Abstract
2-methoxyethanol (2-ME) is an organic solvent widely used in the manufacture of brake fluids, paints, resins, varnish, nail polish, acetate cellulose, wood coloring, and as a plasticizer in plastics manufacturing. We therefore, investigated its effect on the liver, in a time-course study in male Wistar rats. Animals were orally administered 50 mg/kg body weight of 2-ME for a period of 7, 14, and 21 days. Following 7 days of administration of 2-ME, there was a significant increase in the level of Bax, c-Myc, K-Ras, TNF-α, IL-1β, IL-6, MDA and GPx activity, while the levels of Bcl-2, NO and GSH were significantly reduced compared with control. At the end of 14 days exposure, Bcl-2, and GSH levels, as well as GST activity, were significantly decreased, while levels of Bax, c-Myc, K-Ras, caspase-3, TNF-α, IL-1β, IL-6, MDA and NO were significantly increased compared with control. After 21 days of 2-ME administration, Bcl-2, IL-10, and GSH levels, as well as SOD and GST activities, were significantly decreased, while levels of Bax, c-Myc, K-Ras, caspase-3, p53, TNF-α, IL-1β, IL-6, MDA and NO were significantly increased compared with control. Lastly, liver histopathology confirmed and corroborated the biochemical findings reported above. We therefore, advised that exposures to 2-ME should be strictly avoided as it could trigger hepatic damage through the disorganization of the antioxidant system, up-regulation of inflammatory, apoptotic, and oncogenic markers in rats.
Keywords: 2-methoxyethanol, Oxidative stress, Inflammation, Apoptosis, Oncogenes, Liver
Abbreviations: MDA, malondialdehyde; GSH, reduced glutathione; NO, nitric oxide; GPx, glutathione peroxidase; GST, glutathione S-transferase; SOD, superoxide dismutase; CAT, catalase; TNF-α, tumor necrosis factor alpha; IL-1β, interleukin-1 beta; IL-6, interleukin-6; IL-10, interleukin 10; p53, tumor suppressor protein; Bax, Bcl-2 associated X; Bcl-2, B-cell lymphoma 2; c-Myc, myelocytomatosis; K-Ras, Kirsten rat sarcoma viral oncogene
Highlights
-
•
2-ME significantly increased the liver levels of MDA and NO.
-
•
2-ME administration resulted in the disorganization of the redox systems.
-
•
2-ME significantly increased the liver levels of TNF-α, IL-1β, IL-6.
-
•
2-ME significantly increased the liver levels of Bax and caspase-3 and significantly decreased Bcl-2 level.
-
•
2-ME significantly increased the liver levels of c-Myc and K-Ras.
1. Introduction
Ethylene glycol ethers (EGEs) are important constituents of numerous household, industrial, and pharmaceutical products. These EGEs have physicochemical properties, such as solubility in both ethanol and water mixtures as well as low vapor pressure, which makes them a useful solvent with wide applications [1]. EGEs are used in liquid soaps, varnishes, pesticides, herbicides, cooling liquids, household appliances, vaccines, antiseptic specifics, children's toys and many more. Some of the most widely known EGEs are 2-methoxyethanol (2-ME), 2-ethoxyethanol (2-EE), and 2 buthoxyethanol (2-BE). EGEs are highly absorbed following oral, inhalation or dermal exposure and are rapidly distributed throughout the body [2,3]. Numerous in vitro and in vivo studies have demonstrated that these compounds elicit toxic influence on hematological, immunological, developmental and reproductive systems [4,5].
EGEs are mainly metabolized in vivo through oxidation by alcohol dehydrogenase to alkoxyacetaldehyde, followed by conversion to alkoxyacetic acid by aldehyde dehydrogenase [6]. The Toxic effect of EGEs is exerted mainly and predominantly by alkoxyacetic acids [7]. 2-ME and 2-EE predominantly have gonadotoxic effects. In male rats, both 2-ME and 2-EE altered testicular function by lowering the number of spermatocytes and spermatids as well as degeneration of spermatocytes [8]. These compounds also caused a toxic effect majorly on ovarian luteal cells in female rats [9]. Occupational exposure to 2-EE and 2-ME had been reported to reduce sperm count in men and disturbance in the menstrual cycle in women [10]. Contrary to 2-ME and 2-EE, 2-BE exerts potent hemolytic effect in experimental animals and clinical observations of intoxicated patients [5,7,11]. EGEs have been reported to cause bone marrow suppression, reduction of red blood cell, thrombocyte, leukocyte counts and hemoglobin level [12]. The toxicity of EGEs on the immune, hematopoietic, and reproductive system is relatively well reported. Following clinical observations of EGEs intoxicated patients, brain function was affected, while central nervous system depression, disturbed motor coordination, headache, impairment of cognitive function, or convulsions resulted depending on the dose exposed to Ref. [13]. EGEs cross the blood-brain barrier, harming the CNS and therefore triggering the process of neurodegenerative changes. More lipophilic EGEs like BE or 2-phenoxyethanol (2-PHE) are more harmful to neurons than those EGEs with higher hydrophilic properties [14]. In a previous study, the mixture of two EGEs resulted in adverse reactions in the brain in vivo by lowering the total antioxidant capacity, stimulating lipid peroxidation and enhancing caspase-3 activity in hippocampus and frontal cortex of rats [15].
There is still a dearth of information on the hepatotoxic effect of EGEs. Therefore, the present study investigated the time course effect of 2-ME on hepatic markers of lipid peroxidation (MDA), oxidative stress (CAT, SOD, GPx, GST, GSH, and NO), inflammation (TNF-α, IL-1β, IL-6, and IL-10), apoptosis (caspase 3, p53, Bax, and Bcl-2) and proto-oncogenic markers (c-Myc and K-Ras) in male Wistar rats.
2. Materials and methods
2.1. Test materials, chemicals, and kits
2-ME (C3H8O2; CAS# 109-84-4; 99.5% purity), is a product of BDH Laboratory Supplies, Poole, BH15 1TD, England. Rats TNF-α (CSB-E11987r), IL-1β (CSB-E08055r), IL-6 (CSB-E04640r), IL-10 (CSB-E04595r), caspase-3 (CSB-E08857r), p53 (CSB-E08336r), Bax (CSB-EL002573RA), Bcl-2 (CSB-E08854r), c-Myc (CSB-E09260h), and K-Ras (CSB-EL012493h) enzyme-linked immunosorbent assay (ELISA) kits are products of Cusabio Technology Llc, Houston, TX, USA. All other used chemicals and reagents were of analytical grade and were products of Sigma Chemical Co., Saint Louis, MO, USA or BDH Chemical Ltd, Poole, England.
2.2. Oral acute toxicity of 2-ME
The oral median lethal dose (LD50) of 2-ME was determined as described by Lorke [16]. The study was conducted in two phases. In the first phase, three groups of three rats each were orally administered 1000, 2000, and 3000 mg/kg body weight of 2-ME respectively. We administered these doses based on reported findings that LD50 of 2-ME in rats is in the range of 1000 mg/kg body weight or more [17]. The rats were observed for signs of toxicity and possible deaths for a week. In the second phase, another three groups of 1 rat each were orally administered 900, 950 and 980 mg/kg body weight of 2-ME respectively, based on outcomes of the first phase, and were also monitored for toxicity signs and deaths. From the outcomes of the 2 phases, LD50 was determined.
