Abstract
Background
Multisystem inflammatory syndrome in children (MIS-C), also known as pediatric inflammatory multisystem syndrome, is a new dangerous childhood disease that is temporally associated with coronavirus disease 2019 (COVID-19). We aimed to describe the typical presentation and outcomes of children diagnosed with this hyperinflammatory condition.
Methods
We conducted a systematic review to communicate the clinical signs and symptoms, laboratory findings, imaging results, and outcomes of individuals with MIS-C. We searched four medical databases to encompass studies characterizing MIS-C from January 1st, 2020 to July 25th, 2020. Two independent authors screened articles, extracted data, and assessed risk of bias. This review was registered with PROSPERO CRD42020191515.
Findings
Our search yielded 39 observational studies (n = 662 patients). While 71·0% of children (n = 470) were admitted to the intensive care unit, only 11 deaths (1·7%) were reported. Average length of hospital stay was 7·9 ± 0·6 days. Fever (100%, n = 662), abdominal pain or diarrhea (73·7%, n = 488), and vomiting (68·3%, n = 452) were the most common clinical presentation. Serum inflammatory, coagulative, and cardiac markers were considerably abnormal. Mechanical ventilation and extracorporeal membrane oxygenation were necessary in 22·2% (n = 147) and 4·4% (n = 29) of patients, respectively. An abnormal echocardiograph was observed in 314 of 581 individuals (54·0%) with depressed ejection fraction (45·1%, n = 262 of 581) comprising the most common aberrancy.
Interpretation
Multisystem inflammatory syndrome is a new pediatric disease associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that is dangerous and potentially lethal. With prompt recognition and medical attention, most children will survive but the long-term outcomes from this condition are presently unknown.
Funding
Parker B. Francis and pilot grant from 2R25-HL126140. Funding agencies had no involvement in the study
Keywords: MIS-C, PIMS, COVID-19, SARS-CoV-2, Multisystem inflammatory syndrome in children, Coronavirus disease 2019, Pediatric inflammatory multisystem syndrome, Severe acute respiratory syndrome 2, Hyperinflammatory shock, Children, Pediatric
Research in context.
Evidence before this study
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread throughout the world at an alarming rate. Previous reports suggested that children infected with coronavirus disease 2019 (COVID-19), the condition caused by SARS-CoV-2, were highly resilient and had mild symptoms. As of late April 2020, reports from the United Kingdom surfaced describing a new hyperinflammatory disease that is temporally associated with SARS-CoV-2 infection. Since then, several other countries have also reported patients exhibiting similar features, and this phenomenon has subsequently been coined multisystem inflammatory syndrome in children (MIS-C) or pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS). In this context, our goal was to provide a review of published articles focusing on MIS-C.
Added value of this study
This systematic review summarizes the clinical presentation of MIS-C from 662 patients (n = 39 studies). We report the most common signs and symptoms, quantify laboratory findings, and describe imaging characteristics of children with MIS-C. Furthermore, we summate outcomes, treatments, and compare MIS-C to COVID-19.
Implications of all the available evidence
Results from this systematic review represent a comprehensive evaluation of children meeting MIS-C criteria. Our findings will inform clinicians of the signs, markers, and outcomes of children who develop this dangerous and potentially life-threatening hyperinflammatory syndrome. Future research should focus on identifying variables that can prognosticate which pediatric COVID-19 patients will develop MIS-C and which, if any, markers correlate with systemic outcomes.
Alt-text: Unlabelled box
1. Introduction
The rapidly evolving pandemic associated with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has led to more than 19.8 M confirmed cases and over 730,000 global deaths [1]. Previous reports suggest that children infected with coronavirus disease 2019 (COVID-19) are highly resilient and present with a mild upper respiratory illness [2], [3], [4]. For instance, a study of 171 children with confirmed COVID-19 reported that only three cases required intensive care unit admission and only one death was observed [5]. However, in early May 2020, investigators from South Thames Retrieval Service in London, UK published a report describing eight severely ill pediatric patients presenting in hyperinflammatory shock with multiorgan involvement [6] Specifically, the children manifested with high fever, rash, conjunctivitis, peripheral edema, and gastrointestinal symptoms.
The Royal College of Paediatrics and Child Health (RCPCH) referred to this acute condition as pediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS-TS) [7]. As more cases arose globally, the illness was labelled multisystem inflammatory syndrome in children (MIS-C) by the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) [8], [9], [10]. The definition across the organizations is based on 6 principle elements: pediatric age, persistence of fever, presence of laboratory markers of inflammation, manifestation of signs or symptoms of organ dysfunction, lacking an alternative diagnosis, and a temporal relation to COVID-19 infection or exposure. While the RCPCH definition of PIMS-TS recognizes the temporal association with COVID-19, it does not require proof of infection or exposure to meet the case definition like the CDC and WHO criteria.
One of the initial challenges clinicians were facing was differentiating patients with MIS-C versus Kawasaki disease (KD) or toxic shock syndrome (TSS) [9,[11], [12], [13]]. KD is a vasculitis that typically presents with high fever and acute mucocutaneous inflammation in children <5 years of age [14]. Although typically a self-limiting condition, some children may have severe complications including coronary artery aneurysms, myocardial dysfunction, and thrombotic events [15]. KD can be stratified into classic or incomplete, depending on the number of clinical findings characteristic to the disease. Fever for 5 days with ≥4 of the following 5 principle features distinguishes classic KD: (i) conjunctival injection, (ii) rash, (iii) erythema and edema of the hands and feet, (iv) cervical lymphadenopathy, and (v) oral mucosal changes. The diagnosis of incomplete KD includes fever with 2–3 of the principal features [14]. On the other hand, TSS is a potentially lethal disease derived from the release of bacterial toxins. It is depicted by fever, rash, shock, vomiting and diarrhea, and treated by hemodynamic stabilization and antibiotics [16]. A recent publication by Whittaker et al., elegantly compared the age and laboratory findings in patients with MIS-C, KD, and TSS [17].
We sought to conduct a systematic review to provide an overview of the current evidence regarding pediatric patients diagnosed with MIS-C. In addition, we compared features of MIS-C with children with COVID-19. We included patients with COVID-19 to reinforce to the healthcare community and public the differences in the clinical presentation, to highlight the degree of systemic inflammation in MIS-C, and to iterate the differences in treatment and outcome between the two diseases.
2. Methods
2.1. Search strategy and selection criteria
Our methods adhere to the guidelines established by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [18]. Our study protocol was registered with PROSPERO (International Prospective Register of Systematic Reviews) with the following registration number CRD42020191515 [19].
We performed a systematic search in the following databases: PubMed, LitCovid, Scopus, and Science Direct. Additionally, we searched references of included articles and any reviews focusing on MIS-C. Our search terms included “multisystem inflammatory syndrome in children” or “pediatric multisystem inflammatory syndrome”. Search dates were from January 1st, 2020 to July 25th, 2020. Our detailed search with dates can be viewed in Appendix 1.
We included published or in press peer-reviewed articles reporting cases of MIS-C. We accepted the following types of studies: case reports, case-control, case series, cross-sectional studies, and letters to the editors that incorporated clinical, laboratory, imaging, as well as the hospital course of MIS-C patients. Articles were included if the studies met the criteria for hyperinflammatory syndrome (MIS-C or PIMS-TS) as set by the CDC, RCPCH, or the WHO [7,9,10].
Duplicate studies were manually removed from the search results prior to the screening process (AxM, AM). Screening by title and abstract was conducted independently by two investigators (AxM, AM). A third investigator (MA, SA) was consulted to resolve differences of opinion in either phase. Subsequent full-text review and data extraction was conducted by investigators (MA, SA, AxM, SZ, JM, KC, RN, FBM, FB, AT, MP, ME, AH) using Google Sheets (Google, Mountain View, CA, USA). Data retrieved from each article was cross-checked by another independent investigator (MA, SA, AM).
Our goals were twofold: (i) to describe the clinical signs, laboratory findings, imaging characteristics, treatments, and outcomes of patients with MIS-C, and (ii) compare the clinical variables between children with MIS-C to those with COVID-19.
2.2. Data collection and risk of bias assessment
Data collected from the studies included demographics, number of patients, signs and symptoms, laboratory markers, imaging results, medications, and outcomes. Only initial laboratory values were recorded (e.g., at time of admission or first reported lab). If only peak laboratory values were provided, they were not included in the analysis. Signs and symptoms were considered positive if they occurred any time during the patient's hospitalization. All echocardiograms were taken into consideration. More precisely, if any of the echocardiograms reported a depressed ejection fraction, pericarditis, mitral valvular dysfunction, coronary dilation, or coronary aneurysm we recorded this finding as positive. Cardiac dysfunction/depression was defined as an ejection fraction <55% or as a shortening fraction <45% [20,21]. Acute kidney injury was documented according to the definition by the authors. Examples of how acute kidney injury was defined included a serum creatinine ≥1.5 times the upper limit of normal or a serum creatinine above the reference value for age [17,20,21].
Patients were deemed SARS-CoV-2 positive if they had a positive RT-PCR or antibody test. Shock was defined according to the author's criteria, patient requiring inotropic support, or hypotension necessitating volume resuscitation (>20 ml/kg of colloids). Respiratory outcomes were categorized according to level of support (e.g., nasal cannula, noninvasive, mechanical ventilation, ECMO). If the number of intensive care unit (ICU) admissions were not explicitly described, we assumed that respiratory support (>nasal cannula) or need for inotropes was a logical surrogate and entered the higher number of the two into the data collection form. Co-morbidities, signs and symptoms were grouped according to organ system. Reference values for laboratory values were based on the mean age of the whole study population.
Risk of bias for observational studies was appraised through the quality assessment tool published by the National Institutes of Health [22]. Risk of bias was assessed independently by at least two investigators (SZ, JM) and disagreements were resolved by a third researcher (AM). Furthermore, the level of evidence was assessed according to Sackett [23].
