Abstract
Oxytocin is deeply involved in human relations. In recent years, it is becoming clear that oxytocin is also involved in social cognition and social behaviour. Oxytocin receptors are also thought to be present in the hippocampus and amygdala, and the relationship between oxytocin and the structure and function of the hippocampus and amygdala has been reported. However, a few studies have investigated oxytocin and its relationship to hippocampus and amygdala volume in elderly people. The aim of this study is to investigate the association between serum oxytocin levels and hippocampus and amygdala volume in elderly people. The survey was conducted twice in Kurokawa-cho, Imari, Saga Prefecture, Japan, among people aged 65 years and older. We collected data from 596 residents. Serum oxytocin level measurements, brain MRI, Mini–Mental State Examination and Clinical Dementia Rating were performed in Time 1 (2009–11). Follow-up brain MRI, Mini–Mental State Examination and Clinical Dementia Rating were performed in Time 2 (2016–17). The interval between Time 1 and Time 2 was about 7 years. Fifty-eight participants (14 men, mean age 72.36 ± 3.41 years, oxytocin 0.042 ± 0.052 ng/ml; 44 women, mean age 73.07 ± 4.38 years, oxytocin 0.123 ± 0.130 ng/ml) completed this study. We analysed the correlation between serum oxytocin levels (Time 1) and brain volume (Time 1, Time 2 and Times 1–2 difference) using voxel-based morphometry implemented with Statistical Parametric Mapping. Analysis at the cluster level (family-wise error; P < 0.05) showed a positive correlation between serum oxytocin levels (Time 1) and brain volume of the region containing the left hippocampus and amygdala (Time 2). This result suggests that oxytocin in people aged 65 years and older may be associated with aging-related changes in hippocampus and amygdala volume.
Keywords: oxytocin, cognitive function, hippocampus, amygdala, MRI
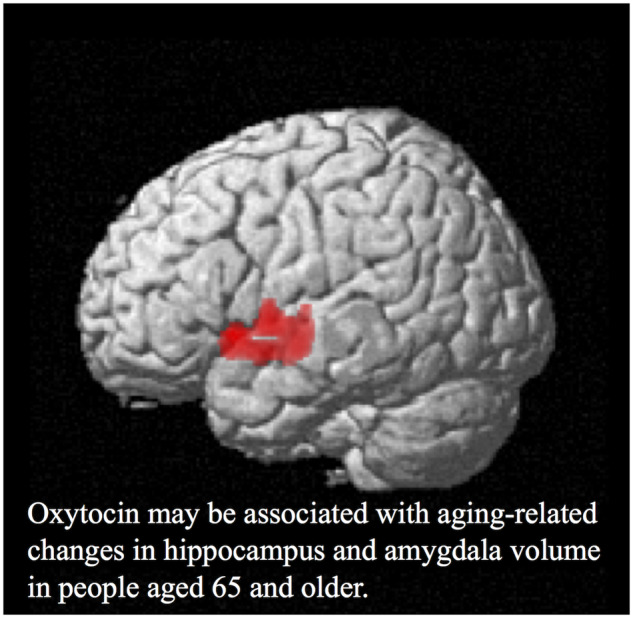
The aim of this study is to investigate the association between serum oxytocin levels and hippocampus and amygdala volume in elderly people. Oxytocin levels in people aged 65 years and older may be associated with aging-related changes in hippocampus and amygdala volume.
Graphical Abstract
Graphical Abstract.
Introduction
Dementia is a major public health problem globally. According to Alzheimer’s Disease International, there were ˜ 46.8 million people with dementia as of 2015, with an accompanying medical cost estimated at 818 billion dollars. Dementia makes it difficult for people to live socially and healthily, and effective treatment methods have not yet been found. The pathological change in Alzheimer’s disease begins ˜ 20 years before the onset of symptoms (Jack et al., 2010). Accordingly, finding advance indicators that predict dementia or cognitive decline will enable treatment and symptom progression prevention, which is extremely beneficial. In this study, we focused on the serum oxytocin levels of elderly people living in a rural community and examined the relationship with brain volume using brain MRI. Healthy elderly people without dementia were examined longitudinally prospectively for 7 years.
Oxytocin promotes labour through contractile action of the uterine muscle and causes milking reflex during breast feeding (Dale, 1906). It is also becoming clear that trust in others is increased by intranasal administration of oxytocin (Kosfeld et al., 2005; Baumgartner et al., 2008). Oxytocin is also involved in the ability to infer others’ mental states from social cues around the eyes (Domes et al., 2007). In addition, oxytocin has been suggested to enhance human social cognition (Heinrichs et al., 2009). This suggests that oxytocin is deeply involved in human relations. Oxytocin is also associated with attachment to others (Rilling and Young, 2014; Bernaerts et al., 2017; Carter, 2017) and promotion of cooperation (De Dreu, 2012; Ten Velden et al., 2017). Thus, oxytocin is considered extremely important for social cognitive ability. It is becoming clear that oxytocin is involved in the foundation of actions and social behaviours such as formation of social relations, attachment behaviour, trust behaviour, anxiety and aggression reduction and stress reduction (Windle et al., 1997; Lee et al., 2009; Bartz et al., 2011; McCall and Singer, 2012). The limbic system, including the hippocampus and amygdala, is an important component of cognitive function that is necessary for social life (Aggleton, 1986; Saunders et al., 1988; Majak and Pitkänen, 2003). Hippocampus and amygdala functions have been clarified in previous reports. The hippocampus has memory functions (Scoville and Milner, 1957), and the amygdala has emotional functions (Klüver and Bucy, 1997). When recognizing or recalling memories, the hippocampus and amygdala coordinate and interact (Fink et al., 1996). Moreover, many oxytocin receptors are present at these sites (Tomizawa et al., 2003; Ophir et al., 2012). These data suggest that oxytocin may mediate different social and cognitive functions by playing a role in neurobiological pathways that involve the hippocampus and amygdala. Regarding the relationship between oxytocin and hippocampus and amygdala structure, plasma oxytocin concentration is inversely correlated with amygdala and hippocampus volume in young adults (Andari et al., 2014) and adult women with early-life maltreatment (Mielke et al., 2018). In terms of functional connectivity, higher levels of salivary oxytocin are associated with a lower degree of functional connectivity between the amygdala and hippocampus regions in adult men with autism spectrum disorder (Alaerts et al., 2019).
