous medium in the presence of IL-Met-cit, which results in the formation of AgNPs after 24 h. Due to plasmonic properties of AgNPs, Uv-Vis spectroscopy could be recognized as valid method of in situ monitoring of synthetic processes. The size and shapes of AgNPs are determining factor in their absorbance wavelenghths. Paramella et al. reported a size and concentration dependent of extinction coefficient of AgNPs. According to this report AgNPs with diameter ranging from 10-40 would appear around wavelengths of 390–412 nm [33]. Herein, formation of AgNPs was monitored at maximum wavelenghth of 410 nm, which was also reported in an other reference [17]. It is obvious that IL-Met-cit could perform two functions of reducing and stabilizing of AgNPs efficiently. FT-IR of IL-Met-Ag shows that AgNPs were capped with organic moiety of IL-Met-cit with average organic layer amount of 2.65%, which is supported by TGA analysis. PSA analysis demonstrates fine distribution of nanoparticles in aqueous medium with average size of 60 nm, while TEM image shows particles with sizes around 10 nm. All data prove the successful formation of AgNPs.
The antimicrobial results, which were assessed in calcium alginate solution of IL-Met-Ag through two types of bacteria including S. aureus as Gram-positive bacteria and P. aeruguinosa as Gram-negative, showed high antimicrobial efficiency of particles, even in low concentration, in comparison with antibiotic discs. The highest efficacy of both bacteria types are observed at 400 μg/ml of IL-Met-Ag. For Gram-positive bacteria, the inhibition efficiency is 100% comparable with antibiotics such as GM, AZM, CP and NOR, while for LOM, nanoaprticles are shown to be work better with 120% efficacy. For Gram-negative bacteria, the 100% efficacy is reached at 300 μg/ml for IPM, CAZ, OX, CC, while for AN it reaches 125% efficacy.
The type of study in this research based on antibacterial inhibitory percent is very specific and used for comparison of IL-Met-Ag efficacy for broad range of antibiotics. The conjugation of metronidazole to AgNPs means that the system has potential to be considered as nano-drug delivery system and can be applied to combat MDR resistance. For example, synergistic antibacterial effect of AgNPs in combination with gentamicin and neomycin antibiotics was investigated on S. aureus bacteria. The results of disc diffusion test showed that AgNPs/gentamicine reduced the resistance to gentamicin to 15% and in AgNPs/neomycin the resistance to neomycin was reduced to 45%. In another study, synergistic effect of AgNPs with gentamicin and chloramphenicol was proved by measuring zone of inhibition via standard disc diffusion method against Enterococcus faecalis [21]. By determining the minimum inhibitory concentration (MIC) of a combinatorial system of AgNPs and tetracycline better antibacterial activity were obtained against S. aureus and E.coli rather than AgNPs alone [22].
The antimicrobial mechanism of AgNPs is still not well understood [34]. However, most of the reports have emphasized the production of silver ions (Ag+) as determining factor in antimicrobial effect. Silver ions can react with the thiol groups of bacteria membrane protein which resulted in bacteria inactivation [34]. Other possible mechanisms are described to be reactive oxygen species (ROS) production by AgNPs and silver ions and direct damage of cell membrane by AgNPs [35]. A detailed mechanistic studies are needed to discuss about possible antibacterial mechanism of our synthesized particles. However, we can propose that IL-Met-Ag with positive charge have potential to penetrate cells and within cytoplasm metronidazole could react with DNA and disrupt cell replication while AgNPs would generate ROS and cause cell death.
The biological safety of metal-oxide nomaterials are highly dependent on the physicochemical properties such as shape and dimensions, surface modifications and surface charge. AgNPs toxicity may depend on the surface coating since having positive surface charge renders them more biocompatible, especially for drug delivery purposes [5].
Synthesized AgNPs could be recognized as potent antimicrobial agent in the preparation of wound care dresses and could provide broad spectrum of antibacterial efficiency in comparison with citrate-capped AgNPs. However, the prepared nanoparticles also have potential efficiency for killing anaerobic bacteria that could not be tested in this work. The variety of different antibiotics applied for comparing efficacy of prepared IL-Met-Ag particles is of great strength in this work.
7. Conclusion
A successful strategy, leading to novel antibiotic like ionic liquid with reduction capability, was introduced. Metronidazolium-based ionic liquid with citrate counter ion are synthesized and characterized. Uv-Vis monitoring shows successful formation of AgNPs after 24 h. TGA diagrams prove that AgNPs have 2.65% organic layer coating. TGA results, in combination with FTIR observation of IL-Met-cit, have verified that IL-Met-cit could successfully play two roles of being both reducing and stabilizing agents. The XRD shows the expected crystallinity of AgNPs and PSA results, while TEM images show AgNPs formation with sizes less than 50 nm. The antimicrobial results, which are assessed in calcium alginate solution of IL-Met-Ag, through two types of bacteria including Gram-positive bacteria Gram-negative demonstrated high antimicrobial efficiency of particles, even in low concentration of AgNPs, in comparison with commercial antibiotic discs. Our results suggest that IL-Met-Ag could be considered as novel antibacterial agents.
