ABSTRACT
We report two outbreaks of Lassa fever that occurred in Benin in 2014 and 2016 with 20 confirmed cases and 50% (10/20) mortality. Benin was not previously considered to be an endemic country for Lassa fever, resulting in a delay to diagnose the disease and its human transmission. Molecular investigations showed the viral genomes to be similar to that of the Togo strain, which is genetically very different from other known strains and confirms the existence of a new lineage. Endemic circulation of Lassa virus in a new territory and the genetic diversity thus confirm that this virus represents a growing threat for West African people. Given the divergence of the Benin strain from the prototypic Josiah Sierra Leone strain frequently used to generate vaccine candidates, the efficacy of vaccine candidates should also be demonstrated with this strain.
KEYWORDS: Lassa fever, Lassa virus, genome, Benin, outbreak, phylogeny, new lineage, serology
Introduction
Lassa fever (LF) is an acute viral haemorrhagic fever caused by an Old-World arenavirus, Lassa virus (LASV) that was discovered in 1969 in Nigeria [1,2]. The disease is prevalent in West Africa, mainly in Nigeria, Sierra Leone, and Liberia, but there have been a few cases in Mali, northern Côte d’Ivoire and Guinea [3–7]. It is estimated that it affects between 100,000 and 300,000 people a year, causing a few thousand deaths [8]. Nevertheless, epidemiological data are scarce and the real incidence of LF is not well known. The incubation period of LF ranges from 6 to 21 days. Symptoms include fever, severe fatigue, myalgia, abdominal pain, diarrhoea, cough, oedema and bleeding [9]. Its clinical course ranges from asymptomatic infection to severe haemorrhagic fever in only 1–2% of cases. LASV is transmitted to humans through exposure to urine or faeces of infected rodents, such as Mastomys spp or Hylomyscus pamfi [10]. Person-to-person infections can also occur, mainly in healthcare settings. A mathematical model suggests that rodent-to-human transmission occurs in 80% of cases and human-to-human transmission in 20% [11]. LF usually leads to seasonal outbreaks in West Africa, with yearly peaks observed between December and March, during the dry season [12]. LASV has a single-stranded bisegmented RNA genome (L and S segment). The genome shows high diversity and seven lineages have thus far been identified. Lineages I, II, and III circulate in Nigeria [13]. Lineage IV is transmitted in Liberia, Guinea, and Sierra Leone [14]. Lineage V includes strains from Mali and the Ivory Coast [5,7,15]. Lineage VI is represented by a few Nigerian sequences and the two sequences recently discovered in Togo have been proposed to represent the seventh lineage [16].
In October 2014, an LF outbreak was reported in Benin, a country in which the virus had not been previously reported. A total of 16 suspected cases were reported in northern Benin. The outbreak was quickly controlled and declared over after one month of viral circulation [17]. A second outbreak occurred in 2016, with a total of 54 suspected cases, including 28 deaths. This second outbreak was larger than that in 2014 and cases were reported in eight regions: Borgou, Donga, Collines, Alibori, Plateau, Ouémé, Atlantique, and Littoral [18]. We aimed to trace the suspected, probable, and confirmed cases to find the localities of origin and possible nosocomial infections. We also describe 15 (10 complete and 5 partial) genomes of LASV from 20 confirmed patients sampled in 2014 and 2016.
Materials and methods
Field investigation and case definition
In September 2016, we consulted the database of the Health Department in Parakou, the Health Zone in Tchaourou, and several Health Centers belonging to these two towns to investigate the origin of the cases. This investigation allowed us to identify the localities of residence of the patients, the notifying hospitals, the dates of symptom onset, consultation, and blood sampling, and the outcome. We traced the chain of transmission using the case definitions as follows:
Suspected cases included patients who had contacts with a confirmed or probable case and had fever or three symptoms compatible with LF (severe fatigue, headache, digestive disorders, chest or muscle pain, etc.) or patients with inexplicable haemorrhage [9,19].
Probable cases consisted of dead patients who had an epidemiological link with a confirmed case in whom a biological test had not performed before death.
Confirmed cases consisted of patients who had a positive IgM serology and/or positive RT-PCR result for LASV.
Blood collection
In December 2014, the Bernhard-Nocht Institute for Tropical Medicine (BNITM, Hamburg, Germany) received six blood samples from Tanguieta in northern Benin, where a cluster of healthcare workers (HCWs) and patients died after suffering from a strong fever and intense fatigue, with haemorrhagic symptoms.
On 21 January 2016, a cluster of HCWs with unexplained fever in the commune of Tchaourou was detected. These HCWs had all provided care to a patient suffering from haemorrhagic fever on 3 January. On 25 January 2016, the National International Health Regulations Focal Point of Benin reported an outbreak of LF. The samples from suspected cases were sent to the Irrua Specialist Teaching Hospital (ISTH, Irrua, Nigeria), the BNITM, the French National Reference Center for Viral Hemorrhagic Fever (Institut Pasteur – INSERM, Lyon, France, abbreviated “Pasteur-INSERM-VHF” in this paper), the Lagos University Teaching Hospital (Lagos, Nigeria), and the Noguchi laboratory (WHO collaborating center, Ghana) to confirm the Lassa infection by PCR. During the course of this outbreak the BNITM received eight samples and the Pasteur-INSERM-VHF received clinical specimens from 26 people from Benin.
Diagnostic assay
Viral RNA was extracted from 100 µl undiluted plasma and 1/10 diluted plasma using the QIAmp viral RNA mini kit (Qiagen) according to the manufacturer’s instructions. LASV screening was performed on all samples with two conventional RT-PCRs: one pan Old-World-arenavirus assay targeting the L gene (OW) [20] and one LASV-specific assay targeting the glycoprotein precursor gene (GPC) [21].
LASV-specific IgM and IgG detection by enzyme-linked immunosorbent assay (ELISA)
IgM was detected using a capture ELISA. The antigens used for LASV-specific IgM detection were obtained from a viral stock of LASV (AV strain, clade V, EMBL accession numbers: FR832710 and FR832711[22]). The wells of 96-well Maxisorp plates (Nunc) were coated with anti-human IgM (µ-specific) (Sigma), followed by sequential washing and incubation with 1:100 and 1:400 dilutions of patient plasma, LASV antigens or negative control antigens, polyclonal LASV-specific mouse ascitic immune fluid, and finally peroxidase-conjugated anti-mouse IgG (Sigma). Tetramethylbenzidine (TMB) (KPL, Eurobio) was then added and the optical density (OD) quantified using a plate reader.
