Abstract
Coronavirus disease 2019 (COVID-19) has become a rapid worldwide pandemic. While COVID-19 primarily manifests as an interstitial pneumonia and severe acute respiratory distress syndrome, severe involvement of other organs has been documented. In this article, we will review the role of non-contrast chest computed tomography in the diagnosis, follow-up and prognosis of patients affected by COVID-19 pneumonia with a detailed description of the imaging findings that may be encountered. Given that patients with COVID-19 may also suffer from coagulopathy, we will discuss the role of CT pulmonary angiography in the detection of acute pulmonary embolism. Finally, we will describe more advanced applications of CT in the differential diagnosis of myocardial injury with an emphasis on ruling out acute coronary syndrome and myocarditis.
Keywords: Computed tomography, COVID-19, Pneumonia, Pulmonary embolism, Myocardial injury
Graphical abstract
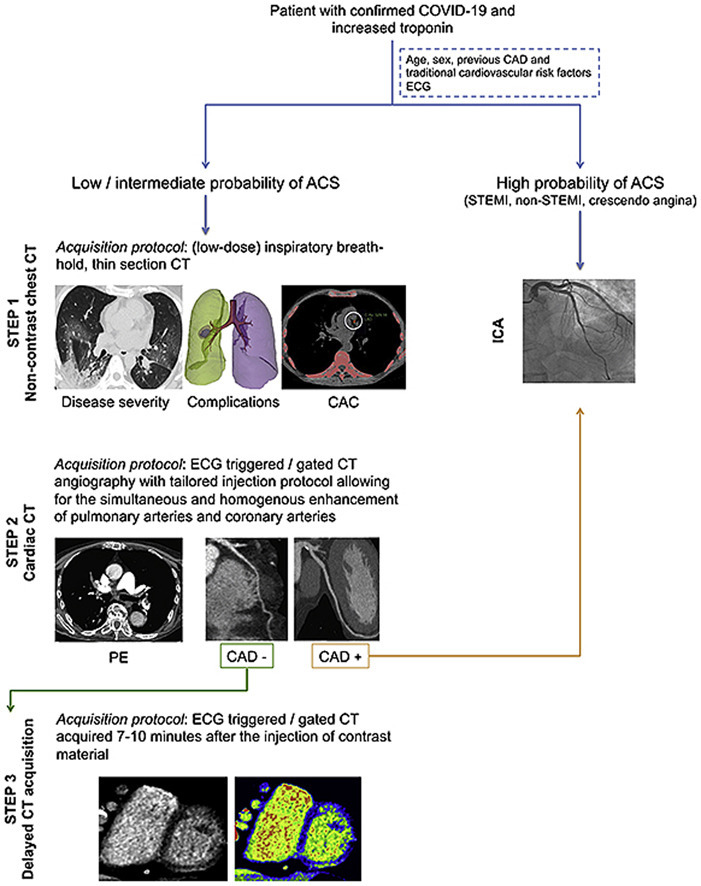
Central Illustration. CT guided diagnostic-work up of COVID-19 patients with elevated troponin. Cardiac CT is reserved to patients with low and intermediate probability of CAD. Timing the acquisition for the simultaneous evaluation of pulmonary and coronary arteries allows the ruling in/out of both pulmonary embolism and CAD. In case of obstructive CAD the patient will be referred to invasive coronary angiography. Conversely, if obstructive CAD is excluded, an additional delayed acquisition might be obtained for the detection of potential myocardial damage. Alternatively to late iodine enhancement imaging, pre and post-contrast CT phase can be analysed to calculate the ECV. Abbreviations: ACS: acute coronary syndrome; CAD: coronary artery disease; CT: computed tomography; ECG: electrocardiogram; ECV: extracellular volume; ICA: invasive coronary angiography; PE: pulmonary embolism; STEMI: ST elevation myocardial infarction.
Abbreviations
- ACS
acute coronary syndrome
- ARDS
acute respiratory distress syndrome
- CAC
coronary artery calcium
- CAD
coronary artery disease
- CMR
cardiac magnetic resonance
- COVID
coronavirus disease
- CPAP
continuous positive airway pressure
- CT
computed tomography
- CTPA
CT pulmonary angiography
- CCTA
coronary CT angiography
- EACVI
European Association of Cardiovascular Imaging
- ECG
electrocardiogram
- ECV
extracellular volume
- ESC
European Society of Cardiology
- GGO
ground glass opacities
- ICA
invasive coronary angiography
- ICU
intensive care unit
- LGE
late gadolinium enhancement
- LIE
late iodine enhancement
- MERS
Middle East Respiratory Syndrome
- PE
pulmonary embolism
- RT-PCR
reverse transcription polymerase chain reaction
- SARS
severe acute respiratory syndrome
- SCCT
Society of Cardiovascular Computed Tomography
- WAL
well-aerated lung
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since the first diagnosis was confirmed in China in December 2019, COVID-19 has rapidly spread worldwide and ravaged the globe. Although COVID-19 is primarily a respiratory disease with involvement of lung parenchyma, several reports have documented that severe forms are associated with a pro-inflammatory cytokine storm leading to systemic inflammation and sepsis with consequent involvement of various other organs, including the cardiovascular system.1 In this scenario, an integrated Computed Tomography (CT) approach can give valuable information on diagnosis, follow-up and prognosis of patients with COVID-19.
In this manuscript, we aim to provide an up to date assessment of cardiothoracic CT applications of COVID-19 through illustrative clinical cases. We will start with a comprehensive review of the role of CT in the evaluation of COVID-19 pneumonia and pulmonary embolism. We will then present an analysis of advanced cardiac CT applications for early risk stratification of severe forms of the disease, through the quantification of coronary artery calcium score, and the differential diagnosis between chest pain coronary artery disease related versus non-ischemic myocardial injury.
