Abstract
Objectives
Although the increasing cancer incidence in older patients is widely recognised, older patients remain underrepresented in clinical cancer trials and eHealth studies. The aim of this research is to identify technological and patient-related barriers to inclusion of this population in a clinical eHealth study.
Material and Methods
This is a retrospective analysis of a prospective cohort study with older patients (≥ 65 years) undergoing cancer-related surgery, who were identified for a perioperative telemonitoring study. Reasons for ineligibility and refusal had been prospectively registered. Characteristics and postoperative outcomes were compared between participants and non-participants.
Results
Between May 2018 and March 2020, 151 patients were assessed for eligibility, resulting in 65 participants and 86 non-participants. The main reason for ineligibility was lack of internet access at home (n = 16), while main reasons for refusal were perceived high mental burden (n = 46) and insufficient digital skills (n = 12). Compared with participants, non-participants were significantly older (mean age 75 vs. 73, p = 0.01); more often female (64% vs. 35%, p = 0.00), unmarried (42% vs. 8%, p = 0.01) living alone (38% vs. 19%, p = 0.02); had a higher ASA classification (43% vs. 19%, p = 0.00); often had polypharmacy (67% vs. 43%, p = 0.00); and were more often discharged to skilled nursing facilities (0% vs. 15%, p = 0.00).
Conclusion
Our results confirm the underrepresentation of older female patients with little support from a partner and higher comorbidity. We should be aware of technological and patient-related barriers to including older adults with cancer, in order to avoid further dividing patients with low and high digital health literacy.
Keywords: Telemedicine, eHealth, Postoperative care, Aged, Cancer, Surgical oncology, Digital health literacy, Preventive health services
1. Introduction
Older patients (over the age of 65) represent the majority of global cancer cases, with a predicted absolute number of 14 million worldwide by 2035 [1]. Surgery is a fundamental part of treatment in more than 80% of cancer cases, as well as for older patients [2]. Higher age alone does not necessarily increase the risk of adverse postoperative events, but the prevalence of age-associated comorbidities and frailty (age-related cumulative decline in multiple physiological systems) does increase this risk [3]. Frail older patients are three to four times more likely to develop postoperative complications compared with non-frail older patients [4,5]. Moreover, the occurrence of complications has a considerable impact on the quality of life and the survival of older patients [6]. Together with the fact that hospital admissions have been shortened due to changes in modern health care [7], this highlights the necessity of prevention and early detection of postoperative complications in this population.
New digital technologies (i.e., eHealth) are emerging rapidly in health care to promote patients' self-management and engagement and improve patient-centred cancer care [8]. The interest in remote care delivery by eHealth has increased even more during the current COVID-19 pandemic, as remote consultation decreases the risk of spreading the virus and could decrease the pressure on health care resources [9,10]. Additionally, eHealth is used to remotely monitor patients' postoperative recovery in surgical wards or at home after hospital discharge [11,12]. This so-called telemonitoring could contribute to timely detection of postoperative complications and therefore potentially decrease the impact of these complications in frail older patients with cancer [13].
Although the increasing incidence of cancer in older patients is widely recognised, these patients remain underrepresented in clinical cancer trials [14,15]. They are excluded from clinical cancer trials because of study restrictions, comorbidity, polypharmacy, or physicians' attitudes [16]. Older patients are also underrepresented in most perioperative eHealth intervention studies. This underrepresentation of older, and often frail, patients leads to a bias in research outcomes, non-generalisable results and inequality in healthcare provided [17]. This poses a real risk that eHealth interventions will remain geared towards a younger, more flexible population, and will result in the exclusion of the population likely to show the greatest benefit. Also, eHealth literacy is known to be lower among older adults with cancer compared with their younger counterparts [18]. The COVID-19 pandemic has further increased the need for new digital solutions in health care and clinical research [9,10]. It is thus of the utmost importance to identify barriers to participation in clinical eHealth trials among the older population. When these barriers are known, both clinical eHealth trials and eHealth applications may be adjusted so that they may benefit the entire oncological population, including frail older patients.
In a prospective cohort study with the aim of assessing feasibility of perioperative telemonitoring of older patients with cancer, we were able to include approximately half of the identified patients. To investigate possible technological and patient-related barriers to participation, we analysed reasons for ineligibility and refusal and differences in characteristics of non-participants and participants. To explore the impact of possible benefits a postoperative telemonitoring intervention could provide for our population, we additionally compared the postoperative outcomes between non-participants and participants.