However, although many facets of the relationship between oxytocin and the hippocampus and amygdala are now becoming clear, few studies have investigated oxytocin and its relationship to hippocampus and amygdala volume in elderly people. Patients with dementia have been shown to have smaller hippocampus (Du et al., 2001; Frisoni et al., 2008) and amygdala (Cavedo et al., 2011) volume compared with healthy controls. Therefore, investigating the association between serum oxytocin levels and hippocampus and amygdala volume in elderly people would be beneficial for people to live socially and healthily.
Materials and methods
Participant characteristics
This study was a longitudinal study. The survey was conducted in Kurokawa-cho, Imari, Saga Prefecture, Japan, among people aged 65 years and older as reported previously (Nabeta et al., 2014; Matsushima et al., 2015; Imamura et al., 2017). Kurokawa-cho is in northwestern Saga Prefecture and is a rural town somewhat removed from urban areas. The area of the town is 26.48 km2. As of 2010, the population of Kurokawa-cho was 3253, with 932 people aged 65 years and older (28.7%). The town had 1134 households, and the population per household was 2.87. The main industries are shipbuilding and primary industries.
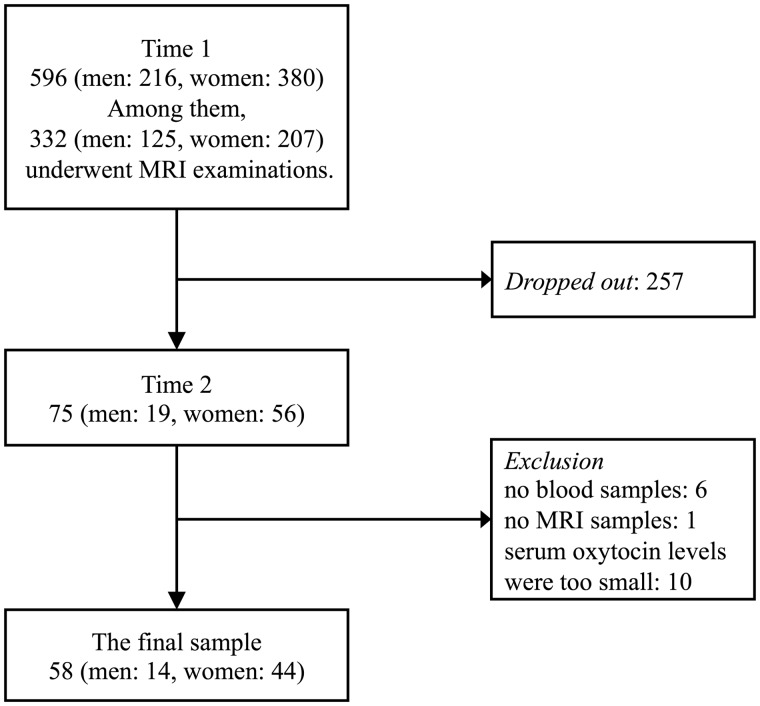
In this study, we collected data from 596 elderly people living in the community. These 596 participants comprised 63.9% of the population of Kurokawa-cho over 65 years old. This survey was conducted twice. First, from October 2009 to March 2011, we conducted a baseline survey that we termed ‘Time 1’. To begin, those diagnosed with dementia, those with a history of depression and those who had received psychiatric treatment were excluded from the survey. Because most of the survey during Time 1 was conducted as part of the national survey of the prevalence of dementia in Japan (Ikejima et al., 2012), not all participants underwent MRI examinations during this time. MRI examinations were optional and executed in cases for which it was necessary for further assessment of dementia or participants themselves requested it. Three hundred thirty-two participants underwent MRI examinations. Finally, 332 participants’ data were used from Time 1. We invited all participants assessed in this survey to a follow-up survey, which we conducted from November 2016 to September 2017 and termed ‘Time 2’. Among the 332 elderly people whose data were used in the Time 1 survey, 75 people agreed to participate in the Time 2 survey. Seventeen participants with no blood or MRI samples or whose serum oxytocin levels were below the detection level were excluded from the final data. Fifty-eight participants (14 men, mean age 72.36 ± 3.41 years; 44 women, mean age 73.07 ± 4.38 years) were included (Fig. 1).
Figure 1.
Flow chart for determining the final sample.
This study was approved by the Ethics Committee of the Faculty of Medicine, Saga University, and all participants agreed in writing to participate in the study according to the Declaration of Helsinki. This research has no financial relationship with any commercial interests.
Cognitive function assessment
The Mini–Mental State Examination (MMSE) is a simple screening index that provides an estimate of elderly people’s cognitive function (Folstein et al., 1975). The Clinical Dementia Rating (CDR) is an indicator for dementia evaluation and severity staging (Hughes et al., 1982; Berg, 1988). All participants underwent MMSE and CDR for cognitive function assessment at Time 1 and Time 2.
Serum samples
Blood samples for serum oxytocin level analysis were collected from participants between 9:00 and 15:00 during Time 1. On the same day, at Saga University, the sample was centrifuged and the serum was extracted and transferred to a container. This was immediately stored at −80°C.
Serum oxytocin assay
Serum was later naturally thawed at room temperature. All samples were analysed in duplicate. The serum oxytocin was analysed using a commercially available peptide enzyme immunoassay (Peninsula Laboratories International, Belmont, CA, USA) with an extraction-free protocol as reported previously (Afinogenova et al., 2016; Imamura et al., 2017; Schmelkin et al., 2017; Aita et al., 2019). A robust correlation has been reported between extracted serum oxytocin levels and unextracted serum oxytocin levels (Lawson et al., 2013). The inter-assay coefficient of variation was 9.77%, and the intra-assay coefficient of variation was 12.98%. Participants whose serum oxytocin levels were below the measurable range and could not be measured were excluded.
MRI acquisition
MRI examinations were performed using a 1.5 Tesla device (Excelart Vantage AGV; Canon Medical Systems, Otawara, Japan). Three-dimensional T1-weighted structural images were acquired for each participant using a field echo three-dimensional method (TR, 21 ms; TE, 5.5 ms; flip angle, 20°; field of view, 240 mm × 240 mm ; matrix, 256 × 256; slice thickness, 1.5 mm; number of slices, 124). The examination conditions were the same for all participants and followed a standardized procedure.
