ABSTRACT
Poorly controlled diabetes mellitus leads to several comorbidities, including susceptibility to infections. Hyperglycemia increases phagocyte responsiveness, however immune cells from people with diabetes show inadequate antimicrobial functions. We and others have shown that aberrant production of leukotriene B4 (LTB4) is detrimental to host defense in models of bacterial infection. Here, we will unveil the consequences of high glucose in the outcome of Leishmania braziliensis skin infection in people with diabetes and determine the role of LTB4 in human phagocytes. We show that diabetes leads to higher systemic levels of LTB4, IL-6 and TNF-α in cutaneous leishmaniasis. Only LTB4 correlated with blood glucose levels and healing time in diabetes comorbidity. Skin lesions of people with leishmaniasis and diabetes exhibit increased neutrophil and amastigote numbers. Monocyte-derived macrophages from these individuals showed higher L. braziliensis loads, reduced production of Reactive Oxygen Species and unbalanced LTB4/PGE2 ratio. Our data reveal a systemic inflammation driven by diabetes comorbidity in opposition to a local reduced capacity to resolve L. braziliensis infection and a worse disease outcome.
KEYWORDS: Diabetes, human leishmaniasis, Leishmania braziliensis, lipid mediators, LTB4, PGE2
Introduction
Diabetes mellitus is a metabolic disorder characterized by hyperglycemia, which occurs when insulin is not produced (Type 1) or inefficiently recognized (Type 2) [1,2]. Both types of diabetes are associated with secondary complications, such as cardiovascular diseases, retinopathy, nephropathy, neuropathy, reduced wound healing and increased susceptibility to infections, particularly in the skin [1–7]. The recent increase in the burden of diabetes [8,9] and reports of abnormal cases of cutaneous leishmaniasis (CL) in individuals with diabetes [10,11] led us to investigate the consequences of association of these diseases. The main goal of this study is to evaluate the influence of diabetes and its inflammatory mediators in the outcome of Leishmania braziliensis skin infection.
Hyperglycemia is thought to cause a state called sterile inflammation, characterized by a low-grade inflammatory response [12]. Although inflammation is crucial to clear pathogens and to induce tissue repair, chronic and sustained inflammatory responses cause tissue damage, leading to immunopathology and worse disease outcome. The tissue damage results from increased production of TNF-α, Interleukin-1β, metalloproteases and recently, we have shown that lipid mediators, such as leukotrienes (LTB4), when produced in abundance, increases susceptibility to skin infection in experimental models of diabetes [13–15]. These inflammatory mediators are also involved in CL caused by Leishmania braziliensis, the causative agent of CL in Brazil, resulting in skin ulcerative lesions [16]. Histological analysis of CL ulcers revealed rare presence of parasites and an intense inflammatory infiltrate, which can lead to a delayed healing process and chronic lesions [17]. In this context, we and others also have shown the participation of lipid inflammatory mediators, including LTB4 and Prostaglandin E2 (PGE2) in phagocyte antimicrobial effector functions upon infection of different pathogens, including Leishmania spp. [18–21].
Lipid mediators are produced from the metabolism of arachidonic acid (AA) present in the cell membrane in response to several stimuli, such as antigens, microbes, cytokines and osmotic challenge [22]. After AA metabolism, leukotrienes (LTs) and prostaglandins (PGs) are produced from lipoxygenase (LO) or cyclooxygenase (COX), respectively [22,23]. Recognition of LTB4 through its cognate receptor Type 1 (BLT1) enhances antimicrobial effector functions and production of cytokines in phagocytic cells [22,24]. Moreover, LTB4 acts as a phagocyte chemoattractant and a trigger of chronic inflammation [24,25]. However, when produced in high amounts, LTB4 can be detrimental to host defense [15]. We and others have shown that aberrant LTB4 production also exacerbate the inflammatory response, promote insulin resistance and delays skin wound healing [13,14,26].
On the other hand, PG elicits a wide range of biological effects associated with inflammation [23]. The synthesis of PGE2 is upregulated and it may signal through four different primary receptors. The concentrations of PGE2 and specific receptor signaling are crucial to induce proinflammatory or regulatory immune responses [23]. Several studies showed that PGE2 impairs the function of innate phagocytes, rendering the host more susceptible to bacterial, fungal, viral infections and protozoan parasites [20,27–29]. Regarding parasite infections, PGE2 has been implicated in the downregulation of ROS production from phagocytes, compromising a protective host response against Leishmania [20,30]. In an opposite way, LTB4 potentiates phagocytes leishmanicidal activity, through increased secretion of ROS [18,19]. As an intracellular parasite, the control of Leishmania infection relies on the balance between LTB4 and PGE2 production by phagocytes. Thus, unbalanced levels of inflammatory lipid mediators can be detrimental to host response, compromising the elimination of the parasite and influencing disease outcome.
Here, we hypothesize that the low-grade inflammation observed in patients with diabetes increases susceptibility to skin infections and compromises the response to treatment. We assessed CL subjects with diabetes and we found that LTB4 correlates with delayed healing time. In addition, macrophages from individuals with diabetes are more susceptible to L. braziliensis infection, due to an unbalanced LTB4/PGE2 production, resulting in inefficient ROS release. Together, our data show that diabetes induces a systemic inflammatory environment causing an inefficient local immune response against L. braziliensis, rendering subjects with diabetes more prone to this infection with a worse prognosis.
Materials and methods
Ethics statement
The Institutional Board for Ethics in Human Research at the Gonçalo Moniz Institute (Oswaldo Cruz Foundation-IGM-FIOCRUZ, Salvador, Bahia-Brazil), approved this study (protocol number: CAAE 95996618.8.0000.0040). Moreover, all data and samples had the consent of the participants.
Study area
The present cross-sectional cohort study was conducted in the Corte de Pedra district, an endemic area for cutaneous leishmaniasis caused by Leishmania braziliensis, located in the municipality of Tancredo Neves (state of Bahia) in northeastern Brazil. The recruitment was conducted between the years 2015–2018, with biweekly visits of our team of physicians to the endemic area.
Sample collection
Prior to therapy, blood and skin biopsy samples were obtained from CL subjects with diabetes (CL + DM) or not (CL). For cell culturing and inflammatory mediator quantification, blood samples (20 mL) were drawn by venipuncture using tubes with Heparin, centrifuged for obtaining of the plasma and Peripheral Blood Mononuclear Cells (PBMCs) using HISTOPAQUE® 1077 (Sigma Aldrich, St Louis, MO). For histopathological analysis, skin biopsies were obtained using a 4 mm punch at borders of topical lesions.
Study population and disease diagnosis
The primary outcome variable was blood glucose levels in individuals with CL. A convenience sample of eight subjects with confirmed CL and 12 individuals with diabetes and CL were enrolled from the Municipal Health Clinic located in Corte de Pedra (CSCP). The statistical power was estimated using Epi infoTM software. This approach was carried out considering a 95% confidence interval (two-sided). The power estimated for each parameter measured in this study was above 80%.
