Abstract
Healthy central nervous system (CNS) development and function require an intricate and balanced bidirectional communication between neurons and glia cells. In this review, we discuss the complementary roles of astrocytes and microglia in building the brain, including in the formation and refinement of synapses. We discuss recent evidence demonstrating how these interactions are coordinated in the transition from healthy physiology towards disease and discuss known and potential molecular mechanisms that mediate this cellular cross-talk.
Keywords: astrocytes, microglia, synapses, brain development, neuroimmune
The bond between astrocytes and microglia
Like many great duos, astrocytes and microglia in the central nervous system (CNS) have a unique bond that constrains and coordinates their functions. The brain is functionally dominated by its most specialized cell type – neurons -- electrically active cells evolved for intercellular communication. Yet as in other organs, brain function depends on a village of cell types whose functions are relatively conserved across multiple tissues, including vascular endothelial cells, pericytes, and myelinating oligodendrocytes. Among these varied cell types are astrocytes and microglia, who, despite their brain-specific names, are close relatives of the macrophages and stromal cells present in most organs. Like macrophages and peripheral stroma, at first glance astrocytes and microglia have little in common. They have different developmental origins: astrocytes are derived from neuroepithelial progenitors, whereas microglia are derived from a hematopoietic common myeloid progenitor that enters the brain during embryogenesis (reviewed in [1] and [2]). From a structural perspective, they differ widely. Most resting astrocytes are tissue embedded, positionally stable, and non-motile [3,4]. In contrast, microglial processes are in constant motion, even under homeostatic conditions [5–7], and when depleted, microglia will migrate broadly to repopulate the CNS [8–10].
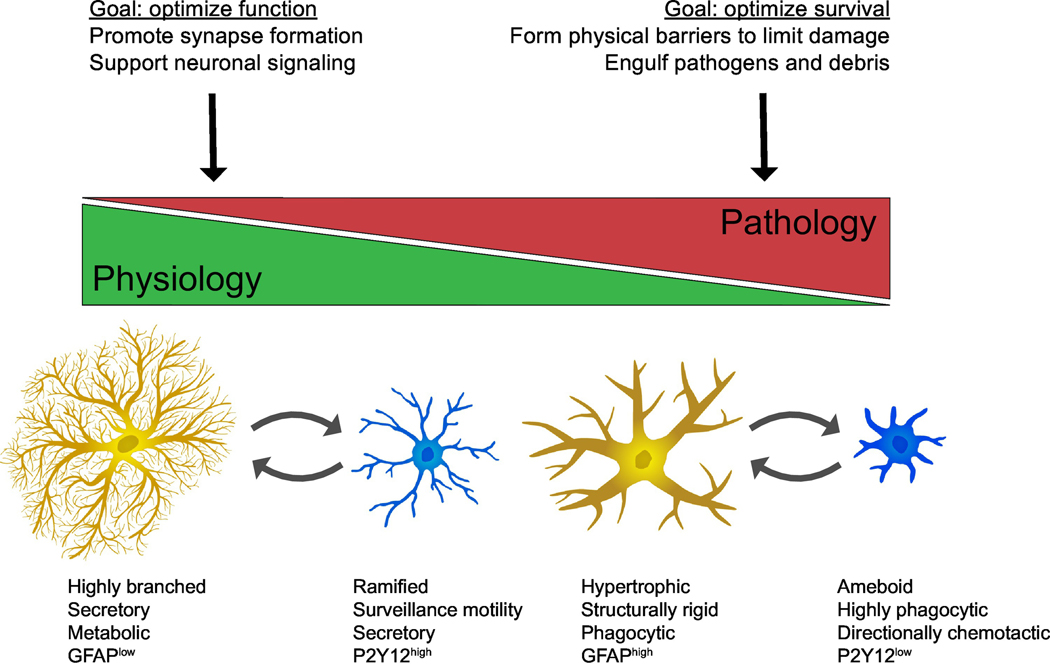
Yet despite their differences, when the brain is perturbed astrocytes and microglia seem to respond almost as one unit. Embedded in any Glial Fibrillary Acidic Protein (GFAP)-rich glial scar of hypertrophied astrocytes are increased numbers of ‘activated’ microglia. After injury, microglia rapidly increase their phagocytic capacity to remove debris, whereas astrocytes become hypertrophic to re-form the blood brain barrier, form a physical barrier to damage [11] and maintain the structural integrity of the brain (reviewed in [12]) (Figure 1). And while this coordination is particularly evident in pathology, there is emerging evidence of its importance in healthy physiology. It is increasingly evident that both astrocytes and microglia play physiologic roles, such as supporting synaptic development and remodeling.
Figure 1: Function of the astrocyte-microglia module from homeostasis to pathology.
Under physiologic conditions astrocytes (ochre) and microglia (blue) support neuronal functions, whereas in pathology they lose some of their supportive functions in favor of optimizing survival. This is accompanied by a change in morphology, secretome and increase in reactive markers and phagocytic activity.
Here we describe ways in which astrocytes and microglia coordinate their functions both in healthy physiology and pathology (Figure 2). We propose that this capacity derives both from direct communication, as well as common responses to shared environmental signals. Rather than providing a comprehensive review of the literature, we highlight examples from recent work where communication between astrocytes and microglia has been directly examined. As these studies are relatively few, we also discuss examples where both astrocytes and microglia have been studied in the same context or disease model, or when a potential signaling interaction clearly implicates both cell types. We hope to highlight the synchronized and complementary functions of astrocytes and microglia in the context of development and disease and inspire questions for future research.
