Abstract
OBJECTIVE.
The purposes of this study were to compile mammographic images in various projections showing commercially available breast biopsy site markers and to provide a standardized nomenclature and marker guide to improve physician communication and patient care.
MATERIALS AND METHODS.
A retrospective review of all breast biopsy markers encountered at one institution was conducted from January 2012 to January 2018. Markers placed at the facility and those placed at outside institutions with the patient subsequently referred to the study institution were included. Additional drawings and photographs and biopsy marker information were compiled from manufacturers and the literature. Intrinsic properties, features, pitfalls, and biopsy marker mimics were recorded from the institution’s experience and the literature.
RESULTS.
Thirty-eight different biopsy marker shapes available from six manufacturers were identified, and mammograms of 37 were compiled and organized by manufacturer. Nomenclature was compiled on the basis of the manufacturer names of each marker. Potential pitfalls and mimics were identified. Manufacturer-reported marker material composition and carrier properties were summarized, including decreased marker migration, enhanced ultrasound visibility, and varying MRI susceptibility.
CONCLUSION.
Variability in the appearance and nomenclature of breast biopsy site markers may contribute to misinterpretation, miscommunication, and possibly removal of the incorrect lesion. A comprehensive guide to breast biopsy marker nomenclature is clinically useful, and standardization is necessary.
Keywords: biopsy clips, biopsy markers, breast biopsy, breast imaging, mammography
As percutaneous breast biopsy techniques evolved from fine-needle aspiration to core needle biopsy for nonsurgical tissue sampling, breast biopsy site markers were introduced to identify the biopsy site [1]. The U.S. Food and Drug Administration first approved biopsy markers for use in soft tissues in 1995 [1, 2]. Since then, breast biopsy marker use has become widespread, and biopsy markers are now commonly placed after biopsies of suspicious lesions [1]. These markers are used to guide preoperative localization when pathologic analysis yields malignant or atypical results, or they remain indefinitely within the breast when pathologic analysis yields benign results, enabling identification and follow-up of benign biopsy sites when necessary [1–4].
Various manufacturers have developed biopsy markers, some of which have distinct advantages, including improved sonographic visualization and reduced susceptibility artifact on MRI [5–10]. Because it is increasingly common to find more than one marker placed within a breast, confusion arises owing to marker redundancy, mimics, and descriptor variability among manufacturers and within postprocedural mammogram reports. Moreover, marker types and nomenclature vary across breast centers locally, nationally, and internationally, further contributing to potential miscommunication and possibly to surgical removal of an incorrect lesion. A standardized comprehensive biopsy marker nomenclature would vastly improve communication and enhance patient care.
Breast biopsy marker literature focuses on indications for use, sonographic visibility, evaluation of postprocedure migration, and assessment of MRI susceptibility artifacts and safety [4, 11–21]. To our knowledge, no publications have addressed the topic of standardization of breast biopsy site marker nomenclature, and no comprehensive guide has been developed. Our aims were to compile mammograms in various projections of all known commercially available breast biopsy site markers and to provide a standardized nomenclature and marker guide to improve physician communication and patient care.
Materials and Methods
Image Review
We conducted an institutional review board–approved HIPAA-compliant retrospective mammogram review at our tertiary cancer center. The requirement for informed consent was waived. We searched our institutional database for all breast biopsy markers encountered at our institution from January 2012 to January 2018. These included markers placed at our facility and those placed at an outside institution after which the patient was referred to our cancer center.
Screening, diagnostic, and postbiopsy mammograms of breast biopsy site markers in different projections were retrieved from and reviewed on the institutional PACS (Centricity, GE Healthcare). Most of the markers were visualized in at least two projections corresponding to the standard two-view mammogram.
Image Correlation With Commercially Available Biopsy Markers
A concurrent review of manufacturer websites was performed to correlate each mammogram with commercially available biopsy markers and to obtain product-specific details. Correlation was done by consensus of the authors—three radiologists specializing in breast imaging, two breast imaging fellows, and one technologist supervisor. Drawings or photographs were obtained with permission from six manufacturers: Argon Medical Devices, BD, Devicor Medical Products (Mammotome), Hologic, Mermaid Medical (Cassi Beacon), and MPM Medical Supply (Somatex). Information was also collected from each manufacturer regarding marker composition, carrier material, and contraindications to use. Standard manufacturer names were compiled for all biopsy site markers.