LASV IgG was quantified by direct ELISA. The wells of 96-well Polysorp plates (Nunc) were coated with recombinant LASV clade IV GPC, C-terminal and N-terminal NP (all from Zalgen Labs, Germantown, MD), or negative control antigens. After washing, 1:100, 1:400, 1:1600, and 1:6400 dilutions of patient plasma were added and the plates incubated, followed by washing and the addition of a peroxidase-conjugated goat polyclonal anti-human IgG (γ-chain specific) (Sigma Aldrich) and further incubation of the plates. Following washing, TMB was added and the OD measured.
LASV sequencing
The two LASV strains from 2014, identified in Hamburg, and LASV strains from 2016, identified in Lyon, were amplified from clinical specimens on Vero cells prior to sequencing by NGS. The sequences of the LASV strains of the 2016 outbreak identified in Hamburg were determined directly from clinical specimens by NGS combined with Sanger sequencing. In Hamburg, both serum and cell culture supernatant samples were filtered through a 0.45-µm filter to remove cell debris and bacteria and treated with a mixture of DNase and RNase to digest unprotected nucleic acids, including host DNA and RNA, prior to RNA extraction. In Lyon, RNA was extracted from the supernatants after three days of culture without RNA carrier and DNase digestion was performed on the nucleic acid extracts (Turbo DNase Ambion). Then, the RNA was converted to double-stranded cDNA. In Hamburg, library preparation was performed using the Nextera XT DNA Library Preparation Kit (Illumina), whereas in Lyon, the libraries were prepared using the NEBNext® fast DNA fragmentation and library prep for Ion Torrent kit (New England BioLabs), with 12 cycles of amplification. Finally, in Hamburg, sequencing was performed on an Illumina MiSeq platform with 250-base paired ends and dual barcoding for each library. In Lyon, sequencing was carried out on a Personal Genome Machine (PGM) using an Ion 316v2 chip and the Ion PGM HiQ OT2 kit (Life Technologies). Automated read data sets provided by both Torrent software suite 5.0 and MiSeq were trimmed according to the quality score and length. In Hamburg, reads were assembled de novo using Geneious 9 and then contigs larger than 100 bp were subjected to mapping using LASV sequences from GenBank. In Lyon, trimmed fastq files were directly mapped onto the LASV reference genome (Togo strain) using bowtie2. Gaps were filled by Sanger sequencing.
Phylogenetic analysis
The nucleotide sequences of full-length glycoprotein precursor (GPC), nucleoprotein (NP), and polymerase (L) were separately aligned in three data sets: 55 sequences for the GPC, 52 sequences for the NP, and 41 sequences for the L gene. There were three more GPC sequences than NP sequences because we added three partial GPC sequences and filled in the missing nucleotides with “N.” The lower number of L sequences was mainly due to the lack of published sequences, notably those derived from rodents and those belonging to lineage III. Nucleotide alignment was performed based on the position of the amino acids in the protein alignment. The phylogeny was inferred using the Bayesian Markov Chain Monte Carlo (MCMC) method implemented in BEAST software, version 1.10.1 [23]. To perform a time-calibrated phylogeny, the parameters were set in BEAUTI as follows: the tip dates to the nearest day, the substitution model as GTR + gamma and codon partition with positions 1,2,3, and the clock model as strict or uncorrelated relaxed. A coalescent tree with the same-sized population was set as the prior. The length of the chain was 10 million, with echo states and log parameters every 10,000 steps. The xml files from BEAUTI were run in BEAST and checked in TRACER. After verifying the effective sample size to be above 200 for all parameters, the consensus trees were obtained using TreeAnnotator and then visualized using FigTree (BEAST packages, https://beast.community/programs).
Results
Description of the outbreaks
2014
The index case was a pregnant woman who died two days after giving birth to a baby girl. She was living near the Nigerian border, where three members of her family died. The father took the baby and went to northern Benin, to a village near Tanguieta, to consult with a traditional healer [24] (Table 1). The baby was finally hospitalized in the St Jean de Dieu Hospital, where she died. As the first health worker in neonatology had symptoms on 21 October 2014, it is likely that she was contaminated while in contact with the baby 1–2 weeks before. Because the mother was sick on 3 October, her infection could have occurred in late September, after 1–2 weeks of incubation. This corresponds to the end of the rainy season. The two spouses of the traditional healer died as well and are therefore considered to be probable cases. For the 2014 epidemic, we were able to identify 2 CCs, 9 PCs, and 2 SPs, with a median age of 27 years (range: 0.04–57). Three times as many females as males were infected (9 vs. 3). In total, five health workers from the hospital were infected, but only two infections were laboratory-confirmed. The most frequently reported symptoms were fever, intense fatigue, headache, nausea, aches and pains, dizziness, vomiting, and diarrhoea.
Table 1. Outbreak of 2014.
| Case | Label | Designation | Sex | Age | Location | Date | Comment |
|---|---|---|---|---|---|---|---|
| 1 | NA | PC | F | NA | Tchaourou commune | 3 October 2014 | Pregnant 1st spouse, died 2 days after giving birth to a baby girl |
| 2 | NA | PC | F | NA | Tchaourou commune | 4 October 2014 | 2nd spouse who took care of the baby when the 1st spouse died, died |
| 3 | NA | SC | F | NA | Tchaourou commune | NA | 3rd spouse was sick, but recovered |
| 4 | NA | PC | M | NA | Tchaourou commune | 4 October 2014 | Child sleeping with the baby, died |
| 5 | NA | PC | F | 2 weeks | Tchaourou commune | 15 October 2014 | Was transported by the father from the Nigerian border to the hamlet of Serhounghè, Cobly village. Kept by the family of a traditional healer. Hospitalized at the Saint Jean de Dieu Hospital in Tanguieta, died |
| 6 | NA | PC | F | NA | Cobly village near Tanguieta | 18 October 2014 | 2nd spouse of the traditional healer, took care of the baby, died |
| 7 | NA | PC | F | NA | Cobly village near Tanguieta | 20 October 2014 | 3rd spouse of the traditional healer, took care of the baby, died |
| 8 | BEN-14-26530 | CC | F | 22 | St Jean de Dieu Hospital, Tanguieta | 21 October 2014 | Health worker in neonatology service, died. Blood sample 23 October 2014 |
| 9 | BEN-14-26529 | CC | F | 29 | St Jean de Dieu Hospital, Tanguieta | 23 October 2014 | Health worker in neonatology service, died. Blood sample 25 October 2014. |
| 10 | NA | PC | M | 32 | St Jean de Dieu Hospital, Tanguieta | 24 October 2014 | Health worker in neonatology service, died |
| 11 | NA | SC | F | 27 | St Jean de Dieu Hospital, Tanguieta | 26 October 2014 | Health worker in neonatology service, recovered |
| 12 | NA | PC | M | 57 | St Jean de Dieu Hospital, Tanguieta | 2 November 2014 | Pediatrician who treated the baby girl, died |
| 13 | NA | PC | NA | 5 months | Cobly village near Tanguieta | 8 November 2014 | Baby of case 6, died |
Notes: Patients are presented chronologically according to the date of symptoms (chain of events related in Sambieni et al. 2016 and data compiled by CKC, WHO Benin). SC: suspected case, PC: probable case, CC: confirmed case. The location indicates where exposure is presumed to have taken place. Coloured rows correspond to laboratory-confirmed cases. NA: not available.
