Abstract
Despite effective therapies that have extended the life expectancy of persons living with HIV, 35–70% of these adults still develop some form of cognitive impairment, and with a growing population of aging adults with HIV, the prevalence of these cognitive deficits is likely to increase. The mechanisms underlying these HIV-associated neurocognitive disorders remain poorly understood but are often accelerated by the aging process and accompanied by disturbances in sensory processing, which may contribute to the observed cognitive decline. The goal of the current study was to identify the impact of aging on HIV-related alterations in inhibitory processing and determine whether such alterations are related to cognitive impairment in neuroHIV. We used magnetoencephalographic imaging, advanced time series analysis methods, and a paired-pulse stimulation paradigm to interrogate inhibitory processing in 87 HIV-infected aging adults and 92 demographically matched uninfected controls (22–72 years old). Whole-brain maps linking age and neural indices were computed for each group and compared via Fisher’s Z transformations. Peak voxel time-series data were also extracted from the resulting images to quantify the dynamics of spontaneous neural activity preceding stimulation onset in each group. Whole-brain analyses using the somatosensory gating index, a metric of inhibitory processing and age distinguished impaired adults with HIV from unimpaired HIV-infected adults and controls. Briefly, younger cognitively impaired adults with HIV strongly utilized the prefrontal cortices to gate somatosensory input, and the role of this region in gating was uniquely and significantly modulated by aging only in impaired adults with HIV. Spontaneous neural activity preceding stimulus onset was also significantly elevated in the prefrontal cortices of those with HIV-associated neurocognitive disorder, and this elevation was significantly related to the CD4 nadir across both HIV-infected groups. This is the first study to examine the impact of aging on inhibitory processing in HIV-infected adults with and without cognitive impairment. Our findings suggest that young adults with HIV-associated neurocognitive disorder utilize the prefrontal cortices to gate (i.e. suppress) redundant somatosensory input, and that this capacity uniquely diminishes with advancing age in impaired adults with HIV.
Keywords: somatosensory gating, gamma, spontaneous neural activity, magnetoencephalography
Younger adults with HIV-related cognitive impairment uniquely utilized prefrontal gamma activity to gate paired-pulse electrical somatosensory stimulation, but this compensatory process became exhausted with increasing age. Spontaneous gamma oscillations in prefrontal cortices were also elevated in these participants relative to their unimpaired counterparts and were predictive of clinical HIV metrics.
Graphical Abstract
Graphical Abstract.
Introduction
HIV-associated neurocognitive impairment remains a major concern in those infected with HIV, despite the availability of combination antiretroviral therapy (Antinori et al., 2007; Robertson et al., 2007; Cysique and Brew, 2009; Heaton et al., 2010, 2011; Lerner et al., 2020). Notably, 35–70% of all persons living with HIV (PLWH) develop some form of cognitive impairment (Robertson et al., 2007; Heaton et al., 2010, 2011; Simioni et al., 2010; Saylor et al., 2016), and with an aging HIV-infected population, this figure will likely remain high or even increase over the next decade (Kamkwalala and Newhouse, 2017). Currently, there are no specific biomarkers that distinguish cognitive impairment amongst PLWH. Thus, examining the neural mechanisms underlying such cognitive dysfunction may enable the identification of dissociable metrics that can ultimately aid in the diagnosis, treatment and understanding of neuroHIV.
Sensory gating is a neurophysiological phenomenon whereby the neural response to the second stimulus in an identical pair is attenuated. This suppression is thought to reflect the capacity of the CNS to filter irrelevant information and preserve its resources for behaviourally relevant input (Cromwell et al., 2008). Recent evidence suggests that healthy older adults exhibit impaired gating in both auditory and somatosensory domains, and that such gating is served by inhibitory processing (Lenz et al., 2012; Cheng and Lin, 2013; Cheng et al., 2015a, b; Spooner et al., 2019). Interestingly, recent studies also indicate that young and middle-aged PLWH exhibit aberrant somatosensory processing and altered sensory gating (Wilson et al., 2015; Spooner et al., 2018). Thus, beyond its potential as a marker of cognitive impairment in neuroHIV, sensory gating (i.e. inhibitory processing) may also be a useful index to determine the potentially compounding, interactive effects of aging with HIV.
In the current study, we used a paired-pulse somatosensory stimulation paradigm to examine whether aging differentially affects sensory gating in PLWH, and to determine whether such age-related alterations in somatosensory processing and gating can distinguish adults with HIV-associated neurocognitive disorder (HAND) from unimpaired PLWH and uninfected controls. Beyond sensory gating and basic somatosensory processing, we also evaluated the strength of spontaneous neural activity in gating-related brain regions, as prior studies have shown such prestimulus spontaneous activity is significantly modulated by aging in healthy controls (Spooner et al., 2019). Finally, we interrogated whether alterations in somatosensory processing (i.e. spontaneous baseline activity, gating) were related to important risk factors for developing neurocognitive impairment, including CD4 nadir and time since diagnosis. Briefly, CD4+ T cells are prominent indicators of immune system stability in PLWH, as their levels dramatically decrease immediately upon infection (Leung et al., 2013; Serrano-Villar et al., 2014). Further, lower CD4 nadir values (i.e. lowest lifetime cell count) have been directly implicated in both the relative age advancement (e.g. DNA methylation) and the likelihood of cognitive impairment in PLWH (Tozzi et al., 2005; Muñoz-Moreno et al., 2008; Ellis et al., 2011; Gross et al., 2016). Thus, we expected our neural markers of somatosensory function to be closely tied to these important clinical measures in PLWH. Our primary hypotheses were that adults with HAND would have: (i) altered sensory gating with increasing age relative to unimpaired PLWH and controls, (ii) elevated spontaneous gamma activity prior to stimulation onset and (iii) that modifications in somatosensory processing would be related to clinical metrics such as CD4 nadir.
