Abstract
Objective
To explore special coagulation characteristics and anticoagulation management in extracorporeal membrane oxygenation (ECMO)–assisted patients with coronavirus disease 2019 (COVID-19).
Design
Single-center, retrospective observation of a series of patients.
Participants
Laboratory-confirmed severe COVID-19 patients who received venovenous ECMO support from January 20–May 20, 2020.
Interventions
This study analyzed the anticoagulation management and monitoring strategies, bleeding complications, and thrombotic events during ECMO support.
Measurements and Main Results
Eight of 667 confirmed COVID-19 patients received venovenous ECMO and had an elevated D-dimer level before and during ECMO support. An ECMO circuit pack (oxygenator and tubing) was replaced a total of 13 times in all 8 patients, and coagulation-related complications included oxygenator thrombosis (7/8), tracheal hemorrhage (5/8), oronasal hemorrhage (3/8), thoracic hemorrhage (3/8), bleeding at puncture sites (4/8), and cannulation site hemorrhage (2/8).
Conclusions
Hypercoagulability and secondary hyperfibrinolysis during ECMO support in COVID-19 patients are common and possibly increase the propensity for thrombotic events and failure of the oxygenator. Currently, there is not enough evidence to support a more aggressive anticoagulation strategy.
Key Words: COVID-19, coronavirus, severe acute respiratory syndrome coronavirus 2, SARS-CoV-2, extracorporeal membrane oxygenation, anticoagulation, thrombosis, ECMO
SEVERE ACUTE respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly in China and across the world, causing a global pandemic as a result of its strong transmissibility and virulence.1 , 2 A majority of coronavirus disease 2019 (COVID-19) patients have mild symptoms and recover completely; however, approximately 5% to 14% become severely or critically ill, with acute respiratory distress syndrome (ARDS) requiring intensive care unit (ICU) admission.3 Rates of invasive mechanical ventilation among patients admitted to the ICU have ranged from 29% to 91%, and the mortality rate was about 80% among patients on mechanical ventilation.4 , 5 Extracorporeal membrane oxygenation (ECMO) could offer life-saving rescue therapy when mechanical ventilation fails to maintain adequate oxygenation in COVID-19 patients. ECMO has been used successfully to manage severe respiratory failure in patients with H1N1 influenza A, H7N9 avian influenza, and Middle East respiratory syndrome.6, 7, 8, 9
However, there are not many reports describing ECMO support for COVID-19 patients, and little is still known about the coagulation characteristics of these patients while on ECMO support. Inflamed lung connective tissue and pulmonary endothelial cells may result in microthrombi formation and contribute to the high incidence of thrombotic complications in severe COVID-19.10 , 11 ECMO also could aggravate the activation of the coagulation cascade and consumption of clotting factors, causing additional coagulation abnormalities. The present study aimed to summarize the coagulation characteristics, anticoagulation management, and complications of COVID-19 patients who received ECMO support in Shanghai, China.
Methods
Patients and Data Collection
All adult patients diagnosed with COVID-19 were admitted to Shanghai Public Health Clinical Center, a designated hospital for COVID-19 treatment in Shanghai, and patients were treated by multidisciplinary teams including ECMO experts from different hospitals.12 The diagnosis of SARS-CoV-2 pneumonia was confirmed by both chest computed tomography (CT) scan and reverse transcription-polymerase chain reaction assays, according to the World Health Organization's interim guidelines and the new coronavirus pneumonia prevention and control program (7th edition) published by the National Health Commission of China.12 , 13 The diagnosis of ARDS was defined with the Berlin definition (3 categories of ARDS were proposed based on the partial pressure of oxygen/fraction of inspired oxygen [PaO2/FiO2] ratio).14 Patients were admitted to the ICU if the PaO2/FiO2 was <100 mmHg with high-flow nasal cannula (FiO2 100%, 30 L/min).
The present study was a retrospective examination of 8 COVID-19 patients who received venovenous ECMO. The demographics, comorbidities, laboratory results, ECMO-related data, and coagulation parameters from the medical records were collected. If there was more than 1 laboratory test in the same day, the most aberrant values were used. All the medical data of the present study were retrieved from the Shanghai Public Health Clinical Center and used with permission.
ECMO Indications, Timing, and Contraindication
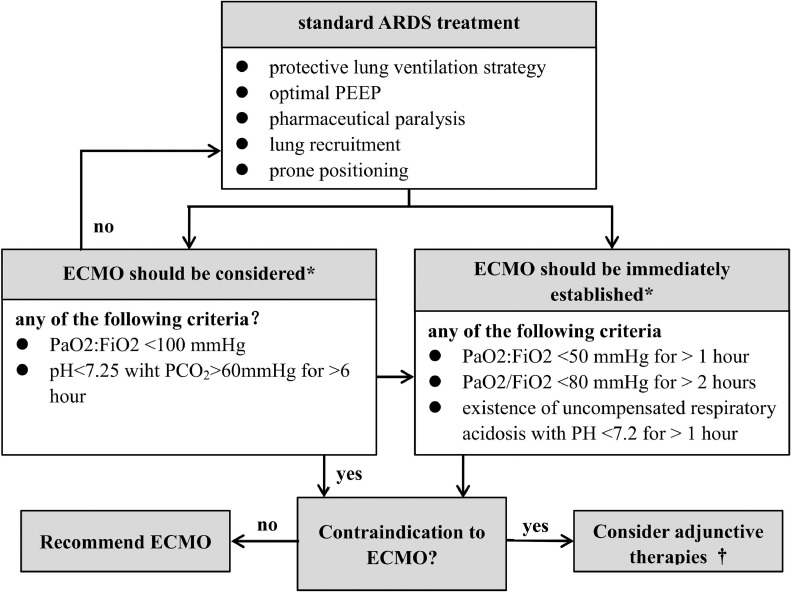
Standard COVID-19 treatment includes protective lung ventilation strategy, optimal positive end-expiratory pressure, sedation, lung recruitment, prone positioning, neuromuscular blockade, and volume optimalization.15 In cases in which a patient showed no substantial improvement, ECMO was initiated according to the protocol at the authors’ institution (Fig 1 ). The decision to provide ECMO support should be individualized and based on the risk and benefit assessment for the patient, but there were some absolute contraindications. According to the exclusion criteria used in the ECMO to Rescue Lung Injury in Severe ARDS trial and Extracorporeal Life Support Organization guidelines for COVID-19,16 , 17 absolute contraindications for venovenous ECMO in the authors’ center included prolonged high ventilatory pressures (ie, end-inspiratory plateau pressures >30 cmH2O for longer than 7 days); an expected difficulty in obtaining vascular access; severe coagulopathy; and any condition or organ dysfunction that would limit the likelihood of overall benefit from ECMO (disseminated malignancy, severe multiple organ failure, and uncontrolled bleeding).
