Abstract
Summary: Coronavirus disease 2019 (COVID-19) is spreading rapidly worldwide. Here, we review recently published studies on COVID-19–associated acute kidney injury (AKI) in China. The pooled incidence of AKI in all reported COVID-19 patients was 6.5%, with a much higher rate in patients from the intensive care unit (32.5%). AKI is associated with the severity of COVID-19 and mortality rates, which is similar to other kidney abnormalities including proteinuria and hematuria. The renal tubule is the main site of injury in COVID-19 patients, and the etiology of renal impairment in COVID-19 patients likely is diverse and multifactorial. Apart from direct viral attack via angiotensin-converting enzyme 2 and transmembrane serine proteases 2, hypoxia and hypercoagulability also may contribute to the occurrence of renal injury. To date, there is only randomized controlled trial evidence to support the use of dexamethasone in patients requiring oxygen therapy and remdesivir for shortening the time to recovery, with no specific treatment for COVID-19–associated AKI. Studies researching kidney pathologies or reporting renal outcome and prognosis are in urgent need. Further studies are urgently warranted to identify risk factors, to predict prognosis and renal outcome, to explore the exact mechanisms of renal injury, and to suggest targeted interventions.
Keywords: COVID-19, acute kidney injury, China, incidence, management
Coronavirus disease 2019 (COVID-19) is a newly discovered contagious disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2 virus). The ongoing outbreak was first reported in Wuhan, China,1 and it is a pandemic that has spread worldwide. The clinical features of COVID-19 vary individually, ranging from no clinical symptoms (asymptomatic) to mild or severe respiratory illness symptoms. Common symptoms of COVID-19 include fever, fatigue, dry cough, and muscle ache, and critical patients may progress rapidly to acute respiratory distress syndrome (ARDS), septic shock, metabolic acidosis, coagulation dysfunction, acute kidney injury (AKI), and death.2
Kidney complications in SARS-CoV-2 infection might occur owing to direct cytopathic effects and secondary damage resulting from the co-existence of a systemic inflammatory response or the use of renal-toxic therapies, respiratory distress syndrome–induced hypoxia, or multiple organ dysfunction. Histologic findings have shown acute tubular necrosis and interstitial inflammation. Clinical manifestations of kidney involvement in COVID-19 include proteinuria, hematuria, and AKI.3, 4, 5
The reported incidence of AKI in SARS-CoV-2 infection has varied substantially from 0.5% to 29%.6, 7, 8 It is believed that the occurrence of AKI is part of the multiorgan dysfunction that develops in critical SARS-CoV-2–infected patients, and the variety of AKI incidence reflects the wide clinical spectrum of COVID-19 in terms of disease severity and outcome. Because this is an emerging infectious disease, there are a paucity of data on the whole picture of kidney involvement in this disease. In this review, we aim to summarize China's initial experience of AKI in patients with COVID-19, with a focus on epidemiology, clinical characteristics, pathophysiology, treatment, and prognosis.
COVID-19: DIAGNOSIS AND CLINICAL CLASSIFICATIONS
According to the Guidance for Corona Virus Disease 2019 released by China's National Health Committee,9 suspected COVID-19 cases are identified via consideration of both epidemiologic histories and clinical manifestations. Confirmed cases are diagnosed with one of the following etiological evidences: a positive result of novel coronavirus 2019 (2019-nCoV) nucleic acid by real-time fluorescence RT-PCR or the virus gene sequence is highly homologous to the known 2019-nCoV. All confirmed cases were required to be admitted to either typical hospitals or temporary shelter hospitals according to the different degrees of disease severity. Disease severity was classified into four categories9: (1) mild: mild clinical manifestation with no unusual imaging data; (2) ordinary: fever, respiratory symptoms, pneumonia manifestation on radiograph or computed tomography; (3) severe (satisfies any of the following): (i) respiratory distress, respiratory rate (RR) of 30 or more breaths/min; (ii) oxygen saturation of 93% at rest state; (iii) arterial partial pressure of oxygen/inspiration O2 of 300 mm Hg or less; (4) critical (satisfies any of the following): (i) respiratory failure that needs mechanical ventilation; (ii) shock; or (iii) complicated with other organ failure that requires intensive care unit (ICU) monitoring and treatment.
EPIDEMIOLOGY OF COVID-19–ASSOCIATED AKI
To better understand the incidence of COVID-19–associated AKI in different regions of China, we selected relevant studies published before July 30, 2020, by searching PubMed using the following search terms: “coronavirus” or “COVID-19” or “SARS-CoV-2” or “2019-nCoV” and “laboratory” or “clinical.” We excluded studies that had a large overlap in enrolled patients, had fewer than 90 patients, or did not have a clear description of kidney function tests.
Finally, we included 25 eligible peer-reviewed studies. The epidemiologic data of COVID-19–associated AKI reported in these studies are summarized in Table 1 . Sixteen of these studies were from various COVID-19–designated hospitals in Wuhan City, which was the most infected area in China.2, 3, 4 , 8 , 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 There was also a nationwide multicenter study supported by the National Health Commission of China that enrolled 1,099 patients from 552 hospitals in 30 provinces.6
