Abstract
With their ‘all-or-none’ action potential responses, single neurons (or units) are accepted as the basic computational unit of the brain. There is extensive animal literature to support the mechanistic importance of studying neuronal firing as a way to understand neuronal microcircuits and brain function. Although most studies have emphasized physiology, there is increasing recognition that studying single units provides novel insight into system-level mechanisms of disease. Microelectrode recordings are becoming more common in humans, paralleling the increasing use of intracranial electroencephalography recordings in the context of presurgical evaluation in focal epilepsy. In addition to single-unit data, microelectrode recordings also record local field potentials and high-frequency oscillations, some of which may be different to that recorded by clinical macroelectrodes. However, microelectrodes are being used almost exclusively in research contexts and there are currently no indications for incorporating microelectrode recordings into routine clinical care. In this review, we summarize the lessons learnt from 65 years of microelectrode recordings in human epilepsy patients. We cover the electrode constructs that can be utilized, principles of how to record and process microelectrode data and insights into ictal dynamics, interictal dynamics and cognition. We end with a critique on the possibilities of incorporating single-unit recordings into clinical care, with a focus on potential clinical indications, each with their specific evidence base and challenges.
Keywords: microelectrode, single units, extracellular action potential, stereoelectroencephalography, subdural grid
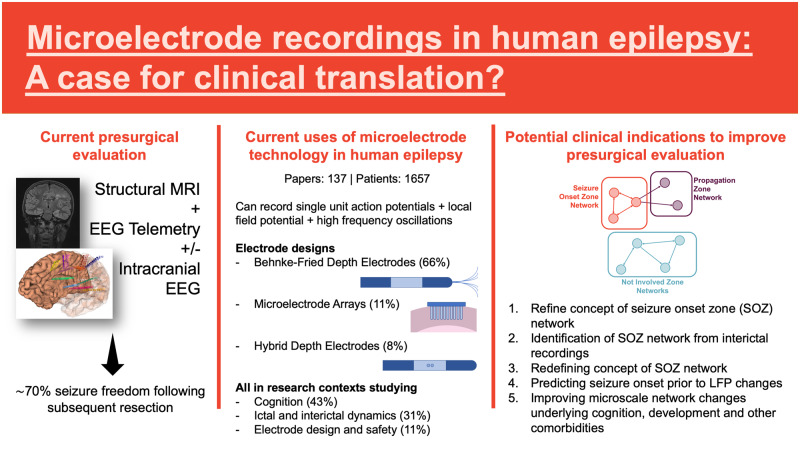
Microelectrode recordings in human epilepsy are becoming more common but are almost exclusively limited to research contexts. This review summarizes the lessons learnt from 65 years of microelectrode recordings in human epilepsy patients. It ends by exploring the potential indications for incorporating single-unit recordings into clinical care.
Graphical Abstract
Graphical Abstract.
Introduction
With their ‘all-or-none’ action potential responses, single neurons (or units) are accepted as the basic computational unit of the brain (McCulloch and Pitts, 1943; Zador, 2000; Buzsáki, 2004; Kreiman, 2004). Spatiotemporal patterns of action potential firing within neuronal microcircuits are system-level mechanisms of brain functions such as movement, memory and sensory perception. Existing literature from animal models affirms the importance of studying neuronal dynamics, evidenced by the award of the Nobel Prize in 2014 for the identification of spatial navigation systems in the rodent brain using single-unit electrophysiological approaches (O’Keefe and Dostrovsky, 1971; O’Keefe, 1976; Buzsáki, 2004).
Perturbations of the brain by disease (gene mutations, brain injury, developmental and degenerative disorders) can lead to changes in the functional architecture of action potential firing with emergent neurological symptoms including seizures and associated morbidities. Therefore, studying patients with epilepsy at the level of microcircuit action potential behaviour has enormous potential for increasing the understanding of mechanisms that predispose to seizures and those that predict adverse cognitive and behavioural outcomes.
Invasive recording techniques associated with the neurosurgical investigation and treatment of focal epilepsy (Jayakar et al., 2014; Rasul et al., 2017; Vakharia et al., 2018) provide an ideal opportunity to record neuronal firing patterns in the human brain. Established techniques of electrophysiological recording during presurgical evaluation [using clinical macroelectrodes in the form of subdural grid and stereoelectroencephalography (SEEG) electrodes] and resective surgical procedures (using electrocorticography) have enabled the concurrent placement of microelectrodes that can sample single-unit activity and multiunit activity (Engel et al., 2005; Abbott, 2009; Rasul et al., 2017). To date, these have been exclusively placed in research contexts in a small proportion of patients undergoing intracranial evaluation (Supplementary Fig. 1A), with the majority of the work assessing neuronal correlates of cognition (Supplementary Fig. 1B).
Sixty-five years after single units were first recorded in the human brain; there remain no established clinical indications for microelectrode recordings in the presurgical evaluation of patients with epilepsy (Cash and Hochberg, 2015). This is contrasted by multiple studies that have demonstrated that, despite the increased use of intracranial electrophysiology in presurgical evaluation, seizure freedom outcomes following epilepsy surgery have remained largely stable around 70% over the last 20 years (Baud et al., 2018; Barba et al., 2020). Therefore, there is a clear need to improve our localization strategies (be that to a specific brain region or network-level change) and we argue that microelectrode recordings have the potential to complement traditional imaging and macroscale electrophysiology by adding an additional layer of data at a different scale that could contribute to improved outcomes.
In this review, we discuss lessons learnt from microelectrode recordings in humans undergoing presurgical evaluation or resective epilepsy surgery, with a focus on how these findings have impacted our biological understanding of epilepsy and cognition. We cover principles of how to record and process microelectrode data and insights into ictal and interictal dynamics. We end with a critique on the possibilities of incorporating single-unit recordings into clinical care, with a focus on potential clinical indications, each with their specific evidence base and challenges.
Historical perspective
Prior to the 1950s, technological limitations precluded the recording of single units in humans during neurosurgical procedures. Intracellular microelectrode recording (Hodgkin et al., 1952) and, later, patch clamping (Sakmann and Neher, 1984) were too cumbersome to be implemented in the time-limited setting of an operating theatre and were necessarily destructive to the tissue that was being recorded. The development of extracellular glass micropipette electrodes (Renshaw et al., 1940) opened the door to single-unit recordings in the live human brain, a technique first reported in 1955 (Ward and Thomas, 1955). In the first account of such recordings, Ward and Thomas reported their ability to record single-unit action potentials in a patient with epilepsy using glass micropipettes filled with 3 M potassium chloride (Ward and Thomas, 1955). They recorded from ‘scar’ and ‘normal’ tissue surrounding a temporo-occipital focus but were unable to make any comparisons between the firing characteristics of neurons in different areas. These glass micropipettes were fragile and could only be used for a limited time in the intraoperative setting and the resultant recordings were confounded by the effects of concurrent or recent anaesthesia (Ward and Thomas, 1955; Ward and Schmidt, 1961; Rayport and Waller, 1967). Subsequently, the routine use of chronic extraoperative implanted electrodes in presurgical evaluation together with a number of microelectrode designs has facilitated successful recording of single-unit activity in awake, asleep, interictal and ictal states from a variety of brain regions (Table 1) (Buzsáki et al., 2012; Tóth et al., 2016; Hong and Lieber, 2019).
Table 1.
