Abstract
Alveolar macrophages (AMs) are highly abundant lung cells with important roles in homeostasis and immunity. Their function influences the outcome of lung infections, lung cancer, and chronic inflammatory disease. Recent findings reveal functional heterogeneity of AMs. Following lung insult, resident AMs can either remain unchanged, acquire new functionality, or be replaced by monocyte-derived AMs. Evidence from mouse models correlates AM function with their embryonic or monocyte origin. We hypothesize that resident AMs are terminally differentiated cells with low responsiveness and limited plasticity, while recruited, monocyte-derived AMs are initially highly immunoreactive but more plastic, able to change their function in response to environmental cues. Understanding cell-intrinsic and -extrinsic mechanisms determining AM function may provide opportunities for intervention in lung disease.
Tidy Past and Messy Present
Classically, immunologists thought of mononuclear phagocytes, including tissue macrophages and circulating monocytes, as bone marrow (BM)-derived cells that act as sentinels against pathogens and damage [1]. Myeloid cells (see Glossary) were considered relatively short lived [2] and therefore unable to maintain over a long period of time any functional changes that arose as a consequence of prior stimulus. With advances in lineage tracing and cell-type-specific depletion methods in recent years, these views were challenged on several levels: First, steady-state tissue-resident macrophages are often long-lived, self-replenishing cell populations of embryonic origin rather than offspring from BM-derived cells [3., 4., 5.]. Second, tissue-resident macrophages are heavily involved in tissue maintenance and repair processes, sometimes at the expense of their immune function [6., 7., 8., 9.]. Third, when the steady state is perturbed – for instance, by infection – recruitment of additional monocyte-derived cells is crucial to accelerate the immune response [10,11]. At the end of the perturbation, recruited cells may remain in the tissue to contribute long term to the pool of macrophages that live permanently in that organ [12]. Finally, a range of long-term changes in the reactivity of innate immune cells following a stimulus have been described under the notion of trained immunity [13].
These novel concepts and observations highlight the notion that mammalian tissue-resident and recruited macrophages are not only of different origins (embryonic versus BM) but can also be functionally different. Besides their origin, important determinants of macrophage responsiveness are the tissue where they reside [14], the time spent in that tissue, and how inflamed or quiescent this environment is. What role does each of these factors play and how do they combine to ultimately fine-tune macrophage function at a given moment in time? What are the underlying mechanisms? Recent studies on murine AMs have started to shed light on some of these questions, through the identification of subsets and their role in health and disease. We propose here an overarching model in which most of the functional alterations to lung immunity derive from recruitment or changes in monocyte-derived AMs, while resident AMs are terminally differentiated and largely unable to modify their function: the old dogs that do not learn new tricks.
The Complexity of AM Subsets
After years of debate, a clear picture of murine tissue macrophage origin is now emerging, with macrophage populations in organs such as the brain (microglia), liver (Kupffer cells), and lung (AMs) being seeded early in ontogeny from an embryonic precursor [15]. These organs are considered ‘closed’, as there is almost no steady-state recruitment of monocyte-derived macrophages [3,5,15]. Other tissues are thought to be ‘open’ for replacement with blood monocytes at either slow (i.e., heart) or fast (i.e., dermis and gut) rates [5,15,16]. Lineage-tracing experiments and BM-chimeric mice (Table 1 ) have shown that tissue-resident AMs self-maintain without further input from monocytes in the steady-state lung [3., 4., 5.], whereas in situations of lung insult, BM-derived cells can contribute to the AM pool [10,12]. Embryonic precursors and blood monocytes are considered highly ‘plastic’ as they are capable of differentiating into many myeloid cell subtypes; by contrast, terminally differentiated tissue macrophages are committed to the phenotype imposed by their specific organ of residence and do not fully acquire the characteristics of AMs when transplanted into the lung [14,17].
Table 1.
Examples of Commonly Used Techniques to Distinguish Tissue-Resident and Monocyte-Derived AMs
| Species | Model | Explanation | Refs |
|---|---|---|---|
| Mouse | Ccr2 knockout (KO) | CCR2 is crucial for monocyte egress from the BM and recruitment from the blood into inflamed tissue. Therefore, in Ccr2 KO mice, changes in AMs can be attributed to the resident AMs, as monocyte recruitment is severely impaired. | [40,75] |
| Busulfan chimeras using Ccr2 KO host | Busulfan is used to ablate BM cells, which are reconstituted with allogeneically marked BM cells (e.g., using CD45.1 or CD45.2 alloantigens). Donor-BM-derived monocytes are recruited into the CCR2-deficient host lung. | [10] | |
| Irradiation chimeras | In host mice, BM cells can be depleted through irradiation while preserving immune cells in the lung if the thorax is shielded. Reconstitution with marked BM cells allows tracing of BM-derived AMs. | [11,12,76] | |
| Parabiotic chimeras | Conjoined mice sharing blood flow develop a high level of chimerism of circulating monocytes. AMs that are marked with the alloantigen from the conjoined mouse must be monocyte derived. | [40,55] | |
| Fate mapping using Cre-loxP system | Granulocyte-monocyte progenitors express high amounts of Ms4a3. Monocytes express high amounts of Mx1, S100a4, and Flt3 as well as high and intermediate amounts of Cx3cr1. Resident AMs do not express these genes. Therefore, the promoter of the above genes is used to drive the expression of Cre recombinase that enables permanent induction of a fluorescent marker in monocyte-derived cells. | [3,4,16] | |
| Labeling with fluorescent antibody/dye | Intravenous injection of fluorescent antibody (e.g., against CD45) or dye (PKH26) labels monocytes and can be traced to monocyte-derived AMs. Dye (PKH26) administration into the lung labels phagocytic cells including resident AMs, so if it is administered before lung insult, newly incoming monocytes and monocyte-derived AMs will not be labeled. These are time-restricted labeling techniques. | [42,76] | |
| Human | Lung transplant HLA mismatch | After lung transplant, donor resident AMs (bearing donor HLA) can be distinguished from host monocyte-derived AMs using mismatched HLA antigens. | [18] |
| Lung transplant sex mismatch | In female recipients of a male-donor lung or vice versa, donor-lung-resident AMs can be distinguished from recipient-monocyte-derived cells using sex-specific transcripts [X-inactive specific transcript (XIST) for females and ribosomal protein S4 Y-linked 1 (RPS4Y1) for males] or fluorescence in situ hybridization (FISH) with X/Y probes. | [19,20] | |
| scRNA-seq | In scRNA-seq, AMs can cluster based on their expression of classical AM genes (potentially resident AMs) or peripheral monocyte-like genes (potentially monocyte-derived AMs). This method is correlative. | [77] |
Until recently, in neither the mouse nor human datasets was it possible to determine AM origin (Table 1). The absence of reliable markers of origin limits our understanding of AM pool composition in healthy humans, but studies in lung transplant recipients use human leukocyte antigen (HLA) or sex mismatch between donor and recipient as markers for the distinction between AMs that were present in the lung prior to the transplantation (resident) or that migrated in later (recruited). These studies have shown that lung macrophages comprise both resident (donor) cells and newly recruited BM-derived (recipient) cells [18., 19., 20.]. In humans, being surrounded by pollutants and undergoing infections or other lung insults multiple times during our lifetime, the AM pool will comprise a mix of macrophages, some of embryonic origin and others of BM origin, with differing arrival times and duration of residence in the lung. Laboratory mice living under specific-pathogen-free (SPF) conditions will not have experienced major lung insults, so the predominance of embryonically derived AMs has not been lost and, as a consequence, naïve laboratory mice contain mostly, if not only, AMs of embryonic origin [3., 4., 5.].