Fig 1.
Flowchart of extracorporeal membrane oxygenation initiation protocol in coronavirus disease 2019 patients. ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; FiO2; fraction of inspired oxygen; PaO2, partial pressure of oxygen; PCO2, partial pressure of carbon dioxide; PEEP, positive-end expiratory pressure.
*Optimal mechanical ventilation: fraction of inspired oxygen ≤50%, tidal volume ≤6 mL/kg, plateau pressure ≤30 cmH2O, and respiratory rate 10 times/min
†Adjunctive therapies: neuromuscular blockade, high positive-end expiratory pressure strategy, inhaled pulmonary vasodilators, recruitment maneuvers, high-frequency oscillatory ventilation.
Establishment of ECMO
Venovenous ECMO was adopted as the chosen mode to improve oxygen supply and carbon dioxide elimination, using the Rotaflow system (Getinge, Rastatt, Germany) equipped with a Quadrox oxygenator (Getinge). The ultrasound-guided Seldinger technique was used for cannulation of the femoral vein as the drainage site and the internal jugular vein as the perfusion site. The tip of the internal jugular vein cannula (outflow cannula) was positioned at the junction between the right atrium and superior vena cava. The tip of the femoral vein cannula (inflow cannula) was advanced into the right atrium approximately 1 cm beyond the inferior vena cava/right atrium junction, and caution was taken to prevent the cannula tip from contacting the interatrial septum, using echocardiographic guidance. The blood flow (50-70 mL/kg) and oxygen flow were set according to the pulse oxygen saturation and blood gas results to maintain a PaO2 of 60-to-80 mmHg and partial pressure of carbon dioxide level of 35-to-45 mmHg. Point-of-care ultrasonography for the lungs, heart, abdomen, vasculature, and chest x-ray was performed on a daily basis. A protective lung ventilation strategy was adopted after ECMO initiation (FiO2 <40%, tidal volume 2-4 mL/kg, plateau pressure <25 cmH2O, and respiratory rate 8-10 times/min). Ventilator parameters and electrical impedance tomography were monitored closely. When necessary, continuous renal replacement therapy was performed using the port on the ECMO oxygenator.
Anticoagulation and Monitoring Strategies
Unfractionated heparin (UFH) was used in all patients, with a bolus at the dose of 50 U/kg 10 minutes before cannulation. If the activated clotting time (ACT) was <180 seconds, a continuous intravenous infusion of UFH was increased at a rate of 2-to-20 U/kg/h, with a target ACT level of 180-to-200 seconds and an activated partial thromboplastin time (aPTT) of 50-to-80 seconds (or 1.5 times of the baseline value). Point-of-care monitoring was conducted for ACT (every 2-4 hours), and the aPTT, thrombin time, prothrombin time, fibrinogen, D-dimer, fibrinogen degradation products (FDP), and platelet count were monitored from the central laboratory every 24 hours. After March 2, antithrombin (AT) activity monitoring was added, and thromboelastography was used whenever necessary to assess the coagulopathy status. When the heparin dose exceeded 20 U/kg/h, the possibility of heparin resistance was considered. Because of the lack of commercial AT agents in China, fresh frozen plasma was supplemented at a dose of 200-to-400 mL/d according to volume status when the AT activity was <70%. Platelets were infused when the platelet count was <80 × 109/L. If there was a significant decrease in platelet counts after continuous heparin infusion, heparin-induced thrombocytopenia (HIT) was suspected. After confirmation by a 4T score and anti-PF4/heparin antibody test, argatroban was used at a dose of 0.2-to-0.7 μg/kg/min, and the target of ACT and aPTT was the same as that of heparin.
When there was significant thrombosis within the oxygenator, accompanied by D-dimer >10 μg/mL, fibrinogen <1.5 g/L, and a sustained decrease in the platelet count, the entire ECMO circuit pack (oxygenator and tubing) was replaced despite satisfactory gas exchange function. In the context of bleeding, secondary hyperfibrinolysis, and fibrinogen consumption, tranexamic acid (10-20 mg/kg via slow injection, followed by a dose of 1,000 mg/d at the rate of 1-2 mg/kg/h for 2-3 days) and fibrinogen (at 1-2 g/d until fibrinogen >1.5 g/L) were infused. If there was significant bleeding or the need for invasive procedures, heparin was reduced or suspended for a short period until the ACT decreased below 150 seconds, and blood products were transfused if necessary.