The groups were paired through age and sex. CL diagnosis was performed from clinical analyzes, PCR for parasite DNA detection in lesion biopsies and Montenegro skin test. Fasting glucose levels were used to determine diabetes status: glucose levels ≥126 mg/dL were considered with diabetes (CL + DM), while values under this cutoff were considered without diabetes (CL). Exclusion criteria: pregnant women, children under 15 years old and PCR negative for parasite detection. All samples were collected after the diagnosis of leishmaniasis and before starting the treatment. As recommended by the guidelines of the Brazilian Ministry of Health, the treatment of CL was performed with Glucantime cycles and patients with diabetes were also treated with Metformin. Clinical and epidemiological information was also obtained from all included individuals, e.g. gender, age, lesion size, number and time to healing (see electronic supplementary Table S1).
Detection of inflammatory mediators
Cytokines TNF-α (Invitrogen, San Diego, CA), IL-1β, IL-6 and IL-10 (all from eBioscience, San Diego, CA) were quantified using sandwich enzyme-linked immunosorbent assays in accordance with the manufacturer’s instructions. To measure LTB4 and PGE2 levels, competitive assays were used (Leukotriene B4 ELISA Kit and Prostaglandin E2, both from Cayman Chemical, Ann Arbor, MI), following the manufacturer’s instructions.
Gene expression in the lesion
The relative expression of Arachidonate 5-Lipoxygenase (ALOX5) and Prostaglandin-Endoperoxide Synthase 2 (PTGS2) was assessed in biopsies of seven patients with CL and four patients with CL + DM available in RNAlater (Thermo Fisher Scientific, IL, USA), for this approach. The tissues were lysed using TRIzol reagent (Invitrogen, San Diego, USA). Subsequently, phenol–chloroform extraction and quantification of total RNA were performed. cDNA was synthesized by mRNA reverse transcription (RT) using a SuperScript® III Reverse Transcriptase kit (Invitrogen, San Diego, CA, USA). Then, cDNA was amplified by quantitative real time PCR (RT-qPCR) using SYBR Green PCR Master Mix (Thermo Fisher Scientific, IL, USA). The fold change of expression between CL and CL + DM patients was calculated using the 2-ΔΔCT method after normalization with the housekeeping gene β-actin. Specific primers for ALOX5 (Hs.PT.56a.28007202.g), PTGS2 (Hs.PT.58.77266) and ACTB (Hs.PT.39a.22214847) were purchased from IDT (Illinois, USA).
Histopathological analysis
Skin biopsies were stained with Hematoxylin and Eosin (H&E) to analyze cell infiltrate and the presence of parasites. Images from five randomly selected fields were captured and scanned using an Olympus Scanner under 40x magnification. Within the software, 50% digital magnification was made to better visualize. The quantification of neutrophils and amastigotes was performed manually using blinded counts, with aid of ImageJ 1.52a software (National Institutes of Health, USA).
Culturing of human macrophages
PBMCs from CL and CL + DM were plated (2 × 106) on 24-well tissue culture plates (TPP, Trasadingen, Switzerlan) using 13 mm round glass coverslips (Perfecta). Plates were incubated at 37°C under 5% CO2 for 30 min. Non-adherent cells were removed by washing and adherent cells were cultured in RPMI 1640 medium (LGC Biotecnologia, São Paulo, Brazil) with 10% autologous serum, 2 mM L-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin and 50 ng/mL M-CSF for 7 days. Glucose was added to cell cultures at the identical concentrations measured in the blood of each patient.
Leishmania braziliensis and macrophages infection
L. braziliensis (MHOM/BR/01/BA788) promastigotes were cultured at 24°C in Schneider’s Insect medium (Sigma Aldrich, St Louis, MO) supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin and 100 mg/mL streptomycin. Cultured macrophages were infected for 4 h with stationary-phase L. braziliensis at a parasite/cell ratio of 10:1. The cells were fixed, stained using the Quick Panoptic (LB Laborclin, Paraná, Brazil) and analyzed by light microscopy under an objective (Nikon) at 100x to determine infection rate and the presence of amastigotes by blinded counts.
Reactive oxygen species production
ROS production was measured following the infection of monocyte-derived macrophages. Cells were incubated for 30 min with 5 μM of CellROX™ Green Reagent (Thermo fisher Scientific, IL, USA) at 37°C under 5% CO2. Next, cells were washed three times with 1x PBS (Gibco), fixed and stained with DAPI and ProLong Gold antifade reagent (Thermo fisher Scientific, IL, USA). Images were captured under a fluorescence microscope (Leica DMi8) with 485/520 excitation. Corrected Total Cell Fluorescence (CTCF) was calculated based on an average of 24 cells (eight randomly selected cells per field) for each individual using ImageJ 1.52a software (National Institutes of Health, USA).
Statistical analysis
The Mann–Whitney test was used to compare groups with unpaired samples, such as systemic inflammatory mediators (LTB4, PGE2, TNF-α, IL-6, IL-1β and IL-10), histological analysis, gene expression and infection rate. Kruskal–Wallis with Dunn’s post-test was used to compare more than two groups (non-infected, infected, CL and CL + DM). For comparisons involving paired samples, such as ROS production, LTB4 and PGE2 from culture supernatant, Wilcoxon’s test was used. Trend analysis was performed using the Chi-squared test for amastigotes number in the lesion. Correlation analysis was employed considering clinical parameters, such as blood glucose, lesion size, healing time and inflammatory mediator levels, obtained from Spearman’s rank test. One individual with missing data (healing time) is shown in electronic supplementary Table S1. All statistical analyses were performed using GraphPad Prism 7.0 (GraphPad, San Diego, CA). Differences were considered statistically significant when p < 0.05.
Results
CL + DM individuals present increased systemic levels of inflammatory mediators
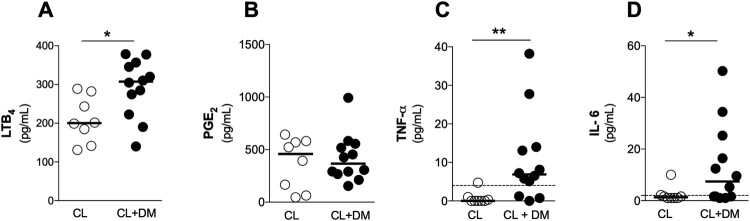
People with diabetes exhibit increased systemic inflammatory cytokines that might be involved in different comorbidities [2,12]. Initially, we obtained plasma samples from individuals with CL and CL + DM to measure systemic levels of inflammatory mediators. CL + DM individuals presented elevated plasma levels of LTB4 (Figure 1(A)), TNF-a (Figure 1(C)) and IL-6 (Figure 1(D)) when compared to CL. However, no differences were found in PGE2 (Figure 1(B)), IL-1β and IL-10 (levels below the detection limit, data not shown) between CL + DM and CL individuals. These findings suggest that diabetes induces an exacerbated and systemic inflammatory profile during CL.