2. Role of CT in COVID-19 pneumonia
In February 2020, Huang et al.2 published the first report describing the use of non-contrast chest CT in 41 patients with confirmed COVID-19. Since then, the scientific evidence on COVID-19 has been rapidly growing and the clinical indications for chest CT are continuously evolving. Although reverse transcription polymerase chain reaction (RT-PCR) is the required laboratory test to confirm the diagnosis of COVID-19, non-contrast chest CT may represent a valid tool in the initial assessment of this patient population. Nevertheless, currently there is no consensus on its role within the major Professional Scientific Societies. For example, in China, during the early phase of the outbreak, CT was widely used as a supporting tool in the diagnosis of COVID-19.3 However, current guidelines from China's National Health Commission do not include imaging findings in the diagnostic criteria.4 Likewise, in the last update released on March 22, 2020, the American College of Radiology did not recommend chest CT as a first line imaging modality to screen for COVID-19 pneumonia. Recommendations advised the use of CT imaging in hospitalized, symptomatic patients with specific clinical conditions such as pulmonary embolism, empyema and co-infection.5 However, in a recent statement, the Fleischner Society identified three main scenarios where imaging may be used as a primary diagnostic tool: (1) patients with mild respiratory features consistent with COVID-19 but with risk factors for disease progression, (2) patients with moderate to severe features of COVID-19, regardless of RT-PCR test results, and (3) patients presenting with moderate-to-severe symptoms within a high prevalence of disease environment and with limited testing resources.6
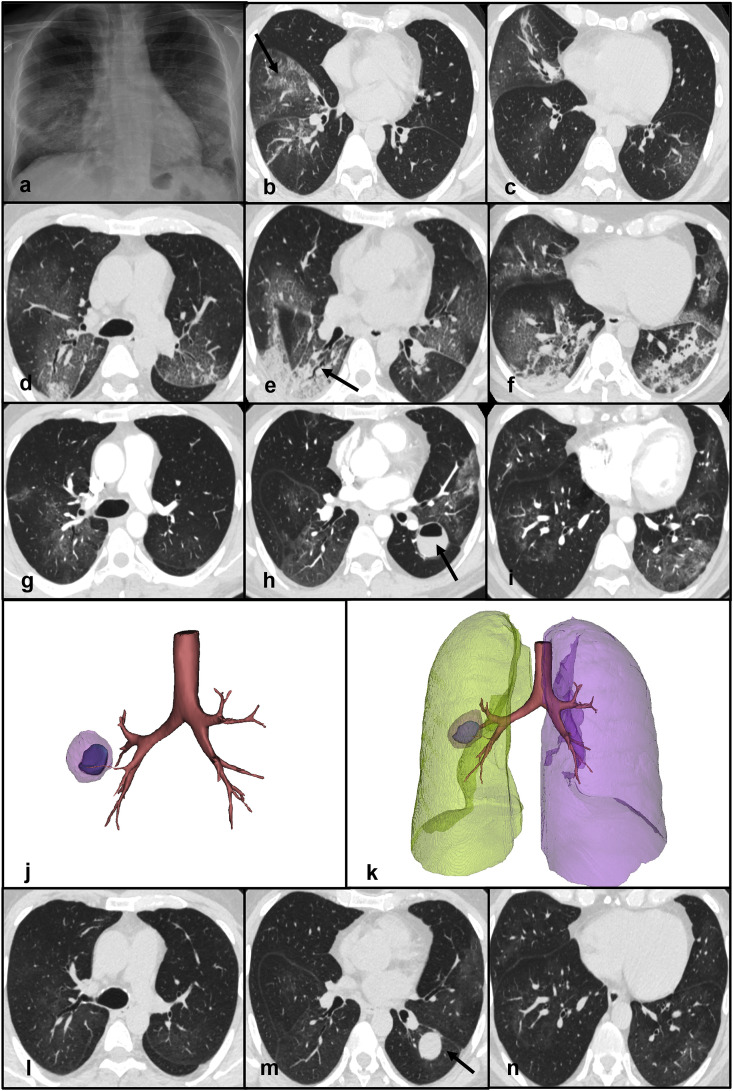
Clinical case 1: A 52-year old male was admitted due to fever and diarrhea. He reported direct contacts with COVID-19 patients. Frontal chest X-ray demonstrated subtle, bilateral opacities involving mainly the mid and lower zones of the lungs and suggested interstitial inflammatory involvement. No pleural effusions were detected. (Fig. 1 - panel a). Because of the local coronavirus epidemic outbreak, a nasopharyngeal swab was obtained confirming SARS-Cov-2 positivity. On the same day, non-contrast chest CT scan was performed documenting the presence of multiple ground glass opacities (GGO) mixed with inter-lobular and intra-lobular thickening in a “crazy-paving” pattern (Fig. 1 - panels b and c). The patient was treated with lopinavir/ritonavir and hydroxychloroquine. Four days after admission, his dyspnea worsened dramatically. Follow-up CT showed increased extent of GGOs mixed to new areas of consolidation in the lower pulmonary lobes with a predominant peripheral distribution (Fig. 1 - panels d, e, f). Because of oxygen desaturation, continuous positive airway pressure (CPAP) was initiated. One month after admission, respiratory gas exchanges were improved but he was still febrile. Therefore, an additional CT was performed after the administration of contrast material documenting the presence of a lung abscess containing an air-fluid level in the superior segment of the left upper lobe. GGOs and consolidative areas were significantly decreased compared to the previous CT scan (Fig. 1 - panels g, h, i, j, k). No acute pulmonary embolism was detected. Pneumococcal urinary antigen test was positive and he was treated with levofloxacin. After improvement of his clinical condition and two negative RT-PCR results he was discharged. A follow-up CT performed after two weeks showed near complete resolution of the interstitial abnormalities. However, the lung abscess was still present (Fig. 1 - panels l, m, n).
Fig. 1.
Panel a - Frontal chest X-ray demonstrating subtle, bilateral opacities mainly involving the mid and lower zones of the lungs. Panels b, c - Non-contrast chest CT scan showing multiple ground glass opacities and “crazy-paving” pattern (arrow - panel b) in the right middle and lower lobes. Panels d, e, f - Non-contrast chest CT scan documenting increased extent of GGOs mixed with new areas of consolidation in the lower pulmonary lobes. An air bronchogram is visible in the right lower lobe (arrow - panel e). Panels g, h, i - Contrast CT scan showing a lung abscess containing an air-fluid level in the superior segment of the left upper lobe (arrow - panel h). GGOs and consolidative areas are significantly decreased compared to the previous CT scan. Panels j, k - 3D reconstructions of the lung abscess. Panels l, m, n - Non-contrast chest CT demonstrating the lung abscess (arrow - panel m) and the near resolution of the parenchymal abnormalitiesAbbreviations
CT: computed tomography; GGO: ground-glass opacities.
2.1. CT features in COVID-19 pneumonia
The CT findings commonly observed in patients with COVID-19 pneumonia are the expression of acute interstitial lung damage, and resulting parenchymal changes caused by the cytokine storm triggered by the internalization of the virus into the pneumocytes.7, 8, 9 Post-mortem studies have evaluated the histological changes in the lungs of COVID-19 patients, revealing the presence of pulmonary edema, hyaline membranes and alveolar cellular exudates.10, 11, 12, 13, 14, 15, 16, 17 These changes are likely the substrate for the most common CT findings detected such as GGO and focal consolidation. In a systematic review including 919 patients with a confirmed diagnosis of COVID-19, GGOs have been reported as the earliest abnormalities, with an occurrence rate up to 88%, whereas consolidations have been described in approximately 32% of the patients.18 While GGOs were documented in both an isolated form and in association with focal areas of consolidations, pure consolidations were a rare finding.12 , 15 , 19 The distribution of the parenchymal lesions was commonly bilateral (88%), multilobar (78%) and peripheral (76%), with frequent involvement of the posterior regions of the lungs (80%).18 Additionally, several other chest CT findings, such as interlobular septal thickening, bronchiectasis, “crazy paving” and halo sign, have been reported with a lower prevalence.18 , 20 By contrast, pleural and pericardial effusions, mediastinal lymphadenopathy and pulmonary nodules have been rarely observed.18 Interestingly, early CT reports described involvement of the vascular structures of the lungs. Specifically, a dilatation of the sub-segmental pulmonary vessels surrounding the parenchymal abnormalities has been documented17 , 21 as a possible effect of the locally released pro-inflammatory factors.20 In the study by Bai et al. this finding was significantly more frequent in patients with COVID-19 pneumonia compared to patients with pneumonia due to other causes (59% vs. 22%, respectively).21
2.2. CT findings at different stages of disease progression
In COVID-19 patients, the evolution of lung abnormalities on chest CT resembles the progression of other forms of acute lung injury due to viral pneumonia, such as the Severe Acute Respiratory Syndrome (SARS)22 , 23 and the Middle East Respiratory Syndrome (MERS).24 The final stage of the disease is usually characterized by an acute respiratory distress syndrome (ARDS) pattern, with findings overlapping with organizing pneumonia.25 Several groups have described this dynamic radiological pattern.11 , 26, 27, 28, 29, 30, 31 In particular, Pan et al.26 delineated four different stages of the disease according to the time from onset of symptoms. In the early phase (0–4 days), the most common abnormality was GGOs. Instead, the hallmark of the progressive phase (5–8 days) was the increasing number and size of GGOs, the gradual transformation of GGOs into multifocal, consolidative areas and the development of a “crazy-paving” pattern. The peak stage (9–13 days) was characterized by a more extensive lung involvement and the presence of dense consolidations. In the absorption stage, consolidations were slowly reabsorbed and repaired lung signs, such as fibrotic bands, appeared.26 In a similar longitudinal study, Wang et al.27 confirmed that pure GGOs were the most common CT findings after symptom onset whereas the prevalence of a mixed pattern of GGOs and irregular linear opacities peaked at 6–11 days. Lung abnormalities usually persist for a long time on chest imaging. Wang et al.27 reported that 94% of the patients had residual CT findings at the time of discharge after a median time from symptom onset and discharge of 25 days. Studies on long-term follow-up imaging are warranted to evaluate possible lung damage including fibrosis, and its impact on pulmonary function, as has been previously seen in patients with SARS and MERS.32
Several semi-quantitative scores have been developed to evaluate the extent of pulmonary involvement33 , 34 and to objectively monitor the course of the disease.15 , 26 , 27 , 29 , 30 Pan et al.26 visually scored the involvement of each of the five lung lobes as follows: 0, indicating no involvement; 1, less than 5%; 2, 5–25%; 3, 26–49%; 4, 50–75%; and 5, 75–100%. They found that the total CT score progressively increased until 10 days from the onset of symptoms with a median peak score of 6. Similarly, in the study by Wang et al.,27 the median CT score peaked at day 10 and slowly decreased thereafter. Remarkably, Dangis et al.35 revealed a high intra- and inter-observer reproducibility for CT scores of lung involvement, highlighting the possible role of CT in longitudinal studies aiming to monitor disease progression in COVID-19 patients. Additional assistance may come from Artificial Intelligence algorithms. Huang et al.36 quantified the lung opacification percentage in 126 COVID-19 patients by using a commercially available deep learning software. Patients were classified in four classes depending on the severity of the disease: mild, moderate, severe and critical. The main finding of the study was that the burden of lung opacification progressively increased with the severity of the disease. In line with the results of the studies by Pan et al.26 , 27 and Wang et al. lung opacification percentage peaked at day 13.
