Abstract
Purpose of the review:
Cognitive impairment is common in kidney transplant recipients and affects quality of life, graft survival, morbidity, and mortality. In this review article we discuss the epidemiology, diagnosis, pathophysiology and future directions for cognitive impairment in kidney transplantation. We describe the potential role of pre-transplant cognition, immunosuppression and peri-transplant factors in post -transplant cognitive impairment.
Recent Findings:
A majority of patients with kidney transplant have cognitive impairment. Cognitive impairment affects both pre-transplant evaluation and post-transplant outcomes. Failure to identify patients with cognitive impairment can withhold appropriate care and timely intervention.
Summary:
Cognitive impairment is common in kidney transplant and affects outcomes. Studies addressing modifiable risk factors and possible interventions to slow cognitive decline in patients with kidney disease are needed.
Keywords: Cognition, Kidney transplant, ESRD
Introduction
Cognitive impairment is common in patients with kidney disease and affects kidney transplant (KT) eligibility and post- KT outcomes. Prior to KT cognitive impairment is associated with a lower likelihood of being listed for KT, and if listed, delays the time to listing.1 After KT cognitive impairment can affect adherence,2 quality of life, health care costs and graft survival.3 The etiology of cognitive impairment in KT recipients is multifactorial. Patients with end stage renal disease (ESRD) have a high prevalence of cognitive impairment that improves post-transplant, but residual deficits persist. These combined with the effects of immunosuppression, low physical activity,4 altered gut microbiome, post-transplant delirium,5 and traditional risk factors for dementia such as hypertension, hypercholesterolemia, metabolic syndrome, diabetes and hyperuricemia likely contribute to cognitive impairment in KT.
Given the significant clinical impact of cognitive impairment in KT, diagnosing and managing cognitive impairment is critical. In this review, we will discuss epidemiology, pathophysiology and future directions for diagnosis and management of cognitive impairment in KT.
Epidemiology
Up to 87% of patients on hemodialysis6 and 75% on peritoneal dialysis7 have cognitive impairment. Of the patients with ESRD, the ones who receive a KT tend to be healthier and have better cognition when compared with the average ESRD population.1,8 We and others have shown that KT improves cognition.9-14 However, prevalence of cognitive impairment post-KT is high and ranges from 22.3% to 58%,15,16 with the wide range reflective of differences among methods used to assess cognition. The prevalence of dementia is approximately 17% among KT recipients aged above 75 years compared to 7.5% in the general population.3 Similar to the general population,17 cognitive impairment in KT recipients increases with age.16 However, unlike the general population, cognitive impatient in KT recipients can affect younger patients.16 Transplant recipients have one functional kidney and by definition a majority of them have chronic kidney disease (CKD). There is, however, a difference in the relationship of renal function with cognition in patients with CKD or KT. In CKD, each 10 ml/min/1.73 m2 decrease in estimated glomerular filtration rate (eGFR) is associated with an 11% increase in the prevalence of cognitive impairment.18 Au contraire, in post-KT patients, cognitive impairment is not associated with eGFR and can affect patients with a successful KT with serum creatinine in the normal range.16
Spectrum of Cognitive impairment: difference between mild cognitive impairment and dementia
Dementia is defined by cognitive impairment that has declined from the past and results in difficulties with activities of daily living interfering with an individual’s independence.19 Moderate to severe dementia is generally recognized clinically and patients are able to receive medical attention. However, mild cognitive impairment (MCI) is often missed as it is not clinically evident. MCI is a term used for cognitive decline that is on the spectrum between normal aging and early dementia.20 Persons with MCI are generally able to function independently. Cognitive impairment tends to be subtle and can easily be missed unless identified in objective neuropsychological tests. Timely diagnosis of MCI is important as it is a precursor to dementia and interventions to slow cognitive decline may be more effective at this stage than after frank dementia has developed. The majority of KT recipients fall under the category of MCI where objective testing is needed for diagnosis and follow up.
Assessment of cognition
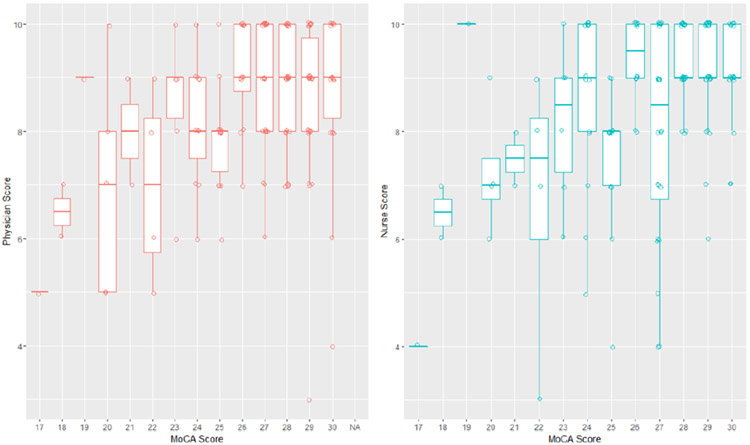
Although KT recipients have frequent interactions with their transplant team, subjective impressions of cognition during a clinic visit may not be adequate to accurately assess full cognitive capacity. We noted significant discrepancies between perceived cognition (both by nurses and physicians) and measured cognition (Figure 1).21 Moreover, frequently, physicians and nurses disagreed in their perception of patients’ cognition. Hence, objective assessment of cognition is needed. There are several tests with established normative values available for both screening and confirmation of cognitive impairment. Available resources (including the ability of the clinic staff to administer the test accurately, the time needed to administer the test and potential management strategies if a patient is diagnosed with cognitive impairment) need to be considered before routine testing of cognition in KT recipients is implemented.