Techniques of microelectrode recording that have been used in human subjects with epilepsy
| Type of electrode | Description | First reported use | Publications using technique | Setting | Schematic diagram |
|---|---|---|---|---|---|
| Glass micropipettes | Glass pipettes pulled to have a small tip diameter of 3–4 μm | Ward and Thomas (1955) | 2 | Intraoperative cortical surface recording | |
| Platinum/iridium/tungsten microwires | Single microwire, often advanced with a hydraulic manipulator attached to the edge of the craniotomy flap | Verzeano et al. (1971) | 12 | Intraoperative cortical surface recording | |
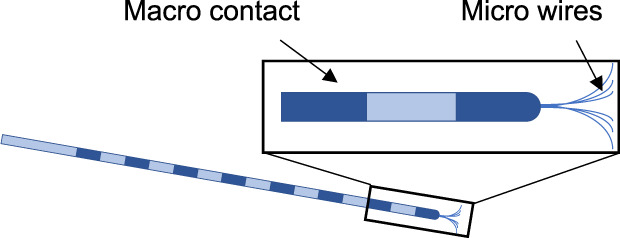
| Microwire bundles at depth of macroelectrode | The most commonly used tool, the ‘Behnke–Fried’ electrode, has exposed platinum/iridium microwires at the tip of a depth macroelectrode | Babb et al. (1973) | 91 | Chronic extraoperative depth monitoring |
|
| Hybrid micro–macro electrode with option for multipoint contacts (e.g. stereotrode, triode or tetrode) | Standard depth electrodes adapted to have micro contacts between macro contacts. Micro contacts can be single or multiple (stereotrode, triode, tetrode) that facilitate better separation of unit activity | Howard et al. (1996) | 11 | Chronic extraoperative depth monitoring |
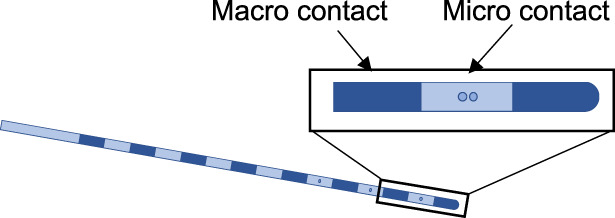
|
| Mircocontact subdural grids | Standard subdural grid adapted to have micro contacts between micro contacts | Van Gompel et al. (2008) | 2 | Chronic extraoperative cortical surface monitoring |
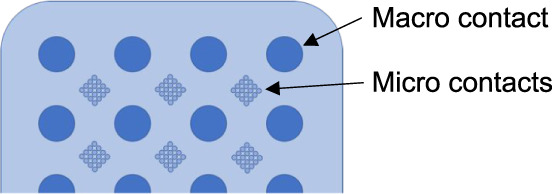
|
| Linear laminar arrays (e.g. linear or depth microarrays) | Narrow electrodes with multiple closely spaced micro contacts that allow the assessment of unit activity in different layers of cortex and resultant current source density (CSD) analysis | Ulbert et al. (2001) | 13 | Chronic extraoperative depth or cortical surface monitoring |
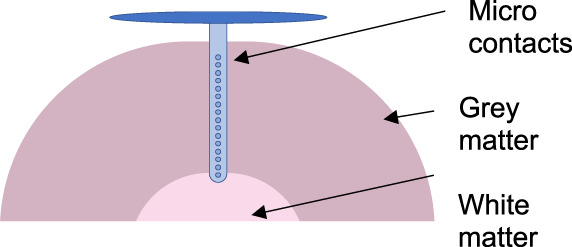
|
| 2D multielectrode arrays (e.g. NeuroPort or Utah arrays) | Small 2D arrays of microelectrodes that allow high-density recordings of units over a small cortical area, facilitating the study of traversing ‘waves’ of activity or patterns of activation in cognitive tasks | House et al. (2006) | 15 | Chronic extraoperative cortical surface monitoring |
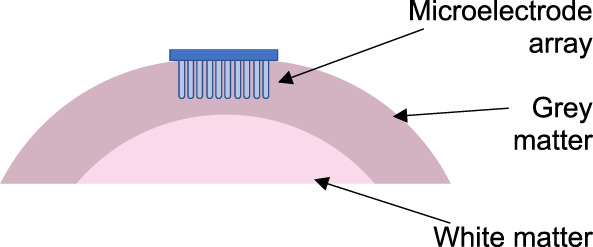
|
2D = 2-dimensional.
Recording techniques and safety
Electrode design and recording
The technical details of how microelectrode recordings are performed are relevant in the context of the proposed clinical translation and are therefore covered briefly. In our systematic literature search (see Supplementary material), chronic extraoperative recordings were more common than intraoperative recordings (86.4% versus 13.6% of studies), with the majority of the intraoperative studies occurring prior to 2000 and in the context of recording from tissue that was to be resected.
The key to microelectrode recording has been the development of small contact sizes (in the region of 20–50 µm) facilitating the delineation of extracellular action potentials from neurons. High-input impedance research amplifier systems are required to optimize power transfer to the recording amplifier and the signal-to-noise ratio is optimized by preamplification systems placed very close to the electrode itself, minimizing signal decay from long cables and interference from other electrical appliances. Recording units from different brain regions has required a number of electrode designs (Table 1) (Prasad and Sanchez, 2012).
Initial techniques were limited to recording single or, at maximum, 10 s of neurons at a time. The development of linear and 2-dimensional microelectrode arrays (MEAs), alongside our ability to handle large datasets, has facilitated the recording of 100–1000 s of individual neurons simultaneously in chronic extraoperative settings (Harris et al., 2016; Hong and Lieber, 2019). Although they present their own unique data processing challenges, these novel devices allow two key functions. First, they allow the recording of unit activity from multiple brain layers or regions, allowing the study of coordinated firing across functional areas. Second, devices that have closely spaced electrodes can detect the same unit from different electrodes, allowing improved resolution of single-unit recordings (Jog et al., 2002). Until non-invasive methods can record coordinated neuronal activity at the whole brain level as has been achieved in larval zebrafish (Yang and Yuste, 2017), neuronal recordings will always be limited by relatively sparse spatial sampling, often determined by clinical need. However, the explicit purpose of studying single units is to characterize microcircuits rather than whole brain networks.
Practical surgical considerations must also be taken into account, the first of which is choice of electrode. Whilst some cortical MEAs and depth electrodes (Behnke–Fried and hybrid design, see Table 1) have regulatory approvals (Federal Drug Administration in the USA and Conformité Européenne marking in the European Union) to record from the brain for up to 30 days, most of the other electrode designs are used purely in research contexts, requiring project-specific ethical approvals. The increasing use of SEEG (Baud et al., 2018) suggests that there is more scope for the incorporation of depth-design electrodes (Behnke–Fried or hybrid design, see Table 1) into routine presurgical evaluation. Although there is potential to sample both cortical and deep areas with hybrid design electrodes, the yield is not as good as with Behnke–Fried electrodes, with units being recorded on only a minority of contacts. Microcontact subdural grids require an open craniotomy for implantation; the indications for subdural grid monitoring seem to be decreasing and the yield of microcontact grids is not clear from the current literature. Linear and 2-dimensional invasive arrays also require a craniotomy and, in addition, cortical penetration; despite reports of safety (see below), it would be difficult to justify implanting these into areas of the brain that were not a high chance of being resected.
Once implanted, successful recording has its own challenge and there is technical literature surrounding the optimization of human microelectrode recordings in the clinical setting, focusing on amplifier technology and noise reduction techniques that maximize signal-to-noise ratio. Groups have optimized protocols for implantation, preamplification, securing connections, grounding the circuit and minimizing interference from other electrical appliances.
In our systematic search (see Supplementary material), none of the studies examining electrode design and implantation safety reported increased complication rates as a result of combining research microelectrode recording with clinical macroelectrodes, illustrating the safety of these techniques (House et al., 2006; Van Gompel et al., 2008; Waziri et al., 2009; Hefft et al., 2013; Misra et al., 2014; Carlson et al., 2018). However, as mentioned above, this does not take into consideration additional cortical penetration of MEAs into the brain; it would be difficult to convince any ethics committee to allow routine recording using these penetrating devices from tissue that was not going to be subsequently resected, especially given the evidence of fine deficits caused by implantation from the animal literature (Goss-Varley et al., 2017).
Methods of analysis
Microelectrode data are sampled at 20–30 kHz, allowing the recording of wideband EEG, termed microelectroencephalography [incorporating traditional local field potential (LFP, 1–100 Hz) and high-frequency oscillations (HFOs, 80–500 Hz)], multiunit activity and single-unit activity (Harris et al., 2016). Wideband microelectroencephalography LFP recordings have been used in numerous studies (Staba et al., 2002, 2004; Schevon et al., 2008; Worrell et al., 2008; Stead et al., 2010), demonstrating an ability to detect subclinical activity that is not detected on adjacent macroelectrodes such as pathological HFOs (specifically in the fast ripple band of 250–500 Hz), microseizures and micro-interictal epileptiform discharges (IEDs) localized to sub-millimetre-scale tissue volumes, all of which are promising biomarkers of epileptogenic regions (Frauscher et al., 2017). In addition to picking out features in these microelectroencephalography data, authors have used line-length analyses to detect changes in activity during pre-ictal and ictal periods in comparison to the baseline (Schevon et al., 2008; Worrell et al., 2008). Linear MEAs have also been used to perform current source density analyses to study the current sources and sinks within cortical layers that provide an indication of the nature and source of inputs (Ulbert et al., 2004).
The isolation of units is usually performed off-line after band pass filtering (usually 300–4000 Hz). Spike sorting applications (freely available or proprietary) are then used to sort action potentials by identifying clusters with similar waveform features. Although these techniques are limited by the so-called ‘cocktail party problem’ of not knowing how many sources are being recorded a priori, it is clear that units can be isolated and analysed in a biologically relevant way. However, it needs to be recognized that it may not be possible to isolate single neurons, especially during the intense firing associated with ictal activity (see ‘Ictal dynamics’ section) and, in this circumstance, it is possible to evaluate multiunit activity. Modern spike sorting applications incorporate the ability to monitor neurons over long periods of time, accounting for changes in action potential shape caused by electrode drift, waveform drift (after prolonged firing or bursting) and deconvolve overlapping ‘collided’ spikes from two or more neurons (Harris et al., 2016; Carlson and Carin, 2019; Merricks et al., 2020).
