Abstract
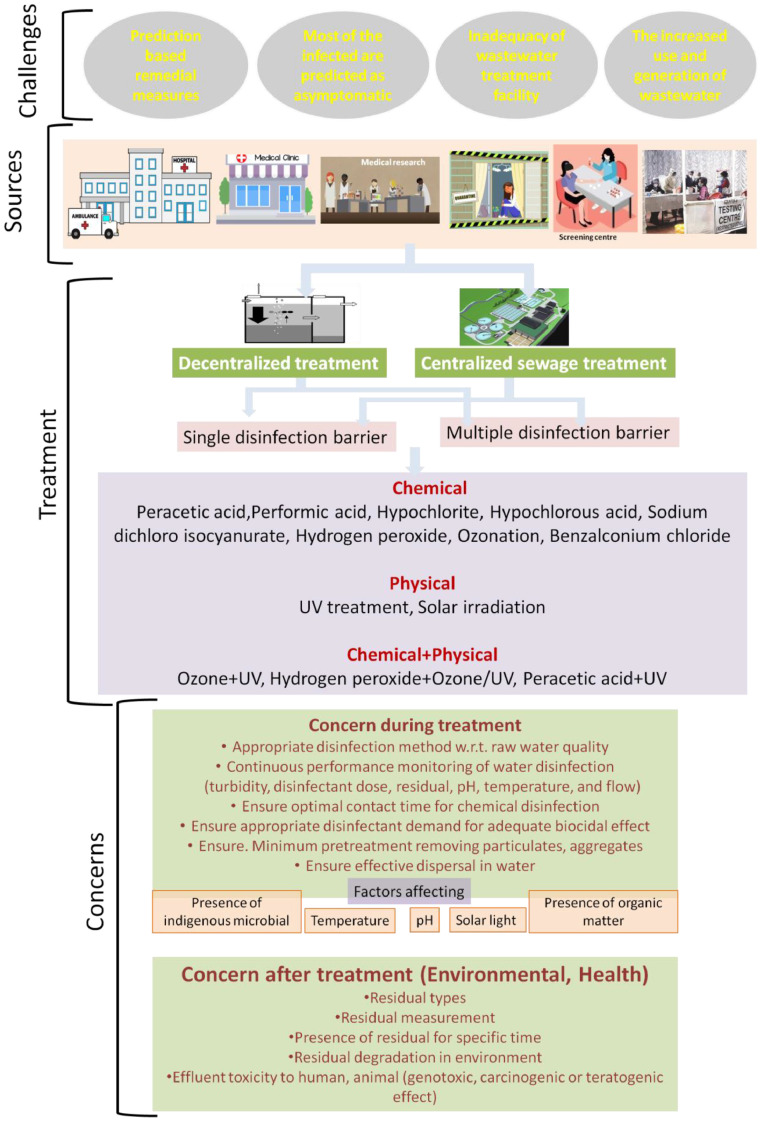
Along with outbreak of the pandemic COVID-19 caused by SARS-CoV-2, the problem of biomedical wastewater disposal has caused widespread public concern, as reportedly the presence is confirmed in wastewater. Keeping in mind (i) available evidence indicating need to better understand potential of wastewater mediated transmission and (ii) knowledge gaps in its occurrence, viability, persistence, and inactivation in wastewater, in this present work, we wanted to re-emphasize some strategies for management of SARS-CoV-2 contaminated wastewater to minimise any possible secondary transmission to human and environment. The immediate challenges to consider while considering wastewater management are uncertainty about this new biothreat, relying on prediction based treatments options, significant population being the latent asymptomatic carrier increased risk of passing out of the virus to sewage network, inadequacy of wastewater treatment facility particularly in populated developing countries and increased generation of wastewater due to increased cleanliness concern. In absence of regulated central treatment facility, installation of decentralized wastewater treatment units with single or multiple disinfection barriers in medical units, quarantine centre, isolation wards, testing facilities seems to be urgent for minimizing any potential risk of wastewater transmission. Employing some emerging disinfectants (peracetic acid, performic acid, sodium dichloro isocyanurate, chloramines, chlorine dioxide, benzalconium chloride) shows prospects in terms of virucidal properties. However, there is need of additional research on coronaviruses specific disinfection data generation, regular monitoring of performance considering all factors influencing virus survival, performance evaluation in actual water treatment, need of augmenting disinfection dosages, environmental considerations to select the most appropriate disinfection technology.