RNA-seq of bulk cell populations in mice have shown a unique tissue-specific transcriptional profile for macrophages, which is distinct from blood monocytes [14]. Recent single-cell transcriptional analyses have indicated a vast variety of lung macrophage clusters in lung cancer patients with their biography of varied lung insults [21,22], but also in mice living under SPF conditions [22]. While there are similarities between mouse and human macrophage populations, these cannot be unequivocally mapped onto each other [22]. As these studies have not determined the embryonic or BM origin of macrophage subsets, future single-cell analysis should be combined with models employing fate-mapping tracing techniques [16] to broaden our understanding of the impact of origin or differing sojourns in the lung on AM functionality.
Increased AM Reactivity Post-Influenza Virus Infection (IVI) May Emerge from Monocyte-Derived Cells
Recent results have shed new light on the link between origin and AM functionality. Using chimeric mice that provide markers to distinguish between adult BM and embryonic origin, our group showed that, following recovery from IVI, the AM pool comprised both embryonically derived ‘resident’ AMs and CCR2-dependent, BM-derived ‘recruited’ AMs, most likely of monocyte origin [10]. The two AM subsets were indistinguishable by surface phenotype and morphology but showed significant transcriptional, epigenetic, and functional differences. While IVI had no impact on the transcriptional profile or functional phenotype of resident AMs, recruited AMs maintained open chromatin at loci controlling the expression of inflammatory genes, including Il6, which made these cells significantly more responsive to stimulation with bacterial Toll-like receptor (TLR) agonists [10] (Figure 1A). It should be noted that resident AMs can be important producers of type I interferons (IFNs) and cytokines post-viral infection [23., 24., 25., 26.], but this response is most prominent on day 1 and is short-lived. On days 3–9 post-infection, resident AMs are often found to be depleted, and monocytes are recruited into the inflamed tissue [10,26., 27., 28., 29.]. In the case of severe IVI, monocytes destined to become recruited AMs arrive in the lung between day 3 and 7 of infection [10]. Even after a 1-month residence in the lung, these BM-derived AMs have not acquired the relatively unresponsive profile of resident AMs as determined by transcriptional and functional analysis [10]. These results highlight a differential responsiveness between resident and recruited AMs and reconcile functional changes at the population level with a lack of functional changes in resident AMs; the new functionality comes in with the newly recruited cells [10]. Similar observations with regard to BM-derived AMs have been made in other lung-insult models, showing that BM-derived AMs provide protection against murine house dust mite (HDM)-induced asthma [11] or can be a driving force for lung fibrosis following mouse lung injury [12,30,31]. Also, similar to IVI, tissue-resident AMs have been reported to remain mostly unchanged following bleomycin treatment [12], consistent with the notion that resident AMs of embryonic origin can play important roles in tissue homeostasis and exhibit reduced functional plasticity [17,32].
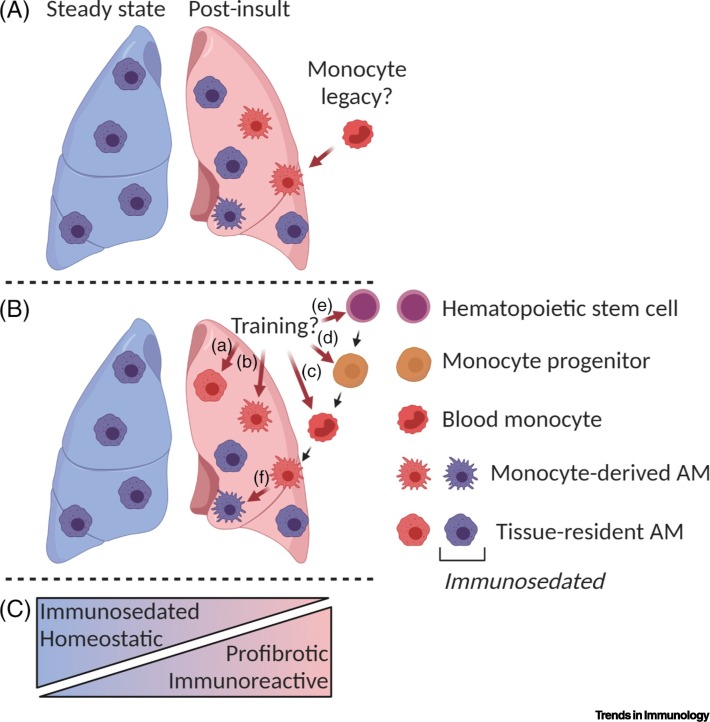
Figure 1.
Model of Changes in Alveolar Macrophage (AM) Phenotype in the Post-Insult Lung.
AMs found in the post-insult lung (A,B) (e.g., in mice) can be profibrotic or immunoreactive, compared with the steady-state, immunosedated resident AMs that are responsible for homeostatic functions (C). This may be explained by (A) monocyte-derived AMs retaining functionality similar to that of monocytes [10] due to their recent recruitment; or by (B) innate training. (a) Resident AMs can be trained to develop a more immunoreactive phenotype [39,40]. Recruited AMs can change functionally as a result of innate training at various stages, including (b) as AMs in the lung, (c) as monocytes in the blood [43], and (d) as monocyte progenitors or (e) hematopoietic stem cells in the BM [44., 45., 46.]. (f) Monocyte-derived AMs can lose reactivity and become immunosedated [10] by anti-inflammatory signals from the uninflamed lung environment. This figure was created using BioRender (https://biorender.com/).
Why Is Proliferation of Resident AMs Not Sufficient to Replenish the AM Pool?
Resident AMs are known to be able to proliferate and contribute to the repopulation of the lung, but some of the AMs found after IVI and other insults are monocyte derived [10., 11., 12.]. Why do resident AMs not fully repopulate the AM pool during recovery from IVI in mice? Why is the contribution of a monocyte-derived AM population needed? The niche model [33] may provide a framework to explain AM dynamics. At the steady state, the lung epithelium can prevent access by monocytes to engraft in this niche. Moreover, the AM niche is unavailable, already occupied with embryonically derived resident cells. Injury such as IVI would lead to AM depletion and render the niche temporarily available. The empty niche may instruct resident AMs to proliferate locally or, if necessary, allow recruited monocytes to engraft [3,10,17]. In vivo studies in mice have highlighted the capacity of resident AMs to self-sustain via proliferation at the steady state and expand after targeted depletion [3]. For instance, accumulation of pleural macrophages was demonstrated in a type 2 immune response induced by infection with the nematode Litomosoides sigmodontis. Here, local proliferation of alternatively activated macrophages was driven via IL-4 signaling, given that it was absent in IL-4-deficient mice, with minimal contribution from recruited macrophages [34,35]. It should be noted that monocyte-derived macrophages could also be driven to proliferate by IL-4 activation, as shown by BrdU incorporation by recruited peritoneal macrophages after IL-4 treatment [34]. The infection-site-restricted IL-4-dependent proliferation of resident macrophages was independent of the M-CSF receptor, in contrast to M-CSF-dependent proliferation at the steady state [35]. In the mouse lung, IL-4-dependent AM proliferation is enhanced by surfactant protein A (SP-A), potentially produced by alveolar epithelial type II cells [36]. By contrast, repopulation of lung macrophages after genotoxic depletion is dependent on both M-CSF and GM-CSF, but independent of IL-4 [3]. Together, these data suggest that proliferative capacity may not be restricted to macrophage precursors, but resident macrophages may be capable of expansion in situ for maintenance in type 2 responses or when restoring tissue homeostasis in situations where the tissue-damage potential of inflammatory cell recruitment is to be avoided.