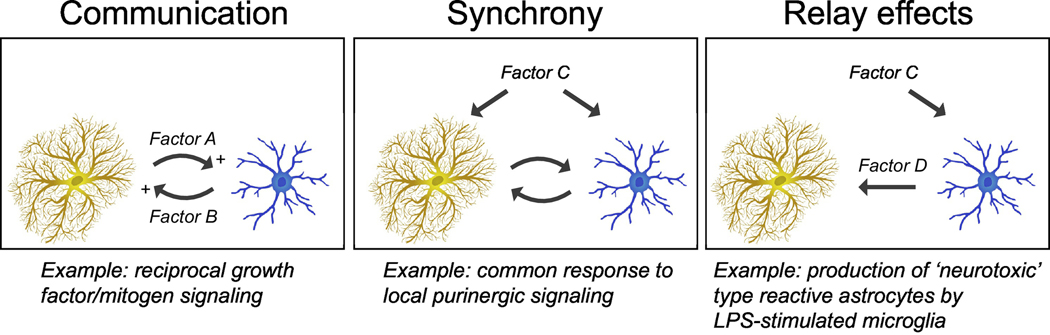
Figure 2: Mechanisms for regulating the astrocyte-microglia module.
Astrocytes (ochre) and microglia (blue) can inter-signal (factors A and B) and thereby amplify or diminish their individual or shared responses (communication). This signaling can be adjusted by ligands (factor C) that target both cell types (synchrony) or that target one cell type that indirectly alters (factor D) the other cell type (relay effects).
Astrocytes and microglia: complementary functions in neuronal development and homeostasis
Astrocytes and microglia promote developmental synapse formation and pruning
Both astrocytes and microglia play critical roles in neural circuit formation during development, particularly in support of synapse formation and remodeling. Astrocytes surround and contact most neuronal synapses, as well as forming the borders of the brain and vasculature. Studies over the past two decades have identified multiple molecules released from astrocytes that promote neuronal synapse formation. These include glypicans 4 and 6, thrombospondin 1 and 2, and hevin (Sparcl1) (reviewed in [4,13]). For example, hevin stabilizes synaptic interactions via binding to neuroligins, adhesion molecules that promote neuronal synapse assembly [14]. As a group, the molecules identified so far have been shown to promote both presynaptic assembly as well as postsynaptic maturation.
While microglia can also promote synapse formation in adulthood and during cortical critical periods, most evidence suggests that their developmental role is primarily phagocytic. They engulf apoptotic neuronal corpses [15,16], phagocytose synapses [17] and other components of the neuropil. Notable examples of their synaptic roles include engulfment of presynaptic afferents in the visual thalamus via complement [18] and removal of dendritic spines in the hippocampus dependent on Trem2 signaling [19]. Interestingly, astrocytes have also been reported to prune synapses and engulf dying cells via the scavenger receptors Multiple EGF Like Domains 10 (Megf10) and MER Receptor Tyrosine Kinase (Mertk) [20], and the drosophila homolog of Megf10 (Draper) that performs analogous roles [21,22]
Do astrocytes and microglia coordinate their synapse formation and pruning functions? One study found that astrocytes can secrete transforming growth factor β (TGF-β) that may positively regulate C1q expression and promote microglial phagocytosis [23]. Work from our group has shown that the cytokine Interleukin-33, produced by developing astrocytes, directly increased microglial phagocytic ability [24]. Astrocyte-IL-33 expression was increased with synaptic maturation suggesting a homeostatic loop by which astrocyte responses to increased synapse numbers promoted microglial synapse pruning. In peripheral tissues, macrophages can direct phagocytosis by non-professional phagocytes by releasing insulin-like growth factor 1 (IGF-1) [25], raising the possibility that microglial IGF-1 [26] could similarly promote astrocyte pruning in some contexts. Mechanisms that regulate direct communication between astrocytes and microglia may be important to appropriately balance synapse numbers during development.
Another potential mediator of astrocyte-microglia-synapse interaction is the extracellular matrix (ECM). Because the ECM constitutes a substantial amount of brain volume (~20%) [27] remodeling of synapses may also involve remodeling of the ECM around them. Macrophages and stromal cells throughout the body coordinate production and remodeling of the ECM (reviewed in [28,29]), raising the question of how microglia and astrocytes (brain resident stroma) might reflect this conserved cellular motif. Interestingly, most of the proteins linked to astrocyte pro-synaptogenic functions are themselves ECM proteins, and astrocytes express many if not most of the ECM forming proteins in the brain [30–32]. However, data regarding microglial involvement in ECM remodeling is sparse. Peripheral macrophages express metalloproteinases that lyse ECM proteins [29], many of which are also expressed by microglia. If microglia do remodel extracellular matrix, their ability to both promote and restrict synapse formation could partly derive from a capacity to clear ECM in a way that might enable new synaptic contacts to form.
The late Roger Tsien proposed that long-term memories may be encoded by the ‘pattern of holes’ in the ECM [33]. If so, synchronization of astrocyte-microglial remodeling functions could be highly relevant for promoting cognition, for counterbalancing the increased tissue stiffness associated with aging [34], and for modulating alterations in how glia support synapses in traumatic brain injury, neurodegeneration, and other pathologies. Directly examining the synchronized function of astrocytes and microglia in the remodeling of brain structure – from synapses to the space outside them -- could yield insight into both physiology and pathology.
Astrocytes and microglia support neurotransmission and adapt to their local environment
In the adult brain, astrocytes and microglia also support homeostatic neuronal function. Astrocytes can sense neurotransmitters through their expression of glutamate receptors, GABA receptors and adrenergic receptors [35] and respond with changes in intracellular Ca2+ (reviewed in [36,37]) and are presumed to reflect responses to neuronal events, although the possible role of the vasculature is too often overlooked [38]. Reducing astrocyte Ca2+ dependent signaling by disrupting intracellular calcium leads to behavioral deficits like repetitive self-grooming due to increased GABA uptake by astrocytes [39]. Astrocytes also reuptake glutamate to regulate neuronal excitability [40], and may respond to other neurotransmitters and neuromodulators. Microglia are continuously scanning the healthy adult CNS [5–7] and form activity-dependent contact with synapses, indicating that like astrocytes, they can sense neuronal activity (reviewed in [41]). Microglia also induce synapse formation after contact with dendrites, and in some cases, are linked to the formation of spine head filopodia-markers of spine plasticity [42,43]. Like astrocytes, microglia have also been shown to impact neuronal activity, in some cases providing a ‘soothing touch’ that quiets neuronal activity [44] (reviewed in [45]). Thus, both astrocytes and microglia respond to neurotransmission and can modulate it.