2.3. Data sources
For our second aim, we compared MIS-C to confirmed pediatric cases of COVID-19 [24]. Justification and rationale for the chosen article included the following: (i) to maintain consistency the selected study was also a systematic review, that was published by our team, and more importantly (ii) the comparative study also provided detailed information regarding clinical signs/symptoms, laboratory markers, outcomes, and treatments. We understand that this approach has inherent bias and therefore our comparison is strictly descriptive and did not conduct statistical analyses between the two diseases.
2.4. Data analysis
Continuous data were summarized as mean ± standard deviation. If results were presented as median with interquartile range, we transformed the data to mean ± standard deviation according to Wan et al. [25]. Categorical data were summarized as counts with percent. Means, standard deviations, counts, and percent were calculated using Excel (Microsoft, Redmond, WA, USA). GetData Graph Digitizer 2.26 (S. Fedorov) was used to extrapolate data from figures when information was not in the text. Figures were created in Excel.
2.5. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
3. Results
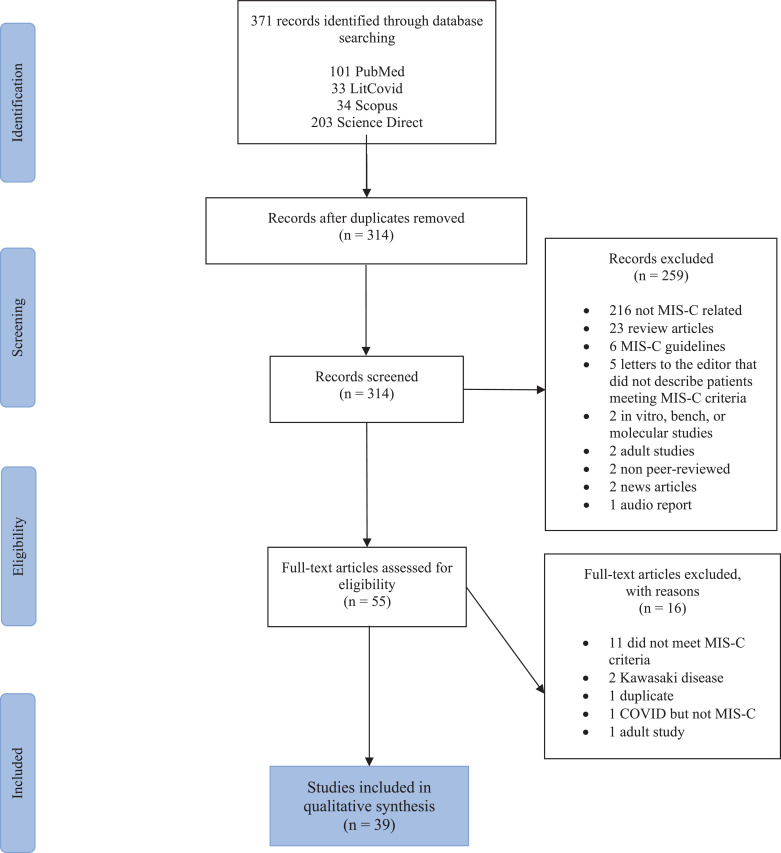
Our search identified 371 articles published from January 1st, 2020 to July 25th, 2020. After removing duplicates and screening the titles and abstracts, 314 studies were evaluated for eligibility. Ultimately, 39 articles were included in this review with a total sample size of 662 children with MIS-C (refer to Fig. 1). All studies were observational and therefore the level of evidence was 5 (1 is highest, 5 is lowest). Appendix 2 depicts that the risk of bias for the studies was moderate to high.
Fig. 1.
PRISMA flow diagram. Delineation of study selection.
Twenty-three (59·0%) of the studies were case series. The two largest studies were from the United States and contributed to 43·0% of the data (n = 285 individuals) [21,26]. The mean age of patients was 9·3 ± 0.5 years and 52·3% of children were male. The total number of deaths was 11 (1·7%). Please see Table 1.
Table 1.
Study details.
| # | First author | Number (% male) | Age (yrs) | Clinical presentation | Shock | Medications | Pulmonary support | Echo findings | Laboratory markers | Died |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Abdel-Mannan, O | 4 (50) |
11.8 | Fever (n = 4), rash (n = 4), dyspnea (n = 2), headache (n = 3), confused (n = 4), muscle weakness (n = 4), hyporeflexia (n = 2) | N = 4 | Anakinra: 2 Rituximab: 1 |
MV: 4 | ↓EF: 1 Pericardial ∆: 1 CAA: 0 |
↑CRP, d-dimer, LDH, ferritin | 0 |
| 2 | Bahrami, A | 1 (0) |
5 | Fever, vomiting, diarrhea, abdominal pain, conjunctivitis, rash, swelling hands, periorbital edema | N = 1 | Vasopressors IVIG ASA Antibiotics |
None | Normal | ↑CRP, hyponatremia | 0 |
| 3 | Balasubramanian, S | 1 (100) |
8 |
Fever, conjunctivitis, rash, mucosal changes, peripheral edema, tongue swelling, cough, dyspnea, sore throat, abdominal pain |
N = 1 |
Tocilizumab Antibiotics IVIG ASA |
NC: 1 | Normal | ↑CRP, ESR, ferritin; ↓Albumin | 0 |
| 4 | Bapst, T | 1 (100) |
13 | Fever, conjunctivitis, rash, sore throat, abdominal pain, chest pain | N = 0 | Antibiotics | None | Normal | ↑CRP, PCT, troponin | 0 |
| 5 | Belhadjer, Z | 35 (46) |
10 | Fever (n = 35), conjunctivitis (n = 31), rash (n = 20), mucosal changes (n = 19), lymphadenopathy (n = 21), dyspnea (n = 33), rhinorrhea (n = 15), myalgia/fatigue (n = 35), gastrointestinal symptoms (n = 29), headache/dizziness (n = 11); 2 children underwent emergency operation for suspected appendicitis which was ultimately mesenteric adenitis | N = 28 | Vasopressors: 28 IVIG: 25 Steroids: 12 Heparin: 23 Anakinra: 3 |
NIV: 11 MV: 22 ECMO: 10 |
↓EF: 35 Pericardial ∆: 3 CAA: 0 |
↑Neutrophil%, CRP, d-dimer, PCT, BNP, troponin, IL-6, NT-proBNP | 0 |
| 6 | Buonsenso, D | 1 (0) |
11 | Fever, myalgias, arthralgia, diffuse skin rash | NR | NR | NR | NR | ↑CRP, fibrinogen, d-dimer, troponin, NT-proBNP, IL-1, IL-6, IL-10, IL-13, TNF α; borderline elevated LDH | 0 |
| 7 | Capone, C | 33 (61) |
8.9 | Fever (n = 33), neurologic symptoms (n = 19), gastrointestinal symptoms (n = 32), respiratory symptoms (n = 17) | N = 25 | Vasopressors: 25 IVIG: 33 Steroids: 23 ASA: 29 |
MV: 6 Required O2 or PPV: 17 |
↓EF: 19 CAA: 5 |
↑CRP, d-dimer, PCT, troponin, fibrinogen, ferritin, LDH, NT-proBNP; ↓Lymphocyte count | 0 |
| 8 | Cheung, E | 17 (47) |
8.5 | Fever (n = 17), conjunctivitis (n = 11), rash (n = 12), mucosal changes (n = 9), lymphadenopathy (n = 6), dyspnea (n = 7), myalgia/fatigue (n = 6), headache/dizziness (n = 8), gastrointestinal symptoms (n = 15) | N = 10 | Vasopressors: 10 IVIG: 13 Steroids: 15 ASA: 4 Tocilizumab: 1 Anticoagulant: 4 |
None | ↓EF: 11 Pericardial ∆: 8 CAA: 1 |
↑Neutrophil%, CRP, d-dimer, PCT, ferritin, troponin, IL-6, NT-proBNP; ↓Lymphocyte%; nml LDH | 0 |
| 9 | Chiotos K | 6 (17) |
8.5 | Fever (n = 6), conjunctivitis (n = 2), rash (n = 2), mucosal changes (n = 3), dyspnea (n = 1), headache/dizziness (n = 1), vomiting/nausea (n = 5), abdominal pain/diarrhea (n = 6), lethargy/altered mental state (n = 3) | N = 6 | Vasopressors: 5 IVIG: 6 Steroids: 5 ASA: 3 Antibiotics: 6 Anakinra: 1 |
NIV: 2 MV: 3 |
↓SF: 3 Pericardial ∆: 0 CAA: 0 |
↓Lymphocyte%, albumin; ↑CRP, d-dimer, PCT, LDH, BNP, troponin | 0 |
| 10 | Dallan, C | 2 (100) |
10.0 | Fever (n = 2), conjunctivitis (n = 2), cough (n = 1), sore throat (n = 1), gastrointestinal symptoms (n = 2) | N = 2 | Vasopressors: 2 Antibiotics: 2 Hydroxychloroquine: 2 |
NIV: 1 MV: 1 |
CAA: 1 | ↑Neutrophil%, CRP, d-dimer, PCT, fibrinogen, ferritin, troponin; ↓Lymphocyte% | 0 |
| 11 | Dasgupta, K | 1 (0) |
8 | Fever, conjunctivitis, rash, mucosal changes, peripheral edema, cough, sore throat, myalgia/fatigue, gastrointestinal symptoms, lethargy | N = 1 | Vasopressors IVIG Steroids ASA Antibiotics |
None | ↓EF: 1 Pericardial ∆: 1 |
↑Neutrophil%, CRP, d-dimer, PCT, BNP, fibrinogen, troponin; ↓Lymphocyte%, albumin; nml LDH | 0 |
| 12 | Dufort, E | 99 (54) |
0–5 years (n = 31) 6–12 years (n = 42) 13–20 years (n = 26) |
Fever (n = 99), conjunctivitis (n = 55), rash (n = 59), mucosal changes (n = 27), peripheral edema (n = 9), lymphadenopathy (n = 6), cough (n = 31), rhinorrhea/congestion (n = 13), sore throat (n = 16), myalgia/fatigue (n = 17), headache/dizziness (n = 29), vomiting/nausea (n = 57), abdominal pain/diarrhea (n = 60), lethargy/altered mental state (n = 2), chest pain (n = 11) | N = 61 | Vasopressors: 61 IVIG: 69 Steroids: 63 Antibiotics: 71 |
NIV: 7 MV: 10 ECMO: 4 |
↓EF: 51 Pericardial ∆: 32 CAA: 9 |