In contrast to our findings describing how depleted AMs are repopulated with both resident AMs and highly immunoreactive recruited AMs following IVI [10], another IVI study in mice found that the reduction of AMs was recovered by local repopulation with resident AMs, independent of monocyte contribution [3]. Similar patterns of AM depletion and proliferation in situ were observed with poly I:C and clodronate liposome administration [3], confirming the ability of resident macrophages to expand in inflammatory settings. Most likely, the contribution of embryonically derived versus monocyte-derived cells to the AM pool post-IVI may depend on the degree of AM depletion during infection, which in turn may depend on the severity of inflammation. This might also contribute to explaining why, in another IVI study, AMs were desensitized for TLR signaling 1–2 months post-recovery [37], reminiscent of the lipopolysaccharide (LPS) tolerance previously described [38].
Is There Trained Immunity of Resident AMs?
Trained immunity in monocytes and macrophages has been described as a stimulus-induced epigenetic alteration leading to long-term functional changes. Where does trained immunity come into play and where is macrophage origin sufficient to explain functional differences? The above results [10., 11., 12.] demonstrate that resident AMs do not change during certain lung insults, in line with their status as terminally differentiated, tissue-adapted cells. However, in other experimental settings, long-lived resident AMs can also show long-term functional alterations (Figure 1Ba). For instance, the Pseudomonas aeruginosa vaccine induces expansion of local AMs independent of monocyte recruitment, providing protection against bacterial challenge in a mouse model of chemotherapy-induced depletion of BM-derived immune cells [39]. Similarly, resident trained AMs provide prolonged antibacterial protection after adenoviral infection independent of monocytes and BM progenitors, as shown in BM chimeras and parabiotic mice ([40]; Table 1). Here, the induction of trained AMs required priming by CD8+ T cells via IFN-γ production [40]. In these studies, increased lung macrophage reactivity did not require newly recruited monocyte-derived cells as it was independent of the monocyte chemoattractant CCL2; instead, increased reactivity was deemed to be dependent on resident cells having changed their functionality [39,40]. It is currently unclear whether this effect is restricted to certain challenge models; also, it is unclear why other viral infections or bleomycin-induced lung insult might instead lead to the strong presence of monocyte-derived AMs with changed functionality. In addition, not all lung insults will lead to strong CD8+ T cell-derived IFN-γ, which appears to be a key cytokine in the imprinting of increased macrophage reactivity in the abovementioned mouse model of adenovirus infection [40]. Given the variety of results obtained in different systems, the jury is still out on the circumstances under which embryonically derived resident AMs adopt long-term functional changes (Table 2 ). It is possible that, depending on the type of insult (e.g., whether it is more local or systemic), different processes may lead to changed reactivity of macrophages, some relying on long-term changes in resident populations, others on recruitment of new cells of differing reactivity.
Table 2.
Recent Studies of Phenotypic Changes in Alveolar Macrophages (AMs) and Their Progenitors after Challenges
| Cell | Species | Challenge | Phenotype | Duration | Refs |
|---|---|---|---|---|---|
| BM-derived AMs | Mouse | Murid herpesvirus (MuHV-4) infection | Decreased IL-4, IL-5, IL-13, and IL-6 production Reduced allergic response to HDM |
28–30 days | [11] |
| Mouse | Bleomycin-induced fibrosis | Increased expression of Adam8, Arg1, Apoe, Itga6, Mfge8, Mmp12, Mmp13, Mmp14, and Pdgfa Drive lung fibrosis |
14–19 days | [12] | |
| Increased expression of Ccl2, Ccl12, Ccl24 Drive lung fibrosis |
7–14 days | [30] | |||
| Increased expression of ApoE Drive lung fibrosis resolution |
8 weeks (fibrosis resolution stage) | [78] | |||
| Mouse | Asbestos-induced fibrosis | Self-sustaining via M-CSF/M-CSFR signaling Increased expression of Pdgfa Drive lung fibrosis |
14 days | [31] | |
| Mouse | Influenza A virus infection | Increased IL-6 production Increased protection against Streptococcus pneumoniae |
28 days (phenotype lost at 2 months) | [10] | |
| Tissue-resident AMs | Mouse | Pseudomonas aeruginosa vaccine | Increased protection against P. aeruginosa pneumonia in chemotherapy settings T cell dependent |
4 weeks | [39] |
| Mouse | Adenovirus infection | Increased production of neutrophil chemokines (MIP-2 and KC) Increased glycolysis CD8+ T cell-dependent priming via IFN-γ Increased protection against S. pneumoniae and Escherichia coli |
4 weeks (up to 16 weeks for S. pneumoniae protection) | [40] | |
| Mouse | E. coli | Reduction of phagocytosis of extracellular bacteria | 7–14 days | [42] | |
| Blood monocytes | Human | BCG vaccine | Increased IL-1β production Reduced yellow fever viremia |
1 month | [43] |
| Increased production of IFNs, IL10, IL-1β, IL-6, IL-1RA, TNF | 14–90 days | [44] | |||
| Severe trauma or sepsis | Reduction of phagocytosis of extracellular bacteria Reduced amounts of SIRPα, CD206, CD14, and CD16 |
Up to 4 weeks post-sepsis Up to 6 months post-trauma |
[42] | ||
| BM cells | Human | BCG vaccine | Enhanced myelopoiesis Increased expression of IFNG, TNF, and IL1B (BMDMs) Increased protection against Mycobacterium tuberculosis |
4 weeks (BMDMs up to 5 months) | [46] |
| Upregulation of myeloid and granulocytic lineage-associated transcripts Upregulation of TFs HNF1A and HNF1B |
90 days | [44] | |||
| Mouse | β-Glucan | Increased G-CSF and IL-1β production Increased glycolysis Enhanced myelopoiesis Increased protection against LPS challenge |
24 h 24 h and 7 days 7–28 days 28 days |
[45] |
Why Are Recruited AMs More Immunoreactive Than Resident AMs?
We argue that the simplest explanation for the increased reactivity of monocyte-derived AMs is that this represents a ‘legacy’ of their former monocyte profile. Recruited monocytes seeded the mouse lung niches early post IVI to contribute to the AM pool [10,28,29]. Nevertheless, when recruited AMs were analyzed 28 days post-infection, they showed great similarities to blood monocytes from naïve animals, transcriptionally and in terms of global chromatin accessibility [10]. A prominent example is the regulation of Il6 gene expression: more IL-6 protein is produced on a bacterial stimulus in recruited AMs than in resident AMs, and this is reflected in open chromatin regions found upstream of the Il6 gene in recruited AMs. The open regions are similar to those in blood monocytes, and absent in resident AMs [10]. In addition, another study in mice found enrichment of genes belonging to the IL-6 signaling pathway after stimulation of inflammatory monocytes and interstitial macrophages, but not of AMs [32]. As interstitial macrophages are located in an open niche, they resemble more blood monocytes, in contrast to the closed niche of AMs, which sets them apart [5,15]. This suggests that the heterogeneous AM population post-IVI in mice [10] might not require major transcriptional, epigenetic, or functional changes of any of the constituent cell subsets. Resident AMs may be programmed to be hyporesponsive; hence, the low IL-6 production. By contrast, recruited AMs, due to their monocyte legacy, might still transcriptionally resemble the blood monocytes they once were, with key proinflammatory genes prone for expression (Figure 1A,C).