ECMO Weaning Procedures and Standards
As described in the authors’ previous study, weaning of ECMO was started when improvements were observed on chest x-ray/CT, arterial blood gas, respiratory mechanics, and other indicators.18 The sweep-to-flow ratio was maintained at 1:1, and the ECMO flow was reduced to 2.5 L/min gradually while the same mechanical ventilation parameters were continued. With the ECMO flow maintained at 2.5 L/min, the ECMO sweep was reduced until gradually there was complete cessation of the sweep. In order to take patients off ECMO, the following criteria were maintained for 24-to-48 hours at ECMO flow rates of 2.5 L/min without sweep: (1) stable hemodynamics; (2) significant improvements in ventilation and gas exchange functions, as evident by chest x-ray, CT, electrical impedance tomography, and pulmonary ultrasound; (3) PaO2/FiO2 >150 mmHg, partial pressure of carbon dioxide ≤50 mmHg, respiratory rate ≤20; (4) body temperature <38°C; (5) Murray index <3; and (6) haematocrit >35%.
Statistics Analysis
SPSS Version 16.0 (SPSS Inc, Chicago, IL) was used for statistical analysis. Data were summarized as frequency for categorical variables and as medians and interquartile ranges for continuous variables. The general linear model repeated measures was used for correlation analysis among factors and the effects of individual factors.
Results
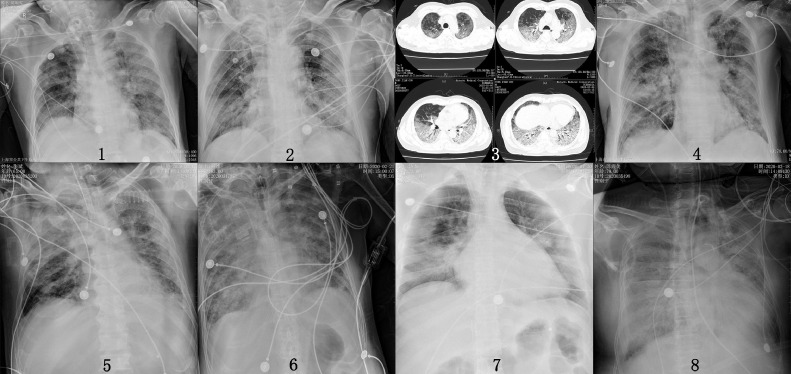
From confirmation of the first case in Shanghai on January 20 until May 20, a total of 667 COVID-19 patients were confirmed in Shanghai. Twenty-two critically ill patients (22/667) with ARDS were admitted to the ICU, and 8 (8/22) of them received venovenous ECMO support because of refractory hypoxemic respiratory failure using traditional therapies. All patients had a history of epidemiologic exposure, tested positive by reverse transcription-polymerase chain reaction detection of SARS-CoV-2 in respiratory secretions, and showed varying degrees of ground-glass opacity in both lungs (Fig 2 ).
Fig 2.
Chest x-ray or computed tomography of 8 patients before extracorporeal membrane oxygenation. Patient 3 did not have a chest x-ray before extracorporeal membrane oxygenation.
Table 1 summarizes the outcomes of 8 ECMO-supported patients included in this analysis. Patient 7 received ECMO initially on January 30 for 8 days and was weaned off support. His condition deteriorated on February 12, and ECMO had to be restarted. He died of pneumothorax and severe bleeding complications 10 days after the reintroduction of ECMO support. Three other patients died of persistent worsening lung consolidation, which was difficult to reverse, and experienced secondary lung infections, with multiple drug-resistant bacteria. Patients 1, 3, and 5 were weaned off ECMO successfully upon meeting the weaning criteria after 40 days, 47 days, and 22 days, respectively, and they were discharged by May 20. Patient 8 was weaned off ECMO successfully and still is receiving rehabilitation treatment. Of the 8 patients, 6 developed acute kidney injury and required continuous renal replacement therapy.
Table 1.