↑Neutrophil%, CRP, d-dimer, PCT, BNP, fibrinogen, ESR, ferritin, IL-6; ↓Lymphocyte%, albumin; nml LDH | 2 |
| 13 | Feldstein, L | 186 (62) |
8.0 | Fever (n = 186), conjunctivitis (n = 103), rash (n = 110), mucosal changes (n = 78), peripheral edema (n = 69), lymphadenopathy (n = 18), gastrointestinal symptoms (n = 170), lethargy/altered mental state (n = 3) | N = 89 | Vasopressors: 89 IVIG: 144 Steroids: 91 Tocilizumab or Siltuximab: 14 Anakinra: 24 Anticoagulants: 87 |
MV: 37 ECMO: 8 |
↓EF: 71 Pericardial ∆: 44 CAA: 15 |
↑Neutrophil%, CRP, ferritin, ESR, INR, BNP; ↓Lymphocyte%, albumin | 4 |
| 14 | Greene, A | 1 (0) |
11 | Fever, rash, sore throat, malaise, pharyngeal erythema, abdominal pain, poor appetite, leg pain | N = 1 | Vasopressors IVIG Steroids Remdesivir Tocilizumab Convalescent plasma Antibiotics Anticoagulants |
NC | ↓SF: 1 Pericardial ∆: 0 CAA: 0 |
↑CRP, d-dimer, PCT, fibrinogen, ferritin, BNP, IL-6, troponin; nml LDH | 0 |
| 15 | Grimaud, M | 20 (50) |
8.9 | Fever (n = 20), conjunctivitis (n = 12), rash (n = 8), mucosal changes (n = 5), lymphadenopathy (n = 4), abdominal pain/diarrhea (n = 20 | N = 20 | Vasopressors: 19 IVIG: 20 Steroids: 2 Anakinra: 1 Tocilizumab: 1 |
NIV: 9 MV: 8 |
↓EF: 20 Pericardial ∆: 4 CAA: 0 |
↑CRP, PCT, fibrinogen, BNP, troponin; ↓Albumin | 0 |
| 16 | Hutchison, L | 1 (100) |
14 | Fever, abdominal pain, rash, hypotension, | N = 1 | Vasopressors IVIG Steroids Anticoagulants Anakinra |
NC | NR | ↑CRP, ESR, d-dimer, ferritin; ↓Leukocyte count | 0 |
| 17 | Jones, V | 1 (0) |
0.5 | Fever, conjunctivitis, rash, mucosal changes, peripheral edema, tongue swelling, congestion, poor appetite, fussiness | N = 0 | IVIG | None | Normal | ↑CRP, ESR; ↓Albumin | 0 |
| 18 | Klocperk, A | 1 (0) |
8 | Fever, headache, abdominal pain, vomiting, diarrhea, rash | NR | IVIG Steroids Anticoagulant Antibiotics |
MV | Normal | ↑Neutrophil count, d-dimer, troponin, NT-proBNP; ↓Lymphocyte count, C3, C4 | 0 |
| 19 | Lee, P | 28 (57) |
8.7 | Fever (n = 28), conjunctivitis (n = 16), gastrointestinal symptoms (n = 15), rash (n = 10), mucositis (n = 7), extremity swelling (n = 6), acute kidney injury (n = 6) | N = 15 | Vasopressors: 7 IVIG: 20 Steroids: 17 Anakinra: 5 Remdesivir: 7 Antibiotics: 15 Anticoagulants: 19 |
NIV: 7 MV: 1 Supplemental O2: 12 |
↓EF: 11 CAA: 4 |
↑CRP, d-dimer, PCT, fibrinogen, LDH, ferritin; ↓Lymphocyte count | 0 |
| 20 | Leon, M | 1 (0) |
6 | Fever, conjunctivitis, rash, peripheral edema, dyspnea, sore throat, poor appetite | N = 1 | Vasopressors IVIG ASA Antibiotics |
ECMO | ↓EF: 21 Pericardial ∆: 0 CAA: 0 |
↑Neutrophil%, CRP, d-dimer, LDH, fibrinogen, ESR, ferritin, troponin; ↓Albumin | 0 |
| 21 | Licciardi, F | 2 (100) |
9.5 |
Fever (n = 2), diarrhea (n = 2), mucocutaneous involvement (n = 2), conjunctivitis (n = 2), abdominal pain (n = 2), vomiting (n = 2), tongue de-epithelialization (n = 1), petechiae (n = 2) | N = 1 | Vasopressors: 1 Steroids: 2 IVIG: 1 |
NIV: 1 | ↓EF: 1 Pericardial ∆: 1 CAA: 0 |
↑CRP, fibrinogen, ferritin; ↓Lymphocyte and Plt count | 0 |
| 22 | Miller, J | 44 (46) |
8.8 | Fever (n = 44), conjunctivitis (n = 23), rash (n = 31), mucosal changes (n = 23), lymphadenopathy (n = 2), dyspnea (n = 11), headache/dizziness (n = 13), vomiting/nausea (n = 25), abdominal pain/diarrhea (n = 33), poor appetite (n = 33), lethargy/altered mental state (n = 13), melena (n = 2), hematemesis (n = 1), constipation (n = 5) | N = 22 | Vasopressors: 22 IVIG: 36 Steroids: 42 Anakinra: 8 Anticoagulants: 40 |
MV: 1 | 22 non-specified abnormalities | ↑CRP, LDH, ESR, IL-6; ↓Albumin | 0 |
| 23 | Moraleda, C | 31 (58) |
7.9 | Fever (n = 31), rash (n = 23), hypotension (n = 15), gastrointestinal symptoms (n = 27), malaise (n = 15), cough (n = 11), dyspnea (n = 8), sore throat (n = 8), myalgia (n = 5), headache (n = 6), altered mental status/confusion (n = 4), lymphadenopathy (n = 4) | N = 20 | IVIG: 20 Steroids: 21 Remdesivir: 2 Lopinavir/ritonavir: 7 Antibiotics: 28 |
MV: 6 | ↓EF: 15 Pericardial ∆: 6 CAA: 1 |
↑CRP, d-dimer, PCT, fibrinogen, ferritin, IL-6, NT-proBNP; ↓Lymphocyte count | 1 |
| 24 | Ng, K | 3 (67) |
15.3 | Fever (n = 3), conjunctivitis (n = 3), rash (n = 2), mucosal changes (n = 1), lymphadenopathy (n = 2), dyspnea (n = 2), sore throat (n = 2), headache/dizziness (n = 1), gastrointestinal symptoms (n = 3), lethargy/altered mental state (n = 1) | N = 3 | Vasopressors: 2 IVIG: 2 Steroids: 1 ASA: 2 Antibiotics: 3 |
NC: 2 MV: 1 |
↓EF: 1 Pericardial ∆: 2 CAA: 0 |
↑Neutrophil%, CRP, d-dimer, PCT, fibrinogen, ferritin, IL-6, NT-proBNP; ↓Lymphocyte%, albumin; Normal troponin | 0 |
| 25 | Paolino, J | 3 (67) |
7.7 | Fever (n = 3), hypotension (n = 3), rash (n = 2), abdominal pain (n = 2), conjunctivitis (n = 2) | N = 3 | NR | NR | ↓EF: 2 | ↑CRP, ferritin, d-dimer, neutrophil/band%; ↓Lymphocyte% | 0 |
| 26 | Pouletty, M | 16 (50) |
9.1 | Fever (n = 16), conjunctivitis (n = 15), rash (n = 13), mucosal changes (n = 14), peripheral edema (n = 11), lymphadenopathy (n = 6), cough (n = 1), dyspnea (n = 2), headache/dizziness (n = 6), gastrointestinal symptoms (n = 2) | N = 11 | Vasopressors: 6 IVIG: 15 Steroids: 4 ASA: 15 Tocilizumab: 1 Anakinra: 1 Hydroxychloroquine: 1 |
NIV: 3 MV: 2 |
↓EF: 11 Pericardial ∆: 4 CAA: 0 |
↑Neutrophil%, CRP, PCT, ferritin, BNP, troponin; ↓Lymphocyte%, albumin | 0 |
| 27 | Rauf, A | 1 (100) |
5 | Fever, conjunctivitis, peripheral edema, abdominal pain, diarrhea, lethargy | N = 1 | Vasopressors IVIG Steroids ASA Antibiotics |
NC | ↓EF: 1 Pericardial ∆ or CAA: 0 |
↑Neutrophil%, ESR, ferritin, BNP, troponin; ↓Lymphocyte%, albumin | 0 |
| 28 | Regev, T | 1 (100) |
16 | Fever, abdominal pain, fatigue, sore throat, rash, followed by headache, nuchal rigidity, warm shock, muscle weakness, and clonus | N = 1 | Vasopressors IVIG ASA |
MV | ↓EF: 1 CAA: 1 |
↑CRP, d-dimer, NT-proBNP, troponin; mild elevation INR; ↓C3, C4 factors; normal fibrinogen | 0 |
| 29 | Riollano-Cruz, M | 15 (73) |
12.1 | Fever (n = 15), conjunctivitis (n = 3), rash (n = 6), mucosal changes (n = 1), cough (n = 3), dyspnea (n = 1), sore throat (n = 3), headache/dizziness (n = 3), vomiting/nausea (n = 11), abdominal pain/diarrhea (n = 12), chest pain (n = 2), scrotal pain (n = 1) | N = 8 | Vasopressors: 8 IVIG: 10 Steroids: 1 Antibiotics: 13 Tocilizumab: 13 Remdesivir: 2 Anakinra: 2 Hydroxychloroquine: 1 Anticoagulation: 13 |
NIV: 5 MV: 2 ECMO: 1 |
↓EF: 7 ↓SF: 4 Pericardial ∆: 7 CAA: 0 |
↑CRP, d-dimer, PCT, ferritin, BNP, troponin, IL-6; ↓Albumin | 1 |
| 30 | Rodriguez-Gonzalez, M | 1 (100) |
0.5 | Fever, severe respiratory distress, hypotension, hypoxemic, irritability, history of nasal congestion and cough | N = 1 | Vasopressors Steroids Antibiotics Tocilizumab Anticoagulants |