Figure 1.
DM increases systemic levels of inflammatory mediators in CL individuals. (A) LTB4, (B) PGE2, (C) TNF-a and (D) IL-6 levels in plasma of individuals with Cutaneous Leishmaniasis, without diabetes (CL) or with diabetes (CL + DM) measured by ELISA. CL, n = 8; CL + DM, n = 12. Data shown in median. Mann-Whitney test. *p < 0.05; **p < 0.01.
Blood glucose positively correlates with inflammatory mediators, worsening the healing time in CL + DM
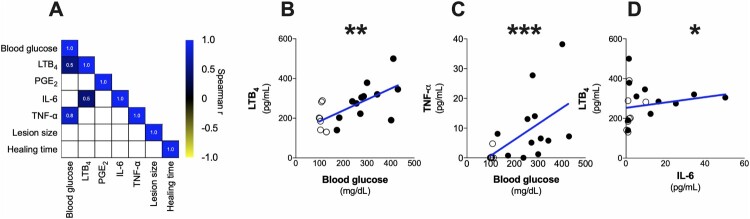
Next, we determined whether the abundance of inflammatory mediators correlate with clinical aspects, such as lesion size, wound healing time as well as blood glucose levels in all patients grouped together (both CL and CL + DM). Despite clinical parameters are statistically similar between CL and CL + DM groups (except plasma glucose levels – see electronic supplementary Table S1), the role of inflammatory mediators could be specific for each condition (with or without diabetes). The matrix summarizes the statistically significant correlations found between these parameters (Figure 2(A)). As shown in Figure 2, the graphs detail all significant correlation found between the parameters, such as blood glucose levels and LTB4 (r = 0.5) (Figure 2(B)), as well as TNF-α (r = 0.8) (Figure 2(C)) regardless of diabetes status in subjects with CL. Furthermore, our results showed that LTB4 production was positively correlated with IL-6 (r = 0.5) (Figure 2(D)). Therefore, these results indicate that glycemic levels and serum LTB4 could contribute to systemic inflammation.
Figure 2.
Blood glucose positively correlates with inflammatory mediators in CL individuals regardless of diabetes. (A) Correlation matrix of the data obtained from CL (open circles) and CL + DM (full circles) individuals grouped together. Detailed correlation between blood glucose and LTB4 (B), TNF-a (C) and between LTB4 and IL-6 (D). Spearman correlation. *p < 0.05; **p < 0.01; ***p < 0.001.
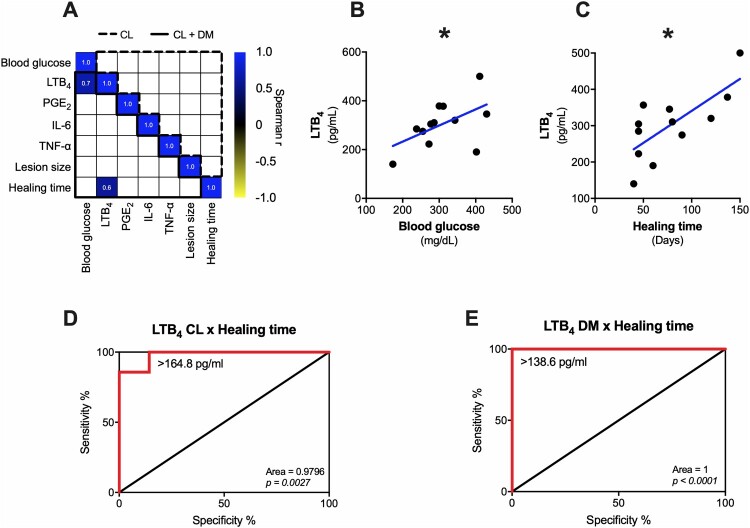
To understand the influence of diabetes on CL, we sought for correlations in CL and CL + DM groups separately (Figure. 3(A)). As shown in Figure 3(A), no correlation was observed in CL subjects (dashed line). However, only CL + DM individuals (full line) show a positive correlation of LTB4 production with blood glucose (r = 0.7) (Figure 3(B)) and healing time (r = 0.6) (Figure 3(C)). We further confirmed that these correlations are not present in CL individuals (see electronic supplementary Figure S1). These results suggest that hyperglycemia-induced LTB4 could drive the delayed healing time in CL + DM individuals, but other mediators might also play a role.
Figure 3.
Only LTB4 positively correlates with healing time and blood glucose in CL + DM individuals. (A) Correlation matrix of clinical data and inflammatory mediators in CL (dashed line) and CL + DM (full line) individuals separately. Detailed correlation between LTB4 and blood glucose (B) and healing time (C) in CL + DM patients. Receiver operating characteristic (ROC) curve of LTB4 participation in healing time of (D) CL and (E) CL + DM individuals. Spearman correlation (A-C). *p < 0.05.
We further performed a Receiver Operating Characteristic curve (ROC) analysis to evaluate the role of systemic LTB4 levels in the lesion outcome (healing time) between CL (Figure 3(D)) and CL + DM (Figure 3(E)) individuals. The results showed that LTB4 systemic levels above 164.8 pg/mL (specificity 100% and sensitivity 85.71%) influence the healing time of CL subjects (Figure 3(D)). On the other hand, for CL + DM individuals, these levels were above 138.6 pg/mL (specificity and sensitivity 100%) (Figure 3(E)).
These findings suggest that the threshold of systemic LTB4 levels in CL + DM individuals is lower than CL subjects to delay the healing time. We can hypothesize that individuals with diabetes are more responsive to LTB4 effects, but further studies with prospective cohorts are necessary to confirm these results.