Statistical analysis
Participants’ basic data were analysed and compared using a commercially available statistical package (JMP 13.1.0; SAS Institute, Cary, NC, USA). Each mean value was compared using Welch’s t-test. Fisher’s exact test was used to compare diabetes, dyslipidaemia, and blood collection time. Multiple regression analysis was used to determine the effect of metabolic status and blood collection time on serum oxytocin levels. Wilcoxon signed-rank test was used to compare MMSE and CDR scores for Time 1 and Time 2. Statistical significance was set at P < 0.05.
Preprocessing of brain MRI and longitudinal voxel-based morphometry analysis
Brain MRI processing steps and analysis were conducted using voxel-based morphometry (Ashburner and Friston, 2000) implemented with Statistical Parametric Mapping (SPM12; Wellcome Department of Cognitive Neurology, London, UK) in MATLAB R2016a (MathWorks, Natick, MA, USA) as reported previously (Good et al., 2001; Yamasue et al., 2003; Loh and Kanai, 2014).
T1-weighted MR images were first segmented for grey matter and white matter using the segmentation procedures implemented in SPM12. The diffeomorphic anatomical registration through exponentiated lie algebra described in SPM12 was performed on the segmented grey matter and white matter images to construct a template for co-registration across participants (Ashburner and Friston, 2000; Ashburner, 2007). The segmented grey matter and white matter images were co-registered to the final diffeomorphic anatomical registration through exponentiated lie algebra template and local volumes ware persevered by modulating the image intensity of each voxel by the Jacobian determinants of the deformation fields computed by diffeomorphic anatomical registration through exponentiated lie algebra. The registered images were smoothed with a Gaussian kernel with full width at half maximum of 8 mm and transformed to Montreal Neurological Institute stereotactic space using affine and nonlinear spatial normalization as implemented in SPM12. Preprocessing was conducted by Araya Brain Imaging (Tokyo, Japan).
Grey matter images were used for this analysis. After preparing the Time 1 and Time 2 images, the Times 1–2 difference images were created by subtracting the Time 2 images from the Time 1 images (Ashburner and Ridgway, 2012). Correlation analyses of MR images were conducted between serum oxytocin levels at baseline (Time 1) and brain volume (Time 1, Time 2 and Times 1–2 differences) using grey matter images and multiple regression design. Analyses were performed on these three patterns; the men and women were analysed together, with age, sex and handedness as covariates. In addition, in the case of Time 1 scans, total brain volume (Time 1) was used as a covariate and, in the case of Time 2 scans, total brain volume (Time 2) was used as a covariate. The masking toolbox was used to create mask images and used for analysis. In the analysis, multiple comparison correction (family-wise error) was performed. The initial voxel threshold was set to P = 0.001 uncorrected. Clusters were considered significant when they fell below a cluster-corrected P (family-wise error) = 0.05. Thus, analyses at the cluster level were performed to identify significant brain regions. After statistically significant brain regions were determined, the anatomical labels were identified using the automated anatomical labelling corresponding to the space of the Montreal Neurological Institute standard coordinate system (Tzourio-Mazoyer et al., 2002). Furthermore, using a similar method, analyses were performed on the association between MMSE or CDR (Times 1–2 difference) and brain volume (Time 1, Time 2 and Times 1–2 difference).
Data availability
Any procedures not provided in the Materials and methods section of this article will be shared at the request of other investigators to enable procedures and results to be replicated. In addition, any anonymized data will be shared upon request from any qualified investigator.
Results
Participant characteristics, serum oxytocin levels and MMSE and CDR scores
Baseline serum oxytocin levels were higher in women (0.123 ± 0.130 ng/ml) than in men (0.042 ± 0.052 ng/ml) as reported previously (Imamura et al., 2017; Marazziti et al., 2019). The educational background was slightly higher in men (11.43 ± 2.53 years) than in women (9.41 ± 1.63 years). Therefore, the results shown in Table 1 are presented separately by sex. There was no sex difference in the interval of brain MRI examinations (men, 6.98 ± 0.81 years; women, 6.93 ± 0.74 years) from Time 1 to Time 2. There was no sex difference in metabolic status due to body mass index, diabetes and dyslipidaemia (Table 2). The blood collection time was defined as ‘AM’ from 9:00 to 12:00 and ‘PM’ from 12:00 to 15:00. Similarly, there were no sex differences (Table 2). According to the previous reports, oxytocin reduces caloric intake and increases insulin sensitivity (Lawson et al., 2015) while oxytocin level and body mass index are positively correlated (Schorr et al., 2017) and are associated with obesity, impaired glucose tolerance (Weingarten et al., 2019) and metabolic status (Lawson, 2017). However, in our data, metabolic status and blood collection time were not related to serum oxytocin levels (Table 3). Overall, MMSE and CDR declined from Time 1 to Time 2, respectively (Table 4). We observed that oxytocin levels (at Time 1) did not correlate with changes (i.e. difference between Time 1 and Time 2) in either MMSE or CDR (data not shown).
Table 1.
Participant demographics
| Overall | Men | Women | Statistical significance | |
|---|---|---|---|---|
| N | 58 | 14 | 44 | |
| Age (years, Time 1) | 72.90 ± 4.15 | 72.36 ± 3.41 | 73.07 ± 4.38 | ns |
| Oxytocin (ng/ml, Time 1) | 0.103 ± 0.120 | 0.042 ± 0.052 | 0.123 ± 0.130 | P = 0.0014 |
| Education (years) | 9.90 ± 2.06 | 11.43 ± 2.53 | 9.41 ± 1.63 | P = 0.0124 |
| MRI interval (years, Times 1–2) | 6.94 ± 0.75 | 6.98 ± 0.81 | 6.93 ± 0.74 | ns |
Welch’s t-test. Values represent mean ± SD.
ns = not significant; SD = standard deviation.
Table 2.