2.3. CT findings and disease severity
Patients with COVID-19 pneumonia may present with different disease severity, from mild to critical forms. Since severe cases can progress to ARDS or death, their identification is of paramount importance to promptly initiate the right treatment. With regards to imaging, in severely ill patients, the most common CT finding is consolidation rather than GGO, with an extensive lung involvement characterized by a bilateral and multilobar distribution.37
Yang et al.33 evaluated the performance of a semi-quantitative score calculating the extent of lung opacification in 20 pulmonary segments as a surrogate for disease burden. A score of 0, 1, and 2 was attributed to each lung opacity depending if parenchymal opacification involved 0%, less than 50%, or equal or more than 50% of each region (total score: 0–40 points). A threshold of 19.5 was identified to discriminate between severe and mild cases with a sensitivity of 83% and a specificity of 94%. Similarly, Li et al.38 employed a semi-quantitative score obtained by summing up the values assigned to each pulmonary lobe on the basis of percent involvement. The median CT-score of the patients group with severe disease was significantly higher compared to the group of patients with mild symptoms (10 versus 5, respectively).
In line with clinical reports which identified older males at higher risk of having a more serious course of the disease,37 , 39 , 40 consolidations were detected more frequently in patients older than 50 years.12 , 34 Moreover, Zhou et al. demonstrated a positive correlation between patient age and involvement of lung parenchyma. Conversely, no significant differences in the distribution of imaging findings were found between hospitalized and in-home isolation patients17 and febrile and afebrile patients.41
2.4. Diagnostic performance of CT in the diagnosis of COVID-19 pneumonia
Several studies have reported the sensitivity of CT using RT-PCR as the reference standard. Values from individual patient cohorts ranged between 61% and 99%.13 , 16 , 35 , 42, 43, 44, 45, 46, 47 Interestingly, Xu B et al.48 demonstrated that CT had a higher sensitivity in Wuhan compared to other regions of China, presumably due to the greater experience of the radiologists in a pandemic area.21 , 48 On the other hand, in a study including 104 subjects from the Diamond Princess cruise ship, the sensitivity of CT was only 61%. Of note, 73% of the subjects were asymptomatic.16 Limiting the inclusion to only symptomatic patients, with proven diagnosis of COVID-19, is a source of selection bias with subsequent distortion of the results of diagnostic accuracy.49 , 50 Extreme caution must be taken when ruling-out COVID-19 disease on the basis of a negative CT scan. Indeed, false negative CT scans have been reported, especially when performed within 48 h from symptom onset.11 , 35
Only few studies reported the specificity of CT in the diagnosis of COVID-19, with values ranging between 24% and 94%.13 , 17 , 21 , 51 There are different reasons that may explain this low specificity. First, a number of factors influence the performance of RT-PCR, including the site of specimen, the stage of disease, the viral load, and the reliability of the testing kit.47 , 52 The sensitivity of RT-PCR ranged from 60% to 89%, depending on the specimen site (throat sample vs. sputum, respectively).53 In particular, a lower sensitivity of RT-PCR has been reported in elderly patients, reflecting the possible poor cooperation of the subjects leading to improper sampling.51 Importantly, in the early phase of the disease, the rate of false negative RT-PCR compared to CT was not negligible.13 , 26 , 54 In the study by Ai et al.13 a sub-group of 258 patients underwent multiple RT-PCR assays. Among the patients in whom RT-PCR turned from negative to positive, 67% showed initial positive chest CT findings.
Second, the CT findings described in the lung parenchyma of patients with COVID-19 are not specific, with a significant overlap with other diseases causing interstitial damage.25 , 55 The differential diagnosis of COVID-19 pneumonia from other forms of viral pneumonia requires a careful evaluation of all clinical information, radiological pattern, and exposure history.56, 57, 58, 59 In this scenario, the implementation of Artificial Intelligence algorithms into radiologist workflow has shown promising results in improving diagnostic outcomes.60 , 61 Nevertheless, the use of chest CT as a screening test in low disease prevalence regions may lead to a high number of false positive results with further unnecessary downstream diagnostic testing, increased healthcare costs and overload of the healthcare system.51
Finally, the low interpretation threshold employed by the radiologists for the diagnosis of COVID-19 pneumonia had a positive effect on sensitivity but a negative impact on specificity.50 The recent introduction of standardized reporting systems (Table 1 )62, 63, 64 represents an attempt to align interpretation thresholds, thus reducing the source of variability and selection bias in further research.50
Table 1.
Imaging reporting systems in COVID-19.
| CT findings | Grade | Level of suspicion | |
|---|---|---|---|
| CO-RADS62 | |||
| Scan technically insufficient for assigning a score | 0 | Not interpretable | |
| Normal or non-infectious | 1 | Very low | |
| Typical for other infection but not COVID-19 | 2 | Low | |
| Features compatible with COVID-19 but also other diseases | 3 | Equivocal/unsure | |
| Suspicious for COVID-19 | 4 | High | |
| Typical for COVID-19 | 5 | Very high | |
| RT-PCR positive for SARS-CoV-2 | 6 | Proven | |
| COVID-RADS63 | |||
| Normal chest CT | 0 | Low | |
| Atypical findings | 1 | Low | |
| Fairly typical findings | 2A | Moderate | |
| Combination of atypical with typical-fairly typical findings | 2B | Moderate | |
| Typical findings | 3 | High | |
Refer to the original manuscripts for the list of imaging findings belonging to each grade.
Abbreviations: COVID: coronavirus disease; CT: computed tomography; RT-PCR: reverse transcription polymerase chain reaction; SARS: severe acute respiratory syndrome.
2.5. Prognostic role of CT in COVID-19 patients
Recent studies reported that older age65 and myocardial injury66 were independent predictors of in-hospital mortality in patients with confirmed diagnosis of COVID-19. As in other fields, CT may provide additional information for better identification of patients who will benefit from more aggressive therapy and close monitoring. However, only limited evidence is available on the role of imaging in the risk stratification of patients with COVID-19. In a selected cohort of 120 consecutive patients, Tabatabaei et al.67 found that non-survivors and patients admitted to the intensive care unit (ICU) had significantly more consolidation, air-bronchograms, “crazy-paving” and central involvement of the lungs compared to other hospitalized patients. Likewise, Yuan et al.68 investigated the characteristics of 27 consecutive patients with confirmed diagnosis of COVID-19. They calculated a CT score which took into account the location, extent, and distribution of each abnormality within the lungs (range: 0–72 points). Mortality rate in this population was 37%. Non-survivors presented a higher CT score at the time of admission, higher rate of consolidation and higher frequency of air bronchograms compared to survivors (30 vs. 12, 40% vs. 6%, 60% vs. 12%, respectively). A cut-off CT score value of 24.5 had a sensitivity of 85% and a specificity of 84% for predicting mortality. Furthermore, Colombi et al.69 investigated the association between the percentage of well-aerated lung (WAL) as a surrogate marker of residual respiratory function and a composite end-point of admission to ICU and death. The percentage of WAL was calculated by visual assessment and by using fully automatic software in 236 patients with COVID-19. The rate of the composite end-point was 46%. Remarkably, they found that the percentage of WAL was an independent predictor of ICU admission/death after adjusting for age and clinical risk factors. Artificial Intelligence will likely expand the role of chest imaging from diagnosis to risk stratification.70
3. Role of CT in the detection of acute pulmonary embolism in COVID-19 patients
Acute infections have been largely associated with a transient status of coagulopathy, potentially leading to disseminated intravascular coagulation and pulmonary embolism (PE).71 , 72 Notably, in a recent meta-analysis including 9 studies and 1105 patients with COVID-19, Xiong et al.73 showed that prothrombin time and D-dimer levels were significantly higher in patients with severe disease compared to patients with a mild course of the disease. Furthermore, Zhou et al. demonstrated that an increased level of D-dimer (>1 μg/ml) was a strong predictor of in-hospital mortality in a retrospective cohort of 191 COVID-19 patients, with an OR of 18.42 (95% confidence intervals: 2.64–128.55). This association was maintained even after adjustment for age, coronary artery disease, sequential organ failure assessment score, and lymphocyte count. In this scenario, CT pulmonary angiography (CTPA) may have a fundamental role in the diagnostic work-up of COVID-19 patients for the diagnosis of acute PE.