Table 1.
Enrolled studies on the incidence of COVID-19 associated AKI in China
| Study | Region | Hospital | Sample Size | Recruitment Time | Male (%) | Age* | CKD (%) | AKI rate (%) | Mortality (%) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | ICU | Non-ICU | Deceased | Total | ||||||||
| Chen et al10 | Wuhan | Jinyintan Hospital | 99 | Jan1-Jan 20,2020 | 67 (67.7) | 55±13 | NA | 3 (3.0) | NA | NA | NA | 11 (11.1) |
| Cheng et al3 | Wuhan | Tongji Hospital | 701 | Jan28-Feb11,2020 | 367 (52.4) | 63 (50-71) | 14 (2.0) | 36 (5.1) | NA | NA | NA | 113 (16.1) |
| Wang et al8 | Wuhan | Tongji Hospital | 344 (ICU) | Jan25-Feb25,2020 | 179 (52.0) | 64 (52-72) | NA | 86 (25.0) | 86 (25.0) | 0 | 80 (60.2) | 133 (38.7) |
| Pei et al4 | Wuhan | Tongji Hospital (Sino-French) | 333 | Jan28-Feb9,2020 | 182 (54.7) | 56±13 | Excluded | 22 (6.6) | NA | NA | NA | 29 (8.7) |
| Cao et al11 | Wuhan | Zhongnan Hospital | 102 | Jan3-Feb1,2020 | 53 (52.0) | 54 (37-67) | 4 (3.9) | 20 (19.6) | NA | NA | 15 (88.2) | 17 (16.7) |
| Shi et al12 | Wuhan | Renmin hospital | 416 | Jan20-Feb10,2020 | 205 (49.3) | 64 (range 21-95) | 14 (3.4) | 8 (1.9) | NA | NA | NA | 57 (13.7) |
| Zhou et al2 | Wuhan | Jinyintan Hospital, Wuhan Pulmonary Hospital | 191 Died or Discharged | Dec29,2019-Jan31,2020 | 119 (62.3) | 56 (46-67) | 2 (1.0) | 28 (14.7) | NA | NA | 27 (50.0) | 54 (28.3) |
| Hu et al13 | Wuhan | Tianyou Hospital | 323 | Jan8-Feb20,2020 | 166 (51.4) | 61 (range 23-91) | 7 (2.2) | 17 (5.3) | NA | NA | NA | 35 (10.8) |
| Yu et al14 | Wuhan | 19 ICUs | 226 (ICU) | Feb26-27,2020 | 139 (61.5) | 64 (57-70) | 3 (1.3) | 57 (25.2) | 57 (25.2) | 0 | NA | 87 (38.5) |
| Zhang et al15 | Wuhan | Union Hospital (West Court) | 258 | Jan29-Feb12,2020 | 138 (53.5) | 64 (range 56-70) | 9 (3.5) | 7 (2.7) | NA | NA | NA | 15 (5.8) |
| Deng et al16 | Wuhan | Tongji Hospital (Hankou, Caidian), Central Hospital (Hankou) | 225 Died or Discharged | Jan1-Feb21,2020 | 124 (55.1) | 54 (95%CI 26-83) | NA | 20 (8.9) | NA | NA | 20 (18.4) | 109 (48.4) |
| Li et al17 | Wuhan | Central Hospital | 134 Died or Discharged | Jan1-Feb20,2020 | 75 (56.0) | 61 (47–69) | NA | 5 (3.7) | NA | NA | NA | 42 (31.3) |
| Zhang et al18 | Wuhan | Zhongnan Hospital, Wuhan Fourth Hospital | 394 | Jan1-Feb1,2020 | 186 (47.2) | 56 (42-67) | NA | 37 (9.4) | NA | NA | 7 (31.8) | 22 (5.6) |
| Xu et al19 | Wuhan | Union Hospital, Jinyintan Hospital, Wuhan Third Hospital | 239 (ICU) | Jan12-Feb3,2020 | 143 (59.8) | 63±13 | NA | 119 (49.8) | 119 (49.8) | 0 | 99 (67.4) | 147 (61.5) |
| Chen et al20 | Wuhan | Tongji Hospital (Three branches) | 3309 | Jan18-Mar27,2020 | 1642 (49.6) | 62 (49-69) | 57 (1.7) | 401 (12.1) | NA | NA | NA | 307 (9.3) |
| Cui et al21 | Wuhan | Zhongnan Hospital, Tongji Hospital (Sino-French) | 116 | Jan5-Mar21,2020 | 66 (56.9) | 59 (95%CI 67-62) | 5 (4.3) | 21 (18.1) | NA | NA | 12 (50.0) | 24 (20.7) |
| Zheng et al5 | Wuhan, Shenzhen | Tongji Hospital (Sino-French), The Third People's Hospital of Shenzhen | 555 | Jan8-Feb28,2020 | 269 (48.5) | 52 (36-64) | 10 (1.8) | 29 (5.6) | NA | NA | 12 (44.4) | 27 (4.9) |
| Zhao et al22 | Jingzhou | Jingzhou Central Hospital | 91 | Jan16-Feb10,2020 | 49 (53.8) | 46 (median) | 1 (1.1) | 5 (5.5) | NA | NA | NA | 2 (2.2) |
| Zhang et al23 | Zhejiang | Multicenter | 645 | Jan17-Feb8,2020 | 328 (50.8) | 40.9 (95%CI 29-52) | 6 (0.9) | 2 (0.3) | NA | NA | NA | 0 (0) |
| Ren et al24 | Shenzhen | The Third People's Hospital | 150 | Jan11-Feb12,2020 | 82 (54.7) | 54 (37-63) | NA | 11 (7.3) | NA | NA | NA | 3 (2.0) |
| Hou et al25 | Beijing | Youan Hospital | 101 | Jan21-Mar9,2020 | 44 (43.6) | 51±20 | NA | 12 (11.9) | NA | NA | NA | 5 (5.0) |
| Wan et al26 | Chongqing | Three Gorges Hospital | 135 | Jan23-Feb8,2020 | 72 (53.3) | 47 (36‐55) | NA | 5 (3.7) | NA | NA | NA | 1 (0.7) |
| Guan et al6 | China | National, multicenter | 1099 | Dec11,2019-Jan 29,2020 | 637 (58.0) | 47 (35-58) | 8 (0.7) | 6 (0.5) | NA | NA | NA | 15 (1.4) |
| Yang et al27 | Yichang | Yichang Central People's Hospital | 200 | Jan30-Feb8,2020 | 98 (49.0) | 55±17 | 3 (1.5) | 24 (12.0) | 12 (41.4) | 12 (7.0) | NA | 15 (7.5) |
| Hong et al28 | Chengdu, Daofu | Public Health Clinical Center of Chengdu, Daofu People's Hospital | 168 | Jan16-Mar13,2020 | 92 (54.2) | 47±18 | 4 (2.4) | 1 (0.6) | NA | NA | NA | 3 (1.8) |
Note: Abbreviation: CKD, chronic kidney disease; ICU, intensive care unit; SD, standard deviation; CI, confidence interval.
mean±SD or median with interquartile range
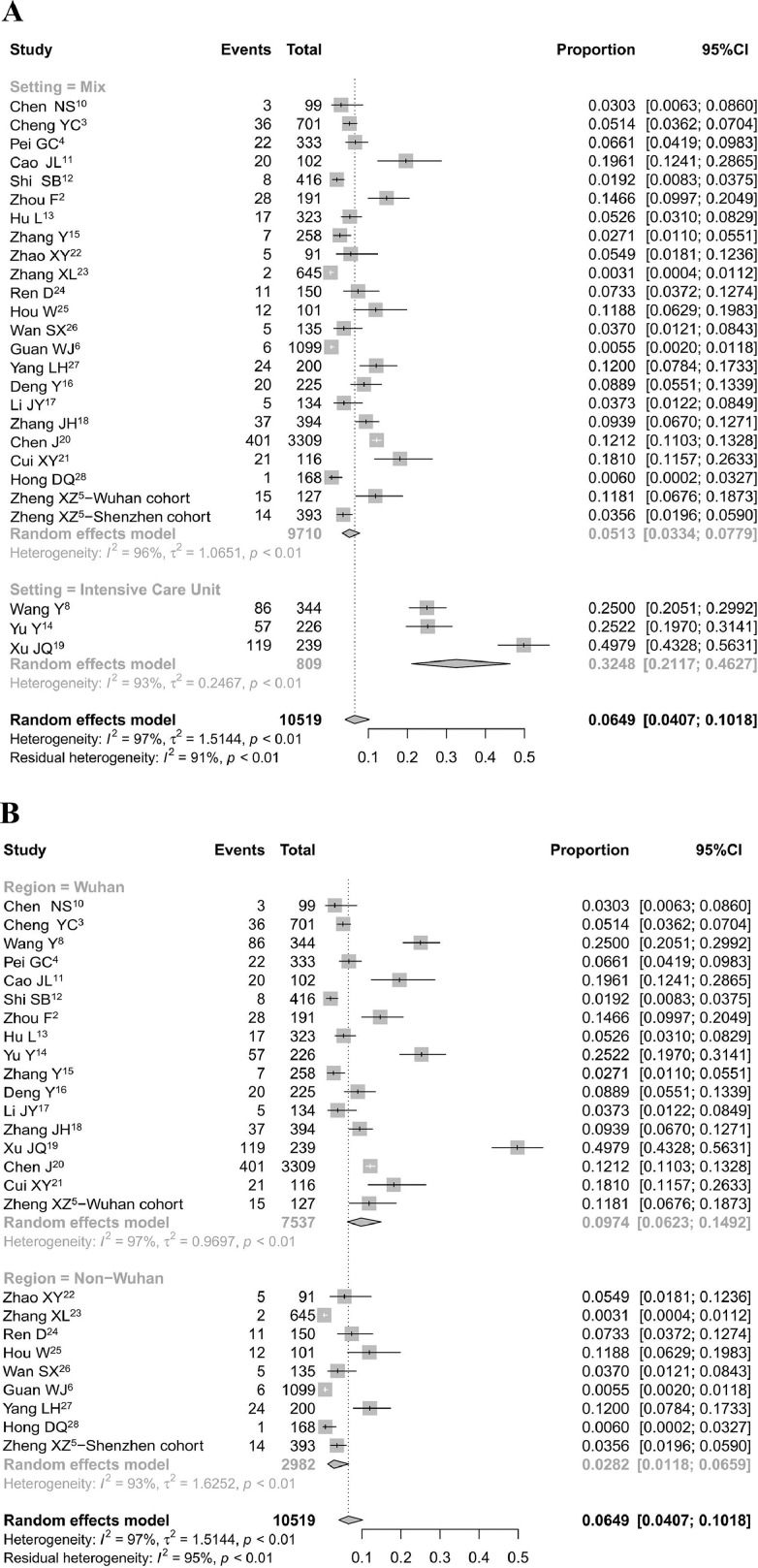
A random-effects model was performed to generate the pooled incidence of AKI and AKI associated mortality. All pooled estimates are provided with 95% confidence intervals (CIs). We performed a meta-analyses using the meta package in R software version 3.6.3 (the R Foundation for Statistical Computing, Vienna, Austria). The pooled incidence of AKI in all reported COVID-19 patients from these 25 studies was 6.5% (95% CI, 4.1%-10.2%), with a much higher rate in patients from the ICU (32.5%; 95% CI, 21.2%-46.3%)8 , 14 , 19 than in patients from mixed departments (5.1%; 95% CI, 3.3%-7.8%)2, 3, 4, 5, 6 , 10, 11, 12, 13 , 15, 16, 17, 18 , 20, 21, 22, 23, 24, 25, 26, 27, 28 (Fig. 1 A). Studies from Wuhan showed a higher AKI incidence (9.7%; 95% CI, 6.2%-14.9%) than studies from outside Wuhan (2.8%; 95% CI, 1.2%-6.6%) (Fig. 1B). However, this regional difference in the AKI rate could be interpreted by the difference in disease severity.