MV | Severe RV systolic dysfunction with supra-systemic pulmonary hypertension | ↑Leukocyte count, CRP, ferritin, PCT, d-dimer, troponin, NT-proBNP, IL-6; ↓Plts; fibrinogen normal | 0 |
| 31 | Rogo, T | 4 (75) |
11.3 | Fever (n = 4), abdominal symptoms (n = 3), chest pain (n = 1), neck pain (n = 1) | N = 2 | Vasopressors: 1 IVIG: 1 Anticoagulants: 3 Convalescent plasma: 1 |
MV: 1 ECMO: 1 |
↓EF: 4 Pericardial ∆: 1 CAA: 0 |
↑Neutrophil%, CRP, troponin, NT-proBNP, d-dimer, ferritin; ↓Lymphocyte% | 1 |
| 32 | Schupper, A | 1 (100) |
5 | Fever (n = 1), cough (n = 1), abdominal pain (n = 1) | N = 1 | NR | ECMO: 1 | NR | NR | 1 |
| 33 | Shenker, J | 1 (100) |
12 | Fever, neck swelling, trismus, loss of smell and taste, difficulty swallowing, mucosal changes, rash, conjunctival injection, followed by altered mental status and status epilepticus | N = 1 | Vasopressors Remdesivir Anakinra Anticoagulants Antibiotics Antiseizures |
MV: 1 | NR | ↑CRP, CK, BNP, d-dimer, ESR, Ferritin, Fibrinogen, IL-6, LDH, PCT | 0 |
| 34 | Toubiana, J | 21 (43) |
9.4 | Fever (n = 11), conjunctivitis (n = 17), rash (n = 16), peripheral edema (n = 10), lymphadenopathy (n = 12), cough (n = 9), headache/dizziness (n = 6), gastrointestinal symptoms (n = 21) | N = 15 | Vasopressors: 15 IVIG: 21 Steroids: 7 ASA: 21 Antibiotics: 18 |
MV: 11 | Myocarditis in 16 individuals with range in EF between 10%−57% Pericardial ∆: 10 CAA: 0 |
↑Neutrophil%, CRP, d-dimer, PCT, BNP, troponin, IL-6; ↓Lymphocyte%, albumin | 0 |
| 35 | Verdoni, L | 10 (70) |
7.5 | Fever (n = 10), conjunctivitis (n = 7), rash (n = 6), mucosal changes (n = 1), peripheral edema (n = 5), tongue swelling (n = 1), lymphadenopathy (n = 1), headache/dizziness (n = 4), abdominal pain/diarrhea (n = 6) | N = 5 | Vasopressors: 2 IVIG: 10 Steroids: 8 ASA: 10 |
NR | ↓EF: 5 Pericardial ∆: 4 CAA: 2 |
↑Neutrophil%, CRP, d-dimer, fibrinogen, ESR, ferritin, troponin, IL-6, NT-proBNP; ↓Lymphocyte%, albumin | 0 |
| 36 | Waltuch, T | 4 (75) |
10 | Fever (n = 4), conjunctivitis (n = 2), rash (n = 2), rash (n = 3), mucosal changes (n = 3), lymphadenopathy (n = 1), myalgia/fatigue (n = 2), pharyngeal erythema (n = 2), headache (n = 1), vomiting (n = 2), diarrhea (n = 4), poor appetite (n = 2) | N = 4 | Vasopressors: 3 IVIG: 3 Tocilizumab: 3 Anticoagulants: 2 |
MV: 1 | ↓EF: 1 Pericardial ∆: 1 CAA: 0 |
↑CRP, ESR, PCT, LDH, BNP, d-dimer, ferritin, fibrinogen, IL-6, IL-8, TNF α; ↓Lymphocyte count; normal IL-1β, INR, Hgb | 0 |
| 37 | Whittaker, E | 58 (43) |
9.4 | Fever (n = 58), conjunctivitis (n = 26), rash (n = 30), mucosal changes (n = 17), peripheral edema (n = 9), lymphadenopathy (n = 9), dyspnea (n = 12), sore throat (n = 6), headache/dizziness (n = 15), vomiting/nausea (n = 26), abdominal pain/diarrhea (n = 31) | N = 29 | Vasopressors: 27 IVIG: 41 Steroids: 37 Anakinra: 3 Infliximab: 8 |
MV: 25 ECMO: 3 |
CAA: 8 | ↑Neutrophil%, CRP, d-dimer, LDH, fibrinogen, ferritin, troponin, NT-proBNP; ↓Lymphocyte%, albumin | 1 |
| 38 | Wolfler, A | 5 (40) |
7.0 | Fever (n = 5), dyspnea (n = 1), myalgia/fatigue (n = 3), vomiting/nausea (n = 3), abdominal pain/diarrhea (n = 5) | N = 4 | Vasopressors: 5 IVIG: 4 Steroids: 1 Antibiotics: 5 Hydroxychloroquine: 1 Anticoagulants: 4 |
NIV: 1 | ↓EF: 3 Pericardial ∆: 0 CAA: 0 |
↑Neutrophil%, CRP, d-dimer, PCT, ferritin, troponin, IL-6, NT-proBNP; ↓Albumin | 0 |
| 39 | Yozgat, C | 1 (0) |
3 | Fever, conjunctivitis, rash, mucosal changes, peripheral edema | N = 0 | IVIG ASA |
NR | NR | ↑CRP, d-dimer, PCT, LDH, ferritin, troponin; ↓Albumin; Normal ESR, lymphocyte% | 0 |
Abbreviations: ASA-aminosalicylate; BNP-brain natriuretic peptide; CAA-coronary artery aneurysm; CRP-C-reactive protein; Echo-echocardiogram; ECMO-extracorporeal membrane oxygenation; ↓EF-ejection fraction ≤50%; ESR-erythrocyte sedimentation rate; Hgb-hemoglobin; INR-international normalized ratio; IL-interleukin; IVIG-intravenous immunoglobulin; LDH-lactate dehydrogenase; MV-mechanical ventilation; NC-nasal cannula; NIV-non-invasive ventilation; NR-not reported; NT-proBNP-N Terminal pro brain natriuretic peptide; O2-oxygen; PCT-procalcitonin; Pericardial ∆ refers to pericardial changes (effusion/pericarditis); Plt-platelet; PPV-positive pressure ventilation; RV-right ventricle; ↓SF-shortening fraction ≤45%; *Shock was defined per author, hypotension requiring fluid resuscitation (>20 ml/kg of colloids), and/or initiation of vasoactive medications.
Data on race/ethnicity was provided for 471 (71·1%) individuals. Children from African American, Afro-Caribbean, or African race/ethnicity represented 34·8% (n = 164) of the population. Five hundred thirty-two (84·7%) children were confirmed SARS-CoV-2 positive. Average length of hospital stay was 7·9 ± 0·6 days. Table 2 offers more details.
Table 2.
Patient characteristics.
| # Patients with available data | N (%) | |
|---|---|---|
| Male gender | 662 | 346 (52.3) |
| Mean age (years) | 528 | 9.3 ± 0.5 |
| Race/Ethnicity | 471 | |
| African American/Afro-Caribbean/African | 164 (34.8) | |
| White/European/Caucasian | 130 (27.6) | |
| Hispanic/Latino | 91 (19.3) | |
| Asian/Indian/Middle Eastern | 38 (8.1) | |
| Other | 48 (10.2) | |
| Co-morbidities* | 558 | 268 (48.0) |
| Overweight/Obese | 136 (50.8) | |
| Respiratory | 71 (26.5) | |
| Immunologic/Allergic | 17 (6.3) | |
| Cardiac | 8 (2.9) | |
| Hematologic | 4 (1.5) | |
| Endocrine/Gastrointestinal | 5 (1.9) | |
| Neurologic/Behavioral | 3 (1.1) | |
| Other | 24 (9.0) | |
| SARS-CoV-2 positive (RT-PCR/antibody) | 628 | 532 (84.7) |
| Number of days symptomatic before presenting to hospital | 294 | 4.8 ± 0.3 |
| Hospital length of stay (days) | 422 | 7.9 ± 0.6 |
| Admission to intensive care unit | 662 | 470 (71.0) |
Continuous data presented as Mean ± SD. Multiple co-morbidities in a subset of patients *. RT-PCR-reverse transcriptase polymerase chain reaction; SARS-CoV-2-severe acute respiratory syndrome coronavirus 2.
Fever (n = 662, 100%), abdominal pain/diarrhea (n = 488, 73·7%), and vomiting (n = 452, 68·3%) were the most common symptoms reported (summarized in Table 3). Like KD and TSS, conjunctivitis (n = 343, 51·8%) and rash (n = 372, 56·2%), were frequently observed. Table 4 summarizes laboratory measurements. The mean neutrophil percent was elevated at 80·7 ± 7·8%, while the mean lymphocyte percent was low at 9·8 ± 0·8%. C-reactive protein (160 ± 6·9 mg/L), ferritin (977 ± 55·8 ng/mL), and procalcitonin (30·5 ± 2·1 ng/mL) were markedly increased. Cardiac markers, troponin, brain natriuretic peptide, and prohormone of brain natriuretic peptide, were extremely elevated at 494 ± 37·6 ng/L, 3604 ± 352 pg/mL, and 5854 ± 743 ng/L, respectively.
Table 3.