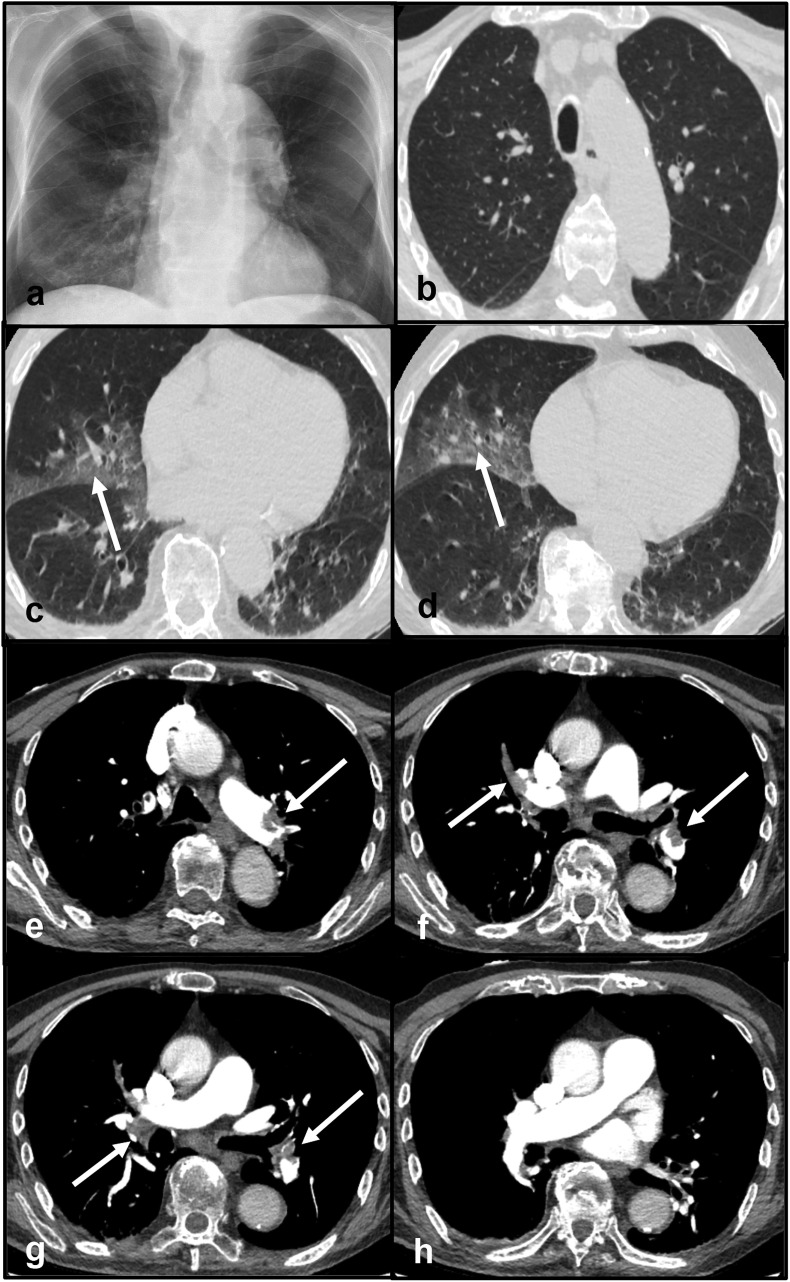
Clinical case 2: An 82-year old male was admitted to the Emergency Department because of severe exertional dyspnea. Neither fever nor cough was registered. ECG and echocardiography were unremarkable. The blood test showed a significant elevation of D-dimer (7334 ng/ml). Oxygen saturation was 94% on room air. Frontal chest X-ray demonstrated a consolidation in the lower zone of the right lung. No pleural effusions were detected (Fig. 2 - panel a). His nasopharyngeal swab tested positive for SARS-CoV-2 infection. A CT scan was performed. The CT protocol consisted of a first acquisition without the administration of contrast material for the evaluation of lung parenchyma and a second acquisition during the administration of contrast material for the evaluation of pulmonary vasculature (CTPA). Despite the presence of breathing artifact negatively affecting image quality, the non-contrast chest CT showed multiple GGOs in both lungs (Fig. 2 - panels b, c, d). CTPA documented the presence of multiple filling defects in the pulmonary arteries (Fig. 2 - panels e, f, g, h). Venous Doppler ultrasound excluded the presence of deep venous thrombosis. He was treated with antiviral therapy and enoxaparin. The final diagnosis was acute PE in addition to early phase COVID-19 pneumonia.
Fig. 2.
Panel a - Frontal chest X-ray demonstrating a consolidation in the lower zone of the right lung. Panels b, c, d - Non-contrast CT scan showing multiple GGOs in both lungs (arrows). Panels e, f, g, h - CT pulmonary angiography documenting multiple filling defects in the pulmonary arteries (arrows).
Abbreviations: CT: computed tomography; GGO: ground-glass opacities.
The overall prevalence of acute PE in COVID-19 patients documented by CTPA ranged between 14% and 30%.74, 75, 76, 77 Of note, despite prophylactic or therapeutic anticoagulation, the rate of PE in COVID-19 patients admitted to ICU was in the range of 25–26% supporting a relationship between the severity of the disease and the prothrombotic phenotype of severely ill patients.78 , 79 Interestingly, pulmonary emboli had more frequent segmental distribution (51%) rather than lobar (31%) or central (13%) involvement.74
A report of the National Institute for Public Health of the Netherlands advises routine D-dimer testing on admission and serially during hospital stay in patients with a proven diagnosis of COVID-19. Instead, CTPA should be reserved to patients with significantly elevated D-dimer on admission (2000–4000 μg/ml) or with significant D-dimer increase during the hospital stay.80 Dual energy CT may be useful for the evaluation of lung perfusion abnormalities in COVID-19 patients not only in the acute setting81 but also to monitor lung sequelae in follow-up scans.
4. Role of CT in the detection of subclinical atherosclerosis
COVID-19 patients with pre-existing cardiovascular disease seems to be at higher risk of in-hospital mortality.3 Based on the well established association between coronary artery calcium (CAC), as index of subclinical atherosclerosis, and fatal and non-fatal outcomes in patients with stable coronary artery disease (CAD),82 two preliminary reports83 , 84 have investigated the impact of CAC on the outcome of patients with COVID-19. In both studies CAC was quantified on non-contrast, non-gated chest CT considering that previous investigations demonstrated good agreement with the reference gated CT.85 Specifically, in a population of 53 patients with proved COVID-19, Fovino et al.84 demonstrated that a high CAC ≥400 was an independent predictor of in-hospital mortality and intensive care unit admission (odds ratio: 7.86, 95% confidence interval: 1.16–53.01) after adjusting for age and sex. Similarly, Dillinger et al.83 showed that CAC was significantly associated with first occurrence of mechanical intubation, extracorporeal membrane oxygenation and in-hospital mortality after adjusting for age, sex and traditional cardiovascular risk factors. Despite these results suggest that CAC might help in the identification of patients who will experience a worse in-hospital prognosis, both studies suffer from similar limitations. First, the use of a composite primary end-point may have introduced a bias due to the competing risk between the endpoints. Second, the value of all potential confounders, especially those related to lung injury, was not evaluated limiting the assessment of CAC as an independent predictor of worse prognosis in this particular population.