2.3. Experimental animals and study design
Twenty (20) male Wistar albino rats of an average weight of 150 g were used for this study. They were obtained from the animal house of the College of Veterinary Medicine, Federal University of Agriculture, Abeokuta, Nigeria. They were sheltered in steel metal cages in the animal house of our Department and were served food and water ad libitum. Experimental protocols were conducted following guidelines of the Institutional Animal Care and Use Committee and were approved by the Animal Ethical Committee of the Department of Biochemistry, Federal University of Agriculture, Abeokuta, Nigeria. After 1 week of acclimatization, the rats were divided randomly into four groups of five animals each. Group I animals served as control and were served only rat chow and water, while groups II, III and IV animals were orally administered 50 mg/kg of 2-ME (1/20th of LD50) based on calculated mean LD50, for 7, 14, and 21 days respectively. Distilled water was used as a vehicle to administer the 2-ME, and it was in the ratio of 1:100.
2.4. Sample collections and preparations
Group 1 animals were sacrificed on day 0 before the commencement of 2-ME administration. 2-ME was orally administered for 7, 14, and 21 days, and 24 h after each of these days (days 7, 14, and 21); animals were sacrificed by cervical dislocation. They were treated following the international guidelines for the care and use of laboratory animals [18]. The liver was harvested, washed in ice-cold saline (0.9% w/v) solution, blotted dry, and weighed. A section of the liver was suspended in ice-cold 0.1 M phosphate buffer (pH 7.4) for disruption using a tissue homogenizer. Homogenization was followed by centrifugation at 5000 rpm for 10 min. The supernatant was aliquoted into Eppendorf tubes and used for the estimations of biochemical parameters.
2.5. Estimation of MDA concentration
The lipid peroxidation marker (MDA) was determined by the method of Buege and Aust [19]. In this procedure, 0.1 mL of the liver sample was added to 2 mL of trichloroacetic acid-thiobarbituric acid-hydrochloric acid (TCA/TBA/HCl) (1:1:1 ratio) reagent, boiled at 100 °C for 15 min, and allowed to cool. Flocculent materials were removed by centrifugation at 3000 rpm for 10 min. The supernatant was removed and the absorbance was read at 532 nm against a blank. MDA concentration was calculated using the molar extinction coefficient for the MDA-TBA complex of 1.55 × 106 M−1cm−1.
2.6. Estimation of NO level
Liver NO concentration was estimated using Griess Reagent [20] that detects nitrite ion. The reaction mixture was made up of 150 mL sulfanilamide,100 mL distilled water and 50 mL of sample. The mixture was incubated for 10 min, and then the addition of 150 mL N-naphthyl ethylenediamine, followed by incubation for another 10 min. The concentration nitrite ion, which represents NO production of the system, was measured at 540 nm.
2.7. Estimation of GSH concentration
The level of liver reduced glutathione (GSH) was determined by the method of Moron et al. [21]. 1:1 of the liver sample and sulphursalicyclic acid were mixed together, and centrifuged at 3000 rpm for 5 min. From the supernatant, 0.5 ml was taken and added to a solution containing 4 ml of 0.1 M phosphate buffer (pH 7.4) and 0.5 mL of Ellman's reagent, and the color developed was read at 412 nm.
2.8. Determination of GPx activity
The liver activity of GPx was determined by the method of Rotruck et al. [22]. The reaction mixture involves 500 μL of phosphate buffer, 100 μL of sodium azide, 200 μL of reduced glutathione, 100 μL of hydrogen peroxide, 500 μL of liver sample, and 600 μL of distilled water. The mixture was incubated for 3 min, followed by addition of 500 μL of TCA. This was centrifuged at 3000 rpm for 10 min and 1000 μL of the resulting supernatant was added to 2000 μL of di-potassium hydrogen phosphate and 1000 μL of Ellman's reagent. The color developed was read at 412 nm.
2.9. Determination of GST activity
Liver glutathione S-transferase (GST) activity was determined by the method of Habig et al. [23] based on enzyme-catalyzed condensation of glutathione with the model substrate, 1-chloro-2,4-dinitrobenzene. Briefly, the reaction mixture involves 30 μL of reduced glutathione, 150 μL of 2,4-dinitrochlorobenzenne (CDNB), 2.79 mL of 0.1 M phosphate buffer (pH 6.5), and 30 μL of liver sample. It was mixed properly and the absorbance was read at 340 nm every minute for 3 min.
2.10. Determination of SOD activity
The activity of liver SOD was determined by the method of Misra and Fridovich [24]. The method is based on the ability of superoxide dismutase to inhibit auto-oxidation of adrenaline to adrenochrome at alkaline pH. Briefly, the reaction mixture involves 2.5 mL of 0.05 M sodium bicarbonate buffer (pH 10.2), 0.3 mL of adrenaline, and 0.2 mL of the liver sample. It was mixed thoroughly and immediately read at 480 nm. The unit of enzyme activity is defined as the enzyme required for 50% inhibition of adrenaline auto-oxidation.
2.11. Estimation of CAT activity
The activity of liver CAT was determined by the method of Sinha [25]. The reaction mixture (2.5 mL) contained 0.01 M phosphate buffer (pH 7.0), tissue homogenate (0.25 mL) and 2 M H2O2 (1 mL). The reaction was stopped by the addition of 0.5 mL dichromate-acetic acid reagent (5% potassium dichromate and glacial acetic acid mixed in 1:3 ratios) at 0, 1, 2, and 3 min, followed by heating in boiling water for 10 min, and then cooled at room temperature. The absorbance was read at 570 nm.
2.12. Estimations of liver levels of TNF-α, IL-1β, IL-6, IL-10, caspase-3, p53, Bax, Bcl-2, c-Myc, and Ras
Protocols in the purchased Cusabio ELISA kits (Cusabio Technology Llc, Houston, TX, USA) were followed. Briefly, 100 μL of samples and standards were added into the wells already pre-coated with an antibody specific for IL-1β, IL-6, TNF-α, IL-10, caspase-3, p53, Bax, Bcl-2, c-Myc, or Ras and incubated for 2 h at 37 °C. Unbound substances were removed, and 100 μL of biotin-conjugated antibody specific for IL-1β, IL-6, TNF-α, IL-10, caspase-3, p53, Bax, Bcl-2, c-Myc, or Ras was added to the well. After washing, 100 μL of avidin conjugated Horseradish Peroxidase (HRP) was added to the wells and incubated for 1 h at 37 °C, followed by addition of 90 μL of TMB substrate solution, followed by incubation for 15–30 min at 37 °C to give a color proportional to the amount of IL-1β, IL-6, TNF-α, IL-10, caspase-3, p53, Bax, Bcl-2, c-Myc, or Ras bound in the initial step. Stop solution was added to each well, the plate was gently tapped for thorough mixing, and the intensity of the color is measured at 450 nm.
2.13. Determination of total protein concentration
The concentration of liver total protein was determined by the method of Gornall et al. [26], and used for the estimations of SOD, CAT, GST, and GPx activities. Briefly, the reaction mixture involves 1 mL of Biuret reagent and 100 μL of the liver sample. The mixture was allowed to incubate for 10 min at room temperature and the absorbance of purple color developed, corresponding to the total protein concentration was measured at 546 nm against reagent blank.