Results
We compiled mammograms of commercially available breast biopsy markers encountered at our institution and subdivided them according to manufacturer. Currently, 38 different biopsy marker shapes are available from six manufacturers. A one-page document was created to summarize mammograms, drawings, and photographs and to standardize nomenclature (Fig. S1). (Fig. S1 can be viewed in the AJR electronic supplement to this article, available at www.ajronline.org.)
Most biopsy markers are compatible for use with stereotactic and ultrasound-guided core biopsy. Some biopsy markers are advertised as specifically designed for MRI with variable amounts of susceptibility artifact [18–20]. Manufacturer-reported MRI-compatible biopsy markers include the BD SenoMark UltraCor M-clip and X-clip, coil, heart, ribbon, and Venus-shaped biopsy markers [6]; Hologic SecurMark buckle, infinity, and stoplight; and Hologic TriMark cork and hourglass [7]. Biopsy markers cause variable MRI susceptibility artifact depending on shape and composition [5–10]. Individual package information should be carefully reviewed for MRI compatibility because reports of MRI conditional markers have appeared [21].
Tables 1–3 show the material composition and carrier properties of each marker. Most biopsy markers are composed of titanium and stainless steel. Other source materials include carbon-coated ceramic and carbon-coated zirconium oxide, heat-resistant polyetherketoneketone polymer, and metal alloys with low nickel content (BioDur 108, Carpenter Technologies; Inconel 625, Special Metals Corporation). The Hologic Professional Tumark and BD UltraCor Twirl markers are composed of nitinol and are contraindicated in patients with severe nickel allergy but enhance visibility under ultrasound imaging, as reported by the manufacturer [6, 7].
TABLE 1:
Breast Biopsy Site Markers Manufactured by Devicor Medical Products (Mammotome)
| Marker Shape | Brand | Composition | Carrier Material |
|---|---|---|---|
|
| |||
| Anchor | MicroMARK | Stainless steel | NA |
| Barbell (2 × 4 mm) | BiomarC | Carbon-coated ceramic (nonmetal) | NA |
| MammoSTAR | Carbon-coated zirconium oxide (nonmetal) | Beta glucan | |
| Tribell (1.5 mm) | BiomarC | Carbon-coated ceramic (nonmetal) | NA |
| MammoSTAR | Carbon-coated zirconium oxide (nonmetal) | Beta glucan | |
| Barrel | HydroMARK | Titanium, stainless steel | PEG-based hydrogel |
| Butterfly | HydroMARK | Titanium | PEG-based hydrogel |
| Open coil | HydroMARK | Titanium, stainless steel | PEG-based hydrogel |
| Bowtie | MammoMARK | Titanium | Bovine collagen |
| Triple twist | MammoMARK | Titanium | Bovine collagen |
| U shape | MammoMARK | Titanium | Bovine collagen |
Note—NA = not applicable, PEG = polyethylene glycol.
TABLE 3:
Breast Biopsy Site Markers Manufactured by Hologic, MPM Medical Supply, Mermaid Medical, and Argon Medical Devices
| Marker Shape | Brand | Composition | Carrier Material |
|---|---|---|---|
|
| |||
| Hologic | |||
| Buckle | SecureMark | Stainless steel | Suturelike netting |
| Cork (3.0 × 1.4 mm) | TriMark (MRI) or CeleroMark (stereotactic) | Titanium | NA |
| Hourglass | TriMark (MRI) or CeleroMark (stereotactic) | Titanium | NA |
| Infinity | SecureMark | Stainless steel | Suturelike netting |
| Mini cork (1.8 × 0.9 mm) | SecureMark | Titanium | Suturelike netting |
| Stoplight | SecureMark | Stainless steel | Suturelike netting |
| Top hat | SecureMark | Titanium | Suturelike netting |
| Tumark Q (10, 12 mm)a | Professional | Nitinol | NA |
| Tumark Flex (7, 10, 12 mm)a | Professional | Nitinol | NA |
| Tumark Vision (10, 12 mm)a | Professional | Nitinol | NA |
| Tumark X (10, 12 mm)a | Professional | Nitinol | NA |
| MPM Medical Supply | |||
| Tumark Eye (10, 12 mm)a | Tumark Eye | Nitinol | NA |
| Mermaid Medical | |||
| Beacon | Beacon | PEKK polymer | NA |
| Cassi Star | Cassi Star | PEKK polymer | NA |
| Argon Medical Devices | |||
| V-Mark | V-Mark | Titanium | NA |
Note—NA = not applicable, PEKK = polyetherketoneketone.