Materials and methods
Participants
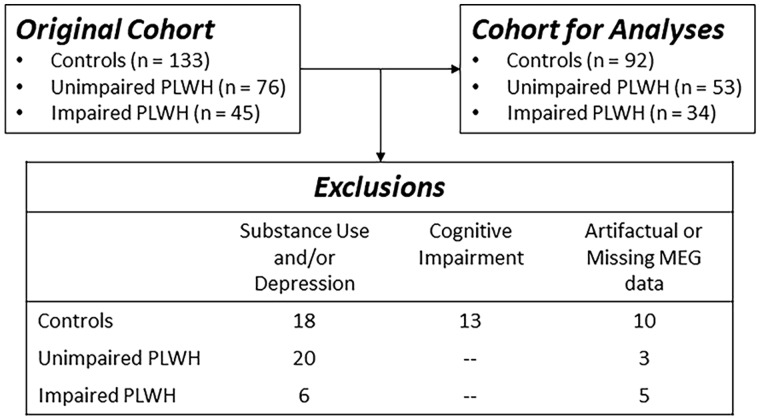
Two hundred and fifty-four adults (range: 22–72 years old; 121 PLWH and 133 controls) were enrolled. All PLWH were receiving effective combination antiretroviral therapy and had complete viral suppression defined as <50 copies/ml. Exclusion criteria included any medical illness affecting CNS function (other than HIV-infection/HAND), any psychiatric or neurological disorder (other than HAND), pregnancy, implanted ferromagnetic objects or orthodonture and a history of head trauma. Uninfected controls were enrolled to demographically match PLWH based on their race/ethnicity, age, sex and handedness. In total, 76 unimpaired PLWH (44 males), 45 participants with HAND (26 males) and 133 unimpaired HIV-negative controls (63 males) were enrolled. Further study-specific exclusion criteria are detailed in Fig. 1 and the Results section, and included active substance abuse, moderate to severe depression according to the Beck Depression Inventory-II (Beck et al., 1996), and missing or artefactual MEG somatosensory gating data or structural magnetic resonance imaging (MRI). HAND diagnoses were based on a thorough neuropsychological assessment consistent with the Frascati criteria (Antinori et al., 2007). The University of Nebraska Medical Center Institutional Review Board approved the study and all participants provided written informed consent.
Figure 1.
CONSORT diagram. Of the 254 total participants enrolled in the project, 179 remained following exclusion for current substance use, moderate to severe depression, cognitive impairment (in controls) and artefactual or missing MEG data.
Experimental paradigm
Participants were seated in a nonmagnetic chair with their head positioned within the magnetoencephalography (MEG) helmet-shaped sensor array. Electrical stimulation was applied to the right median nerve using external cutaneous stimulators connected to a Digitimer DS7A constant-current stimulator system (Digitimer Limited, Letchworth Garden City, UK). For each participant, we collected at least 80 paired-pulse trials with an inter-stimulation interval of 500 ms and an inter-pair interval that randomly varied between 4500 and 4800 ms. Each pulse generated a 0.2 ms constant-current square wave that was set to a limit of 10% above the motor threshold required to elicit a subtle twitch of the thumb.
MEG data acquisition and coregistration with structural MRI
All recordings were performed in a one-layer magnetically shielded room with active shielding engaged for environmental noise compensation. With an acquisition bandwidth of 0.1–330 Hz, neuromagnetic responses were sampled continuously at 1 kHz using an Elekta MEG system (Elekta, Helsinki, Finland) with 306 magnetic sensors, including 204 planar gradiometers and 102 magnetometers. Throughout data acquisition, participants were monitored using a real-time audio-video feed from inside the magnetically shielded room. MEG data from each participant were individually corrected for head motion and subjected to noise reduction using the signal space separation method with a temporal extension (Taulu and Simola, 2006). Each participant’s MEG data were coregistered with their structural T1-weighted MRI data prior to imaging analyses using BESA MRI (Version 2.0). Structural MRI data were aligned parallel to the anterior and posterior commissures and transformed into standardized space. After beamformer analysis (see below), each subject’s functional images were transformed into standardized space using the transform that was previously applied to the structural MRI volume and spatially resampled.
MEG preprocessing, time-frequency transformation and sensor-level statistics
Cardiac artefacts were removed from the data using signal-space projection and the projection operator was accounted for during source reconstruction (Uusitalo and Ilmoniemi, 1997). Epochs were of 3700 ms duration, with 0 ms defined as the onset of the first stimulation and the baseline being the −700 to −300 ms window. Of note, we shifted our baseline away from the period immediately preceding stimulus onset to avoid potential contamination by any anticipatory responses, although there was no evidence of such anticipatory responses in our final analyses. Epochs containing artefacts were rejected based on a fixed threshold method, supplemented with visual inspection. On average, 70 trials per participant were used for further analysis and the average number of trials accepted did not statistically differ by group (Controls: M = 71.3, SD = 5.0; HIV: M = 69.1, SD = 7.0; HAND: M = 71.0, SD = 6.0).