2. Material and Methods
2.1. Study Design
This study is a retrospective analysis of a prospective cohort study with older patients undergoing cancer-related surgery, who were identified for a perioperative telemonitoring study (Netherlands trial registration number: NL 8253) [19]. The prospective telemonitoring study was conducted in a tertiary referral hospital in the north of the Netherlands and approved by the local medical ethical committee (registration number: 2017/286). In consultation with legal officers at our local medical ethical committee we obtained permission to collect additional routine data on care of all identified patients. The principal reason was to collect reasons why candidates did not participate, to identify potential modifiable factors to improve on this situation for future studies. Also, it was evaluated that obtaining additional consent was perceived too burdensome for patients and/or carers.
2.2. Setting and Patients
We had identified patients over the age of 65 with an indication for oncological resection of a solid malignant tumour. Patients had been approached at the hospital's outpatient clinic or by telephone in the period between May 2018 and March 2020, after they were identified for the study by a surgical nurse or surgeon from the treatment team. Patients were eligible if they had internet access at home. Exclusion criteria were severe auditory, visual and cognitive impairment that were expected to impair the ability to use digital technologies or hear/understand the explanation by telephone; being wheelchair- or bed-ridden; having contact dermatitis; insufficient understanding of Dutch; and emergency surgery.
Participants had been assessed at three moments in time: before surgery, before hospital discharge and at three months after surgery. Participants had used a mobile application connected to various electronic monitoring devices. Physical activity had been measured using an accelerometer-based wearable activity monitor (Fitbit Charge 2, Fitbit Inc., San Francisco, CA, USA) during the entire study period. For two weeks after hospital discharge, postoperative recovery had been monitored using the mobile application and additional devices to measure temperature, blood pressure, heart rate, pain, and the occurrence of other postoperative symptoms. Due to the observational character of the study, no intervention followed when a deviation had been detected in monitored data. Patients had only been contacted by telephone by the research physician if no data was transferred or if alarming parameters had been observed. Following the latter, the treating physician would have been contacted if there was a need for medical consultation.
We had implemented several strategies in our study design to minimise refusal, based on solutions presented in previous studies for approaching older patients [20]. First, we recognised the importance of adequate communication, especially with older patients. We preferred face-to-face contact to inform patients, offered clearly written study information and emphasized that the study case manager in charge was easily available by telephone for any questions during the study period. Second, we involved patients' family members in the recruitment process, as family members have a major influence on the decision to participate. The study information at the outpatient clinic was preferably provided with a family member present. The supporting role of the family member was emphasized before the start of the study, and if the patient preferred that communication about study participation or technological explanation was given to a family member, this family member was approached by telephone. Third, we decided to plan follow-up visits with patients at home or schedule appointments to coincide with planned hospital visits because additional hospital visits discourage patients from participating [20]. These strategies to minimise refusal were also meant to promote study completion. Family members were involved in technical actions. Technology support was provided by the case manager throughout the whole study period by telephone and if necessary, at home or coinciding with planned hospital visits [19].
2.3. Data Collection and Handling
Reasons for ineligibility and refusal had been prospectively registered in a database by the case manager directly after assessing eligibility or after approaching patients for the prospective telemonitoring study. Relevant demographics, preoperative indicators of frailty, surgical data and postoperative complications of participants were prospectively collected in face-to-face assessments and from hospital medical records. Routine care data about non-participants was retrospectively collected from hospital medical records to evaluate health outcomes. No additional non-consented patient data was collected outside routine care. Collected data on the somatic domain of frailty included preoperative physical status assessed by an anaesthesiologist (American Society of Anesthesiologists [21] [ASA classification]), comorbidity (Charlson Comorbidity Index [22]) and, polypharmacy (>4 different types of medication [23]). Nutritional status was assessed using body mass index (BMI). Marital status and housing data were collected to indicate social status. Data on the psychological domain was collected from the routine consultation with a nurse at admission and registered in the medical records; including i) concerns about hospital admission, ii) anxiety that influenced daily life and, iii) the use of any psychiatric medication. Functional status had been determined using the reported Katz Activities of Daily Living (ADL [24]) score.