Various techniques have been used to analyse spike trains extracted from these spike sorting algorithms, including measures of:
Rate dynamics: rate of neuronal firing in relation to a particular stimulus (in the form of, e.g. peristimulus time histogram or event filters);
Timing dynamics: timing of neuronal firing in relation to the underlying LFP phase (in the form of, e.g. autocorrelograms, post-spike filters or phase locking with respect to concurrent LFP spectra); and
Population dynamics: timing of neuronal firing in relation to other nearby/distant neurons (in the form of, e.g. crosscorrelograms or coupling filters).
Abnormalities in these domains have been identified in animal models of brain disease, supporting the rationale for examining neuronal dynamics in humans.
Another important aspect of analysing and making inferences from spike data is ascertainment of cell type. Traditional approaches differentiated putative interneurons and excitatory neurons based on characteristic shapes of the action potential waveform and interspike interval (ISI) histograms. More recent work has taken this a step further, distinguishing functionally different cell types using data-driven approaches (Buccino et al., 2018; Ghaderi et al., 2018; Trainito et al., 2019; Mosher et al., 2020), although it remains to be seen whether these classifiers are helpful in distinguishing pathological microcircuit dynamics associated with diseases such as epilepsy.
Neuronal dynamics of epilepsy
Ictal dynamics
The cardinal symptom of epilepsy is seizures, and therefore, it is unsurprising that many human studies have studied single units in the context of seizures, attending to the pre-ictal, ictal onset, propagating and terminal phases of ictal events. These dynamics were first described in 1976 when 12 focal seizures were recorded from mesial temporal structures in 7 patients with mesial temporal lobe epilepsy. Although quantitative analyses were not performed, it was noted that neuronal firing rate at seizure onset decreased with high-amplitude EEG activity and increased with low-voltage fast EEG activity. Later in the seizure, during the spike-wave activity, neuronal firing increased during the spike and remained silent during the slow wave whilst, postictally, there was variable neuronal activity, with firing rates returning to baseline within 1 minute of the end of the seizure (Babb and Crandall, 1976).
Since then, studies have independently confirmed sparse and variable activation patterns of neuronal firing at seizure onset, including many neurons that do not change firing rates at all; together, these suggest that seizure initiation at a particular recording site may be more complex than an instantaneous transition from interictal firing to coordinated burst firing (Babb et al., 1987; Truccolo et al., 2011, 2014; Bower et al., 2012; Schevon et al., 2012; Jiruska et al., 2013; Weiss et al., 2016; Lambrecq et al., 2017). It is likely that the seizure state is part of the neural dynamic repertoire in patients with epilepsy and that the patterns of action potential firing at local and more distributed scales during seizure initiation have particular characteristics that have not yet been fully elucidated. Despite some evidence that single-unit activity is stereotyped between seizures in the same patient (Truccolo et al., 2011), other data have shown that multiunit activity firing patterns differ between mesial temporal seizures within the same patient despite similar LFP activity (Bower et al., 2012), suggesting that these networks may be dynamically changing. Others have shown an increase in inhibitory interneuronal firing associated with low-voltage fast activity at seizure onset, which occurs before an increase in excitatory cell firing, further contradicting the coordinated synchronous excitatory firing hypotheses of seizure onset (Weiss et al., 2016; Elahian et al., 2018). Ictal onset HFOs recorded on microelectrodes have shown an increasing spectral power in the fast ripple band (200–600 Hz) prior to macroelectrode seizure onset in certain subtypes of mesial temporal lobe seizures (Weiss et al., 2016). The future in this area revolves around developing models that unify and explain these diverse changes at seizure onset.
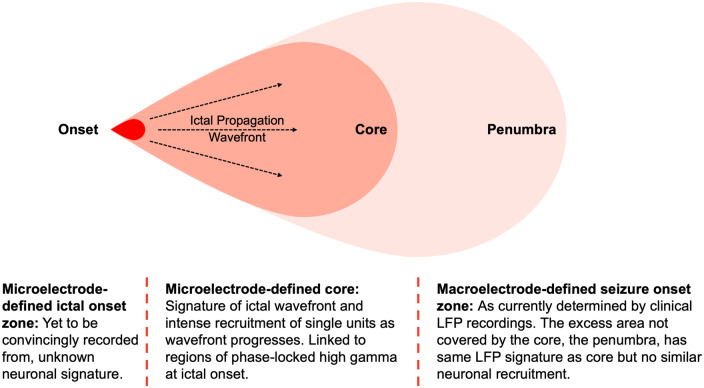
MEA recordings have also investigated the mechanisms behind ictal propagation (Fig. 1). An ‘ictal wavefront’ triggers coordinated recruitment of unit activity, which is stereotyped between seizures in the same patient (Schevon et al., 2012; Weiss et al., 2013). This coordinated unit activity has been shown to occur in only a subset of areas affected by ictal LFP activity recorded on macroelectrodes (the ‘core’), indicating that many areas of ictal LFP activity may not correspond to actual seizure activity at the unit level. Unit firing in the core is also characterized by changes in the spike waveform (Merricks et al., 2020). This is of clinical significance as it indicates a potential to refine our EEG-defined concepts of seizure onset zone (SOZ) localization (Schevon et al., 2012; Jiruska et al., 2013; Weiss et al., 2013; Smith et al., 2016). Phase-locked high-gamma activity (80–150 Hz activity phase locked to low-frequency 4–30 Hz activity) has been found to be a surrogate of this ‘core’ territory, and the resection of a larger proportion of contacts with phase-locked high-gamma activity has been shown to be associated with better outcomes (Weiss et al., 2013, 2015).
Figure 1.
Concepts of the ictal ‘core’ and ‘penumbra’. Schematic representation illustrating the concepts of the ictal ‘core’ and ‘penumbra’, both areas of ictal activity on macroelectrode LFP recordings that lie within the clinically defined SOZ. The ictal onset zone has not been convincingly recorded on MEA recordings but propagation from that onset area to the core territory is facilitated by the ‘ictal wavefront’. The core is characterized by intense unit activity as it becomes recruited, whilst the penumbra may display the same LFP activity but corresponding intense unit activity is not present. Phase-locked high-gamma oscillations have been shown to be a marker of the ‘core’, with the resection of this region correlated with outcome. Adapted from Weiss et al. (2013) with permission (Schevon et al., 2012; Smith et al., 2016).
A number of studies have shown increased unit firing rates mimicking coordinated hypersynchronous excitatory activity towards the end of a seizure, followed by a period of decreased firing (almost silence) that lasts many seconds before a slow recovery to baseline levels (Truccolo et al., 2011; Weiss et al., 2016; Lambrecq et al., 2017). Patterns of cessation have been shown to be inconsistent with depolarizing block caused by changes in neurotransmitter or electrolyte concentrations following rapid firing, suggesting that the hypersynchronous activity and sudden cessation could be emergent properties of large-scale networks themselves.
From a methodological perspective, these studies have demonstrated our ability to consistently record from the same units before, during and after a seizure, although the intense spiking activity in the pre-termination phase may preclude spike sorting and unit isolation (Merricks et al., 2015, 2020; Smith et al., 2016; Lambrecq et al., 2017). Despite these significant advances, recording unit activity in the seizure focus itself is challenging to perform and our understanding of this process therefore remains poor (Cash and Hochberg, 2015).
To date, studies have evaluated changes in firing rate without evaluating changes in firing patterns with respect to oscillations and with respect to the other recorded neurons. Studying the timing and population dynamics may allow a more complete understanding of microcircuit behaviour during seizures.
Interictal dynamics
Although features of conventional macroelectrode interictal recordings (such as IEDs and HFOs) have been found to overlap with the epileptogenic zone, presurgical evaluation of patients still relies upon ictal recordings to identify the SOZ (Bartolomei et al., 2017, 2018). However, the epilepsies are fundamentally brain diseases that disrupt neural circuitry. The behaviour of these disrupted microcircuits is likely to be manifest as disruptions to single-unit firing properties, even in the periods between seizures. The study of unit activity in humans therefore holds the promise of being able to identify the SOZ (or its network correlates) purely from interictal activity, but this has yet to be realized.