Figure 1.
AKI incidence in COVID-19 patients in China. Studies are subgrouped by (A) settings and (B) regions. Abbreviation: CI, confidence interval.
Recently published studies on COVID-19 worldwide reported AKI rates in hospitalized patients of 17.9% to 72.7% in Italy,29 , 30 9.2% to 18.3% in Korea,31 , 32 19.7% to 69.2% in Spain,33 , 34 5.8% to 56.9% in the United States,35 , 36 52.2% to 74.6% in Germany,37 , 38 and 4.7% to 55.9% in France and Belgium,39 , 40 which are much higher than the rates in China. The difference may be explained by the fact that only very sick COVID-19 patients were admitted to hospitals in those countries compared with the admission of less sick patients in China. Health care systems and policies for hospitalization and assigning levels of care (eg, ICU admission) are different across the world, and the admission rate of COVID-19 patients varied among different countries. Therefore, it is difficult to compare AKI rates based on the number of hospitalized patients.
COVID-19–Associated AKI in All Hospitalizations
Chen et al10 reported 99 patients with COVID-19 from January 1 to January 20, 2020, in Jinyintan Hospital, Wuhan. Three cases (3.0%) presented with AKI on admission. Cheng et al3 conducted a prospective cohort study of 701 COVID-19 patients from January 28, 2020, to February 11, 2020, in Tongji Hospital, Wuhan, and 5.1% (36 of 701) of the overall patients developed AKI during hospitalization. Hu et al13 observed a similar finding that 17 of 323 COVID-19 patients developed AKI in Tianyou Hospital, Wuhan, which is an AKI incidence of 5.3%.
A few subsequent studies described COVID-19 patients outside Wuhan. Zhang et al23 reported findings from 645 patients with COVID-19 in Zhejiang Province. The incidence of AKI was as low as 0.3% (2 of 645). Low rates of ICU admission (0.6%) and mortality (0%) also were observed in this study. Wan et al26 reported findings from 135 patients with COVID-19 in Chongqing City, where 5 subjects developed AKI (3.7%) and 1 died (0.7%). In the study by Guan et al6 regarding 1,099 COVID-19 patients from 552 hospitals across China, 6 subjects experienced AKI (0.5%), and 4 of them were critically ill. A relatively low AKI incidence (2% for in-hospital AKI, and 1% for prehospital AKI) also was reported in the overall population of COVID-19 patients in Shenzhen City.5
The prevalence of AKI increases in parallel with the disease severity of COVID-19.4 , 5 , 13 In the study by Hu et al,13 AKI occurred in 1.3% (2 of 151) of nonsevere patients, in 3.4% (5 of 146) of severe patients, and in 38.5% (10 of 26) of critical patients. Similar findings were reported by Zheng et al,5 who found that the incidence of AKI in nonsevere, severe, and critical patients was 1.0% (3 of 297), 6.8% (13 of 190), and 39.4% (13 of 33), respectively.
COVID-19–Associated AKI in ICU/Critically Ill Cases
Evidence has shown that AKI is more common in ICU patients. In the study by Huang et al,1 of the first reported 41 COVID-19 patients in Jinyintan Hospital, 13 patients were admitted to the ICU, 3 developed AKI, and the presence of AKI was documented in 23.1% (3 of 13).1 Subsequently, Yang et al7 reported findings from 52 ICU cases admitted to the same hospital from December 24, 2019, to January 26, 2020, and the results suggested that AKI, which was the most common extrapulmonary complication, was observed in 15 patients (28.8%). This finding is consistent with the experience of a study8 from Tongji Hospital that documented the presence of AKI in 25.0% of 344 ICU patients with COVID-19 from January 25, 2020, to February 25, 2020, and a cross-sectional study in 19 ICUs in Wuhan in which 25.2% of 252 ICU cases developed AKI.14
RISK FACTORS FOR AKI IN COVID-19
Observational studies from China have suggested that older age,18 hypertension,18 cardiovascular disease,18 and COVID-19 grade4 , 21 are associated with the development of AKI in patients with COVID-19. Patients with AKI are more likely to require mechanical ventilation.21 More than two thirds of the in-hospital AKI episodes were reported to develop after the patients reached critical illness.5 Increased markers of inflammation, such as ferritin, C-reactive protein, and D-dimer,4 , 5 suggest a role for inflammation in the underlying disease state. However, there are no data available on the association between medications and procedures (eg, surgeries, contrast administration) and the development of AKI.
Similarly, male sex,41 older age,30 , 41 diabetes,41 hypertension,41 black race,41 cardiovascular disease (coronary artery disease, heart failure, peripheral vascular disease),41 , 42 respiratory disease (asthma and chronic obstructive pulmonary disease),41 premorbid chronic kidney diseae,30 and the need for ventilator support or vasopressor drug treatment also were reported as risk factors in the United States, Italy, and France. In addition, a higher body mass index42 and greater baseline levels of inflammatory markers, including ferritin, C-reactive protein, procalcitonin, and lactate dehydrogenase,43 were observed in COVID-19 patients with AKI than in non-AKI patients, which suggests the risks for developing AKI and AKI stage escalation.
The rate of AKI varies considerably among different regions and countries. Data from China suggest that AKI is less common in China than in the United States, and the reported cases from China had lower rates of comorbidities such as diabetes and hypertension, and a lower proportion of cases with severe respiratory disease/ARDS than the reported cases from the United States.21 , 41 To date, there are no data on risk factors between different hospital settings (eg, academic versus community, rural versus urban).
CLINICAL CHARACTERISTICS OF COVID-19–ASSOCIATED AKI
The studies of kidney involvement in COVID-19 patients show that abnormal urine findings, including proteinuria and hematuria, are more common than AKI.3 ,5In a prospective cohort study of 701 COVID-19 patients in Wuhan, 43.9% of the patients had proteinuria, 26.7% had hematuria, and 5.1% had AKI.3 These findings were confirmed by the study by Zheng et al5 describing 555 patients from two separate COVID-19 cohorts, one from Wuhan and one from Shenzhen city, where the incidence of proteinuria, hematuria, and AKI was 33.5%, 21.5%, and 5.6%, respectively.