One potential molecule that could mediate this synchrony in astrocyte-microglial function is norepinephrine, a neuromodulator which is essential to arousal, wakefulness, and sympathetic activation [46]. Multiple noradrenergic receptors are expressed by glia. Astrocytes express α−1 adrenergic receptors that are necessary for calcium activity [47] and β−1 receptors that promote astrocyte process growth [48]. Microglia, in contrast, express extremely high levels of the β−2 adrenergic receptor [24,30]. Norepinephrine acutely inhibits microglial homeostatic motility [49,50] and may instead increase the formation of fine actin-based filopodia [51]. The functional implications of these various motility, morphology, and calcium changes are unclear. However, the impact of norepinephrine on both astrocytes and microglia emphasizes that studying these cells as a functional unit in response to shared signaling molecules may yield novel clues about how environment shapes brain function.
As is clear from the examples above, astrocytes and microglia both sense and respond to their neural environment. As such, heterogeneity in neuronal function may in part drive glial heterogeneity. Astrocytes are heterogeneous at the transcriptomic and functional level [52–55] partly in response to neuron-derived molecules such as sonic hedgehog [56]. Signals like TGF-beta [57,58] and other molecules [59–62] also shape the microglial transcriptome as distinct from peripheral macrophages. However, microglial heterogeneity within the homeostatic adult brain has so far been less evident at the transcriptomic level [63–65] even though evidence that microglia have region-specific functions strongly suggest that they can respond to their local environment in a context-dependent manner [66,67]. Importantly, however, regional cues that determine glial heterogeneity can be very rapidly lost with isolation, particularly when cells are cultured ex vivo [57,60,68,69]. It is possible that microglial identity is dynamic enough that current transcriptional profiling via cell isolation cannot accurately capture it. And of course, our understanding of the proteome and other important indicators of function lags far behind. However, given at least two examples of how astrocytes regulate microglial function during development (IL-33 and TGF-β), it is also interesting to speculate that astrocyte heterogeneity in response to neuronal cues could be one mechanism that drives heterogeneous responses of adult microglia in vivo.
Balancing cellular proportions
How are astrocyte and microglia numbers maintained? As positionally stable stromal cells of the brain, astrocytes are an often-overlooked source of tissue resident signals. Microglial depletion pharmacologically or genetically leads to a robust proliferative and migratory response that normalizes cell numbers within weeks [8–10]. These new microglia closely resemble their native counterparts, but can only fully adopt a microglial identity when expanding from a local source or yolk sac derived macrophages [70–74]. Are astrocytes a source of local cues that drive microglial repopulation? One study proposes that fibroblasts (in vitro) determine macrophage numbers via the release of the essential mitogen colony-stimulating factor 1 (CSF-1), and that a negative feedback loop from macrophages limits proliferation when a stable ratio is achieved [75]. Another study in the retina found that microglia control astrocyte numbers by actively engulfing them, although the molecular mediators are not clear [76]. If reciprocal signaling between brain astrocytes and microglia maintains their relative proportions, one might expect that astrocytes would dynamically respond to the loss of microglia. Transcriptional profiling of astrocytes after microglial depletion would be a straightforward first step to investigating this.
Molecular mechanisms of astrocyte microglial communication in pathology
Recent work has begun to reveal several molecular mechanisms by which astrocyte and microglial function is synchronized, and more often than not, these mechanisms are studied in the context of pathology. In the sections below, we will review some of the signaling molecules that coordinate the function of astrocytes and microglia across a range of perturbations (Figure 3). We focus on selected examples where recent literature indicates direct interactions between astrocytes and microglia, or where both cell types have been studied in the same model system, providing clues to how they might synchronize pathologic responses.
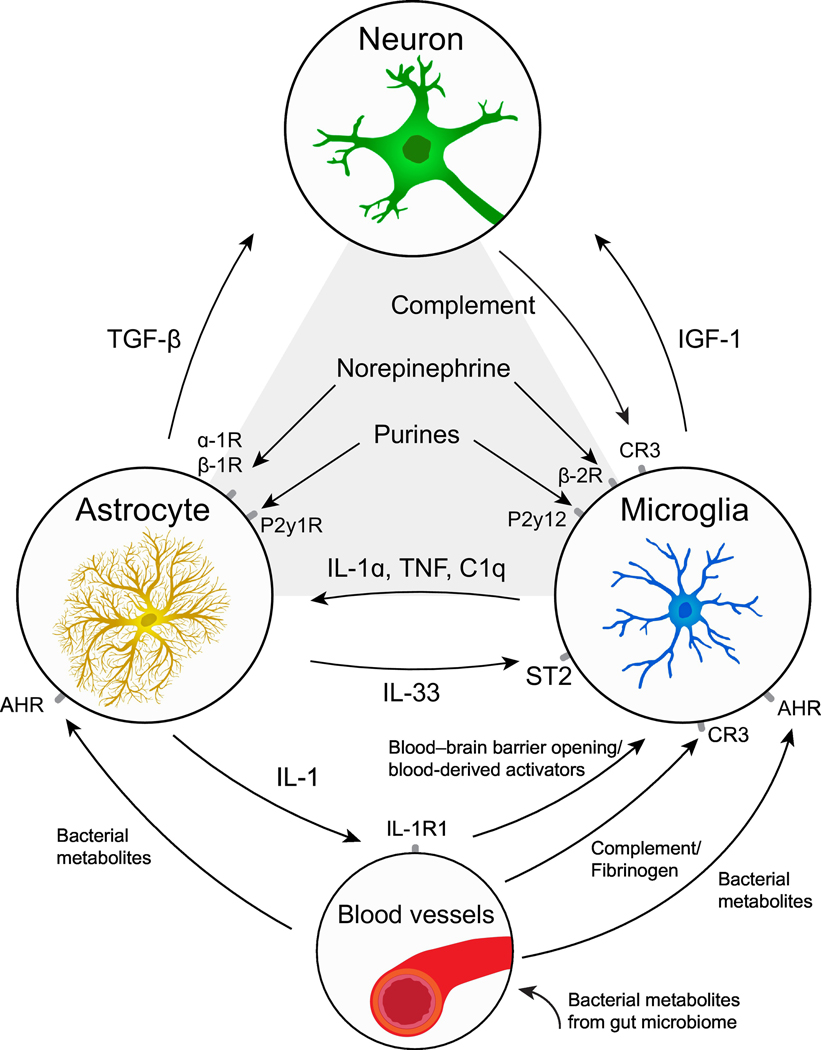
Figure 3: Molecules involved in astrocyte-microglia communication.