2016
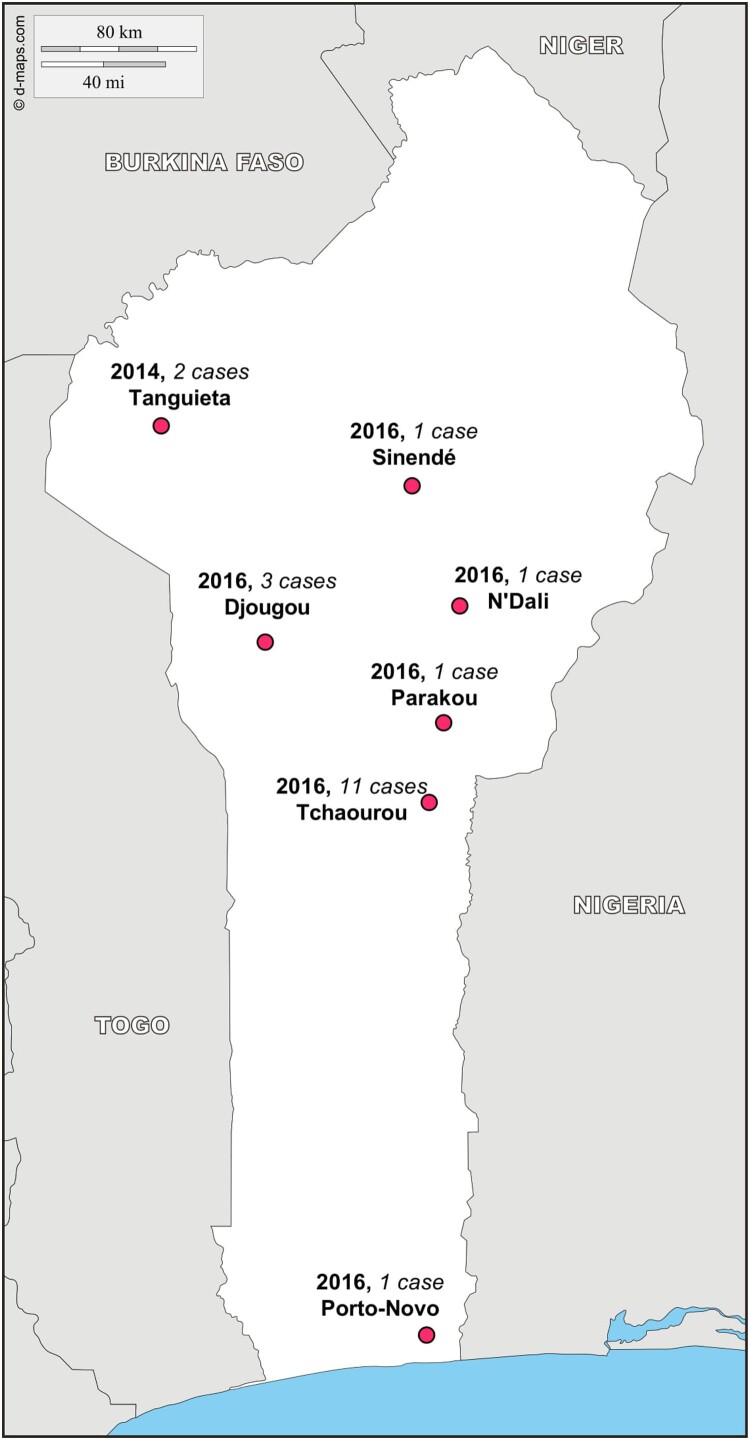
The index case was a pregnant woman from a village near the Nigerian border. She was hospitalized at the nearest hospital, Saint Martin de Papané, where a chain of nosocomial transmission was initiated. As of 2014, four health workers from this hospital were infected (Table 2). Given that the index case occurred in late December and the last case in March, the second outbreak occurred entirely in the dry season. For the 2016 epidemic, we were able to identify 18 CCs, 3 PCs, and no SPs, with a median age of 28 years (range: 0.3–60). Two times as many females as males were infected (14 vs. 7). The symptoms were similar as those reported in 2014 and the median sample collection time post-onset was seven days (range: 0–19, Table 3). The case fatality rate, based on CCs only, was approximately 44% (8/18). The 18 CC patients came from three regions: Borgou (14 cases), Donga (3 cases), and Ouémé (1 case) (Figure 1).
Table 2. Outbreak of 2016 according to our investigation in the Department of Health in Parakou, the Health Zone in Tchaourou, and Health Center of Kassouala and personal data (GKC, WHO Benin).