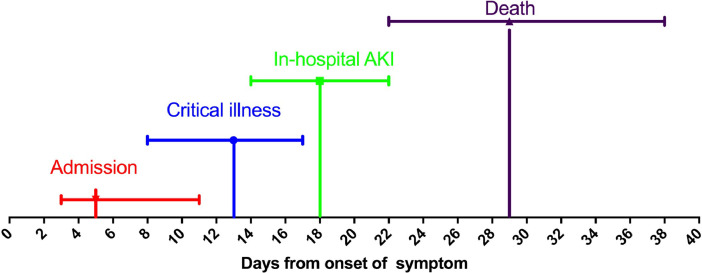
As reported by Zheng et al,5 altogether 13.5% (75/555) of the patients were categorized as critical illness, and 29 patients were defined as having AKI (5.6%%), of whom 21 cases were recognized as in-hospital AKI (4.0%), and 8 (1.5%) as prehospital AKI. The median time of COVID-19 symptoms onset with respect to in-hospital AKI onset was 18 days (25% to 75% percentile, 14-22). The duration from symptom onset to critical illness and death was 13 days (8-17) and 29 days (22-38), respectively (Fig. 2 ). The peak stages of in-hospital AKI were stage 1 in 38% (8/21), stage 2 in 19% (4/21), and stage 3 in 43% (9/21).5 In the study by Pei et al,4 of the 22 COVID-19 patients with overall AKI, 18.2%, 31.8%, and 50.0% were staged as AKI stage 1, stage 2, and stage 3, respectively. The rate of renal replacement therapy (RRT) in COVID-19–associated AKI was rarely reported. In a preprint study from 11 designated ICUs in Wuhan, 21.1% (26/123) of the COVID-19 AKI patients required RRT.44
Figure 2.
Timeline of admission, occurrence of critical illness, onset of in-hospital acute kidney injury (AKI), and death from onset of symptom. The median time was 5 days (25% to 75% percentile, 3-11) for admission (n = 555), 13 days (25% to 75% percentile, 8-17) for critical illness (n = 75), 18 days (25% to 75% percentile, 14-22) for in-hospital AKI (n = 21), and 29 days (25% to 75% percentile, 22-38) for death (n = 27). Data adapted with permission from Zheng et al.5
PATHOLOGIC CHANGES IN COVID-19–ASSOCIATED AKI
To date, more than 4,600 patients have died from COVID-19 in China.45 The first postmortem tissue biopsy report of kidney pathologic presentation was from 26 COVID-19 patients, and it described extensive acute tubular injury and endothelial injury.46 Among these 26 patients, acute tubular lesions were significant and diffuse, including the loss of brush border, vacuolar degeneration, and dilatation of the tubular lumen with cellular debris, but only 9 patients showed clinical signs of kidney injury, which included increased serum creatinine concentrations and/or new-onset proteinuria. Five patients presented with severe pathologic tubular injury with increased serum creatinine concentrations, and two of these patients showed multiple foci of bacteria and diffuse polymorphonuclear casts in the lumen of tubules, which was consistent with the pathologic findings in the lungs. Occasional hemosiderin granules in the tubular epithelium were identified in four patients with hematuria using a dipstick. Another common morphologic finding was erythrocyte stagnation in the peritubular and glomerular capillary loops without distinct fragmentation of erythrocytes, platelets, or fibrin thrombi. Angiotensin-converting enzyme 2 (ACE2) expression was prominent in proximal tubular cells, particularly in areas with severe acute tubular injury. Immunostaining with a SARS-CoV nucleoprotein antibody was positive in tubules, and electron microscopic examination showed clusters of coronavirus particles with distinctive spikes in the tubular epithelium and podocytes, showing a direct invasion of SARS-CoV-2 into kidney tissue.
Similar results were observed in a preprint study that reported postmortem kidney biopsy findings from six COVID-19 patients with AKI.47 Varying degrees of acute tubular necrosis, luminal brush-border sloughing, and vacuole degeneration were observed in all six renal specimens, and an accumulation of SARS-CoV-2 viral nucleoprotein antigen in kidney tubules was detected by immunohistochemistry. The earlier-described findings are consistent with kidney injury secondary to SARS-CoV and the Middle East respiratory syndrome coronavirus infections, in which viral invasion also was detected in renal tubular epithelial cells.48 , 49
ETIOPATHOGENESIS OF COVID-19–ASSOCIATED AKI
The mechanism of the development of AKI in SARS-CoV-2 infection may be multifactorial and vary among cases and different disease conditions. In vitro Vero E6 and 293T cell experiments showed that the glycoprotein spikes on the outer surface of SARS-CoV are responsible for the attachment and entry of the virus into host cells.50 Recently, another in vitro study used human kidney and airway epithelial cells and suggested that the spike protein of SARS-CoV-2 binds to ACE2 for entry and that the spike protein is activated and cleaved by cellular transmembrane serine protease 2 (TMPRSS2), allowing the virus to release fusion peptides for membrane fusion.51 A study based on single-cell transcriptome analysis showed that the co-expression of the receptor ACE2 and TMPRSS genes in kidney cells was no less than that in the lung, esophagus, small intestine, and colon, and relatively high co-expression of ACE2 and TMPRSS genes was observed in podocytes and proximal straight tubule cells.52 These findings suggest that the kidney is an important target organ for SARS-CoV-2, with podocytes and proximal straight tubule cells as candidate host cells. Recently, some researchers successfully isolated SARS-CoV-2 virus particles from the urine of COVID-19 patients.53, 54, 55 Virus particles also were identified in kidney specimens of AKI patients.46 , 47 These results show that SARS-CoV-2 is a cytopathic virus that can directly infect human renal tubules and podocytes and consequently lead to AKI and proteinuria in COVID-19 patients. Clinical findings showed exuberant inflammatory responses during SARS-CoV-2 infection, further resulting in uncontrolled pulmonary inflammation, likely a leading cause of case fatality,1 , 8 suggesting that a possible cytokine storm may be involved in the occurrence and development of AKI. Other putative mechanisms include the use of renal-toxic therapies, respiratory distress syndrome–induced hypoxia, hypovolemia, and hypotension.
COVID-19 IN SPECIAL KIDNEY DISEASE POPULATIONS
Patients With End-Stage Kidney Disease on Maintenance Hemodialysis
In a large cohort of patients with end-stage kidney disease and undergoing maintenance hemodialysis (MHD) in Wuhan, 154 of 7,154 patients had laboratory-confirmed COVID-19, making the incidence of COVID-19 in MHD patients 2.15%, which is much higher than that of the general population (approximately 0.5% during the same period in Wuhan).56 Therefore, the MHD population is highly susceptible to COVID-19, and hemodialysis centers are a high-risk clinical setting for the spread of SARS-CoV-2 infection.
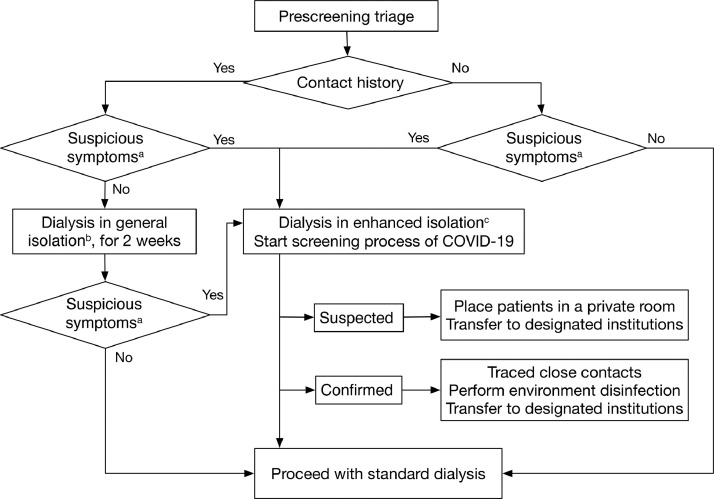
Management of MHD patients in the context of an epidemic poses several challenges. Patients usually require transportation from home to the dialysis units, and dialysis care delivery requires close contact and multiperson attendance. In response to this emerging threat, the Chinese Medical Association Nephrology Branch released guidance documents for the prevention and control of COVID-19 infection in hemodialysis settings,57 such as strict entrance screening of temperatures and symptoms and select nucleic acid tests and computed tomography scans. In addition, all patients and staff were required to wear medical masks during dialysis and in public places. Patients undergoing MHD who were suspected to have COVID-19 were quickly isolated and transferred to a fever clinic for further examination. Figure 3 showed patient screening and disposition for COVID-19 in hemodialysis settings recommended by the Chinese Society of Blood Purification Administration.58 The effectiveness of comprehensive intervention measures in controlling the development of the epidemic in patients undergoing MHD was verified because disease onset continued to decrease from 10 per day on January 30, 2020, to 4 per day on February 11, 2020. No new cases have occurred between February 26, 2020 and March 10, 2020.56
Figure 3.