2.14. Histopathological analysis
Briefly, sections of the liver were fixed in phosphate-buffered formalin solution for 48 h. After dehydration in an increasing concentration of alcohol and cleared twice in xylene, the tissues were embedded in paraffin, cut into sections, stained with hematoxylin-eosin dye, and finally observed at ×400 magnification under a Nikon light microscope.
2.15. Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA), followed by Tukey test for multiple comparisons among the groups of rats using Graph Pad Prism program version 6.0. Data were expressed as mean ± standard error of the mean. P values less than 0.05 were considered statistically significant.
3. Results
3.1. Oral acute toxicity test
After about 12 h of 2-ME administration, animals showed signs of toxicity which include decreased food and water consumption and death. In phase one, mortality was recorded following administration of 1000 and 3000 mg/kg of 2-ME (Table 1). In the second phase, mortality was not recorded by 900, 950, and 980 mg/kg of 2-ME (Table 1). Based on these, oral LD50 of 2-ME was calculated using the formula: LD50 = (Do x D100), and was found to be 990 mg/kg in rat, where D0 = highest dose that gave no mortality (980 mg/kg) and D100 = lowest dose that produced mortality (1000 mg/kg).
Table 1.
Records of mortality in phases 1 and 2 of the oral acute toxicity study.
| Phase 1 (n = 3 per group) |
Phase 2 (n = 1 per group) |
|||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 1 | Group 2 | Group 3 | |
| 2 ME (mg/kg) | 1000 | 2000 | 3000 | 900 | 950 | 980 |
| Mortality | 2 | 0 | 1 | 0 | 0 | 0 |
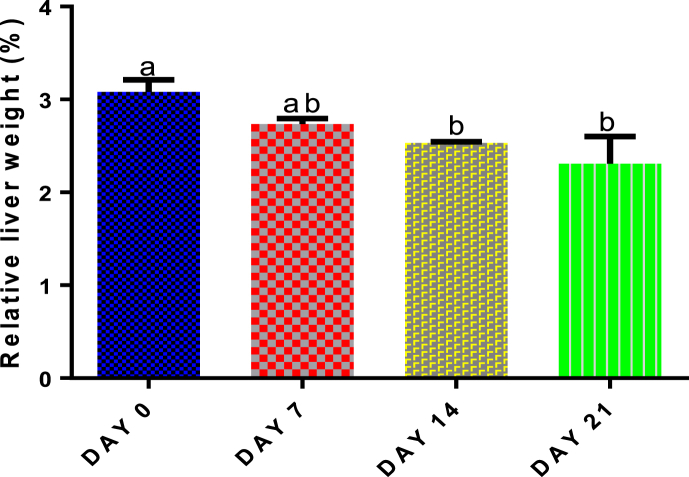
3.2. Time course effect of 2-ME on liver relative weight
Compared with control, only administration of 50 mg/kg body weight of 2-ME for 14 and 21 days significantly (p < 0.05) decreased liver relative weight (Fig. 1).
Fig. 1.
Time course effect of 2-ME on relative liver weight. Values are expressed as mean ± standard error of the mean (n = 5). Bars labeled with different letters are statistically significant (p < 0.05).
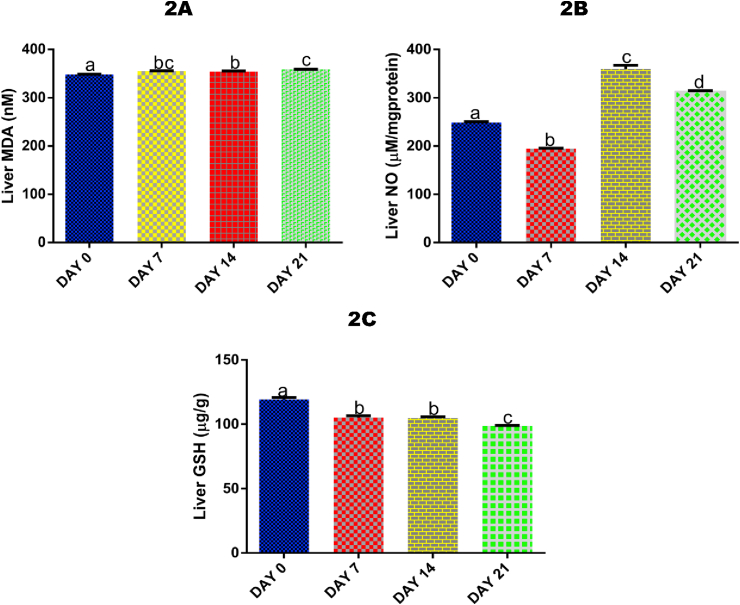
3.3. Time course effect of 2-ME on liver MDA level
There was a significant (p < 0.05) increase in hepatic MDA level after 7 (by 1.91%), 14 (by 1,72%), and 21 days (by 2.82%) of 2-ME administration compared with control (Fig. 2A).
Fig. 2.
Time course effect of 2-ME on liver MDA (2A), NO (2B), and GSH (2C) concentrations. Values are expressed as mean ± standard error of the mean (n = 5). Bars labeled with different letters are statistically significant (p < 0.05).
3.4. Time course effect of 2-ME on liver NO level
Administrations of 2-ME for 7 days resulted in a significant (p < 0.05) decrease in hepatic NO level (by 27.65%) compared with control, while administrations for 14 and 21 days significantly (p < 0.05) increased NO level compared with 7 days (by 45.88% and 37.77% respectively) and control (by 30.92% and 20.56% respectively) (Fig. 2B).
3.5. Time course effect of 2-ME on liver GSH level
Administrations of 2-ME for 7, 14, and 21 days significantly (p < 0.05) decreased liver level of GSH compared with control by 13.40%, 14.05%, and 20.96% respectively. Also, exposures after 21 days significantly (p < 0.05) decreased liver GSH compared with exposures after 7 (by 6.66%) and 14 days (by 6.05%) (Fig. 2C).
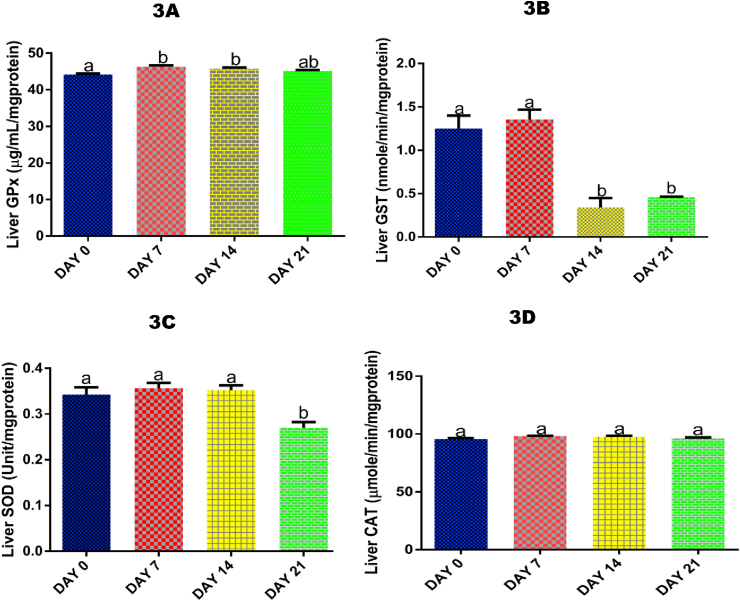
3.6. Time course effect of 2-ME on liver activity of GPx
Following 7 and 14 days of 2-ME administrations, there was a significant (p < 0.05) increase in liver activity of GPx by 4.58% and 3.60% respectively compared with control (Fig. 3A).