| Case | Label | Designation | Sex | Age | Location | Date | Comment |
|---|---|---|---|---|---|---|---|
| 1 | NA | PC | F | 24 | Kassouala village, Tchaourou, Borgou | 12 December 2015 | Pregnant, was received at the Health Center of Kassouala on 31 December 2015 and was transferred to St Martin de Papané Hospital 3 January 2016. Died on 24 January 2016 |
| 2 | BEN-16-3490 | CC | M | 44 | St Martin de Papané Hospital, Tchaourou, Borgou | 8 January 2016 | Health worker at the St Martin de Papané Hospital who took care of case 1 at arrival. Blood sample 21 January 2016. Recovered, but still suffering from hearing loss and headaches one year after the onset. He remembers that the case 1 was spitting blood at arrival |
| 3 | BEN-16081 | CC | F | 32 | NA | 11 January 2016 | Merchant coming from Lagos, was hospitalized in Porto Novo, in the department of Ouémé, blood sample 30 January 2016, died |
| 4 | NA | CCa | F | 34 | Tchaourou, Borgou | 11 January 2016 | Merchant working in Tchaourou, blood sample 21 January 2016, recovered |
| 5 | BEN-16-3489 | CC | M | 25 | St Martin de Papané Hospital, Tchaourou, Borgou | 12 January 2016 | Health worker at the St Martin de Papané Hospital, blood sample 21 January 2016, recovered |
| 6 | BEN-16-3488 | CC | F | 24 | St Martin de Papané Hospital, Tchaourou, Borgou | 13 January 2016 | Health worker at the hospital St Martin de Papané Hospital, blood sample 21 January died |
| 7 | NA | CC | F | 25 | St Martin de Papané Hospital, Tchaourou, Borgou | 13 January 2016 | Health worker at the St Martin de Papané Hospital, blood sample 21 January 2016, recovered |
| 8 | NA | CC | F | 28 | Tchaourou, Borgou | 19 January 2016 | Housewife, Blood sample 25 and 30 January 2016, recovered |
| 9 | NA | PC | F | 39 | Kassouala, Tchaourou, Borgou | 22 January 2016 | Sister of case 1, consultation 24 January 2016, died |
| 10 | BEN 16-22 | CC | F | 0.3 | St Martin de Papané Hospital, Tchaourou, Borgou | 22 January 2016 | Baby of case 7, blood sample 28 January and 9 March 2016, recovered |
| 11 | BEN-16070 | CC | F | 30 | Banikanni, N’Dali, Borgou | 23 January 2016 | Merchant, blood sample 25 and 29 January 2016, recovered |
| 12 | NA | CC | F | 3 | Tekparou, Tchaourou, Borgou | 29 January 2016 | Blood sample 5 February 2016, died |
| 13 | BEN-16-15 | CC | F | 30 | Kadjola near Kassouala, Tchaourou, Borgou | 2 February 2016 | Merchant working in Tchaourou, blood sample 5 February 2016, died |
| 14 | NA | PC | M | 18 | Sekere Sinendé, Borgou | NA | Linked with case 15, consultation 29 January 2016, died 7 February 2016 |
| 15 | BEN-16071 | CC | M | 12 | Sekere Sinendé, Borgou | 3 February 2016 | Blood sample 7 February 2016, recovered |
| 16 | BEN 16-16 | CC | F | 25 | Tchatchou, Parakou Borgou | 5 February 2016 | Merchant working in Tchaourou, blood sample 5 February 2016, died |
| 17 | BEN-16069 | CC | F | 60 | Village near Papané, Tchaourou, Borgou | 16 February 2016 | Merchant working in Tchaourou, blood sample 23 February and 10 March 2016, recovered |
| 18 | BEN-16082 | CC | M | 30 | Djougou, Donga | 26 February 2016 | Blood sample 5 March 2016, died |
| 19 | NA | CC | M | 32 | Djougou, Donga | 1 March 2016 | Farmer, blood sample 5 March 2016, recovered |
| 20 | BEN-16090 | CC | M | 34 | Djougou, Donga | 12 March 2016 | Merchant working in Djougou, blood sample 13 March 2016, died |
| 21 | BEN-16131 | CC | F | 25 | Boukouro village, Tchaourou, Borgou | 28 March 2016 | Merchant working in Tchaourou, blood sample 10 April 2016, died |
Notes: Patients are presented chronologically according to the date of symptoms. PC: probable case, CC: confirmed case. The location indicates where exposure is presumed to have taken place. Coloured rows correspond to laboratory-confirmed cases. NA: not available.
aDetected positive by q-PCR in using the prototype Altona kit V1-02, but negative by conventional PCR.
Table 3. Lassa serology and sequences obtained in 20 laboratory-confirmed samples from patients during the outbreaks in 2014 and 2016.
| Label | Lab ref N° BNITM |
RT-PCR | Lab ref N° Pasteur-INSERM-VHF |
RT-PCR | Date | IgM | IgG | Days after onset | Sequence | Accession N° |
|---|---|---|---|---|---|---|---|---|---|---|
| BEN-14-26530 | 14-26530 | + | NA | NA | 21 October 2014 | NA | NA | 2 | S and L complete | MT193284 MT199233 |
| BEN-14-26529 | 14-26529 | + | NA | NA | 23 October 2014 | NA | NA | 2 | S and L complete | MT193285 MT199234 |
| BEN-16-3490 | 16-3490 | + | 16-74 | + | 8 January 2016 | 1:400 | 1:400 | 13 | S complete | MT193286 |
| BEN-16081 | 16-11 | + | 16-81 | + | 11 January 2016 | NA | NA | 19 | S and L complete | MT193283 MT199232 |
| NA | 16-3485 | + | NA | NA | 11 January 2016 | NA | NA | 10 | NA | NA |
| BEN-16-3489 | 16-3489 | + | NA | NA | 12 January 2016 | NA | NA | 9 | S complete | MT193287 |
| BEN-16-3488 | 16-3488 | + | NA | NA | 13 January 2016 | NA | NA | 8 | S and L complete | MT193288 MT199235 |
| NA | 16-3493 | +a | 16-73 | + | 13 January 2016 | 1:400 | 1:6,400 | 8 | NA | NA |
| NA | NA | NA | 16-76 | + | 19 January 2016 | 1:400 | NA | 11 | NA | NA |
| BEN-16-22 | 16-22 | + | 16-92 | − | 22 January 2016 | 1:400 | 1:400 |
6 47 |
GP 1 kb | MT248264 |
| BEN-16070 | 16-10 | + | 16-70 | + | 23 January 2016 | NA- | NA |
2 6 |
S and L complete | MT193278 MT199227 |
| NA | NA | NA | 16-78 | + | 29 January 2016 | 1:400 | 1:1,600 | 7 | NA | NA |
| BEN-16-15 | 16-15 | + | NA | NA | 2 February 2016 | NA | NA | 3 | GP 1 kb | MT248262 |
| BEN-16071 | NA | NA | 16-71 | + | 3 February 2016 | NA | 1:400 | 4 | S and L complete | MT193279 MT199228 |
| BEN-16-16 | 16-16 | + | NA | NA | 5 February 2016 | NA | NA | 0 | GP 1 kb | MT248263 |
| BEN-16069 | 16-14425 | + |
16-69 16-91 |
+ − |
16 February 2016 |
NA 1:400 |
1:400 1:1,600 |
7 22 |
S and L complete | MT193277 MT199226 |
| BEN-16082 | NA | NA | 16-82 | + | 26 February 2016 | NA | 1:100 | 7 | S and L complete | MT193280 MT199229 |
| NA | NA | NA | 16-88 | − | 1 March 2016 | 1:100 | 1:1,600 | 4 | NA | NA |
| BEN-16090 | 16-14432 | - | 16-90 | + | 12 March 2016 | NA | 1:400 | 1 | S and L complete | MT193281 MT199230 |
| BEN-16131 | 16-14433 | + | 16-131 | + | 28 March 2016 | 1:400 | 1:400 | 13 | S and L complete | MT193282 MT199231 |
Note: Days after onset represents the period between the date of onset and the diagnosis.
aDetected positive by q-PCR using the prototype Altona kit V1-02, but negative by conventional PCR. NA: not available.