Patient screening and disposition for coronavirus disease 2019 (COVID-19) in hemodialysis settings. aSuspicious symptoms include fever, cough, sore throat, chest pain, shortness of breath, fatigue, headache, conjunctivitis, and muscle soreness. bDialysis in general isolation should be the last shift of the day in a separate treatment room. cDialysis in enhanced isolation should be the last additional shift after daily routine treatment. Adapted with permission from the Chinese Journal of Blood Purification.58
Kidney Transplant Patients
Kidney transplant recipients appear to be at higher risk for hospitalization and death from COVID-19. However, there is not much information on the mortality rate of kidney recipients co-infected with COVID-19 in China. The reported mortality rate in this population was as high as 28% (10 of 36) in a single institution in New York.59
The Transplantation Technology Branch of Chinese Medicinal Biotechnology Association developed a recommendation on the prevention and treatment of COVID-19 kidney transplant recipients,60 which suggested determining an optimal follow-up schedule.60 By timely adjusting working methods and procedures and implementing the emergency prevention and control measures during the epidemic, none of the kidney transplant inpatients and outpatients were infected with COVID-19 in a single institution in Henan Province, China.61 The recommended treatment of kidney transplant recipients with COVID-19 infection is to tailor treatment options based on the patient's clinical status, duration of transplant, and severity of illness.60 The reduction or suppression of antimetabolic agents (eg, mycophenolate mofetil) is the first approach in mild cases. Immunosuppressive therapy (eg, a calcineurin inhibitor) and rapamycin also should be reduced in moderate-severe cases. For severe patients, withdrawal of all immunosuppressive therapies, except corticosteroids, is required to restore an adequate immune response.
PREVENTION AND CONTROL MANAGEMENT OF THE COVID-19 EPIDEMIC
Controlling the source and cutting off the route of transmission are the fundamental measures for the prevention and control of infectious diseases. After the outbreak of COVID-19, extreme disease control practices were adopted by the Chinese government to promote active case finding and case management in suppression and containment strategies. Wuhan, the epicenter of COVID-19 in China, suspended all transportation into and out of the city from January 23, 2020, to April 8, 2020. Hospitals were required to report confirmed or suspected cases within 2 hours. Laboratories reported test results within 12 hours, and local Centers for Disease Control and Prevention completed case investigations within 24 hours.9"?> Door-to-door and individual-to-individual universal symptom surveys were initiated to single out presumptive cases in the community.
All confirmed cases were hospitalized and transferred to isolation wards in COVID-19–designated hospitals, regardless of disease severity. Presumptive cases, that is, those with fever or respiratory symptoms, and close contacts of confirmed cases, were allocated to centralized isolation sites with protective conditions. To combat the shortage of hospital beds, 16 Fangcang shelter hospitals were built rapidly in Wuhan, which isolated more than 12,000 patients with mild and moderate COVID-19.62 Three mobile physical protection level 3 laboratories were transported to Wuhan to help enhance the capability and efficiency of nucleic acid testing.63 These hospitals and laboratories helped ensure that every suspected case could be tested, treated, and isolated, and that their close contacts could be traced and isolated in a timely manner.
According to China's national guidelines for the prevention and control of COVID-19,9 medical staff who provided diagnosis and treatment services to COVID-19 patients at designated medical and health institutions were required to keep effective personal protection. Individual protective equipment, including protective clothing, medical protective masks or powered air filter respirators, and protective face screens or goggles, were necessary. All of the medical staff lived in isolation, worked as a team to limit exposure to the infection, and were prohibited from working in non–COVID-19 wards during the same time. After finishing their work in the isolation wards, medical staff also were required to undergo centralized isolation medical observation. Patients with RRT indications were transferred to designated wards managed by a special team with significant expertise in catheter placement, management of RRT, and critical care. The use of at least double-layered protection was recommended for the personnel placing dialysis catheters.
The containment strategies, including the implemented nonpharmaceutical public health measures, were effective in China. The reconstruction of the full transmission dynamics of COVID-19 in Wuhan, on the basis of 32,583 laboratory-confirmed cases between December 8, 2019, and March 8, 2020, showed that the proportion of severe and critical cases decreased from 53.1% to 10.3% over time and that the effective reproduction number fluctuated above 3.0 before January 26, 2020, decreased to less than 1.0 after February 6, 2020, and decreased further to less than 0.3 after March 1, 2020.64
TREATMENT OF COVID-19–ASSOCIATED AKI
General Management
To date, the care strategy for patients with COVID-19 and AKI largely is supportive because of the lack of specific treatments. Oxygen delivery is optimized to maintain a high level of oxygenation parameters and to prevent tissue hypoxia and worsening of AKI under severe infection.65 Appropriate fluid management is essential because patients with fever tend to be hypovolemic, and mechanical ventilation with high positive end-expiratory pressure maneuvers might exacerbate the condition. However, avoiding volume overload and pulmonary edema are equally important.66 The discontinuation of all potentially nephrotoxic drugs and dose adaptation of drugs excreted by the kidney are pivotal to the patient's renal function.66 Protein-calorie malnutrition is associated with mortality in patients with AKI, and the nutritional management of AKI patients with COVID-19 is vital. In addition, there has been no evidence in recent studies showing that renin-angiotensin-aldosterone system inhibitors would affect the risk of COVID-19 or mortality.67, 68, 69"?> Therefore, cardiovascular societies recommend against the addition or cessation of renin-angiotensin-aldosterone system inhibitors.
Antiviral Therapies
The use of antiviral drugs in treating COVID-19 patients varies from 22% to 93% in different studies.1, 2, 3 , 6 , 10 , 69, 70, 71 Guan et al6 retrospectively reviewed 1,099 patients with COVID-19 and found that 35.8% of the patients had received oseltamivir therapy. The proportion of patients reaching the composite outcome (including ICU admission, need for mechanical ventilation, and death) was higher in patients with oseltamivir administration than in those patients without oseltamivir therapy (9.2% versus 4.4%). This result could be interpreted by the fact that antiviral agents were more likely to be used in severe patients (46.2% in severe patients versus 33.8% in nonsevere patients) who tended to have a high viral load and a long virus-shedding period.
Currently, remdesivir appears to be the only antiviral agent with randomized controlled trial (RCT) evidence for shortening the time to recovery in COVID-19 patients. In an initial RCT in Wuhan, remdesivir use showed a nonsignificant trend toward reduced time to clinical improvement.72 However, this trial did not reach its target enrollment because of marked reductions in new patient presentations. A subsequent RCT enrolling 1,063 patients from 60 sites worldwide (45 in the United States) found accelerated recovery in patients receiving remdesivir (median time to recovery, 11 days in patients receiving remdesivir versus 15 days in patients receiving placebo; P < .001).73 In addition, mortality at 14 days was 7.1% with remdesivir and 11.9% with placebo (hazard ratio for death, 0.7; 95% CI, 0.47-1.04). Subgroup analysis found no benefit in patients on high-flow oxygen, noninvasive ventilation, or invasive ventilation, suggesting that antivirals such as remdesivir will be of limited efficacy in the late disease stage, during which the pathology likely is inflammatory in origin.