Keywords: SARS CoV-2, COVID-19, Virus, Wastewater, Disinfection, Treatment
1. Introduction
The newly recognized SARS-CoV-2 virus is the seventh corona virus known to infect humans after HCoV-OC43, HCoV-HKU1, HCoV-229E, HCoV-NL63, MERS-CoV, SARS-CoV (Hasöksüz et al., 2020). Typically, a corona virus is pleomorphic RNA virus having crown shape peplomers with a size of 80–160 nM and 27–32 kb positive polarity (Sahin et al., 2020). The genome sequence of SARS-CoV-2 has a similarity of 96.2% to that of bat coronavirus (BatCoV RaTG13 (Yan et al., 2020). Though SARS-CoV-2 has a low mortality rate (around 2%), which is significantly lower than that of severe acute respiratory syndrome SARS (9.6%) and Middle East respiratory syndrome MERS (35%), the former is reported to have high transmission rate among humans with an incubation period of up to 24 days (Yan et al., 2020). World Health Organization (WHO) reported that, as of August 16.8.2020, 21,260,760 cases of COVID-19 have been confirmed, including 761,018 deaths (WHO COVID Dashboard, 2020). The pandemic has caused nationwide lockdowns in many countries and other restrictions such as stay-at-home orders, shelter-in-place orders as a prevention of the spread of the disease. Though research community across globe is working on this new biothreat, many uncertainties still remain to be elucidated with regard to virus-host interaction, its mechanisms of transmission, the clinical spectrum, diagnostics, and prevention and therapeutic strategies (Cascella et al., 2020).
However, along with outbreak of this menace, the problem of biomedical waste and wastewater disposal has caused widespread public concern (IWA, 2020). Concern of disease transmission during wastewater management has already been highlighted during latest disease events caused by emerging pathogens such as SARS-CoV-1, Ebola virus, pandemic influenza, about which presently little information on transmission is available (Chattopadhyay and Taft, 2018). Recent scientific investigations indicate potential risk of waterborne transmission of the coronavirus, as already a body of literature has confirmed the presence of SARS-CoV-2 in sewage treatment plants (Quilliam et al., 2020). Such occurrences have intensified the need for generation of more information on its transmission pathways through various environmental exposures, including the wastewater pathway. This is because wastewater has been known as a major source of pathogen transmission and pathogen contaminated water should carefully be treated. Possibility of such transmission might be a major concern in areas that do not have adequate sanitation and water treatment facilities, as discharge of wastewater without appropriate treatment would expose the public for infection (Wang et al., 2020; Usman et al., 2020). Recently, it was urged by The Basel, Rotterdam and Stockholm Convention (BRS) Executive Secretary to decision makers to treat waste management (medical, household as well as hazardous waste) as an essential public service to ensure minimum impacts on human and environment in the battle with COVID-19 (Basel International, 2020). However, unfortunately till now, immediate response to the global pandemic has focused primarily upon preventing person to person transmission. Potential threat from contaminated wastewater exposure has started to perturb the scientific community only recently, though not much importance is being given at ground level. This is due to the fact that, daily more numbers of quarantine centres, isolation wards, dedicated testing centres, hospitals, research centres are being developed globally to facilitate detection of the infected, to accommodate the infected, to test the suspect, to carry out research about this new threat to mankind. It is obvious that, such facilities would increase the generation of virus contaminated wastewater posing threat. Though it has been reported that, the existing disinfection is expected to kill the virus in water, the fate of coronavirus in wastewater treatment plants or in the water environment is yet to be elucidated (Nghiem et al., 2020; IWA, 2020). Further, availability of water will be a crucial determinant for a successful outcome in this war against the new global enemy, as there is inflation in water consumption owing to increase in consciousness towards cleanliness (Singhal, 2020). Consequently, there is increased concern to treat the generated wastewater to ensure that there is minimal public exposure to untreated wastewater. Hence, in this work, with the COVID-19 pandemic spreading day by day, we wanted to re-emphasize and draw attention about the seriousness of treating the wastewater with virus contamination to reduce any possible secondary impacts upon human and environment. Keeping in mind (i) the available evidence indicating need to better understand potential role of wastewater in disease transmission and (ii) our knowledge gaps in occurrence, persistence, and removal of SARS-CoV-2 in wastewater, present study attempts to highlight some strategies for management of SARS-CoV-2 contaminated wastewater disinfection, which is of great significance during the occurrence of this pandemic.