Lesions from CL + DM individuals present a neutrophilic infiltrate and reduced 5-LO expression
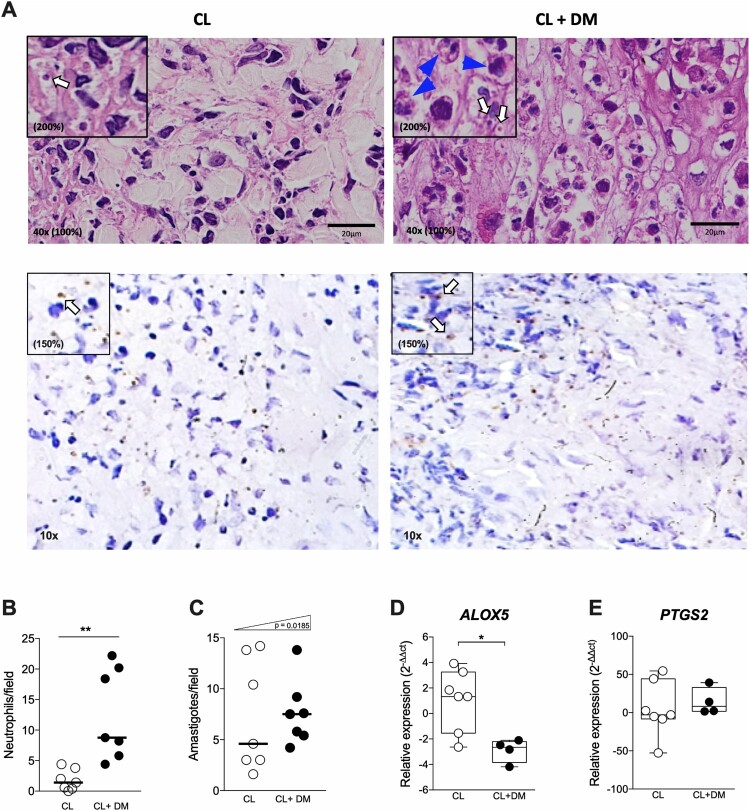
Next, we sought to locally identify the inflammatory profile in CL and CL + DM lesions, since the production of lipid mediators and the cellular infiltrate could define susceptibility to Leishmania spp. [20,31]. Representative images of CL lesions (Figure 4(A)) show the cellular infiltrate of CL subjects (left) and CL + DM individuals (right). We observed a significant increase of neutrophil infiltrate in CL + DM compared with CL lesions (Figure 4(B)). Furthermore, we found a trend toward higher numbers of amastigotes per field in CL + DM lesions (Figure 4(C)). The gene expression of enzymes that produce inflammatory lipid mediators, such as LTB4 (ALOX5 encodes 5-LO enzyme) and PGE2 (PTGS2 encodes COX-2 enzyme), was assessed in these lesions. Surprisingly, the results revealed a reduction in the expression of ALOX5 in CL + DM compared to CL (Figure 4(D)). On the other hand, PTGS2 gene expression was not altered in patients with diabetes (Figure 4(E)). Together, these findings suggest that diabetes locally alters the inflammatory infiltrate with a reduction of 5-LO expression in the lesion and increased susceptibility to CL.
Figure 4.
DM individuals are more prone to CL infection. (A) Histological images of a representative CL (left) and CL + DM (right) lesions in HE (above) and IHQ (below). Quantification of neutrophils (B) and amastigotes for field (C) in the lesions of CL and CL + DM individuals. Expression of ALOX5 (D) and PTGS2 (E) in the lesion of CL and CL + DM individuals detected by RT-qPCR. Blue arrows: neutrophils; white arrows: amastigotes. Scale bar: 20 µm. 40x objective with 200% digital magnification in HE and 150% in IHQ. Data shown in median (B,C). Box and whiskers with min to max (D,E). Mann-Whitney test (B,D). Chi-squared test for trend (C). *p < 0.05; **p < 0.01.
Macrophages from CL + DM individuals are more susceptible to L. braziliensis infection
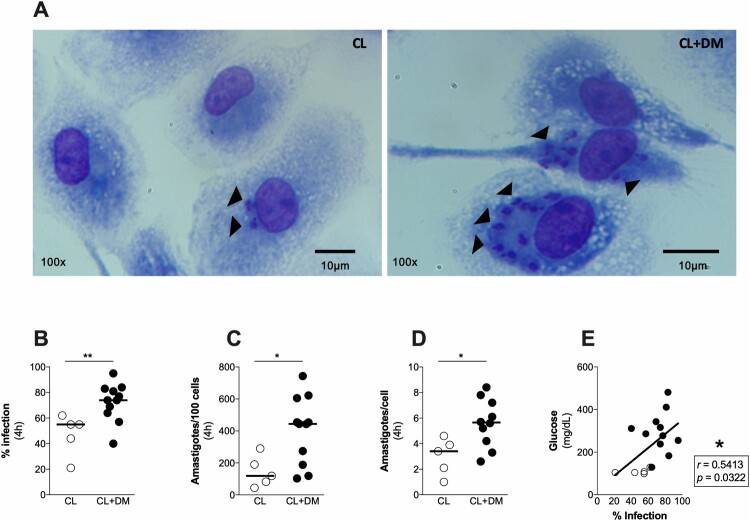
Since we found an increased susceptibility to L. braziliensis in the lesions of CL + DM, we assessed the response of macrophages from these individuals in vitro (Figure 5(A)). It is well-established that Leishmania parasites reside in macrophages, which control the infection through the production of reactive oxygen species (ROS), cytokines and chemokines [18]. We found an increased infection rate (Figure 5(B)), total number of intracellular amastigotes (Figure 5(C)) and amastigotes per macrophage (Figure 5(D)) in CL + DM compared to CL macrophages. Furthermore, a positive correlation was observed between blood glucose levels and infection rate of macrophages (Figure 5(E)), suggesting that hyperglycemia leads to susceptibility to L. braziliensis in macrophages.
Figure 5.
Monocyte-derived macrophages from individuals with CL + DM are more susceptible to Leishmania braziliensis. Representative images of cultured macrophages from CL and CL + DM individuals after L. braziliensis infection (A). Quantification of infection rate (B), amastigotes in 100 cells (C) and amastigotes per cell (D) in macrophages from CL and CL + DM individuals, after infection with L. braziliensis. (E) Spearman correlation between blood glucose levels and infection rate in the CL (open circles) and CL + DM (full circles). Arrows indicate the presence of amastigotes. Data shown in median. Mann-Whitney test (B-D). Spearman correlation (E). *p < 0.05; **p < 0.01.