Contraindicated in patients with severe nickel allergy.
Biopsy markers are sometimes associated with carrier materials that are embedded, deployed with, or interwoven into the biopsy marker. The combination of biopsy marker composition and carrier material properties determines manufacturer-designated strengths for which they are marketed and given different brand names. Carrier materials include beta glucan, polyethylene glycol (PEG)-based hydrogel, bovine collagen, polyvinyl alcohol (PVA) polymer, polyglycolic acid (PGA) microfiber pad, PGA microfiber–PVA polymer combinations, starch pellets, polylactic acid–PGA pellets, and suturelike netting. The Mammotome HydroMARK device contains PEG-based hydrogel, which affords a 12- to 15-month increase in long-term visibility under ultrasound guidance [17, 22]. Bovine collagen in the Mammotome MammoMARK device prevents marker migration [23]. BD PGA microfiber pads and Hologic SecureMARK suturelike netting are reported to reduce marker migration and improve sonographic visibility [6, 7]. BD PVA polymers permanently enhance visibility under ultrasound guidance [6]. BD starch pellets promote hemostasis [6].
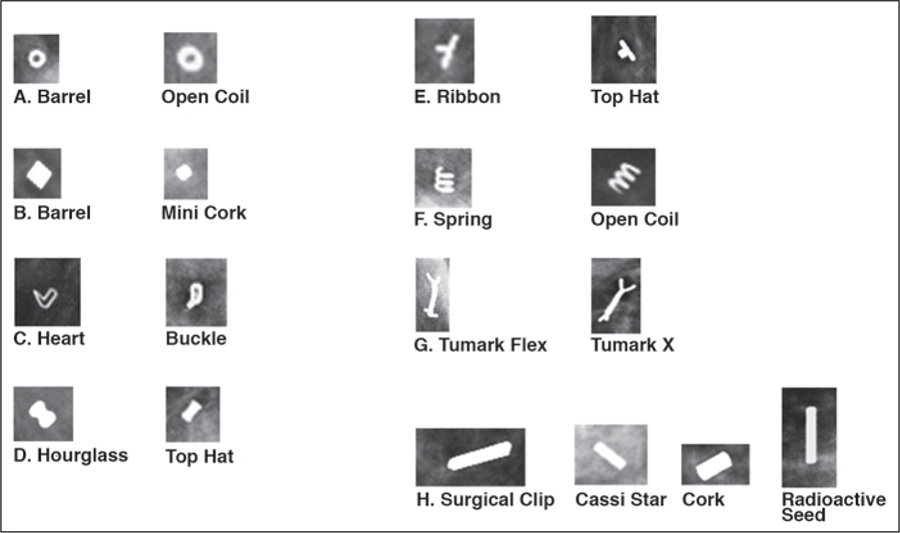
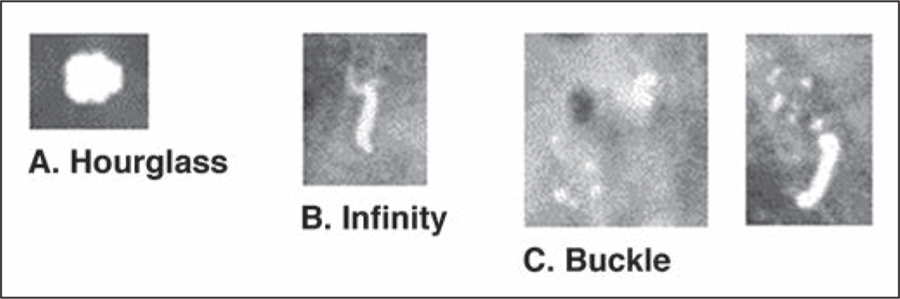
We noted potential pitfalls and mimics related to biopsy markers. Different marker shapes appear similar depending on the angle of image acquisition, possibly leading the radiologist to confuse one marker for another marker or for radioactive seeds and surgical clips (Fig. 1). Biopsy markers can also be confused for calcifications and calcifications for biopsy markers (Fig. 2). Biopsy marker shape may not be clear on tomosynthesis images. We also found examples of the same biopsy marker referred to by multiple names in different reports, highlighting a lack of standardization and a potential additional source of confusion.
Fig. 1—
Mammograms show biopsy site marker pitfalls and mimics.