Participants’ metabolic status (BMI, diabetes, dyslipidaemia) and blood collection time at Time 1
| Overall | Men | Women | Statistical significance | |
|---|---|---|---|---|
| BMI (kg/m2), mean ± SD | 23.89 ± 3.27 | 24.06 ± 2.43 | 23.84 ± 3.51 | nsa |
| Diabetes, n (%) | 8 (14.0) | 1 (7.1) | 7 (16.3) | nsb |
| Dyslipidaemia, n (%) | 26 (45.6) | 4 (28.6) | 22 (51.2) | nsb |
| Blood collection time, n (%) | ||||
| AM | 27 (46.6) | 7 (50.0) | 20 (45.4) | nsb |
| PM | 31 (53.4) | 7 (50.0) | 24 (54.6) | |
Missing data: BMI (N = 2), diabetes (N = 1) and dyslipidaemia (N = 1). The blood collection time was defined as ‘AM’ from 9:00 to 12:00 and ‘PM’ from 12:00 to 15:00.
Welch’s t-test.
Fisher’s exact test.
BMI = body mass index; ns = not significant.
Table 3.
Multiple regression analysis between serum oxytocin levels (Time 1) as the dependent variable with age, sex, BMI, diabetes, dyslipidaemia and blood collection time as the independent variables
| Estimate | T | P | Lower 95% CI | Upper 95% CI | β | |
|---|---|---|---|---|---|---|
| Age (years) | −0.002 | −0.49 | 0.623 | −0.0096 | 0.0058 | −0.070 |
| Sex (women) | 0.036 | 1.99 | 0.052 | −0.0003 | 0.0720 | 0.278 |
| BMI | −0.003 | −0.73 | 0.469 | −0.0128 | 0.0059 | −0.101 |
| Diabetes (yes) | 0.007 | 0.32 | 0.749 | −0.0368 | 0.0508 | 0.045 |
| Dyslipidaemia (yes) | 0.008 | 0.51 | 0.609 | −0.0239 | 0.0403 | 0.074 |
| Blood collection time (AM) | −0.010 | −0.70 | 0.487 | −0.0412 | 0.0199 | −0.097 |
R 2 = 0.119.
BMI = body mass index; CI = confidence interval; β = standard partial regression coefficient.
Table 4.
MMSE and CDR at Time 1 and Time 2
| Time 1 | Time 2 | Statistical significance | |
|---|---|---|---|
| MMSE, mean ± SD | 28.55 ± 1.39 | 26.31 ± 3.67 | P < 0.0001 |
| CDR, n (%) | |||
| 0 | 56 (96.6) | 51 (88.0) | P = 0.016 |
| 0.5 | 2 (3.4) | 6 (10.3) | |
| 1 | 1 (1.7) | ||
Wilcoxon signed-rank test.
Voxel-based morphometry findings
First, we analysed the correlation between serum oxytocin levels (Time 1) and brain volume (Time 1). No correlation existed between serum oxytocin levels and brain volume in any region.
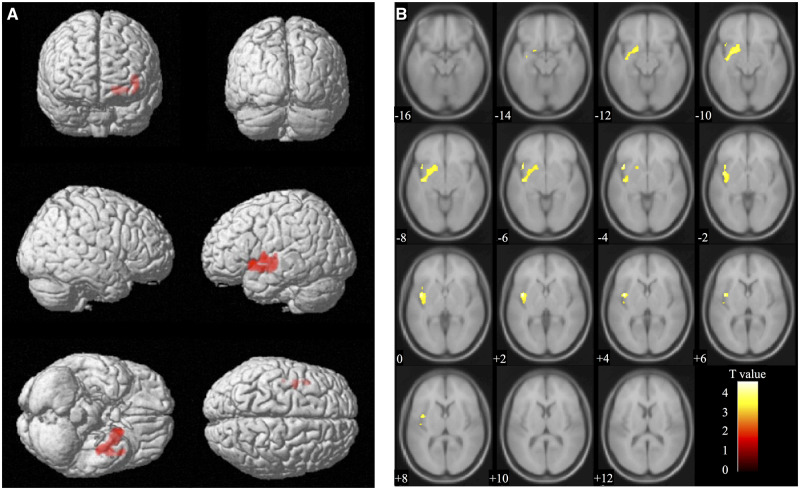
We next analysed the correlation between serum oxytocin levels (Time 1) and brain volume (Time 2). Analyses at the cluster level applying multiple comparison correction (family-wise error; significance level, P < 0.05) showed a positive correlation with the brain volume of the region containing the left hippocampus (coordinates −38, −12, −9) and the left amygdala (coordinates −29, 2, −9). The thresholds for statistics were set to T = 3.25 for the height threshold and k = 1225 voxels for the extent threshold. In addition, this cluster contains the left superior temporal gyrus and the left insular cortex regions. Table 5 shows details of the P, T and cluster size (number of voxels) values for the region in which correlations were found. The voxel-based morphometry findings on the significant cluster containing the left hippocampus and the left amygdala are shown in Fig. 2 using standard brain MR images. Furthermore, a scatterplot of serum oxytocin levels (Time 1) and the voxel values (Time 2) of the region containing the left hippocampus (coordinates −38, −12, −9) and the left amygdala (coordinates −29, 2, −9) are shown in Fig. 3.
Table 5.
VBM findings
| Cluster level |
Peak level |
MNI coordinates |
Anatomical region | |||||
|---|---|---|---|---|---|---|---|---|
| P FWE-corr | k, cluster size (voxels) | P uncorr | T | P uncorr | X (mm) | Y (mm) | Z (mm) | |
| 0.013 | 1225 | 0.002 | 4.61 | <0.001 | −41 | 0 | 3 | Temporal_Sup_L |
| 4.30 | <0.001 | −42 | 14 | −5 | Insula_L | |||
| 4.01 | <0.001 | −38 | −12 | −9 | Hippocampus_L | |||
| 3.92 | <0.001 | −29 | 2 | −9 | Amygdala_L | |||
Positive correlation between serum oxytocin levels (Time 1) and brain volume (Time 2) by multiple regression applying multiple comparison correction. The cluster size is 1225 voxels and contains the left hippocampus and left amygdala. Height threshold, T = 3.25. Extent threshold, k = 1225 voxels. Expected voxels per cluster, k = 110.160. Degrees of freedom = [1.0, 52.0]. FWHM = 15.6 mm × 15.5 mm × 14.9 mm, 10.4 × 10.3 × 9.9 (voxels). Volume, 1 258 524 = 372 896 voxels = 302.6 resels. Voxel size, 1.5 mm × 1.5 mm × 1.5 mm (resel = 1070.18 voxels).
corr = corrected; FWE = family-wise error; FWHM = full width at half maximum; L = left; MNI = Montreal Neurological Institute; sup = superior; uncorr = uncorrected; VBM = voxel-based morphometry. Labels are marked using automated anatomical labelling.