Figure 1.
Location of the 20 confirmed cases, 2 in 2014 and 18 in 2016, by commune of notification. Tanguieta is located in the department of Atacora, Sinendé, N’Dali, Parakou, and Tchaourou in the department of Borgou Departement, Djougou in the department of Donga, and Porto Novo in the department of Ouémé.
Laboratory diagnoses
LASV infection was confirmed by the detection of LASV RNA in plasma by RT-PCR, together with LASV-specific IgM and IgG for some patients (Table 3). LF was confirmed for patients with a positive RT-PCR and/or LASV IgM. Patients with only LASV IgG were not considered to be positive cases. We detected LASV RNA up to 19 days after disease onset, whereas LASV IgM was still present 47 days after onset.
Phylogeny
The BNITM obtained two full-length virus sequences from the 2014 outbreak. They also obtained one complete genome, one full-length S segment, eight partial sequences of the GPC gene, and five partial sequences of the L gene from the 2016 outbreak. The Pasteur-INSERM-VHF produced seven near complete genomes from the 2016 outbreak. Four LASVs were sequenced by both the BNITM and Pasteur-INSERM-VHF groups (Table 3). The sequences were submitted to Genbank under the accession numbers MT193277–MT193288, MT199226–MT199235 and MT248262–MT248264.
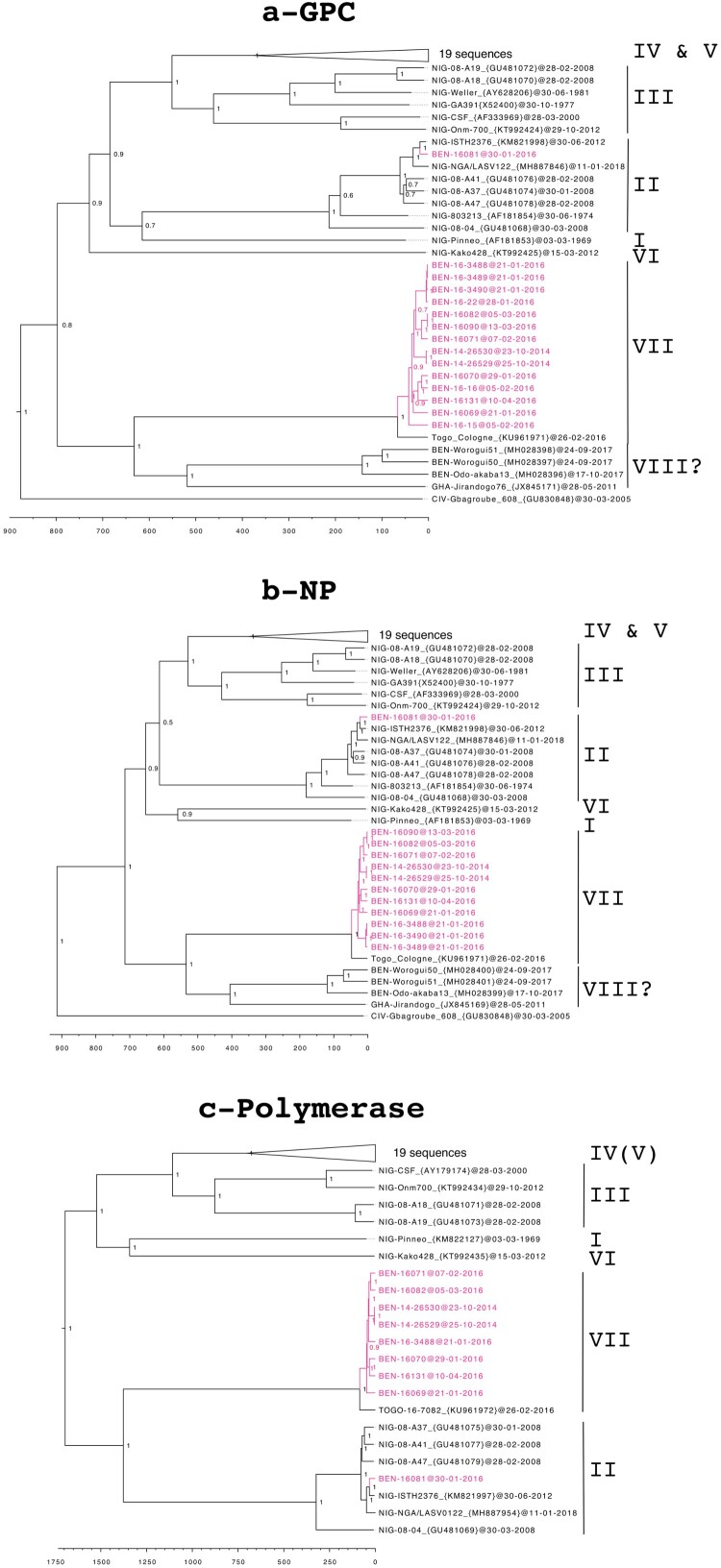
Phylogenetic analysis, including publicly complete genomes of LASV, indicated that all but one of our Benin genomes belong to the putative new lineage VII, closely related to the Togo strain (Figure 2). As observed for the Togo strain, the L sequences are close to those of lineage II, whereas the GPC and NP sequences form a cluster separate from all the other lineages. Furthermore, the long branches observed for all genes confirm the distant relationship with the other LASV strains. This is supported by the low percentage of homology between the Benin lineage and the other lineages (i.e. 68.1–71.0% for the polymerase, Table 4). The only exception is for the virus isolated from one patient (BEN-16081), for which the genome falls within lineage II, including the Nigeria strains. This patient lived in Porto Novo, close to the border with Nigeria, indicating that it was probably an imported case.
Figure 2.
Time-calibrated phylogeny of the Lassa virus strains from Benin, 2014–2016 (in pink), showing the trees created using the glycoprotein precursor (a), the nucleoprotein (b), and the polymerase (c). The trees are based on nucleotide sequences and posterior support values are shown at the nodes. Lineages are noted to the right of each tree and the scale axis represents the time in years. The GPC tree was inferred from 55 sequences, the NP tree from 52 sequences, and the L free from 41 sequences. The 19 sequences belonging to lineages IV and V have been collapsed, as they appear to be phylogenetically very distant, to make the figure more readable. However, the detailed list of the sequences used in this study are available in supplementary information.