Initial studies focused upon ‘epileptic’ and ‘nonepileptic’ neurons in mesial temporal lobe epilepsy based on the laterality of recording, identifying increasing bursting activity and synchronization on the side of seizure onset (Babb et al., 1987; Isokawa-Akesson et al., 1987, 1989). Since then, only one study has attempted to predict the clinical SOZ from interictal unit recordings, using logistic regression approaches. This model incorporated the normalized peak height of bursts, burst ISI ratio (ratio of ISIs <10 ms/ISIs >10 ms) and cellular yield in each mesial temporal lobe area. Although they found convincing results in certain regions (e.g. there was a 12× increase in the likelihood of being within the SOZ for each unit increase in the burst ISI ratio in the left hippocampus), these were not consistent between regions and therefore not generalizable (Valdez et al., 2012). More sophisticated analyses are likely to generate novel pathophysiological hypotheses on the nature of background networks that are able to generate spontaneous recurrent seizures.
In addition to predicting the SOZ, authors have attempted to explain the neuronal dynamics generating interictal epileptiform activity. IEDs have been linked to increased neuronal spiking prior to onset, although this relationship is not always consistent (Altafullah et al., 1986; Keller et al., 2010; Schevon et al., 2010; Alvarado-Rojas et al., 2013; Despouy et al., 2019). Others have used laminar arrays to perform current source density analysis, identifying distinct patterns of current sources and sinks of IEDs that are locally generated compared to those that are propagated from elsewhere (Ulbert et al., 2004; Fabó et al., 2008). It has also been noted that unit firing activity may be modulated more in fast ripples in epileptic mesial temporal lobe, furthering the theory that fast ripples are pathogenic markers of epileptogenic tissue (Bragin et al., 1999, 2002). These findings add further weight to the possibility of understanding the epileptogenic zone by recording interictal unit activity.
Neuronal dynamics and cognition
The majority of microelectrode recording studies in human epilepsy have evaluated the neural basis of cognition, most of them via the use of Behnke–Fried electrodes in the mesial temporal structures. There is a vast literature evaluating neural dynamics and cognition in animal models. Chronic electrode implantation over several days provides a window for patients to perform cognitive tasks and several of the concepts derived from animal models have now been tested in humans, leading to a number of ground-breaking discoveries. Although the specifics of many of these studies have little relevance to the presurgical evaluation of focal epilepsy, they highlight that the principles of coding via rate, populating and timing dynamics are applicable to human single-unit recordings too. They act as a proof of concept of how these analyses may be applied to unit recordings in the context of epilepsy.
Mesial temporal lobe neurons in the amygdala and hippocampus are involved in the encoding and retrieval of memories and timing dynamics have been shown to be particularly important in this context (Cameron et al., 2001; Rutishauser et al., 2006, 2008, 2010; Jacobs et al., 2007; Rey et al., 2018). In agreement with animal data, a number of human studies have confirmed the importance of phase-locked firing of neurons to the underlying theta and delta rhythms in cognitive processes, with further attentional elements coded through phase locking to the trough of gamma oscillations (Fries, 2005; Jacobs et al., 2007; Rutishauser et al., 2010).
The mesial temporal lobe has also been found to sparsely encode features through rate coding. A seminal example is the concept cell (the ‘Jennifer Aniston neuron’) that responds to different pictures and written or spoken name of the same famous individual (Quiroga et al., 2005; Quiroga, 2012). These responses may be modulated by perceptual decisions (attention) and controlled by the patient using real-time neurofeedback (Kreiman et al., 2002; Cerf et al., 2010; Ison et al., 2011; Quian Quiroga et al., 2014). Analysis of latency and selectivity reveals that the mesial structures (hippocampus, amygdala, entorhinal cortex) have the longest time delay to peak response and are more selective compared to parahippocampal and inferotemporal areas (Mormann et al., 2008; Quiroga, 2012), implicating the mesial temporal lobe structures at the top of this cognitive processing hierarchy. However, it is clear that there is some redundancy/compensatory reserve in these systems as temporal lobe resections do not often lead to deficits in facial recognition (Ojemann et al., 1992). Authors have also found the human correlates of place cells (hippocampus) and grid/path cells (entorhinal cortex); interestingly, these cells have also been found to respond during memory retrieval, illustrating their role in providing a spatial context (Ekstrom et al., 2003; Jacobs et al., 2010, 2013; Miller et al., 2013, 2015).
These studies should be interpreted in the context of supporting evidence from animal and non-invasive human studies as, despite contributing significantly to our understanding of the neural basis of perception and cognition, they have a number of limitations. Electrodes are placed only where is clinically indicated and current techniques have been limited mostly to sampling mesial temporal structures. Despite testing to ensure normal cognitive function, one must consider that these recordings are occurring in individuals with epilepsy, whose memory networks may be affected by the underlying abnormal dynamics, which are the cause and/or consequence of the epilepsy (Bower et al., 2015).
Although these cognitive studies are currently of no relevance in terms of clinical decision-making, they represent important concepts to consider in the future, when alternative neuromodulatory strategies to alter neuronal networks may become available, with the aim of optimizing both seizure and cognitive outcomes simultaneously.
The future: single-unit recordings in the clinical context
Despite the immense gains in the understanding of neurobiology in health and disease, microelectrode recordings in human epilepsy remain limited to research contexts. This is partly due to the added infrastructure and cost associated with performing microelectrode recordings, including the costs of high-frequency amplifier systems, MEAs and hybrid electrodes and the computing power required to record and process such large volumes of data.
Justifying the monetary, logistical and computational costs of incorporating unit recordings into clinical practice requires the identification of clear indications. We discuss the evidence in five potential areas where unit recordings may become clinically relevant in the investigation and treatment of focal epilepsy:
Refining the concept of the SOZ network: Although specific patterns of neuronal firing within and outside the SOZ have not firmly been established, existing evidence suggests that unit firing in the ‘core’ of the SOZ may be different to that in the ‘penumbra’ (Fig. 1) (Schevon et al., 2012; Weiss et al., 2013), indicating that the microelectrode-defined SOZ could be significantly smaller than (or different to) that defined by macroscale recordings. However, it is unlikely that this will form the core of the rationale for clinical translation for a number of reasons. First, these recordings have all been performed using cortically implanted MEAs that require a craniotomy for implantation; the increasing use of SEEG, which does not require a craniotomy, makes implanting MEAs more difficult. Microelectrodes attached to SEEG electrodes are limited by low yield (hybrid design) or being able to sample deep structures only (Behnke–Fried design). Second, markers of this ‘core’ have been identified in ictal LFP recordings through the phase-locked high-gamma measure. The resection of contacts where such activity is present has been shown to correlate well with outcomes (Weiss et al., 2013, 2015) and it therefore may not be necessary to record unit activity to define this ‘core’.
Identification of the SOZ network from interictal recordings: Current invasive evaluation of focal epilepsy relies upon the patient having seizures during the recording period, requiring long periods of monitoring and the reduction in antiepileptic medication. Analysis of IEDs, which constitute the ‘irritative zone’ (Lüders et al., 2006), HFOs and functional and seizure stimulation tests have provided important adjunctive information with which to guide tailored resections. In the last few years, quantitative signal analysis methods have attempted to objectively determine the SOZ from ictal recordings (Bartolomei et al., 2008; David et al., 2011; Andrzejak et al., 2015). Despite these, intracranial electrode-guided resections are not always successful, with seizure freedom rates in the range of 50–80% (Wellmer et al., 2012; Gonzalez-Martinez et al., 2013; Garcia-Lorenzo et al., 2019; Thorsteinsdottir et al., 2019).
Given that the vast majority of intracranial recordings are in the interictal state, it would seem logical to try and utilize these ‘steady-state’ recordings for clinical localization (Goodale et al., 2020), a concept that is supported at the neuronal level by animal literature showing interictal neuronal network abnormalities in animal models. Early work in interictal microelectrode recordings attempted to ascertain interictal properties of ‘epileptic’ and ‘nonepileptic’ neurons in the interictal state (Ward and Schmidt, 1961; Isokawa-Akesson et al., 1987, 1989), mainly in mesial temporal structures. Establishing whether there are signatures of neuronal firing in the interictal period that reliably predicts the ictally defined SOZ would revolutionize intracranial monitoring in human epilepsy as it would obviate the need to wait for spontaneous seizures and ictal recordings. The assessment of ‘normal’ and ‘abnormal’ dynamics of a particular area will be aided by the production of normal atlases of function in different brain regions, something that has been successfully achieved for macroelectrode SEEG recordings (Frauscher et al., 2018).
However, there are significant hurdles that must be overcome. SEEG electrodes currently allow for (an albeit limited) 3-dimensional sampling of the brain. Microelectrode data have mostly been recoded from depth Behnke–Fried electrodes (which sample deep structures) or cortical MEAs (Table 1). Hybrid design electrodes, which have the microelectrode contacts between the macro contacts (Table 1), would allow the construction of 3-dimensional neuronal networks but have only been used in limited contexts in the literature so far and the yield of units is purported to be low using these constructs.