Baseline and Clinic Characteristics of 8 COVID-19 Patients on ECMO (up to May 20, 2020)
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Date of admission | 2 Feb | 28 Jan | 30 Jan | 1 Feb | 1 Feb | 22 Jan | 29 Jan | 5 Mar |
| Sex | Male | Male | Male | Male | Male | Female | Male | Male |
| Age (y) | 64 | 81 | 62 | 75 | 65 | 63 | 25 | 75 |
| Weight/BMI (kg) | 76/24.5 | 72/23.8 | 75/24.3 | 67/22.4 | 62/20.8 | 65/24.2 | 125/40.8 | 67/22.4 |
| Comorbidities | ||||||||
| Hypertension | Yes | Yes | Yes | Yes | ||||
| Diabetes | Yes | Yes | ||||||
| Cardiovascular disease | Yes | |||||||
| Malignancy | BC | |||||||
| Cerebrovascular disease | CI | |||||||
| COPD | Yes | |||||||
| Chronic kidney disease | MN | |||||||
| Murry index | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| RT-PCR | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive |
| WBC count (×10⁹/L) | 8.03 | 8.40 | 9.92 | 9.56 | 5.26 | 11.31 | 4.06 | 15.64 |
| Neutrophil count (×10⁹/L) | 6.91 | 7.56 | 8.82 | 9.03 | 4.96 | 9.82 | 3.21 | 14.58 |
| Lymphocyte (×10⁹/L) | 0.64 | 0.62 | 0.61 | 0.29 | 0.18 | 0.74 | 0.65 | 0.63 |
| Plt (×10⁹/L) | 337 | 148 | 159 | 144 | 151 | 161 | 162 | 203 |
| ALT (U/L) | 19 | 70 | 64 | 20 | 16 | 63 | 57 | 84 |
| AST (U/L) | 18 | 61 | 53 | 30 | 33 | 44 | 121 | 54 |
| Creatinine (μmol/L) | 81.40 | 139.70 | 70.80 | 162.31 | 184.69 | 61.85 | 140.00 | 95.07 |
| Troponin I (pg/mL) | 0.022 | 0.067 | 0.033 | 0.257 | 0.080 | 0.072 | 0.038 | 0.087 |
| NT-proBNP (pg/mL) | 58 | 1251 | 675 | 227 | 1313 | 6312 | 34 | 62.63 |
| Procalcitonin (ng/mL) | 2.09 | 0.55 | 0.14 | 0.3 | 16.39 | 3.56 | 0.07 | 0.68 |
| C-reactive protein (mg/L) | 83.09 | 57.69 | 63.57 | 88.65 | 155.28 | 92.05 | 39.95 | 111.32 |
| CD4 (cell/ul) | 386 | 68 | 63 | 53.5 | 373 | 132 | 143 | 92 |
| CD8 (cell/uL) | 125 | 31 | 63 | 83 | 166 | 55 | 143 | 17 |
| IL-6 (pg/mL) | 61.37 | 481.00 | 70.63 | 182.50 | 199.28 | 53.95 | 230.16 | 387.82 |
| Time of mechanical ventilation before ECMO | 4 | 10 | 12 h | 13 | 4 | 21 | 5 h* 13* |
11 h |
| P/F before ECMO | 67 | 66 | 64 | 75 | 76 | 70 | 54 | 78 |
| Lactate (mmol/L) | 2.4 | 2.8 | 3.1 | 4.0 | 1.3 | 2.4 | 3.1 | 3.2 |
| Time of ECMO (d) | 40 | 47 | 47 | 37 | 22 | 33 | 8/10 | 16 |
| State by now | Discharged | Died | Discharged | Died | Discharged | Died | Died | Weaned† |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BC, bladder cancer; CI, cerebral infarction; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; FiO2, fraction of inspired oxygen; IL-6, interleukin 6; MN, membranous nephropathy; NT-proBNP, N-terminal-pro hormone B-type natriuretic peptide; PaO2, partial pressure of oxygen; P/F, partial pressure of oxygen/fraction of inspired oxygen ratio; Plt, platelet; RT-PCR, reverse transcription-polymerase chain reaction.
Patient 7 received extracorporeal membrane oxygenation twice, on January 30 and February 12.
Patient 8 weaned from extracorporeal membrane oxygenation and ventilated.
The ages of patients ranged from 25-to-81 years, and the body mass index ranged from 20.8 to 24.5. Before the initiation of ECMO, mechanical ventilation duration was between 5 hours to 21 days, and the PaO2/FiO2 ratio was <80 (54-76) for all patients. The authors’ previous report18 and Table 1 provide detailed clinical data of demographics, laboratory results, ventilator parameters, and ECMO-related data for each patient. All patients were sedated and provided with analgesics (Richmond Agitation-Sedation Scale <–3) during ECMO.
As indicated by the coagulation parameters in Table 2 , most patients had elevated D-dimer (7/8), FDP (5/8), and fibrinogen (3/8) levels but with normal aPTT, prothrombin time, and thrombin time before ECMO support. Despite standard anticoagulation prophylaxis, both D-dimer and FDP still were maintained at high levels, with an average D-dimer level of 15.43 μg/mL and FDP level of 41.84 μg/mL. In these 8 patients, the average heparin infusion rate was 10.21 U/kg/h; average ACT and aPTT were maintained at 197 seconds and 61.48 seconds, respectively; the average platelet count was 116.94 × 109/L; and the lactate level was 2.32 mmol/L. The clinical observations showed a relationship among D-dimer, FDP level, and oxygenator thrombotic events or the need to replace the ECMO circuit, but statistical correlations were not observed with general linear models.
Table 2.