However, these findings in IVI do not exclude the possibility that in other situations, greater reactivity of recruited AMs is imprinted. Where could this happen? One possibility is that inflammatory conditions in the lung during the monocyte-to-macrophage transition imprint enhanced reactivity onto these cells (Figure 1Bb), similar to what has been described in vitro when human blood monocytes were kept in culture for time periods of several days [41]. Alternatively, prior to arrival in the lung, monocytes might receive a cytokine-mediated signal in the blood imprinting their functionality (Figure 1Bc), or monocyte precursors might be imprinted even earlier in the BM (Figure 1Bd,e). The latter hypotheses are testable as they predict that, post-lung injury, the function of monocytes or monocyte-derived macrophages might be changed in peripheral organs in addition to the lung, because presumably, blood monocytes or their precursors would be trained. Inversely, how systemic insults such as sepsis and trauma modify monocyte and macrophage function locally [42] is an important area of research that we do not cover here.
Bacillus Calmette–Guérin (BCG)-induced long-term epigenetic changes were demonstrated in humans, with consequent enhanced innate immune responses to yellow fever vaccination or restimulation with Candida albicans in blood monocytes [43,44] concomitant with changes in the hematopoietic progenitor compartment [44]. The hypothesis that these alterations are due to upstream changes induced in BM precursors is also supported by mouse data showing changes in hematopoiesis and the possibility of adoptive transfer of hematopoietic precursors that transfer reactivity [45,46]. In addition, earlier studies have shown an IFN- or IL-12-mediated instructive signal to BM cells during peripheral infection that set up antiviral or regulatory programs, respectively, in nascent monocytes [47,48]. While the underlying mechanisms are yet to be fully understood at the transcriptional and epigenetic levels, it seems clear that murine myeloid progenitors can be trained with BCG or β-glucan exposure, although the potential persistence of these stimuli must be taken into account [44., 45., 46.]. Whether different types of lung insult can cause reprogramming in the BM to generate trained recruited AMs remains to be determined; if so, whether these are cells trained differently depending on the specific challenge is also unknown. In conclusion, increased immunoreactivity of recruited AMs may sometimes be the result of imprinting along the developmental path from BM precursors to recruited AMs (via blood monocytes). We propose that, in many instances, a simpler explanation is that the specific functionality of recruited AMs is a remnant of the chromatin landscape and transcriptional profile of the former monocytic identity of these cells.
Maintaining or Losing Monocyte Immunoreactivity in Recruited AMs
If monocyte legacy determines the high reactivity of recruited AMs, why, then, do other, less inflammatory interventions to recruit AMs into the lung, such as clodronate liposomes or transfer into GM-CSFR-deficient mice, yield less-reactive recruited AMs [10,11,17,49]? The monocyte legacy model would hypothetically explain this as follows. There is no proinflammatory signal required to imprint high reactivity in monocyte-derived cells: monocytes themselves are highly reactive cells, so this profile simply needs to be maintained. However, the lung will start to exert environmental imprinting on newly recruited cells, and the degree of lung inflammation may make the difference. For example, exposure to tumor necrosis factor (TNF) is known to suppress the LPS-triggered expression of IL6 and other genes encoding proinflammatory cytokines in human monocyte-derived macrophages [50], but this effect can be abolished by type I IFNs present during TNF priming [51]. IFNs can render cells refractory to a wide range of immunosuppressive signals, such as glucocorticoids, IL-4, and IL-10 [52]. Moreover, IFN-γ and IL-4 can inhibit each other’s polarization programs [53]. Therefore, while recovering from IVI, the lung may not send the ‘immunosedative’ signals required to turn off inflammatory programs in recruited cells, or such calming signals may be counterbalanced by activating signals coming from the still-inflamed lung. This might result in a prolonged state of monocyte-like high reactivity in recruited AMs (Figure 2 ). By contrast, a largely uninflamed lung environment, as found in sterile AM depletion models, might result in faster and more efficient establishment of an ‘immunosedated’ state in incoming monocytes, relative to those recruited into a highly inflamed lung. At 1 month post-IVI, recruited murine AMs retain a significant epigenetic and transcriptional resemblance to monocytes; after longer periods of lung residence post-IVI, recruited AMs become transcriptionally more similar to resident AMs, suggesting that, given sufficient time, the recovered lung does impose an immunosedated state in the recruited cells [10]. To settle these questions conclusively, BM progenitors, monocytes, and recruited macrophages will have to be compared under different recruitment regimens in careful kinetic studies, paying special attention to the signals given by the lung environment.
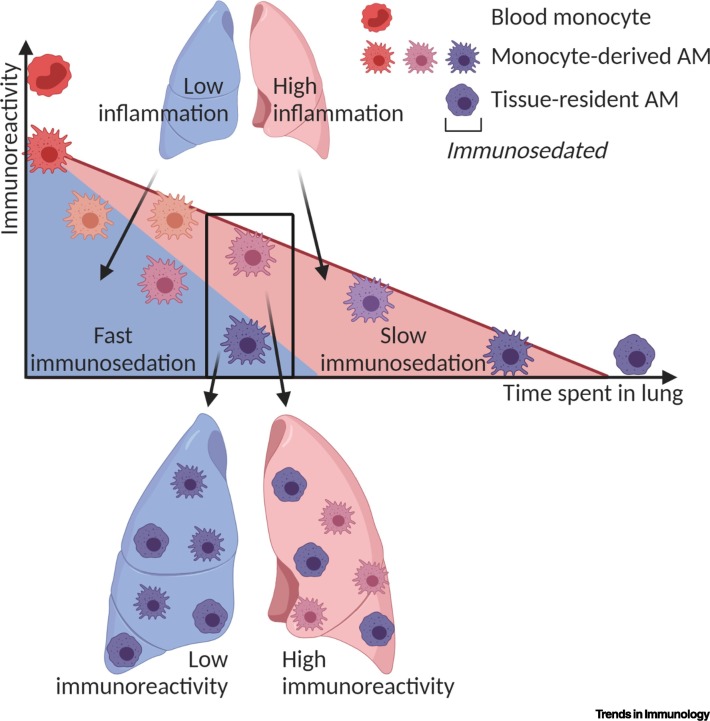
Figure 2.
Model of Kinetics of Alveolar Macrophage (AM) Immunosedation in Different Mouse Lung Environments.
In this model, the immunoreactivity of monocyte-derived AMs is determined by the lung environment and the duration spent in the lung. After lung insult or depletion of AMs, highly reactive monocytes are recruited into the lung and lose reactivity over time (immunosedation). In a non-inflamed lung (blue), the signals from the environment may be anti-inflammatory and therefore immunosedate these cells fast and efficiently, while activating stimuli are largely absent. In a still-inflamed lung environment (red), both pro- and anti-inflammatory signals may be present, leading to a slower rate of immunosedation. This might explain the comparably low immunoreactivity of monocyte-derived AMs after sterile depletion and the higher immunoreactivity for a longer time period following an inflammatory insult [10., 11., 12.,49]. This figure was created using BioRender (https://biorender.com/).
What Signals Are Given by the Lung Environment to Establish AM Functionality in Recruited Cells?