Examples of the types of shared signaling pathways described in Figure 2 are shown here. Molecules that induce synchronous responses in both astrocytes and microglia via distinct receptors include norepinephrine and purines. More complex interactions involving intermediary cells have also been described- for example, astrocyte-derived TGF-beta leads to neuronal release of complement, which acts on microglia. Astrocytes and microglia can directly impact one another via molecules like IL1a, TNF, and IL-33. Finally, blood derived signals, such as circulating bacterial metabolites and likely others can act on both astrocytes and microglia through shared receptors. Again, intermediate cell types can be relevant, for example astrocyte-derived IL-1 could alter permeability of the blood brain barrier, thereby enabling microglial activation.
Cytokine and growth factors in injury and inflammation
Cytokines are the primary lexicon of immune communication and are also an important mechanism of astrocyte-microglia cross-talk in homeostasis and disease. Although many cytokines are produced by immune cells, there are subsets of stromal or epithelial cytokines that are produced locally within tissues and play important roles in immune homeostasis. Our group has found that astrocyte-derived IL-33 has physiologic roles in synapse pruning that are required to limit excitatory synapse numbers during development [24]. This is a direct cytokine-mediated mechanism by which astrocyte-derived signals regulate microglial synaptic function. However, IL-33 is perhaps best studied as an ‘alarmin’- a signal released from dying cells that orchestrates a reparative and remodeling immune response, critical to wound healing, and implicated in the pathologic remodeling that takes place in tissue fibrosis [77]. Several recent studies have shown that IL-33 functions as an alarmin in the adult CNS, where in some cases it can be beneficial, promoting recovery in spinal cord injury, Alzheimer’s disease models, and experimental stroke [78–81].
Data from IL-33 and other contexts makes clear an important distinction between homeostatic and reactive functions of astrocyte-microglial signaling—the recruitment of other myeloid cells. As an alarmin, one of IL-33’s most potent effects is to robustly increase chemokine expression in microglia and drive recruitment of peripheral macrophages into the brain [78]. It may be that during injury, a more effective and rapid strategy for modulating microglial function is to recruit non-native macrophages into the brain. These may serve a temporary role and become functionally identical to the resident population with time. Defining the heterogeneity of myeloid cells at different levels of IL-33 signaling could yield insight into the delicate balance between healthy physiology and pathology [82].
Cytokines also mediate communication from microglia to astrocytes in inflammation. In one of the first examples of the heterogeneity of reactive glia, it was found that lipopolysaccharide (LPS) injection or ischemic injury via middle cerebral artery occlusion (MCAO) led to distinct transcriptional responses in astrocytes (termed “A1”/neurotoxic and “A2”/protective) [83]. A subsequent paper showed that the transcriptional hallmarks of these LPS induced reactive astrocytes can be recapitulated in vitro by a cocktail of IL-1α, TNF and C1q, cytokines that are released by microglia after LPS stimulation [84]. This cocktail is sufficient to induce an astrocyte phenotype that has a diminished ability to support synaptogenesis, neuronal outgrowth and survival. Unlike human astrocytes, mouse astrocytes do not express TLR-4, which is required to sense LPS [31], so it is not clear whether this mechanism is universally applicable. However, the concept that inflammation can either drive neurotoxicity or engage feedback mechanisms that promote repair has important experimental and therapeutic implications.
Purinergic signaling in physiologic and pathologic remodeling
ATP and its reaction products ADP, AMP and adenosine (collectively known as purines [85]) are released from synaptic vesicles and have been long proposed as neurotransmitters and regulators of neuron-glial communication [86,87]. Several studies have shown that astrocytes and microglia both respond to purinergic signaling. The metabotropic ATP receptor P2Y12 is one of the top microglial markers and is required for experience-dependent plasticity in the visual cortex after monocular deprivation [88]. In astrocytes, the metabotropic P2Y1 receptor promotes a propagating calcium wave [89]. These data suggest that purines may play physiologic remodeling roles in both astrocytes and microglia.
Studies of purinergic signaling in pathology are some of the few that have examined microglia and astrocytes as a functional unit. Similar to IL-33, ATP is also a well-described damage associated molecular pattern (DAMP) which is released from dead or stressed cells after injury [90]. After traumatic injury P2Y12 and ATP release are required for directed microglial movement towards the lesion and closure of the blood brain barrier [91–93], although P2Y12 is dispensable for the homeostatic ‘surveillance’ motility of microglia [7]. Acute activation of microglia by lipopolysaccharide (LPS) drives ATP release which increases spontaneous excitatory synaptic currents in hippocampal slices within minutes, an effect that requires P2Y1 receptor on astrocytes [94]. However, in the days after traumatic brain injury, activated microglia promote downregulation of P2Y1R on astrocytes. This downregulation is necessary for appropriate scar formation and to protect the remaining tissue [95]. It is interesting that both astrocytic P2Y1 and microglial P2Y12 are strongly downregulated within days after LPS and other activating stimuli [24,91]. This suggests a potential feedback loop whereby acute sensing of purines leads to a coordinated functional change in both astrocytes and microglia.