ROS production is unaltered by L. braziliensis infection in CL + DM macrophages
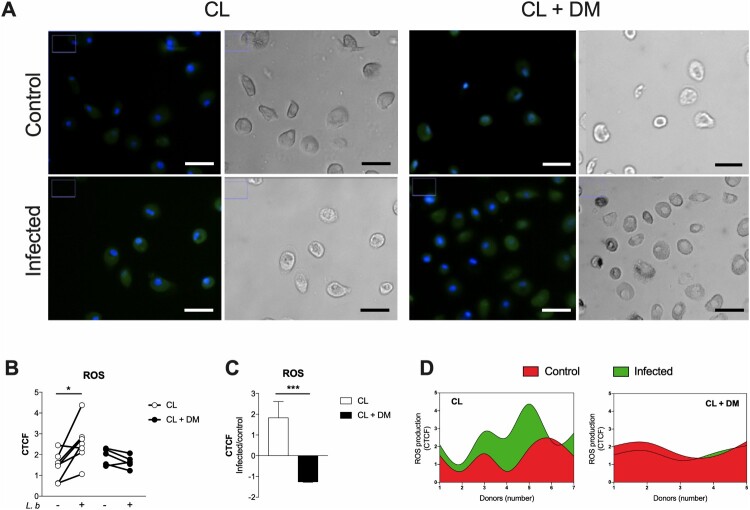
To investigate the mechanisms underlying the increased susceptibility of CL + DM macrophages to L. braziliensis, we measured the production of ROS. Representative images of cultured macrophages before and after L. braziliensis infection show ROS labelling in green (Figure 6(A)) comparing CL (left) and CL + DM (right). We found that ROS production of macrophages from CL + DM individuals (dark circles) is unaltered after L. braziliensis infection (Figure 6(B)). On the other hand, macrophages from CL (clear circles) subjects efficiently respond to the infection with increased ROS production. Moreover, the ratio of ROS production between infected and control macrophages revealed higher ROS production in CL subjects (Figure 6(C)). The production of ROS is also showed individually through Cubic spline analysis (Figure 6(D)), confirming that CL + DM macrophages do not alter its production after L. braziliensis infection. Taken together, these findings suggest that, under diabetes, macrophages exhibit a reduced capacity to produce ROS in response to L. braziliensis infection.
Figure 6.
Unaltered production of ROS after L. braziliensis infection of monocyte-derived macrophages from CL + DM individuals. Representative images of ROS production in monocyte-derived macrophages from CL and CL + DM individuals, before (control) and after infection by L. braziliensis (infected) (A). Quantification of ROS production in monocyte-derived macrophages from CL (open circles) and CL + DM (full circles) individuals after L. braziliensis infection (B). Ratio of ROS production between infected and control samples of CL and CL + DM individuals (C). Cubic spline analysis of donor-specific ROS production in monocyte-derived macrophages before (red) and after (green) L. braziliensis infection from CL and CL + DM individuals. CTCF: Corrected Total Cell Fluorescence. Green: ROS production; blue: cell nucleus. 40x magnification. Scale bar = 10 µm. Data represent individual values before and after infection (B). Data shown in median with interquartile range (C). Wilcoxon’s test (B). Mann-Whitney test (C). *p < 0.05; ***p < 0.001.
CL + DM macrophages produce more PGE2 than LTB4 in response to L. braziliensis infection
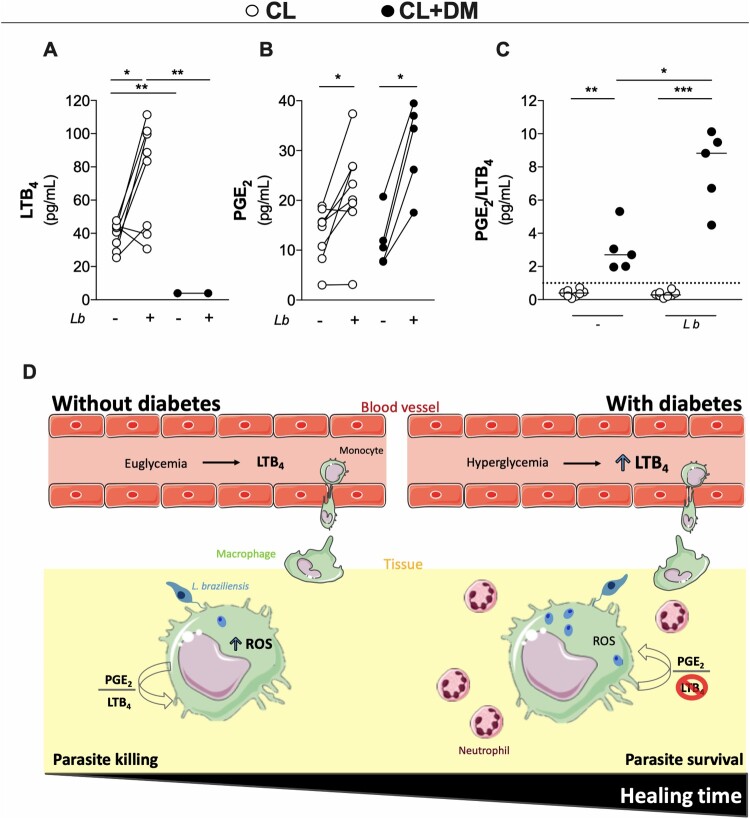
To investigate whether ROS release could be a consequence of lipid mediators production, we measured LTB4 and PGE2 in the culture of macrophages from CL and CL + DM individuals. It is well established that the balance between LTB4 and PGE2 can influence the outcome of Leishmania spp. infection [32]. Our findings revealed negligible levels of LTB4 in CL + DM macrophages in response to L. braziliensis infection, while levels of LTB4 in CL macrophages increased after infection (Figure 7(A)). By contrast, both CL + DM and CL macrophages produced increased levels of PGE2 after infection (Figure 7(B)). To define the predominance of these mediators in the microenvironment, the ratio between PGE2 and LTB4 was calculated in both conditions, before and after infection. Interestingly, PGE2 was the more predominant lipid mediator in CL + DM macrophages, regardless of infection status (Figure 7(C)). Together, these findings show that diabetes imbalances PGE2/LTB4 ratio locally produced from macrophages after L. braziliensis infection probably through a negative feedback from increased systemic inflammation.
Figure 7.
LTB4 production of monocyte-derived macrophages from CL + DM individuals is abolished after L. braziliensis infection. Production of LTB4 (A) and PGE2 (B) in the supernatant of cultured macrophages from CL and CL + DM individuals after infection with L. braziliensis. Ratio between PGE2 and LTB4 production in the supernatant of uninfected or infected macrophages (C). Graphical abstract (D). Data represent individual values before and after infection. Wilcoxon’s test and Kruskal-Wallis with Dunn’s post-test. *p < 0.05; **p < 0.01.
In sum, this study assessed systemically and locally the effects of diabetes in CL outcome. We found basal levels of LTB4 in the bloodstream of CL subjects and increased LTB4 over PGE2 production from macrophages after L. braziliensis infection, resulting in ROS release of and parasite killing. On the other hand, CL + DM individuals show increased levels of LTB4 in the bloodstream. Furthermore, diabetes abolished LTB4 production from their macrophages driving PGE2/LTB4 ratio toward PGE2 predominance. This imbalance prevents the production of ROS, favouring parasite survival, increasing the number of neutrophils in the lesion and delays CL healing time (Figure 7(D)).
Discussion
In the current study, we show that diabetes induces a systemic inflammatory profile in individuals with CL. We found that glycemic status and LTB4 levels directly affect the healing time of CL lesions. More importantly, macrophages obtained from CL + DM individuals are more susceptible to L. braziliensis infection.