Artefact-free epochs were transformed into the time-frequency domain using complex demodulation, and the resulting spectral power estimations per sensor were averaged over trials to generate time–frequency plots of mean spectral density. The sensor-level data per time–frequency bin were normalized using the mean power per frequency during the −700 to −300 ms baseline period. The specific time–frequency windows used for imaging were determined by statistical analysis of the sensor-level spectrograms across all participants. Each data point in the spectrogram was initially evaluated using a mass univariate approach based on the general linear model (GLM). To reduce the risk of false-positive results while maintaining reasonable sensitivity, a two-stage procedure was followed to control for Type 1 error (Maris and Oostenveld, 2007). Briefly, paired-sample t-tests comparing the mean baseline amplitude per frequency bin with each post-stimulation time–frequency bin were conducted and the resulting spectrogram of t-values was thresholded at P < 0.05 to define time–frequency bins containing potentially significant oscillatory responses across all participants. In stage two, time–frequency bins that survived this threshold were clustered with temporally and/or spectrally neighbouring bins that were also significant, and a cluster value was derived by summing all of the t-values of all data points in the cluster. Non-parametric permutation testing was then used to derive a distribution of cluster values and the significance level of the observed clusters (from stage one) were tested directly using this distribution (Ernst, 2004; Maris and Oostenveld, 2007). For each comparison, at least 10 000 permutations were computed to build a distribution of cluster values. Further details of this method and our processing pipeline can be found in recent papers (Kurz et al., 2017; Wiesman et al., 2017; Spooner et al., 2018, 2019).
MEG beamformer imaging
Cortical networks were imaged through the dynamic imaging of coherent sources beamformer (Van Veen et al., 1997; Gross et al., 2001), which employs spatial filters in the time–frequency domain to calculate source power for the entire brain volume. The resulting functional images reflect noise-normalized power differences (i.e. active versus passive) per voxel and are typically in pseudo-t units. MEG preprocessing and imaging used the Brain Electrical Source Analysis (Version 6.1; BESA) software. Normalized source power was computed for the selected time-frequency periods (see Results section) over the entire brain volume per participant at 4.0 mm × 4.0 mm × 4.0 mm resolution. The resulting beamformer images were then averaged across all participants to assess the neuroanatomical basis of the significant oscillatory responses identified through the sensor-level analysis.
Statistical analyses
To evaluate changes in somatosensory gating as a function of age, ‘gating maps’ were computed using whole-brain reconstructions of the first stimulation compared to the second (i.e. stimulation 1 – stimulation 2). The resulting maps reflected the individual’s capacity to ‘gate’ the second stimulus in an identical pair, with smaller values being indicative of worse gating (i.e. worse suppression of redundant stimuli). To identify regions where somatosensory gating was significantly modulated by age, whole-brain correlation maps were computed using the voxel-wise gating maps and their respective ages. These gating-by-age interaction maps were computed for each group individually (i.e. uninfected adults, unimpaired PLWH and those with HAND). From these maps, whole-brain bivariate correlation coefficient comparisons were computed using Fisher’s Z-transformation, which provided a voxel-wise map of z-scores representing the normalized difference between each group in the age/gating relationship.
To examine the effects of disease-related indices on spontaneous activity during the baseline period (i.e. −700 to −300 ms), voxel time-series data (‘virtual sensors’) were extracted from each participant’s MEG data using the peak voxel coordinate derived from the whole-brain interaction image. To compute these virtual sensors, we applied the sensor weighting matrix derived through the forward computation to the preprocessed signal vector, which yielded a time series for the specific coordinate in source space. Note that virtual sensor extraction was done per participant, once the coordinates of interest were known. Using these virtual sensor time series, we computed the envelope of the spectral power in the frequency bin used in the beamforming analysis. One-way analysis of covariance (ANCOVA: controlling for years of education) and post hoc comparisons were then conducted to evaluate changes in absolute baseline power as a function of disease. Finally, multiple regressions (controlling for years of education) were conducted among spontaneous neural activity and indices of clinical status, including CD4 nadir in unimpaired PLWH and those with HAND.
Data availability statement
All data will be deposited into the National NeuroAIDS Tissue Consortium data archive at the conclusion of the study. Further anonymized data can be made available to qualified investigators upon reasonable request to the corresponding author.
Results
Of the 254 participants who were originally enrolled, 44 were excluded at the evaluation phase due to moderate or severe depression and/or current substance abuse. In addition, 13 controls scored in the impaired range on our neuropsychological battery and were excluded, and an additional 18 participants were excluded due to missing or artefactual imaging data. The remaining sample of 179 participants was comprised of 53 unimpaired PLWH, 34 adults with HAND and 92 uninfected controls (see Fig. 1). The groups did not significantly differ by age (P = 0.372), sex (P = 0.964) or handedness (P = 0.577), but did differ in education level (P < 0.001) and we controlled for this variable in all analyses. Time since diagnosis, CD4 and CD4 nadir were collected at the time of the MEG session for unimpaired PLWH and adults with HAND (see Table 1). The HIV-infected groups did not significantly differ in time since diagnosis (P = 0.225), CD4 nadir (P = 0.553) or current CD4 count (P = 0.926). Finally, of the participants with HAND, 23 met diagnostic criteria for asymptomatic neurocognitive impairment, 7 for mild neurocognitive disorder and 4 for HIV-associated dementia.
Table 1.