Complement cascade in Alzheimer’s Disease and viral infection
In some forms of developmental synapse elimination, the complement cascade plays a physiologic role in promoting synapse elimination by microglia [96], partly in response to astrocyte-derived signals [18,23,96]. However, complement is also an important link between the innate and adaptive immune system [97], and abundant evidence of complement involvement in neuroinflammation and neurodegeneration suggests that it may also be important in the transition from homeostatic to inflammatory microglia function. For example, inhibiting the complement cascade in mouse models of Alzheimer’s Disease (AD) reduced cognitive decline and synapse loss in mice [98,99]. Astrocytes may accelerate this transition. Astrocytes exposed to β-amyloid plaques (a pathologic hallmark of AD) release complement component C3 [100,101], suggesting that they are one source of complement that accelerates spine loss. However, complement receptors may have different ligands, including the blood protein fibrinogen, which induces spine elimination by microglia in a mouse model of AD [102]. This suggests that astrocyte-derived complement could be particularly relevant during early phases of disease when the BBB is relatively intact. Once barriers are breached and higher levels of blood derived complement ligands become available, other mechanisms may predominate.
Data from murine models of West Nile Virus (WNV) infections also suggest links between astrocytes, microglia, and the blood brain barrier. Complement-mediated removal of presynaptic terminals by microglia promotes cognitive impairment after WNV infection [103]. In parallel, West Nile virus was found to lead to IL-1 release from astrocytes which decreased adult neurogenesis, and deficiency of the IL-1 receptor enhanced recovery from WNV infection [104]. Interestingly, endothelial cells are a major source of the IL-1 receptor at rest [30] and are required for IL-1 induced microglial activation, although astrocyte sensing of IL-1 also plays direct roles in modulating neurogenesis [105]. Taken together, these data suggest two possible mechanisms of astrocyte-microglial synchrony in response to IL-1. One is that astrocytes are directly activated by IL-1 to impact neurogenesis. A second is that astrocyte derived IL-1, by permeabilizing the BBB, can lead to microglial activation which accelerates synapse loss. Again in this context, feedforward mechanisms involving breaches of the blood brain barrier that amplify an inflammatory response.
Circulating metabolites implicating the gut microbiome in Multiple Sclerosis
Once barriers are breached and blood derived factors enter the brain, a host of other modulators of the astrocyte-microglial unit come into play. These include circulating metabolites derived from the gut microbiome. It was first observed that microglia from germ free mice lack a mature phenotype, and had diminished responses to injection of LPS partly driven by short-chain fatty acids from bacteria [106,107]. In experimental autoimmune encephalomyelitis (EAE), a mouse model of Multiple Sclerosis (MS), astrocyte and microglial function may be linked by shared protective responses mediated by gut metabolites via the aryl hydrocarbon receptor (AHR). Type I interferon signaling, a known therapeutic in MS, increases astrocytic expression of AHR and is protective in EAE [108]. In parallel, AHR signaling in microglia limited inflammation in astrocytes by increased production of anti-inflammatory TGF-α and decreased production of pro-inflammatory VEGF-β [109]. Thus, shared expression of immune-modulating receptors may be another mechanism by which astrocyte and microglial inflammatory functions are coordinated.
Concluding Remarks
Recent advances in single-cell analysis have changed the landscape of science and have the potential to reveal synchronized responses among multiple cell types in the brain. Single-cell data has already opened up new approaches to understanding microglia and revealed novel microglial and myeloid subsets in inflammatory conditions, including a Disease Associate Microglial (DAM) signature that may be conserved in several different pathologies [63–65,110]. This subset represented 7% of cells in the original study, and would likely be missed by bulk profiling studies (see Box 1). Defining astrocyte heterogeneity at the single-cell level in different pathologies may complement recent studies in microglia and define new molecules mediating astrocyte-microglial communication (see Outstanding Questions). In addition, the examples we discuss above likely cover only a small subset of potentially coordinated roles of astrocytes and microglia, which may include the formation of myelin, remodeling of blood vessels, and regulation of transport across the blood brain barrier. As such, future work focused on astrocytes and microglia as a functional unit in all of their varied roles has the potential to address the complexity of biology in new ways and reveal novel therapeutic avenues in disease.
Text Box 1. M1/M2, A1/A2: should we call the whole thing off?
There has been increasing appreciation of the diversity of astrocyte and microglial responses to their environment. However, attempts to categorize these changers have been challenging, leading to controversy and confusion. Below we provide our perspective on how these proposed categories have both helped and hindered the field.
The concept of “M1/M2” microglia was loosely based on the concept that stimuli associated with Type 1 and Type 2 immune responses in vivo produced distinct patterns of macrophage activation in vitro. For example, the bacterial lipopolysaccharide (LPS), induced a more proinflammatory type 1 or “M1” profile, whereas the type 2 cytokines IL-4/13 led to expression of markers like Arginase 1, Mannose receptor C-type 1, and others associated with type 2 responses. Since the proposal of this paradigm, the field has evolved dramatically. Experiments in vivo have revealed the many limitations of the in vitro environment. The advent of transcriptomics has uncovered gene expression changes much beyond what can be appreciated with a handful of markers. Our understanding of immune responses themselves has changed, with the identification for instance of Th17 and regulatory responses as major axes of the immune response.
It has become apparent that the M1/M2 concept does not adequately capture the heterogeneity of macrophage responses in vivo, as clarified by some of the original authors of the proposal [111]. One could argue that it is particularly poorly suited to describing microglia, which occupy a distinct immunologic niche. The literature has been slow however to catch up with these evolving views. Investigators continue to use the expression of M1/M2 markers as a surrogate for a genuine mechanistic understanding of how microglial function changes in each particular pathology. This is not to say that these subsets do not exist at all. Single cell sequencing has revealed small populations of Arg1-positive microglia in vivo, for example [63]. The challenge is to determine what these subsets mean for microglial function.