Figure 1:
Difference in perceived and measured cognition in kidney transplant recipients. Used with permission from Karger.
Screening tests:
There are several neuropsychological tests available to screen for cognitive impairment. The majority of these tests were developed for assessing cognitive decline with aging and Alzheimer’s disease (AD), the most common type of dementia. Some of these may not be well suited for measurement of cognition in kidney disease where the etiology of cognitive impairment is thought to be largely vascular in nature. The mini mental scale exam (MMSE) is one of the most common screening tests used worldwide. It is quick and easy-to-use, comprises of 30 questions and takes 5-10 minutes to administer. It assesses domains of orientation, attention, calculation, recall, language and motor skills.22 Cutoff scores for MCI and dementia are adjusted based on education and age.23 The Montreal Cognitive Assessment (MoCA)24 another commonly used test, is more sensitive in measuring executive function, a domain commonly affected in vascular dementia. Therefore, the MoCA is rapidly gaining popularity in the measurement of cognition in kidney disease.25,26 The MoCA is a one-page test with 30 questions and takes about 10 minutes to be administered. It assesses multiple domains of cognition: visuospatial/executive, naming, memory, attention, language, abstraction, delayed recall and orientation.24 The final score is adjusted for the level of education by adding one point to the score for ≤12 years of education. To ensure consistency and proper implementation with the increasing use of the test, there is now a mandatory training and certification required for persons who administer the MoCA. The training may be accessed via their website, https://www.mocatest.org/. The MoCA has a higher sensitivity and specificity compared to the MMSE, however, the cut off score of 26 may have a high number of false positive results.27 Some have, therefore, proposed a lower cut off score of 24 to decrease the number of false positives.28 Another screening test commonly used in KT is the mini-cog test29 which takes roughly three minutes to administer. While it has the advantage of being more efficient, there is some concern that the mini-cog test might not perform as well as others, such as the MMSE.30 The Modified Mini-Mental State (or 3 MS) test extends the scope of the MMSE with the addition of testing long-term memory, abstract thinking, category fluency and delayed recall. The 3 MS has improved sensitivity and specificity in screening for MCI compared to the MMSE.31 Most of the screening tests are relatively short and could be administered in the setting of a busy transplant clinic. We have successfully used the MoCA in our transplant clinic for the past six years after training medical assistants and coordinators on how to administer the test.
Confirmation of cognitive impairment:
If a screening test is positive for cognitive impairment, patients should undergo a thorough evaluation with additional neuropsychological tests.20 Complete neuropsychological testing can take several hours. In the case of KT recipients, this assessment should preferably be done by a neuropsychologist with expertise in vascular dementia so that appropriate neuropsychological tests are selected for confirmation of the diagnosis of cognitive impairment, quantification of the degree of cognitive impairment and determination of domain-specific impairment.
Pathophysiology
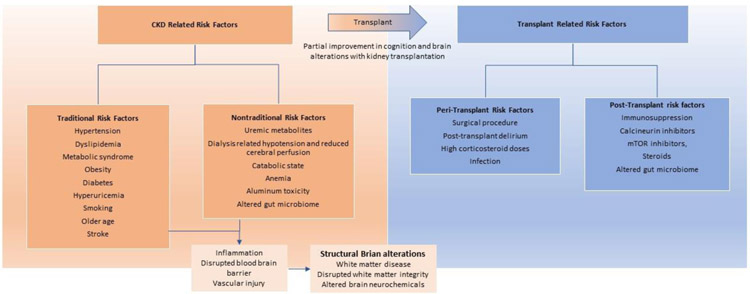
Understanding the etiology of cognitive impairment in KT recipients is important and may enable development of strategies to prevent cognitive decline, and maintain, or even improve current level of cognition. To understand the pathophysiology of cognitive impairment in KT recipients, it is important to understand the etiology of cognitive impairment prior to KT, modification of pre-KT risk factors with KT, and additional peri-operative and post-transplant factors influencing cognition (Figure 2).