Clinical signs and symptoms.
| # Patients with available data | N (%) | |
|---|---|---|
| CONSTITUTIONAL | ||
| Fever | 662 | 662 (100.0) |
| Myalgia, fatigue | 662 | 89 (13.4) |
| Lymphadenopathy | 662 | 92 (13.9) |
| GASTROINTESTINAL | ||
| Abdominal pain, diarrhea | 662 | 488 (73.7) |
| Vomiting | 662 | 452 (68.3) |
| Loss of appetite | 662 | 73 (11.0) |
| HEAD, EYES, EARS, NOSE, THROAT | ||
| Conjunctivitis | 662 | 343 (51.8) |
| Cheilitis | 662 | 216 (32.6) |
| Tongue swelling | 662 | 31 (4.7) |
| Sore throat | 662 | 59 (8.9) |
| RESPIRATORY | ||
| Dyspnea, shortness of breath | 662 | 121 (18.3) |
| Cough | 662 | 86 (13.0) |
| Rhinorrhea, nasal congestion | 662 | 47 (7.1) |
| NEUROLOGIC | ||
| Headache, dizziness | 662 | 129 (19.5) |
| Somnolence, altered mental status, lethargy, fussy | 662 | 66 (10.0) |
| DERMATOLOGIC | ||
| Rash | 662 | 372 (56.2) |
| Edema to extremities | 662 | 128 (19.3) |
Table 4.
Laboratory measures.
| # Patients | Mean ± SD | Ref. range | |
|---|---|---|---|
| HEMATOLOGY | |||
| White blood cell count (103/µL) | 395 | 13.2 ± 0.8 | 4.0–12.0 |
| Neutrophil (%) | 276 | 80.7 ± 7.8 | 54–62 |
| Lymphocyte (%) | 306 | 9.8 ± 0.8 | 25–33 |
| Hemoglobin (g/dL) | 211 | 10.2 ± 0.8 | 11.5–14.5 |
| Platelets (103/µL) | 394 | 215 ± 11.4 | 150–450 |
| LIVER and RENAL FUNCTION | |||
| Albumin (g/dL) | 337 | 2.8 ± 0.2 | 4.0–5.3 |
| Creatinine (mg/dL) | 158 | 0.9 ± 0.1 | 0.22–0.59 |
| Alanine transaminase (U/L) | 226 | 59.8 ± 4.1 | 5–45 |
| Aspartate aminotransferase (U/L) | 145 | 57.3 ± 5.3 | 15–50 |
| INFLAMMATORY MARKERS | |||
| C-reactive protein (mg/L) | 439 | 160 ± 7.0 | Male 0.6–7.9 Female 0.5–10.0 |
| Ferritin (ng/mL) | 303 | 977 ± 55.8 | 10–60 |
| Procalcitonin (ng/mL) | 312 | 30.5 ± 2.1 | ≤0.15 |
| Lactate dehydrogenase (U/L) | 300 | 478 ± 45.4 | 150–500 |
| Interleukin-6 (pg/mL) | 257 | 184 ± 15.6 | ≤1.8 |
| Creatine kinase (U/L) | 49 | 135 ± 46.0 | 5–130 |
| COAGULATION | |||
| D-dimer (mg/L) | 349 | 3.5 ± 0.4 | <0.4 |
| Fibrinogen (mg/dL) | 267 | 499 ± 58.3 | 220–440 |
| Erythrocyte sedimentation rate (mm/h) | 191 | 59.4 ± 9.1 | 0–20 |
| CARDIAC* | |||
| Troponin (ng/L) | 281 | 494 ± 38.3 | <10 |
| Brain natriuretic peptide (pg/mL) | 147 | 3604 ± 352 | 0–100 |
| Prohormone of brain natriuretic peptide (ng/L) | 164 | 5854 ± 743 | 0–450 |
Reference (Ref.) ranges were obtained from Nelson Textbook of Pediatrics (we chose eight years as the age category provided the overall mean of included patients). *Data from Kaushik et al. was used for the normal cardiac values.
Pesce, M. Reference ranges for laboratory tests and procedures. In: Kliegman RM, Behrman R, Jenson H, Stanton B, editors. Nelson Textbook of Pediatrics. 18th edition. Philadelphia: WB Saunders; 2011. pp. 2943–2949.
Kaushik S, Aydin SI, Derespina KR, et al. Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Multi-institutional Study from New York City [published online ahead of print, 2020 Jun 14]. J Pediatr. 2020;S0022–3476(20)30,747–2. doi:10.1016/j.jpeds.2020.06.045.
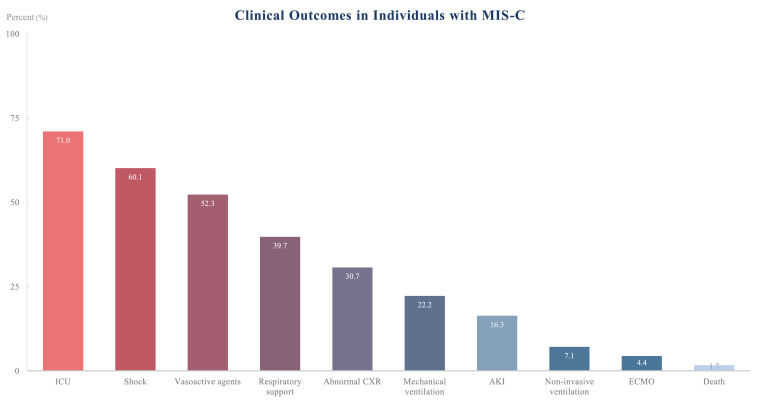
Fig. 2 depicts clinical outcomes. Four hundred sixty-nine (71·0%) children diagnosed with MIS-C were admitted into the ICU. Mechanical ventilation and extracorporeal membrane oxygenation were required in 147 (22·2%) and 29 (4·4%) patients, respectively. Acute kidney injury (AKI) occurred in 108 (16·3%) patients.
Fig. 2.
Overall clinical outcomes in individuals with MIS-C. All 662 patients were considered in these findings. ICU-intensive care unit, CXR-chest x-ray, AKI-acute kidney injury, ECMO-extracorporeal membrane oxygenation.
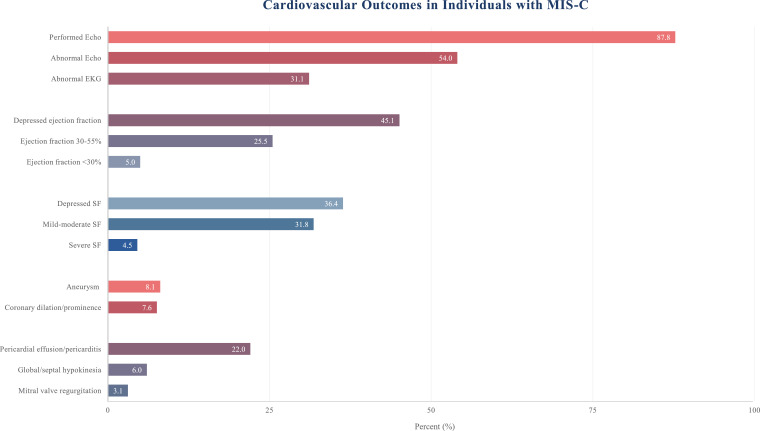
Cardiac outcomes are graphically presented in Fig. 3. Echocardiograms were performed in 581 of 662 patients (87·8%). Three hundred fourteen (54·0%) individuals had an abnormal echocardiogram. The most common abnormality was depressed left ventricular ejection fraction (n = 262, 45·1%). Aneurysms occurred in 47 patients (8·1%). Appendix 3 and 4 summate non-cardiac imaging findings.
Fig. 3.
Cardiovascular complications in individuals with MIS-C. Performed Echo (echocardiogram) is out of the whole population, n = 662. The denominator in electrocardiogram (EKG) was 89 and shortening fraction (SF) was 22, as this was the number of individuals reported to have those studies conducted or variables reported in their echocardiogram, respectively. The remaining cardiac findings included a sample of 581 pediatric cases of MIS-C (87•8% of 662 individuals).
Table 5 summarizes information regarding treatments administered. Intravenous immunoglobulin (IVIG) therapy was the most common medication (n = 506, 76·4%), followed by vasoactive agents (n = 347, 52·3%), and corticosteroids (n = 347, 52·3%).
Table 5.
Medications.
| Total n = 662 | N (%) |
|---|---|
| Intravenous immunoglobulin | 506 (76.4) |
| Vasoactive support | 347 (52.3) |
| Corticosteroids | 347 (52.3) |
| Antibiotics | 108 (16.3) |
| Anticoagulants | 172 (25.9) |
| Aspirin | 111 (16.8) |
| Interleukin-1ra inhibitor | 56 (8.5) |
| Interleukin-6 inhibitor | 40 (6.0) |
| Remdesivir | 6 (0.9) |
| Hydroxychloroquine | 5 (0.8) |
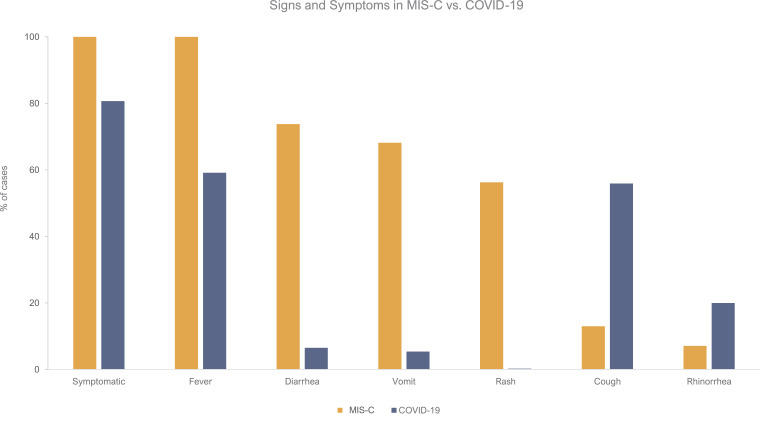
Differences in clinical signs and symptoms between MIS-C and COVID-19 can be visualized in Fig. 4. The overlap of presentation can be appreciated, but rash, vomiting, and diarrhea are more common in MIS-C. In contrary, COVID-19 has more upper respiratory symptoms (e.g., cough, rhinorrhea).
Fig. 4.