Herein, we assessed circulating levels of inflammatory mediators in the blood of CL and CL + DM individuals. We found an increase in TNF-α, IL-6 and LTB4 systemic levels of CL + DM. It has been shown that LTB4 is produced immediately in response to infections, and is one of those responsible for the production of proinflammatory cytokines in experimental model of diabetes [33–35]. Given that inflammatory lipid mediators, such as LTB4 and PGE2, have been scarcely studied in human with diabetes, these findings represent an initial result of diabetes-induced LTB4 production in response to a protozoan parasite in humans. Interestingly, although our data show an increased production of different inflammatory mediators in CL + DM individuals, only LTB4 positively correlates with blood glucose levels and healing time of CL lesion. Similar results were described in an experimental model of diabetes, where LTB4 was found to promote insulin resistance [33]. Accordingly, mice deficient for 5-lipoxygenase (5-LO), presented faster healing time of a sterile skin wound compared to wild type mice, indicating that LTB4 may be an important target to improve cutaneous healing [36]. Moreover, a previous study demonstrated that exaggerated LTB4 signaling correlated with larger nonhealing lesion areas and increased bacterial loads in diabetic mice infected with methicillin-resistant Staphylococcus aureus (MRSA) in the skin [15]. We therefore expanded these findings to humans, confirming that diabetes induces a systemic inflammatory profile and delayed healing of a skin lesion caused by an infectious agent.
The healing of an injured tissue is a complex process, involving several inflammatory mediators and immune cells, such as neutrophils, the first leukocytes recruited, followed by monocytes and lymphocytes [15,36–38]. Enhanced neutrophil chemotaxis is dependent on complement fragments, chemokines and exogenous mediators produced by pathogens, as well as the presence of LTB4. More specifically, LTB4 signaling can increase actin polymerization and myosin phosphorylation, which are crucial to neutrophil migration [25,38]. Our findings indicate that diabetes induces an increased neutrophil infiltrate to the skin lesion caused by L. braziliensis. Similarly, high levels of LTB4 were associated with a predominant neutrophil infiltrate toward a sterile injury in the skin of diabetic mice [34]. Regarding skin lesions caused by an infectious agent, it was observed an inefficient abscess formation against S. aureus in an experimental model of diabetes, where LTB4 was associated with altered neutrophil migration [15]. Taken together, these findings indicate that LTB4 orchestrates an exaggerated systemic inflammatory response with neutrophil predominance in the infiltrate, which could be related with the delayed healing process observed in skin lesions of individuals with diabetes.
Diabetes is also known to promote susceptibility to infections by extracellular bacteria, fungus and mycobacteria [39–41]. However, the susceptibility to a parasitic infection remains poorly understood. Indeed, we found a trend towards higher numbers of amastigotes in the lesions of CL + DM individuals. With an increment in the number of lesion samples, we should detect statistically differences between the groups. In addition to the small number of patients included, our study is also limited by the lack of a molecular tool to quantify parasite burden in lesions in order to confirm increased susceptibility in CL + DM individuals. However, direct blinded counts of amastigotes in lesions stained with HE represents a reliable alternative approach. Further investigation will be necessary with increased sample sizes to quantify parasite load by molecular methods to confirm whether diabetes favours L. braziliensis survival.
In accordance with these findings in the lesion, locally expression of ALOX gene, which encodes 5-LO enzyme [22], is reduced in CL + DM individuals, compared to CL samples, indicating a local down-modulation of LTB4 mediated response. It is important to consider that diabetes involves chronic low-grade inflammation, triggered by endogenous molecules [42]. The accumulation of metabolic products, such as glucose, can induce “sterile inflammation” through the engagement of TLRs [43] or inflammasome activation [44]. Despite the inflamed environment, this immunological dysfunction renders individuals with diabetes more prone toward infectious diseases [40]. Since the main pathogenic mechanism of increased susceptibility in diabetes is a hyperglycemic/inflamed systemic environment that reduces the production of mediators locally in response to infection, this implies the reduction of chemotaxis, phagocytic activity and immobilization of polymorphonuclear leukocytes [40].
We speculate that LTB4 may be provoking a counterintuitive effect in the context of diabetes, i.e. the excessive production of this mediator is associated with a deficient phagocyte response to pathogens [13,15]. While the mechanisms involved in this association remain unclear, it is theoretically possible that chronic exposition to hyperglycemia could lead to alterations in epigenetic marks or to trained immunity, causing macrophages to respond to subsequent restimulation in a sensitized and non-specific manner [45].
During inflammation, the production of leukotrienes is increased, as already reported for diabetic mice, that exhibited high serum levels of LTB4 [46]. However, the source of this increased systemic levels of LTB4 is a matter of debate. The production of leukotrienes is largely confined to leukocytes, mainly neutrophils and monocytes/macrophages [22], but other cell types can take up products from arachidonic acid and metabolize it into bioactive leukotrienes through transcellular biosynthesis, such as endothelial cells and keratinocytes [26]. Considering that diabetes and CL are chronic diseases, these individuals probably produce LTB4 in a constitutive manner [47]. Although we and others have studied LTB4 production by macrophages [13], other cell types could be involved, such as neutrophils. The CL lesion is initially composed of neutrophils that are later replaced by an intense macrophage infiltrate. Nevertheless, the source of LTB4 in diabetes and CL remains to be determined.
The main mechanism described to resolve Leishmania infection is through the production of ROS by different cell types, especially macrophages [18,20,32]. Surprisingly, we did not find increased ROS production by macrophages from CL + DM individuals after in vitro infection, despite their systemic inflammatory status. On the other hand, macrophages from CL subjects showed higher levels of ROS in response to the infection, resulting in parasite killing. Similarly, CL lesions have increased ROS production compared to healthy control skin [48]. Our results indicate that ROS production by macrophages is compromised during diabetes, rendering these individuals more susceptible to a parasite infection. However, phagocytes from diabetic conditions were shown to be able produce increased levels of ROS compared to non-diabetics [13], it was also reported that cells from diabetic patients with tuberculosis produce reduced levels of ROS [49]. Similarly, we showed that, after L. braziliensis infection, macrophages from CL + DM patients did not exhibit increased ROS production compared to cells from CL patients, yet basal levels (before infection) were similar. Our findings suggest that locally absent LTB4 production by macrophages is reflective of reduced ROS production by these cells, since the LTB4/PGE2 balance is known to modulate ROS production [18–20].