5. Role of cardiac CT in COVID-19 patients with myocardial injury
In the setting of COVID-19, myocardial injury has been usually defined as an increase of troponin level above the 99th percentile of the upper reference limit during the course of the disease.86 Recent data suggested that the prevalence of myocardial injury in COVID-19 patients ranged between 7% and 36%.2 , 65 , 66 , 87, 88, 89 Of note, patients with elevated cardiac troponin were proved at increased risk of morbidity and mortality compared to patients who did not developed cardiac injury.89 The causes leading to myocardial injury in COVID-19 are multiple90 and they can be summarised as follows:
-
(1)
Acute coronary syndrome (ACS) due to either cytokine mediated plaque instability or inflammatory prothrombotic stage;91
-
2)
Myocardial mismatch between myocardial demand and consumption of oxygen leading to type 2 myocardial infarction;92, 93, 94
-
(3)
Direct cardiac damage mediated by the membrane protein angiotensin converting enzyme 2;95
-
(4)
Inflammatory myocarditis due to the massive release of interleukin-1 and interluking-6, occurring in the advanced stage of the disease.96
Due to this complex scenario and due to the overlapping of clinical presentation, interpretation of elevated troponin may be extremely challenging.86 Therefore, additional cardiac imaging is often required to clarify the diagnosis, to guide the therapy and to establish the prognosis of these patients (Central Illustration). In line with the current recommendations from the European Society of Cardiology (ESC), the European Association of Cardiovascular Imaging (EACVI) and the Society of Cardiovascular Computed Tomography (SCCT), cardiac CT in COVID-19 patients should be limited to hospitalized patients where it is likely to change patient management or be lifesaving.97, 98, 99
5.1. Coronary CT angiography in ruling-out ACS
The prevalence of ACS in patients infected by SARS-CoV-2 is still unknown. In a recent study, Stefanini et al.94 evaluated angiographic findings and outcome of all COVID-19 patients with ST-segment elevation myocardial infarction within Lombardy during the first 6 weeks of the COVID-19 outbreak. A total of 28 patients were included in the analysis. Remarkably, 39% of the patients did not present with obstructive coronary artery disease (CAD) at invasive coronary angiography (ICA). It is worth noting that type-2 myocardial infarction is a common subtype in patients with acute infections.1 , 91 Therefore, the usefulness of invasive coronary angiography (ICA) with the aim of coronary revascularization may be limited and an alternative non-invasive diagnostic work-up may be considered.1
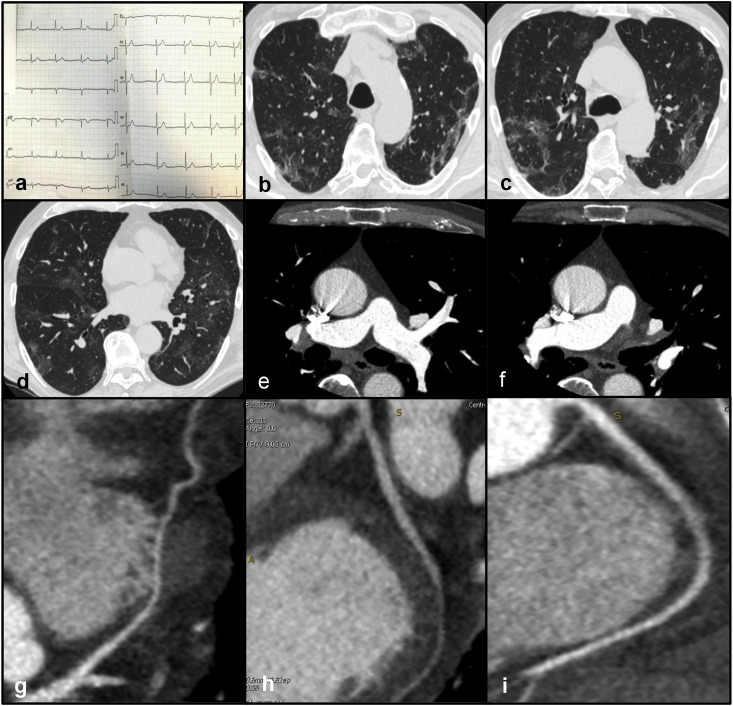
Clinical case 3: A 74-year old male was admitted to the Emergency Department due to dyspnea and fever for three days. The high-sensitivity troponin curve was 9.70 μg/L (time: 9am) » 33.80 μg/L (time: 1pm) » 49.00 μg/L (time: 5pm). The electrocardiogram (ECG) did not demonstrate significant abnormalities (Fig. 3 - panel a). Echocardiography was normal. Nasopharyngeal swab tested positive for SARS-CoV-2 infection. A CT scan consisting of a non-contrast acquisition followed by an ECG-triggered CCTA was performed. The non-contrast chest CT documented multiple, patchy GGOs in both lungs consisting with viral pneumonia (Fig. 3 – panels b, c, d). CCTA excluded the presence of pulmonary embolism (Fig. 3 - panels e, f) and showed normal coronary arteries (Fig. 3 – panels g, h, i). The final diagnosis was myocardial injury with non-obstructive CAD.
Fig. 3.
Panel a - Normal ECG. Panels b, c, d - Non-contrast CT documenting multiple, patchy GGOs in both lungs consistent with viral pneumonia. Panels e, f - CCTA excluding acute pulmonary embolism. Panels g, h, d – CCTA showing absence of coronary artery disease in the left anterior descending artery (panel g), left circumflex (panel h) and right coronary artery (panel i)
Abbreviations: CCTA: coronary computed tomography angiography; ECG: electrocardiogram.
According to the recently published EACVI position paper,86 the diagnostic work-up of COVID-19 patients with myocardial injury has to be guided by the pre-test probability of CAD based on symptoms, age, sex, cardiovascular risk factor, and previous history of CAD (Central Illustration). In particular, in patients with high probability of ACS, such as in the presence of ST-elevation myocardial infarction (STEMI) or high-risk non-STEMI, ICA should be considered. On the other hand, CCTA should be reserved to patients at low and intermediate risk of ACS with equivocal presentation where it can replace ICA in ruling-out ACS thanks to its excellent negative predictive value.100 , 101 This may help avoid unnecessary exposure to all members of the cardiac catheterization laboratory, decrease personal protective equipment utilization and limit patient procedural risk.99
5.2. Role of CT in the diagnosis of acute myocarditis in COVID-19 patients
When myocardial injury is detected in the absence of obstructive CAD, myocarditis should be considered as a possible differential diagnosis.102 Nowadays, cardiac magnetic resonance (CMR) is considered the imaging modality of choice for diagnosing myocarditis.103 Typical CMR features of acute viral myocarditis include diffuse myocardial edema on T2-weighted imaging, hyperemia on early gadolinium enhancement imaging, areas of myocardial fibrosis with a non-ischemic distribution on late gadolinium enhancement (LGE), increased signal on native T1 and T2 mapping,104 and increased extracellular volume (ECV).105 Interestingly, preliminary studies in which CMR was performed in patients with active COVID-19 or who recovered from COVID-19 showed that the most common finding was myocardial edema (54%–100%) while fibrosis on LGE was present in a lower proportion of patients (30%–41%). These results support the theory of inflammation as the primary mechanism of myocardial injury in patients with COVID-19.106, 107, 108 Nevertheless, the use of CMR may be limited during a pandemic due to contamination issues of personnel and patients and due to reduced availability related to long environmental cleaning time.99 In this scenario cardiac CT may represent a valid alternative to CMR, facilitating the differential diagnosis in COVID-19 patients with myocardial injury109 (Central Illustration).