In this context, it has been proposed that there are subtypes of reactive astrocytes, termed by some groups A1/A2, that are themselves loosely based on the M1/M2 paradigm. This classification followed the discovery that LPS-exposed astrocytes have a distinct gene expression profile from astrocytes isolated after ischemic stroke [83]. Astrocytes expressing A1 markers in vitro had clearly neurotoxic effects [84]. These discoveries were important because they clearly laid out the concept that reactive astrocytes are heterogeneous and suggested possible new markers to sort out this heterogeneity. However, the notion that “LPS astrocytes” or “stroke astrocytes” should or could be identified in pathologies ranging from viral infection, to neurodegeneration, to cancer, is unlikely to bear fruit. The identification of these subtypes has no agreed upon definition, further adding to the confusion. In some cases, cells have been labeled “A1 astrocytes” in human tissues based on expression of a single marker. In others, they are defined based on expressing somewhat more A1 than A2 genes from the original study. As with the M1/M2 paradigm, the danger of this approach is that it may be used as a shortcut to imply a mechanistic understanding.
One thing is clear from these examples: both astrocytes and microglia are exquisitely sensitive to their environment. Unlike neuronal or T-cell subsets, which retain a relatively stable lineage identity, astrocytes and microglia are in a constant state of flux. Their subtypes are defined less by lineage-determining transcription factors, and more by state-dependent transcription factors (e.g. NF-κB, STAT) [59]. This makes it more likely that subtypes will shift with time, with distance from the lesion, and with other variables not yet identified. As, such, any method of categorization, whether it consists of two groups, 20 groups, or a continuum, is useful only to the degree that it advances the following goals: 1) To identify novel astrocyte and microglial functional states that impact CNS function. 2) To rigorously define the impact of those molecules on other CNS cell types. 3) To discover ways to promote or inhibit these functions in healthy physiology or disease.
OUTSTANDING QUESTIONS.
Single-cell profiling studies have identified new myeloid-cell subsets. How do these recently identified subsets modulate astrocyte identity and astrocyte-microglial communication? Does microglial heterogeneity induce astrocyte heterogeneity?
How does environment shape the emergence of microglial and astrocyte subsets? Do specific subsets drive astrocyte-microglial communication?
Which molecules secreted from astrocytes can regulate microglia numbers, and do microglia provide feedback in this regulatory loop (and if so, how)?
To what degree does the periphery and non-myeloid immune cells (including in the brain meninges) modulate astrocytes and microglia under steady state conditions?
How might therapeutics targeted against one cell type in the microglia-astrocytic unit shift the phenotype of the other cell type? Could therapeutics targeted against microglia, for instance, be used to shift astrocyte phenotypes in beneficial ways, such as to minimize glial scarring after brain injury?
HIGHLIGHTS.
Astrocytes and microglia perform complementary roles during brain development and physiology. Among the best studied of these are their roles in supporting synapse development and responding to neuronal signals. Astrocytes and microglia may coordinate their supportive functions in other, less studied physiologic processes, including myelination, blood brain barrier regulation, and angiogenesis.
In response to injury, inflammation, and degenerative diseases, context-specific signals can shape both astrocyte and microglial responses. This type of synchrony in the astrocytic-microglial unit has been demonstrated in mouse models of Alzheimer’s Disease, Multiple Sclerosis, and encephalitis.
Molecular mechanisms that regulate astrocyte-microglia communication include direct signaling through cytokines and other molecules, as well as distinct but coordinated responses to shared environmental signals such as purines and norepinephrine. In pathology, blood-derived factors help to synchronize the astrocyte-microglia unit.
Acknowledgements:
Thanks to Ari Molofsky and members of the Anna Molofsky lab for helpful discussion and comments on the manuscript, and to funding from NIMH (R01 MH119349), the Pew Charitable Trusts, and the Burroughs Wellcome Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Ginhoux F and Prinz M (2015) Origin of microglia: Current concepts and past controversies. Cold Spring Harb. Perspect. Biol. 7, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molofsky AV and Deneen B (2015) Astrocyte development: A Guide for the Perplexed. Glia 63, 1320–1329 [DOI] [PubMed] [Google Scholar]
- 3.Foo LC et al. (2011) Development of a method for the purification and culture of rodent astrocytes. Neuron 71, 799–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen NJ and Eroglu C (2017) Cell Biology of Astrocyte-Synapse Interactions. Neuron 96, 697–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davalos D et al. (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758 [DOI] [PubMed] [Google Scholar]
- 6.Nimmerjahn A et al. (2005) Neuroscience: Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 [DOI] [PubMed] [Google Scholar]
- 7.Madry C et al. (2018) Microglial Ramification, Surveillance, and Interleukin-1b Release Are Regulated by the Two- Pore Domain K+ Channel THIK-1. Neuron 97, 299–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruttger J et al. (2015) Genetic Cell Ablation Reveals Clusters of Local Self-Renewing Microglia in the Mammalian Central Nervous System. Immunity 43, 92–107 [DOI] [PubMed] [Google Scholar]
- 9.Najafi AR et al. (2018) A limited capacity for microglial repopulation in the adult brain. Glia 66, 2385–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y et al. (2018) Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat. Neurosci. 21, 530–540 [DOI] [PubMed] [Google Scholar]
- 11.Silver J and Miller JH (2004) Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5, 146–156 [DOI] [PubMed] [Google Scholar]
- 12.Sofroniew MV (2014) Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist 20, 160–172 [DOI] [PubMed] [Google Scholar]
- 13.Clarke LE and Barres BA (2013) Emerging roles of astrocytes in neural circuit development. Nat. Rev. Neurosci. 14, 311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh SK et al. (2016) Astrocytes Assemble Thalamocortical Synapses by Bridging NRX1α and NL1 via Hevin. Cell 164, 183–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marín-Teva JL et al. (2004) Microglia Promote the Death of Developing Purkinje Cells. Neuron 41, 535–547 [DOI] [PubMed] [Google Scholar]
- 16.Peri F and Nüsslein-Volhard C (2008) Live Imaging of Neuronal Degradation by Microglia Reveals a Role for v0-ATPase a1 in Phagosomal Fusion In Vivo. Cell 133, 916–927 [DOI] [PubMed] [Google Scholar]
- 17.Paolicelli RC et al. (2011) Synaptic Pruning by Microglia Is Necessary for Normal Brain Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science 333, 1456–1459 [DOI] [PubMed] [Google Scholar]
- 18.Schafer DP et al. (2012) Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 74, 691–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filipello F et al. (2018) The Microglial Innate Immune Receptor TREM2 Is Required for Synapse Elimination and Normal Brain Connectivity. Immunity 48, 979–991 [DOI] [PubMed] [Google Scholar]