An open-label RCT of 150 hospitalized patients in China compared hydroxychloroquine with the standard of care and showed no difference in viral clearance at 28 days and a significantly higher risk of adverse events (30% in the hydroxychloroquine group vs 8.8% in the standard-of-care group; P = .001).74 Similar findings were observed in a press release from the Randomised Evaluation of COVID-19 therapy trial, which was conducted at 176 National Health Service hospital organizations in the United Kingdom. In total, 1,561 patients randomly allocated to receive hydroxychloroquine were compared with 3,155 patients concurrently allocated to usual care, and hydroxychloroquine was not associated with reductions in 28-day mortality (26.8% versus 25.0% usual care; RR, 1.09; 95% CI, 0.96-1.23; P = .18) but was associated with a lower probability of discharge alive within 28 days (60.3% versus 62.8% usual care; RR, 0.92; 95% CI, 0.85-0.99).75
Corticosteroids
China's National Guidance on COVID-19 recommends the use of systematic corticosteroid treatment (methylprednisolone, 1-2 mg/kg body weight, for 3-5 days) in patients with disease development that manifests as uncontrollable high fever, exacerbation of dyspnea, progressive deterioration of oxygenation index, rapid progress on imaging, and a sharp increase in cytokine levels.9 Huang et al1 reported that 46% of ICU cases and 11% of non-ICU cases were given systemic corticosteroids. Subsequently, Guan et al6 reported similar findings from 1,099 cases, with 60% of ICU cases receiving corticosteroids. These results immediately raised concerns about whether patients would benefit from corticosteroid therapy because of the risk of inhibiting immune responses and impairing pathogen clearance.76
Several observational studies in China failed to show solid evidence for the influence of corticosteroids on either severe77 or nonsevere78 COVID-19 patients. Two clinical trials aimed to explore their effectiveness and safety in the treatment of COVID-19 (NCT04273321 and NCT04263402). Both studies have been completed, but the results are not yet known. The recent results of the Randomised Evaluation of COVID-19 therapy trial in the United Kingdom indicate that at the doses tested, the benefits of steroid treatment outweighed the potential harm. A total of 2,104 patients were randomized to receive 6 mg dexamethasone once per day for 10 days and were compared with 4,321 patients randomized to usual care alone.79 Dexamethasone reduced mortality by 35% in patients on mechanical ventilation (29.3% versus 41.4%; RR, 0.64; 95% CI, 0.51-0.81) and by 20% in patients treated with oxygen (23.3% versus 26.2%; RR, 0.82; 95% CI, 0.72-0.94); however, a trend toward worse survival was observed in mild cases not requiring oxygen (17.8% versus 14.0%; RR, 1.19; 95% CI, 0.91-1.55). Therefore, the associations between corticosteroid use and disease severity and/or death that were found in observational studies might reflect a greater propensity to use corticosteroids in severe cases.1 , 6 , 77 , 80 , 81
Traditional Chinese Medicine
Traditional Chinese medicine (TCM), one of the oldest healing practices, mainly includes natural medication, acupuncture, and physiotherapy, and has been used in China for centuries to treat various diseases, including viral infections. The integration of TCM and Western medicine is recommended in the national guidelines for COVID-19 treatment,9 , 83 with different TCM plans (lianhuaqingwen capsules, jinhuaqinggan granules, xuebijing injections, and so forth) based on patients’ clinical manifestations. Adjusting therapies according to patients’ physical conditions under the supervision of a TCM physician also is recommended.
In vitro, liushen capsules showed antiviral and anti-inflammatory abilities against SARS-CoV-2 via suppression of the nuclear factor-κB signaling pathway.83 Lianhuaqingwen capsules significantly inhibited SARS-CoV-2 replication in Vero E6 cells and markedly reduced proinflammatory cytokine production at the messenger RNA level.84 Furthermore, several observational studies showed that TCM, as an adjuvant therapy for COVID-19, played a role in promoting recovery by controlling fever symptoms and accelerating the absorption of lung lesions.85, 86, 87
A multicenter open-label RCT on lianhuaqingwen capsules enrolled 284 COVID-19 patients (142 each) from 23 hospitals in nine provinces throughout mainland China. Patients who received lianhuaqingwen capsules were more likely to achieve complete recovery of clinical symptoms such as fever, fatigue, and coughing (91.5% versus 82.4% in controls; P = .022). lianhuaqingwen capsules also shortened the time to symptom recovery (median, 7 versus 10 days in controls; P < .001) and improved the recovery of chest radiologic abnormalities (83.8% versus 64.1%; P < .001).88 There is another ongoing, multicenter, open-label RCT in China on the efficacy and safety of anluohuaxian, a proprietary Chinese medicine that improves hepatic fibrosis in patients with chronic hepatitis B virus infection, in the treatment of pulmonary fibrosis in severe COVID-19 patients (NCT04334265).89 Further well-designed RCTs are required to evaluate the efficacy and safety of TCM for COVID-19 patients.
Extracorporeal Blood Purification for COVID-19–Associated AKI
Extracorporeal blood purification with continuous renal replacement therapy (CRRT), as the most common technique in clinical practice, played an important role in the rescue of SARS, MERS, and other sepsis-associated AKI.90 Approximately 0.5% to 2.0% of COVID-19 patients required CRRT, with AKI as the main reason.6 , 12 , 91 The proportion increased to 5.6% to 23.0% in critically ill patients in the ICU.2 , 3 , 7 , 10 , 69 The national guidelines published by the Chinese National Health Commission82 proposed blood purification therapies for critically ill COVID-19 patients with AKI and increased inflammatory factors. Based on Chinese experts' consensus on blood purification treatment of severe COVID-19,92 , 93 hemoperfusion, hemoadsorption, and plasma exchange were performed in addition to conventional CRRT to clear crucial inflammatory mediators in patients with sepsis and ARDS. Wang and Hu94 reported a patient with severe SARS-CoV-2 infection who recovered from a cytokine storm after treatment with the combination of a double plasma molecular adsorption system (BS330 and HA330II; Jafron, Zhuhai, China) and plasma exchange (2,000 mL each). The potential effect of blood purification therapy on reducing the cytokine storm also was described in the report by Ma et al95 of three critically ill COVID-19 patients. Although RCT evidence is lacking, multidisciplinary efforts should be made to maximize the availability of blood purification therapy for indicated patients.
PROGNOSIS OF COVID-19–ASSOCIATED AKI
As of August 6, 2020, the case-fatality ratio of COVID-19 was 6.6% (4,512 of 68,138) in Hubei Province,96 and 0.8% (172 of 20,666) in all other regions of China.45 According to the Johns Hopkins Coronavirus Resource Center, the global case-fatality ratio of COVID-19 was 3.8% (707,666 of 18,810,382), with higher mortality rates reported in Yemen (28.7%; 506 of 1760), the United Kingdom (15.1%; 46,295 of 307,256), and Italy (14.2%; 35,171 of 248,419).97 The differences in the mortality of COVID-19 in various countries may be owing to the differences in the testing policy, the efficacy of disease recognition, and the degree of overwhelmed health care facilities.
The mortality of COVID-19–associated AKI was reported mainly in studies from Wuhan.2 , 4 5 , 8 , 11 16 18 19 , 21 The pooled mortality rate was 77.2% (95% CI, 51.9%-91.4%) in patients with AKI versus 9.0% (95% CI, 3.6%-20.7%) in non-AKI COVID-19 total hospital admissions, and the rate correlated with AKI severity stage (75.0% for AKI stage 1, 85.7% for AKI stage 2, and 90.9% for AKI stage 3).4 Given the lack of information, it is difficult to compare mortality rates among AKI patients requiring RRT in COVID-19 across studies. Nonetheless, an extremely high mortality rate was observed in COVID-19 patients requiring RRT. Yang et al7 included 52 COVID-19 patients in their study and found that 8 of 9 subjects who required CRRT did not survive. In another study including 191 COVID-19 patients, all the 10 subjects who required CRRT did not survive.2
Information on renal recovery in COVID-19–associated AKI is very limited. In the study by Pei et al,4 18% (4 of 22) of patients with AKI achieved complete remission of kidney function in 3 weeks after the onset of infection, and the critical type of COVID-19 was associated independently with nonrecovery of AKI (odds ratio, 0.03; 95% CI, 0.004-0.32). More information on renal recovery and short- and long-term renal outcomes of COVID-19–associated AKI is needed.