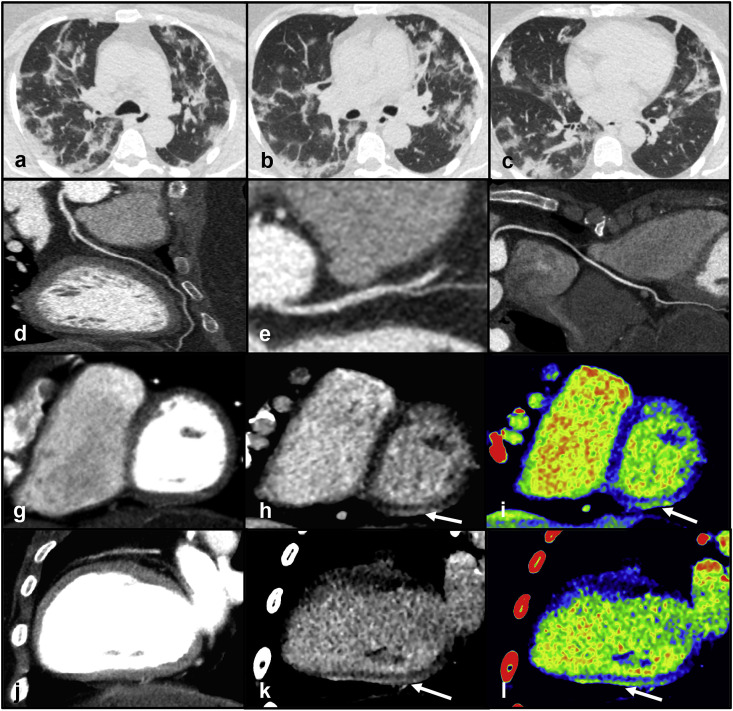
Clinical case 4: A 39-year old female presented to the Emergency Department because of dyspnea and burning chest pain exacerbated by inspiration. She reported a history of fever, rhinorrhea, and pharyngodynia three days before the admission. Blood tests showed an increased level of C-reactive protein (81 mg/L). The high-sensitivity troponin curve was 24 μg/L (time: 5am) » 51 μg/L (time: 9am) » 84 μg/L (time: 1pm). Oxygen saturation was 94% on room air. ECG and echocardiography were unremarkable. The nasopharyngeal swab tested positive for SARS-CoV-2 infection. Considering her young age, clinical presentation and low pre-test probability of CAD the initial hypothesis was myocarditis. She underwent a dedicated CT examination to avoid contamination of the only MR scanner in the department. The dedicated CT protocol included a non-contrast acquisition, an ECG-triggered CCTA and a delayed acquisition after 7 min from contrast administration. The non-contrast chest CT demonstrated the presence of multiple, bilateral GGOs and multiple areas of consolidation with a predominant sub-pleural distribution in both lungs (Fig. 4 - panels a, b, c). CCTA excluded coronary artery disease (Fig. 4 - panels d, e, f) and perfusion abnormalities in the myocardium (Fig. 4 - panels g, j). The delayed CT acquisition documented a sub-epicardial area of hyperdensity in the basal, infero-lateral wall of the left ventricle (Fig. 4 - panels h, k, i, l). Imaging was suggestive of myocarditis in addition to early-progressive COVID-19 pneumonia.
Fig. 4.
Panels a, b, c - Non-contrast chest CT demonstrating the presence of multiple, bilateral GGOs mixed with areas of consolidation with a predominant sub-pleural distribution in both lungs. Panels d, e, f - CCTA showing absence of coronary artery disease in the left anterior descending artery (panel d), left circumflex (panel e) and right coronary artery (panel f). Panels g, j - CCTA excluding perfusion defects in the left ventricular myocardium. Panels h, k, i, l - Delayed post-contrast scan (panels h, k) and corresponding color-maps (panels i, l) documenting a sub-epicardial area of hyperdensity in the basal, infero-lateral wall of the left ventricleAbbreviations
CCTA: coronary computed tomography angiography; CT: computed tomography; GGO: ground-glass opacities. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
In myocarditis myocardial fibrosis can occur as focal areas of replacement fibrosis following myocyte death or as diffuse fibrosis due to the expansion of the interstitium surrounding the myocytes.110 Despite the different molecular structures, iodine contrast material and gadolinium present similar kinetic and ECV distribution.111 Therefore, imaging of myocardial fibrosis by CT is feasible with good agreement with CMR, as documented in the detection and quantification of acute and chronic myocardial infarction.111 Specifically, late iodine enhancement (LIE) CT allows the identification of focal fibrosis whereas ECV CT permits the detection of diffuse myocardial injury. Currently, only little data is available on the performance of LIE CT in patients with acute myocarditis.109 , 112 Of note, the largest analysis including 20 patients with CMR-proven acute myocarditis was performed with spectral CT.113 In this proof-of-concept study, LIE spectral CT showed a sensitivity of 100% in the detection of myocardial fibrosis on a per-patient based-analysis compared to LGE CMR. Additional evaluation of pre-contrast and post-contrast CT images allows myocardial ECV assessment.114 This may be of interest in patients with myocarditis since they usually present higher ECV compared to controls, as demonstrated in a previous CMR study.115 Also, adding ECV to the diagnostic algorithm may improve the overall accuracy of CT in the diagnosis of myocarditis when focal fibrosis is not present.115 Although these preliminary results support the role of CT in the evaluation of myocardial damage in patients with suspected myocarditis, further larger studies are warranted before its implementation in the clinical arena.
6. Conclusions
During SARS-Cov-2 pandemic, CT may be used as a comprehensive, non-invasive imaging modality which allows for the evaluation of lung parenchyma, patency of pulmonary and coronary arteries and myocardial damage. The era of the “quadruple rule-out” has just begun.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Guzik T.J., Mohiddin S.A., Dimarco A., et al. COVID-19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.2648. In press. [DOI] [PubMed] [Google Scholar]
- 4.General Office of the National Health Commission and the Office of the National Administration of Traditional Chinese Medicine . 2020. Diagnosis and Treatment Protocol for COVID-19 (Trial Version 7) [Google Scholar]
- 5.ACR . 2020. ACR Recommendations for the Use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. [Google Scholar]
- 6.Rubin G.D., Ryerson C.J., Haramati L.B., et al. The role of chest imaging in patient management during the COVID-19 pandemic: A multinational consensus statement from the fleischner society. Radiology. 2020;296:172–180. doi: 10.1148/radiol.2020201365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felsenstein S., Herbert J.A., McNamara P.S., Hedrich C.M. COVID-19: Immunology and treatment options. Clin Immunol. 2020;215:108448. doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000 e1003. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin C., Zhou L., Hu Z., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung M., Bernheim A., Mei X., et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song F., Shi N., Shan F., et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295:210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ai T., Yang Z., Hou H., et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ming-Yen Ng E.Y.L., Jin Yang, Yang Fangfang, et al. Imaging profile of the COVID-19 infection: Radiologic findings and literature review. Radio: Cardiothoracic Imag. 2020;2 doi: 10.1148/ryct.2020200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernheim A., Mei X., Huang M., et al. Chest CT findings in coronavirus disease-19 (COVID-19): Relationship to duration of infection. Radiology. 2020;295:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shohei Inui A.F., Jitsu Motoyuki, Kunishima Naoaki, et al. Chest CT findings in cases from the cruise ship “Diamond princess” with coronavirus disease 2019 (COVID-19) Radiol: Cardiothoracic Imag. 2020;2 doi: 10.1148/ryct.2020204002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caruso D., Zerunian M., Polici M., et al. Chest CT features of COVID-19 in rome, Italy. Radiology. 2020;296:E79–E85. doi: 10.1148/radiol.2020201237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): A systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215:87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 19.Shi H., Han X., Jiang N., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye Z., Zhang Y., Wang Y., Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): A pictorial review. Eur Radiol. 2020;30:4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai H.X., Hsieh B., Xiong Z., et al. Performance of radiologists in differentiating COVID-19 from non-COVID-19 viral pneumonia at chest CT. Radiology. 2020;296:E46–E54. doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ooi G.C., Khong P.L., Muller N.L., et al. Severe acute respiratory syndrome: temporal lung changes at thin-section CT in 30 patients. Radiology. 2004;230:836–844. doi: 10.1148/radiol.2303030853. [DOI] [PubMed] [Google Scholar]
- 23.Wong K.T., Antonio G.E., Hui D.S., et al. Severe acute respiratory syndrome: radiographic appearances and pattern of progression in 138 patients. Radiology. 2003;228:401–406. doi: 10.1148/radiol.2282030593. [DOI] [PubMed] [Google Scholar]
- 24.Ajlan A.M., Ahyad R.A., Jamjoom L.G., Alharthy A., Madani T.A. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: chest CT findings. AJR Am J Roentgenol. 2014;203:782–787. doi: 10.2214/AJR.14.13021. [DOI] [PubMed] [Google Scholar]
- 25.Kligerman S.J., Franks T.J., Galvin J.R. From the radiologic pathology archives: Organization and fibrosis as a response to lung injury in diffuse alveolar damage, organizing pneumonia, and acute fibrinous and organizing pneumonia. Radiographics. 2013;33:1951–1975. doi: 10.1148/rg.337130057. [DOI] [PubMed] [Google Scholar]