Figure 2:
Hypothesized pathophysiology of cognitive impairment in kidney transplantation
Etiology of cognitive impairment in CKD
Vascular risk factors
The association between uremia and cognitive impairment has been well known for several years.32,33 CKD, even without clinical uremia, is associated with increased risk of cognitive impairment.18,34 The brain and the kidney share common vascular characteristics. Both organs have low vascular resistance and receive high blood flow and have similar vasoregulatory systems and hemodynamic changes in the vascular network. With these similarities, small vessel disease in one organ could be a sign of disease in the other.35 CKD and dementia share common risk factors such as hypertension, dyslipidemia, metabolic syndrome, obesity, diabetes, smoking, high homocysteine levels. All of these are risk factors for chronic inflammation,36,37 oxidative stress,38,39 endothelial dysfunction, atherosclerotic vascular disease and increased sympathetic activity leading to vascular endothelial injury and disruption of the blood brain barrier, exacerbating the effects on cognition. Protein-bound metabolites and middle molecules such as indoxyl sulfate and p-cresyl sulfate are not easily dialyzable, have high plasma concentrations in ESRD and are associated with cognitive impairment.40 There is also an increased production of these substances due to altered gut microbiome.41 Other biomarkers such as homocysteine and uric acid have also been associated with small-vessel disease, progression of white matter lesions42 and cognitive decline.43,44
Structural brain changes in CKD
CKD is associated with increased white matter hyperintensities, cerebral atrophy, microbleeds, microinfarctions, altered brain metabolites, cerebral blood flow, and white matter integrity suggesting vascular causes to play an important role in cognitive impairment.45,46 We have an ongoing longitudinal study assessing cognition and brain alterations before and after KT (ClinicalTrials.gov identifier NCT01883349).
White matter changes:
Brain imaging studies showed that ESRD is associated with increased white matter disease, clinical and subclinical strokes, and cerebral atrophy.47 A novel imaging technique, diffusion tensor imaging (DTI), evaluates structural integrity of white matter by mapping the diffusion of water molecules restricted to specific tissue. In organized tissues such as the white matter in the brain where water molecules diffuse more freely along an axonal fiber tract than across it, DTI can detect subtle but functionally significant ultrastructural abnormalities not visible during traditional MRI imaging. Altered DTI metrics, a lower fractional anisotropy (FA) and a higher mean diffusivity (MD) are seen in ESRD48 and are associated with cognitive impairment.49
Alterations in Cerebral Blood Flow:
ESRD is associated with an increase in cerebral blood flow.50 Inflammation, vascular disease, and disruption of cerebral autoregulation may play a role. Cerebral blood flow decreases after KT.51 This may be considered a normalization of blood flow after KT, but for unclear reasons, blood flow decreases lower than the cerebral blood flow in normal persons without kidney disease.52 Calcineurin inhibitors (CNIs), which are inherently vasoconstrictive and used commonly for maintenance immunosuppression in KT may play a role. Further research is needed to understand if the vasoconstrictor effect of CNIs is responsible for the decrease in cerebral blood flow after KT and the consequences of this decrease in blood flow.
Alterations in Brain Metabolites:
ESRD is associated with alterations in brain metabolites, which are associated with cognitive impairment in other disease states. Magnetic resonance spectroscopy (MRS) allows evaluation of cerebral neurochemicals providing in vivo information on neurons, energy and metabolism in the brain. Choline/creatine and myo-inositol/creatine ratios are increased in ESRD,53 and as major brain osmolytes may reflect changes in osmotic pressure. Myo-inositol is also a glial marker and may reflect increased gliosis. Both choline/creatine and myo-inositol/creatine ratios decrease after KT. 51 Alterations in N-Acetylaspartate/ creatine ratios remain controversial in ESRD.54
Perioperative factors
Post-transplant delirium is associated with subsequent diagnosis dementia.55 Whether delirium causes changes in the brain or is a biomarker of decreased brain reserve making one susceptible to future dementia is unclear.56 Whether exposure to a surgical procedure and general anesthesia, and post-operative infections increases the risk of cognitive impairment remains controversial.57-59 Changes in the gut microbiome with KT may also play a role in cognitive changes- further research is needed in this area.60
Role of immunosuppression
Immunosuppression used in KT can affect cognition. For example, CNIs have a known side effect of neurotoxicity. Occipital white matter appears to be uniquely susceptible to the CNI neurotoxicity and cases of posterior reversible leukoencephalopathy syndrome have been reported, presumably from injury to cerebral vasculature through hypoperfusion or ischemia.61,62 Commonly used CNIs, tacrolimus and cyclosporine, do not readily cross the blood brain barrier, but in the presence of kidney disease and hypertension this blood brain barrier can be disrupted.63 CNIs in the brain inhibit calcineurin resulting in altered neurotransmission, calcium homeostasis and gene expression, thereby, impairing response to ischemic injury and neuronal plasticity and affecting cognition. Intracerebral tacrolimus concentrations do not correlate well with blood concentrations and show high inter-individual variability but are likely dependent on peak levels. In animal models, comparing continuous vs intermittent tacrolimus dosing, intracerebral levels were lower with continuous dosing despite higher area under the curve.64 These data might favor usage of extended release preparations of tacrolimus. Trials to test effects of immediate release vs extended release tacrolimus on cognition are in progress. As alluded to earlier, CNI’s are also inherent vasoconstrictors,65 which may also contribute to some of observed neurological side effects. Cerebral vasoconstriction also has the potential to alter the cerebrovascular kinetics in response to exercise. Given the brain’s lack of oxygen stores and intolerance to anoxia,66 increase in cerebral blood flow and oxygen delivery is necessary to match the brain’s metabolic requirements. Inability to increase cerebral blood flow may impact long term cerebrovascular outcomes. Although CNIs seem to impact cognition, it remains unclear whether the neurotoxicity of CNIs is due to vasoconstriction vs other direct drug effects.