2. Concern is already raised: “presence is confirmed in sewage treatment plants”
As per the report published till now, it has been indicated that there is a possibility of the virus to become widespread through wastewater network (Naddeo and Liu, 2020). The risk of exposure via the faecal-oral route due to its excretion into sewage has also been highlighted in areas with inadequate sanitation facility (Quilliam et al., 2020; Packman, 2020; Amirian, 2020). Though the infectivity of such virus is not known, presence of the virus is confirmed in human faeces up to 33 days after the patient is tested negative for COVID-19 (Quilliam et al., 2020). In China, in the clinical guideline “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7)” possibility of faecal-oral transmission of the virus is officially added and it was urged for attention to faeces or urine contaminated environment to check any possible transmission via this route (National Health Commission, China, 2020). The notice stipulated all designated medical institutions, temporary quarantine centres and research institutions to adhere to the “Water Pollutant Discharge Standards for Medical Institutions” (GB 18466–2005). Reports on presence of the SARS-CoV-2 at sewage/wastewater treatment plants (WTP) around the world are being sought up increasingly. Sewage samples from Chennai, India recently showed presence of SARS-CoV-2 RNA (www.hindu.com 2020). Recent report from China indicated possibility of contamination of the drainage system, as wastewater effluent from COVID-19 designated hospital was found SARS-CoV-2 RNA positive (Chinawaterrisk.org 2020). Ahmed et al., 2020 reported SARS-CoV-2 RNA from untreated wastewater in a catchment in Australia. Reports on presence of RNA of SARS-CoV-2 in wastewater have been published from the Netherlands (Medema et al., 2020; Lodder and Husman, 2020). Wurtzer et al. (2020) detected SARS-CoV-2 genomes in raw or treated wastewater from major WTP in France and confirmed proportional increase of genome units in raw wastewaters to number of COVID-19 cases. Similarly Randazzo et al. (2020) reported the occurrence of SARS-CoV-2 RNA in six wastewater treatments plants in Spain. Similarly, Nemudryi et al. (2020) from the United States confirmed SARS-CoV-2 RNA in wastewater. In particular, presence of coronaviruses in water increases the possibility for the virus to become aerosolised (Casanova et al., 2009), particularly during wastewater pumping (Quilliam et al., 2020). Though till now there is no report on SARS-CoV-2 in aerosols from WTPs, studies by Fears et al. (2020) shows its infectivity in aerosols for up to 16 h, and studies by El Baz and Imziln (2020) indicates its potential risk if found in wastewater aerosol.
3. The immediate challenges of water dynamics during COVID-19
3.1. Uncertainty about the virus and prediction based remedial approaches
The main challenge in this regard is the absence of SARS-CoV-2 specific data. Predictions about behaviour of coronavirus in wastewater is mostly based on related similar virus SARS, MERS. Since SARS and MERS are from the same family of coronaviruses, SARS-CoV-2 is expected to have similar physical and biochemical characteristics and transmission pathway as SARS and MERS. At the same time concern has been also raised about its potentially different behaviour in aquatic environment due to its different structural makeup specifically the lipid envelope compared to other viruses typically found in the intestine (Economictimes, India, 2020). Development of rapid SARS-CoV-2 countermeasure depends on the availability of robust, scalable and readily deployable surrogate virus, since dealing with SARS involves significant challenge and requires specially trained personnel employed in BSL-3 laboratory containment (Casanova et al., 2009; Dieterle et al., 2020). The use of appropriate surrogate viruses can overcome the challenges of working with SARS-CoV and to enhance our understanding on the environmental survival and persistence of the virus (Casanova et al., 2009; Hulkower et al., 2011). Identification of suitable surrogate for SARS-CoV-2 is underway. ASTM in its draft guidance on selection of surrogate SARS-CoV-2 virus included Human Coronoavirus 229E. NL63, OC43, Murine hepatitis virus, Transmissible gastroenteritis virus, Feline infectious peritonitis virus, Canine coronavirus, Porcine respiratory coronavirus, Influenza A virus (Strain H1N1) based upon primary criteria of enveloped virus, respiratory type, public availability of virus cell line, mammalian origin with a preference for human viruses and viruses that are categorized as BSL2 (ASTM, 2020). Casanova et al. (2009) recommended transmissible gastroenteritis virus (TGEV) and mouse hepatitis virus (MHV) as surrogate for coronavirus. In a recent study, Dieterle et al. (2020) developed recombinant vesicular stomatitis virus encoding the SARS-CoV- 2 spike protein, which could serve as surrogate for the later. Both bovine coronavirus and the avian infectious bronchitis virus may serve as surrogates for SARS-CoV considering their resemblance (Steinmann, 2004).
3.2. Most of the population is predicted to be latent asymptomatic carrier
It has been forecast that most of the people will develop only mild symptoms of the disease whilst others will be only latent asymptomatic carrier of the virus (Quilliam et al., 2020). More than 80% of the people infected are expected to recover from the disease without going for medical treatment. The state of latency of the virus in asymptomatic carrier or people experiencing mild symptoms and not seeking medical attention increase the risk of spreading of the virus through sewer systems. This fraction of people will be the source by directly discharging the virus through faeces, sputum and nasal secretion (Kam et al., 2020; Zhang et al., 2020).