- 26.Pan F., Ye T., Sun P., et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y., Dong C., Hu Y., et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: A longitudinal study. Radiology. 2020;296:E55–E64. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan Y., Guan H., Zhou S., et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020;30:3306–3309. doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J., Chen T., Yang H., et al. Clinical and radiological changes of hospitalised patients with COVID-19 pneumonia from disease onset to acute exacerbation: A multicentre paired cohort study. Eur Radiol. 2020 doi: 10.1007/s00330-020-06916-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou S., Zhu T., Wang Y., Xia L. Imaging features and evolution on CT in 100 COVID-19 pneumonia patients in Wuhan, China. Eur Radiol. 2020 doi: 10.1007/s00330-020-06879-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang T., Liu Z., Wu C.C., et al. Evolution of CT findings in patients with mild COVID-19 pneumonia. Eur Radiol. 2020;30:4865–4873. doi: 10.1007/s00330-020-06823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosseiny M., Kooraki S., Gholamrezanezhad A., Reddy S., Myers L. Radiology perspective of coronavirus disease 2019 (COVID-19): Lessons from severe acute respiratory syndrome and Middle East respiratory syndrome. AJR Am J Roentgenol. 2020;214:1078–1082. doi: 10.2214/AJR.20.22969. [DOI] [PubMed] [Google Scholar]
- 33.Ran Yang X.L., Liu Huan, Zhen Yanling, et al. Chest CT severity score: An imaging tool for assessing severe COVID-19. Radiol: Cardiothoracic Imag. 2020;2 doi: 10.1148/ryct.2020200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Z., Guo D., Li C., et al. Coronavirus disease 2019: initial chest CT findings. Eur Radiol. 2020;30:4398–4406. doi: 10.1007/s00330-020-06816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anthony Dangis C.G., De Bruecker Yves, Janssen Lode, et al. Accuracy and reproducibility of low-dose submillisievert chest CT for the diagnosis of COVID-19. Radiol: Cardiothoracic Imag. 2020;2 doi: 10.1148/ryct.2020200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu Huang R.H., Tao Ai, Yu Pengxin, Han Kang, Qian Tao, Xia Liming. Serial quantitative chest CT assessment of COVID-19: deep-learning approach. Radiol: Cardiothoracic Imag. 2020;2 doi: 10.1148/ryct.2020200075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minhua Yu D.X., Lan Lan, Tu Mengqi, et al. Thin-section chest CT imaging of coronavirus disease 2019 pneumonia: comparison between patients with mild and severe disease. Radiol: Cardiothoracic Imag. 2020;2 doi: 10.1148/ryct.2020200126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li K., Fang Y., Li W., et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30:4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang R., Ouyang H., Fu L., et al. CT features of SARS-CoV-2 pneumonia according to clinical presentation: a retrospective analysis of 120 consecutive patients from Wuhan city. Eur Radiol. 2020;30:4417–4426. doi: 10.1007/s00330-020-06854-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J.J., Dong X., Cao Y.Y., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 43.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y., Xia L. Coronavirus disease 2019 (COVID-19): Role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020;214:1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 45.Liu K.C., Xu P., Lv W.F., et al. CT manifestations of coronavirus disease-2019: A retrospective analysis of 73 cases by disease severity. Eur J Radiol. 2020;126:108941. doi: 10.1016/j.ejrad.2020.108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu X., Yu C., Qu J., et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imag. 2020;47:1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fang Y., Zhang H., Xie J., et al. Sensitivity of chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020;296:E115–E117. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu B., Xing Y., Peng J., et al. Chest CT for detecting COVID-19: A systematic review and meta-analysis of diagnostic accuracy. Eur Radiol. 2020 doi: 10.1007/s00330-020-06934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams H.J.A., Kwee T.C., Kwee R.M. COVID-19 and chest CT: Do not put the sensitivity value in the isolation room and look beyond the numbers. Radiology. 2020:201709. doi: 10.1148/radiol.2020201709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eng J., Bluemke D.A. Imaging publications in the COVID-19 pandemic: Applying new research results to clinical practice. Radiology. 2020:201724. doi: 10.1148/radiol.2020201724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim H., Hong H., Yoon S.H. Diagnostic performance of CT and reverse transcriptase polymerase chain reaction for coronavirus disease 2019: A meta-analysis. Radiology. 2020;296:E145–E155. doi: 10.1148/radiol.2020201343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goyal N., Chung M., Bernheim A., et al. Computed tomography features of coronavirus disease 2019 (COVID-19): A review for radiologists. J Thorac Imag. 2020;35:211–218. doi: 10.1097/RTI.0000000000000527. [DOI] [PubMed] [Google Scholar]
- 53.Yang Yang M.Y., Shen Chenguang, Wang Fuxiang, et al. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. medRxiv. 2020 02.11.20021493. 2020. [Google Scholar]
- 54.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for typical coronavirus disease 2019 (COVID-19) pneumonia: Relationship to negative RT-PCR testing. Radiology. 2020;296:E41–E45. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franquet T. Imaging of pulmonary viral pneumonia. Radiology. 2011;260:18–39. doi: 10.1148/radiol.11092149. [DOI] [PubMed] [Google Scholar]
- 56.Chen X., Tang Y., Mo Y., et al. A diagnostic model for coronavirus disease 2019 (COVID-19) based on radiological semantic and clinical features: A multi-center study. Eur Radiol. 2020;30:4893–4902. doi: 10.1007/s00330-020-06829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X., Fang X., Bian Y., Lu J. Comparison of chest CT findings between COVID-19 pneumonia and other types of viral pneumonia: A two-center retrospective study. Eur Radiol. 2020 doi: 10.1007/s00330-020-06925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu M., Zeng W., Wen Y., Zheng Y., Lv F., Xiao K. COVID-19 pneumonia: CT findings of 122 patients and differentiation from influenza pneumonia. Eur Radiol. 2020 doi: 10.1007/s00330-020-06928-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang H., Wei R., Rao G., Zhu J., Song B. Characteristic CT findings distinguishing 2019 novel coronavirus disease (COVID-19) from influenza pneumonia. Eur Radiol. 2020;30:4910–4917. doi: 10.1007/s00330-020-06880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bai H.X., Wang R., Xiong Z., et al. Artificial intelligence augmentation of radiologist performance in distinguishing COVID-19 from pneumonia of other origin at chest CT. Radiology. 2020;296:E156–E165. doi: 10.1148/radiol.2020201491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li L., Qin L., Xu Z., et al. Using artificial intelligence to detect COVID-19 and community-acquired pneumonia based on pulmonary CT: Evaluation of the diagnostic accuracy. Radiology. 2020;296:E65–E71. doi: 10.1148/radiol.2020200905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prokop M., van Everdingen W., van Rees Vellinga T., et al. CO-RADS: A categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation. Radiology. 2020;296:E97–E104. doi: 10.1148/radiol.2020201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19) imaging reporting and data system (COVID-RADS) and common lexicon: a proposal based on the imaging data of 37 studies. Eur Radiol. 2020;30:4930–4942. doi: 10.1007/s00330-020-06863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scott Simpson F.U.K., Abbara Suhny, Bhalla Sanjeev, et al. Radiological society of north America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the society of thoracic Radiology, the American College of Radiology, and RSNA. Radiol: Cardiothoracic Imag. 2020;2 doi: 10.1148/ryct.2020200152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seyed Mohammad Hossein Tabatabaei H.T., Moghaddas Fahimeh, Hamid Rajebi. Computed tomographic features and short-term prognosis of coronavirus disease 2019 (COVID-19) pneumonia: A single-center study from kashan. Radiology: Cardiothoracic Imag. 2020;2 doi: 10.1148/ryct.2020200130. Iran. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yuan M., Yin W., Tao Z., Tan W., Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PloS One. 2020;15 doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colombi D., Bodini F.C., Petrini M., et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. 2020;296:E86–E96. doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shinjini Kundu H.E., Gichoya Judy W., Kahn Charles E., Jr. vol. 2. Artificial Intelligence; 2020. (How Might AI and Chest Imaging Help Unravel COVID-19's Mysteries? Radiology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levi M., Keller T.T., van Gorp E., ten Cate H. Infection and inflammation and the coagulation system. Cardiovasc Res. 2003;60:26–39. doi: 10.1016/s0008-6363(02)00857-x. [DOI] [PubMed] [Google Scholar]