Data on tumour location, recurrence of disease, primary malignancy, neoadjuvant therapy, and anaesthesia time was collected. Postoperative outcome measures found in the medical records of the individual treatment centre, were collected from its administration. Postoperative outcome measures included postoperative ICU (intensive care unit) admission, length of hospital stay, complications related to surgery in-hospital and within 30 days after discharge (Clavien–Dindo classification ≥2 [25]), unplanned hospital readmission to the individual treatment centre and outside the treatment centre within 30 days after discharge, referral to a nursing home or skilled nursing facility (SNF) post-discharge, and overall survival at three and twelve months.
2.4. Statistical Analysis
We compared characteristics and outcomes from non-participants and participants using an independent sample t-test for parametric continuous data, Mann-Whitney U test for non-parametric continuous data, and Pearson's chi-squared or Fisher's exact test for categorical data. A p-value <0.05 was considered statistically significant. Data on baseline characteristics was only used for analysis if it was available for more than 90% of both groups. We compared postoperative outcomes for all patients and per subgroup, classified by type of primary malignancy (gastro-intestinal, gynaecological, or other oncology). The participants and non-participants who underwent surgery were included in overall survival analyses using the Kaplan-Meier with log-rank testing. Data was analysed with IBM SPSS Statistics version 23 (IBM Corporation, Armonk, NY).
3. Results
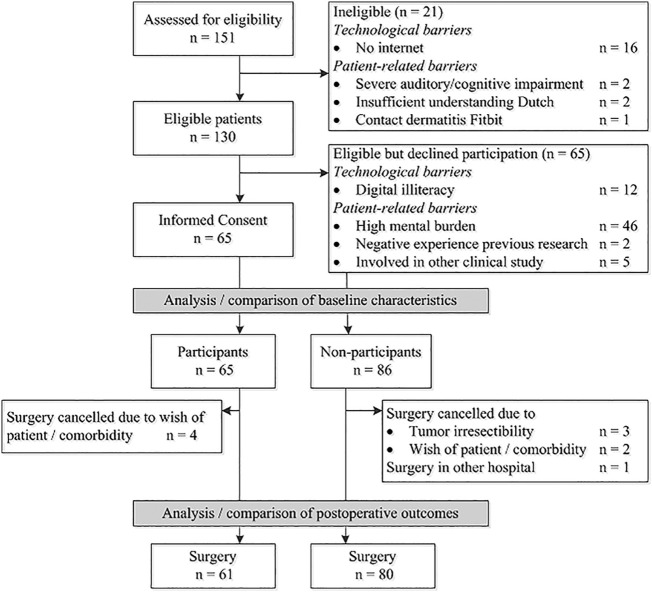
Out of 151 patients who were assessed for eligibility, 65 patients consented to participate, and 86 patients did not participate (Fig. 1 ). Of the 86 non-participants, 21 patients were not eligible for participation and 65 patients did not want to participate. Technological barriers to participation were lack of internet access at home (n = 16) and the perceived inability to work with electronic devices and mobile applications (digital illiteracy, n = 12). The main patient-related barrier was a perceived high mental burden (n = 46). Baseline characteristics of participants and non-participants are presented in Table 1 . Compared with participants, non-participants were significantly older and more often female (Table 1). In addition, non-participants had a significantly higher ASA classification, more polypharmacy and less social support based on data regarding marital status and housing circumstances. Non-participants were more often ADL-dependent compared with participants, although this difference was not statistically significant.
Fig. 1.
Study flowchart.
Table 1.
Patient and tumour characteristics.