CONCLUSIONS
SARS-CoV-2 infection is spreading rapidly and causing daily mortality worldwide. Studies have shown that AKI is prevalent in critically ill COVID-19 patients. Kidney involvement is associated with poor outcomes. Early detection and appropriate management should be instituted as soon as possible. Prevention is the critical aspect in the management of this disease. Extreme disease control practices effectively contains the spread of the disease. Several vaccines and promising treatments are undergoing clinical trials with the hope of finding a cure for this global crisis soon.
Acknowledgments
X.Z. and Y.Z. contributed equally to this article.
Footnotes
Financial support: Supported by grants from the National Natural Science Foundation of China (91742205 and 81625004), the Beijing Young Scientist Program (BJJWZYJH01201910001006), and the Peking University Clinical Scientist Program by the Fundamental Research Funds for the Central Universities.
Conflict of interest statement: none.
REFERENCES
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pei G, Zhang Z, Peng J, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31:1157–1165. doi: 10.1681/asn.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng X, Yang, H, Li, et al. X. Prevalence of kidney injury and associations with critical illness and death in patients with COVID-19. Clin J Am Soc Nephrol. 2020. In press doi: 10.2215/CJN.04780420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:457–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Lu X, Chen H, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201:1430–1434. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Health Commission (NHC) of the People's Republic of China . People's Medical Publishing House; Beijing: 2020. Guidance for corona virus disease 2019: prevention, control, diagnosis and management. [Google Scholar]
- 10.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/s0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:748–755. doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu L, Chen S, Fu Y, et al. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa539. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Y, Xu D, Fu S, et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020;24:219. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Cui Y, Shen M, et al. Association of diabetes mellitus with disease severity and prognosis in COVID-19: a retrospective cohort study. Diabetes Res Clin Pract. 2020;165 doi: 10.1016/j.diabres.2020.108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J. 2020;133:1261–1267. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Li M, Zheng S, et al. Plasma albumin levels predict risk for nonsurvivors in critically ill patients with COVID-19. Biomark Med. 2020;14:827–837. doi: 10.2217/bmm-2020-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Li J, Su L, et al. [Clinical characteristics and risk factors of acute kidney injury in coronavirus disease 2019] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32:407–411. doi: 10.3760/cma.j.cn121430-20200302-00198. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Yang X, Yang L, et al. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan, China. Crit Care. 2020;24:394. doi: 10.1186/s13054-020-03098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Bai H, Liu J, et al. Distinct clinical characteristics and risk factors for mortality in female COVID-19 inpatients: a sex-stratified large-scale cohort study in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa920. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui X, Yu X, Wu X, et al. Acute kidney injury in patients with the coronavirus disease 2019: a multicenter study. Kidney Blood Press Res. 2020;45:612–622. doi: 10.1159/000509517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao XY, Xu XX, Yin HS, et al. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020;20:311. doi: 10.1186/s12879-020-05010-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Cai H, Hu J, et al. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int J Infect Dis. 2020;94:81–87. doi: 10.1016/j.ijid.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren D, Ren C, Yao RQ, Feng YW, Yao YM. Clinical features and development of sepsis in patients infected with SARS-CoV-2: a retrospective analysis of 150 cases outside Wuhan, China. Intensive Care Med. 2020;46:1630–1633. doi: 10.1007/s00134-020-06084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou W, Zhang W, Jin R, Liang L, Xu B, Hu Z. Risk factors for disease progression in hospitalized patients with COVID-19: a retrospective cohort study. Infect Dis. 2020;52:498–505. doi: 10.1080/23744235.2020.1759817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, Lei Y, Zhang R, et al. Epidemiological and clinical features of 200 hospitalized patients with corona virus disease 2019 in Yichang, China: a descriptive study,J Clin Virol. 129, 2020, 104475. 10.1016/j.jcv.2020.104475. [DOI] [PMC free article] [PubMed]
- 28.Hong D, Long L, Wang AY, et al. Kidney manifestations of mild, moderate and severe coronavirus disease 2019: a retrospective cohort study. Clin Kidney J. 2020;13:340–346. doi: 10.1093/ckj/sfaa083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piano S, Dalbeni A, Vettore E, et al. Abnormal liver function tests predict transfer to intensive care unit and death in COVID-19. Liver Int. 2020;40:2394–2406. doi: 10.1111/liv.14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fominskiy EV, Scandroglio AM, Monti G, et al. Prevalence, characteristics, risk factors, and outcomes of invasively ventilated COVID-19 patients with acute kidney injury and renal replacement therapy. Blood Purif. Published online 28 July 2020 doi: 10.1159/000508657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong KS, Lee KH, Chung JH, et al. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in Daegu, South Korea: a brief descriptive study. Yonsei Med J. 2020;61:431–437. doi: 10.3349/ymj.2020.61.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim J-H, Park S-H, Jeon Y, et al. Fatal outcomes of COVID-19 in patients with severe acute kidney injury. J Clin Med. 2020;9:1718. doi: 10.3390/jcm9061718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uribarri A, Nez-Gil InJ, Aparisi A, et al. Impact of renal function on admission in COVID-19 patients: an analysis of the international HOPE COVID-19 (Health Outcome Predictive Evaluation for COVID 19) registry. J Nephrol. 2020;33:737–745. doi: 10.1007/s40620-020-00790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trujillo H, Caravaca-Font F, Sevillano N, et al. SARS-CoV-2 infection in hospitalized patients with kidney disease. Kidney Int Rep. 2020;5:905–909. doi: 10.1016/j.ekir.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imam Z, Odish F, Gill I, et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J Intern Med. 2020;288:469–476. doi: 10.1111/joim.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher M, Neugarten J, Bellin E, et al. AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol. 2020;31:2145–2157. doi: 10.1681/ASN.2020040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnold F, Westermann L, Rieg S, et al. Superior anticoagulation strategies for renal replacement therapy in critically ill patients with COVID-19: a cohort study. MedRxiv. 2020. In press. 10.1101/2020.06.26.20140699. [DOI] [PMC free article] [PubMed]
- 38.Husain-Syed F, Wilhelm J, Kassoumeh S, et al. Acute kidney injury and urinary biomarkers in hospitalized patients with coronavirus disease 2019. Nephrol Dial Transplant. 2020;35:1271–1274. doi: 10.1093/ndt/gfaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oussalah A, Gleye S, Urmes IC, et al. Follow-up of multi-organ dysfunction and inflammation using biomarker kinetics in patients with severe COVID-19 disease and association with disease outcomes: results from a referral center cohort in the north east of France. SSRN. 2020. In press. 10.2139/ssrn.3590489. [DOI]
- 40.Grimaldi D, Aissaoui N, Blonz G, et al. Characteristics and outcomes of acute respiratory distress syndrome related to COVID-19 in Belgian and French intensive care units according to antiviral strategies. The COVADIS multicenter observational study. MedRxiv. 2020. In press. 10.1101/2020.06.28.20141911. [DOI] [PMC free article] [PubMed]
- 41.Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin S, Orieux A, Prevel R, et al. Characterization of acute kidney injury in critically ill patients with severe coronavirus disease 2019. Clin Kidney J. 2020;13:354–361. doi: 10.1093/ckj/sfaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohamed MM, Lukitsch I, Torres-Ortiz AE, et al. Acute kidney injury associated with coronavirus disease 2019 in urban New Orleans, Kidney360.1, 2020, 614–622. 10.34067/KID.0002652020. [DOI] [PMC free article] [PubMed]
- 44.Zhang X, Guo W, Hua J, et al. The incidence, risk factors and clinical outcomes of acute kidney injury in critically ill patients with COVID-19: a multicenter study. SSRN. 2020. In press. 10.2139/ssrn.3572908. [DOI]
- 45.2020. Daily briefing on novel coronavirus cases in China.http://en.nhc.gov.cn/2020-08/01/c_81268.htm [cited 2020 August 6]. Available from: [Google Scholar]