Comparison of the signs and symptoms of individuals with MIS-C versus COVID-19. Pediatric cases of MIS-C are depicted in gold, while children with COVID-19 are the solid blue bars. All 662 MIS-C patients were included in this analysis. The sample size for COVID-19 patients was 2445 patients for all the signs/symptoms, except for symptomatic (n = 2367).
Table 6 compares demographics, laboratory findings, treatments, and outcomes between MIS-C and COVID-19. Children with MIS-C had a higher a percentage of neutrophils and lower percentage of lymphocytes. The level of inflammation experienced in this new childhood disease surpasses COVID-19. For instance, ferritin is 18-fold greater in MIS-C (977 ± 55·8 ng/mL vs. 51·6 ± 13·2 ng/mL). Procalcitonin was also much higher in MIS-C compared to COVID-19 (30·5 ± 2·1 ng/mL vs. 0·25 ± 0·0).
TABLE 6.
Differences between children with MIS-C and COVID-19.
| MIS-C | COVID-19 | |
|---|---|---|
| GENERAL INFORMATION | ||
| Total number of patients | 662 | 7780 |
| Dates included | January 1, 2020 - July 25, 2020 | December 1, 2019 - May 14, 2020 |
| Number of studies | 39 | 131 |
| Data source | Multi-national | Multi-national |
| DEMOGRAPHICS | ||
| Age | 9.3 ± 0.5 | 8.9 ± 0.5 |
| mean ± SD | [n = 528] | [n = 4517] |
| Male gender% | 52.3 | 55.6 |
| [n = 662] | [n = 4640] | |
| Comorbidity% | 48.0 | 35.6 |
| [n = 558] | [n = 655] | |
| LABORATORY MARKERS | ||
| Complete blood count mean ± SD | ||
| Leukocytes (103/μL) | 13.2 ± 0.8 | 7.1 ± 0.3 |
| [n = 395] | [n = 811] | |
| Neutrophil (%) | 80.7 ± 7.8 | 44.4 ± 2.7 |
| [n = 276] | [n = 512] | |
| Lymphocyte (%) | 9.8 ± 0.8 | 39.9 ± 2.0 |
| [n = 306] | [n = 672] | |
| Hemoglobin (g/dL) | 10.2 ± 0.8 | 12.9 ± 0.9 |
| [n = 211] | [n = 211] | |
| Platelets (103/µL) | 215 ± 11.4 | 273 ± 8.5 |
| [n = 394] | [n = 115] | |
| Liver and renal function mean ± SD | ||
| Alanine transaminase (U/L) | 59.8 ± 4.1 | 19.5 ± 1.0 |
| [n = 226] | [n = 656] | |
| Aspartate aminotransferase (U/L) | 57.3 ± 5.3 | 29.4 ± 2.2 |
| [n = 145] | [n = 469] | |
| Creatinine (mg/dL) | 0.9 ± 0.1 | 0.3 ± 0.0 |
| [n = 158] | [n = 449] | |
| Inflammatory markers mean ± SD | ||
| C-reactive protein (mg/L) | 160 ± 7.0 | 9.4 ± 0.5 |
| [n = 439] | [n = 643] | |
| Ferritin (ng/mL) | 977 ± 55.8 | 51.6 ± 13.2 |
| [n = 303] | [n = 22] | |
| Procalcitonin (ng/mL) | 30.5 ± 2.1 | 0.25 ± 0.0 |
| [n = 312] | [n = 259] | |
| Lactate dehydrogenase (U/L) | 478 ± 45.4 | 277 ± 25.9 |
| [n = 300] | [n = 404] | |
| Creatine kinase (U/L) | 135 ± 46.0 | 197 ± 23.1 |
| [n = 49] | [n = 193] | |
| Interleukin-6 (pg/mL) | 184 ± 15.6 | 26.1 ± 3.7 |
| [n = 257] | [n = 92] | |
| Coagulation mean ± SD | ||
| D-dimer (mg/L) | 3.5 ± 0.4 | 0.7 ± 0.1 |
| [n = 349] | [n = 285] | |
| Fibrinogen (mg/dL) | 499 ± 58.3 | 224 ± 1.3 |
| [n = 267] | [n = 179] | |
| Erythrocyte sedimentation rate (mm/h) | 59.4 ± 9.1 | 14.1 ± 3.4 |
| [n = 191] | [n = 134] | |
| OUTCOME | ||
| Length of hospitalization mean ± SD | 7.9 ± 0.6 | 11.6 ± 0.3 |
| [n = 423] | [n = 652] | |
| Intensive care unit admission | 470 (71.0) | 116 (3.3) |
| In (%) | [n = 662] | [n = 3564] |
| Shock | 398 (60.1) | 19 (0.24) |
| In (%) | [n = 662] | [n = 7780] |
| Mechanical ventilation | 147 (22.2) | 42 (0.54) |
| In (%) | [n = 662] | [n = 7780] |
| Aneurysm | 47 (7.1) | |
| In (%) | [n = 662] | – |
| Death | 11 (1.7) | 7 (0.09) |
| In (%) | [n = 662] | [n = 7780] |
| TREATMENT | ||
| IVIG | 506 (76.4) | 19 (3.1) |
| In (%) | [n = 662] | [n = 614] |
| Corticosteroid | 347 (52.3) | 25 (4.1) |
| In (%) | [n = 662] | [n = 614] |
Data presented as mean ± standard deviation (SD). Brackets under data signify sample size for that variable. Refer to reference Hoang et al. [Ref. 24] for data source for COVID-19. COVID-19-coronavirus disease 2019, IVIG-intravenous immunoglobulin, MIS-C-multisystem inflammatory syndrome in children.
4. Discussion
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread throughout the world at an alarming rate [1]. Previous reports suggested that children infected are highly resilient to the disease and generally progress with a mild course [2,4]. However, as of late April 2020, a new and potentially life-threating childhood disease, referred to as MIS-C or PIMS-TS, emerged [6,8]. Our systematic review focused on describing the clinical presentation and short-term outcomes of this novel disease.
As described by Riphagen et al., hyperinflammatory shock is a common element in MIS-C [6]. These findings are substantiated in our review as 60·1% of children required vasopressor support and/or fluid resuscitation, in addition to 71·0% of children were admitted to the ICU. Although children were critically ill and had extraordinary inflammation, most responded to prompt administration of anti-inflammatory agents, namely IVIG and corticosteroids. A notable finding was that 11 of 662 individuals (1·7%) did not survive. The death rate in this review is comparable to that observed in adults with severe COVID-19 between the ages of 55–64 years (1% to 3%) [29]. While low, it is much higher than the 0.09% mortality rate observed in children with COVID-19 [24]. While writing this manuscript a new study was published involving 570 US patients with MIS-C [28]. The percentage of deaths for the cohort was comparable to the one observed in this review (n = 10, 1·8%).
Among the more concerning findings was that children could still develop MIS-C despite an asymptomatic course of coronavirus 2019 disease [20], [29], [30], [31]. The literature reports that MIS-C typically manifests 3–4 weeks after SARS-CoV-2 infection [21,28]. This may explain why many children had positive antibodies to SARS- CoV-2, but negative RT-PCR at the time of MIS-C evaluation [17,[32], [33], [34], [35], [36]]. An added matter of trepidation was that 52·0% of individuals (n = 290 of 558) who developed the inflammatory syndrome did not have any underlying medical conditions.
More research is needed to understand why some children may be more susceptible to developing MIS-C. For instance, our review noticed that there was a higher rate of MIS-C in the African American/Afro-Caribbean population. This may explain the disproportionate rate of deaths in African American US children between the ages of 5–17 [27,28,37]. A similar finding is observed in African American US adults, wherein they are more likely to be hospitalized compared to individuals of White race [38].
Another population that requires further investigation are children who are overweight or obese. In this review, children who were overweight or obese accounted for 50·8% (n = 136 of 268) of children with co-morbidities. Proposed mechanisms explaining why obesity may be a risk factor for COVID-19/MIS-C include: an accumulation of inflammatory cells in adipose tissue, fat tissue-associated cytokines are proinflammatory, impaired respiratory function, and adipose cells have more SARS-CoV-2 binding receptors [39], [40], [41], [42].
While children with COVID-19 present with upper respiratory symptoms, MIS-C is distinguishable by fever (100%), vomiting (68·2%), and abdominal pain/diarrhea (73·8%) [24] The abdominal pain in MIS-C can be so severe that in several cases patients were presumed to have appendicitis. For instance, patients in studies by Belhadjer et al. and Dasgupta received urgent abdominal surgery, but ultimately found the patients had mesenteric lymphadenitis [43,44]. In this review, further imaging of the abdomen (ultrasound, computed tomography, or magnetic resonance imaging) was performed in 14 of the 39 studies. Only one of those studies had a normal abdominal evaluation [45]. More common findings included ascites (n = 27 individuals), intestinal/colonic inflammation (n = 16 individuals), and mesenteric adenopathy (n = 15 individuals) [20,26,29,31,32,34,[44], [45], [46], [47], [48], [49], [50], [51]]. On one occasion, an 11-year old was diagnosed with pancreatitis secondary to SARS-CoV-2 [52]. This highlights the vast spectrum of gastrointestinal pathology in MIS-C.
Per the definition of MIS-C, neutrophilia and lymphocytopenia were frequent. The average percentage of neutrophils was 80·7%, a finding that echoed that reported in 99 children from New York (average = 82·3%) [26]. Given the standard deviation of 7·8% in this report, this implies that 95% of the 662 cases (2 standard deviations) had an elevated neutrophil percent. In contrast, Feldstein et al. reported neutrophilia in 67·7% [21] Inconsistency is most likely a reflection of how neutrophilia was defined: we used neutrophil percentage versus Feldstein et al. used an absolute number (>7700/μL). Of note, laboratory data from Feldstein et al. was not used for the calculations in this review as they described their data in a binary format (e.g. above/below a set number) [21]. Likewise, our lymphocyte percentage aligned with that seen in the study by Dufort et al. (average = 9·8% vs. 10·3%) [26] A meta-analysis of 4911 COVID-19 patients demonstrated that severe patients present with neutrophilia and lymphocytopenia [53]. The degree of separation between the two cell lines may coincide with the severity of inflammation. Evidence to support this assumption comes from COVID-19 studies that found an association between the percentage of neutrophils, lymphocytes, and the neutrophil to lymphocyte ratio to severity of disease [54,55].