| Variables | Participants N = 65 | Non-Participants N = 86 | p-value |
|---|---|---|---|
| General patient characteristics | |||
| Age, mean in years (SD) | 72.8 (5.4) | 75.1 (5.7) | 0.01* |
| Gender, n (%) | |||
| Male | 42 (64.6) | 31 (36.0) | |
| Female | 23 (35.4) | 55 (64.0) | 0.00* |
| Nationality, n (%) | |||
| Dutch | 64 (98.5) | 81 (94.2) | 0.24 |
| Domains of frailty | |||
| Somatic - Comorbidity | |||
| ASA-classification,n (%) | |||
| ASA 1–2 | 53 (81.5) | 49 (57.0) | |
| ASA 3–4 | 12 (18.5) | 37 (43.0) | 0.00* |
| Charlson Comorbidity Index, median (IQR) | 4.0 (2.0–6.0) | 3.0 (2.0–6.0) | 0.88 |
| Polypharmacy (≥ 4), n (%) | 28 (43.1) | 58 (67.4) | 0.00* |
| Nutritional status | |||
| BMI, mean (SD) | 26.9 (4.2) | 28.0 (6.0) | 0.18 |
| Social status | |||
| Marital status, n (%) | |||
| Married | 53 (81.5) | 50 (58.1) | |
| Widow(er) | 9 (13.9) | 24 (27.9) | |
| Divorced | 1 (1.5) | 5 (5.8) | |
| Single | 2 (3.1) | 7 (8.1) | 0.01* |
| Housing,n (%) | |||
| Independent, alone | 12 (18.8) | 33 (38.4) | |
| Independent, with others | 52 (81.3) | 51 (59.3) | |
| Nursing home | 0 | 2 (2.3) | 0.02* |
| Psychological status | |||
| Concerns about hospital admission, n (%) a | 17 (27.9) | 27 (34.2) | 0.47 |
| Anxiety that influences daily life, n (%) a | 4 (6.6) | 4 (5.1) | 0.73 |
| Use of psychiatric medication? n (%)b | 6 (9.8) | 8 (10.3) | 1.00 |
| Functional status | |||
| Impaired ADLc (Katz ≥1), n (%) | 4 (6.2) | 14 (16.7) | 0.05 |
| Participation in other research | |||
| - Yes, n (%) | 34 (52.3) | 39 (45.3) | 0.42 |
| Tumour characteristics | |||
| Tumour location,n (%) | |||
| Intracavitary | 54 (83.1) | 64 (74.4) | |
| Superficial | 11 (16.9) | 22 (25.6) | 0.20 |
| Primary Malignancy | |||
| Gastro-intestinal oncology d | 48 (73.8) | 53 (61.6) | |
| Gynaecological oncology e | 7 (10.8) | 19 (22.1) | |
| Other oncology f | 10 (15.4) | 14 (16.3) | 0.17 |
| Recurrent disease, yes, n (%) | 18 (27.7) | 35 (40.7) | 0.12 |
| Neoadjuvant therapy, yes, n (%) | 24 (36.9) | 36 (41.9) | 0.54 |
SD: standard deviation; ASA: American Society of Anesthesiologists [19]; BMI: body mass index; ADL: activities of daily living; ⁎Statistically significant, p < 0.05.
Data missing for four participants and seven non-participants.
Data missing for four participants and eight non-participants.
Data missing for two non-participants.
Mostly colorectal cancer (n = 77).
Vulva carcinoma (n = 20) and ovarium carcinoma (n = 6).
Mostly sarcoma (n = 12).
From the 65 patients who consented to participate, seven patients were excluded before surgery and 43 patients completed the study. Reasons for study drop-out were cancellation of surgery, logistic issues regarding baseline assessment, or the combination of a high burden of disease and treatment and performing measurements at home. Results of our feasibility study demonstrated that the compliance of performing vital sign measurements and completing electronic health questionnaires was lower than synchronising physical activity (Fitbit-)data, suggesting that these aspects were challenging for the patients [19].
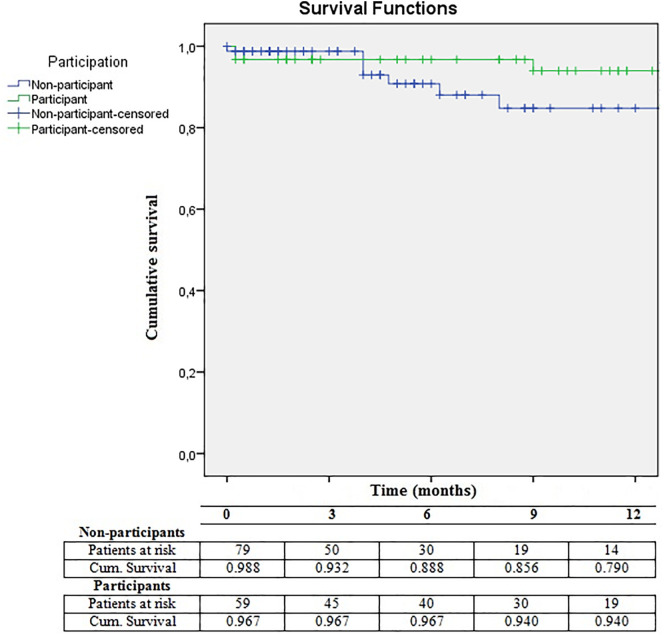
Surgery was cancelled for four participants and six non-participants, resulting in analysis of postoperative outcomes of 61 participants and 80 non-participants (Fig. 1; Table 2 ). Compared with participants, non-participants had similar complication rates. Difference in readmission rates were not statistically significant (23% vs. 15%, p = 0.27). In sub-analysis, these differences in postoperative adverse event rates tended to be larger in the patients who underwent gastro-intestinal oncological surgery, although the difference remained not statistically significant. Non-participants were significantly more often discharged to an SNF compared with participants. The twelve patients who were discharged to an SNF were significantly older (mean age 79.0 versus 73.6 years old [p = 0.01]), had a higher ASA classification (ASA 3–4 58% versus 29% [p = 0.05]), used more medication (% polypharmacy 92% versus 50% [p = 0.00]) and were more often living alone or in a nursing home before surgery (50% versus 30%, 17% versus 0% [p = 0.00]). The survival analysis in Fig. 2 demonstrates no difference in survival between three and twelve months for non-participants compared with participants (p = 0.37).