Table 4. Pairwise identities of nucleotides and amino acids between different lineages of Lassa virus.
| Cluster comparison | GPC | NP | L | |||
|---|---|---|---|---|---|---|
| nt | aa | nt | aa | nt | aa | |
| BEN-intra | 95.5–100 | 98.4–100 | 96.3–100 | 98.2–100 | 95.7–99.7 | 98.1–99.6 |
| BEN vs. TOGO | 93.1–94.1 | 97.6–98.4 | 94.6–95.1 | 98.6–99.3 | 93.2–93.7 | 96.2–96.8 |
| BEN-human vs. BEN-rodent* | 77.7–79.9 | 90.1–92.3 | 76.9–78.2 | 89.7–90.8 | NA | NA |
| BEN vs. clade I | 77.6–78.2 | 94.1–94.3 | 75.9–76.7 | 89.8–90.5 | 68.4–68.7 | 72.9–73.2 |
| BEN vs. clade II | 77.1–78.0 | 92.1–93.7 | 76.4–77.9 | 90.7–92.3 | 69.8–71.0 | 76.5–77.5 |
| BEN vs. clade III | 76.9–79.4 | 91.6–95.1 | 76.4–78.8 | 88.6–92.6 | 69.1–69.7 | 73.3–74.5 |
| BEN vs. clade IV (V) | 76.4–78.9 | 92.1–94.5 | 76.1–78.6 | 89.3–91.9 | 68.4–69.6 | 73.7–75.3 |
| BEN vs. clade VI | 77.7–78.8 | 89.2–90.7 | 76.1–76.7 | 88.2–88.8 | 68.1–68.7 | 73.2–73.5 |
*The GPC and NP sequences were respectively truncated at 1400 and 1650 nt to fit the length of rodent-derived LASV and establish this comparison.
The nucleotide homology between the Benin LASV genomes (except BEN-16081) ranged from 95.5% to 100% for GPC, 96.3% to 100% for NP, and 95.7% to 99.7% for the L gene (Table 4). The sequences BEN 14-26529 and BEN 14-26530 are identical and confirm nosocomial transmission in the St Jean de Dieu Hospital in 2014 among the health workers. Similarly, the sequences BEN 16-3488, BEN 16-3489, BEN 16-3490, and BEN 16-22 are identical (Figure 2(a,b)) and confirm the nosocomial transmission in the St Martin de Papané Hospital in 2016. Phylogenic analysis confirms that cases in Tanguieta were closely linked with those in Tchaourou and that the index cases came from the same area, in villages near the Nigerian border. The time of the most recent common ancestor (TMRCA) in the Benin clade is estimated to be 66 (44–102) years based on GPC, 47 (34–66) years based on NP, and 85 (63–112) years based on the L gene.
The LASV sequences observed in pygmy mice (Worogui50, Worogui51, and Odo-akaba13 in Figure 2(a,b)) captured in villages around Tchaourou are grouped with those observed in humans. However, they diverged more than 600 years (421–969 by GPC analysis) ago. The similarity of nucleotides between rodent- and human-derived LASV only reaches 77.7–79.9% for GPC and 76.9–78.2% for NP (Table 4). This leads to amino-acid identities ranging from 89.7% to 92.3% for GPC and NP, similar to those between the LASV lineages (Table 4).
Discussion
This analysis clearly shows that the Lassa strains which circulate in Benin are the same as those which have been recently described in Togo [16]. This information reinforces the existence of the new lineage VII. In addition, a father and his newborn baby travelled between Saint Martin de Papané and Mango in Togo after his wife passed away from LF in February 2017 [25]. This indicates interrelations between the two regions. As the Benin strains predate that of Togo (2014 vs. 2016), we suggest adopting the Benin lineage rather than the Togo lineage. This lineage is very different from those observed in Nigeria and these strains can be considered to be specific to Benin. However, it is possible that this strain also exists in western Nigeria, particularly in Kwara state, as there is frequent contact between the two countries. The two recent cases in Baruten in Kwara state, but diagnosed in Nigeria in January 2019, are consistent with this hypothesis [26]. Indeed, local people exchange a large volume of goods on board pirogues loaded with rice from Benin or fuel from Nigeria. More recently, an asphalt road and a bridge were built in 2017 in the highlands of Kassouala to facilitate trade (pers. obs.). In January 2016, we also observed the importation of an LF case from Nigeria, but the individual was infected with lineage II, corroborating the initial results obtained at the Lagos laboratory [27]. In August 2019, the border with Nigeria was closed and no LF cases have been recorded in Benin since. This supports the hypothesis of human connectivity acting as a driver of viral spread, as indicated by the modelling carried out by Pigott et al. [28].
With the cases described in 2014, Benin discovered the presence of LF in its territory. However, an old study conducted by Saltzmann in 1977 [29] showed that a case had been discovered in Bembereke. It concerned a laboratory technician (Elsbeth Lenherr, 23 years old) who was allegedly infected at the Evangelical Hospital in April 1977. Although presenting a clinical picture suggestive of fulminant hepatitis, her serum tested LASV-positive due to isolation of the virus at the CDC in Atlanta (Frame in [29]). We suggest that this woman may have been infected with both viruses simultaneously, leading to her death on 12 May 1977 in Jos, Nigeria, where she had been transferred. A serological survey of hospital staff conducted in May 1977 showed two positive cases out of 85 (Frame in [29]). LF can thus be considered to not be completely new to Benin, which is confirmed by the TMRCA values of between 50 and 85 years. The rarity of the disease combined with poor sanitation and barrier nursing has led to nosocomial transmission. Such human-to-human transmission taking place in hospitals has already been reported during the first epidemics in Nigeria in the 1980s [30]. This was confirmed here by the similarity of sequences among hospital staff in Tanguieta and Tchaourou.