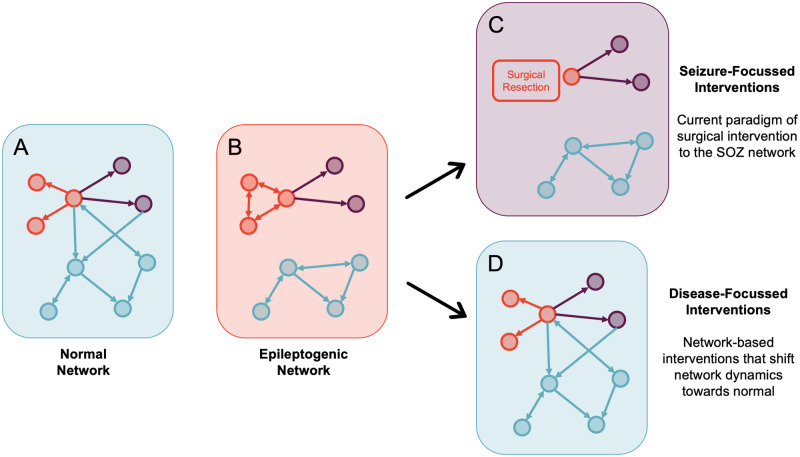
Redefining the concept of the SOZ network: For many years, seizures and epilepsy have been viewed as a network disorder rather than a focal disorder and significant effort has been invested into the modelling of structural, functional and effective connectivity networks in epilepsy using structural imaging, functional imaging and electrophysiology (Scott, 2016; Wendling et al., 2016; Bartolomei et al., 2017; Jirsa et al., 2017; Scott et al., 2018) (Fig. 2). However, current surgical treatment of focal epilepsy still relies largely on the resection of specific foci (the SOZ) rather than modifying more widespread ictogenic (SOZ and propagation) networks.
It is possible that studying unit-level connectivity, both ictally and interictally, will provide further insight into microcircuit network-level disturbances in focal epilepsy. Specifically, the interaction between neurons at a population level (Fig. 2B) may add to LFP-level connectivity measures, although the rationale for this is currently purely speculative. In addition, building a 3-dimensional understanding of these networks would require hybrid design electrodes that were able to sample deep and superficial structures in the brain, which, as mentioned above, have their own limitations in terms of yield.
To be clinically useful this has to translate into tailored network-modifying interventions that disrupt the pathological network architecture that results in a susceptibility to seizures. This may not necessarily be in the form of resective surgical intervention, and it is plausible that targeted medical and neuromodulatory therapies may hold more promise in modifying these network dynamics.
Predicting seizure onset prior to LFP changes: A number of studies have revealed that unit-level changes can occur prior to LFP-defined seizure onset (Truccolo et al., 2011; Mormann and Jefferys, 2013). Given the increase in neuromodulatory strategies for the treatment of epilepsy such as responsive neurostimulation (Matias et al., 2019), microelectrode recordings could be incorporated into such real-time feedback systems, improving seizure detection algorithms and resultant patient outcomes.
Normalizing cognition and development in epilepsy: The cognitive, behavioural and psychological impairments associated with epilepsy are key determinants of the quality of life and must form a key focus of our treatments of this condition (Lenck-Santini and Scott, 2015). Current surgical treatments are largely focused on seizure freedom, although it is increasingly being recognized that effective surgical treatment positively impacts cognitive and developmental outcomes in children (Veersema et al., 2019; Braun, 2020). There is a need to shift the focus of intervention in epilepsy, and specifically epilepsy surgery, from purely seizure-based interventions to disease-based interventions that look to normalize brain network dynamics (e.g. neuronal dynamics) to address both seizures and associated comorbidities (Fig. 3).
Studying the neuronal bases of cognitive processes and how these may be perturbed in epilepsy may additionally allow a tailoring of invasive and non-invasive neuromodulatory strategies designed to optimize cognitive and developmental outcomes.
Figure 2.
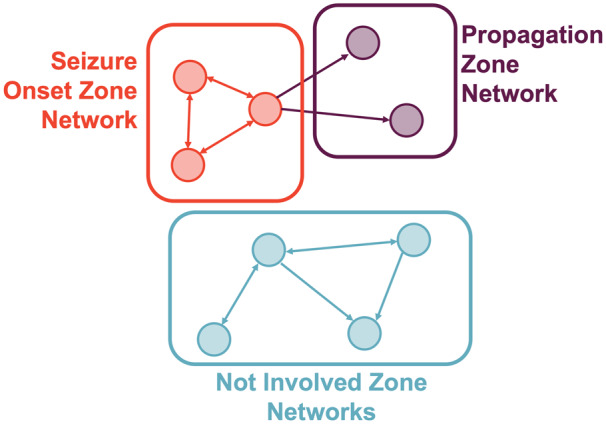
Current understanding of network concepts in focal epilepsy. Schematic representation of our current understanding of network concepts in focal epilepsy, which has largely been informed by network analyses of imaging and intracranial EEG recordings. The schema breaks down the brain regions (nodes) into groups based on a hierarchical classification of epileptogenicity into the SOZ networks (areas involved in seizure generation), propagation networks (less epileptogenic areas involved in seizure spread) and not involved networks. Adapted from Bartolomei et al. (2017) with permission.
Figure 3.
Paradigms of network concepts in epilepsy surgery. Schematic representation of current paradigms of network concepts of epilepsy surgery, illustrating a normal network (A), an epileptogenic network (B) as in Fig. 2 that results in both seizures and associated comorbidities including cognitive, psychological and social problems. In terms of treatments, current surgical treatments (C) are focused heavily on addressing the seizures, which may have some impact on the cognitive and developmental aspects but we envisage a future where surgical and non-surgical treatments are individually tailored to push the network towards normal dynamics (D), concurrently addressing all facets of the disease. Adapted from Bartolomei et al. (2017) with permission (Lenck-Santini and Scott, 2015; Scott, 2016).
All the aforementioned potential clinical indications will be aided by advances in technology. These include the development of novel electrode designs that facilitate recording from a growing number of neurons simultaneously, although none of these devices are as yet licenced for human use (Viventi et al., 2011; Khodagholy et al., 2015; Jun et al., 2017; Musk and Neuralink, 2019). Accompanying advances in our ability to process large datasets means that there is potential to meaningfully record from and understand the behaviour of thousands of neurons at a time. Novel analyses will also be aided by endeavours to ensure that open datasets are available and organized in a homogenous fashion, ensuring that the maximum can be garnered from data that are often difficult to acquire (Holdgraf et al., 2019; Miller, 2019).
However, there remain significant challenges in making microelectrode recordings mainstream in patients with epilepsy. In addition to the equipment costs mentioned previously, routine microelectrode recordings would result in increased demand on infrastructure and computational resources, which may all be justifiable in the context of improved outcomes. In addition, incorporating microelectrodes into contexts outside presurgical evaluation (e.g. implantable closed-loop neuromodulation systems) requires the assessment of long-term stability of these recordings (Chung et al., 2019) and mitigation of factors such as the foreign body reaction (Lotti et al., 2017) and much of this work so far has been conducted in animals only.
Limitations
The methodology for this review is covered in the Supplementary material. Although we registered the review and examined the literature systematically identifying 137 studies examining microelectrode recordings in human patients undergoing surgery for epilepsy, we did not have two reviewers to independently extract data from each study and the choice of studies to include in the manuscript was based on the opinions of the authors. Of note, the ‘cognition’ field of study, which had the most number of papers (Supplementary Fig. 1B), was only superficially addressed as this review was specifically focused on clinical translation from the perspective of presurgical evaluation for epilepsy surgery. The review also did not follow the PRISMA guidance for reporting, and the risk of bias of the studies was not systematically assessed. The lack of intervention/diagnostic studies and focus on biological understanding of human epilepsy and cognition from these studies to date made it difficult to follow a clinical systematic review structure and we felt that our approach gave us the flexibility to discuss evidence towards the potential novel indications we have proposed for microelectrode recordings in the presurgical evaluation of patients with focal epilepsy.
Conclusions
Over the last 65 years, intracranial microelectrode recordings in human epilepsy have significantly advanced our understanding of the neuronal mechanisms of human epilepsy and cognition. Theories of microcircuit dynamics developed from the enormous literature on unit dynamics in animal models have been translated in many of these studies. Increasing the number of patients who undergo single-unit evaluations will allow us to demonstrate which concepts derived from animal models actually apply in humans. In addition, novel hypotheses about human cognition and clinical hypotheses about brain dysfunction in epilepsy are likely to be generated.
There remains a lack of clinical indications for routine clinical microelectrode recordings. In addition to advancing our understanding of the neuronal bases of cognition, future research must focus on optimizing these recordings to refine our understanding of both the SOZ and seizure networks to develop treatments that maximize both rates of seizure freedom and cognitive/developmental outcomes in patients with medically intractable focal epilepsy.