Several studies have also demonstrated that ROS-dependent elimination of Leishmania spp. can be mediated by the lipid mediators LTB4 and PGE2 [18–20]. Accordingly, we found that macrophages from CL + DM individuals produce negligible levels of LTB4 after L. braziliensis infection compared to CL macrophages. Moreover, PGE2/LTB4 ratio revealed the predominance of PGE2 in CL + DM macrophages both before and after the infection. A similar study described that the balance between these inflammatory mediators dictates the state of susceptibility or resistance to different species of Leishmania [32]. In addition, it has already been demonstrated that increased levels of PGE2 produced by macrophages in comparison to LTB4 impairs ROS production and L. infantum killing [20]. This has also been observed in autoimmune disease, such as rheumatoid arthritis [50] and atherosclerosis [51], even in the absence of an infective pathogen. In the context of diabetes, we suggest that systemic chronic low-grade inflammation creates a localized imbalance in eicosanoid production geared towards a predominance of PGE2, which leads to unaltered ROS production and the survival of L. braziliensis parasites. Taken together, these results suggest that PGE2/LTB4 balance dictates the production of ROS and, consequently, the control of an infection.
In summary, the current study revealed that diabetes induces a systemic pro-inflammatory profile in individuals with CL, orchestrated by LTB4, which correlates with impaired healing process and ability to eliminate parasites. These findings suggest that LTB4 play a central role in the innate immune response against pathogens during diabetes. In particular, due to its capacity to induce ROS production, LTB4 has significant influence in the disease outcome, defining a host as resistant or susceptible to Leishmania. Moreover, this point out LTB4 as a potential target for future interventions designed to minimize the impact of leishmaniasis in the context of human with diabetes.
Acknowledgments
The authors thank all individuals who participated this study and all the clinical group that recruited eligible participants. We also thank C. P. Figueira, A. Lanfredi and M. F. Santos from the Microscopy facility – RPT (FIOCRUZ). A. K. Miranda and I. N. Coelho for secretariat assistance.
Funding Statement
This work was supported by [Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) #1] under Grant [number PET0009/2016]; and [Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES) #2] under Finance [Code 001].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Katsarou A, Gudbjörnsdottir S, Rawshani A, et al. . Type 1 diabetes mellitus. Nat Rev Dis Prim. 2017;doi: 10.1038/nrdp.2017.16. [DOI] [PubMed] [Google Scholar]
- 2.Donath MY, Shoelson SE.. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925 [DOI] [PubMed] [Google Scholar]
- 3.Bowling FL, Rashid ST, Boulton AJM.. Preventing and treating foot complications associated with diabetes mellitus. Nat Rev Endocrinol. 2015;11:606–616. doi: 10.1038/nrendo.2015.130 [DOI] [PubMed] [Google Scholar]
- 4.Ezzati M. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010 : a comparative risk assessment. LANCET Diabetes Endocrinol. 2014;2:634–647. doi: 10.1016/S2213-8587(14)70165-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golden SH, Anderson C, Bray GA, et al. . Update on Prevention of cardiovascular disease in Adults With Type 2 diabetes mellitus in light of recent Evidence : A Scienti fi c Statement from the American Heart association and the American diabetes association. Diabetes Care. 2015;38:1777–1803. doi: 10.2337/dci15-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent AM, Callaghan BC, Smith AL, et al. . Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7:573–583. doi: 10.1038/nrneurol.2011.137 [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Quan Y, Liu Y, et al. . Hyperglycaemia inhibits REG3A expression to exacerbate TLR3-mediated skin inflammation in diabetes. Nat Commun. 2016;7:13393. doi: 10.1038/ncomms13393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Federation ID. IDF Diabetes Atlas - 2019. 2019 doi: 10.1289/image.ehp.v119.i03. [DOI]
- 9.World Health Organization . Estimates for the year 2000 and projections for 2030. World Health. 2004;27:1047–1053. [Google Scholar]
- 10.Chiheb S, Oudrhiri L, Zouhair K, et al. . Unusual clinical presentation of cutaneous leishmaniasis in three diabetic patients | Leishmanioses cutanées d’aspect clinique inhabituel chez trois patients diabétiques. Ann Dermatol Venereol. 2012;139:542–545. doi: 10.1016/j.annder.2012.05.013 [DOI] [PubMed] [Google Scholar]
- 11.Join-Lambert O, Fraitag S, Ribadeau-Dumas F, et al. . Is granulomatous mastitis a localized form of hidradenitis suppurativa? Eur J Dermatology. 2009;19:513–514. doi: 10.1684/ejd.2009.0731 [DOI] [PubMed] [Google Scholar]
- 12.Kono H, Onda A, Yanagida T.. Molecular determinants of sterile inflammation. Curr Opin Immunol. 2014;26:147–156. doi: 10.1016/j.coi.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 13.Filgueiras LR, Brandt SL, Wang S, et al. . Leukotriene B 4 -mediated sterile inflammation favors susceptibility to sepsis in murine type 1 diabetes HHS Public Access. Sci Signal. 2015;8, doi: 10.1126/scisignal.2005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramalho T, Filgueiras L, Silva-Jr IA, et al. . Impaired wound healing in type 1 diabetes is dependent on 5-lipoxygenase products. Sci Rep. 2018;8:14164. doi: 10.1038/s41598-018-32589-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandt SL, Wang S, Dejani NN, et al. . Excessive localized leukotriene B4 levels dictate poor skin host defense in diabetic mice. J Clin Invest. 2018;3(17):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maspi N, Abdoli A, Ghaffarifar F.. Pro- and anti-inflammatory cytokines in cutaneous leishmaniasis: a review. Pathog Glob Health. 2016;110:247–260. doi: 10.1080/20477724.2016.1232042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saldanha MG, Queiroz A, Machado PRL, et al. . Characterization of the histopathologic features in patients in the early and late phases of cutaneous leishmaniasis. Am J Trop Med Hyg. 2017;96:645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morato CI, da Silva IA, Borges AF, et al. . Essential role of leukotriene B4 on Leishmania (Viannia) braziliensis killing by human macrophages. Microbes Infect. 2014; doi: 10.1016/j.micinf.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Tavares N, Afonso L, Suarez M, et al. . Degranulating neutrophils promote leukotriene B 4 production by infected macrophages To Kill Leishmania amazonensis parasites. J Immunol. 2016;196:1865–1873. doi: 10.4049/jimmunol.1502224 [DOI] [PubMed] [Google Scholar]