Fig. 3.
Time course effect of 2-ME on liver GPx (3A), GST (3B), SOD (3C), and CAT (3D) activities. Values are expressed as mean ± standard error of the mean (n = 5). Bars labeled with different letters are statistically significant (p < 0.05).
3.7. Time course effect of 2-ME on liver GST activity
For GST, 14 and 21 days of exposure to 2-ME significantly (p < 0.05) decreased the hepatic activity of the antioxidant enzyme compared with control (by 268.53% and 172.39% respectivvely) and 7 days (by 299.41% and 195.21% respectively) of exposure (Fig. 3B).
3.8. Time course effect of 2-ME on liver SOD activity
Liver SOD activity was only significantly (p < 0.05) decreased by 26.84% following 21 days of 2-ME administrations compared with control (Fig. 3C).
3.9. Time course effect of 2-ME on liver CAT activity
For liver CAT activity, no significant (p > 0.05) effect was recorded after 7, 14, and 21 days of 2-ME administrations compared with control (Fig. 3D).
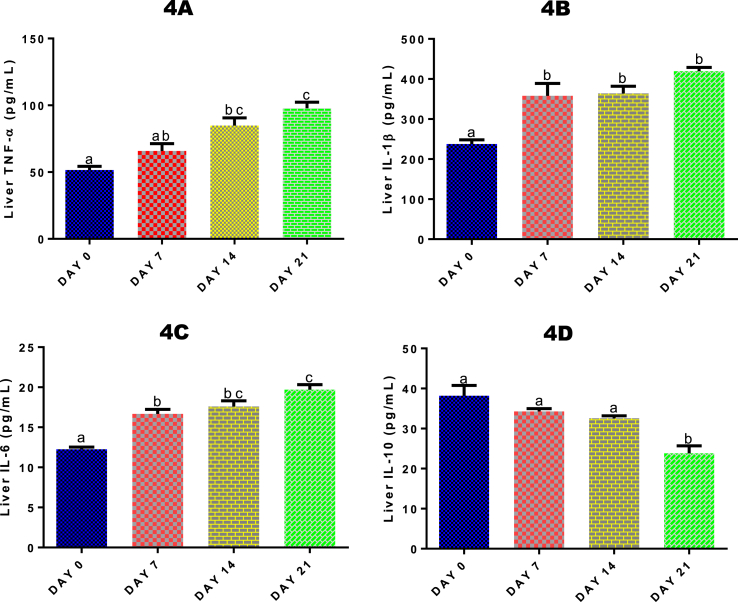
3.10. Time course effect of 2-ME on liver TNF-α, IL-1β, IL-6 and IL-10 levels
Liver TNF-α level was significantly (p < 0.05) increased after 14 (by 39.22%) and 21 (by 47.30%) days of 2-ME administrations compared with control (Fig. 4A). Also, there was a significant (p < 0.05) increase in TNF- α level after 21 days of 2-ME administrations compared with 7 days of administrations. For liver IL-1β (Fig. 4B) and IL-6 (Fig. 4C) levels, there was a significant (p < 0.05) increase following 7, 14 and 21 days of 2-ME administrations. The significant increase was 33.63%, 34.69% and 43.31% respectively for IL-1β, and 26.51%, 30.36%, and 37.79% respectively for IL-6. For IL-10 (Fig. 4D), there was a significant (p < 0.05) decreased after 21 days only compared with control.
Fig. 4.
Time course effect of 2-ME on liver TNF-α (4A), IL-1β (4B), IL-6 (4C), and IL-10 (4D) levels. Values are expressed as mean ± standard error of the mean. Bars labeled with different letters are statistically significant (p < 0.05).
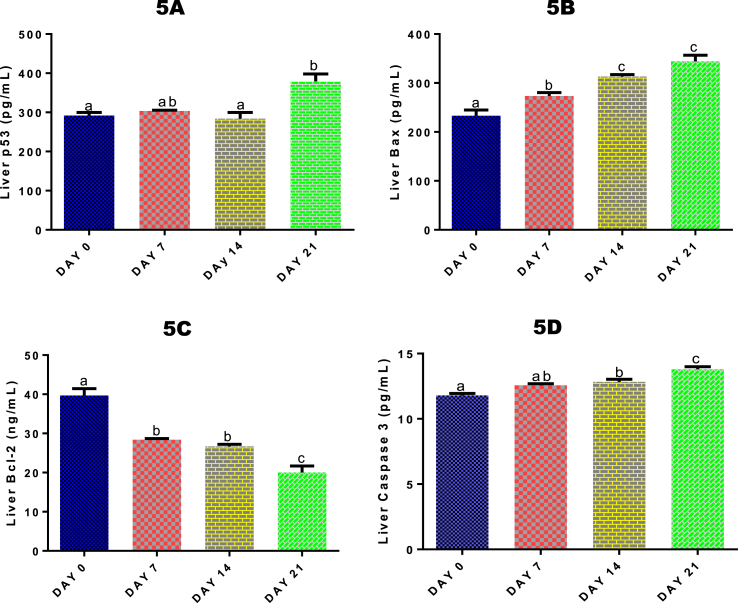
3.11. Time course effect of 2-ME on liver p53, Bax, Bcl-2, caspase-3 levels
For liver p53, a significant (p < 0.05) increase by 22.94% was only recorded after 21 days of 2-ME exposure (Fig. 5A) compared with control. Hepatic Bax level (Fig. 5B) was significantly (p < 0.05) increased after 7, 14 and 21 days of 2-ME administrations by 14.77%, 25.54%, and 32.27% respectively, while liver Bcl-2 level (Fig. 5C) was significantly (p < 0.05) decreased after 7, 14 and 21 days of 2-ME administrations by 39.74%, 48.63%, and 98.20% respectively compared with control. After 14 and 21 days of 2-ME administrations, hepatic level of caspase-3 was significantly (p < 0.05) increased by 8.11% and 14.57% respectively compared with control (Fig. 5D).
Fig. 5.
Time course effect of 2-ME on liver p53 (5A), Bax (5B), Bcl-2 (5C) and caspase-3 (5D) levels. Values are expressed as mean ± standard error of the mean. Bars labeled with different letters are statistically significant (p < 0.05).
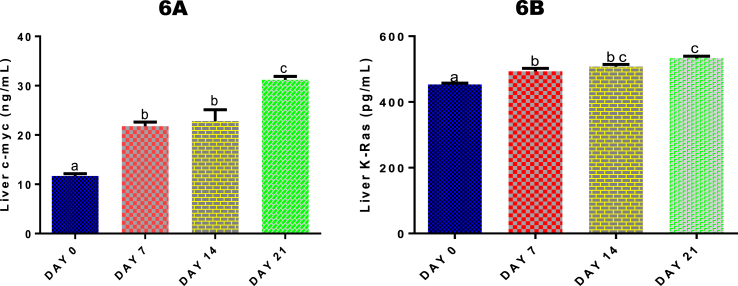
3.12. Time course effect of 2-ME on liver levels of c-Myc and K-Ras
Both hepatic levels of c-Myc (Fig. 6A) and K-Ras (Fig. 6B) were significantly (p < 0.05) increased by 2-ME after 7, 14 and 21 days of administrations compared with control. The increase in c-Myc level compared with control was 46.44%, 48.84%, and 62.57% respectively, while for K-Ras, it was 8.15%, 10.70%, and 14.94% respectively.