Figure 2.
Voxel-based morphometry findings. Positive correlation between serum oxytocin levels (Time 1) and brain volume (Time 2) shown by multiple regression. The significant cluster (cluster size, 1225 voxels) containing the left hippocampus (coordinates −38, −12, −9) and the left amygdala (coordinates −29, 2, −9) is shown in (A) whole brain images and (B) axial images. T value applies to (B) axial images.
Figure 3.
Correlation between serum oxytocin levels and voxel values. Scatterplot of serum oxytocin levels (Time 1) and voxel values (Time 2) of the region containing the left hippocampus and amygdala. (A) The left hippocampus (coordinates −38, −12, −9) and (B) the left amygdala (coordinates −29, 2, −9). Serum oxytocin levels and voxel values were adjusted during the analysis. Fitted value = the voxel value − (the averaged voxel value + the error). Plus error = the voxel value − the averaged voxel value.
Finally, we analysed the correlation between serum oxytocin levels (Time 1) and brain volume (Time 1–2 difference). However, no correlation existed between the two in any region.
Analyses at the cluster level applying multiple comparison correction (family-wise error; significance level, P < 0.05) showed no association between MMSE difference (Times 1–2 difference) and brain volume. However, lower CDR scores were associated with decreased brain volume (Time 2) of the region containing the left hippocampus. The thresholds for statistics were set to T = 3.25 for the height threshold and k = 2461 voxels for extent threshold (Supplementary Table 1). The voxel-based morphometry findings on the significant cluster containing the left hippocampus are shown in Supplementary Fig. 1 using standard brain MR images.
Discussion
Principal findings
In this study, we focused on the correlation between serum oxytocin levels and brain volume in people aged over 65 years. The two were not correlated in any region at baseline. However, they were positively correlated with left hippocampus and amygdala volume 7 years after baseline at the cluster level.
The hippocampus and amygdala comprise part of the limbic system and have important roles in memory formation, cognition and emotion activation. The limbic system network is dense, and the hippocampus and amygdala do not only function individually but also interact and operate with other areas (Aggleton, 1986; Saunders et al., 1988; Fink et al., 1996; Majak and Pitkänen, 2003). Oxytocin receptors are widely distributed in the brain (Gimpl and Fahrenholz, 2001). They are also thought to be abundantly present in the limbic system, including the hippocampus (Tomizawa et al., 2003) and amygdala (Ophir et al., 2012). Oxytocin-producing neurons originating in the hypothalamus are widely projected to other brain regions. It also acts on the limbic system, including the hippocampus and amygdala, and has neuromodulatory effects (Meyer-Lindenberg et al., 2011). Therefore, oxytocin is closely related to the hippocampus and amygdala. We found a notable correlation with the hippocampus and amygdala in people aged over 65 years. Therefore, we will focus on the relationship between oxytocin and the hippocampus and amygdala volume and discuss our results with reference to previous studies.
Oxytocin and the hippocampus and amygdala
There was no correlation between serum oxytocin levels and brain volume at baseline. However, the fact that a positive correlation with the hippocampus and amygdala volume was found after ˜ 7 years suggests that oxytocin in people aged 65 years and older may be associated with aging-related changes in hippocampus and amygdala volume.
To date, there have been reports on volume, neuroplasticity and neurogenesis related to the hippocampus. For example, oxytocin promotes neurogenesis in the hippocampus and is involved in neural plasticity in mice. Consequently, it is considered to have an important role in learning and memory (Tomizawa et al., 2003; Lin et al., 2017). In addition, intranasal administration of oxytocin acted on the oxytocin receptor and reduced the effects of stress on synaptic plasticity and memory in the hippocampus of rats (Lee et al., 2015). Until now, it was thought that neurogenesis does not occur in adulthood. However, as these reports show, neurogenesis occurs over a lifetime in specific brain regions such as the dentate gyrus of the hippocampus (Ming and Song, 2005). Neurogenesis in the dentate gyrus of the hippocampus also forms a network of nerves and plays an important role in the formation of new memories (Deng et al., 2010; Sahay et al., 2011). In addition, neurogenesis in the dentate gyrus of the hippocampus requires the presence of neural stem and progenitor cells; the presence of these in the dentate gyrus of the hippocampus has been confirmed not only in animals but also in humans (Eriksson et al., 1998). Certainly, atrophy or deterioration of the brain including the hippocampus may occur because of aging (Salat et al., 2004), but it may be possible to increase the size of the hippocampus by continuing appropriate exercise for 1 year (Erickson et al., 2011). According to Erickson et al., appropriate exercise increased hippocampus volume and improved spatial memory. Furthermore, a study on London taxi drivers found that time spent driving was positively correlated with hippocampus volume and that a change in plasticity occurred (Maguire et al., 2000). Therefore, neurogenesis or change in plasticity of the brain may occur because of external stimulation by exercise or environmental demand.
Regarding the relationship between oxytocin and amygdala, activation of oxytocin-producing neurons in the hypothalamus acts on the oxytocin receptor of the amygdala and promotes social memory (Takayanagi et al., 2017). In addition, research on facial expression cognition confirmed changes in amygdala activity due to oxytocin administration (Kirsch et al., 2005). Furthermore, oxytocin administration suppresses amygdala activity, alleviates fear and anxiety and promotes trust activities to others (Kosfeld et al., 2005; Baumgartner et al., 2008). Considering these examples and our results, oxytocin is an internal stimulus for the hippocampus and amygdala and may be associated with aging-related changes in hippocampus and amygdala volume.