Apart from CNI’s, other immunosuppressive agents can also affect cognition by affecting neuronal function with alteration of immune function by inhibiting interleukin-2 (IL-2). IL-2 is essential for neurogenesis and cognition67 and mediates innervation of dopamine and acetylcholine68 involved in executive function tasks69 CNI’s and mammalian target of rapamycin (mTOR) inhibitors, commonly used post-KT, affect the production and action of IL-2.70,71 High doses of corticosteroids commonly used for induction and treatment of rejection, are associated with cognitive impairment, as well as psychotic symptoms.72 Prolonged hypercortisolemia is associated with cognitive impairment and decreased hippocampal volume on brain imaging.73 With recent availability of new immunosuppressive agents in KT, determining the effects of specific immunosuppressive agents in KT on cognition has important clinical implications.
Improvement in cognition after KT
Longitudinal studies indicate improvement in cognition after KT.9-14 This reversibility in cognition is not seen with dialysis.74 A recent meta-analysis evaluating cognitive function in KT included four longitudinal studies that evaluated pre- to post-KT cognition and showed moderate to large improvements in global cognition and domains of information and motor speed, spatial reasoning, verbal memory and visual memory post KT.75 In addition, scores in these domains were better when compared to patients with ESRD. Depression also improves with KT76 and likely improves performance on the neuropsychological tests. However, KT recipients performed below healthy controls without kidney disease in domains of executive function, verbal fluency and language. Some domains such as attention, executive function, verbal fluency and language did not improve after KT. This may reflect irreversible brain alterations or impact of other factors described above. Other smaller studies have indicated improvement in memory and executive function.13 This improvement in cognition with KT may be diminished by the neurotoxicity associated with calcineurin inhibitors.77 However, the improvement in cognition post-KT appears to be durable for one78 year or even two10 years after KT.10,78 That being said, there are only six longitudinal studies assessing pre- to- post KT cognition.9,10,12-14,78 Most have a small sample size, while some have short-term follow-up or limited neurophysiological testing. Table 1 summarizes all the published longitudinal studies assessing cognition pre- to post- KT.
Table 1:
Longitudinal studies assessing cognitive function in kidney transplantation
| Study | Number tested after KT |
Neuropsychological tests performed (domain of cognition tested) |
Time after KT |
Main findings | Limitations |
|---|---|---|---|---|---|
| Kramer, 1996 | 15 | MMSE (global cognition) TMT-A (attention, planning, visual scanning, motor speed) |
14 months | Trend towards improvement in TMT. No significant change in cognition. |
Small sample size and lack of more sensitive tests |
| Griva, 2006 | 28 | TMT-A and B (attention, planning, visual scanning, motor speed) Symbol Digit Modalities Test (visual attention, coordination) RAVLT (total learning, delayed recall, recognition) Grooved Pegboard (fine motor coordination, manual dexterity) Benton Visual Retention Test (visual memory, vasoconstrictive abilities) |
6 months | Improvement in RAVLT (p < 0.001) |
Small sample size with limited power to detect changes in multiple tests |
| Harciarek, 2009 and 2011 | 27 | MMSE (global cognition) RAVLT (total learning, delayed recall, recognition) BVMT-R (memory and recall) Digit span (attention, working memory) Tapping (motor abilities) TMT-A and B (attention, planning, visual scanning, psychomotor speed) Digit Symbol (psychomotor speed, executive function) Similarities (abstract reasoning) Verbal fluency (verbal letter, category) Vocabulary (semantic knowledge) |
34 days (range 12-57), 1 year | Psychomotor speed improved by 20.6%, motor abilities by 9.3%, abstract reasoning by 12.4% and memory by 27.40% | Small sample size with limited power to detect changes in multiple tests |
| Radic, 2011 | 21 | Complex Reactiometer Drenovac (spatial reasoning, memory, and executive function) | 20.5 months | Improvement in processing speed, attention, memory, and executive function | Small sample size |
| Gupta, 2016 | 11 | MMSE (global cognition) Digit Span (attention, working memory) Logical Memory I and II (memory and recall) Category Fluency (Verbal Fluency) TMT-A and B (attention, planning, visual scanning, psychomotor speed) Digit Symbol (psychomotor speed, executive function) Block Design (Special visualization and motor skill) Stroop (Attention and processing speed) Free and Cued Recall (Visual perception and memory) |
3 months, 12 months | Improvement in logical memory I (p = 0.004), logical memory II (p = 0.003) and digit span (p = 0.007) | Small sample size |
| Chu, 2019 | 665 | 3MS (global cognition) | 18 months (range 1-48) | Improvement in 3 MS score |
Specific domains of cognition not assessed |
Abbreviations: BVMT-R; Brief Visual Memory Test-Revised, KT; kidney transplant, MMSE; Mini-Mental State Examination, RAVLT; Rey Auditory Verbal Learning Test, RCF; Rey-Osterrieth Complex Figure, TMT; Trail Making Test.