Fig. 6.
Time course effect of 2-ME on liver c-Myc (6A) and K-Ras (6B) levels. Values are expressed as mean ± standard error of mean. Bars labeled with different letters are statistically significant (p < 0.05).
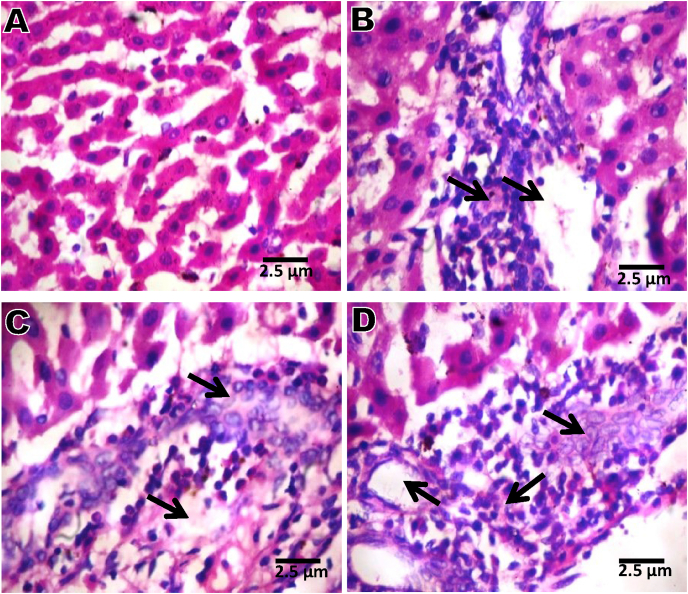
3.13. Time course effect of 2-ME on liver histopathology
Results of liver histopathology (Fig. 7) revealed micro-vesucular steatosis, severe periportal infiltration by inflammatory cells and severe infiltration by inflammatory cells in groups administered 2-ME for 7, 14, and 21 days compared with day 0 that revealed normal architecture.
Fig. 7.
Liver microphotographs (x 400) showing normal appearance (A); microvesucular steatosis, severe periportal infiltration by inflammatory cells and severe infiltration by inflammatory cells (B, C and D). A = Day 0; B = Day 7; C = Day 14; D = Day 21.
4. Discussion
EGEs have high solubility in both ethanol and water mixtures which makes them a useful solvent with wide applications [1]. EGEs including 2-ME are used in liquid soaps, varnishes, pesticides, herbicides, cooling liquids, household appliances, vaccines, antiseptic specifics, children's toys and many more. As a result, humans are unavoidably and inadvertently exposed to them. In this present study, we investigated the time-course effect of 2-ME exposure in male Wistar rats for 21 days. In the oral acute toxicity study conducted, LD50 of 2-ME was calculated to be 990 mg/kg body weight. Mortality was recorded following administration of 1000 and 3000 mg/kg, and not 2000 mg/kg body weight of 2-ME. The reason for the observed non-mortality effect of 2000 mg/kg 2-ME may be due to genetic variation whereby the genes responsible for the metabolism of 2-ME may not have been adequately expressed, and therefore limiting the amount of the active intermediate that is responsible for liver toxicity in that particular rat. Following exposures, relative liver weight was significantly decreased after 14 and 21 days of 2-ME administration, an indication of liver toxicity in the rats over time (Fig. 1). Also, the liver being the major site of biotransformation of 2-ME to 2-methoxyacetaldehyde (2-MAD), and then 2-methoxyacetic acid (2-MAA), by alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) respectively, may be responsible for the significant decrease in relative liver weight of the rats [27,28].
Measurement of thiobarbituric acid reactive substance (TBARS) is usually used to assess lipid peroxidation and indirectly, oxidative stress in vitro and in vivo [29]. Lipid oxidation results in the disruption of the bilayer and cell integrity, leading to leakage of cellular content from the damaged organ into the bloodstream [[30], [31], [32]]. The significant increase in MDA concentration after 7, 14 and 21 days of 2-ME administrations (Fig. 2A) may be attributed to the generation of reactive oxygen species that may have attacked the electron-rich unsaturated fatty acid components of cell membranes, causing their oxidation and destruction and thereby jeopardizing the cellular integrity and functions [32,33].
NO is a strong mediator of inflammation, a low level is known to play an important role in cellular signaling under normal physiological conditions. Excessive production in the cell can lead to the generation of peroxynitrite which subsequently can damage the tissue [34,35]. The high level of liver NO level reported in this study (Fig. 2B) may be an indication of nitrosative stress and hepatic damage following 2-ME administration.
Oxidative stress, characterized by an increase in the production of reactive oxygen or nitrogen species due to insufficient antioxidant defense [36] has been reported in clinical and experimental studies to play a key role in the etiology of many diseases. Oxidative stress adds to the pathological processes of diseases such as diabetes mellitus, cardiovascular diseases, cancer, rheumatoid arthritis, and neurological disorders such as Parkinson and Alzheimer's [37]. From the findings of this study, the significant decrease in hepatic GSH (Fig. 2C) levels, GST (Fig. 3B) and SOD (Fig. 3C) activities, as well as increased activity of GPx (Fig. 3A) following 2-ME administrations can be attributed to cellular response to 2-ME-induced free radical generation and oxidative stress. SOD is responsible for catalytic dismutation of highly reactive and potentially toxic superoxide radicals to hydrogen peroxide (H2O2) and O2, while CAT is responsible for the catalytic decomposition of H2O2 to molecular oxygen and water [38]. In the reaction catalyzed by GPx, GSH serves as a substrate, and as H2O2 is being detoxified, there is concomitant oxidation of GSH to GSSG [39]. GST is a phase two drug-metabolizing enzyme and catalyzes the release and transfer of GSH to xenobiotic for their detoxification. All these may have been the cause or responsibility for the observed results in this study. Their roles in the detoxification and mopping of generated free radicals may be the cause of the up-regulation or depletion of antioxidant systems [32,33,39].