Declarations
Author contribution statement
Fatemeh Farjadian: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Amin Reza Akbarizadeh: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Lobat Tayebi: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Funding statement
Fatemeh Farjadian was supported by Vice-Chancellor for Research, Shiraz University of Medical Sciences (94-01-36-9980).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Nikalje A.P. Nanotechnology and its applications in medicine. Med. Chem. 2015;5(2):81–89. [Google Scholar]
- 2.Farjadian F., Ghasemi A., Gohari O., Roointan A., Karimi M., Hamblin M.R. Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities. Nanomedicine. 2018 doi: 10.2217/nnm-2018-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farjadian F., Roointan A., Mohammadi-Samani S., Hosseini M. Mesoporous silica nanoparticles: synthesis, pharmaceutical applications, biodistribution, and biosafety assessment. Chem. Eng. J. 2019;359:684–705. [Google Scholar]
- 4.Farjadian F., Moghoofei M., Mirkiani S., Ghasemi A., Rabiee N., Hadifar S. Bacterial components as naturally inspired nano-carriers for drug/gene delivery and immunization: set the bugs to work? Biotechnol. Adv. 2018;36(4):968–985. doi: 10.1016/j.biotechadv.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baptista P.V., McCusker M.P., Carvalho A., Ferreira D.A., Mohan N.M., Martins M. Nano-strategies to fight multidrug resistant bacteria-"A Battle of the Titans". Front. Microbiol. 2018;9:1441. doi: 10.3389/fmicb.2018.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmadi S., Rabiee N., Bagherzadeh M., Elmi F., Fatahi Y., Farjadian F. Stimulus-responsive sequential release systems for drug and gene delivery. Nano Today. 2020;34:100914. doi: 10.1016/j.nantod.2020.100914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Entezar-Almahdi E., Mohammadi-Samani S., Tayebi L., Farjadian F. Recent advances in designing 5-fluorouracil delivery systems: a stepping stone in the safe treatment of colorectal cancer. Int. J. Nanomed. 2020;15:5445–5458. doi: 10.2147/IJN.S257700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajawat S., Malik M.M. American Society of Mechanical Engineers; 2018. Silver Nanoparticles: Properties, Synthesis Techniques, Characterizations, Antibacterial and Anticancer Studies. [Google Scholar]
- 9.Guzman M., Dille J., Godet S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012;8(1):37–45. doi: 10.1016/j.nano.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Rai M., Yadav A., Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009;27(1):76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X.-F., Liu Z.-G., Shen W., Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016;17(9):1534. doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaqoob A.A., Umar K., Ibrahim M.N.M. Silver nanoparticles: various methods of synthesis, size affecting factors and their potential applications–a review. Appl. Nanosci. 2020;10(5):1369–1378. [Google Scholar]
- 13.Iravani S., Korbekandi H., Mirmohammadi S.V., Zolfaghari B. Synthesis of silver nanoparticles: chemical, physical and biological methods. Res. Pharmaceut. Sci. 2014;9(6):385. [PMC free article] [PubMed] [Google Scholar]
- 14.Srikar S.K., Giri D.D., Pal D.B., Mishra P.K., Upadhyay S.N. Green synthesis of silver nanoparticles: a review. Green Sustain. Chem. 2016;6(1):34. [Google Scholar]
- 15.Li C.-C., Chang S.-J., Su F.-J., Lin S.-W., Chou Y.-C. Effects of capping agents on the dispersion of silver nanoparticles. Colloid. Surface. Physicochem. Eng. Aspect. 2013;419:209–215. [Google Scholar]
- 16.Flores C.Y., Miñán A.G., Grillo C.A., Salvarezza R.C., Vericat C., Schilardi P.L. Citrate-capped silver nanoparticles showing good Bactericidal effect against both planktonic and sessile bacteria and a low cytotoxicity to osteoblastic cells. ACS Appl. Mater. Interfaces. 2013;5(8):3149–3159. doi: 10.1021/am400044e. [DOI] [PubMed] [Google Scholar]
- 17.Er H., Yasuda H., Harada M., Taguchi E., Iida M. Formation of silver nanoparticles from ionic liquids comprising N-alkylethylenediamine: effects of dissolution modes of the silver (I) ions in the ionic liquids. Colloid. Surface. Physicochem. Eng. Aspect. 2017;522:503–513. [Google Scholar]