If the above monocyte legacy model is correct, incoming monocytes would receive signals derived from structural cells turning them slowly into less-inflammatory cells, focused towards homeostatic functions and much resembling embryonically derived resident AMs. What might these signals be? As murine adult blood monocytes have the capacity to develop into AMs [17], it may be instructive to consider those signals driving the ontogeny of resident AMs as possible candidates to promote the monocyte-to-AM transition. Important cytokines involved in AM development are transforming growth factor beta (TGF-β) and GM-CSF (Figure 3 ) [54,55]. The absence of or inability to signal via either of them leads in humans and mice to the development of pulmonary alveolar proteinosis (PAP), a disease arising when AMs do not develop to fulfill their key maintenance role of clearing surfactant and lipids from the alveolar space [54., 55., 56., 57., 58., 59.]. TGF-β acts in an autocrine manner, and signaling via the TGF-β receptor upregulates the master transcription factor (TF) peroxisome proliferator-activated receptor gamma (PPAR-γ) in fetal monocytes and is essential for the development of these embryonic precursors as well as BM-derived cells into AMs in mice [54]. GM-CSF is secreted by lung epithelial cells as well as resident macrophages and plays a vital role in inducing murine fetal monocyte development into macrophages via PPAR-γ in a paracrine manner [58]. Downstream of GM-CSF, the TF PU.1 also appears to be important: GM-CSF induces PU.1, while forced expression of PU.1 in GM-CFS-deficient Csf2 −/- AMs rescues their ability to catabolize surfactants [60]. In the absence of PPAR-γ, murine Pparg −/- macrophages exhibit increased proinflammatory and reduced anti-inflammatory phenotypes [61]. PPAR-γ is also highly expressed in adipocytes in mice and regulates a lipid metabolic program in these cells [62]. A similar PPAR-γ-driven metabolic program is likely to promote the key lung maintenance function of AMs by keeping the balance of surfactant via constant removal of these lipid-rich molecules, constitutively produced by lung epithelia.
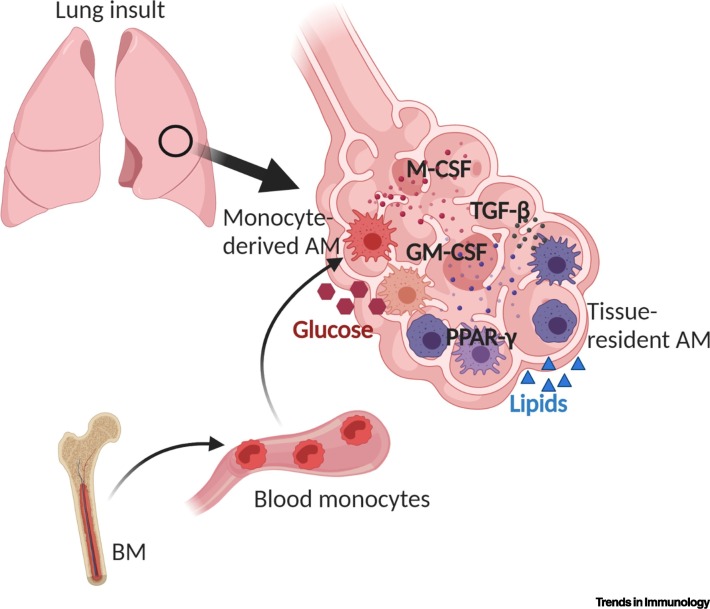
Figure 3.
Model of How Signals in the Mouse Lung Shape Newly Recruited Alveolar Macrophages (AMs).
The functionality of monocyte-derived AMs can be shaped by various signals in the lung environment, including colony-stimulating factors (CSFs) (GM-CSF and M-CSF) and transforming growth factor beta (TGF-β), acting in a paracrine or autocrine manner. In the lung, monocyte-derived AMs are exposed to different amounts of glucose and fatty acids, which, together with other stimuli, may establish over time the transition from an immunoreactive phenotype initially to the subsequent immunosedation of recruited AMs expressing the signature transcription factor peroxisome proliferator-activated receptor gamma (PPAR-γ). This figure was created using BioRender (https://biorender.com/).
To summarize, the AM TF signature regulates lipid metabolism and the primary tissue maintenance function of AMs is the degradation of lipid-rich surfactants in humans and mice [57,58,63]. Lung alveoli are not only rich in lipids but also thought to be low in glucose [64], suggesting that the low immunoreactivity of AMs may be strongly dictated by the metabolic adaption to their maintenance role and their environment. Generally, macrophage function is known to be tightly linked to their metabolic profile, with a distinction, largely based on in vitro work, between LPS-stimulated inflammatory macrophages mostly depending on glycolysis and alternatively activated macrophages employing oxidative phosphorylation to generate energy [65,66]. However, it is becoming increasingly clear that, in addition to increased fatty acid oxidation and oxidative phosphorylation, glycolysis is also essential for the latter phenotype [67]. Glycolysis inhibition studies have highlighted the importance of glucose consumption in determining the alternatively activated phenotype in murine [67,68] and human [69] monocyte-derived macrophages. This fits with the finding that AMs are hyporesponsive to IL-4-induced type 2 inflammation in vivo [59]. Of note, removed from their niche and cultured in vitro, murine AMs regain responsiveness to IL-4 in a glycolysis-dependent and fatty acid oxidation-independent manner, whereas peritoneal macrophages transferred to the lung become less responsive to IL-4 [59]. Given the now-recognized importance of glycolysis for macrophage functions, combined with the scarcity of glucose in the lung due to active epithelial removal processes [64], the low responsiveness of AMs might be a consequence of their metabolic profile of near-absent glycolysis.
How is metabolism linked with gene expression patterns? A novel link between macrophage transcriptome and metabolism is lactate, a product of glycolysis. The addition of a lactyl group (lactylation) to a lysine residue on histones serves as an epigenetic modification in murine BM-derived macrophages (BMDMs) [70]. The degree of histone lactylation is dependent on the endogenous production rate of lactate via glycolysis, with inflammatory, but not alternatively activated, macrophages having elevated amounts of lactate and increased histone lactylation [70]. Although lowering of lactate concentrations by depletion of lactate dehydrogenase A in BMDMs was shown to be not essential for the inflammatory phenotype, histone lactylation seems to serve as a molecular clock that ensures a late switch from the expression of proinflammatory genes to gene signatures associated with a homeostatic macrophage phenotype [70]. We speculate that similar metabolism-driven epigenetic modifications might contribute to shaping the functional profile of AMs. Presumably, newly recruited monocyte-derived AMs might highly depend on glycolysis, through activation or induced during training [71,72]. Increased glycolysis might set in motion a lactate clock leading to a switch towards a homeostatic phenotype. As lung glucose concentrations are increased in inflammatory conditions and rise transiently during infections [73], recruited cells might initially have access to glucose, which might become less available over the course of recovery. Once settled in the healing lung, where glucose supply is limited [64], recruited AMs would have to downregulate glycolysis, resulting in the cessation of glycolysis-driven histone modifications important for macrophage polarization. Together with a variety of other links between cell metabolism and gene expression (reviewed in [74]), a mechanism as delineated hypothetically here might contribute to the establishment of the unique immunosedated phenotype of AMs in the long term. This means that homeostatic requirements and immunosedation might be intimately linked, opening new potential therapeutic intervention strategies in lung diseases.