Participant demographics
| Controls | PLWH | HAND | P-value | |
|---|---|---|---|---|
| N | 92 | 53 | 34 | |
| Age (years) | 45.4 | 47.6 | 49.1 | 0.372 |
| Gender (n males) | 50 | 31 | 18 | 0.964 |
| Handedness (n right-handed) | 78 | 48 | 31 | 0.577 |
| Time since diagnosis (years) | 11.3 | 13 | 0.225 | |
| CD4 (cells/μl) | 750 | 759 | 0.926 | |
| CD4 nadir (cells/μl) | 237.5 | 218 | 0.553 |
Sensor- and source-level analyses
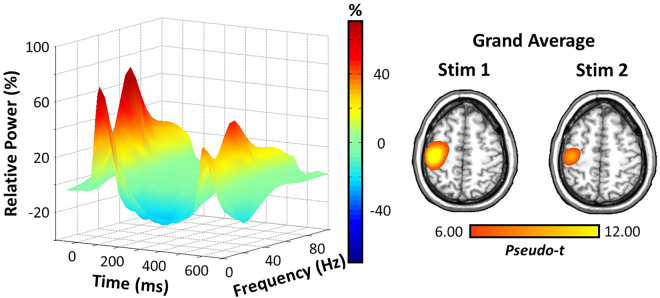
Robust broadband (10–90 Hz) synchronizations were found across many MEG sensors near the sensorimotor and parietal regions during the first 100 ms after the onset of each electrical stimulation (P < 0.001, corrected; Fig. 2). The strongest high-frequency responses (i.e. > 20 Hz) were clearly during the first 50 ms after stimulus onset. Thus, we focused our beamformer analyses on the higher 20–75 Hz frequency range and utilized two 50 ms time intervals in which the neural response to stimulation was the strongest (0–50 ms and 500–550 ms). Note that our main analyses were limited on the low end to 20 Hz, as this was the lowest frequency that we could precisely resolve using a time window of 50 ms, and to 75 Hz on the high end because relative power sharply decreased thereafter, especially in response to the second stimulus. Beamformer images revealed peak responses in the contralateral somatosensory hand region of the postcentral gyrus, with virtually identical peak locations in response to the first and second stimulations across participants (Fig. 2).
Figure 2.
Neural responses to somatosensory stimulation of the right median nerve. (Left) A 3D spectrogram of a MEG sensor near the sensorimotor cortices illustrating the somatosensory spectral responses to paired-pulse electrical stimulation. Time is shown on the x-axis (ms), relative power (%) on the y-axis and frequency (Hz) is shown on the z-axis. All signal power data are expressed as percent change from baseline (−700 to −300 ms), and the corresponding colour scale bar is displayed to the right of the graphic. (Right) Grand-averaged beamformer images (pseudo-t) for stimulation 1 and stimulation 2 across all groups. Strong increases in power were found in virtually identical areas of the contralateral hand region of the somatosensory cortex for both stimulations.
Disease-related alterations in somatosensory processing as a function of age
To identify brain regions where neuroHIV and cognitive status modulated the relationship between somatosensory gating and age, we computed voxel-wise ‘gating maps’ (i.e. stimulation 1 – stimulation 2) and performed whole-brain correlations using these maps and participant age for each group. We then applied Fisher’s Z-transformation to these whole-brain correlation maps and found that there was a stronger negative correlation between somatosensory gating and age in the left DLPFC of adults with HAND compared to both unimpaired PLWH and uninfected controls (P < 0.05, corrected; Fig. 3). The results also indicated significant gating/age correlations in the left postcentral gyrus (i.e. S1 cortices) across all three groups (P < 0.05, corrected), but this relationship did not differ by neuroHIV or cognitive status.
Figure 3.
Correlation between gating and age. Gating maps (stimulation 1 − stimulation 2) were computed for each participant and these were correlated with age in each group. Fisher’s Z-transformation was then used to identify regions where the correlation between gating and age significantly differed by group. In each plot, age in years is plotted on the x-axis and the gating index in pseudo-t units is plotted on the y-axis, with the line of best-fit overlaid on each graph for each group. (Left) Adults with HAND (red) exhibited a strong negative correlation between gating and age in the left DLPFC, and this correlation statistically differed from controls (blue). (Middle) Same as the left except now the significant difference between adults with HAND (red) and unimpaired PLWH (black) is shown. (Right) Same as the previous two maps/plots, except the data for unimpaired PLWH and controls is shown; these groups did not differ.
Alterations in spontaneous activity in HIV-infected adults
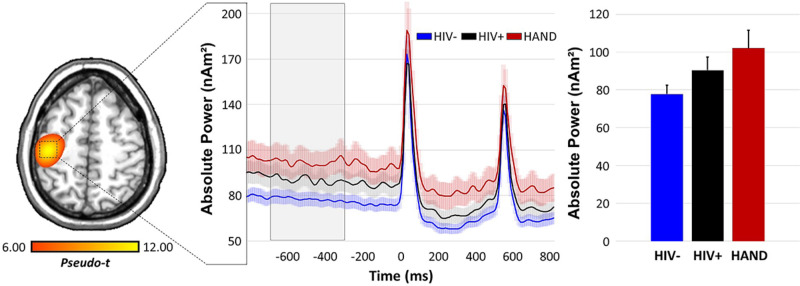
To evaluate whether spontaneous neural activity preceding stimulus onset differed as a function of group, we computed the absolute power time series (i.e. not baseline corrected; Fig. 4) for the grand-averaged peak voxel in the contralateral somatosensory cortex, as well as the same left DLPFC voxels as were observed in the whole-brain correlational analyses described above. We then computed the mean power during the baseline period (−700 to −300 ms) in each region per participant and conducted a one-way ANCOVA per region to examine group effects controlling for years of education. In the somatosensory cortex, this ANCOVA was significant [F(177) = 5.65, P = 0.004] and post hoc comparisons revealed that 20–75 Hz spontaneous gamma power was significantly stronger in participants with HAND relative to controls, t(125) = 2.53, P = 0.013, but not elevated compared to unimpaired PLWH, t(86) = −0.99, P = 0.322; further, controls and unimpaired PLWH did not statistically differ, t(144) = –1.56, P = 0.121 (Fig. 4). In regard to the left DLPFC, a one-way ANCOVA using the peak voxel from the unimpaired PLWH/HAND comparison above indicated that there were significant group differences in 20–75 Hz spontaneous gamma in this region [F(177) = 6.74, P = 0.002], and post hoc comparisons revealed that spontaneous gamma was elevated in adults with HAND compared to unimpaired PLWH, t(86) = 2.32, P = 0.023, and uninfected controls, t(125) = −3.60, P < 0.001 (Fig. 5). Spontaneous gamma activity in this region did not statistically differ in the two unimpaired groups (PLWH and controls), t(144) = 1.20, P = 0.232.