- 18.Fuller F.W., Parrish M., Nance F.C. A review of the dosimetry of 1% silver sulfadiazine cream in burn wound treatment. J. Burn Care Rehabil. 1994;15(3):213–223. doi: 10.1097/00004630-199405000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Kora A.J., Rastogi L. Enhancement of antibacterial activity of capped silver nanoparticles in combination with antibiotics, on model gram-negative and gram-positive bacteria. Bioinorgan. Chem. Appl. 2013;2013 doi: 10.1155/2013/871097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalinowska-Lis U., Felczak A., Chęcińska L., Zawadzka K., Patyna E., Lisowska K. Synthesis, characterization and antimicrobial activity of water-soluble silver (I) complexes of metronidazole drug and selected counter-ions. Dalton Trans. 2015;44(17):8178–8189. doi: 10.1039/c5dt00403a. [DOI] [PubMed] [Google Scholar]
- 21.Katva S., Das S., Moti H.S., Jyoti A., Kaushik S. Antibacterial synergy of silver nanoparticles with gentamicin and chloramphenicol against Enterococcus faecalis. Phcog. Mag. 2018;13(Suppl 4):S828–S833. doi: 10.4103/pm.pm_120_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Djafari J., Marinho C., Santos T., Igrejas G., Torres C., Capelo J.L. New synthesis of gold- and silver-based nano-tetracycline composites. Chem. Open. 2016;5(3):206–212. doi: 10.1002/open.201600016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee K.Y., Mooney D.J. Alginate: properties and biomedical applications. Prog. Polym. Sci. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahimi M., Noruzi E.B., Sheykhsaran E., Ebadi B., Kariminezhad Z., Molaparast M. Carbohydrate polymer-based silver nanocomposites: recent progress in the antimicrobial wound dressings. Carbohydr. Polym. 2020:231. doi: 10.1016/j.carbpol.2019.115696. [DOI] [PubMed] [Google Scholar]
- 25.Choudhury H., Pandey M., Lim Y.Q., Low C.Y., Lee C.T., Marilyn T.C.L. Silver nanoparticles: advanced and promising technology in diabetic wound therapy. Mater. Sci. Eng. C. 2020:112. doi: 10.1016/j.msec.2020.110925. [DOI] [PubMed] [Google Scholar]
- 26.Naskar A., Kim K.S. Recent advances in nanomaterial-based wound-healing therapeutics. Pharmaceutics. 2020;12(6) doi: 10.3390/pharmaceutics12060499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghasemi S., Farjadian F., Tamami B. Biaryl formation via Suzuki and Stille coupling reactions using palladium nanoparticle/polymeric N-heterocyclic carbene grafted silica as recyclable and efficient catalyst. Appl. Organomet. Chem. 2016;30(10):818–822. [Google Scholar]
- 28.Tamami B., Farjadian F., Ghasemi S., Allahyari H. Synthesis and applications of polymeric N-heterocyclic carbene palladium complex-grafted silica as a novel recyclable nano-catalyst for Heck and Sonogashira coupling reactions. New J. Chem. 2013;37(7):2011–2018. [Google Scholar]
- 29.Yang X., Du Y., Li D., Lv Z., Wang E. One-step synthesized silver micro-dendrites used as novel separation mediums and their applications in multi-DNA analysis. Chem. Commun. 2011;47(38):10581–10583. doi: 10.1039/c1cc11374g. [DOI] [PubMed] [Google Scholar]
- 30.Ng L.Y., Mohammad A.W., Rohani R., Hairom N.H.H. Development of a nanofiltration membrane for humic acid removal through the formation of polyelectrolyte multilayers that contain nanoparticles. Desalination Water Treat. 2016;57(17):7627–7636. [Google Scholar]
- 31.Karthik L., Kumar G., Kirthi A.V., Rahuman A., Rao K.B. Streptomyces sp. LK3 mediated synthesis of silver nanoparticles and its biomedical application. Bioproc. Biosyst. Eng. 2014;37(2):261–267. doi: 10.1007/s00449-013-0994-3. [DOI] [PubMed] [Google Scholar]
- 32.Naveed S., Qamar F. Simple UV spectrophotometric assay of Metronidazole. Open Access Libr. J. 2014;1(6):1. [Google Scholar]
- 33.Paramelle D., Sadovoy A., Gorelik S., Free P., Hobley J., Fernig D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst. 2014;139(19):4855–4861. doi: 10.1039/c4an00978a. [DOI] [PubMed] [Google Scholar]
- 34.Durán N., Durán M., de Jesus M.B., Seabra A.B., Fávaro W.J., Nakazato G. Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016;12(3):789–799. doi: 10.1016/j.nano.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Marambio-Jones C., Hoek E.M. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanoparticle Res. 2010;12(5):1531–1551. [Google Scholar]