Consistent with the diagnosis of MIS-C, multiple inflammatory markers were elevated. Examining the trends of some of these values may provide biologic insight to the disease or may serve as potential predictors of MIS-C outcomes. In particular, the following analytes were extremely elevated- procalcitonin (203-fold upper limit of normal, ULN), interleukin-6 (103-fold ULN), and troponin (49-fold ULN). We provide brief descriptions of each inflammatory marker and their potential use as future prognosticators.
Procalcitonin is a glycoprotein that is typically produced by the thyroid gland, but during severe systemic infections it can also be produced by other tissues [56]. In the past, procalcitonin was used to distinguish between bacterial and viral infection, where the thought was that higher levels correlated more with bacterial infections, while viral infections maintained a normal or slightly elevated procalcitonin level [56]. In detail, patients with bacterial septic shock classically had a procalcitonin level >0.10 ng/mL [57]. An average procalcitonin level of 30.5 ± 2.1 ng/mL puts into perspective the level of systemic inflammation seen in MIS-C cases.
In a recent study, Italian physicians evaluated the prognostic value of interleukin-6 (IL-6) for severe COVID-19 and in-hospital mortality [58]. They found that the mean level of IL-6 was greater in patients meeting their combined outcome (134·3 ± 19·5 pg/mL vs. 15·6 ± 14·8 pg/mL, p<0.01). Area under the receiver operator characteristic curve for IL-6 for in-hospital mortality was 0·90 (95% CI 0·81–0·95), using a cut-off of 25 pg/mL. Providing this context, the average IL-6 level in patients with MIS-C was 185 ± 15·6 pg/mL. Thus, it is logical why patients who did not respond to intravenous immunoglobulin and/or corticosteroids also received immunomodulatory agents known to target the IL-6 receptor.
The predominance of cardiac manifestations in children with multisystem inflammatory syndrome was striking. Many of the patients in this review had an initial echocardiogram that was normal and a few days later showed depressed ejection fraction or dilation/aneurysm of the coronary arteries. We found that the most common cardiac abnormality, on echocardiogram, was a depressed ejection fraction (45·0%). In line with these findings, a recent study revealed that adults who recently recovered from COVID-19 had ongoing cardiac involvement and myocardial inflammation [59]. Accordingly, children undergoing evaluation for MIS-C should have a baseline echocardiogram, electrocardiogram, and repeat imaging to follow cardiac function and artery changes. Close follow-up will be important as the long-term implications of MIS-C cardiac involvement are currently unknown.
Provided the abundance of angiotensin-converting enzyme 2 (ACE2) receptors in the heart, and the myocardial changes observed in cardiac imaging, it is no surprise troponin was among the most abnormal MIS-C markers [60]. Elevated troponin levels in individuals with COVID-19 are independent prognostic markers of poor outcome [61]. However, it is unknown if troponin levels correlate or can prognosticate specific cardiac abnormalities (e.g., myocardial dysfunction, coronary dilation, aneurysm) in patients with MIS-C. Future studies may provide more clarity regarding whether high troponin levels are a reflection of systemic inflammation, direct myocardial changes, vascular stress, or a combination of these injuries. On a positive note, many of the MIS-C patients had a down trending troponin and were able to recover their myocardial function by the time of discharge.
While MIS-C has overlapping features with KD and TSS, the inflammatory storm observed in MIS-C is much more intense. Distinguishing clinical characteristics found in MIS-C includes age, vomiting, diarrhea, and abdominal pain. Another important difference to highlight between KD and MIS-C is that approximately 5% of children with
Kawasaki's disease presented with cardiovascular collapse [62]. Conversely, 60·2% of children with MIS-C presented with shock. The rate of coronary artery aneurysms in 615 Japanese children with KD was 1·3% compared to 7·1% in this review of MIS-C [62]. To date, it is unclear if MIS-C patients are at more risk for aneurysms, or if there are key factors, such as genetic predisposition, playing a role in vascular changes.
Kawasaki disease been implicated in young children <5 years of age, whereas the mean age in MIS-C was 9·3 ± 0.4 years [14]. Although KD can affect young children of all ethnic backgrounds, there is a clear predilection towards Asian populations and young males [63]. In contrast, there was no obvious gender preference in MIS-C, yet individuals with African, African American, or Afro-Caribbean may have a higher risk.
The overlapping features between these syndromes suggests that they may share similar pathophysiology and likely explains why these patients respond to similar therapies. As described in this review, most children recovered with standard KD therapies, IVIG and glucocorticoids. Second IVIG administrations occurred in 20·9% (n = 39 of 186) of cases in the study by Feldstein et al., in 62·5% of patients in Pouletty et al. (n = 10 of 16), 23·8% in the study by Toubiana et al. (n = 5 of 21), and 12·8% in the study by Godfred-Cato et al. (n = 73 of 570) [21,28,32], [64]. Even with second dosing of IVIG, only a small number of individuals required/were given immunomodulating agents, such as IL-1 or IL-6 antagonists (8·5% and 6·0%, respectively). Future longitudinal studies will help delineate if there is a subset of patients with MIS-C that would benefit from such medications. For example, patients who may have therapeutic improvement from immunomodulators may include those with persistent elevation in IL-1 and/or IL-6 despite initial and/or redosing of initial therapies. Furthermore, subsequent studies should attempt to examine different features (e.g. demographic, biomarkers) that can predict which patients will need a second dose. A final note, in a study of 26,691 Japanese children with KD, the number of deaths was 4, giving a mortality rate of 0·01% which is much lower than the 1·7% observed in MIS-C [65].
Our review has several limitations. First, numerous case reports and case series were included and therefore the level of evidence is low. Along the same lines, the risk of bias varied among studies. Second, we converted data to mean and standard deviations, which may skew data with outliers. Although our PROSPERO protocol does not explicitly state that we were going to include letters to the editor, we did incorporate them. This protocol deviation was performed to offer a more complete portrayal of individuals with MIS-C and to optimize the number of patients in this review. However, we did not include letters to the editor that were perspectives or commentary pieces that did not describe pediatric cases of MIS-C. We were rigorous in our search but there is a possibility we may have missed studies. We removed patients and/or studies that did not describe patients presenting with fever, a cardinal characteristic of MIS-C [66], [67], [68]. In addition, to reduce duplication of information we removed studies in which individuals were or may have been included in larger or studies that were more comprehensive [6,[69], [70], [71], [72]]. Our review is mainly descriptive, and inferential statistical conclusions cannot be drawn from it. We are cognizant of the numerous listed limitations, but we believe the summative data is clear, concise, and communicates our intent to describe the clinical picture of patients with MIS-C. Most importantly, we hope this report is clinically useful and may impact patient care.
We reviewed and summarized the clinical presentation of a new childhood disease that is most likely linked to SARS-CoV-2 infection. MIS-C is a dangerous systemic infection characterized by extreme inflammation, fever, abdominal symptoms, conjunctivitis, and rash. Children will typically show signs/symptoms of MIS-C three to four weeks after COVID-19 infection and many will progress rapidly into shock and cardiorespiratory failure. Families should seek immediate medical care as children with this condition decompensate quickly and most children will need management in an intensive care unit. Overall, children will survive this hyperinflammatory condition with administration of IVIG, steroids, a multidisciplinary team of healthcare providers, and in some cases immunomodulatory agents. MIS-C is rare but the potential long-term sequelae from this disease are currently unknown.
Acknowledgments
Data sharing statement
The authors declare that the data collected was gathered from publicly available databases and is available upon reasonable request.
Declaration of Competing Interest
We declare no competing interests.
Funding
Funding sources: Parker B. Francis; Pilot grant 2R25-HL126140. Funding agencies had no role in the writing of the manuscript or the decision to submit.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100527.
Appendix. Supplementary materials
References
- 1.Johns Hopkins University and Medicine. Coronavirus resource center. Coronavirus.jhu.edu/map.html (accessed August 5, 2020).
- 2.Castagnoli R., Votto M., Licari A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020:1467. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101623. March 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu X., Zhang L., Du H. SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riphagen S, Gomez X, Gonzales-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic Lancet 2020; May 7. doi: 10.106/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed]
- 7.Royal College of Paediatrics and Child Health. Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19. www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19 (accessed August 5, 2020).
- 8.Verdoni L., Mazza A., Gerasoni A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)31103-X. May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Emergency preparedness and response: health alert network. Published May 14, 2020. emergency.cdc.gov/han/2020/han00432.asp (accessed August 5, 2020).
- 10.World Health Organization. Multisystem inflammatory syndrome in children and adolescents with COVID-19. Published May 15, 2020. www.who.int/publications-detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19(accessed August 5, 2020).
- 11.American Academy of Pediatrics. CDC details COVID-19-related inflammatory syndrome in children.www.aappublications.org/news/2020/05/14/covid19inflammatory051420(accessed August 6, 2020).
- 12.Centers for Disease Control and Prevention. Toxic shock syndrome 2011 case definition. wwwn.cdc.gov/nndss/conditions/toxic-shock-syndrome-other-than-streptococcal/case-definition/2011/(accessed August 6, 2020).
- 13.American Academy of Pediatrics. multisystem inflammatory syndrome in children (MIS-C) interim guidance. https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/multisystem-inflammatory-syndrome-in-children-mis-c-interim-guidance/(accessed August 6, 2020).
- 14.Correction to Diagnosis, treatment, and long-term management of kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2019;140(5):e181–e184. doi: 10.1161/CIR.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 15.Dietz S.M., van Stijn D., Burgner D. Dissecting Kawasaki disease: a state-of-the-art review. Eur J Pediatr. 2017;176(8):995–1009. doi: 10.1007/s00431-017-2937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb M., Long B., Koyfman A. The evaluation and management of toxic shock syndrome in the emergency department: a review of the literature. J Emerg Med. 2018;54(6):807–814. doi: 10.1016/j.jemermed.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 17.Whittaker E., Bamford A., Kenny J. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with sars-cov-2. JAMA. 2020 doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group PRISMA. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institute for Health Research. PROSPERO-International prospective register of systematic reviews. www.crd.york.ac.uk/prospero/.