Improvement in cognition post-KT may reflect improved clearance of middle molecules and inflammatory mediators in ESRD such as IL-1β, IL-6, tumor necrosis factor (TNF) that have limited clearance with dialysis.79 In addition, structural brain alterations in ESRD also seem to improve post-KT. We were the first group to show that FA increases and MD decreases after KT.13 Notably, these changes were more prominent in white matter tracts associated with memory and executive function, the two domains of cognition that improve after KT.13 These results are encouraging as they indicate at least partial reversibility in cognition and brain alterations seen in CKD. Case reports indicate that some white matter lesions may be reversible, however, it remains unknown whether the global white matter lesions associated with CKD improve after KT. Functional MRI studies also show recovery in select resting-state networks after KT.80,81 Further studies are needed to understand if the decrease in cerebral blood flow below normal after transplant is due to improvement in cerebral autoregulation or due to cerebral vasoconstriction due to calcineurin inhibitors. Longitudinal studies assessing MRS and cerebral blood flow pre- to post- KT are ongoing.
Future directions
Assessment of cognition in KT is important. Studies are needed to confirm the etiology of cognitive impairment in both ESRD and KT and to mitigate the risk for cognitive decline. The effect of modifiable risk factors such as post-KT weight gain, metabolic syndrome and new onset diabetes, hypertension, low physical activity and immunosuppression on long-term cognition needs to be better elucidated. Transplantation is a unique life changing event which reverses cognitive impairment and brain alterations. However, since some brain alterations during CKD and ESRD may be irreversible even after KT, efforts for preserving pre-transplant cognition should be maximized. Additionally, the effect of dialysis and other co-morbidities on pre-KT cognitive decline need to be further studied.
Conclusion:
Cognitive impairment in KT remains a significant but underappreciated clinical problem affecting a majority of our patients with CKD or ESRD. Brain imaging and neuropsychological tests point to a vascular etiology of cognitive impairment, exacerbated with some KT specific issues such as immunosuppression. The etiology of cognitive impairment in KT is unique and further studies are needed in the area as extrapolated results from other causes of cognitive impairment may not hold true for this population.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
Compliance with Ethical Standards Conflict of Interest: Dr. Gupta reports grants from Novartis, Veloxsis and NIH and consultancy from Novartis during the conduct of the study. None of these affect the work submitted by the authors.
References:
- 1.**.Gupta A, Montgomery RN, Bedros V, et al. Subclinical Cognitive Impairment and Listing for Kidney Transplantation. Clinical journal of the American Society of Nephrology: CJASN. 2019;14(4):567–575.This study highlights the importance of subclinical cognitive impairment in the selection of patients for kidney transplantation. Patients with cognitive impairment have a lower liklihood of being listed for kidney transplant and take longer to get listed.
- 2.Patzer RE, Serper M, Reese PP, et al. Medication understanding, non-adherence, and clinical outcomes among adult kidney transplant recipients. Clinical transplantation. 2016;30(10):1294–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.*.McAdams-DeMarco MA, Bae S, Chu N, et al. Dementia and Alzheimer's Disease among Older Kidney Transplant Recipients. J Am Soc Nephrol. 2017;28(5):1575–1583.This study reflects the high burden of dementia in older patients with kidney transplant.
- 4.van den Ham EC, Kooman JP, Schols AM, et al. Similarities in skeletal muscle strength and exercise capacity between renal transplant and hemodialysis patients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(8):1957–1965. [DOI] [PubMed] [Google Scholar]
- 5.Haugen CE, Mountford A, Warsame F, et al. Incidence, Risk Factors, and Sequelae of Post-kidney Transplant Delirium. J Am Soc Nephrol. 2018;29(6):1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67(2):216–223. [DOI] [PubMed] [Google Scholar]
- 7.Kalirao P, Pederson S, Foley RN, et al. Cognitive impairment in peritoneal dialysis patients. Am J Kidney Dis. 2011;57(4):612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haugen CE, Chu NM, Ying H, et al. Frailty and Access to Kidney Transplantation. Clinical journal of the American Society of Nephrology : CJASN. 2019;14(4):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griva K, Thompson D, Jayasena D, Davenport A, Harrison M, Newman SP. Cognitive functioning pre- to post-kidney transplantation--a prospective study. Nephrol Dial Transplant. 2006;21(11):3275–3282. [DOI] [PubMed] [Google Scholar]
- 10.Radic J, Ljutic D, Radic M, Kovacic V, Dodig-Curkovic K, Sain M. Kidney transplantation improves cognitive and psychomotor functions in adult hemodialysis patients. Am J Nephrol. 2011;34(5):399–406. [DOI] [PubMed] [Google Scholar]
- 11.Ozcan H, Yucel A, Avsar UZ, et al. Kidney Transplantation Is Superior to Hemodialysis and Peritoneal Dialysis in Terms of Cognitive Function, Anxiety, and Depression Symptoms in Chronic Kidney Disease. Transplant Proc. 2015;47(5):1348–1351. [DOI] [PubMed] [Google Scholar]
- 12.Kramer L, Madl C, Stockenhuber F, et al. Beneficial effect of renal transplantation on cognitive brain function. Kidney Int. 1996;49(3):833–838. [DOI] [PubMed] [Google Scholar]
- 13.*.Gupta A, Lepping RJ, Yu AS, et al. Cognitive Function and White Matter Changes Associated with Renal Transplantation. Am J Nephrol. 2016;43(1):50–57.In this study there was an improvement in cognition and changes in white matter integrity after kidney transplantation. Furthermore, the tracts with improvement in diffusion tensor imaging metrics were associated with domains of cognition that improved after transplant.