A, Barrel mimics open coil.
B, Barrel mimics mini cork.
C, Heart mimics buckle.
D, Hourglass mimics top hat.
E, Ribbon mimics top hat.
F, Spring (opposite endpoints) mimics open coil (parallel endpoints).
G, Tumark Flex mimics Tumark X (both Hologic).
H, Surgical clip, Cassi Star device, and cork mimic radioactive seed.
Fig. 2—
Mammograms show biopsy site markers mimicking calcifications.
A, Hourglass can be mistaken for dystrophic calcification.
B, Infinity marker can be mistaken for suture calcification.
C, Buckle marker mimics rim or suture calcification (left). Orthogonal projection shows shape is clearly buckle marker. Residual adjacent pleomorphic microcalcifications are evident. Biopsy revealed ductal carcinoma in situ.
Discussion
A complete pictorial review of commercially available breast biopsy markers in different orthogonal projections is shown in Figure S1 (which can be viewed in the AJR electronic supplement to this article, available at www.ajronline.org). The literature thus far has centered on the use of biopsy markers, postbiopsy migration, ultrasound visibility, and MRI susceptibility. To our knowledge, however, no publications have attempted to standardize breast biopsy site marker nomenclature for effective communication among practitioners and for optimal patient care.
Thomassin-Naggara et al. [4] justified deploying breast biopsy markers in all percutaneous breast biopsies. They highlighted the importance of markers in facilitating patient care among radiologists, surgeons, and pathologists. At our institution, approximately 7000 screening, 20,500 diagnostic, and 2300 outside-hospital mammographic examinations are reviewed per year. Outside reports often do not mention biopsy marker shape, or they provide different descriptors for the same-shaped biopsy marker. For example, the cork has been referred to as a bar, cylinder, and rectangle. However, this name can be misinterpreted for other biopsy markers with similar shapes, such as mini cork, barrel, and buckle.
Clip nomenclature is not standardized in the literature. Data regarding stereotactic core needle biopsies and marker migration reported by Jain et al. [14] focused on contributory and noncontributory factors related to breast biopsy marker migration. Biopsy marker names in the article include rod, spiral, T, and elongated ring. Although in the methods section Jain et al. attributed these markers to specific manufacturers’ brands, the use of nonspecific nomenclature can be misleading and confusing to readers. In addition, only a postprocedure report without the correlating mammograms may be available at consultation. If such a report does not indicate the biopsy site marker shape or provides varying and unclear nomenclature, misidentification can result.
Certain biopsy site marker shapes are similar and can mimic one another depending on the image projection. This potential pitfall of the use of particular biopsy markers can be a source of perplexity and result in inefficient patient care. This highlights the importance of looking closely at biopsy markers in different projections and having a widely available standardized reference source. Furthermore, although different in appearance and manufacturer, the names “open coil” and “coil” are so similar that the need for accuracy in reporting is crucial for avoiding error. Because it is an increasingly common occurrence to have more than one biopsy marker within the same breast, uniformity by use of a standardized nomenclature minimizes confusion and improves accuracy in communication and preoperative localization. In addition, commonly seen surgical clips and radioactive seeds in the breast [24] can be confused with biopsy markers, especially the Cassi Star device (Mermaid Medical) given the thin linear configuration. The 6-mm surgical clip is longer than the Cassi Star device (5 mm) and radioactive seeds (5 mm) (Fig. 1H).
Limitations of our study were that only biopsy site markers and radioactive seeds encountered at our institution were included; other markers are potentially currently available, including magnetic markers. Additional markers will undoubtedly be manufactured in the future, and we anticipate that our guide will serve as a dynamic document on which to add future markers as they are produced. We do not expect that manufacturers will change the current names provided. We obtained information directly from manufacturers, which introduces bias because manufacturers presumably highlight self-reported strengths rather than weaknesses of various markers. Research to objectively evaluate and compare different biopsy marker properties continues to be important [25]. Various publications have described marker migration [14], variability in ultrasound visibility [15–17], and the effects of MRI susceptibility artifact [18, 19].
Our institution has adopted the universal nomenclature and placed a quick reference guide (Fig. S1) at each radiology reading station and distributed it to surgeons.
Conclusion
We provide a comprehensive guide to standardizing nomenclature for breast biopsy site markers to improve patient care, increase accuracy for breast imaging and intervention, and facilitate communication among practitioners. Implementation of uniform nomenclature in radiologist reports, clinician discussions, and published literature will improve communication and patient care.