Hematologic Laboratory Parameters of 8 COVID-19 Patients on ECMO (up to May 20, 2020)
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Mean |
|---|---|---|---|---|---|---|---|---|---|
| Baseline before ECMO | |||||||||
| Dose of UFH (U/kg/H) | \ | \ | \ | \ | \ | \ | \ | \ | \ |
| ACT (s) | \ | \ | \ | \ | \ | \ | \ | \ | \ |
| aPTT (31.5-43.5 s) | 47.6 | 46.2 | 33.6 | 39.3 | 41.7 | 45.8 | 45.3 | 35.9 | \ |
| PT (11-13.7 s) | 15.7 | 15.1 | 13.5 | 14.7 | 15.2 | 16.0 | 13.9 | 13.0 | \ |
| TT (14-21 s) | 21.2 | 24.0 | 17.5 | 16.8 | 15.7 | 16.4 | 17.9 | 19.0 | \ |
| FIB (2-4 g/L) | 4.17 | 3.17 | 5.32 | 4.54 | 4.82 | 6.51 | 4.2 | 6.95 | \ |
| FDP (0-5 μg/mL) | 17.19 | 22.05 | 4.8 | 24.6 | 24.2 | 47.35 | 0.95 | 3.11 | \ |
| D-dimer (0-0.5 μg/mL) | 5.29 | 6.24 | 0.80 | 4.80 | 4.20 | 10.52 | 0.39 | 1.09 | \ |
| AT (80-120%) | \ | \ | \ | \ | \ | \ | \ | \ | \ |
| Plt (×10⁹/L) | 324 | 141 | 166 | 138 | 142 | 123 | 157 | 152 | \ |
| During ECMO support (min-max) | |||||||||
| Dose of UFH (U/kg/H) | 2.1-31.2 | 2.1-15.0 | 8.2-9.2 | 1.3-25.0 | 0-11.7 | 0-25.0 | 12.6-15.2 | 2.4-22.3 | 10.21 ± 7.28 |
| ACT (s) | 182-230 | 189-225 | 165-216 | 169-202 | 170-408 | 175-221 | 174-229 | 166-238 | 197 ± 42 |
| aPTT (31.5-43.5 s) | 42.3-148.6 | 31.3-113.8 | 33.6-117.4 | 40.6-128.0 | 42.6-139.4 | 39.8-154.6 | 39.2-127.3 | 41.1-113.2 | 61.48 ± 23.6 |
| PT (11-13.7 s) | 13.8-19.1 | 14.8-19.4 | 13.9-20.9 | 13.9-15.5 | 13.4-19.6 | 13.2-14.5 | 15.2-27.7 | 14.8-24.3 | 16.24 ± 3.17 |
| TT (14-21 s) | 13.4-68.5 | 13.1-58.9 | 15.1-159.4 | 13.9-201.1 | 16-172.1 | 13.1-200.0 | 10-206.1 | 15.6-176.3 | 57.68 ± 18.4 |
| FIB (2-4 g/L) | 0.90-5.84 | 1.97-4.68 | 0.92-7.28 | 1.58-4.43 | 4.17-7.41 | 1.24-4.38 | 1.46-4.46 | 1.23-4.75 | 3.45 ± 1.98 |
| FDP (0-5 μg/mL) | 5.96-116.01 | 7.33-90.44 | 3.11-Hlimit | 2.92-28.58 | 3.63-47.04 | 4.961-Hlimit | 3.95-Hlimit | 3.63-79.8 | 41.84 ± 21.4 |
| D-dimer (0-0.5 μg/mL) | 2.70-Hlimit | 2.90-Hlimit | 1.30-Hlimit | 1.47-8.19 | 2.52-15.93 | 1.75-Hlimit | 1.37-Hlimit | 2.12-17.42 | 15.43 ± 4.12 |
| AT (80%-120%) | 42-66 | 46-62 | 58-72 | 74-89 | 64-81 | 72-88 | \ | 61-81 | 67 ± 14 |
| Plt (×10⁹/L) | 110-267 | 40-112 | 88-189 | 68-116 | 58-197 | 81-199 | 46-181 | 76-169 | 116 ± 23 |
Abbreviations: ACT, activated coagulation time; aPTT, activated partial thromboplastin time; AT, antithrombin Ⅲ; ECMO, extracorporeal membrane oxygenation; FDP, fibrin degradation product; FIB, fibrinogen; Hlimit, >150 μg/mL in FDP and >20 μg/mL in D-dimer; Plt, platelet; PT, prothrombin time; TT, thrombin time; UFH, unfractionated heparin.
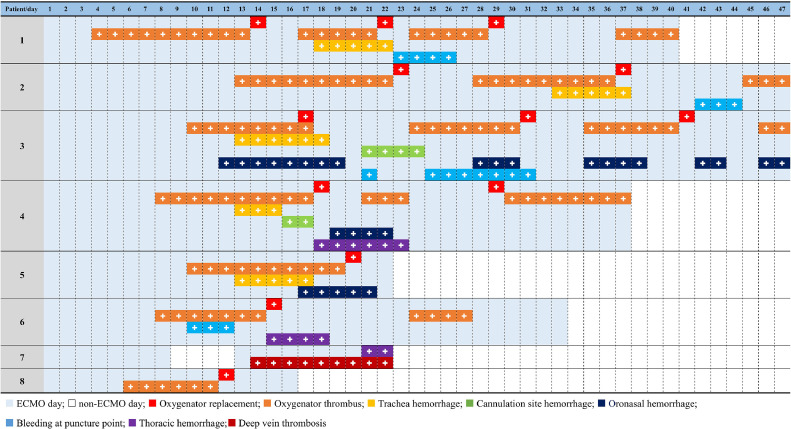
Figure 3 presents the thrombosis and bleeding events in the 8 patients during ECMO support (which lasted between 18 and 47 days) and coagulation-related complications, including oxygenator thrombosis (7), tracheal hemorrhage (5), oronasal hemorrhage (3), thoracic hemorrhage (3), bleeding at puncture sites (4), cannulation site hemorrhage (2), deep vein thrombosis (1), and HIT.1 The ECMO circuit pack was replaced a total of 13 times in all 8 patients.
Fig 3.
Thrombosis and bleeding events of 8 patients (up to May 20, 2020).
Most patients had a low AT level (<80%) during ECMO support (patient 7 died before AT level monitoring was added on March 2), and the effect of plasma therapy had an unsatisfactory effect at 200-to-400 mL/d. However, there is no commercially available AT supply in China. HIT was confirmed 6 days after the start of the second initiation of ECMO support, and argatroban was used instead of heparin in patient 7.