Figure 4.
Spontaneous neural activity preceding stimulation distinguishes groups. (Left) Absolute voxel time-series data were extracted from the contralateral primary somatosensory cortex of each participant using the peak voxel in the grand-averaged image. (Middle) These time series were averaged by group and are plotted as a function of time. (Right) Neural activity during the baseline period (−700 to −300 ms) was averaged in each participant, and independent samples t-tests were conducted. These revealed significantly elevated gamma activity during the baseline period in HAND participants (shown in red) compared to controls (P = 0.013, blue), but not unimpaired PLWH (P = 0.322, black), and no differences between unimpaired PLWH and control participants (P = 0.121).
Figure 5.
Disease-related alterations in prefrontal spontaneous gamma activity. (Left) Peak voxel extraction from Fisher’s Z comparisons of the gating/age relationship in unimpaired PLWH and those with HAND. Independent samples t-tests revealed significantly elevated gamma activity during the baseline period (−700 to −300 ms) in HAND participants (shown in red) compared to controls (P < 0.001, blue) and unimpaired PLWH (P = 0.023, black). Baseline gamma power was not significantly different between unimpaired PLWH and uninfected controls (P = 0.232). (Right) Pearson correlation revealed a significant association among spontaneous neural activity in the left DLPFC and CD4 nadir such that, as spontaneous gamma activity increased, CD4 nadir count decreased across all PLWH (with and without HAND).
Relationship to HIV metrics
Finally, to determine if neural metrics of somatosensory processing were related to HIV clinical history, multiple regressions (controlling for years of education) were conducted among CD4 nadir count, time since diagnosis, gating index and spontaneous gamma activity preceding stimulus onset in both the contralateral primary somatosensory cortex and the left DLPFC. In regard to sensory gating, there was no significant association between gating and CD4 nadir (P’s > 0.888) or time since diagnosis (P’s > 0.193) in the somatosensory or prefrontal cortices. In regard to spontaneous gamma activity, there was a marginally significant negative association among baseline gamma in the primary somatosensory cortex and CD4 nadir (P = 0.097), as well as a robust relationship amongst these variables in the left DLPFC, [R(85) = −0.33, P = 0.009; Fig. 5]. In both cases, higher baseline gamma was associated with lower CD4 nadir across both HIV-infected groups. Lastly, no association among spontaneous gamma and time since diagnosis was evident in the somatosensory cortex (P = 0.663) or left DLPFC (P = 0.846).
Discussion
The goal of the current study was to evaluate the differential impact of aging on inhibitory processing in PLWH and uninfected controls. Through analysis of whole-brain and voxel time-series data, we observed robust somatosensory responses in the gamma range (20–75 Hz) following electrical stimulation of the right median nerve. Interestingly, the impact of age on gating was similar across all three groups in the primary somatosensory (S1) cortices, but significantly differed in those with HAND relative to both unimpaired PLWH and controls in the prefrontal cortices. In addition, given recent findings of altered spontaneous gamma activity preceding stimulus onset in aging adults, we evaluated this metric and found elevations in baseline gamma power in participants with HAND, and determined that these elevations were significantly related to CD4 nadir. The implications of these novel findings are discussed below.
As stated in the Introduction section, the neurophysiological signature of sensory gating is the attenuated neural response to repetitive sensory input and this is thought to reflect the capacity of the CNS to filter irrelevant information and preserve neural resources for behaviourally relevant stimuli (Cromwell et al., 2008). This process has been studied extensively in the auditory and somatosensory domains, with recent work focused on its modulation by the healthy aging process (Spooner et al., 2019). Such studies have suggested that older adults exhibit reduced gating of irrelevant or identical information relative to healthy younger adults (Lenz et al., 2012; Cheng and Lin, 2013; Spooner et al., 2019). Interestingly, emerging evidence in the neuroHIV literature suggests that PLWH may exhibit accelerated aging phenotypes (Appay and Rowland-Jones, 2002; Rickabaugh et al., 2015; Gross et al., 2016). For example, in a recent DNA methylation study of recent and chronic HIV-infection, PLWH showed a relative age advancement of ∼5 years compared to uninfected controls regardless of disease duration, and further, this biological age advancement was significantly related to indices of disease progression (i.e. CD4/CD8 T cell ratio) (Gross et al., 2016). Given such data, one might expect the age-related decline in sensory gating observed in healthy adults to be accentuated in PLWH, and this was a major factor in motivating the current study, which used whole-brain correlational analyses to identify regions where age-related changes in somatosensory gating uniquely distinguished PLWH from controls. Of particular interest, we found that gating decreased with age across all participants in the left somatosensory cortex, and that such age-related changes in the DLPFC significantly differed by group. Specifically, we found that sensory gating in the DLPFC was robust only in younger participants with HAND, and that this gating sharply declined with age. In contrast, gating in this brain region was much weaker in unimpaired PLWH and controls and was not significantly modulated by age. These findings may indicate that younger adults utilize potential compensatory mechanisms such as top-down modulation via DLPFC to effectively gate redundant stimuli. In other words, DLPFC hyperactivity in the gamma range may allow young participants with HAND to gate somatosensory responses, while this mechanism may become exhausted as they get older.