The epidemics of 2014 and 2016 occurred in the rainy season (April to October) and dry season (November to March), indicating that there is no preferential season. However, the new cases that appeared in 2017, 2018, and 2019 suggest an upsurge in the dry season [25,26,31]. The question arises as to why people are more infected in the dry season. One possibility is that there is more contact between rodents and humans in this season. An ecological investigation carried out in September 2017 in six villages around Tchaourou revealed the commensal rodents to be the soft-furred mouse, Praomys daltoni, which constituted 91% (196/216) of the rodent community living inside the houses [32]. This situation, in which the non-reservoir species (here P. daltoni) lives with humans was also observed in Ghana in six villages and in Nigeria in two localities [33,34]. The two Nigerian localities, located in Edo state, (Egare-Egoro and Ekpoma) showed that only the Natal multimammate mouse, Mastomys natalensis, was infected by LASV. In Benin, only two of six villages showed the presence of M. natalensis, of which a very small number were trapped either inside (n = 3) or outside (n = 3). None were infected with LASV [32]. On the other hand, this study showed that certain pygmy mice, Mus baoulei, circulating in the fields around the houses were carriers of the LASV virus belonging to the same cluster as the Beninese strains, but genetically very distant. This is specific to this new lineage, because when humans are infected with strains circulating in rodents in their environment, phylogenetic analysis shows some trees with very close “rodent” branches and “human” branches [35–37]. For example, the GP-LASV sequences observed in M. natalensis and Homo sapiens living in the Ekpoma zone of Nigeria showed an amino-acid similarity of approximately 95–99% [36], whereas we observed a similarity of approximately 90–92%. The Beninese human cluster is therefore very distant from the Benin murine cluster, showing that speciation occurred long ago. We suggest that humans may have been contaminated by other reservoir rodents that are yet to be discovered.
LASV IgM and IgG specific for the Benin strain were detected using LASV antigens derived from clade V and IV, respectively, confirming cross-reactivity between these divergent strains. We demonstrated that our ELISA, based on Josiah LASV antigens, cross-reacts with IgG specific for these divergent LASV strains using Benin-infected cynomolgus monkey samples. Indeed, we observed similar titres as for assays using homologous antigens (unpublished results). We sometimes observed discrepancies in samples obtained a few days after disease onset, with positive RT-PCR and LASV IgG but negative IgM responses. This unexpected absence of IgM detection probably resulted more from the low sensitivity of our assay rather than a lack of cross-reactivity, as LASV IgM was found in patients infected with similar strains, as shown in the phylogenetic tree. These results are reassuring and confirm that the current diagnostic assays we use can detect LASV strains that are highly divergent from the reference Josiah or AV strains used to produce the antigens.
The recent emergence of LASV in Benin is worrying, as it indicates an expansion of the area of active circulation and transmission of LASV to humans. As LF is the most frequently imported VHF in Europe and because sporadic LASV infection can occur outside of outbreaks, this new risk should be considered when a patient returns from Benin to Europe with compatible clinical signs. In any case, this new LASV strain has already been involved in an imported case of LF, with further autochthonous transmission in Germany in 2016 [16]. In addition, the emergence of this new LASV strain shows an increase in LASV genetic diversity and may have consequences for vaccine development. Indeed, a major issue for LASV vaccine design is the necessity to protect against the entire LASV diversity. Given the divergence of this strain from the prototypic Josiah strain frequently used to generate vaccine candidates, the efficacy of vaccine candidates should also be demonstrated with this strain.
Supplementary Material
Acknowledgements
We thank Yahannon Martial and Mama Yaya Abdou Matinou for facilitating access to the epidemiological data from the department of Borgou. We are grateful to Anne Bocquin and Héloïse Thomasset from INSERM – Jean Mérieux BSL4Laboratory and Institut Pasteur for their technical assistance. AY, CP, SG, SB, and EFC conceived the study; CP, SB, and EFC wrote the manuscript; CP, TR, and DC acquired genome data by NGS and Sanger sequencing; TR and DT performed virus isolation on Vero cells; LO, BBZ, AJ, SMe, SMu, and CP performed the identification of LASV from the samples by RT-PCR and ELISA; TR, LO, DP, and SB validated the results of the diagnosis; MP managed the data and supported the logistics in Benin and Germany; ECK and EOJ coordinated the collection of samples and epidemiological data in Tchaourou and Parakou; AY, FL, HB, FG coordinated the shipment of samples to Europe; LK, RS, CGK, MDS, SK, ALK, and PM coordinated the outbreak response within the country; and EFC gathered the field data and performed the phylogenetic analysis.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Funding Statement
This research was funded by the German Research Foundation [grant numbers DFG FI 1781/2-1 and GU 883/4-1], the European Commission through the Horizon 2020 project EVAg (European Virus Archive goes global [grant number 653316], and the Global Health protection programme supported by the Federal Ministry of Health [grant number ZMVI1-2516-GHP-704]. Institut Pasteur and INSERM – Jean Mérieux BSL4 Laboratory were funded by Santé Publique France.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Buckley SM, Casals J, Downs WG.. Isolation and antigenic characterization of Lassa virus. Nature. 1970;227:174. doi: 10.1038/227174a0 [DOI] [PubMed] [Google Scholar]
- 2.Frame JD, Baldwin JMJ, Gocke DJ, et al. Lassa fever, a new virus disease of man from West Africa. Am J Trop Med Hyg. 1970;19(4):670–676. doi: 10.4269/ajtmh.1970.19.670 [DOI] [PubMed] [Google Scholar]
- 3.Bausch DG, Demby AH, Coulibaly M, et al. Lassa fever in Guinea: I. epidemiology of human disease and clinical observations. Vector Borne Zoonotic Dis. 2001;1(4):269–281. doi: 10.1089/15303660160025903 [DOI] [PubMed] [Google Scholar]