Cytokines are small non-structural proteins, which include tumor necrosis factors, interleukins, interferons, and chemokines, having a multitude of pleiotropic effects in different organs of the host [40]. These pleiotropic effects of individual cytokines give them the ability to exert multiple actions, and particularly in vitro, various cytokines have overlapping actions [41,42]. The significant increase in the levels of hepatic TNF-α (Fig. 4A), IL-1β (Fig. 4B), and IL-6 (Fig. 4C), as well as decreased level of IL-10 (Fig. 4D) as a result of 7, 14 and 21 days of 2-ME administrations, is an indication of 2-ME-induced liver injury or infection causing their secretion and recruitment predominantly by the helper T cells and macrophages to the site of injury or infection where they promote inflammation and trigger pathological pain [43]. In liver damage, Kupffer cells (a type of immune cell) become activated, leading to increased and rapid cytokine generation [44]. The resulting cytokine generations mediate the regeneration of damaged liver tissue. Therefore, the significant increase in the pro-inflammatory cytokines recorded in this study can also be as a result of the cellular inflammatory response in the liver that is required to start the healing process [44], due to 2-ME-induced hepatotoxicity. IL-10 has potent anti-inflammatory properties capable of blocking the formation of inflammatory cytokines like TNF-α, IL-6, and IL-1β by activated macrophages [45]. IL-10 down-regulates pro-inflammatory cytokine and up-regulates endogenous anti-cytokines receptors, causing it to counter-regulate the formation and function of pro-inflammatory cytokines at different levels [45]. In our previous study, we reported that administration of methyl cellosolve significantly increase the renal levels of inflammatory cytokines after 7, 14 and 21 days of administration in rats [46]. Also administration of camphor was reported to increase the hepatic expressions of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) and two chemokines called regulated upon activation normal T cell expressed and secreted (RANTES), and monocyte chemo-attractant protein 1 (MCP-1) in a dose dependent manner in rats [47].
Induction of apoptosis helps prevent tumorigenesis by eliminating damaged cells [48]. It has been identified long ago that tumor suppressor gene p53 is induced by DNA damage [49]. An increase in p53 leads either to the induction of cell cycle arrest or apoptosis [50,51]. Therefore, apoptosis is a fundamental cellular activity occurring under a wide range of physiologic and pathologic conditions [[52], [53], [54]]. In this study, the significant increase in hepatic p53 (Fig. 5A) level following 21 days of 2-ME administration is an indication of hepatic damage. 2-ME-induced hepatic oxidative stress and inflammation recorded in this study may have promoted the activation of p53, which signals the induction of cell cycle arrest and activates the apoptotic genes to initiate apoptosis. Many pathways are involved in p53-induced apoptosis, and one of these makes use of the Bcl-2 and Bax proteins. Bax protein is a target of p53 and a pro-apoptotic member of the Bcl-2 family [55,56]. The Bcl-2 family consists of both pro-apoptotic and anti-apoptotic members that cause opposing effects on mitochondria. Bax can stimulate the release of cytochrome c into the cytosol from mitochondria, which in turn activates caspase-3, one of the major executioners of apoptosis and poly (ADP-ribose) polymerase (PARP) [57]. The anti-apoptotic proteins such as Bcl-2 and Bcl-xl, which are transcriptionally under-regulated by p53, protect the integrity of the mitochondria [58]. This hinders the release of cytochrome c that activates the executors of apoptosis [59]. In this study, the significant increase in liver Bax (Fig. 5B) level and a significant decrease in liver Bcl-2 (Fig. 5C) suggest p53-induced apoptosis, since Bax and Bcl-2 are the targets of p53. In response to cellular damage, up-regulated p53 may have stimulated Bax expression and down-regulated Bcl-2. The increased level of free Bax may have eventually bound to the mitochondrial membrane, creating pores in it, causing mitochondrial membrane damage and the release of cytochrome c that subsequently initiates cellular apoptosis. Majorly, the ratio of pro- and anti-apoptotic protein expression, such as Bax/Bcl-2, is critical for the initiation of apoptosis, and the ratio of Bax/Bcl-2 determines a cell's susceptibility to embark on apoptosis [58]. Change in the ratio of Bax/Bcl-2 promotes the release of cytochrome c from mitochondria into the cytosol. Cytosolic cytochrome c interacts with apoptotic protease-activating factor-1 (Apaf-1) and results in the activation of caspase-3 and PARP that are key to the induction of apoptosis [60,61]. The above may, therefore, explain the increased level of liver caspase-3 (Fig. 5D) after 14 and 21 days of 2-ME administrations. The released cytochrome c following Bax attack on the mitochondrial membrane may have interacted with downstream apoptotic mediators (Apaf-1, caspase-9) to form an apoptosome that cleaved the executioner caspases including caspase-3, that facilitate the programmed cell death. In a related study, administration of methyl cellosolve in rats led to the significant increase in renal caspase-3 after 14 and 21 days in rats [46].
c-Myc, a proto-oncogene, is a strong pleiotropic transcription factor known to coordinate cell cycle growth, progression, adhesion, differentiation, proliferation, apoptosis, and metabolism [[62], [63], [64]]. c-Myc is linked with more than 70% of cancers [65,66]. In hepatocellular carcinoma, c-Myc was one of the first oncogenes known for its high levels of expression [67]. Ras on the other hand, is one of the most common and often mutated oncogenes in human cancer but the frequency and distribution of mutations are not uniform [68,69]. The significant increase in liver c-Myc (Fig. 6A) and K-Ras (Fig. 6B) levels after 7, 14 and 21 days of 2-ME administrations may be an indication of 2-ME-induced interference with these oncogenes by amplification or translocation, resulting into their activations and subsequent generation of reactive oxygen species that may have caused DNA damage. Also, activation of these oncogenes may, therefore, explains the marked increase in the levels of apoptotic players (p53, Bax, caspase-3) recorded in this study that facilitated apoptosis and boycotted tumor initiation and progression. Following genetic analyses, overexpression of c-Myc is commonly caused by genomic amplification at 8q24.1 and found in about 70% of viral and alcohol-related hepatocellular carcinoma [70].
Our results on liver histopathology (Fig. 7) revealing microvesicular steatosis and severe periportal infiltration by inflammatory cells are a confirmation of the 2-ME-induced up-regulation of the pro-inflammatory cytokines reported in this study.
In conclusion, this study has demonstrated, shown, and added to the existing knowledge of the toxic effect of 2-ME. It has been revealed in this study that exposure to 2-ME over time can aggravate and exacerbate its toxicity, and it is therefore advised that exposures to 2-ME should be strictly avoided to the barest minimum as continuous exposure to it could trigger hepatic damage through the disorganization of the antioxidant system, up-regulation of inflammatory, apoptotic, and oncogenic markers in rats.
Author statement
The contribution(s) of each author are stated below.
Oluwatobi Somade (Corresponding Author/Chief Investigator): Conceptualization, methodology, validation, formal analysis, investigation, resources, writing original draft, review and editing, supervision, project administration.
Babajide Ajayi: Methodology, investigation, resources, supervision, project administration.
Oyinkansola Olunaike: Methodology, investigation, resources, project administration.
Latifah Jimoh: Methodology, investigation, resources, project administration.
Declaration of competing interest
None to declare.
Footnotes
Transparency document related to this article can be found online at https://doi.org/10.1016/j.bbrep.2020.100806.