However, studies in young adults have reported an inverse correlation between plasma oxytocin concentration and amygdala and hippocampus volume (Andari et al., 2014). Similar results have also been reported in studies in adult women with early-life maltreatment (Mielke et al., 2018). The results on these structural aspects may differ from the results of our study on elderly people. In adult men with autism spectrum disorder, higher levels of salivary oxytocin are associated with a lower degree of functional connectivity between the amygdala and hippocampus regions (Alaerts et al., 2019). Moreover, intranasal administration of oxytocin reduces the functional connection between the amygdala and brainstem regions (Kirsch et al., 2005) and hippocampus (Alaerts et al., 2019). These studies were conducted on relatively young adults, those with early-life maltreatment and those with autism spectrum disorder, but their results should be considered when discussing our results. Furthermore, no uniform pattern of suppressed amygdala activity has been identified for the effect of intranasal administration of oxytocin (Grace et al., 2018). In task-based fMRI studies, left amygdala activity was suppressed in women (Rilling et al., 2014) while was potentiated in men (Rilling et al., 2012) during the Prisoners Dilemma task. Similarly, in the iterated Prisoners Dilemma game with same-sex human and computer partners, amygdala activity decreased in both men and women, while there was a difference in the game situation between men and women (Chen et al., 2016). In response to facial threat, amygdala activity was potentiated in women (Domes et al., 2010) while was suppressed in men (Kanat et al., 2015). In addition, Frijling et al. (2016) reported that post-traumatic men and women showed no sex differences in amygdala responses to fearful faces. Thus, individual differences such as age, sex and underlying psychopathology and differences in methods of stimulation appear to have diverse effects of intranasal administration of oxytocin on amygdala activity (Grace et al., 2018; Seeley et al., 2018).
Reports of hippocampus and amygdala volume in elderly people have shown that elderly patients with Alzheimer’s disease have significantly smaller hippocampus volume than healthy elderly controls (Du et al., 2001; Frisoni et al., 2008). Moreover, similar results have been reported for amygdala volume (Cavedo et al., 2011). Deterioration of the hippocampus occurs prior to memory impairment in late adulthood and leads to disability (Raz et al., 2005). A similar study found that healthy elderly people without dementia who later developed dementia had decreases in hippocampus and amygdala volume before onset (den Heijer et al., 2006). In addition, the hippocampus volume in elderly women can be an index for associative memory processing in aging and an index for the early detection of associative memory deterioration (Zheng et al., 2017). Hippocampus volume and amygdala volume are useful as indicators for predicting memory and cognitive decline in advance. Based on these points, when thinking about dementia and cognitive function of elderly people, our results may be associated with elderly people’s cognitive function. Oxytocin may not be positively associated with hippocampus and amygdala volume in non-elderly humans. However, oxytocin in those aged 65 years and older may be positively associated with hippocampus and amygdala volume.
Altogether, the limbic system is deeply involved in human cognitive function. These systems allow people to live socially and healthily. Oxytocin may be one factor of social health maintenance. Therefore, it is necessary to further investigate brain volume and oxytocin action.
Oxytocin and elderly health
Oxytocin also reduces oxidative stress and inflammation (Szeto et al., 2008). Alzheimer’s disease is related to inflammation and treating chronic systemic inflammation may be effective in the prevention and intervention of Alzheimer’s disease (Tao et al., 2018). Oxytocin is also involved in bone formation and osteoporosis improvement (Colaianni et al., 2014). A lack of oxytocin can negatively affect muscle regeneration and lead to aging (Elabd et al., 2014). Thus, oxytocin has anti-aging effects in various aspects. It is possible to promote the secretion of oxytocin; for example blood oxytocin levels rise when trying to help and support partners or others in need. In other words, oxytocin increases when communicating warmly with others (Grewen et al., 2005). In addition, oxytocin is created by remembering the experience of a good connection with a partner and others or when feeling a warm connection (Crockford et al., 2014). These reports show that connection and communication with others are important anti-aging activities for elderly people living in a rural community. We hope that the results of our research will lead to self-care behaviour for preventing cognitive decline and anti-aging in elderly people in the future.
Limitations
Some caveats should be noted. The evaluation of participants in Time 1 was conducted as a part of a national survey of the prevalence of dementia in Japan. Importantly, MRI examinations were optional and were executed when necessary for further assessment of dementia or when participants themselves requested it. Thus, in our study, the cohort may be biased and may not reflect the characteristics of a general rural elderly population. In this study, serum oxytocin was analysed using a commercially available peptide enzyme immunoassay with an extraction-free protocol. Oxytocin measurement using ELISA has been discussed in several studies. Extraction removes some of the interfering substances (Szeto et al., 2011). However, the discarded substances may include oxytocin bound to proteins (Brandtzaeg et al., 2016). Absolute oxytocin levels cannot be compared across studies because of differences in assays, but measurements can be useful for comparing relative levels of peripheral oxytocin between groups (Lawson et al., 2020). Furthermore, the relationship between peripheral and central oxytocin levels is an ongoing debate and a positive correlation between peripheral and central oxytocin levels has been reported (Valstad et al., 2017). However, there are reports that there is no significant association between peripheral and central concentrations (Kagerbauer et al., 2013).
Conclusions
In this study, we focused on the correlation between serum oxytocin levels and brain volume in elderly people living in a rural community. We found that, for people aged over 65 years, serum oxytocin levels correlated positively with the left hippocampus and amygdala volume after 7 years. This result suggests that oxytocin may be associated with aging-related changes in hippocampus and amygdala volume in people aged 65 years and older.
Funding
This study was supported by grants from the Japan Society for the Promotion of Science—Scientific Research (to Y.M. and A.M.) and Challenging Exploratory Research (to Y.M. and Y.I.). We all appreciate Hiroko Kunitake for her technical support.
Competing interests
The authors report no competing interests.