- 14.**.Chu NM, Gross AL, Shaffer AA, et al. Frailty and Changes in Cognitive Function after Kidney Transplantation. Journal of the American Society of Nephrology. 2019;30(2):336.This study shows an association between change in cognitive impairment and frailty pre- to- post- kidney transplantation.
- 15.Nohre M, Bauer-Hohmann M, Klewitz F, et al. Prevalence and Correlates of Cognitive Impairment in Kidney Transplant Patients Using the DemTect-Results of a KTx360 Substudy. Front Psychiatry. 2019;10:791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.**.Gupta A, Mahnken JD, Johnson DK, et al. Prevalence and correlates of cognitive impairment in kidney transplant recipients. BMC nephrology. 2017;18(1):158.This study highlights the high prevalence of cognitive impairmnt in kidney transplant recipients.
- 17.Unverzagt FW, Gao S, Baiyewu O, et al. Prevalence of cognitive impairment. Data from the Indianapolis Study of Health and Aging. 2001;57(9):1655–1662. [DOI] [PubMed] [Google Scholar]
- 18.Kurella Tamura M, Wadley V, Yaffe K, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis. 2008;52(2):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knopman DS, Petersen RC. Mild cognitive impairment and mild dementia: a clinical perspective. Mayo Clin Proc. 2014;89(10): 1452–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. Jama. 2014;312(23):2551–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.*.Gupta A, Thomas TS, Klein JA, et al. Discrepancies between Perceived and Measured Cognition in Kidney Transplant Recipients: Implications for Clinical Management. Nephron. 2018;138(1):22–28.This study indicates that an objective assessment of cognition is required and perceived assessment of a patient’s cogniton is often inaccurate.
- 22.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 23.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. Jama. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
- 24.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 25.Ciesielska N, Sokolowski R, Mazur E, Podhorecka M, Polak-Szabela A, Kedziora-Kornatowska K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr Pol. 2016;50(5):1039–1052. [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Cho A, Min YK, Lee YK, Jung S. Comparison of the montreal cognitive assessment and the mini-mental state examination as screening tests in hemodialysis patients without symptoms. Ren Fail. 2018;40(1):323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solias A, Skapinakis P, Degleris N, Pantoleon M, Katirtzoglou E, Politis A. [Mini Mental State Examination (MMSE): determination of cutoff scores according to age and educational level]. Psychiatriki. 2014;25(4):245–256. [PubMed] [Google Scholar]
- 28.Creavin ST, Wisniewski S, Noel-Storr AH, et al. Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst Rev. 2016(1):Cd011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michieletto F, Binkin N, Saugo M, Boorson S, Scanlan J. [Use of the Mini-Cog test as a screening method for dementia in the Italian population: the Argento Study results]. Ig Sanita Pubbl. 2006;62(2):159–172. [PubMed] [Google Scholar]
- 30.Carnero-Pardo C, Cruz-Orduna I, Espejo-Martinez B, Martos-Aparicio C, Lopez-Alcalde S, Olazaran J. Utility of the mini-cog for detection of cognitive impairment in primary care: data from two Spanish studies. Int J Alzheimers Dis. 2013;2013:285462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bland RC, Newman SC. Mild dementia or cognitive impairment: the Modified Mini-Mental State examination (3MS) as a screen for dementia. Can J Psychiatry. 2001;46(6):506–510. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe K, Watanabe T, Nakayama M. Cerebro-renal interactions: impact of uremic toxins on cognitive function. Neurotoxicology. 2014;44:184–193. [DOI] [PubMed] [Google Scholar]
- 33.Seifter JL, Samuels MA. Uremic encephalopathy and other brain disorders associated with renal failure. Semin Neurol. 2011;31(2):139–143. [DOI] [PubMed] [Google Scholar]
- 34.Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52(11):1863–1869. [DOI] [PubMed] [Google Scholar]
- 35.Ito S, Nagasawa T, Abe M, Mori T. Strain vessel hypothesis: a viewpoint for linkage of albuminuria and cerebro-cardiovascular risk. Hypertens Res. 2009;32(2):115–121. [DOI] [PubMed] [Google Scholar]
- 36.Elewa U, Sanchez-Nino MD, Martin-Cleary C, Fernandez-Fernandez B, Egido J, Ortiz A. Cardiovascular risk biomarkers in CKD: the inflammation link and the road less traveled. Int Urol Nephrol. 2012;44(6):1731–1744. [DOI] [PubMed] [Google Scholar]
- 37.Kaysen GA. The microinflammatory state in uremia: causes and potential consequences. J Am Soc Nephrol. 2001; 12(7): 1549–1557. [DOI] [PubMed] [Google Scholar]