Supplementary Material
TABLE 2:
Breast Biopsy Site Markers Manufactured by BD
| Marker Shape | Brand | Composition | Carrier Material |
|---|---|---|---|
|
| |||
| Coil | UltraClip | Low-nickel stainless steel alloya | NA |
| UltraClip Dual Trigger | Low-nickel stainless steel alloya | PVA polymer | |
| SenoMark UltraCor | Low-nickel stainless steel alloya | PGA microfiber pad and PVA polymer | |
| SenoMark Ultra | Low-nickel stainless steel alloya | PGA microfiber pad and PVA polymer | |
| Heart | UltraClip Dual Trigger | Titanium | PVA polymer |
| SenoMark UltraCor | Titanium | PGA microfiber pad and PVA polymer | |
| M clip | SenoMark and SenoMark UltraCor MRI | Stainless steel | PGA microfiber pads |
| O clip | SenoMark | Titanium | PGA microfiber pads |
| Omega | Gel Mark UltraCor | Stainless steel | PLA-PGA pellets |
| Gel Mark Ultra | Stainless steel | PLA-PGA pellets | |
| SenoMark | Stainless steel | PGA microfiber pads | |
| StarchMark | Stainless steel | Starch pellets | |
| Ribbon | UltraClip | Titanium | NA |
| UltraClip and UltraClip Dual Trigger | Titanium | PVA polymer | |
| SenoMark UltraCor | Titanium | PGA microfiber pad and PVA polymer | |
| SenoMark Ultra | Titanium | PGA microfiber pad and PVA polymer | |
| Twirl ring (same as nitinol O twist)b | UltraCor Twirl | Nitinol | NA |
| S clip | Gel Mark UltraCor | Titanium | PLA-PGA pellets |
| Gel Mark Ultra | Titanium | PLA-PGA pellets | |
| SenoMark | Titanium | PGA microfiber pads | |
| Spring | UltraCor | Stainless steel | PEG plugs |
| V clip | StarchMark UltraCor | Stainless steel | Starch pellets |
| StarchMark | Stainless steel | Starch pellets | |
| Venus | UltraClip Dual Trigger | Low-nickel stainless steel alloya | PVA polymer |
| SenoMark UltraCor | Low-nickel stainless steel alloya | PGA microfiber pad and PVA polymer | |
| Wing | UltraClip | Nickel-chromium alloyc | NA |
| UltraClip Dual Trigger | Nickel-chromium alloyc | PVA polymer | |
| X clip | SenoMark or UltraCor MRI | Titanium | PGA microfiber pads |
Note—NA = not applicable, PVA = polyvinyl alcohol, PGA = polyglycolic acid, PEG = polyethylene glycol.
BioDur 108 (Carpenter Technologies).
Contraindicated in patients with severe nickel allergy.
Inconel 625 (Special Metals Corporation).
Acknowledgments
We thank Joanne Chin for editorial assistance and Elijah D. Kreider, Leica Biosystems, for support in gathering material composition and carrier property data.
Supported by MSK Cancer Center support grant/core grant P30 CA008748, Breast Cancer Research Foundation.
Footnotes
Based on presentation at the Society of Breast Imaging 2018 annual meeting, Las Vegas, NV.
Supplemental Data
Available online at www.ajronline.org.