- 46.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diao B, Wang C, Wang R, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv. 2020 doi: 10.1101/2020.03.04.20031120. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poissy J, Goffard A, Parmentier-Decrucq E, et al. Kinetics and pattern of viral excretion in biological specimens of two MERS-CoV cases. J Clin Virol. 2014;61:275–278. doi: 10.1016/j.jcv.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glowacka I, Bertram S, Muller MA, et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122–4134. doi: 10.1128/jvi.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan XW, Xu D, Zhang H, Zhou W, Wang LH, Cui XG. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 2020;46:1114–1116. doi: 10.1007/s00134-020-06026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun J, Zhu A, Li H, et al. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg Microbes Infect. 2020;9:991–993. doi: 10.1080/22221751.2020.1760144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng L, Liu J, Xu W, et al. SARS-CoV-2 can be detected in urine, blood, anal swabs and oropharyngeal swabs specimens. J Med Virol. 2020;92:1676–1680. doi: 10.1002/jmv.25936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang L, Li X, Chen H, et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51:343–348. doi: 10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiong F, Tang H, Liu L, et al. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol. 2020;31:1387–1397. doi: 10.1681/ASN.2020030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chinese Medical Association Nephrology Branch Recommendations for prevention and control of novel coronavirus infection in blood purification center (room) from the Chinese Medical Association Nephrology Branch. Chin J Nephrol. 2020;36:82–84. doi: 10.3760/cma.j.issn.1001-7097.2020.02.002. [DOI] [Google Scholar]
- 58.Chinese Society of Blood Purification Administration Recommendation for prevention of COVID-19 virus infection in blood purification center. Chin J Blood Purif. 2020;19:73–76. doi: 10.3969/j.issn.1671-4091.2020.02.001. [DOI] [Google Scholar]
- 59.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Transplantation technology branch of China Medicinal Biotechnology Association Expert recommendation for postoperative follow-up and prevention and treatment of infection in organ transplanted recipients during the COVID-19 epidemic (trial version 1) Chin J Transplant. 2020;14:1–5. doi: 10.3877/cma.j.issn.1674-3903.2020.01.001. [DOI] [Google Scholar]
- 61.Li Y, Yang N, Li X, Wang J, Yan T. Strategies for prevention and control of the 2019 novel coronavirus disease in the Department of Kidney Transplantation. Transplant Int. 2020;33:1040–1045. doi: 10.1111/tri.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen S, Zhang Z, Yang J, et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395:1305–1314. doi: 10.1016/s0140-6736(20)30744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.2020. China.org.cn, China dispatches over 20,000 medics to aid anti-virus battle in Hubei.http://www.china.org.cn/china/2020-02/13/content_75702194.htm [cited 2020 August 6]. Available from: [Google Scholar]
- 64.Hao X, Cheng S, Wu D, Wu T, Lin X, Wang C. Reconstruction of the full transmission dynamics of COVID-19 in Wuhan. Nature. 2020;384:420–424. doi: 10.1038/s41586-020-2554-8. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Z. Biomarkers, diagnosis and management of sepsis-induced acute kidney injury: a narrative review. Heart Lung Vessels. 2015;7:64–73.. [PMC free article] [PubMed] [Google Scholar]
- 66.Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012;2:1–138. [Google Scholar]
- 67.Reynolds HR, Adhikari S, Pulgarin C, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kui L, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;133:1025–1031. doi: 10.1097/cm9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. In press. [DOI] [PubMed] [Google Scholar]
- 74.Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Horby P, Mafham M, Linsell L, et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial, MedRxiv. 2020. 10.1101/2020.07.15.20151852. In press. [DOI]
- 76.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/s0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu X, Chen T, Wang Y, Wang J, Yan F. Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Crit Care. 2020;24:241. doi: 10.1186/s13054-020-02964-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuan M, Xu X, Xia D, et al. Effects of corticosteroid treatment for non-severe COVID-19 pneumonia: a propensity score-based analysis. Shock. 2020 doi: 10.1097/shk.0000000000001574. In press. [DOI] [PubMed] [Google Scholar]
- 79.Horby P, Lim WS, Emberson J, et al. Dexamethasone in hospitalized patients with COVID-19- preliminary report. N Engl J Med. 2020. 10.1056/NEJMoa2021436. In press. [DOI] [PMC free article] [PubMed]
- 80.Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020;81:e13–e20. doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gong Y, Guan L, Jin Z, Chen S, Xiang G, Gao B. Effects of methylprednisolone use on viral genomic nucleic acid negative conversion and CT imaging lesion absorption in COVID-19 patients under 50 years old. J Med Virol. 2020 doi: 10.1002/jmv.26052. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.National Health Committee of the People's Republic of China. The diagnosis and treatment plan for the novel coronavirus disease (tentative, the Seventh version). 2020.
- 83.Ma Q, Pan W, Li R, et al. Liu shen capsule shows antiviral and anti-inflammatory abilities against novel coronavirus SARS-CoV-2 via suppression of NF-kappaB signaling pathway. Pharmacol Res. 2020;158 doi: 10.1016/j.phrs.2020.104850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Runfeng L, Yunlong H, Jicheng H, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol Res. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luo E, Zhang D, Luo H, et al. Treatment efficacy analysis of traditional Chinese medicine for novel coronavirus pneumonia (COVID-19): an empirical study from Wuhan, Hubei Province, China. Chin Med. 2020;15:34. doi: 10.1186/s13020-020-00317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ni L, Zhou L, Zhou M, Zhao J, Wang DW. Combination of western medicine and Chinese traditional patent medicine in treating a family case of COVID-19 in Wuhan. Front Med. 2020;14:210–214. doi: 10.1007/s11684-020-0757-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ren JL, Zhang AH, Wang XJ. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res. 2020;155 doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu K, Guan WJ, Bi Y, et al. Efficacy and safety of lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153242. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang C, Li J, Wu Z, et al. Efficacy and safety of anluohuaxian in the treatment of patients with severe coronavirus disease 2019- a multicenter, open label, randomized controlled study: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:495. doi: 10.1186/s13063-020-04399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang J, Tian J, Sun H, et al. How does continuous renal replacement therapy affect septic acute kidney injury? Blood Purif. 2018;46:326–331. doi: 10.1159/000492026. [DOI] [PubMed] [Google Scholar]
- 91.Wu F, Zhou Y, Wang Z, et al. Clinical characteristics of COVID-19 infection in chronic obstructive pulmonary disease: a multicenter, retrospective, observational study. J Thorac Dis. 2020;12:1811–1823. doi: 10.21037/jtd-20-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chinese Research Hospital Association of Critical Care Medicine. Youth Committee of Chinese Research Hospital Association of Critical Care Medicine [Chinese experts' consensus on diagnosis and treatment of severe and critical coronavirus disease 2019 (revised edition)] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32:269–274. doi: 10.3760/cma.j.cn121430-20200218-00188. [DOI] [PubMed] [Google Scholar]
- 93.Liu J, Zhou Y, Wang M, et al. Application of continuous renal replacement therapy in coronavirus disease 2019. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32:618–621. doi: 10.3760/cma.j.cn121430-20200508-00369. [DOI] [PubMed] [Google Scholar]
- 94.Wang Q, Hu Z. Successful recovery of severe COVID-19 with cytokine storm treating with extracorporeal blood purification. Int J Infect Dis. 2020;96:618–620. doi: 10.1016/j.ijid.2020.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma J, Xia P, Zhou Y, et al. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin Immunol. 2020;214 doi: 10.1016/j.clim.2020.108408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.2020. People's Government of Hubei Province. Daily briefing on novel coronavirus cases in Hubei province.https://www.hubei.gov.cn/zhuanti/2020/dqssl/qwtb/202008/t20200807_2752605.shtml [cited 2020 August 6]. Available from: [Google Scholar]
- 97.Johns Hopkins University & Medicine Coronavirus Resource Center. Daily briefing on novel coronavirus cases worldwide. [cited 2020 August 6]. Available from: https://coronavirus.jhu.edu/.