Discussion
COVID-19 surfaced in China in December 2019 and now is a worldwide pandemic. To the authors’ knowledge, several drugs currently are being tested, but there is still a lack of consensus or recommendations for any antiviral drug or drug combination, and traditional ARDS therapies may fail in critically ill patients.19 Indeed, recent trials have shown that ECMO could be used as an alternative therapeutic regimen, prolong survival time, and reduce the mortality for patients with severe ARDS.16 , 20 However, when it comes to COVID-19 patients complicated by severe ARDS, existing experience is limited and it is necessary to elucidate the indications, intervention time, therapeutic efficacy, and complications of ECMO in these complex patients. After an expert consensus statement and guidelines from Shanghai and the United States, the authors’ center has provided venovenous ECMO support to 8 patients since May 20.9 , 21 Traditional ECMO indications might lead to prolonged hypoxia and multiple organ failure in COVID-19 patients. Therefore, early ECMO support was adopted when mechanical ventilation was insufficient to correct hypoxia in these patients.18 In the present study, it was found that the clinical characteristics of COVID-19 patients were different from those of other viral pneumonia patients in terms of ECMO anticoagulation management and coagulation-related complications.
Severe COVID-19 patients manifest abnormal inflammatory responses and immune system damage, characterized by the rise of interleukin-6 levels and the decline in lymphocyte count, which is correlated with the severity of pneumonia.22 , 23 All 8 critically ill patients on ECMO in the present study exhibited a cytokine storm syndrome, with high interleukin-6 levels. As other reports have described, the inflammatory storm could activate the coagulation cascade and cause secondary hyperfibrinolysis or disseminated intravascular coagulation (DIC) in severe COVID-19 patients.24, 25, 26 It has become evident from published evidence that SARS-CoV-2 infection itself promotes immunologic response, which is unseen with seasonal influenza.27 Through the renin-angiotensin system, the SARS-CoV-2 virus may attack the endothelium, possibly leading to its activation and systemic prothrombotic state.28 Chen et al. revealed that nearly 20% of COVID-19 patients experienced coagulopathy, and nearly all severe and critically ill patients experienced different extents of coagulopathy.29 , 30 This was confirmed by venous thromboembolism and microthrombosis in the lungs associated with SARS-CoV-2 infection.31 , 32 The hypercoagulability observed in COVID-19 patients could arise from pulmonary vascular endothelial cell injuries, inflammatory responses, exocytosis of unusually large von Willebrand factor multimers, and platelet activation.33 The present study's cohort of ECMO-supported critically ill COVID-19 patients also showed certain degrees of coagulation activation and hyperfibrinolysis before ECMO initiation. The D-dimer, FDP, and fibrinogen levels had increased significantly in most of the patients. Chest CT performed 6 days after hospitalization revealed new cerebral infarction in patient 5. These clinical manifestations were consistent with the findings from Tang et al. that severe COVID-19 patients showed a significant increase in D-dimer and FDP levels and a higher incidence of DIC.27 , 30
In addition, supraphysiologic shear stress and interactions between foreign material and blood components during ECMO cause systemic activation of coagulation and inflammation pathways that, in extreme conditions, may lead to thrombosis and DIC.34 All the present study's patients consistently showed high levels of D-dimer and FDP throughout, high levels of fibrinogen at the early stage, and consumption of fibrinogen and platelets at the later stage during ECMO support. From clinical observation in the authors’ medical center, the D-dimer and FDP levels stayed consistently high and correlated with oxygenator thrombosis and ECMO circuit pack replacement. After the ECMO circuit pack was replaced, D-dimer and FDP levels decreased significantly for a short period but increased again as thrombi formed on the new oxygenator. Several patients experienced multiple ECMO circuit pack replacements (see Fig 3). Shortly after replacement, oxygenator thrombus was observed again in most patients and often was accompanied by D-dimer and FDP near limit values (FDP >150 μg/mL and D-dimer level >20 μg/mL, respectively). In a standardized anticoagulation regimen with ACT maintained at around 200 seconds, frequent oxygenator thrombosis events and hyperfibrinolysis were rarely seen in previous ECMO-supported patients.35 In a retrospective study of 201 COVID-19 patients, Wu et al. found that the increase of D-dimer level was an independent risk factor of death.36 In another multicenter, retrospective cohort study, elevated D-dimer levels were strongly associated with in-hospital death, even after multivariate adjustment.37 However, whether this is associated with poor prognosis in ECMO-supported COVID-19 patients still needs further research. According to Granja et al., activation of GPIIb/IIIa and increased release of platelet microparticles in venovenous ECMO suggests that ARDS-related inflammatory responses may lead to activation of platelets and enhancement of fibrin polymerization, which may promote thrombosis.38 Consumption of coagulation factors after thrombosis events was obvious. The need for fresh frozen plasma at a dose of 200-to-400 mL/day and platelets at an average of 0.8 U/day to restore coagulation function was necessary in the present study's patients.
In cases of oxygenator thrombosis with D-dimer >10 μg/mL, fibrinogen <1 g/L, and a decrease in platelet count, the entire ECMO circuit pack should be replaced regardless of gas exchange function. In addition to aggressive ECMO circuit pack replacement, the intensity of anticoagulation was increased moderately and the deficiency of platelets and fibrinogen was corrected. If there was only hyperfibrinolysis or DIC (International Society on Thrombosis and Haemostasis score >539) without oxygenator thrombosis, the coagulation pathway was enhanced moderately and tranexamic acid therapy was provided. Although the Extracorporeal Life Support Organization does not recommend conventional antifibrinolytic therapy, the authors of the present study believe that it is beneficial for blood protection in hypercoagulation status.39 There is no evidence to support the increase of anticoagulation intensity from standard anticoagulation prophylaxis to intermediate-intensity prophylaxis in COVID-19 patients who experience recurrent clotting of extracorporeal circuits despite prophylactic anticoagulation. Any decision to increase the intensity of anticoagulation should consider the individual patient's bleeding risk.