- 20.Chung W et al. (2013) Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuentes-Medel Y et al. (2009) Glia and muscle sculpt neuromuscular arbors by engulfing destabilized synaptic boutons and shed presynaptic debris. PLoS Biol. 7, e1000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tasdemir-Yilmaz OE and Freeman MR (2014) Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev. 28, 20–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bialas AR and Stevens B (2013) TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat. Neurosci. 16, 1773–1782 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Vainchtein ID et al. (2018) Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 1273, 1269–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han CZ et al. (2016) Macrophages redirect phagocytosis by non-professional phagocytes and influence inflammation. Nature 539, 570–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueno M et al. (2013) Layer V cortical neurons require microglial support for survival during postnatal development. Nat. Neurosci. 16, 543–551 [DOI] [PubMed] [Google Scholar]
- 27.Tønnesen J et al. (2018) Super-Resolution Imaging of the Extracellular Space in Living Brain Tissue. Cell 172, 1108–1121 [DOI] [PubMed] [Google Scholar]
- 28.Bonnans C et al. (2014) Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wynn TA and Vannella KM (2016) Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 44, 450–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y et al. (2014) An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J. Neurosci. 34, 11929–11947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y et al. (2016) Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 89, 37–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelley KW et al. (2018) Variation among intact tissue samples reveals the core transcriptional features of human CNS cell classes. Nat. Neurosci. 21, 1171–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsien RY (2013) Very long-term memories may be stored in the pattern of holes in the perineuronal net. Proc. Natl. Acad. Sci. 110, 12456–12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segel M et al. (2019) Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature 573, 130–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poskanzer KE and Molofsky AV (2018) Dynamism of an Astrocyte In Vivo: Perspectives on Identity and Function. Annu. Rev. Physiol 801615, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bazargani N and Attwell D (2016) Astrocyte calcium signaling: The third wave. Nat. Neurosci. 19, 182–189 [DOI] [PubMed] [Google Scholar]
- 37.Shigetomi E et al. (2016) Probing the Complexities of Astrocyte Calcium Signaling. Trends Cell Biol. 26, 300–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stobart JL et al. (2018) Cortical Circuit Activity Evokes Rapid Astrocyte Calcium Signals on a Similar Timescale to Neurons. Neuron 98, 726–735 [DOI] [PubMed] [Google Scholar]
- 39.Yu X et al. (2018) Reducing Astrocyte Calcium Signaling In Vivo Alters Striatal Microcircuits and Causes Repetitive Behavior. Neuron 99, 1170–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poskanzer KE and Yuste R (2016) Astrocytes regulate cortical state switching in vivo. Proc. Natl. Acad. Sci. U. S. A. 113, E2675–E2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eyo UB and Wu L-J (2019) Progress in Neurobiology Microglia : Lifelong patrolling immune cells of the brain. Prog. Neurobiol. 179, 101614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyamoto A et al. (2016) Microglia contact induces synapse formation in developing somatosensory cortex. Nat. Commun. 7, 12540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinhard L et al. (2018) Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat. Commun. 9, 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y et al. (2012) Reciprocal Regulation between Resting Microglial Dynamics and Neuronal Activity In Vivo. Dev. Cell 23, 1189–1202 [DOI] [PubMed] [Google Scholar]
- 45.Panatier A and Robitaille R (2012) The Soothing Touch: Microglial Contact Influences Neuronal Excitability. Dev. Cell 23, 1125–1126 [DOI] [PubMed] [Google Scholar]
- 46.España RA et al. (2016) Norepinephrine at the nexus of arousal, motivation and relapse. Brain Res. 1641, 207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paukert M et al. (2014) Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82, 1263–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sherpa AD et al. (2016) Activation of β-adrenergic receptors in rat visual cortex expands astrocytic processes and reduces extracellular space volume. Synapse 70, 307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stowell RD et al. (2019) Noradrenergic signaling in wakeful states inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. Nat. Neurosci. 22, 1782–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu YU et al. (2019) Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat. Neurosci. 22, 1771–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernier L et al. (2019) Nanoscale Surveillance of the Brain by Microglia via cAMP-Regulated Filopodia. Cell Rep. 27, 2895–2908 [DOI] [PubMed] [Google Scholar]
- 52.John Lin CC et al. (2017) Identification of diverse astrocyte populations and their malignant analogs. Nat. Neurosci. 20, 396–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bayraktar OA et al. (2018) Single-cell in situ transcriptomic map of astrocyte cortical layer diversity. bioRxiv DOI: 10.1101/432104. [DOI] [Google Scholar]
- 54.Chai H et al. (2017) Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 95, 531–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molofsky AV et al. (2014) Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature 509, 189–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farmer TW et al. (2016) Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Science 351, 849–54 [DOI] [PubMed] [Google Scholar]
- 57.Butovsky O et al. (2014) Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 17, 131–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zöller T et al. (2018) Silencing of TGFβ signalling in microglia results in impaired homeostasis. Nat. Commun. 9, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gosselin D et al. (2014) Environment Drives Selection and Function of Enhancers Controlling Tissue-Specific Macrophage Identities. Cell 159, 1327–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gosselin D et al. (2017) An environment-dependent transcriptional network specifies human microglia identity. Science 356, 6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lavin Y et al. (2014) Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ayata P et al. (2018) Epigenetic regulation of brain region-specific microglia clearance activity. Nat. Neurosci. 21, 1049–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hammond TR et al. (2019) Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 50, 253–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Q et al. (2019) Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing. Neuron 101, 207–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keren-shaul H et al. (2017) A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease Article A Unique Microglia Type Associated with Restricting Development of Alzheimer ‘s Disease. Cell 169, 1276–1290 [DOI] [PubMed] [Google Scholar]