References
- 1.Burbank F, Forcier N. Tissue marking clip for stereotactic breast biopsy: initial placement accuracy, long-term stability, and usefulness as a guide for wire localization. Radiology 1997; 205:407–415 [DOI] [PubMed] [Google Scholar]
- 2.Parker SH, Burbank F. A practical approach to minimally invasive breast biopsy. Radiology 1996; 200:11–20 [DOI] [PubMed] [Google Scholar]
- 3.Liberman L, Dershaw DD, Morris EA, Abramson AF, Thornton CM, Rosen PP. Clip placement after stereotactic vacuum-assisted breast biopsy. Radiology 1997; 205:417–422 [DOI] [PubMed] [Google Scholar]
- 4.Thomassin-Naggara I, Lalonde L, David J, Darai E, Uzan S, Trop I. A plea for the biopsy marker: how, why and why not clipping after breast biopsy? Breast Cancer Res Treat 2012; 132:881–893 [DOI] [PubMed] [Google Scholar]
- 5.Devicor Medical Products website. Mammotome breast biopsy markers. www.mammotome.com/breast-biopsy-markers/. Accessed December 18, 2017
- 6.CR Bard website. 2019. product list. www.crbard.com/Biopsy/BardBiopsy/files/b7/b7a4941c-43e6-47ff-af50-4cff5248d9e0.pdf. Accessed January 14, 2019
- 7.Hologic website. Biopsy site marking solutions: breast biopsy markers. www.hologic.com/hologic-products/breast-skeletal/breast-biopsy-markers. Accessed January 14, 2019
- 8.Scion Medical Technologies website. Cassi Beacon marker. scionmedtech.com/products/breast-biopsy/cassi-beacon/. Accessed March 26, 2018
- 9.Mermaid Medical website. Cassi Star breast tissue marker. www.mermaidmedical.dk/introducing-cassi-star-marker/. Accessed March 26, 2018
- 10.Argon Medical Devices website. V-Mark breast biopsy site marker. www.argonmedical.com/products/vmark-breast-biopsy-site-marker. Accessed December 18, 2017
- 11.Rosen EL, Vo TT. Metallic clip deployment during stereotactic breast biopsy: retrospective analysis. Radiology 2001; 218:510–516 [DOI] [PubMed] [Google Scholar]
- 12.Whaley DH, Adamczyk DL, Jensen EA. Sono graphically guided needle localization after stereotactic breast biopsy. AJR 2003; 180:352–354 [DOI] [PubMed] [Google Scholar]
- 13.Pinkney DM, Mychajlowycz M, Shah BA. A prospective comparative study to evaluate the displacement of four commercially available breast biopsy markers. Br J Radiol 2016; 89:20160149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain A, Khalid M, Qureshi MM, et al. Stereotactic core needle breast biopsy marker migration: an analysis of factors contributing to immediate marker migration. Eur Radiol 2017; 27:4797–4803 [DOI] [PubMed] [Google Scholar]
- 15.Seow JH, Phillips M, Taylor D. Sonographic visibility of breast tissue markers: a tissue phantom comparison study. Australas J Ultrasound Med 2012; 15:149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakamoto N, Ogawa Y, Tsunoda Y, Fukuma E. Evaluation of the sonographic visibility and sonographic appearance of the breast biopsy marker (UltraClip®) placed in phantoms and patients. Breast Cancer 2017; 24:585–592 [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto N, Fukuma E, Tsunoda Y, Teraoka K, Koshida Y. Evaluation of the dislocation and long-term sonographic detectability of a hydrogel-based breast biopsy site marker. Breast Cancer 2018; 25:575–582 [DOI] [PubMed] [Google Scholar]
- 18.Shellock FG. Metallic marking clips used after stereotactic breast biopsy: ex vivo testing of ferromagnetism, heating, and artifacts associated with MR imaging. AJR 1999; 172:1417–1419 [DOI] [PubMed] [Google Scholar]
- 19.Genson CC, Blane CE, Helvie MA, Waits SA, Chenevert TL. Effects on breast MRI of artifacts caused by metallic tissue marker clips. AJR 2007; 188:372–376 [DOI] [PubMed] [Google Scholar]
- 20.Kubota K, Gomi N, Wakita T, Shibuya H, Kakimoto M, Osanai T. Magnetic resonance imaging of the metal clip in a breast: safety and its availability as a negative marker. Breast Cancer 2004; 11:55–59 [DOI] [PubMed] [Google Scholar]
- 21.Cronenweth CM, Shellock FG. Assessment of MRI issues at 3 Tesla for a new metallic tissue marker. Int J Breast Cancer 2015; 2015:823759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devicor Medical Products website. Mammotome breast biopsy markers: HydroMARK. www.mammotome.com/breast-biopsy-markers/hydromark/. Accessed December 18, 2017
- 23.Devicor Medical Products website. Mammotome breast biopsy markers: MammoMARK/CorMARK. www.mammotome.com/breast-biopsy-markers/mammomark-cormark/. Accessed December 18, 2017
- 24.Sung J, King V, Thornton C, et al. Safety and efficacy of radioactive seed localization with I-125 prior to lumpectomy and/or excisional biopsy. Eur J Radiol 2013; 82:1453–1457 [DOI] [PubMed] [Google Scholar]
- 25.Shah AD, Mehta AK, Talati N, Brem R, Margolies LR. Breast tissue markers: why? What’s out there? How do I choose? Clin Imaging 2018; 52:123–136 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.