UFH is required for ECMO but could aggravate coagulopathy further in ECMO-supported COVID-19 patients. ECMO-associated coagulopathy is a multifactorial, rapidly developing syndrome which, based on platelet and coagulation disorders before ECMO, deteriorates with ECMO-required anticoagulation and may cause bleeding and thrombotic complications.40 The hypercoagulability and secondary hyperfibrinolysis during ECMO support also caused coagulopathy and manifested as coexistence of bleeding and thrombotic events. Common bleeding complications mainly involve airway, cannulation sites, invasive procedures or puncture wounds, skin or oronasal mucosae, gastrointestinal tracts, and lungs. Seventeen bleeding events occurred in 8 of the present study's patients, including tracheal hemorrhage, oronasal hemorrhage, thoracic hemorrhage, and puncture or cannulation site hemorrhage (see Fig 3). Given the complex nature of this problem, early consultation with hematologists may be prudent as part of a multidisciplinary team. The varying and dynamic heparin requirements can be difficult to monitor and manipulate.33 Granja et al. suggested that for the purpose of identifying high-risk factors of ECMO-associated coagulopathy in individual patients, bedside whole blood coagulation detection, platelet aggregation assay, and von Willebrand factor determination were necessary.38 In addition, cannulation site hemorrhage occurred in 2 patients 1 week after ECMO support. This was mainly because of hardening of the vascular walls and formation of subcutaneous tissue fistula at cannulation sites. In cases like this, compression is less effective and suture ligation should be performed. It is necessary to minimize iatrogenic bleeding caused by airway care, bronchoscopy, and invasive procedures if possible. It is also worth noting that when the intensity of anticoagulation is decreased for concerns about bleeding, it may aggravate anticoagulation insufficiency in the hypercoagulable state of COVID-19 patients, resulting in hyperfibrinolysis, DIC, and potentially more bleeding. Hemorrhage in this context is very difficult to manage because the circuit remains prothrombotic while the patient is bleeding.
AT plays an important role in the continuous endothelial activation because it is more exposed on the endothelium when the cells are activated, and there is increased release in the blood, with consequent rapid consumption with the use of high-dose heparin.28 In ECMO patients, acquired AT deficiency is a result of hemodilution, initiation of coagulation cascade, and consumption as a result of the use of heparin. AT supplementation is necessary to restore adequate anticoagulation. Criteria for AT supplementation in adult ECMO patients are not well-defined. Even though AT is frequently exogenously supplemented to restore therapeutic anticoagulation, when AT activity is deficient, this practice varies widely among institutions. One concern about supplementing AT in the presence of large doses of heparin is increased risk of bleeding.41 After March 2, the AT activity level was obtained in the authors’ center and AT supplementation using continuous infusion plasma was recommended because of the lack of commercial AT recombinant product in China. The effect of plasma therapy was unsatisfactory at 200-to-400 mL/d, and most patients had a low AT level during ECMO support. The authors’ goal for AT was at least 70% of normal values.
Conclusion
This was a single-center study based on a small number of patients. The coagulation properties of ECMO support in this cohort may not be representative; therefore, more comprehensive clinical studies are needed to confirm these findings. In summary, hypercoagulability and secondary hyperfibrinolysis during ECMO support in COVID-19 patients were common and possibly increased the propensity for thrombotic events and oxygenator membrane failure. Careful management of the anticoagulation regimen, along with the recruitment of highly experienced teams are necessary. Currently, there is insufficient evidence to support a more aggressive anticoagulation regimen for COVID-19 patients on ECMO support.
Acknowledgments
Acknowledgments
The authors acknowledge Guo Zhen, Sun Lin, Li Bailing, Tian Rui, Zhang Zhongwei, and Li Xin who currently are working as ECMO experts at Shanghai Public Health Clinical Center for COVID-19 patients. The authors also extend special thanks to Gao Yuan (RenJi Hospital, Shanghai Jiao Tong University, School of Medicine); Qu Hongping (RuiJin Hospital, Shanghai Jiao Tong University, School of Medicine); Li Yingchuan (Shanghai Sixth People's Hospital, Shanghai Jiao Tong University), Yu Kanglong (Shanghai General Hospital, Shanghai Jiao Tong University); and many staff members at Shanghai Public Health Clinical Center for their hard and meticulous work during the diagnosis and treatment of COVID-19 patients in Shanghai.
Conflicts of Interest
None.
Footnotes
This work was funded by the Science and Technology Commission of Shanghai Municipality, Medical Guidance Funded Project Number 17411971300.