Funding
A.C. is supported by a Great Ormond Street Hospital (GOSH) Children’s Charity Surgeon Scientist Fellowship. This work has been supported by the GOSH-National Institute of Health Research Biomedical Research Centre.
Competing interests
The authors have no competing interests to declare.
Supplementary Material
Glossary
- HFO
high-frequency oscillation
- IED
interictal epileptiform discharge
- ISI
interspike interval
- LFP
local field potential
- MEA
microelectrode array
- SEEG
stereoelectroencephalography
- SOZ
seizure onset zone
References
- Abbott A. Neuroscience: opening up brain surgery. Nature 2009; 461: 866–8. [DOI] [PubMed] [Google Scholar]
- Altafullah I, Halgren E, Stapleton JM, Crandall PH.. Interictal spike-wave complexes in the human medial temporal lobe: typical topography and comparisons with cognitive potentials. Electroencephalogr Clin Neurophysiol 1986; 63: 503–16. [DOI] [PubMed] [Google Scholar]
- Alvarado-Rojas C, Lehongre K, Bagdasaryan J, Bragin A, Staba R, Engel JJ, et al. Single-unit activities during epileptic discharges in the human hippocampal formation [Internet]. Front Comput Neurosci 2013; 7 Available from: https://www.frontiersin.org/articles/10.3389/fncom.2013.00140/full (31 January 2020, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrzejak RG, David O, Gnatkovsky V, Wendling F, Bartolomei F, Francione S, et al. Localization of epileptogenic zone on pre-surgical intracranial EEG recordings: toward a validation of quantitative signal analysis approaches. Brain Topogr 2015; 28: 832–7. [DOI] [PubMed] [Google Scholar]
- Babb TL, Carr E, Crandall PH. Analysis of extracellular firing patterns of deep temporal lobe structures in man. Electroenceph Clin Neurophysiol 1973; 34: 247–57. [DOI] [PubMed] [Google Scholar]
- Babb TL, Crandall PH.. Epileptogenesis of human limbic neurons in psychomotor epileptics. Electroencephalogr Clin Neurophysiol 1976; 40: 225–43. [DOI] [PubMed] [Google Scholar]
- Babb TL, Wilson CL, Isokawa-Akesson M.. Firing patterns of human limbic neurons during stereoencephalography (SEEG) and clinical temporal lobe seizures. Electroencephalogr Clin Neurophysiol 1987; 66: 467–82. [DOI] [PubMed] [Google Scholar]
- Barba C, Cross JH, Braun K, Cossu M, Klotz KA, De Masi S, et al. Trends in pediatric epilepsy surgery in Europe between 2008 and 2015: country-, center-, and age-specific variation. Epilepsia 2020; 61: 216–27. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Chauvel P, Wendling F.. Epileptogenicity of brain structures in human temporal lobe epilepsy: a quantified study from intracerebral EEG. Brain 2008; 131: 1818–30. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Lagarde S, Wendling F, McGonigal A, Jirsa V, Guye M, et al. Defining epileptogenic networks: contribution of SEEG and signal analysis. Epilepsia 2017; 58: 1131–47. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Nica A, Valenti-Hirsch MP, Adam C, Denuelle M.. Interpretation of SEEG recordings. Neurophysiol Clin 2018; 48: 53–7. [DOI] [PubMed] [Google Scholar]
- Baud MO, Perneger T, Rácz A, Pensel MC, Elger C, Rydenhag B, et al. European trends in epilepsy surgery. Neurology 2018; 91: e96–e106. [DOI] [PubMed] [Google Scholar]
- Bower MR, Stead M, Bower RS, Kucewicz MT, Sulc V, Cimbalnik J, et al. Evidence for consolidation of neuronal assemblies after seizures in humans. J Neurosci 2015; 35: 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower MR, Stead M, Meyer FB, Marsh WR, Worrell GA.. Spatiotemporal neuronal correlates of seizure generation in focal epilepsy. Epilepsia 2012; 53: 807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Engel J Jr., Wilson CL, Fried I, Mathern GW.. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia 1999; 40: 127–37. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, Engel J.. Interictal high-frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol 2002; 52: 407–15. [DOI] [PubMed] [Google Scholar]
- Braun K. Influence of epilepsy surgery on developmental outcomes in children. Eur J Paediatr Neurol 2020; 24: 40–2. [DOI] [PubMed] [Google Scholar]
- Buccino AP, Ness TV, Einevoll GT, Cauwenberghs G, Hafliger PD.. A deep learning approach for the classification of neuronal cell types. Conf Proc Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf 2018; 2018: 999–1002. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Large-scale recording of neuronal ensembles. Nat Neurosci 2004; 7: 446–51. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C.. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat Rev Neurosci 2012; 13: 407–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron KA, Yashar S, Wilson CL, Fried I.. Human hippocampal neurons predict how well word pairs will be remembered. Neuron 2001; 30: 289–98. [DOI] [PubMed] [Google Scholar]
- Carlson AA, Rutishauser U, Mamelak AN.. Safety and utility of hybrid depth electrodes for seizure localization and single-unit neuronal recording. Stereotact Funct Neurosurg 2018; 96: 311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson D, Carin L.. Continuing progress of spike sorting in the era of big data. Curr Opin Neurobiol 2019; 55: 90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash SS, Hochberg LR.. The emergence of single neurons in clinical neurology. Neuron 2015; 86: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf M, Thiruvengadam N, Mormann F, Kraskov A, Quiroga RQ, Koch C, et al. On-line, voluntary control of human temporal lobe neurons. Nature 2010; 467: 1104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JE, Joo HR, Fan JL, Liu DF, Barnett AH, Chen S, et al. High-density, long-lasting, and multi-region electrophysiological recordings using polymer electrode arrays. Neuron 2019; 101: 21–31.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David O, Blauwblomme T, Job A-S, Chabardès S, Hoffmann D, Minotti L, et al. Imaging the seizure onset zone with stereo-electroencephalography. Brain 2011; 134: 2898–911. [DOI] [PubMed] [Google Scholar]
- Despouy E, Curot J, Denuelle M, Deudon M, Sol J-C, Lotterie J-A, et al. Neuronal spiking activity highlights a gradient of epileptogenicity in human tuberous sclerosis lesions. Clin Neurophysiol 2019; 130: 537–47. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, et al. Cellular networks underlying human spatial navigation. Nature 2003; 425: 184–8. [DOI] [PubMed] [Google Scholar]
- Elahian B, Lado NE, Mankin E, Vangala S, Misra A, Moxon K, et al. Low-voltage fast seizures in humans begin with increased interneuron firing. Ann Neurol 2018; 84: 588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Moll CKE, Fried I, Ojemann GA.. Invasive recordings from the human brain: clinical insights and beyond. Nat Rev Neurosci 2005; 6: 35–47. [DOI] [PubMed] [Google Scholar]
- Fabó D, Maglóczky Z, Wittner L, Pék A, Eross L, Czirják S, et al. Properties of in vivo interictal spike generation in the human subiculum. Brain J Neurol 2008; 131: 485–99. [DOI] [PubMed] [Google Scholar]
- Frauscher B, Bartolomei F, Kobayashi K, Cimbalnik J, van ‘T Klooster MA, Rampp S, et al. High-frequency oscillations: the state of clinical research. Epilepsia 2017; 58: 1316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauscher B, von Ellenrieder N, Zelmann R, Doležalová I, Minotti L, Olivier A, et al. Atlas of the normal intracranial electroencephalogram: neurophysiological awake activity in different cortical areas. Brain 2018; 141: 1130–44. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci 2005; 9: 474–80. [DOI] [PubMed] [Google Scholar]
- Garcia-Lorenzo B, del Pino-Sedeño T, Rocamora R, López JE, Serrano-Aguilar P, Trujillo-Martín MM.. Stereoelectroencephalography for refractory epileptic patients considered for surgery: systematic review, meta-analysis, and economic evaluation. Neurosurgery 2019; 84: 326–38. [DOI] [PubMed] [Google Scholar]
- Ghaderi P, Marateb HR, Safari M-S.. Electrophysiological profiling of neocortical neural subtypes: a semi-supervised method applied to in vivo whole-cell patch-clamp data. Front Neurosci 2018; 12: 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Martinez J, Bulacio J, Alexopoulos A, Jehi L, Bingaman W, Najm I.. Stereoelectroencephalography in the ‘difficult to localize’ refractory focal epilepsy: early experience from a North American epilepsy center. Epilepsia 2013; 54: 323–30. [DOI] [PubMed] [Google Scholar]