Transparency document
References
- 1.Boatman R.J., Knaak J.B. Ethers of ethylene glycol and derivatives. In: Bingham E., Cohressen B., Powell C.H., editors. Patty's Toxicology. fifth ed. Wiley; New York: 2001. 73–270. [Google Scholar]
- 2.Lockley D.J., Howes D., Williams F.M. Percutaneous penetration and metabolism of 2-ethoxyethanol. Toxicol. Appl. Pharmacol. 2002;180(2):74–82. doi: 10.1006/taap.2002.9373. [DOI] [PubMed] [Google Scholar]
- 3.Udden M.M. Rat erythrocyte morphological changes after gavage dosing with 2 butoxyethanol: a comparison with the in vitro effects of butoxyacetic acid on rat and human erythrocytes. J. Appl. Toxicol. 2000;20(5):381–387. doi: 10.1002/1099-1263(200009/10)20:5<381::AID-JAT701>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 4.Johanson G. Toxicity review of ethylene glycol monomethyl ether and its acetate ester. Crit. Rev. Toxicol. 2000;30(3):307–345. doi: 10.1080/10408440091159220. [DOI] [PubMed] [Google Scholar]
- 5.Starek A., Szymczak W., Zapor L. Hematological effects of four ethylene glycol monoalkyl ethers in short-term repeated exposure in rats. Arch. Toxicol. 2008;82(2):125–136. doi: 10.1007/s00204-007-0236-z. [DOI] [PubMed] [Google Scholar]
- 6.Ma H., An J., Hsie A.W., Au W.W. Mutagenicity and cytotoxicity of 2-methoxyethanol and its metabolites in Chinese hamster cells (the CHO/HPRT and AS52/GPT assays) Mutat. Res. Genet. Toxicol. Test. Biomonit. Environ. Occup. Expos. 1993;298(3):219–225. doi: 10.1016/0165-1218(93)90044-e. [DOI] [PubMed] [Google Scholar]
- 7.Ghanayem B.I., Sullivan C.A. Assessment of the haemolytic activity of 2-butoxyethanol and its major metabolite, butoxyacetic acid, in various mammals including humans. Hum. Exp. Toxicol. 1993;12(4):305–311. doi: 10.1177/096032719301200409. [DOI] [PubMed] [Google Scholar]
- 8.Adedara I.A., Farombi E.O. Induction of oxidative damage in the testes and spermatozoa and hematotoxicity in rats exposed to multiple doses of ethylene glycol monoethyl ether. Hum. Exp. Toxicol. 2010;29(10):801–812. doi: 10.1177/0960327109360115. [DOI] [PubMed] [Google Scholar]
- 9.Davis B.J., Almekinder J.L., Flagler N., Travlos G., Wilson R., Maronpot R.R. Ovarian luteal cell toxicity of ethylene glycol monomethyl ether and methoxy acetic acid in vivo and in vitro. Toxicol. Appl. Pharmacol. 1997;142(2):328–337. doi: 10.1006/taap.1996.8035. [DOI] [PubMed] [Google Scholar]
- 10.Gold E.B., Eskenazi B., Hammond S.K., Lasley B.L., Samuels S.J., Rasor M.O., Hines C.J., Overstreet J.O., Schenker M.B. Prospectively assessed menstrual cycle characteristics in female water-fabrication and non-fabrication semiconductor employees. Am. J. Ind. Med. 1995;28:799–816. doi: 10.1002/ajim.4700280613. [DOI] [PubMed] [Google Scholar]
- 11.Starek A., Szabla J., Kiec-Kononowicz K., Szymczak W. Comparison of the in vitro hemolytic effects produced by alkoxyacetic acids on human and rat erythrocytes. Int. J. Occup. Med. Environ. Health. 2008;21(2):147–155. doi: 10.2478/v10001-008-0009-9. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y., Lee N., Sakai T., Kim K., Yang J.S., Park S., Lee C.R., Cheong H., Moon Y. Evaluation of exposure to ethylene glycol monoethyl ether acetates and their possible haematological effects on shipyard painters. Occup. Environ. Med. 1999;56(6):378–382. doi: 10.1136/oem.56.6.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morton W.E. Occupational phenoxyethanol neurotoxicity: a report of three cases. J. Occup. Med. 1990;32(1):42–45. doi: 10.1097/00043764-199001000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Regulska M., Pomierny B., Basta-Kaim A., Starek A., Filip M., Lason W., Budziszewska B. Effects of ethylene glycol ethers on cell viability in the human neuroblastoma SH-SY5Y cell line. Pharmacol. Rep. 2010;62(6):1243–1249. doi: 10.1016/s1734-1140(10)70389-3. [DOI] [PubMed] [Google Scholar]
- 15.Pomierny B., Starek A., Krzyzanowska W., Starek-Swiechowicz B., Smaga I., Pomierny-Chamiolo L., Regulska M., Budziszewska B. Potential neurotoxic effect of ethylene glycol ethers mixtures. Pharmacol. Rep. 2013;65(5):1415–1421. doi: 10.1016/s1734-1140(13)71501-9. [DOI] [PubMed] [Google Scholar]
- 16.Lorke D. A new approach to tropical acute toxicity testing. Arch. Toxicol. 1983;53:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 17.ECETOC The toxicology of glycol ethers and its relevance to man. Brussels, Eur. Cent. Ecotoxicol. Toxicol. Chem. 1995:350. ECETOC Technical Report No. 64) [Google Scholar]
- 18.NRC . National Academy Press; Washington, DC: 1996. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 19.Buege J.A., Aust S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 20.Green L.C., Wagner D.A., Glogowski J., Skiper P.L., Wishnock J.S., Tannenbaum S.R. Analysis of nitrate, nitrite and [15 N] nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 21.Moron M.S., Depierre J.W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 22.Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W.G. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 23.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 24.Misra H.P., Fridovich I. The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 25.Sinha A.K. Colorimetric assay of catalase. Anal. Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 26.Gornall A.G., Bardawill C.J., David M.M. Determination of serum protein by biuret method. J. Biol. Chem. 1949;117:751–766. [PubMed] [Google Scholar]
- 27.Aasmoe L., Winberg J.O., Aarbakke J. The role of liver alcohol dehydrogenase isoenzymes in the oxidation of glycolethers in male and female rats. Toxicol. Appl. Pharmacol. 1998;150:86–90. doi: 10.1006/taap.1998.8410. [DOI] [PubMed] [Google Scholar]
- 28.Welsch F. The mechanism of ethylene glycol ether reproductive and developmental toxicity and evidence for adverse effects in humans. Toxicol. Lett. 2005;156(1):13–28. doi: 10.1016/j.toxlet.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Beltowski J., Wojcicka G., Gorny D., Marciniak A. The effect of dietary-induced obesity on lipid peroxidation, antioxidant enzymes and total plasma antioxidant capacity. J. Physiol. Pharmacol. 2000;51:883–896. [PubMed] [Google Scholar]
- 30.Ologundudu A., Ologundudu A.O., Oluba O.M., Omotuyi I.O., Obi F.O. Effect of Hibiscus sabdariffa anthocyanins on 2,4-dinitrophenyl hydrazine-induced tissue damage in rabbits. J. Toxicol. Environ. Health Sci. 2010;2:1–6. [Google Scholar]
- 31.Hamed A.N.E., Wahid A. Hepatoprotective activity of Borago officinalis extract against CCl4-induced hepatotoxicity in rats. J. Nat. Prod. 2015;8:113–122. [Google Scholar]
- 32.Somade O.T., Olorode S.K., Olaniyan T.O., Faokunla O. Quercetin, a polyphenolic phytochemical prevents sodium azide-induced extrahepatic oxidative stress in rats. Cog. Biol. 2016;2:1200798. [Google Scholar]
- 33.Somade O.T., Akinloye O.A., Adeyeye M.O., Fabunmi G.D., Idowu O.O., Badmus F.O., Salaudeen B.O. Quercetin, a natural phytochemical and antioxidant protects against sodium azide-induced hepatic and splenic oxidative stress in rats. J. Invest. Biochem. 2015;4:69–74. [Google Scholar]