These results align nicely with a well-known extension of the compensation hypothesis of neurocognitive aging (i.e. the compensation-related utilization of neural circuits hypothesis) (Reuter-Lorenz and Cappell, 2008). The compensation-related utilization of neural circuits hypothesis suggests that in times of lower cognitive demand, older adults recruit larger volumes of tissue or increase the degree of activation to aid in task performance relative to their younger counterparts. In contrast, during times of higher cognitive demand, older adults have exhausted such resources, leading to poorer task performance while younger adults engage the same mechanisms to support the increased task demands. This hypothesis has been corroborated by a host of neuroimaging studies showing age-related compensation in prefrontal cortices across a variety of tasks (Cabeza et al., 2002; Mattay et al., 2006; Cappell et al., 2010). Of note, the involvement of the DLPFC during sensory processing and gating is not new, as lesion studies have linked impaired function of the prefrontal cortex with impaired processing and suppression of sensory information (Yamaguchi and Knight, 1990; Knight et al., 1999). Further, task-based neuroimaging studies in healthy adults have revealed that increases in prefrontal and somatosensory activity coincide with directing attention to tactile stimuli (Staines et al., 2002; Schaefer et al., 2005). There is also a previous MEG investigation that found aberrant prefrontal activity in PLWH relative to uninfected controls during the processing of tactile stimulation (Wilson et al., 2015). The latter study did not divide PLWH by cognitive status (i.e. presence or absence of HAND) nor investigate aging, but their findings are of a major interest given the current data. Finally, it is worth noting that prefrontal generators have also been a well-characterized component of auditory gating (Grunwald et al., 2003; Williams et al., 2011; Cheng et al., 2015a). Taken together, these findings implicate the prefrontal cortices in the active processing and gating of sensory responses, and our study is the first to report age-related alterations in such activity that differ based on cognitive status in neuroHIV.
A final novel contribution to this literature is our investigation of spontaneous neural activity as a function of cognitive impairment and clinical status in HIV-infection. Most investigations that have examined this neural metric have examined its modulation by healthy aging. Briefly, two recent studies reported elevated spontaneous beta activity in the primary motor cortices prior to movement onset in healthy aging adults, and further, this elevation in baseline power was significantly linked to movement-related beta oscillatory activity and behavioural performance (Rossiter et al., 2014; Heinrichs-Graham and Wilson, 2016). A third study found a similar relationship among spontaneous gamma activity and healthy aging in the somatosensory cortex, such that older adults had significantly elevated baseline power relative to younger adults (Spooner et al., 2019). Importantly, this study was the first to provide evidence that spontaneous gamma power preceding stimulus onset directly modulated age-related declines in somatosensory processing and gating (Spooner et al., 2019). Finally, another recent study probed the impact of HIV-infection on spontaneous neural activity preceding somatosensory stimulation and found that young and middle-aged PLWH exhibited elevated baseline gamma power, which was reminiscent of the levels previously reported in much older controls (Spooner et al., 2018; Spooner et al., 2019). In conjugate, these studies suggest that spontaneous neural activity in sensorimotor cortices increases with healthy aging, and that such increases happen earlier in PLWH, which provides new support to the notion of premature or accelerated aging in neuroHIV. Further, these modifications in spontaneous cortical activity appear to be directly linked to task-relevant neural oscillations across the sensorimotor network (Rossiter et al., 2014; Heinrichs-Graham and Wilson, 2016). Expanding upon previous work, the current study also found alterations in spontaneous power within the left DLPFC, where significant gating/age relationships were observed, and this had not been previously reported. Importantly, this increase was not only modulated by HIV-infection, but was specifically related to CD4 nadir across all PLWH. With increasing evidence suggesting that CD4 nadir is predictive of cognitive impairment in these participants (Tozzi et al., 2005; Muñoz-Moreno et al., 2008; Ellis et al., 2011; Panel on Antiretroviral Guidelines for Adults and Adolescents, 2017), the need for early diagnosis and treatment initiation is clear and of utmost importance.