Table 2.
Number of participants and non-participants with postoperative adverse outcomes, in total and per type of surgery.
| Participants | Non-participants | p-value | |
|---|---|---|---|
| Surgery performed | N = 61 | N = 80 | |
| As planned | 60 (98.4) | 77 (96.3) | |
| Irresectable tumour | 1 (1.6) | 3 (3.7) | 0.63 |
| Type of surgery,n (%) | |||
|
44 (72.1) | 48 (60.0) | |
|
7 (11.5) | 19 (23.8) | |
|
10 (16.4) | 13 (16.3) | 0.17 |
| In-hospital | |||
| Postoperative ICU,n (%) | 13 (21.3) | 24 (30.0) | 0.33 |
|
12 (27.3) | 21 (43.8) | 0.13 |
|
0 | 2 (10.5) | 1.00 |
|
1 (10.0) | 1 (7.7) | 1.00 |
| Median length of hospital stays, days (IQR) | 8.0 (4.0–21.0) | 10.0 (5.0–17.0) | 0.92 |
|
13.0 (7.0–22.0) | 15.0 (9.0–20.0) | 0.30 |
|
2.0 (2.0–3.0) | 2.0 (2.0–5.0) | 0.50 |
|
4.0 (2.5–13.0) | 6.0 (4.0–10.5) | 0.24 |
| In-hospital complications,an (%) | 19 (31.1) | 28 (35.0) | 0.63 |
|
16 (36.4) | 25 (52.1) | 0.13 |
|
0 | 1 (5.3) | 1.00 |
|
3 (30.0) | 2 (15.4) | 0.62 |
| In-hospital mortality, n (%) | 2 (3.3) | 1 (1.3) | 0.58 |
|
1 (2.3) | 1 (2.1) | 1.00 |
|
0 | 0 | – |
|
1 (10.0) | 0 | 0.44 |
| After hospital discharge | |||
| Referral skilled nursing facility, n (%) | 0 | 12 (15.2) | 0.00* |
|
0 | 6 (12.8) | 0.03* |
|
0 | 4 (21.1) | 0.55 |
|
0 | 2 (15.4) | 0.50 |
| Complications at home, a, bn (%) | 12 (20.3) | 17 (21.5) | 0.87 |
|
7 (16.3) | 11 (23.4) | 0.40 |
|
1 (14.3) | 1 (5.3) | 0.47 |
|
4 (44.4) | 5 (38.5) | 1.00 |
| Unplanned readmissions, bn (%) | 9 (15.3) | 18 (22.8) | 0.27 |
|
5 (11.6) | 13 (27.7) | 0.06 |
|
0 | 1 (5.3) | 1.00 |
|
4 (44.4) | 4 (30.8) | 0.66 |
ICU: intensive care unit; IQR: interquartile range; ⁎Statistically significant, p < 0.05.
Complications classified as Clavien–Dindo 2 or higher.
Complications and unplanned readmissions within 30 days post-discharge.
Fig. 2.
Survival analyses of non-participants and participants.
4. Discussion
In this prospective cohort study, we investigated technological and patient-related barriers to participation of older patients with cancer-related surgery in a perioperative telemonitoring study. Main inclusion barriers were ineligibility due to lack of internet access at home, refusal due to digital illiteracy (the perceived inability to work with electronic devices and mobile applications), and a perceived high mental burden. Non-participants were older, were more often female, had a higher ASA classification, used more medication, and were more often living alone compared with participants. About one fifth of participants and non-participants experienced a serious complication after hospital discharge. In addition, we observed significantly more SNF referrals for non-participants compared with participants. No statistical differences were observed in other postoperative outcomes between participants and non-participants.