- 34.Ajayi B.O., Adedara A.I., Farombi E.O. Benzo(a)pyrene induces oxidative stress, pro-inflammatory cytokines, expression of nuclear factor-kappa B and deregulation of wnt/beta-catenin signaling in colons of BALB/c mice. Food Chem. Toxicol. 2016;95:42–51. doi: 10.1016/j.fct.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Ignarro L.J., Cirino G., Casini A., Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J. Cardiovasc. Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 37.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Tomlin C.D. tenth ed. The British Crop Protection Council; Surrey, UK: 1994. The E-Pesticide Manual. [Google Scholar]
- 39.Somade O.T., Adeniji K.D., Adesina A.A., Olurinde O.J. Oral acute toxicity study as well as tissues oxidative stress and histopathological disorders in edible camphor administered rats. Exp. Toxicol. Pathol. 2017;69:99–108. doi: 10.1016/j.etp.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Dinarello C. Historical insights into cytokines. Eur. J. Immunol. 2007;37(Suppl. 1):S34–S45. doi: 10.1002/eji.200737772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paul W.E. Pleiotropy and redundancy; T cell-derived lymphokines in the immune response. Cell. 1989;57:521–524. doi: 10.1016/0092-8674(89)90121-9. [DOI] [PubMed] [Google Scholar]
- 42.Leonard W.J. The defective gene in X-linked severe combined immunodeficiency encodes a shared interleukin receptor subunit: implications for cytokine pleiotropy and redundancy. Curr. Opin. Immunol. 1994;6(4):631–635. doi: 10.1016/0952-7915(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 43.Somade O.T., Ajayi B.O., Adeyi O.E., Aina B.O., David B.O., Sodiya ID I.D. Activation of NF-kB mediates up-regulation of cerebellar and hypothalamic pro-inflammatory chemokines (RANTES and MCP-1) and cytokines (TNF-α, IL-1β, IL-6) in acute edible camphor administration. Sci. Afri. 2019;5 doi: 10.1016/j.toxrep.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mannaa F.A., Abdel-Wahhab K.G. Physiological potential of cytokines and liver damages. Hepatoma Res. 2016;2:131–143. [Google Scholar]
- 45.Zhang J.M., An J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007;45(2):27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Somade O.T., Ajayi B.O., Olushola M.O., Omoseebi E.O. Methyl cellosolve-induced renal oxidative stress and time-dependent up-regulation of pro-inflammatory cytokines, apoptotic, and oncogenic markers in rats. Toxicol. Rep. 2020;7:779–787. doi: 10.1016/j.toxrep.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Somade O.T., Ajayi B.O., Tajudeen N.O., Atunlute E.M., James A.S., Kehinde S.A. Camphor elicits up-regulation of hepatic and pulmonary pro-inflammatory cytokines and chemokines via activation of NFkB in rats. Pathophysiology. 2019;26:305–313. doi: 10.1016/j.pathophys.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Leibowitz B., Yu J. Mitochondrial signaling in cell death via the Bcl-2 family. Canc. Biol. Ther. 2010;9(6):417–422. doi: 10.4161/cbt.9.6.11392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kastan M.B., Onyekwere O., Sidransky D., Vogelstein B., Craig R.W. Participation of p53 protein in the cellular response to DNA damage. Canc. Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 50.Liebermann D.A., Hoffman B., Steinman R.A. Molecular controls of growth arrest and apoptosis: p53-dependent and independent pathways. Oncogene. 1995;11:199–210. [PubMed] [Google Scholar]
- 51.Mass P., Hoffmann K., Gambichler T., Altmeyer P., Mannherz H.G. Premature keratinocyte death and expression of marker proteins of apoptosis in human skin after UVB exposure. Arch. Dermatol. Res. 2003;295:71–79. doi: 10.1007/s00403-003-0403-x. [DOI] [PubMed] [Google Scholar]
- 52.Ellis R.E., Yuan J., Horvitz H.R. Mechanisms and functions of cell death. Annu. Rev. Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- 53.Wyllie A.H., Kerr F.R., Currie A.R. Cell death: the significance of apoptosis. Int. Rev. Cytol. 1980;68:251–270. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 54.Raff M.C. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1992;356:397–400. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- 55.Yin C., Knudson C.M., Korsmeyer S.J., Van Dyke T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 1997;385:637–640. doi: 10.1038/385637a0. [DOI] [PubMed] [Google Scholar]
- 56.McCurrach M.E., Connor T.M., Knudson C.M., Korsmeyer S.J., Lowe S.W. Bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2345–2349. doi: 10.1073/pnas.94.6.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reed J.C. Regulation of apoptosis by Bcl-2 family proteins and its role in cancer and chemoresistance. Curr. Opin. Oncol. 1995;7:541–546. doi: 10.1097/00001622-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 58.Ryan K.M., Phillips A.C., Vousden K.H. Regulation and function of the p53 tumor suppressor protein. Curr. Opin. Cell Biol. 2001;13:332–337. doi: 10.1016/s0955-0674(00)00216-7. [DOI] [PubMed] [Google Scholar]
- 59.Donovan M., Cotter T.G. Control of mitochondrial integrity by Bcl-2 family members and caspase-independent cell death. Biochim. Biophys. Acta. 2004;1644:133–147. doi: 10.1016/j.bbamcr.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 60.Yang J., Liu X., Bhalla K., Kim C.N., Ibrado A.M., Cai J., Peng T.I., Jones D.P., Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 61.Kluck R.M., Bossy-Wetzel E., Green D.R., Newmeyer D.D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 62.Whitfield J.R., Soucek L. Tumor microenvironment: becoming sick of myc. Cell. Mol. Life Sci. 2012;69:931–934. doi: 10.1007/s00018-011-0860-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meyer N., Penn L.Z. Reflecting on 25 years with myc. Nat. Rev. Canc. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 64.Miller D.M., Thomas S.D., Islam A., Muench D., Sedoris K. C-myc and cancer metabolism. Clin. Canc. Res. 2012;18:5546–5553. doi: 10.1158/1078-0432.CCR-12-0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y., Casey S.C., Felsher D.W. Inactivation of myc reverses tumorigenesis. J. Intern. Med. 2014;276:52–60. doi: 10.1111/joim.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nesbit C.E., Tersak J.M., Prochownik E.V. Myc oncogenes and human neoplastic disease. Oncogene. 1999;18:3004–3016. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- 67.Peng S.Y., Lai P.L., Hsu H.C. Amplification of the c-myc gene in human hepatocellular carcinoma: biologic significance. J. Formos. Med. Assoc. 1993;92:866–870. [PubMed] [Google Scholar]
- 68.Cox A.D., Fesik S.W., Kimmelman A.C., Luo J., Der C.J. Drugging the undruggable RAS: mission possible? Nat. Rev. Drug Discov. 2014;13:828–851. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prior I.A., Lewis P.D., Mattos C. A comprehensive survey of Ras mutations in cancer. Canc. Res. 2012;72:2457–2467. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schlaeger C., Longerich T., Schiller C., Bewerunge P., Mehrabi A., Toedt G., Kleeff J., Ehemann V., Eils R., Lichter P., Schirmacher P., Radlwimmer B. Etiology-dependent molecular mechanisms in human hepatocarcinogenesis. Hepatology. 2008;47:511–520. doi: 10.1002/hep.22033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.