- 4.Fichet-Calvet E, Rogers DJ.. Risk maps of Lassa fever in West Africa. PLoS Negl Trop Dis. 2009;3(3):e388. doi: 10.1371/journal.pntd.0000388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunther S, Emmerich P, Laue T, et al. Imported Lassa fever in Germany: molecular characterization of a new Lassa virus strain. Emerg Infect Dis. 2000 Sep-Oct;6(5):466–476. doi: 10.3201/eid0605.000504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mylne AQ, Pigott DM, Longbottom J, et al. Mapping the zoonotic niche of Lassa fever in Africa. Trans R Soc Trop Med Hyg. 2015 Aug;109(8):483–492. doi: 10.1093/trstmh/trv047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safronetz D, Lopez JE, Sogoba N, et al. Detection of Lassa virus, Mali. Emerg Infect Dis. 2010 Jul;16(7):1123–1126. doi: 10.3201/eid1607.100146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCormick JB, Fisher-Hoch SP.. Lassa fever. Curr Top Microbiol Immunol. 2002;262:75–109. [DOI] [PubMed] [Google Scholar]
- 9.Khan SH, Goba A, Chu M, et al. New opportunities for field research on the pathogenesis and treatment of Lassa fever. Antiviral Res. 2008 Apr;78(1):103–115. doi: 10.1016/j.antiviral.2007.11.003 [DOI] [PubMed] [Google Scholar]
- 10.Olayemi A, Cadar D, Magassouba N, et al. New hosts of the Lassa virus. Sci Rep. 2016 May 03;6:25280. doi: 10.1038/srep25280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo Iacono G, Cunningham AA, Fichet-Calvet E, et al. A unified framework for the infection dynamics of zoonotic spillover and spread. PLoS Negl Trop Dis. 2016 Sep;10(9):e0004957. doi: 10.1371/journal.pntd.0004957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asogun DA, Adomeh DI, Ehimuan J, et al. Molecular diagnostics for Lassa fever at Irrua specialist teaching hospital, Nigeria: lessons learnt from two years of laboratory operation. PLoS Negl Trop Dis. 2012 Sep;6(9):e1839. doi: 10.1371/journal.pntd.0001839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowen MD, Rollin PE, Ksiazek TG, et al. Genetic diversity among Lassa virus strains. J Virol. 2000;74(15):6992–7004. doi: 10.1128/JVI.74.15.6992-7004.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiley MR, Fakoli L, Letizia AG, et al. Lassa virus circulating in Liberia: a retrospective genomic characterisation. Lancet Infect Dis. 2019 Dec;19(12):1371–1378. doi: 10.1016/S1473-3099(19)30486-4 [DOI] [PubMed] [Google Scholar]
- 15.Manning JT, Forrester N, Paessler S.. Lassa virus isolates from Mali and the Ivory Coast represent an emerging fifth lineage. Front Microbiol. 2015;6:1037. doi: 10.3389/fmicb.2015.01037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitmer SLM, Strecker T, Cadar D, et al. New lineage of Lassa virus, Togo, 2016. Emerg Infect Dis. 2018 Mar;24(3):599–602. doi: 10.3201/eid2403.171905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO . Lassa fever – Benin; 2015. Available from: http://www.who.int/features/2015/benin-lassa-fever/en/
- 18.WHO . Lassa fever – Benin; 2016. Available from: http://www.who.int/csr/don/13-june-2016-lassa-fever-benin/en/
- 19.Ogbu O, Ajuluchukwu E, Uneke CJ.. Lassa fever in West African sub-region: an overview. J Vector Borne Dis. 2007 Mar;44(1):1–11. [PubMed] [Google Scholar]
- 20.Vieth S, Drosten C, Lenz O, et al. RT-PCR assay for detection of Lassa virus and related old world arenaviruses targeting the L gene. T Roy Soc Trop Med H. 2007 Dec;101(12):1253–1264. doi: 10.1016/j.trstmh.2005.03.018 [DOI] [PubMed] [Google Scholar]
- 21.Olschlager S, Lelke M, Emmerich P, et al. Improved detection of Lassa virus by reverse transcription-PCR targeting the 5′ region of S RNA. J Clin Microbiol. 2010 Jun;48(6):2009–2013. doi: 10.1128/JCM.02351-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carnec X, Baize S, Reynard S, et al. Lassa virus nucleoprotein mutants generated by reverse genetics induce a robust type I interferon response in human dendritic cells and macrophages. J Virol. 2011 Nov;85(22):12093–12097. doi: 10.1128/JVI.00429-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drummond AJ, Suchard MA, Xie D, et al. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012 Aug;29(8):1969–1973. doi: 10.1093/molbev/mss075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambieni NE, Danko N, Ridde V.. La fièvre hémorragique à virus Lassa au bénin en 2014 en contexte d'Ebola: une épidémie révélatrice de la faiblesse du système sanitaire. Anthropologie & Santé. 2015.. DOI: 10.4000/anthropologiesante.1772 [DOI] [Google Scholar]
- 25.WHO . Lassa fever – Benin, Togo and Brurkina Faso; 2017 Mar 10. Available from: http://www.who.int/csr/don/10-march-2017-lassa-fever-benin-togo-burkina-faso/en/
- 26.ProMED-mail . Lassa fever – West Africa (04): Nigeria (Plateau, Kwara ex Benin); 2019. Available from: https://promedmail.org/promed-post/?id=6264610
- 27.Salu OB, James AB, Bankole HS, et al. Molecular confirmation and phylogeny of Lassa fever virus in Benin Republic 2014–2016. Afr J Lab Med. 2019;8(1):803. doi: 10.4102/ajlm.v8i1.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pigott DM, Deshpande A, Letourneau I, et al. Local, national, and regional viral haemorrhagic fever pandemic potential in Africa: a multistage analysis. Lancet. 2017 Dec 16;390(10113):2662–2672. doi: 10.1016/S0140-6736(17)32092-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saltzmann S. La fièvre de Lassa. Neuchâtel: Editions des Groupes Missionnaires; 1978. [Google Scholar]
- 30.Fisher-Hoch SP, Tomori O, Nasidi A, et al. Review of cases of nosocomial Lassa fever in Nigeria: the high price of poor medical practice. Br Med J. 1995;311:857–859. doi: 10.1136/bmj.311.7009.857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Attinsounon CA, Ossibi Ibara BR, Alassani A, et al. Report of a fatal case of Lassa fever in Parakou in 2018: clinical, therapeutic and diagnostic aspects. BMC Infect Dis. 2018 Dec 17;18(1):667. doi: 10.1186/s12879-018-3587-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yadouleton A, Agolinou A, Kourouma F, et al. Lassa virus in pygmy mice, Benin, 2016–2017. Emerg Infect Dis. 2019 Oct;25(10):1977–1979. doi: 10.3201/eid2510.180523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kronmann KC, Nimo-Paintsil S, Guirguis F, et al. Two novel arenaviruses detected in pygmy mice, Ghana. Emerg Infect Dis. 2013 Nov;19(11):1832–1835. doi: 10.3201/eid1911.121491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olayemi A, Obadare A, Oyeyiola A, et al. Small mammal diversity and dynamics within Nigeria, with emphasis on reservoirs of the Lassa virus. Syst Biodivers. 2018;15:1–10. [Google Scholar]
- 35.Andersen KG, Shapiro BJ, Matranga CB, et al. Clinical sequencing uncovers origins and evolution of Lassa virus. Cell. 2015 Aug 13;162(4):738–750. doi: 10.1016/j.cell.2015.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olayemi A, Obadare A, Oyeyiola A, et al. Arenavirus diversity and phylogeography of Mastomys natalensis rodents, Nigeria. Emerg Infect Dis. 2016 Apr;22(4):687–690. doi: 10.3201/eid2204.150155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olayemi A, Adesina AS, Strecker T, et al. Determining ancestry between rodent- and human-derived virus sequences in endemic foci: towards a more integral molecular epidemiology of Lassa fever within West Africa. Biology. 2020;9:26. doi: 10.3390/biology9020026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.