- 20.Araújo-Santos T, Prates DB, França-Costa J, et al. . Prostaglandin E 2/leukotriene B 4 balance induced by Lutzomyia longipalpis saliva favors Leishmania infantum infection. Parasites and Vectors. 2014;7:1–8. doi: 10.1186/s13071-014-0601-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaves MM, Marques-da-Silva C, Monteiro APT, et al. . Leukotriene B4 Modulates P2X7 receptor-mediated Leishmania amazonensis elimination in Murine macrophages. J Immunol. 2014;192:4765–4773. doi: 10.4049/jimmunol.1301058 [DOI] [PubMed] [Google Scholar]
- 22.Peters-golden M, Henderson WR.. Leukotrienes. N Engl J Med. 2007;357:1841–1854. doi: 10.1056/NEJMra071371 [DOI] [PubMed] [Google Scholar]
- 23.Ricciotti E, Fitzgerald AG.. [Eicosanoid neuroinflammation] prostaglandins and inflammation. Arter Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serezani CH, Lewis C, Jancar S, et al. . Leukotriene B4 amplifies NF-??B activation in mouse macrophages by reducing SOCS1 inhibition of MyD88 expression. J Clin Invest. 2011. doi: 10.1172/JCI43302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afonso P V, Janka-Junttila M, Lee YJ, et al. . LTB4 is a signal-Relay Molecule during neutrophil chemotaxis. Dev Cell. 2012;22:1079–1091. doi: 10.1016/j.devcel.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandt SL, Serezani CH.. Too much of a good thing: How modulating LTB 4 actions restore host defense in homeostasis or disease. Semin Immunol. 2017;33:37–43. doi: 10.1016/j.smim.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nataraj C, Thomas DW, Tilley SL, et al. . Receptors for prostaglandin E2 that regulate cellular immune responses in the mouse. J Clin Invest. 2001;108:1229–1235. doi: 10.1172/JCI200113640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogawa M, Suzuki J-I, Kosuge H, et al. . The mechanism of Anti-inflammatory effects of Prostaglandin E2 receptor 4 activation in Murine Cardiac Transplantation. Transplantation. 2009;87:1645–1653. doi: 10.1097/TP.0b013e3181a5c84c [DOI] [PubMed] [Google Scholar]
- 29.Gill SK, Yao Y, Kay LJ, et al. . The anti-inflammatory effects of PGE2 on human lung macrophages are mediated by the EP4 receptor. Br J Pharmacol. 2016;173:3099–3109. doi: 10.1111/bph.13565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Díaz-Gandarilla JA, Osorio-Trujillo C, Hernández-Ramírez VI, et al. . PPAR activation induces m1 macrophage polarization via cPLACOX-2 inhibition, activating ros production against Leishmania Mexicana. Biomed Res Int. 2013;2013, doi: 10.1155/2013/215283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amorim CF, Novais FO, Nguyen BT, et al. . Variable gene expression and parasite load predict treatment outcome in cutaneous leishmaniasis. Sci Transl Med. 2019;4204:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaves MM, Canetti C, Coutinho-Silva R.. Crosstalk between purinergic receptors and lipid mediators in leishmaniasis. Parasit Vectors. 2016;9:489. doi: 10.1186/s13071-016-1781-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li P, Oh DY, Bandyopadhyay G, et al. . LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat Med. 2015;21:239–247. doi: 10.1038/nm.3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saiwai H, Ohkawa Y.. The LTB4-BLT1 Axis Mediates neutrophil Infiltration and secondary injury in experimental Spinal Cord injury. Am J Pathol. 2010;176:2352–2366. doi: 10.2353/ajpath.2010.090839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serezani CH, Lewis C, Jancar S, et al. . Leukotriene B 4 amplifies NF- κ B activation in mouse macrophages by reducing SOCS1 inhibition of MyD88 expression. J Clin Invest. 2011;121:671–682. doi: 10.1172/JCI43302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guimarães FR, Sales-Campos H, Nardini V, et al. . The inhibition of 5-lipoxygenase (5-LO) products leukotriene B4 (LTB4) and cysteinyl leukotrienes (cysLTs) modulates the inflammatory response and improves cutaneous wound healing. Clin Immunol. 2018;190:74–83. doi: 10.1016/j.clim.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 37.Simpson DM, Ross R.. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J Clin Invest. 1972;51:2009–2023. doi: 10.1172/JCI107007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonald B, Kubes P.. Chemokines: Sirens of neutrophil recruitment-but Is It Just One Song? Immunity. 2010;33:148–149. doi: 10.1016/j.immuni.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 39.Benfield T, Jensen JS, Nordestgaard BG.. Influence of diabetes and hyperglycaemia on infectious disease hospitalisation and outcome. Diabetologia. 2007;50:549–554. doi: 10.1007/s00125-006-0570-3 [DOI] [PubMed] [Google Scholar]
- 40.Alves C, Casqueiro J, Casqueiro J.. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab. 2012;16:27. doi: 10.4103/2230-8210.94253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neto AF, Dell’Armelina Rocha PR, Perez EC, et al. . Diabetes mellitus increases the susceptibility to encephalitozoonosis in mice. PLoS One. 2017;12:1–18. doi: 10.1371/journal.pone.0181612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robbins GR, Wen H, Ting JPY.. Inflammasomes and metabolic disorders: Old genes in modern diseases. Mol Cell. 2014;54:297–308. doi: 10.1016/j.molcel.2014.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lakhani CM. Toll-Like receptor 9 Promotes Steatohepatitis by Induction of Interleukin-1β in mice. Physiol Behav. 2019;176:139–148. [Google Scholar]
- 44.Wen H, Gris D, Lei Y, et al. . Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thiem K, Stienstra R, Riksen NP, et al. . Trained immunity and diabetic vascular disease. Clin Sci. 2019;133:195–203. doi: 10.1042/CS20180905 [DOI] [PubMed] [Google Scholar]
- 46.Filgueiras LR, Brandt SL, Wang S, et al. . Leukotriene B 4 – mediated sterile inflammation promotes susceptibility to sepsis in a mouse model of type 1 diabetes. Sci Signal. 2015;8:1–10. doi: 10.1126/scisignal.2005568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boizel R, Bruttmann G, Benhamou PY, et al. . Regulation of oxidative stress and inflammation by glycaemic control: Evidence for reversible activation of the 5-lipoxygenase pathway in type 1, but not in type 2 diabetes. Diabetologia. 2010;53:2068–2070. doi: 10.1007/s00125-010-1822-9 [DOI] [PubMed] [Google Scholar]
- 48.Novais FO, Nguyen BT, Beiting DP, et al. . Human Classical monocytes control the intracellular Stage of Leishmania braziliensis by reactive oxygen species. J Infect Dis 2014;209, doi: 10.1093/infdis/jiu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chao WC, Yen CL, Wu YH, et al. . Increased resistin may suppress reactive oxygen species production and inflammasome activation in type 2 diabetic patients with pulmonary tuberculosis infection. Microbes Infect. 2015;17:195–204. doi: 10.1016/j.micinf.2014.11.009 [DOI] [PubMed] [Google Scholar]
- 50.Zheng LX, Li KX, Hong FF, et al. . Pain and bone damage in rheumatoid arthritis: role of leukotriene B4. Clin Exp Rheumatol 2019;37:872–878. [PubMed] [Google Scholar]
- 51.Geng S, Zhang Y, Lee C, et al. . Novel reprogramming of neutrophils modulates inflammation resolution during atherosclerosis. Sci Adv. 2019;5, doi: 10.1126/sciadv.aav2309. [DOI] [PMC free article] [PubMed] [Google Scholar]