- 38.Jing W, Jabbari B, Vaziri ND. Uremia induces upregulation of cerebral tissue oxidative/inflammatory cascade, down-regulation of Nrf2 pathway and disruption of blood brain barrier. Am J Transl Res. 2018;10(7):2137–2147. [PMC free article] [PubMed] [Google Scholar]
- 39.Vaziri ND. Oxidative stress in uremia: nature, mechanisms, and potential consequences. Semin Nephrol. 2004;24(5):469–473. [DOI] [PubMed] [Google Scholar]
- 40.Kurella Tamura M, Chertow GM, Depner TA, et al. Metabolic Profiling of Impaired Cognitive Function in Patients Receiving Dialysis. Journal of the American Society of Nephrology. 2016;27(12):3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohtsuki S, Asaba H, Takanaga H, et al. Role of blood-brain barrier organic anion transporter 3 (OAT3) in the efflux of indoxyl sulfate, a uremic toxin: its involvement in neurotransmitter metabolite clearance from the brain. J Neurochem. 2002;83(1):57–66. [DOI] [PubMed] [Google Scholar]
- 42.Kloppenborg RP, Geerlings MI, Visseren FL, et al. Homocysteine and progression of generalized small-vessel disease: the SMART-MR Study. Neurology. 2014;82(9):777–783. [DOI] [PubMed] [Google Scholar]
- 43.van den Kommer TN, Dik MG, Comijs HC, Jonker C, Deeg DJ. Homocysteine and inflammation: predictors of cognitive decline in older persons? Neurobiol Aging. 2010;31(10):1700–1709. [DOI] [PubMed] [Google Scholar]
- 44.Vannorsdall TD, Jinnah HA, Gordon B, Kraut M, Schretlen DJ. Cerebral ischemia mediates the effect of serum uric acid on cognitive function. Stroke. 2008;39(12):3418–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61(10):1531–1534. [DOI] [PubMed] [Google Scholar]
- 46.Iadecola C The pathobiology of vascular dementia. Neuron. 2013;80(4):844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drew DA, Bhadelia R, Tighiouart H, et al. Anatomic brain disease in hemodialysis patients: a cross-sectional study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;61(2):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drew DA, Koo BB, Bhadelia R, et al. White matter damage in maintenance hemodialysis patients: a diffusion tensor imaging study. BMC Nephrol. 2017;18(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Voineskos AN, Rajji TK, Lobaugh NJ, et al. Age-related decline in white matter tract integrity and cognitive performance: a DTI tractography and structural equation modeling study. Neurobiol Aging. 2012;33(1):21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lau WL, Huisa BN, Fisher M. The Cerebrovascular-Chronic Kidney Disease Connection: Perspectives and Mechanisms. Transl Stroke Res. 2017;8(1):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.*.Gupta A, Sarnak M, Montgomery R, Mahanken J, Lepping R, Brooks W, Burns J. Changes in Cognition, Cerebral Blood flow and Brain Neurochemicals before and after Kidney Transplantation. Alzheimer's Association International Conference 2019.This study demostrates change in brian blood flow and neurochemicals after kidney transplant indicating potenial reversibility of some of the bran alterations seen in kidney disease.
- 52.Kamano C, Komaba Y, Sakayori O, Iino Y, Katayama Y. Decreased cerebral blood flow in renal transplant recipients. Intern Med. 2002;41(9):677–683. [DOI] [PubMed] [Google Scholar]
- 53.Michaelis T, Videen JS, Linsey MS, Ross BD. Dialysis and transplantation affect cerebral abnormalities of end-stage renal disease. Journal of Magnetic Resonance Imaging. 1996;6(2):341–347. [DOI] [PubMed] [Google Scholar]
- 54.Tryc AB, Alwan G, Bokemeyer M, et al. Cerebral metabolic alterations and cognitive dysfunction in chronic kidney disease. Nephrology Dialysis Transplantation. 2011;26(8):2635–2641. [DOI] [PubMed] [Google Scholar]
- 55.Haugen CE, Mountford A, Warsame F, et al. Incidence, Risk Factors, and Sequelae of Post-kidney Transplant Delirium. Journal of the American Society of Nephrology : JASN. 2018;29(6):1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK. The interface between delirium and dementia in elderly adults. The Lancet Neurology. 2015;14(8):823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aiello Bowles EJ, Larson EB, Pong RP, et al. Anesthesia Exposure and Risk of Dementia and Alzheimer's Disease: A Prospective Study. Journal of the American Geriatrics Society. 2016;64(3):602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu L, Zhao H, Weng H, Ma D. Lasting effects of general anesthetics on the brain in the young and elderly: "mixed picture" of neurotoxicity, neuroprotection and cognitive impairment. Journal of anesthesia. 2019;33(2):321–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.H OB, N OL, Scarlett S, C OH, Kenny RA. Hospitalisation and surgery: are there hidden cognitive consequences? Evidence from The Irish Longitudinal study on Ageing (TILDA). Age and ageing. 2018;47(3):408–415. [DOI] [PubMed] [Google Scholar]