- 66.Bisht K et al. (2016) Dark microglia: A new phenotype predominantly associated with pathological states. Glia 64, 826–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Biase LM et al. (2017) Local Cues Establish and Maintain Region-Specific Phenotypes of Basal Ganglia Microglia. Neuron 95, 341–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bohlen CJ et al. (2017) Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures. Neuron 94, 759–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guttenplan KA and Liddelow SA (2019) Astrocytes and microglia: Models and tools. J. Exp. Med. 216, 71–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mildner A et al. (2007) Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 10, 1544–53 [DOI] [PubMed] [Google Scholar]
- 71.Ajami B et al. (2011) Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 14, 1142–9 [DOI] [PubMed] [Google Scholar]
- 72.Shemer A et al. (2018) Engrafted parenchymal brain macrophages differ from microglia in transcriptome, chromatin landscape and response to challenge. Nat. Commun. 9, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cronk JC et al. (2018) Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia. J. Exp. Med. 215, 1627–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bennett FC et al. (2018) A Combination of Ontogeny and CNS Environment Establishes Microglial Identity. Neuron 98, 1170–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou X et al. (2018) Circuit Design Features of a Stable Two-Cell System. Cell 172, 744–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puñal VM et al. (2019) Large-scale death of retinal astrocytes during normal development is non-apoptotic and implemented by microglia. PLoS Biol. 17, e3000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Molofsky AB et al. (2015) Review Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity 42, 1005–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gadani SP et al. (2015) The Glia-Derived Alarmin IL-33 Orchestrates the Immune Response and Promotes Recovery following CNS Injury. Neuron 85, 703–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pomeshchik Y et al. (2015) Interleukin-33 treatment reduces secondary injury and improves functional recovery after contusion spinal cord injury. Brain. Behav. Immun. 44, 68–81 [DOI] [PubMed] [Google Scholar]
- 80.Fu AKY et al. (2016) IL-33 ameliorates Alzheimer’s disease-like pathology and cognitive decline. Proc. Natl. Acad. Sci. 113, E2705–E2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo Q et al. (2018) Interleukin-33 Protects Ischemic Brain Injury by Regulating Specific Microglial Activities. Neuroscience 385, 75–89 [DOI] [PubMed] [Google Scholar]
- 82.Chovatiya R and Medzhitov R (2014) Stress, inflammation, and defense of homeostasis. Mol. Cell 54, 281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zamanian J et al. (2012) Genomic Analysis of Reactive Astrogliosis. J. Neurosci. 32, 6391–6410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liddelow SA et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Junger WG (2011) Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 11, 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fields RD and Burnstock G (2006) Purinergic signalling in neuron–glia interactions. Nat. Rev. Neurosci. 7, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mutafova-yambolieva VN and Durnin L (2014) THE PURINERGIC NEUROTRANSMITTER REVISITED : A SINGLE SUBSTANCE OR MULTIPLE PLAYERS ? Pharmacol. Ther. 144, 162–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sipe GO et al. (2016) Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat. Commun. 7, 10905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shigetomi E et al. (2018) Role of Purinergic Receptor P2Y1 in Spatiotemporal Ca2+ Dynamics in Astrocytes. J. Neurosci. 38, 1383–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vénéreau E et al. (2015) DAMPs from cell death to new life. Front. Immunol. 6, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haynes SE et al. (2006) The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 9, 1512–1519 [DOI] [PubMed] [Google Scholar]
- 92.Lou N et al. (2016) Purinergic receptor P2RY12-dependent microglial closure of the injured blood–brain barrier. Proc. Natl. Acad. Sci. 113, 1074–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eyo UB et al. (2018) P2Y12R-Dependent Translocation Mechanisms Gate the Changing Microglial Landscape. Cell Rep. 23, 959–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pascual O et al. (2012) Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc. Natl. Acad. Sci. 109, E197–E205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shinozaki Y et al. (2017) Transformation of Astrocytes to a Neuroprotective Phenotype by Microglia via P2Y1 Receptor Downregulation. Cell Rep. 19, 1151–1164 [DOI] [PubMed] [Google Scholar]
- 96.Hammond TR et al. (2018) Microglia and the Brain: Complementary Partners in Development and Disease. Annu. Rev. Cell Dev. Biol. [DOI] [PubMed] [Google Scholar]
- 97.Hajishengallis G et al. (2017) Novel mechanisms and functions of complement. Nat. Immunol. 18, 1288–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shi Q et al. (2017) Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP / PS1 mice. Sci. Transl. Med. 9, 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hong S et al. (2016) Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lian H et al. (2015) NFκB-Activated Astroglial Release of Complement C3 Compromises Neuronal Morphology and Function Associated with Alzheimer’s Disease. Neuron 85, 101–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lian H et al. (2016) Astrocyte-Microglia Cross Talk through Complement Activation Modulates Amyloid Pathology in Mouse Models of Alzheimer’s Disease. J. Neurosci. 36, 577–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Merlini M et al. (2019) Fibrinogen Induces Microglia-Mediated Spine Elimination and Cognitive Impairment in an Alzheimer’s Disease Model. Neuron 101, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vasek MJ et al. (2016) A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature 534, 538–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garber C et al. (2018) Astrocytes decrease adult neurogenesis during virus-induced memory dysfunction via IL-1 article. Nat. Immunol. 19, 151–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu X et al. (2019) Cell-Type-Specific Interleukin 1 Receptor 1 Signaling in the Brain Regulates Distinct Neuroimmune Activities. Immunity 50, 317–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matcovitch-Natan O et al. (2016) Microglia development follows a stepwise program to regulate brain homeostasis. Science 353, aad8670 [DOI] [PubMed] [Google Scholar]
- 107.Erny D et al. (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rothhammer V et al. (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22, 586–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rothhammer V et al. (2018) Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Masuda T et al. (2019) Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 [DOI] [PubMed] [Google Scholar]
- 111.Martinez FO and Gordon S (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]