Supplementary Material
Glossary
- CDR =
Clinical Dementia Rating
- MMSE =
Mini–Mental State Examination
References
- Afinogenova Y, Schmelkin C, Plessow F, Thomas JJ, Pulumo R, Micali N, et al. Low fasting oxytocin levels are associated with psychopathology in anorexia nervosa in partial recovery. J Clin Psychiatry 2016; 77: e1483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP. A description of the amygdalo-hippocampal interconnections in the macaque monkey. Exp Brain Res 1986; 64: 515–26. [DOI] [PubMed] [Google Scholar]
- Aita C, Mizoguchi Y, Yamamoto M, Seguch IY, Yatsuga C, Nishimura T, et al. Oxytocin levels and sex differences in autism spectrum disorder with severe intellectual disabilities. Psychiatry Res 2019; 273: 67–74. [DOI] [PubMed] [Google Scholar]
- Alaerts K, Bernaerts S, Vanaudenaerde B, Daniels N, Wenderoth N.. Amygdala-hippocampal connectivity is associated with endogenous levels of oxytocin and can be altered by exogenously administered oxytocin in adults with autism. Biol Psychiatry Cogn Neurosci Neuroimaging 2019; 4: 655–63. [DOI] [PubMed] [Google Scholar]
- Andari E, Schneider FC, Mottolese R, Vindras P, Sirigu A.. Oxytocin’s fingerprint in personality traits and regional brain volume. Cereb Cortex 2014; 24: 479–86. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007; 38: 95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ.. Voxel-based morphometry–the methods. Neuroimage 2000; 11: 805–21. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Ridgway GR.. Symmetric diffeomorphic modeling of longitudinal structural MRI. Front Neurosci 2012; 6: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN.. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci 2011; 15: 301–9. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E.. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 2008; 58: 639–50. [DOI] [PubMed] [Google Scholar]
- Berg L. Clinical Dementia Rating (CDR). Psychopharmacol Bull 1988; 24: 637–9. [PubMed] [Google Scholar]
- Bernaerts S, Prinsen J, Berra E, Bosmans G, Steyaert J, Alaerts K.. Long-term oxytocin administration enhances the experience of attachment. Psychoneuroendocrinology 2017; 78: 1–9. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg OK, Johnsen E, Roberg-Larsen H, Seip KF, MacLean EL, Gesquiere LR, et al. Proteomics tools reveal startlingly high amounts of oxytocin in plasma and serum. Sci Rep 2016; 6: 31693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. The role of oxytocin and vasopressin in attachment. Psychodyn Psychiatry 2017; 45: 499–517. [DOI] [PubMed] [Google Scholar]
- Cavedo E, Boccardi M, Ganzola R, Canu E, Beltramello A, Caltagirone C, et al. Local amygdala structural differences with 3T MRI in patients with Alzheimer disease. Neurology 2011; 76: 727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Hackett PD, DeMarco AC, Feng C, Stair S, Haroon E, et al. Effects of oxytocin and vasopressin on the neural response to unreciprocated cooperation within brain regions involved in stress and anxiety in men and women. Brain Imaging Behav 2016; 10: 581–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaianni G, Tamma R, Di Benedetto A, Yuen T, Sun L, Zaidi M, et al. The oxytocin-bone axis. J Neuroendocrinol 2014; 26: 53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockford C, Deschner T, Ziegler TE, Wittig RM.. Endogenous peripheral oxytocin measures can give insight into the dynamics of social relationships: a review. Front Behav Neurosci 2014; 8: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale HH. On some physiological actions of ergot. J Physiol 1906; 34: 163–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CK. Oxytocin modulates cooperation within and competition between groups: an integrative review and research agenda. Horm Behav 2012; 61: 419–28. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Geerlings MI, Hoebeek FE, Hofman A, Koudstaal PJ, Breteler MM.. Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Arch Gen Psychiatry 2006; 63: 57–62. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH.. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 2010; 11: 339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC.. Oxytocin improves “mind-reading” in humans. Biol Psychiatry 2007; 61: 731–3. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, et al. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology 2010; 35: 83–93. [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2001; 71: 441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, et al. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun 2014; 5: 4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 2011; 108: 3017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med 1998; 4: 1313–7. [DOI] [PubMed] [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD.. Cerebral representation of one’s own past: neural networks involved in autobiographical memory. J Neurosci 1996; 16: 4275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR.. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98. [DOI] [PubMed] [Google Scholar]
- Frijling JL, van Zuiden M, Koch SB, Nawijn L, Veltman DJ, Olff M.. Effects of intranasal oxytocin on amygdala reactivity to emotional faces in recently trauma-exposed individuals. Soc Cogn Affect Neurosci 2016; 11: 327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoni GB, Ganzola R, Canu E, Rüb U, Pizzini FB, Alessandrini F, et al. Mapping local hippocampal changes in Alzheimer’s disease and normal ageing with MRI at 3 Tesla. Brain 2008; 131: 3266–76. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F.. The oxytocin receptor system: structure, function, and regulation. Physiol Rev 2001; 81: 629–83. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS.. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001; 14: 21–36. [DOI] [PubMed] [Google Scholar]
- Grace SA, Rossell SL, Heinrichs M, Kordsachia C, Labuschagne I.. Oxytocin and brain activity in humans: a systematic review and coordinate-based meta-analysis of functional MRI studies. Psychoneuroendocrinology 2018; 96: 6–24. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC.. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom Med 2005; 67: 531–8. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G.. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol 2009; 30: 548–57. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RA.. new clinical scale for the staging of dementia. Br J Psychiatry 1982; 140: 566–72. [DOI] [PubMed] [Google Scholar]
- Ikejima C, Hisanaga A, Meguro K, Yamada T, Ouma S, Kawamuro Y, et al. Multicentre population-based dementia prevalence survey in Japan: a preliminary report. Psychogeriatrics 2012; 12: 120–3. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Mizoguchi Y, Nabeta H, Haraguchi Y, Matsushima J, Kojima N, et al. An association between belief in life after death and serum oxytocin in older people in rural Japan. Int J Geriatr Psychiatry 2017; 32: 102–9. [DOI] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 2010; 9: 119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagerbauer SM, Martin J, Schuster T, Blobner M, Kochs EF, Landgraf R.. Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J Neuroendocrinol 2013; 25: 668–73. [DOI] [PubMed] [Google Scholar]
- Kanat M, Heinrichs M, Schwarzwald R, Domes G.. Oxytocin attenuates neural reactivity to masked threat cues from the eyes. Neuropsychopharmacology 2015; 40: 287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci 2005; 25: 11489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüver H, Bucy PC.. Preliminary analysis of functions of the temporal lobes in monkeys. J Neuropsychiatry Clin Neurosci 1997; 9: 606–20. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E.. Oxytocin increases trust in humans. Nature 2005; 435: 673–6. [DOI] [PubMed] [Google Scholar]