Concluding Remarks
To protect its delicate structure and allow gas exchange, the lung is under normal circumstances an immunologically quiet, or immunosedated, site, as not every inhaled particle should trigger inflammation. Non-inflammatory defenses such as the epithelial barrier and the mucus layer are in place for protection. Embryonically derived, long-lived resident AMs are a crucial part of this environment and may be ‘terminally sedated’, retaining some, but minimal, immunoreactivity. Newly arriving myeloid cells, recruited rapidly on insult, are initially highly immunoreactive due either to their proinflammatory monocyte legacy or to training at various stages of recruitment, as determined in mouse models. Recruited AMs might also become immunosedated over time and develop a functional profile similar to that of embryonically derived AMs, centered around the homeostatic role of lipid catabolism to remove surfactants from the alveoli. This functional change is reflected in alterations to the transcriptional and chromatin landscape. Many questions remain regarding the precise order of events, the signals and TFs at work, and the underlying molecular mechanisms (see Outstanding Questions), but some answers are beginning to emerge. Generally, blood monocytes may be an all-purpose weapon to be deployed in divergent contexts, explaining their unique combination of high immunoreactivity and high plasticity. Monocyte-derived recruited AMs retain this plasticity for some time and therefore may be the macrophages that determine outcomes in circumstances such as cancer, allergy, infections [including IVI and severe acute respiratory syndrome (SARS)-CoV-2 (Box 1 )], and chronic lung conditions such as chronic obstructive pulmonary disease (COPD).
Outstanding Questions.
Why do tissue-resident AMs remain unchanged after various lung insults? What is specific to those challenges that do induce changes in resident AMs? Understanding cell-intrinsic or -extrinsic factors and processes that control tissue-resident AM plasticity could allow targeted modulation.
How do short-lived BM-derived monocytes become long-lived, self-sustaining, homeostatic macrophages in the lung? How does the lung environment drive this process? This transition appears to be the reverse of many biological processes and understanding it may be useful when lung homeostasis needs to be re-established.
What are the functions of resident and recruited AMs in resolving a primary lung insult? Are the functions specific, complementary, or overlapping? How are these initial differences reflected during subsequent challenges?
Are the beneficial or detrimental roles of locally recruited AMs merely a reflection of their monocyte origin or does innate training of precursors in BM or blood change macrophage phenotypes systemically? This could have important implications for vaccine strategies and other interventions to reprogram macrophages organism wide.
Under what conditions are monocyte-derived AMs recruited into the human lung? How can we identify resident and monocyte-derived AMs in humans? What is the ratio of resident to recruited AMs in health and disease? Are functional changes specifically in resident or recruited AMs linked to human diseases? Such knowledge may open new avenues for targeted intervention in humans.
Do monocyte-derived AMs accumulate with age across species? If so, are they responsible (or partially responsible) for ‘inflammaging’? If true, this might help to explain why certain diseases disproportionately affect elderly people.
Do the specific metabolic constraints in the lung change AM polarization? Do differently programmed macrophages change their metabolic status? Better knowledge might open possibilities for the (re)programming of AM function in disease through metabolic intervention.
Alt-text: Outstanding Questions
Box 1. Lessons from the SARS-CoV-2 Infection.
Cytokine storm and related immunopathology are hallmarks of severe COVID-19, caused by SARS-CoV-2 infection (reviewed in [79]). Symptoms include profound lymphopenia and loss of HLA-DR expression on immune cells [80., 81., 82., 83.]. Infiltration of inflammatory monocytes and macrophages (IMMs) has been previously reported in fatal cases of SARS [84] and IMMs were shown to be responsible for the increased expression of proinflammatory cytokines, including IL-6 and IL-1β, in the SARS mouse model [85,86]. Single-cell RNA-seq (scRNA-seq) has revealed an altered macrophage composition in the lungs of patients with moderate or severe COVID-19, with a drastically reduced resident AM population and emerging populations expressing monocyte-like markers, but also AMs with a reparative but potentially profibrotic phenotype [77,87]. Also, peripheral blood classical monocytes can express increased amounts of IL-6 and IL-1β [80,88,89]. Therefore, proinflammatory cytokines produced by IMMs might be promising candidate targets for blockade that could hypothetically contribute to the prevention of COVID-19 pathology [80,90,91]. For example, administration of an IL-6-blocking antibody might partially revert immune dysregulation in some COVID-19 patients by increasing circulating lymphocytes, restoring HLA-DR expression on CD14 monocytes [80].
Another potential but complex therapeutic target for SARS-CoV-2 is GM-CSF signaling (reviewed in [92]). On the one hand, increased GM-CSF was reported in the plasma of COVID-19 patients [93], suggesting that IMMs are exposed to proinflammatory GM-CSF produced by peripheral blood T cells and monocytes [94] and might potentially be targeted with GM-CSF inhibitors [79]. On the other hand, GM-CSF can contribute to improvement of epithelial repair in humans [95] and regulate AM differentiation in mice [60]. Therefore, GM-CSF treatment might accelerate the differentiation of monocyte-derived AMs. Because GM-CSF is essential for the repopulation of resident AMs after insults by inducing proliferation [3], one might speculate that this results in the expansion of resident AMs with low immunoreactivity, which in turn might reduce the niche for recruited proinflammatory AMs [94]. Overexpression of GM-CSF post-IVI in an inducible transgenic mouse model resulted in increased survival and reduced expression of proinflammatory genes in AMs [96]. These studies provide a rationale for plans to administer GM-CSF to COVID-19 patients [79,92]. Evidently, robust studies in this regard are still awaited.
In the long term, many patients will have recovered from severe cases of SARS-CoV-2 infection. Within 7 days post-recovery, blood monocytes still express high amounts of inflammatory genes, such as IL-1β, but after 14 days this expression is reduced [88]. However, based on animal studies with various challenges [10,12], we predict that patients who have recovered from COVID-19 might bear a mixed AM population in the lung, which is likely to have a strong component of monocyte-derived AMs recruited during a massive inflammatory episode. In patients with disease, macrophages accumulate in alveolar cavities and are associated with lung lesions [97,98], whereas in vitro macrophage co-stimulation with poly I:C and serum from patients with severe COVID-19 induces endothelial barrier disruption [99]. In recovered patients, the role of AMs in short- and long-term lung damage, as well as potentially altered AM phenotypes, should be further investigated and taken into account when treating subsequent diseases.
Alt-text: Box 1
Acknowledgments
We are thankful to Stefania Crotta, Helena Aegerter, Julie Rappe, Jack Major, and other former and current members of the Immunoregulation Laboratory of the Francis Crick Institute for thoughtful comments on the manuscript. This work was supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001206), the UK Medical Research Council (FC001206), and the Wellcome Trust (FC001206).
Glossary
- Alternatively activated macrophages
induced by IL-4, IL-10, or IL-13 to perform mainly tissue-repair functions.
- Alveolar epithelial type II cells
cells in alveoli, secreting lipoproteins called surfactants.
- Alveolar macrophages (AMs)
specialized lung macrophage population present mainly in alveoli, whose main function in lung maintenance is the removal of surfactant and whose immunoreactivity is comparably low.
- Bacillus Calmette–Guérin (BCG)
live attenuated Mycobacterium strain used for tuberculosis vaccination.
- Bleomycin
drug commonly administered to mice to study post-injury pulmonary fibrosis.
- Clodronate liposomes
lipid vesicles encapsulating clodronate, used to specifically deplete macrophages and other phagocytic cells by apoptosis without causing much inflammation.
- Immunosedation
a term we have coined to describe the effect of the non-inflamed, steady-state lung environment to induce low reactivity in immune cells present in the lung.
- Inflammaging
chronic low-level, age-associated inflammation.
- Inflammatory macrophages
generated on IFN-γ and LPS stimuli; they rely on glycolysis to perform host-defense functions, including secretion of IL-6, IL-1, IL-12, IL-23, TNFα, and other proinflammatory cytokines.