References
- 1.Wang C., Horby P.W., Hayden F.G. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo Y., Huang Y.M., Huang J. [COVID-19 pandemic: Global epidemiological trends and China's subsequent preparedness and responses] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:642–647. doi: 10.3760/cma.j.cn112338-20200301-00222. [DOI] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiersinga W.J., Rhodes A., Cheng A.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 6.Alshahrani M.S., Sindi A., Alshamsi F. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care. 2018;8:3. doi: 10.1186/s13613-017-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraaij P.L., Wildschut E.D., Houmes R.J. Severe acute respiratory infection caused by swine influenza virus in a child necessitating extracorporeal membrane oxygenation (ECMO), the Netherlands, October 2016. Euro Surveill. 2016;21:30416. doi: 10.2807/1560-7917.ES.2016.21.48.30416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthay M.A. ECMO in severe acute respiratory distress syndrome. Lancet Respir Med. 2019;7:106–108. doi: 10.1016/S2213-2600(18)30507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelson D.P., Sasson C., Chan P.S. Interim guidance for basic and advanced life support in adults, children, and neonates with suspected or confirmed COVID-19: From the Emergency Cardiovascular Care Committee and Get With The Guidelines-Resuscitation Adult and Pediatric Task Forces of the American Heart Association. Circulation. 2020;141 doi: 10.1161/CIRCULATIONAHA.120.047463. e933-933e943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bilaloglu S., Aphinyanaphongs Y., Jones S. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324:799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helms J., Tacquard C., Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanghai Municipal Health Commission. Shanghai Novel Coronavirus Pneumonia Prevention and Control Program (5th Edition). Available at:http://wsjkw.sh.gov.cn/jbfk2/20200313/19d2f5ed7e174e0db5b895da0a1e0b20.html.
- 13.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected-interim guidance. 13 March 2020. 13 March 2020. Available at: https://apps.who.int/iris/handle/10665/331446?search-result=true&query=10665%2F331446&scope=&rpp=10&sort_by=score&order=desc.
- 14.Ranieri V.M., Rubenfeld G.D., Thompson B.T. Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 15.Abrams D., Ferguson N.D., Brochard L. ECMO for ARDS: From salvage to standard of care. Lancet Respir Med. 2019;7:108–110. doi: 10.1016/S2213-2600(18)30506-X. [DOI] [PubMed] [Google Scholar]
- 16.Combes A., Hajage D., Capellier G. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 17.Shekar K., Badulak J., Peek G. Extracorporeal Life Support Organization coronavirus disease 2019 interim guidelines: A consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO J. 2020;66:707–721. doi: 10.1097/MAT.0000000000001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X., Guo Z., Li B. Extracorporeal membrane oxygenation for coronavirus disease 2019 in Shanghai, China. ASAIO J. 2020;66:475–481. doi: 10.1097/MAT.0000000000001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peek G.J., Mugford M., Tiruvoipati R. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 21.Shanghai Clinical Treatment Expert Group for Corona Virus Disease 2019 Comprehensive treatment and management of corona virus disease 2019: Expert consensus statement from Shanghai [e-pub ahead of print] Chin J Infect Dis. 2020 doi: 10.3760/cma.j.issn.1000-6680.2020.0016. Accessed Published 2020-03-01. [DOI] [Google Scholar]
- 22.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chambers R.C. Procoagulant signalling mechanisms in lung inflammation and fibrosis: Novel opportunities for pharmacological intervention. Br J Pharmacol. 2008;153(Suppl 1):S367–S378. doi: 10.1038/sj.bjp.0707603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panigada M., Bottino N., Tagliabue P. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang X., Du R.H., Wang R. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. 2020;158:195–205. doi: 10.1016/j.chest.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowalewski M., Fina D., Słomka A. COVID-19 and ECMO: The interplay between coagulation and inflammation-a narrative review. Crit Care. 2020;24:205. doi: 10.1186/s13054-020-02925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arachchillage D.R.J., Laffan M. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1233–1234. doi: 10.1111/jth.14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Q., Wang R.S., Qu G.Q. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi. 2020;36:21–23. doi: 10.12116/j.issn.1004-5619.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Zhai Z., Li C., Chen Y. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: A consensus statement before guidelines. Thromb Haemost. 2020;120:937–948. doi: 10.1055/s-0040-1710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yusuff H., Zochios V., Brodie D. Thrombosis and coagulopathy in COVID-19 patients requiring extracorporeal membrane oxygenation. ASAIO J. 2020;66:844–846. doi: 10.1097/MAT.0000000000001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von B.V., Millar J.E., Malfertheiner M.V. Mesenchymal stem cells may ameliorate inflammation in an ex vivo model of extracorporeal membrane oxygenation. Perfusion. 2019;34:15–21. doi: 10.1177/0267659119830857. [DOI] [PubMed] [Google Scholar]
- 35.Mazzeffi M.A., Tanaka K., Roberts A. Bleeding, thrombosis, and transfusion with two heparin anticoagulation protocols in venoarterial ECMO patients. J Cardiothorac Vasc Anesth. 2019;33:1216–1220. doi: 10.1053/j.jvca.2018.07.045. [DOI] [PubMed] [Google Scholar]
- 36.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Granja T., Hohenstein K., Schüssel P. Multi-modal characterization of the coagulopathy associated with extracorporeal membrane oxygenation. Crit Care Med. 2020;48:e400–e408. doi: 10.1097/CCM.0000000000004286. [DOI] [PubMed] [Google Scholar]
- 39.Dalainas I. Pathogenesis, diagnosis, and management of disseminated intravascular coagulation: A literature review. Eur Rev Med Pharmacol Sci. 2008;12:19–31. [PubMed] [Google Scholar]
- 40.Kreyer S., Muders T., Theuerkauf N. Hemorrhage under veno-venous extracorporeal membrane oxygenation in acute respiratory distress syndrome patients: A retrospective data analysis. J Thorac Dis. 2017;9:5017–5029. doi: 10.21037/jtd.2017.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrisette M.J., Zomp-Wiebe A., Bidwell K.L. Antithrombin supplementation in adult patients receiving extracorporeal membrane oxygenation. Perfusion. 2020;35:66–72. doi: 10.1177/0267659119856229. [DOI] [PubMed] [Google Scholar]