In our study, 11% of all patients who were assessed for eligibility could not participate because they had no internet access at home. This corresponds with statistics provided by the Dutch Central Bureau of Statistics [26]. Although access to the internet in the Netherlands has improved considerably in the past decade, in 2019 6% of the Dutch population aged 65–75 and 23% of people aged over 75 still had no internet access at home [26]. Another 8% of all patients who were assessed for eligibility refused because they thought they possessed insufficient digital skills or felt uncomfortable with acquiring these skills for study purposes. Studies have confirmed that the main reason people refuse to learn new technologies is anxiety about using them [27]. In addition, ageing causes a decrease in self-efficacy, memory and speed of learning [27]. However, if the perceived advantages of new digital technologies are large and relevant enough and family or peer support is present, older adults are able to overcome their fears and start learning to use new technology [28,29].
One of the main reasons for refusal was a perceived high mental burden, which might be related to technological barriers as well. An inclusion rate of 50% (65/130) was achieved through several strategies in our study design such as face-to-face contact, involving family members in the recruitment process and, flexible home study visits [20]. The difference in characteristics of participants and non-participants in our study corresponds with previous studies [15,17,18,30]. Previous eHealth studies have also demonstrated that older, unmarried, less educated, and lower-income patients use health applications for self-management less frequently than their younger counterparts [30]. Unfortunately, we did not have sufficient data on education level and social-economic status in our population. However, data on social status, housing, and referral to SNFs suggests that non-participants had less social support. Also, the two patients who were residing in a SNF both refused participation. We believe that improving social support would decrease both technological barriers and refusal rates due to a perceived high mental burden.
The acceptance and implementation of new digital technologies has been accelerated by the COVID-19 pandemic, as remote consultation and monitoring decrease the risk of spreading the virus [9]. These changes will lead to a more prominent and perhaps permanent role for telemedicine in future health care and underline the urgency of improving digital technology skills in specific populations such as older adults [9]. Because learning new digital skills takes time and energy [27], it is best to empower older adults to do so when they are relatively healthy and not when they have just been diagnosed with cancer or scheduled for surgery. Furthermore, it is essential that people who have insufficient social support can rely on professional or peer support provided by, for example, older adult advocacy groups or the government [31].
A limitation of this study is that we did not have information on the patients' socio-economic status, educational level, geriatric assessment, or impact of complications on functional recovery and quality of life. This is inherent to the retrospective analysis of a prospective cohort study. Approximately one fifth of all patients experienced a serious complication within 30 days after hospital discharge, and hospital readmission rates were 15% for participants and 23% for non-participants. Because we retrospectively collected data regarding non-participants from hospital medical records, complications and readmissions outside our hospital might have been missed; on the other hand, for participants, data on complications and readmissions were complemented with self-reported data at three months follow-up. In addition, participation in the telemonitoring study might have led to identification of more complications. Nonetheless, these results demonstrate a high incidence of postoperative complications post-discharge for all patients. More referrals to SNFs among non-participants also suggest that complications have a larger impact on this group. Additional parameters to measure the impact of complications, such as functional recovery, quality of life and long-term survival, are needed in future research. Subsequent telemonitoring studies with older adults should consider various logistical problems in usability and acceptability [19]. When considering the technological and mental barriers described in this study, studies could be even more inclusive. For example, WiFi hotspots could be provided at home for the patients without internet access at home. A technical ‘buddy’ could be assigned or technological support materials developed to decrease the fear of new technologies and enrol patients with digital illiteracy.
5. Conclusion
The main barriers to older adults' participation in a perioperative telemonitoring study were lack of internet access at home, digital illiteracy, and a perceived high mental burden. Non-participants were older and more often female, had a higher ASA classification and more polypharmacy, and more often lived alone without a partner compared with participants. The complication rate was high in both participants and non-participants, with a seemingly greater impact of those complications in non-participants. This demonstrates the need for inclusion of underrepresented patients, who are at a high risk for severe postoperative complications and who experience a large impact of these complications. We should be aware of the barriers to participation of this population in order to avoid further dividing patients with low and high digital health literacy. Solutions to improve this situation are needed on a societal level and include improving internet accessibility, teaching digital skills and expanding social support for older people.