- Lymphopenia
condition of reduced numbers of lymphocytes in the blood.
- Monocyte legacy
epigenetic and transcriptional features in monocyte-derived macrophages, which may be remnants from their prior monocyte identity.
- Myeloid cells
monocytes, macrophages, dendritic cells, granulocytes, mast cells, megakaryocytes, and erythrocytes; arise from a common myeloid progenitor in hematopoiesis.
- Niche model
according to this model, local macrophage development is determined by niche accessibility (is there a barrier?), niche availability (is it unoccupied?), and precursor plasticity (is there a more-suited precursor?).
- Plasticity
capacity to respond to various environmental cues by developing different functional profiles.
- Polarization
functional profile of macrophages developed in response to environmental stimuli. The extremes of polarization defined in vitro are inflammatory and alternatively activated macrophages, but in vivo, intermediate and independent profiles exist.
- Poly I:C
synthetic analog of double-stranded RNA used to mimic RNA-virus infections.
- Pulmonary alveolar proteinosis (PAP)
pulmonary disease due to impaired clearance of surfactants by AMs, leading to surfactant accumulation in alveoli.
- Recruited macrophages
develop from circulating monocytes that originated in the BM and were recruited into a peripheral organ. They can develop self-renewal capacity, similar to tissue-resident macrophages. The terms ‘recruited’, ‘monocyte-derived’, and ‘BM-derived’ macrophages are used interchangeably in this opinion article.
- Resident, or tissue-resident, macrophages
population of sessile macrophages that self-renew in situ by proliferation. They originate from fetal precursors and colonize organs during development. ‘Embryonically derived macrophages’ is used synonymously.
- Specific pathogen free (SPF)
term applied to laboratory animals that are proven to be free of particular pathogens that might cause interference with experimentation.
- Surfactant
complex of lipids and proteins lowering the surface tension in alveoli.
- Toll-like receptor (TLR) agonist
substance that stimulates immune responses by TLR activation.
- Trained immunity
changes in the immunoreactivity of innate immune cells resulting from previous exposure to a stimulus. Here, we use it to discuss medium- to long-term changes in monocyte/macrophage reactivity due to stimulus-induced epigenetic reprogramming.
- Type 2 immune response
triggered by helminths, allergens, and other stimuli; characterized by cytokine production, including IL-4, IL-5, and IL-13.
References
- 1.van Furth R., et al. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull. World Health Organ. 1972;46:845–852. [PMC free article] [PubMed] [Google Scholar]
- 2.Janssen W.J., et al. Myeloid cell turnover and clearance. Microbiol. Spectr. 2016;4:1–16. doi: 10.1128/microbiolspec.MCHD-0005-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashimoto D., et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yona S., et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bain C.C., et al. Long-lived self-renewing bone marrow-derived macrophages displace embryo-derived cells to inhabit adult serous cavities. Nat. Commun. 2016;7:11852. doi: 10.1038/ncomms11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts A.W., et al. Tissue-resident macrophages are locally programmed for silent clearance of apoptotic cells. Immunity. 2017;47:913–927.e6. doi: 10.1016/j.immuni.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uderhardt S., et al. 12/15-Lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity. 2012;36:834–846. doi: 10.1016/j.immuni.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe S., et al. The role of macrophages in the resolution of inflammation. J. Clin. Invest. 2019;129:2619–2628. doi: 10.1172/JCI124615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aegerter H., et al. Influenza-induced monocyte-derived alveolar macrophages confer prolonged antibacterial protection. Nat. Immunol. 2020;21:145–157. doi: 10.1038/s41590-019-0568-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machiels B., et al. A gammaherpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nat. Immunol. 2017;18:1310–1320. doi: 10.1038/ni.3857. [DOI] [PubMed] [Google Scholar]
- 12.Misharin A.V., et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017;214:2387–2404. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Netea M.G., et al. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9:355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Lavin Y., et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginhoux F., Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z., et al. Fate mapping via Ms4a3-expression history traces monocyte-derived cells. Cell. 2019;178:1509–1525.e19. doi: 10.1016/j.cell.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 17.van de Laar L., et al. Yolk sac macrophages, fetal liver, and adult monocytes can colonize an empty niche and develop into functional tissue-resident macrophages. Immunity. 2016;44:755–768. doi: 10.1016/j.immuni.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Nayak D.K., et al. Long-term persistence of donor alveolar macrophages in human lung transplant recipients that influences donor-specific immune responses. Am. J. Transplant. 2016;16:2300–2311. doi: 10.1111/ajt.13819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eguíluz-Gracia I., et al. Long-term persistence of human donor alveolar macrophages in lung transplant recipients. Thorax. 2016;71:1006–1011. doi: 10.1136/thoraxjnl-2016-208292. [DOI] [PubMed] [Google Scholar]
- 20.Byrne A.J., et al. Dynamics of human monocytes and airway macrophages during healthy aging and after transplant. J. Exp. Med. 2020;217 doi: 10.1084/jem.20191236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavin Y., et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell. 2017;169:750–765. doi: 10.1016/j.cell.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zilionis R., et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity. 2019;50:1317–1334. doi: 10.1016/j.immuni.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peiró T., et al. Neutrophils drive alveolar macrophage IL-1β release during respiratory viral infection respiratory infection. Thorax. 2018;73:546–556. doi: 10.1136/thoraxjnl-2017-210010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pribul P.K., et al. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. J. Virol. 2008;82:4441–4448. doi: 10.1128/JVI.02541-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumagai Y., et al. Alveolar macrophages are the primary interferon-α producer in pulmonary infection with RNA viruses. Immunity. 2007;27:240–252. doi: 10.1016/j.immuni.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Goritzka M., et al. Alveolar macrophage-derived type I interferons orchestrate innate immunity to RSV through recruitment of antiviral monocytes. J. Exp. Med. 2015;212:699–714. doi: 10.1084/jem.20140825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghoneim H.E., et al. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J. Immunol. 2013;191:1250–1259. doi: 10.4049/jimmunol.1300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawson T.C., et al. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am. J. Pathol. 2000;156:1951–1959. doi: 10.1016/S0002-9440(10)65068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin K.L., et al. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J. Immunol. 2008;180:2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- 30.McCubbrey A.L., et al. Deletion of c-FLIP from CD11bhi macrophages prevents development of bleomycin-induced lung fibrosis. Am. J. Respir. Cell Mol. Biol. 2018;58:66–78. doi: 10.1165/rcmb.2017-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joshi N., et al. A spatially restricted fibrotic niche in pulmonary fibrosis is sustained by M-CSF/M-CSFR signalling in monocyte-derived alveolar macrophages. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00646-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sajti E., et al. Transcriptomic and epigenetic mechanisms underlying myeloid diversity in the lung. Nat. Immunol. 2020;21:221–231. doi: 10.1038/s41590-019-0582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guilliams M., Scott C.L. Does niche competition determine the origin of tissue-resident macrophages? Nat. Rev. Immunol. 2017;17:451–460. doi: 10.1038/nri.2017.42. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins S.J., et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenkins S.J., et al. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J. Exp. Med. 2013;210:2477–2491. doi: 10.1084/jem.20121999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minutti C.M., et al. Local amplifiers of IL-4Ra-mediated macrophage activation promote repair in lung and liver. Science. 2017;356:1076–1080. doi: 10.1126/science.aaj2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Didierlaurent A., et al. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J. Exp. Med. 2008;205:323–329. doi: 10.1084/jem.20070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foster S.L., et al. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 39.Kamei A., et al. Exogenous remodeling of lung resident macrophages protects against infectious consequences of bone marrow-suppressive chemotherapy. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E6153–E6161. doi: 10.1073/pnas.1607787113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao Y., et al. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell. 2018;175:1634–1650. doi: 10.1016/j.cell.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 41.Saeed S., et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345 doi: 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roquilly A., et al. Alveolar macrophages are epigenetically altered after inflammation, leading to long-term lung immunoparalysis. Nat. Immunol. 2020;21:636–648. doi: 10.1038/s41590-020-0673-x. [DOI] [PubMed] [Google Scholar]