- Goodale SE, González HFJ, Johnson GW, Gupta K, Rodriguez WJ, Shults R, et al. Resting-state SEEG may help localize epileptogenic brain regions. Neurosurgery 2020; 86: 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss-Varley M, Dona KR, McMahon JA, Shoffstall AJ, Ereifej ES, Lindner SC, et al. Microelectrode implantation in motor cortex causes fine motor deficit: implications on potential considerations to Brain Computer Interfacing and Human Augmentation. Sci Rep 2017; 7: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Quiroga RQ, Freeman J, Smith SL.. Improving data quality in neuronal population recordings. Nat Neurosci 2016; 19: 1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefft S, Brandt A, Zwick S, von Elverfeldt D, Mader I, Cordeiro J, et al. Safety of hybrid electrodes for single-neuron recordings in humans. Neurosurgery 2013; 73: 78–85. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF, Katz B.. Measurement of current–voltage relations in the membrane of the giant axon of Loligo. J Physiol 1952; 116: 424–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdgraf C, Appelhoff S, Bickel S, Bouchard K, D’Ambrosio S, David O, et al. iEEG-BIDS, extending the Brain Imaging Data Structure specification to human intracranial electrophysiology. Sci Data 2019; 6: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong G, Lieber CM.. Novel electrode technologies for neural recordings. Nat Rev Neurosci 2019; 20: 330–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House PA, MacDonald JD, Tresco PA, Normann RA.. Acute microelectrode array implantation into human neocortex: preliminary technique and histological considerations. Neurosurg Focus 2006; 20: 1–4. [DOI] [PubMed] [Google Scholar]
- Howard MA, Volkov IO, Granner MA, Damasio HM, Ollendieck MC, Bakken HE. A hybrid clinical—research depth electrode for acute and chronic in vivo microelectrode recording of human brain neurons. J Neurosurg 1996; 84: 129–32. [DOI] [PubMed] [Google Scholar]
- Isokawa-Akesson M, Wilson CL, Babb TL.. Structurally stable burst and synchronized firing in human amygdala neurons: auto- and cross-correlation analyses in temporal lobe epilepsy. Epilepsy Res 1987; 1: 17–34. [DOI] [PubMed] [Google Scholar]
- Isokawa-Akesson M, Wilson CL, Babb TL.. Inhibition in synchronously firing human hippocampal neurons. Epilepsy Res 1989; 3: 236–47. [DOI] [PubMed] [Google Scholar]
- Ison MJ, Mormann F, Cerf M, Koch C, Fried I, Quiroga RQ.. Selectivity of pyramidal cells and interneurons in the human medial temporal lobe. J Neurophysiol 2011; 106: 1713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Kahana MJ, Ekstrom AD, Fried I.. Brain oscillations control timing of single-neuron activity in humans. J Neurosci 2007; 27: 3839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Kahana MJ, Ekstrom AD, Mollison MV, Fried I.. A sense of direction in human entorhinal cortex. Proc Natl Acad Sci USA 2010; 107: 6487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Weidemann CT, Miller JF, Solway A, Burke JF, Wei X-X, et al. Direct recordings of grid-like neuronal activity in human spatial navigation. Nat Neurosci 2013; 16: 1188–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakar P, Gaillard WD, Tripathi M, Libenson MH, Mathern GW, Cross JH; The Task Force for Paediatric Epilepsy Surgery, Commission for Paediatrics, and the Diagnostic Commission of the International League Against Epilepsy. Diagnostic test utilization in evaluation for resective epilepsy surgery in children. Epilepsia 2014; 55: 507–18. [DOI] [PubMed] [Google Scholar]
- Jirsa VK, Proix T, Perdikis D, Woodman MM, Wang H, Gonzalez-Martinez J, et al. The virtual epileptic patient: individualized whole-brain models of epilepsy spread. NeuroImage 2017; 145: 377–88. [DOI] [PubMed] [Google Scholar]
- Jiruska P, de Curtis M, Jefferys JGR, Schevon CA, Schiff SJ, Schindler K.. Synchronization and desynchronization in epilepsy: controversies and hypotheses: Synchronization in epilepsy. J Physiol 2013; 591: 787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jog MS, Connolly CI, Kubota Y, Iyengar DR, Garrido L, Harlan R, et al. Tetrode technology: advances in implantable hardware, neuroimaging, and data analysis techniques. J Neurosci Methods 2002; 117: 141–52. [DOI] [PubMed] [Google Scholar]
- Jun JJ, Steinmetz NA, Siegle JH, Denman DJ, Bauza M, Barbarits B, et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 2017; 551: 232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CJ, Truccolo W, Gale JT, Eskandar E, Thesen T, Carlson C, et al. Heterogeneous neuronal firing patterns during interictal epileptiform discharges in the human cortex. Brain 2010; 133: 1668–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodagholy D, Gelinas JN, Thesen T, Doyle W, Devinsky O, Malliaras GG, et al. NeuroGrid: recording action potentials from the surface of the brain. Nat Neurosci 2015; 18: 310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiman G. Neural coding: computational and biophysical perspectives. Phys Life Rev 2004; 1: 71–102. [Google Scholar]
- Kreiman G, Fried I, Koch C.. Single-neuron correlates of subjective vision in the human medial temporal lobe. Proc Natl Acad Sci USA 2002; 99: 8378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecq V, Lehongre K, Adam C, Frazzini V, Mathon B, Clemenceau S, et al. Single-unit activities during the transition to seizures in deep mesial structures. Ann Neurol 2017; 82: 1022–8. [DOI] [PubMed] [Google Scholar]
- Lenck-Santini P-P, Scott RC.. Mechanisms responsible for cognitive impairment in epilepsy. Cold Spring Harb Perspect Med 2015; 5: a022772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotti F, Ranieri F, Vadalà G, Zollo L, Pino GD.. Invasive intraneural interfaces: foreign body reaction issues. Front Neurosci 2017; 11: 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüders HO, Najm I, Nair D, Widdess-Walsh P, Bingman W.. The epileptogenic zone: general principles. Gen Princ 2006; 8: 9. [PubMed] [Google Scholar]
- Matias CM, Sharan A, Wu C.. Responsive neurostimulation for the treatment of epilepsy. Neurosurg Clin N Am 2019; 30: 231–42. [DOI] [PubMed] [Google Scholar]
- McCulloch WS, Pitts W.. A logical calculus of the ideas immanent in nervous activity. Bull Math Biophys 1943; 5: 115–33. [PubMed] [Google Scholar]
- Merricks EM, Smith EH, Emerson RG, Bateman LM, McKhann GM, Goodman RR, et al. Neuronal firing and waveform alterations through ictal recruitment in humans. bioRxiv 2020: 2020.01.11.902817. [DOI] [PMC free article] [PubMed]
- Merricks EM, Smith EH, McKhann GM, Goodman RR, Bateman LM, Emerson RG, et al. Single unit action potentials in humans and the effect of seizure activity. Brain J Neurol 2015; 138: 2891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JF, Fried I, Suthana N, Jacobs J.. Repeating spatial activations in human entorhinal cortex. Curr Biol CB 2015; 25: 1080–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JF, Neufang M, Solway A, Brandt A, Trippel M, Mader I, et al. Neural activity in human hippocampal formation reveals the spatial context of retrieved memories. Science 2013; 342: 1111–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ. A library of human electrocorticographic data and analyses. Nat Hum Behav 2019; 3: 1225–35. [DOI] [PubMed] [Google Scholar]
- Misra A, Burke JF, Ramayya AG, Jacobs J, Sperling MR, Moxon KA, et al. Methods for implantation of micro-wire bundles and optimization of single/multi-unit recordings from human mesial temporal lobe. J Neural Eng 2014; 11: 026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormann F, Jefferys J.. Neuronal firing in human epileptic cortex: the ins and outs of synchrony during seizures. Epilepsy Curr 2013; 13: 100–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormann F, Kornblith S, Quiroga RQ, Kraskov A, Cerf M, Fried I, et al. Latency and selectivity of single neurons indicate hierarchical processing in the human medial temporal lobe. J Neurosci 2008; 28: 8865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher CP, Wei Y, Kamiński J, Nandi A, Mamelak AN, Anastassiou CA, et al. Cellular classes in the human brain revealed in vivo by heartbeat-related modulation of the extracellular action potential waveform. Cell Rep 2020; 30: 3536–51.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musk E, Neuralink An integrated brain–machine interface platform with thousands of channels. bioRxiv 2019: 703801. [DOI] [PMC free article] [PubMed]
- Ojemann JG, Ojemann GA, Lettich E.. Neuronal activity related to faces and matching in human right nondominant temporal cortex. Brain 1992; 115: 1–13. [DOI] [PubMed] [Google Scholar]
- O’Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol 1976; 51: 78–109. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J.. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 1971; 34: 171–5. [DOI] [PubMed] [Google Scholar]
- Prasad A, Sanchez JC.. Quantifying long-term microelectrode array functionality using chronic in vivo impedance testing. J Neural Eng 2012; 9: 026028. [DOI] [PubMed] [Google Scholar]