Author Contributions
Conceptualization: LTJ, MMHL, SF, GHdB, BLvL; Data curation: LTJ, MMHL, MHMO; Formal analysis : LTJ, MMHL, SF, GHdB, BLvL; Funding acquisition: MMHL; Formal analysis : LTJ, MMHL, SF, GHdB, BLvL; Funding acquisition: MMHL; Investigation: LTJ; Methodology: LTJ, MMHL, SF, GHdB, BLvL; Project administration: LTJ, MMHL; Resources: LTJ, MMHL; Software: LTJ; Supervision: MMHL, GHdB, BLvL; Validation: LTJ, MMHL; Visualization: LTJ; Writing - review & editing: MMHL, SF, MHMO, GHdB, BLvL.
Funding
The prospective telemonitoring study was funded by European Union's Horizon 2020 Research & Innovation Program (project grant agreement number 689802, CONNECARE). The funding source had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; nor in the decision to submit the paper for publication.
Declaration of Competing Interest
LTJ: no conflicts of interest to declare; MMH: no conflicts of interest to declare; SF: no conflicts of interest to declare; MHMO: no conflicts of interest to declare; GHdB: no conflicts of interest to declare; BLvL: no conflicts of interest to declare.
Acknowledgements
We wish to express our gratitude to all colleagues in the Connecare consortium for providing input during the development and supporting of the IT systems and connected devices used in the prospective telemonitoring study.
References
- 1.Pilleron S., Sarfati D., Janssen-Heijnen M. Global cancer incidence in older adults, 2012 and 2035: a population-based study. Int. J. Cancer. 2019;144(1):49–58. doi: 10.1002/ijc.31664. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan R., Alatise O.I., Anderson B.O. Global cancer surgery: delivering safe, affordable, and timely cancer surgery. Lancet Oncol. 2015;16(11) doi: 10.1016/S1470-2045(15)00223-5. 1193–24. [DOI] [PubMed] [Google Scholar]
- 3.Lin H., Watts J.N., Peel N.M., Hubbard R.E. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16(1):157. doi: 10.1186/s12877-016-0329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan K.Y., Kawamura Y.J., Tokomitsu A., Tang T. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. Am. J. Surg. 2012 Aug;204(2):139–143. doi: 10.1016/j.amjsurg.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Kristjansson S.R., Nesbakken A., Jordhøy M.S. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study. Crit. Rev. Oncol. Hematol. 2010 Dec;76(3):208–217. doi: 10.1016/j.critrevonc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Weerink L.B.M., Gant C.M., van Leeuwen B.L., de Bock G.H., Kouwenhoven E.A., Faneyte I.F. Long-term survival in octogenarians after surgical treatment for colorectal Cancer: prevention of postoperative complications is key. Ann. Surg. Oncol. 2018 Dec;25(13):3874–3882. doi: 10.1245/s10434-018-6766-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regenbogen S.E., Cain-Nielsen A.H., Norton E.C., Chen L.M., Birkmeyer J.D., Skinner J.S. Costs and consequences of early hospital discharge after major inpatient surgery in older adults. JAMA Surg. 2017 May 17;152(5) doi: 10.1001/jamasurg.2017.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penedo F.J., Oswald L.B., Kronenfeld J.P., Garcia S.F., Cella D., Yanez B. The increasing value of eHealth in the delivery of patient-centred cancer care. Lancet Oncol. 2020 05/01 doi: 10.1016/S1470-2045(20)30021-8. e240–e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bashshur R., Doarn C.R., Frenk J.M., Kvedar J.C., Woolliscroft J.O. Telemedicine and the COVID-19 pandemic, lessons for the future. Telemed. J. E Health. 2020 May;26(5):571–573. doi: 10.1089/tmj.2020.29040.rb. [DOI] [PubMed] [Google Scholar]
- 10.Khairat S., Meng C., Xu Y., Edson B., Gianforcaro R. Interpreting COVID-19 and virtual care trends: cohort study. JMIR Public Health Surveill. 2020 Apr 15;6(2) doi: 10.2196/18811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downey C., Randell R., Brown J., Jayne D.G. Continuous versus intermittent vital signs monitoring using a wearable, wireless patch in patients admitted to surgical wards: pilot cluster randomized controlled trial. J. Med. Internet Res. 2018 Dec 11;20(12) doi: 10.2196/10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheper H., Derogee R., Mahdad R. A mobile app for postoperative wound care after arthroplasty: ease of use and perceived usefulness. Int. J. Med. Inform. 2019 Sep;129:75–80. doi: 10.1016/j.ijmedinf.