In conclusion, the current study quantified the adult lifespan trajectory of disease-related alterations in somatosensory processing and gating in unimpaired PLWH, adults with HAND, and healthy controls. Our most important finding was that young participants with HAND recruit additional neural resources (i.e. the prefrontal cortex) to gate redundant sensory stimuli, and that this probable compensatory mechanism becomes exhausted by middle age. This mechanism not only implicates the prefrontal cortex in age-related alterations of sensory gating but also in the differentiation of HIV-infected adults living with and without cognitive impairment. While previous work has implicated prefrontal involvement in sensory processing (Yamaguchi and Knight, 1990; Knight et al., 1999; Staines et al., 2002; Schaefer et al., 2005; Wilson et al., 2015), the current study was the first to link this activity to gating in the context of HIV, and to identify its unique role in those with HAND versus unimpaired PLWH. Additionally, we observed significant increases in spontaneous gamma activity within sensorimotor cortices as a function of age, which was accentuated in PLWH. Of note, spontaneous gamma activity in the left DLPFC distinguished participants with HAND from PLWH who were unimpaired, and thus may hold promise as a potential neural marker of HAND. Such a marker could help address current limitations in the diagnosis and treatment of cognitive dysfunction in PLWH, but future studies are definitely needed to assess the sensitivity and specificity of this metric before moving forward. Before closing, it is important to acknowledge several possible limitations of the study. First, neural processing of somatosensory input like that used in our study includes relays in the thalamus and primary somatosensory cortex, as well as the potential involvement of the basal ganglia through cortical-basal ganglia-cortical loops (McCormick and Bal, 1994; Seki and Fetz, 2012; Colder, 2015; Lei et al., 2018; Conte et al., 2020; D’Antonio et al., 2019). Given this, we cannot rule out that gating like that observed in the primary somatosensory and DLPFC of this study also occurs in subcortical regions such as the basal ganglia. While we did not find evidence of this using our whole-brain beamforming approach, we cannot definitely rule it out either and future studies should keep this in mind. In addition, there is evidence of altered temporal discrimination abilities in some neurological conditions (e.g. D’Antonio et al., 2019), and such altered processing could contribute to gating and similar aberrations in these populations. Future studies should focus on teasing apart these inter-related constructs. To close, about half of the PLWH in the United States are over 50 years old (Kamkwalala and Newhouse, 2017), and this number is expected to grow. Thus, investigations of aging phenotypes, and mechanisms of cognitive and accelerated aging are paramount to increase our understanding of neural processing and cognitive decline in neuroHIV.
Funding
This work was primarily supported by the National Institute of Mental Health (MH103220, MH116782, MH118013, and MH062261), the National Institute for Aging (AG055332), the National Institute of General Medical Sciences (GM130447), and the National Science Foundation (#1539067). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests
The authors report no competing interests.
Acknowledgements
The authors would like to acknowledge the enormous contribution of Kevin R. Robertson, PhD, professor of neurology and director of the AIDS Neurological Center at the University of North Carolina at Chapel Hill. Dr. Robertson designed and analysed all the neuropsychological testing, and sadly died during the conduct of the study. We would also like to thank Kathryn L. Losh for her help with the graphical abstract. Finally, we would like to thank our participants for volunteering.
Glossary
- cART =
combination antiretroviral therapy
- HAND =
HIV-associated neurocognitive disorder
- MEG =
magnetoencephalography
- MRI =
magnetic resonance imaging
- PLWH =
persons living with HIV
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69: 1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Rowland-Jones SL.. Premature ageing of the immune system: the cause of AIDS? Trends Immunol 2002; 23: 580–5. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK.. Beck depression inventory-II, Vol. 78 San Antonio: Psychological Corporation; 1996. p. 9. [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR.. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage 2002; 17: 1394–402. [DOI] [PubMed] [Google Scholar]
- Cappell KA, Gmeindl L, Reuter-Lorenz PA.. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex 2010; 46: 462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CH, Baillet S, Lin YY.. Region-specific reduction of auditory sensory gating in older adults. Brain Cogn 2015. a; 101: 64–72. [DOI] [PubMed] [Google Scholar]
- Cheng CH, Chan PY, Baillet S, Lin YY.. Age-related reduced somatosensory gating is associated with altered alpha frequency desynchronization. Neural Plast 2015. b; 2015: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CH, Lin YY.. Aging-related decline in somatosensory inhibition of the human cerebral cortex. Exp Brain Res 2013; 226: 145–52. [DOI] [PubMed] [Google Scholar]
- Colder B. The basal ganglia select the expected sensory input used for predictive coding. Front Comput Neurosci 2015; 9: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte A, Giannì C, Belvisi D, Cortese A, Petsas N, Tartaglia M, et al. Deep grey matter involvement and altered sensory gating in multiple sclerosis. Mult Scler 2020; 26: 786–94. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Mears RP, Wan L, Boutros NN.. Sensory gating: a translational effort from basic to clinical science. Clin EEG Neurosci 2008; 39: 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ.. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychol Rev 2009; 19: 169–85. [DOI] [PubMed] [Google Scholar]
- D’Antonio F, De Bartolo MI, Ferrazzano G, Trebbastoni A, Amicarelli S, Campanelli A, et al. Somatosensory temporal discrimination threshold in patients with cognitive disorders. J Alzheimers Dis 2019; 70: 425–32. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, et al. ; CHARTER Group. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 2011; 25: 1747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MD. Permutation methods: a basis for exact inference. Statist Sci 2004; 19: 676–85. [Google Scholar]