- 43.Arts R.J.W., et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23:89–100. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Cirovic B., et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe. 2020;28:322–334.e5. doi: 10.1016/j.chom.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitroulis I., et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell. 2018;172:147–161. doi: 10.1016/j.cell.2017.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaufmann E., et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell. 2018;172:176–190. doi: 10.1016/j.cell.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 47.Hermesh T., et al. Antiviral instruction of bone marrow leukocytes during respiratory viral infections. Cell Host Microbe. 2010;7:343–353. doi: 10.1016/j.chom.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Askenase M.H., et al. Bone-marrow-resident NK cells prime monocytes for regulatory function during infection. Immunity. 2015;42:1130–1142. doi: 10.1016/j.immuni.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibbings S.L., et al. Transcriptome analysis highlights the conserved difference between embryonic and postnatal-derived alveolar macrophages. Blood. 2015;126:1357–1366. doi: 10.1182/blood-2015-01-624809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park S.H., et al. Tumor necrosis factor induces GSK3 kinase-mediated cross-tolerance to endotoxin in macrophages. Nat. Immunol. 2011;12:607–615. doi: 10.1038/ni.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park S.H., et al. Type I interferons and the cytokine TNF cooperatively reprogram the macrophage epigenome to promote inflammatory activation. Nat. Immunol. 2017;18:1104–1116. doi: 10.1038/ni.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barrat F.J., et al. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 2019;20:1574–1583. doi: 10.1038/s41590-019-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piccolo V., et al. Opposing macrophage polarization programs show extensive epigenomic and transcriptional cross-talk. Nat. Immunol. 2017;18:530–540. doi: 10.1038/ni.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu X., et al. The cytokine TGF-β promotes the development and homeostasis of alveolar macrophages. Immunity. 2017;47:903–912. doi: 10.1016/j.immuni.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Guilliams M., et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dranoff G., et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 57.Bonfield T.L., et al. Peroxisome proliferator-activated receptor-γ is deficient in alveolar macrophages from patients with alveolar proteinosis. Am. J. Respir. Cell Mol. Biol. 2003;29:677–682. doi: 10.1165/rcmb.2003-0148OC. [DOI] [PubMed] [Google Scholar]
- 58.Schneider C., et al. Induction of the nuclear receptor PPAR-γ 3 by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat. Immunol. 2014;15:1026–1037. doi: 10.1038/ni.3005. [DOI] [PubMed] [Google Scholar]
- 59.Svedberg F.R., et al. The lung environment controls alveolar macrophage metabolism and responsiveness in type 2 inflammation. Nat. Immunol. 2019;20:571–580. doi: 10.1038/s41590-019-0352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shibata Y., et al. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001;15:557–567. doi: 10.1016/s1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]
- 61.Heming M., et al. Peroxisome proliferator-activated receptor-γ modulates the response of macrophages to lipopolysaccharide and glucocorticoids. Front. Immunol. 2018;9:893. doi: 10.3389/fimmu.2018.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma X., et al. Deciphering the roles of PPARγ in adipocytes via dynamic change of transcription complex. Front. Endocrinol. (Lausanne) 2018;9:473. doi: 10.3389/fendo.2018.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bates S.R., Fisher A.B. Surfactant protein A is degraded by alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 1996;271:L258–L266. doi: 10.1152/ajplung.1996.271.2.L258. [DOI] [PubMed] [Google Scholar]
- 64.Garnett J.P., et al. Sweet talk: insights into the nature and importance of glucose transport in lung epithelium. Eur. Respir. J. 2012;40:1269–1276. doi: 10.1183/09031936.00052612. [DOI] [PubMed] [Google Scholar]
- 65.O’Neill L.A.J.J., et al. Nature Publishing Group; 2016. A Guide to Immunometabolism for Immunologists. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Viola A., et al. The metabolic signature of macrophage responses. Front. Immunol. 2019;10:1462. doi: 10.3389/fimmu.2019.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang S.C.C., et al. Metabolic reprogramming mediated by the mTORC2–IRF4 signaling axis is essential for macrophage alternative activation. Immunity. 2016;45:817–830. doi: 10.1016/j.immuni.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tan Z., et al. Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. J. Immunol. 2015;194:6082–6089. doi: 10.4049/jimmunol.1402469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suzuki H., et al. Glycolytic pathway affects differentiation of human monocytes to regulatory macrophages. Immunol. Lett. 2016;176:18–27. doi: 10.1016/j.imlet.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 70.Zhang D., et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574:575–580. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng S-C.C., et al. MTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345 doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arts R.J.W., et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. 2016;17:2562–2571. doi: 10.1016/j.celrep.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mallia P., et al. Role of airway glucose in bacterial infections in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018;142:815–823. doi: 10.1016/j.jaci.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Phan A.T., et al. Metabolic and epigenetic coordination of T cell and macrophage immunity. Immunity. 2017;46:714–729. doi: 10.1016/j.immuni.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsou C-L., et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Invest. 2007;117:902. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murphy J., et al. The prolonged life-span of alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 2008;38:380–385. doi: 10.1165/rcmb.2007-0224RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liao M., et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 78.Cui H., et al. Monocyte-derived alveolar macrophage apolipoprotein E participates in pulmonary fibrosis resolution. JCI Insight. 2020;5 doi: 10.1172/jci.insight.134539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giamarellos-Bourboulis E.J., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu J., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu Z., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;2600:19–21. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nicholls J.M., et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Channappanavar R., et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu X., et al. Single-cell analysis reveals macrophage-driven T cell dysfunction in severe COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.05.23.20100024. Published online May 26, 2020. [DOI] [Google Scholar]
- 88.Wen W., et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020;6:31. doi: 10.1038/s41421-020-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee J.S., et al. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci. Immunol. 2020;5:1554. doi: 10.1126/sciimmunol.abd1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu B., et al. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020;111 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang C., et al. The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lang F.M., et al. GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat. Rev. Immunol. 2020;20:507–514. doi: 10.1038/s41577-020-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou Y., et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Herold S., et al. Inhaled granulocyte/macrophage colony-stimulating factor as treatment of pneumonia-associated acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2014;189:609–611. doi: 10.1164/rccm.201311-2041LE. [DOI] [PubMed] [Google Scholar]
- 96.Halstead E.S., et al. GM-CSF overexpression after influenza a virus infection prevents mortality and moderates M1-like airway monocyte/macrophage polarization. Respir. Res. 2018;19 doi: 10.1186/s12931-017-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carsana L., et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30434-5. Published online June 8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang C., et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBioMedicine. 2020;57 doi: 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoepel W., et al. Anti-SARS-CoV-2 IgG from severely ill COVID-19 patients promotes macrophage hyper-inflammatory responses. bioRxiv. 2020 doi: 10.1101/2020.07.13.190140. Published online July 13, 2020. [DOI] [Google Scholar]