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Branowicki P.M., Vessey J.A., Graham D.A. Meta-analysis of clinical trials that evaluate the effectiveness of hospital-initiated Postdischarge interventions on hospital readmission. J. Healthc. Qual. Nov/Dec 2017;39(6):354–366. doi: 10.1097/JHQ.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 14.Hutchins L.F., Unger J.M., Crowley J.J., Coltman C.A., Albain K.S. Underrepresentation of patients 65 years of age or older in Cancer-treatment trials. N. Engl. J. Med. 1999;341(27):2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 15.Ford J.G., Howerton M.W., Lai G.Y. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008 Jan 15;112(2):228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 16.Aapro M.S., Köhne C., Cohen H.J., Extermann M. Never too old? Age should not be a barrier to enrollment in Cancer clinical trials. Oncologist. 2005 Mar;10(3):198–204. doi: 10.1634/theoncologist.10-3-198. [DOI] [PubMed] [Google Scholar]
- 17.Downing A., Morris E.J., Corrigan N. High hospital research participation and improved colorectal cancer survival outcomes: a population-based study. Gut. 2017 Jan;66(1):89–96. doi: 10.1136/gutjnl-2015-311308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoogland A.I., Mansfield J., Lafranchise E.A., Bulls H.W., Johnstone P.A., HSL Jim. eHealth literacy in older adults with cancer. J. Geriatr. Oncol. 2020 Jan 6;S1879–4068(19) doi: 10.1016/j.jgo.2019.12.015. 30438–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonker L.T., Plas M., de Bock G.H. Remote home monitoring of older surgical cancer patients: Perspective on study implementation and feasibility. Ann. Surg. Oncol. 2020 June 29 doi: 10.1245/s10434-020-08705-1. Published online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hempenius L., Slaets J.P.J., Boelens M.A.M. Inclusion of frail elderly patients in clinical trials: solutions to the problems. J. Geriatr. Oncol. 2013 Jan;4(1):26–31. doi: 10.1016/j.jgo.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Owens W.D., Felts J.A., Spitznagel E.L., J. ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978 Oct;49(4):239–243. doi: 10.1097/00000542-197810000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Peters L.L., Boter H., Buskens E., Slaets J.P. Measurement properties of the Groningen frailty Indicator in home-dwelling and institutionalized elderly people. J. Am. Med. Dir. Assoc. 2012 Jul;13(6):546–551. doi: 10.1016/j.jamda.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Katz S., Ford A.B., Moskiwitz R.W., Jackson B.A., Jaffe M.W. Studies of illness in the aged the index of adl: a standardized measure of biological and psychosocial function. JAMA. 1963 Sep 21;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 25.Clavien P.A., Barkun J., de Oliveira M.L. The Clavien-Dindo classification of surgical complications: five-year experience. Ann. Surg. 2009 Aug;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 26.Dutch Central Bureau of Statistics . 2020, April. Internet Acces at Home in the Netherlands. Available at: https://www.cbs.nl/nl-nl/nieuws/2020/14/453-duizend-nederlanders-hadden-in-2019-thuis-geen-internet. Accessed 23/4, 2020. [Google Scholar]
- 27.Haederle M.A.B. 2011. Technology Fear Stops Older Adults From Logging On: But Scientists are Breaking the Computer Block. Available at: http://www. aarp.org/technology/innovations/info-08-2011/elderly-fear-of-technology.3.html. Accessed April/23, 2020. [Google Scholar]
- 28.Mitzner T.L., Bailey J.B., Fausset C.B. Older adults talk technology: technology usage and attitudes. Comput. Hum. Behav. 2010 Nov 1;26(6):1710–1721. doi: 10.1016/j.chb.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai H.S., Shillair R., Cotten S.R. Social support and “playing around”: an examination of how older adults acquire digital literacy with tablet computers. J. Appl. Gerontol. 2017 Jan;36(1):29–55. doi: 10.1177/0733464815609440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang Y., West B.T., Barton D.L., Harris M.R. Acceptance and use of eHealth/mHealth applications for self-management among Cancer survivors. Stud. Health Technol. Inform. 2017;245:131–135. [PMC free article] [PubMed] [Google Scholar]
- 31.Berkowsky R.W., Cotten S.R., Yost E.A., Winstead V.P. Attitudes towards and limitations to ICT use in assisted and independent living communities: findings from a specially-designed technological intervention. Educ. Gerontol. 2013 Nov;1:39(11). doi: 10.1080/03601277.2012.734162. [DOI] [PMC free article] [PubMed] [Google Scholar]