- 60.Swarte JC, Douwes RM, Hu S, et al. Characteristics and Dysbiosis of the Gut Microbiome in Renal Transplant Recipients. Journal of Clinical Medicine. 2020;9(2):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song T, Rao Z, Tan Q, et al. Calcineurin Inhibitors Associated Posterior Reversible Encephalopathy Syndrome in Solid Organ Transplantation: Report of 2 Cases and Literature Review. Medicine (Baltimore). 2016;95(14):e3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Q, Marescaux C, Wolff V, et al. Tacrolimus-associated posterior reversible encephalopathy syndrome after solid organ transplantation. Eur Neurol. 2010;64(3):169–177. [DOI] [PubMed] [Google Scholar]
- 63.Stolp HB, Dziegielewska KM, Ek CJ, Potter AM, Saunders NR. Long-term changes in blood-brain barrier permeability and white matter following prolonged systemic inflammation in early development in the rat. Eur J Neurosci. 2005;22(11):2805–2816. [DOI] [PubMed] [Google Scholar]
- 64.Sakamoto Y, Makuuchi M, Harihara Y, Imamura H, Sato H. Higher intracerebral concentration of tacrolimus after intermittent than continuous administration to rats. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2001;7(12):1071–1076. [DOI] [PubMed] [Google Scholar]
- 65.Lanese DM, Conger JD. Effects of endothelin receptor antagonist on cyclosporine-induced vasoconstriction in isolated rat renal arterioles. J Clin Invest. 1993;91(5):2144–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirsch S, Reichold J, Schneider M, Szekely G, Weber B. Topology and hemodynamics of the cortical cerebrovascular system. J Cereb Blood Flow Metab. 2012;32(6):952–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Snyder SH, Lai MM, Burnett PE. Immunophilins in the nervous system. Neuron. 1998;21(2):283–294. [DOI] [PubMed] [Google Scholar]
- 68.Alonso A, Klink R. Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. J Neurophysiol. 1993;70(1):128–143. [DOI] [PubMed] [Google Scholar]
- 69.Koenigs M, Barbey AK, Postle BR, Grafman J. Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci. 2009;29(47):14980–14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoon KH. Efficacy and cytokine modulating effects of tacrolimus in systemic lupus erythematosus: a review. J Biomed Biotechnol. 2010;2010:686480–686480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rabinovitch A, Suarez-Pinzon W, Shapiro AMJ, Rajotte R, Power R. Combination Therapy With Sirolimus and Interleukin-2 Prevents Spontaneous and Recurrent Autoimmune Diabetes in NOD Mice. Diabetes. 2002;51:638–645. [DOI] [PubMed] [Google Scholar]
- 72.Keenan PA, Jacobson MW, Soleymani RM, Mayes MD, Stress ME, Yaldoo DT. The effect on memory of chronic prednisone treatment in patients with systemic disease. Neurology. 1996;47(6):1396–1402. [DOI] [PubMed] [Google Scholar]
- 73.Brown ES, D JW, Frol A, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid therapy. Biol Psychiatry. 2004;55(5):538–545. [DOI] [PubMed] [Google Scholar]
- 74.Dixon BS, VanBuren JM, Rodrigue JR, et al. Cognitive changes associated with switching to frequent nocturnal hemodialysis or renal transplantation. BMC Nephrol. 2016;17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joshee P, Wood AG, Wood ER, Grunfeld EA. Meta-analysis of cognitive functioning in patients following kidney transplantation. Nephrol Dial Transplant. 2018;33(7):1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gupta A PT, Johnson D, Burns J. Sustained Improvement in Depression after Renal Transplantation. American Society of Nephrology Week. 2016.
- 77.Martinez-Sanchis S, Bernal MC, Montagud JV, et al. Effects of immunosuppressive drugs on the cognitive functioning of renal transplant recipients: a pilot study. J Clin Exp Neuropsychol. 2011;33(9):1016–1024. [DOI] [PubMed] [Google Scholar]
- 78.Harciarek M, Biedunkiewicz B, Lichodziejewska-Niemierko M, Debska-Slizien A, Rutkowski B. Continuous cognitive improvement 1 year following successful kidney transplant. Kidney Int. 2011;79(12):1353–1360. [DOI] [PubMed] [Google Scholar]
- 79.Castell JV, Geiger T, Gross V, et al. Plasma clearance, organ distribution and target cells of interleukin-6/hepatocyte-stimulating factor in the rat. Eur J Biochem. 1988;177(2):357–361. [DOI] [PubMed] [Google Scholar]
- 80.*.Chen HJ, Wen J, Qi R, et al. Re-Establishing Brain Networks in Patients with ESRD after Successful Kidney Transplantation. Clinical journal of the American Society of Nephrology : CJASN. 2018;13(1):109–117.This paper demostrates reversibility of brain networks with a successful kidney transplant.
- 81.*.Zhang LJ, Wen J, Liang X, et al. Brain Default Mode Network Changes after Renal Transplantation: A Diffusion-Tensor Imaging and Resting-State Functional MR Imaging Study. Radiology. 2016;278(2):485–495.This study demostrates reversibility of diffusion tensor imaging and brain network alterations with a successful kidney transplant.