- Gross AM, Jaeger PA, Kreisberg JF, Licon K, Jepsen KL, Khosroheidari M, et al. Methylome-wide analysis of chronic HIV infection reveals five-year increase in biological age and epigenetic targeting of HLA. Mol Cell 2016; 62: 157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R.. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci USA 2001; 98: 694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald T, Boutros NN, Pezer N, von Oertzen J, Fernández G, Schaller C, et al. Neuronal substrates of sensory gating within the human brain. Biol Psychiatry 2003; 53: 511–9. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, et al. ; For the CHARTER Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75: 2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. ; for the CHARTER and HNRC Groups. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW.. Is an absolute level of cortical beta suppression required for proper movement? Magnetoencephalographic evidence from healthy aging. Neuroimage 2016; 134: 514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamkwalala A, Newhouse P.. Mechanisms of cognitive aging in the HIV-positive adult. Curr Behav Neurosci Rep 2017; 4: 188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RT, Staines WR, Swick D, Chao LL.. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychol (Amst) 1999; 101: 159–78. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Wiesman AI, Coolidge NM, Wilson TW.. Children with cerebral palsy hyper-gate somatosensory stimulations of the foot. Cereb Cortex 2017; 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Ozdemir RA, Perez MA.. Gating of sensory input at subcortical and cortical levels during grasping in humans. J Neurosci 2018; 38: 7237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz M, Tegenthoff M, Kohlhaas K, Stude P, Höffken O, Gatica Tossi MA, et al. Increased excitability of somatosensory cortex in aged humans is associated with impaired tactile acuity. J Neurosci 2012; 32: 1811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner AM, Eisinger RW, Fauci AS.. Comorbidities in persons with HIV: the lingering challenge. JAMA 2020; 323: 19. [DOI] [PubMed] [Google Scholar]
- Leung V, Gillis J, Raboud J, Cooper C, Hogg RS, Loutfy MR, et al. ; the CANOC Collaboration. Predictors of CD4:CD8 ratio normalization and its effect on health outcomes in the era of combination antiretroviral therapy. PLoS One 2013; 8: e77665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R.. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 2007; 164: 177–90. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, et al. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett 2006; 392: 32–7. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Bal T.. Sensory gating mechanisms of the thalamus. Curr Opin Neurobiol 1994; 4: 550–6. [DOI] [PubMed] [Google Scholar]
- Muñoz-Moreno JA, Fumaz CR, Ferrer MJ, Prats A, Negredo E, Garolera M, et al. Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res Hum Retroviruses 2008; 24: 1301–7. [DOI] [PubMed] [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Human Services. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- Reuter-Lorenz PA, Cappell KA.. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci 2008; 17: 177–82. [Google Scholar]
- Rickabaugh TM, Baxter RM, Sehl M, Sinsheimer JS, Hultin PM, Hultin LE, et al. Acceleration of age-associated methylation patterns in HIV-1-infected adults. PLoS One 2015; 10: e0119201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 2007; 21: 1915–21. [DOI] [PubMed] [Google Scholar]
- Rossiter HE, Davis EM, Clark EV, Boudrias MH, Ward NS.. Beta oscillations reflect changes in motor cortex inhibition in healthy ageing. Neuroimage 2014; 91: 360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens A, Sacktor N, Haughey N, Slusher B, Pletnikov M, et al. HIV-associated neurocognitive disorder—pathogenesis and prospects for treatment. Nat Rev Neurol 2016; 12: 234–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M, Heinze HJ, Rotte M.. Task-relevant modulation of primary somatosensory cortex suggests a prefrontal-cortical sensory gating system. Neuroimage 2005; 27: 130–5. [DOI] [PubMed] [Google Scholar]
- Seki K, Fetz EE.. Gating of sensory input at spinal and cortical levels during preparation and execution of voluntary movement. J Neurosci 2012; 32: 890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014; 10: e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 2010; 24: 1243–50. [DOI] [PubMed] [Google Scholar]
- Spooner RK, Wiesman AI, Mills MS, O'Neill J, Robertson KR, Fox HS, et al. Aberrant oscillatory dynamics during somatosensory processing in HIV-infected adults. Neuroimage Clin 2018; 20: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Wiesman AI, Proskovec AL, Heinrichs-Graham E, Wilson TW.. Rhythmic spontaneous activity mediates the age-related decline in somatosensory function. Cereb Cortex 2019; 29: 680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staines WR, Graham SJ, Black SE, McIlroy WE.. Task-relevant modulation of contralateral and ipsilateral primary somatosensory cortex and the role of a prefrontal-cortical sensory gating system. Neuroimage 2002; 15: 190–9. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J.. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 2006; 51: 1759–68. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Lorenzini P, Bellagamba R, Galgani S, Corpolongo A, et al. Prevalence and risk factors for human immunodeficiency virus-associated neurocognitive impairment, 1996 to 2002. J Neurovirol 2005; 11: 265–73. [DOI] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ.. Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput 1997; 35: 135–40. [DOI] [PubMed] [Google Scholar]
- Van Veen B, van Drongelen W, Yuchtman M, Suzuki A.. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 1997; 44: 14. [DOI] [PubMed] [Google Scholar]
- Wiesman AI, Heinrichs-Graham E, Coolidge NM, Gehringer JE, Kurz MJ, Wilson TW.. Oscillatory dynamics and functional connectivity during gating of primary somatosensory responses. J Physiol 2017; 595: 1365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Nuechterlein KH, Subotnik KL, Yee CM.. Distinct neural generators of sensory gating in schizophrenia. Psychophysiology 2011; 48: 470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Becker KM, Aloi J, Robertson KR, Sandkovsky U, et al. Multimodal neuroimaging evidence of alterations in cortical structure and function in HIV-infected older adults. Hum Brain Mapp 2015; 36: 897–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT.. Gating of somatosensory input by human prefrontal cortex. Brain Res 1990; 521: 281–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data will be deposited into the National NeuroAIDS Tissue Consortium data archive at the conclusion of the study. Further anonymized data can be made available to qualified investigators upon reasonable request to the corresponding author.