- 20.Chiotos K., Bassiri H., Behrens E.M. multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: a case series. J Pediatric Infect Dis Soc. 2020;9(3):393–398. doi: 10.1093/jpids/piaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldstein L.R., Rose E.B., Horwitz S.M. multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021680. NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institutes of Health (2014). National Heart, Lung, and Blood Institute. Quality assessment tool for observational cohort and cross-sectional studies. Nhlbi.nih.gov/health-topics/study-quality-assessment-tools(accessed June 15, 2020).
- 23.Sackett D.L. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest. 1989;95:2S–4S. [PubMed] [Google Scholar]
- 24.Hoang A., Chorath K., Moreira A. COVID-19 in 7780 pediatric patients: a systematic review. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dufort E.M., Koumans E.H., Chow E.J. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021756. NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention Severe outcomes among patients with coronavirus disease 2019 (COVID-19) — United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2externalicon. (accessed August 8, 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godfred-Cato S, Bryant B, Leung J, et al. COVID-19-associated multisystem inflammatory syndrome in children-United States, March-July 2020. MMWR Morb Mortal Wkly Rep. ePub:7 August 2020. DOI: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed]
- 29.Hutchison L., Plichta A.M., Lerea Y., Madora M., Ushay H.M. Neuropsychiatric symptoms in an adolescent boy with multisystem inflammatory syndrome in children. Psychosomatics. 2020 doi: 10.1016/j.psym.2020.06.015. S0033-3182(20)30201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones V.G., Mills M., Suarez D. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10(6):537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 31.Waltuch T., Gill P., Zinns L.E. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department [published online ahead of print, 2020 May 23] Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.05.058. S0735-6757(20)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toubiana J., Poirault C., Corsia A. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020 doi: 10.1136/bmj.m2094. 369:m2094. Published 2020 Jun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riollano-Cruz M., Akkoyun E., Briceno-Brito E. multisystem inflammatory syndrome in children (mis-c) related to COVID-19: a New York City experience. J Med Virol. 2020 doi: 10.1002/jmv.26224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller J., Cantor A., Zachariah P., Ahn D., Martinez M., Margolis K. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children (MIS-C) that is related to COVID-19: a single center experience of 44 cases. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.079. doi: 10.1053/j.gastro.2020.05.079. [published online ahead of print, 2020 Jun 4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimaud M., Starck J., Levy M. Vol. 10. 2020. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children; p. 69. (Ann intensive care). Published 2020 Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capone C.A., Subramony A., Sweberg T. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory disease of childhood (MIS-C) associated with SARS-CoV-2 infection. J Pediatr. 2020 doi: 10.1016/j.jpeds.2020.06.044. S0022-3476(20)30746-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.CDC COVID Data tracker. Demographic trends of COVID-19 cases and deaths in the US reported to CDC. cdc.gov/covid-data-tracker/index.html#demographics(accessed August 8, 2020).
- 38.Killerby M.E., Link-Gelles R., Haight S.C. characteristics associated with hospitalization among patients with COVID-19 — Metropolitan Atlanta, Georgia, March–April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:790–794. doi: 10.15585/mmwr.mm6925e1externalicon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson M.R., Geleris J., Anderson D.R. Body mass index and risk for intubation or death in SARS-CoV-2 infection: a retrospective cohort study [published online ahead of print, 2020 Jul 29] Ann Intern Med. 2020:M20–3214. doi: 10.7326/M20-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrakis D., Margină D., Tsarouhas K. Obesity - a risk factor for increased COVID-19 prevalence, severity and lethality (Review) Mol Med Rep. 2020;22(1):9–19. doi: 10.3892/mmr.2020.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16(7):341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iannelli A., Favre G., Frey S. Obesity and COVID-19: ACE 2, the missing tile. Obes Surg. 2020:1–3. doi: 10.1007/s11695-020-04734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belhadjer Z., Méot M., Bajolle F. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 44.Dasgupta K., Finch S.E. A case of pediatric multisystem inflammatory syndrome temporally associated with COVID-19 in South Dakota. S D Med. 2020;73(6):246–251. [PubMed] [Google Scholar]
- 45.Rodriguez-Gonzalez M., Rodríguez-Campoy P., Sánchez-Códez M., Gutiérrez-Rosa I., Castellano-Martinez A., Rodríguez-Benítez A. New onset severe right ventricular failure associated with COVID-19 in a young infant without previous heart disease. Cardiol Young. 2020:1–4. doi: 10.1017/S1047951120001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dallan C., Romano F., Siebert J., Politi S., Lacroix L., Sahyoun C. Septic shock presentation in adolescents with COVID-19. Lancet Child Adolesc Health. 2020;4(7):e21–e23. doi: 10.1016/S2352-4642(20)30164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bahrami A., Vafapour M., Moazzami B., Rezaei N. Hyperinflammatory shock related to COVID-19 in a patient presenting with multisystem inflammatory syndrome in children: first case from Iran. J Paediatr Child Health. 2020 doi: 10.1111/jpc.15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Regev T., Antebi M., Eytan D. Pediatric inflammatory multisystem syndrome with central nervous system involvement and hypocomplementemia following SARS-COV-2 infection. Pediatr Infect Dis J. 2020;39(8):e206–e207. doi: 10.1097/INF.0000000000002804. [DOI] [PubMed] [Google Scholar]
- 49.Lee P.Y., Day-Lewis M., Henderson L.A. Distinct clinical and immunological features of SARS-COV-2-induced multisystem inflammatory syndrome in children. J Clin Invest. 2020 doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Licciardi F., Pruccoli G., Denina M. SARS-CoV-2-induced Kawasaki-like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics. 2020;146(2) doi: 10.1542/peds.2020-1711. [DOI] [PubMed] [Google Scholar]
- 51.Klocperk A., Parackova Z., Dissou J. Case report: systemic inflammatory response and fast recovery in a pediatric patient with COVID-19. Front Immunol. 2020;11:1665. doi: 10.3389/fimmu.2020.01665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blondiaux E., Parisot P., Redheuil A. Cardiac MRI of Children with Multisystem Inflammatory Syndrome (MIS-C) Associated with COVID-19: case Series [published online ahead of print, 2020 Jun 9] Radiology. 2020 doi: 10.1148/radiol.2020202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng X., Li S., Sun Q. Immune-inflammatory parameters in COVID-19 cases: a systematic review and meta-analysis. Front Med (Lausanne) 2020;7:301. doi: 10.3389/fmed.2020.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y., Du X., Chen J. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81(1):e6–e12. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu Z., Cai T., Fan L. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332–339. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reinhart K., Karzai W., Meisner M. Procalcitonin as a marker of the systemic inflammatory response to infection. Intens Care Med. 2000;26(9):1193–1200. doi: 10.1007/s001340000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harbarth S., Holeckova K., Froidevaux C. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001;164(3):396–402. doi: 10.1164/ajrccm.164.3.2009052. [DOI] [PubMed] [Google Scholar]
- 58.Grifoni E., Valoriani A., Cei F. Interleukin-6 as prognosticator in patients with COVID-19. J Infect. 2020;81(3):452–482. doi: 10.1016/j.jinf.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puntmann V.O., Carerj M.L., Wieters I. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu H., Zhong L., Deng J. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen Y., Corre F., Honsel V. A nomogram to predict the risk of unfavourable outcome in COVID-19: a retrospective cohort of 279 hospitalized patients in Paris area. Ann Med. 2020:1–23. doi: 10.1080/07853890.2020.1803499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kil H.R., Yu J.W., Lee S.C., Rhim J.W., Lee K.Y. Changes in clinical and laboratory features of Kawasaki disease noted over time in Daejeon, Korea. Pediatr Rheumatol Online J. 2017;15(1):60. doi: 10.1186/s12969-017-0192-y. Published 2017 Aug 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burns J.C. The riddle of Kawasaki disease. N Engl J Med. 2007;356(7):659–661. doi: 10.1056/NEJMp068268. [DOI] [PubMed] [Google Scholar]
- 64.Pouletty M., Borocco C., Ouldali N. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79(8):999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Makino N., Nakamura Y., Yashiro M. Descriptive epidemiology of Kawasaki disease in Japan, 2011-2012: from the results of the 22nd nationwide survey. J Epidemiol. 2015;25(3):239–245. doi: 10.2188/jea.JE20140089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dolinger M.T., Person H., Smith R. Pediatric crohn disease and multisystem inflammatory syndrome in children (MIS-C) and COVID-19 treated with infliximab. J Pediatr Gastroenterol Nutr. 2020;71(2):153–155. doi: 10.1097/MPG.0000000000002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hameed S., Elbaaly H., Reid C.E.L. Spectrum of imaging findings on chest radiographs, US, CT, and MRI images in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. Radiology. 2020 doi: 10.1148/radiol.2020202543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cook J., Harman K., Zoica B., Verma A., D'Silva P., Gupta A. Horizontal transmission of severe acute respiratory syndrome coronavirus 2 to a premature infant: multiple organ injury and association with markers of inflammation. Lancet Child Adolesc Health. 2020;4(7):548–551. doi: 10.1016/S2352-4642(20)30166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramcharan T., Nolan O., Lai C.Y. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol. 2020:1–11. doi: 10.1007/s00246-020-02391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Belot A., Antona D., Renolleau S. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25(22) doi: 10.2807/1560-7917.ES.2020.25.22.2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blondiaux E., Parisot P., Redheuil A. Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19: case series. Radiology. 2020 doi: 10.1148/radiol.2020202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaushik S., Aydin S.I., Derespina K.R. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection: a multi-institutional study from New York City. J Pediatr. 2020 doi: 10.1016/j.jpeds.2020.06.045. S0022-3476(20)30747-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.