- Lawson EA. The effects of oxytocin on eating behaviour and metabolism in humans. Nat Rev Endocrinol 2017; 13: 700–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson EA, Ackerman KE, Estella NM, Guereca G, Pierce L, Sluss PM, et al. Nocturnal oxytocin secretion is lower in amenorrheic athletes than nonathletes and associated with bone microarchitecture and finite element analysis parameters. Eur J Endocrinol 2013; 168: 457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson EA, Marengi DA, DeSanti RL, Holmes TM, Schoenfeld DA, Tolley CJ.. Oxytocin reduces caloric intake in men. Obesity (Silver Spring) 2015; 23: 950–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson EA, Olszewski PK, Weller A, Blevins JE.. The role of oxytocin in regulation of appetitive behaviour, body weight and glucose homeostasis. J Neuroendocrinol 2020; 32: e12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS.. Oxytocin: the great facilitator of life. Prog Neurobiol 2009; 88: 127–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Park SH, Chung C, Kim JJ, Choi SY, Han JS.. Oxytocin protects hippocampal memory and plasticity from uncontrollable stress. Sci Rep 2015; 5: 18540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Chen CC, Huang CC, Nishimori K, Hsu KS.. Oxytocin stimulates hippocampal neurogenesis via oxytocin receptor expressed in CA3 pyramidal neurons. Nat Commun 2017; 8: 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh KK, Kanai R.. Higher media multi-tasking activity is associated with smaller gray-matter density in the anterior cingulate cortex. PLoS One 2014; 9: e106698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA 2000; 97: 4398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majak K, Pitkänen A.. Projections from the periamygdaloid cortex to the amygdaloid complex, the hippocampal formation, and the parahippocampal region: a PHA-L study in the rat. Hippocampus 2003; 13: 922–42. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Baroni S, Mucci F, Piccinni A, Moroni I, Giannaccini G, et al. Sex-related differences in plasma oxytocin levels in humans. Clin Pract Epidemiol Ment Health 2019; 15: 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima J, Kawashima T, Nabeta H, Imamura Y, Watanabe I, Mizoguchi Y, et al. Association of inflammatory biomarkers with depressive symptoms and cognitive decline in a community-dwelling healthy older sample: a 3-year follow-up study. J Affect Disord 2015; 173: 9–14. [DOI] [PubMed] [Google Scholar]
- McCall C, Singer T.. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat Neurosci 2012; 15: 681–8. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M.. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci 2011; 12: 524–38. [DOI] [PubMed] [Google Scholar]
- Mielke EL, Neukel C, Bertsch K, Reck C, Möhler E, Herpertz SC.. Alterations of brain volumes in women with early life maltreatment and their associations with oxytocin. Horm Behav 2018; 97: 128–36. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H.. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 2005; 28: 223–50. [DOI] [PubMed] [Google Scholar]
- Nabeta H, Mizoguchi Y, Matsushima J, Imamura Y, Watanabe I, Tateishi T, et al. Association of salivary cortisol levels and later depressive state in elderly people living in a rural community: a 3-year follow-up study. J Affect Disord 2014; 158: 85–9. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Gessel A, Zheng DJ, Phelps SM.. Oxytocin receptor density is associated with male mating tactics and social monogamy. Horm Behav 2012; 61: 445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 2005; 15: 1676–89. [DOI] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Chen X, Gautam P, Stair S, et al. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology 2014; 39: 237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, et al. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology 2012; 37: 447–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Young LJ.. The biology of mammalian parenting and its effect on offspring social development. Science 2014; 345: 771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 2011; 472: 466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex 2004; 14: 721–30. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Rosene DL, Van Hoesen GW.. Comparison of the efferents of the amygdala and the hippocampal formation in the rhesus monkey: II. Reciprocal and non-reciprocal connections. J Comp Neurol 1988; 271: 185–207. [DOI] [PubMed] [Google Scholar]
- Schmelkin C, Plessow F, Thomas JJ, Gray EK, Marengi DA, Pulumo R, et al. Low oxytocin levels are related to alexithymia in anorexia nervosa. Int J Eat Disord 2017; 50: 1332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorr M, Marengi DA, Pulumo RL, Yu E, Eddy KT, Klibanski A, et al. Oxytocin and its relationship to body composition, bone mineral density, and hip geometry across the weight spectrum. J Clin Endocrinol Metab 2017; 102: 2814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B.. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 1957; 20: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley SH, Chou YH, O’Connor MF.. Intranasal oxytocin and OXTR genotype effects on resting state functional connectivity: a systematic review. Neurosci Biobehav Rev 2018; 95: 17–32. [DOI] [PubMed] [Google Scholar]
- Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, et al. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom Med 2011; 73: 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto A, Nation DA, Mendez AJ, Dominguez-Bendala J, Brooks LG, Schneiderman N, et al. Oxytocin attenuates NADPH-dependent superoxide activity and IL-6 secretion in macrophages and vascular cells. Am J Physiol Endocrinol Metab 2008; 295: E1495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Takashima A, Takanami K, Yoshida S, Nishimori K, et al. Activation of supraoptic oxytocin neurons by secretin facilitates social recognition. Biol Psychiatry 2017; 81: 243–51. [DOI] [PubMed] [Google Scholar]
- Tao Q, Ang TFA, DeCarli C, Auerbach SH, Devine S, Stein TD, et al. Association of chronic low-grade inflammation with risk of Alzheimer disease in ApoE4 carriers. JAMA Netw Open 2018; 1: e183597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Velden FS, Daughters K, De Dreu C.. Oxytocin promotes intuitive rather than deliberated cooperation with the in-group. Horm Behav 2017; 92: 164–71. [DOI] [PubMed] [Google Scholar]
- Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, et al. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci 2003; 6: 384–90. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15: 273–89. [DOI] [PubMed] [Google Scholar]
- Valstad M, Alvares GA, Egknud M, Matziorinis AM, Andreassen OA, Westlye LT, et al. The correlation between central and peripheral oxytocin concentrations: a systematic review and meta-analysis. Neurosci Biobehav Rev 2017; 78: 117–24. [DOI] [PubMed] [Google Scholar]
- Weingarten MFJ, Scholz M, Wohland T, Horn K, Stumvoll M, Kovacs P, et al. Circulating oxytocin is genetically determined and associated with obesity and impaired glucose tolerance. J Clin Endocrinol Metab 2019; 104: 5621–32. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD.. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology 1997; 138: 2829–34. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, et al. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci USA 2003; 100: 9039–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Li R, Xiao F, He R, Zhang S, Li J.. Sex matters: hippocampal volume predicts individual differences in associative memory in cognitively normal older women but not men. Front Hum Neurosci 2017; 11: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any procedures not provided in the Materials and methods section of this article will be shared at the request of other investigators to enable procedures and results to be replicated. In addition, any anonymized data will be shared upon request from any qualified investigator.