- 72.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:E438–E440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiong M., Liang X., Wei Y.D. Changes in blood coagulation in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Br J Haematol. 2020;189:1050–1052. doi: 10.1111/bjh.16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology. 2020;296:E186–E188. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klok F.A., Kruip M., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leonard-Lorant I., Delabranche X., Severac F., et al. Acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to d-dimer levels. Radiology. 2020;296:E189–E191. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poyiadji N., Cormier P., Patel P.Y., et al. Acute pulmonary embolism and COVID-19. Radiology. 2020:201955. doi: 10.1148/radiol.2020201955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poissy J., Goutay J., Caplan M., et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 80.Oudkerk M., Buller H.R., Kuijpers D., et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the national Institute for public Health of The Netherlands. Radiology. 2020:201629. doi: 10.1148/radiol.2020201629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Min Lang A.S., Carey Denston, Reid Nicholas, et al. Pulmonary vascular manifestations of COVID-19 pneumonia. Radiology: Cardiothoracic Imag. 2020;2 doi: 10.1148/ryct.2020200277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Budoff M.J., Mayrhofer T., Ferencik M., et al. Prognostic value of coronary artery calcium in the PROMISE study (prospective multicenter imaging study for evaluation of chest pain) Circulation. 2017;136:1993–2005. doi: 10.1161/CIRCULATIONAHA.117.030578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dillinger J.G.B.F., Pezel T., Voicu S., et al. JACC Cardiovasc Imaging. 2020. On behalf ofCOVID research group of lariboisiere hospital CAC and C in P with C-19. Coronary artery calcification and complications in patients with COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nai Fovino L., Cademartiri F., Tarantini G. Subclinical coronary artery disease in COVID-19 patients. Eur Heart J Cardiovasc Imag. 2020;21:1055–1056. doi: 10.1093/ehjci/jeaa202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie X., Zhao Y., de Bock G.H., et al. Validation and prognosis of coronary artery calcium scoring in nontriggered thoracic computed tomography: systematic review and meta-analysis. Circ Cardiovasc Imag. 2013;6:514–521. doi: 10.1161/CIRCIMAGING.113.000092. [DOI] [PubMed] [Google Scholar]
- 86.Cosyns B., Lochy S., Luchian M.L., et al. The role of cardiovascular imaging for myocardial injury in hospitalized COVID-19 patients. Eur Heart J Cardiovasc Imag. 2020;21:709–714. doi: 10.1093/ehjci/jeaa136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lala A., Johnson K.W., Januzzi J.L., et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Agricola E., Beneduce A., Esposito A., et al. Heart and lung multimodality imaging in COVID-19. JACC Cardiovasc Imaging. 2020;13:1792–1808. doi: 10.1016/j.jcmg.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Musher D.M., Abers M.S., Corrales-Medina V.F. Acute infection and myocardial infarction. N Engl J Med. 2019;380:171–176. doi: 10.1056/NEJMra1808137. [DOI] [PubMed] [Google Scholar]
- 92.Bangalore S., Sharma A., Slotwiner A., et al. ST-segment elevation in patients with covid-19 - a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feuchtner G.M., Barbieri F., Luger A., et al. Myocardial injury in COVID-19: the role of coronary computed tomography angiography (CTA) J Cardiovasc Comput Tomogr. 2020 doi: 10.1016/j.jcct.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stefanini G.G., Montorfano M., Trabattoni D., et al. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. 2020;141:2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tavazzi G., Pellegrini C., Maurelli M., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sala S., Peretto G., Gramegna M., et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.The European Society for Cardiology . 2020. ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. (Last update: 10 June 2020) [Google Scholar]
- 98.Choi A.D., Abbara S., Branch K.R., et al. Society of cardiovascular computed tomography guidance for use of cardiac computed tomography amidst the COVID-19 pandemic endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr. 2020;14:101–104. doi: 10.1016/j.jcct.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Skulstad H., Cosyns B., Popescu B.A., et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imag. 2020;21:592–598. doi: 10.1093/ehjci/jeaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Linde J.J., Kelbaek H., Hansen T.F., et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2020;75:453–463. doi: 10.1016/j.jacc.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 101.Smulders M.W., Kietselaer B., Wildberger J.E., et al. Initial imaging-guided strategy versus routine care in patients with non-ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2019;74:2466–2477. doi: 10.1016/j.jacc.2019.09.027. [DOI] [PubMed] [Google Scholar]
- 102.Peretto G., Sala S., Caforio A.L.P. Acute myocardial injury, MINOCA, or myocarditis? Improving characterization of coronavirus-associated myocardial involvement. Eur Heart J. 2020;41:2124–2125. doi: 10.1093/eurheartj/ehaa396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ferreira V.M., Schulz-Menger J., Holmvang G., et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: Expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 104.Demirkiran A., Everaars H., Amier R.P., et al. Cardiovascular magnetic resonance techniques for tissue characterization after acute myocardial injury. Eur Heart J Cardiovasc Imag. 2019;20:723–734. doi: 10.1093/ehjci/jez094. [DOI] [PubMed] [Google Scholar]
- 105.Esposito A., Palmisano A., Barbera M., et al. Cardiac computed tomography in troponin-positive chest pain: sometimes the answer lies in the late iodine enhancement or extracellular volume fraction map. JACC Cardiovasc Imag. 2019;12:745–748. doi: 10.1016/j.jcmg.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 106.Esposito A., Palmisano A., Natale L., et al. Cardiac magnetic resonance characterization of myocarditis-like acute cardiac syndrome in COVID-19. JACC Cardiovasc Imag. 2020 doi: 10.1016/j.jcmg.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang L., Zhao P., Tang D., et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imag. 2020 doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Axsom K., Lin F., Weinsaft J.W., Min J.K. Evaluation of myocarditis with delayed-enhancement computed tomography. J Cardiovasc Comput Tomogr. 2009;3:409–411. doi: 10.1016/j.jcct.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 110.Mewton N., Liu C.Y., Croisille P., Bluemke D., Lima J.A. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gerber B.L., Belge B., Legros G.J., et al. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast-enhanced magnetic resonance. Circulation. 2006;113:823–833. doi: 10.1161/CIRCULATIONAHA.104.529511. [DOI] [PubMed] [Google Scholar]
- 112.Pontone G., Baggiano A., Conte E., et al. Quadruple rule-out" with computed tomography in a COVID-19 patient with equivocal acute coronary syndrome presentation. JACC Cardiovasc Imag. 2020;13:1854–1856. doi: 10.1016/j.jcmg.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bouleti C., Baudry G., Iung B., et al. Usefulness of late iodine enhancement on spectral CT in acute myocarditis. JACC Cardiovasc Imag. 2017;10:826–827. doi: 10.1016/j.jcmg.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 114.Scully P.R., Bastarrika G., Moon J.C., Treibel T.A. Myocardial extracellular volume quantification by cardiovascular magnetic resonance and computed tomography. Curr Cardiol Rep. 2018;20:15. doi: 10.1007/s11886-018-0961-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Radunski U.K., Lund G.K., Stehning C., et al. CMR in patients with severe myocarditis: diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC Cardiovasc Imag. 2014;7:667–675. doi: 10.1016/j.jcmg.2014.02.005. [DOI] [PubMed] [Google Scholar]