- Quian Quiroga R, Kraskov A, Mormann F, Fried I, Koch C.. Single-cell responses to face adaptation in the human medial temporal lobe. Neuron 2014; 84: 363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga RQ. Concept cells: the building blocks of declarative memory functions. Nat Rev Neurosci 2012; 13: 587–97. [DOI] [PubMed] [Google Scholar]
- Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I.. Invariant visual representation by single neurons in the human brain. Nature 2005; 435: 1102–7. [DOI] [PubMed] [Google Scholar]
- Rasul FT, Bal J, Pereira EA, Tisdall M, Themistocleous M, Haliasos N.. Current surgical options for patients with epilepsy. CPD 2017; 23: 6508–23. [DOI] [PubMed] [Google Scholar]
- Rayport M, Waller HJ, Technique and results of micro-electrode recording in human epileptogenic foci. Electroencephalogr Clin Neurophysiol 1967. Suppl 25:p.143+. [PubMed] [Google Scholar]
- Renshaw B, Forbes A, Morison BR.. Activity of isocortex and hippocampus: electrical studies with micro-electrodes. J Neurophysiol 1940; 3: 74–105. [Google Scholar]
- Rey HG, De Falco E, Ison MJ, Valentin A, Alarcon G, Selway R, et al. Encoding of long-term associations through neural unitization in the human medial temporal lobe. Nat Commun 2018; 9: 4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U, Mamelak AN, Schuman EM.. Single-trial learning of novel stimuli by individual neurons of the human hippocampus–amygdala complex. Neuron 2006; 49: 805–13. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Ross IB, Mamelak AN, Schuman EM.. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature 2010; 464: 903–7. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Schuman EM, Mamelak AN.. Activity of human hippocampal and amygdala neurons during retrieval of declarative memories. Proc Natl Acad Sci USA 2008; 105: 329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B, Neher E.. Patch clamp techniques for studying ionic channels in excitable membranes. Annu Rev Physiol 1984; 46: 455–72. [DOI] [PubMed] [Google Scholar]
- Schevon CA, Goodman RR, McKhann GJ, Emerson RG.. Propagation of epileptiform activity on a submillimeter scale. J Clin Neurophysiol 2010; 27: 406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Ng SK, Cappell J, Goodman RR, McKhann G, Waziri A, et al. Microphysiology of epileptiform activity in human neocortex. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc 2008; 25: 321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Weiss SA, McKhann G, Goodman RR, Yuste R, Emerson RG, et al. Evidence of an inhibitory restraint of seizure activity in humans. Nat Commun 2012; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RC. Network science for the identification of novel therapeutic targets in epilepsy. F1000 Res 2016; 5: 893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RC, Menendez de la Prida L, Mahoney JM, Kobow K, Sankar R, de Curtis M.. WONOEP APPRAISAL: the many facets of epilepsy networks. Epilepsia 2018; 59: 1475–83. [DOI] [PubMed] [Google Scholar]
- Smith EH, Liou J, Davis TS, Merricks EM, Kellis SS, Weiss SA, et al. The ictal wavefront is the spatiotemporal source of discharges during spontaneous human seizures. Nat Commun 2016; 7: 11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J.. Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol 2002; 88: 1743–52. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J.. High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol 2004; 56: 108–15. [DOI] [PubMed] [Google Scholar]
- Stead M, Bower M, Brinkmann BH, Lee K, Marsh WR, Meyer FB, et al. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain 2010; 133: 2789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsteinsdottir J, Vollmar C, Tonn J-C, Kreth F-W, Noachtar S, Peraud A.. Outcome after individualized stereoelectroencephalography (sEEG) implantation and navigated resection in patients with lesional and non-lesional focal epilepsy. J Neurol 2019; 266: 910–20. [DOI] [PubMed] [Google Scholar]
- Tóth E, Fabó D, Entz L, Ulbert I, Erőss L.. Intracranial neuronal ensemble recordings and analysis in epilepsy. J Neurosci Methods 2016; 260: 261–9. [DOI] [PubMed] [Google Scholar]
- Trainito C, von Nicolai C, Miller EK, Siegel M. Extracellular spike waveform dissociates four functionally distinct cell classes in primate cortex [Internet]. Curr Biol 2019. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0960982219309315 (31 January 2020, date last accessed). [DOI] [PubMed]
- Truccolo W, Ahmed OJ, Harrison MT, Eskandar EN, Cosgrove GR, Madsen JR, et al. Neuronal ensemble synchrony during human focal seizures. J Neurosci 2014; 34: 9927–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truccolo W, Donoghue JA, Hochberg LR, Eskandar EN, Madsen JR, Anderson WS, et al. Single-neuron dynamics in human focal epilepsy. Nat Neurosci 2011; 14: 635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbert I, Halgren E, Heit G, Karmos G. Multiple microelectrode-recording system for human intracortical applications. J Neurosci Methods 2001; 106: 69–79. [DOI] [PubMed] [Google Scholar]
- Ulbert I, Heit G, Madsen J, Karmos G, Halgren E.. Laminar analysis of human neocortical interictal spike generation and propagation: current source density and multiunit analysis in vivo. Epilepsia 2004; 45: 48–56. [DOI] [PubMed] [Google Scholar]
- Vakharia VN, Duncan JS, Witt J-A, Elger CE, Staba R, Engel J.. Getting the best outcomes from epilepsy surgery. Ann Neurol 2018; 83: 676–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez AB, Hickman EN, Treiman DM, Smith KA, Steinmetz PN.. A statistical method for predicting seizure onset zones from human single-neuron recordings. J Neural Eng 2012; 10: 016001. [DOI] [PubMed] [Google Scholar]
- Van Gompel JJ, Stead SM, Giannini C, Meyer FB, Marsh WR, Fountain T, et al. Phase I trial: safety and feasibility of intracranial electroencephalography using hybrid subdural electrodes containing macro- and microelectrode arrays. Neurosurg Focus 2008; 25: E23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veersema TJ, van Schooneveld MMJ, Ferrier CH, van Eijsden P, Gosselaar PH, van Rijen PC, et al. Cognitive functioning after epilepsy surgery in children with mild malformation of cortical development and focal cortical dysplasia. Epilepsy Behav EB 2019; 94: 209–15. [DOI] [PubMed] [Google Scholar]
- Verzeano M, Crandall PH, Dymond A. Neuronal activity of the amygdala in patients with psychomotor epilepsy. Neuropsychologia 1971; 9: 331–44. [DOI] [PubMed] [Google Scholar]
- Viventi J, Kim D-H, Vigeland L, Frechette ES, Blanco JA, Kim Y-S, et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci 2011; 14: 1599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AA, Schmidt RP.. Some properties of single epileptic neurons. Arch Neurol 1961; 5: 308–13. [DOI] [PubMed] [Google Scholar]
- Ward AA, Thomas LB.. The electrical activity of single units in the cerebral cortex of man. Electroencephalogr Clin Neurophysiol 1955; 7: 135–6. [DOI] [PubMed] [Google Scholar]
- Waziri A, Schevon CA, Cappell J, Emerson RG, McKhann GM, Goodman RR.. Initial surgical experience with a dense cortical microarray in epileptic patients undergoing craniotomy for subdural electrode implantation. Neurosurgery 2009; 64: 540–5; discussion 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SA, Alvarado‐Rojas C, Bragin A, Behnke E, Fields T, Fried I, et al. Ictal onset patterns of local field potentials, high frequency oscillations, and unit activity in human mesial temporal lobe epilepsy. Epilepsia 2016; 57: 111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SA, Banks GP, McKhann GM, Goodman RR, Emerson RG, Trevelyan AJ, et al. Ictal high frequency oscillations distinguish two types of seizure territories in humans. Brain J Neurol 2013; 136: 3796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SA, Lemesiou A, Connors R, Banks GP, McKhann GM, Goodman RR, et al. Seizure localization using ictal phase-locked high gamma: a retrospective surgical outcome study. Neurology 2015; 84: 2320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmer J, Groeben F V D, Klarmann U, Weber C, Elger CE, Urbach H, et al. Risks and benefits of invasive epilepsy surgery workup with implanted subdural and depth electrodes. Epilepsia 2012; 53: 1322–32. [DOI] [PubMed] [Google Scholar]
- Wendling F, Benquet P, Bartolomei F, Jirsa V.. Computational models of epileptiform activity. J Neurosci Methods 2016; 260: 233–51. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain J Neurol 2008; 131: 928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Yuste R.. In vivo imaging of neural activity. Nat Methods 2017; 14: 349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zador AM. The basic unit of computation. Nat Neurosci 2000; 3: 1167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.