3.3. Inadequacy of wastewater treatment facility in developing countries
Parallel to the potential spreading of the virus through asymptomatic carrier, poor waste management strategies, absence of appropriate sewage treatment framework can worsen the impending through wastewater particularly in developing countries (Usman et al., 2020; Barcelo, 2020). According to UN's World Water Development Report, 2017, globally 80% of wastewater (>95% in developing countries) is released to the environment without sufficient treatment (Usman et al., 2020). For e.g. in India, out of the 61,754 million litre of sewage generated per day, it has a treatment capacity for only about 22,963 million litre per day, which is mostly underperforming due to operation and maintenance problem (Sulabhenvis, 2020). Thus, around 70% of sewage generated in urban India is not treated and there is a huge gap between generation and existing wastewater treatment capacity. Risk is higher in parts of the world with high magnitude of open defecation. UNICEF data shows that worldwide 892 million people still go for open defecation (UNICEF, 2018). In a recent survey National Statistical Office, India, November 2019, claimed that about 28.7% of rural households across India still lack access to any form of latrines (National Statistical Office, 2019). Such ground truth realities cannot be denied and should be made into account to understand possible transmission of the virus through unregulated wastewater network.
3.4. The increased use and generation of wastewater
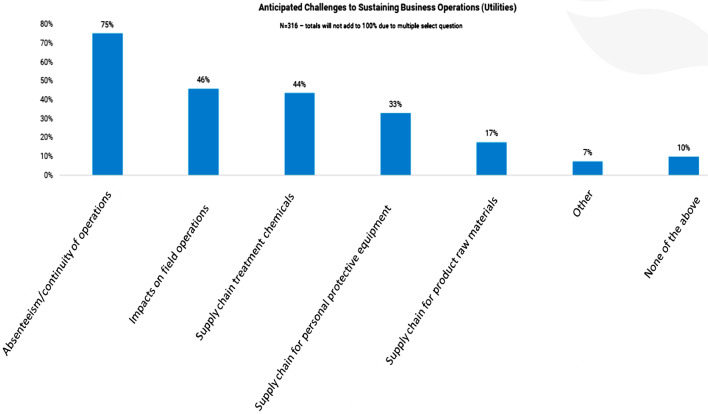
It is to be mentioned that, due to mass transmission of SARS-CoV-2, globally government of most of the countries are making large number of quarantine centres, screening centre, isolation wards for keeping the infected. Additionally significant numbers of testing and sample collection facilities are being raised within a very short time dedicated for COVID-19 related testing. As of June 3, 2020, India has 480 government testing laboratories and 208 private laboratories assigned to fight against the virus (Statista.com 2020). This implies that substantial quantities of wastewater would be generated from such facilities which may pose a threat if not treated before release. It is especially challenging for temporarily requisitioned COVID-19 dedicated centres where toilets are connected directly to the municipal sewage network, as there is no residence time and disinfectants addition is not easy (Xu, 2020). Further, as there is inflation in water consumption owing to increase in consciousness towards cleanliness, this will add to additional generation of wastewater from all sectors. According to a recent study in India, during this pandemic, around 20–40 litres of water per person is being used every day which is five times higher than the average, which will lead to 25% increase in water demand and wastewater generation (Rohila, 2020). The American Water Works Association (AWWA) conducted a survey on AWWA member about expected business operations challenges in wastewater sector caused due to the pandemic. Absenteeism/ continuity of operations and impacts on field operations were found as the top challenges in this sector (Fig. 1 ) (AWWA, 2020). The direct impact of COVID-19 is expected to reflect on market of Global Water and Wastewater Treatment Chemicals, as per the report published by TechnaVio, UK (Technavio, 2020). As per the assessment, the water and wastewater treatment chemicals market is poised to progress at a compound annual growth rate of over 6% during the forecast period 2020–2024.
Fig. 1.
Survey results conducted by AWWA on anticipated business operations challenges caused by COVID-19 in wastewater sector (source: awwa.org).
4. The anticipatory fact: we have some background knowledge
The genome of the SARS-CoV-2 virus is phylogenetically most similar to bat SARS related coronaviruses (84% nucleotide similarity with bat-SL-CoVZC45 coronavirus), and the spike protein has a 78% nucleotide similarity with the human SARS-CoV-1 (Chan et al., 2020). Therefore, SARS-CoV-2 should also be susceptible to environmental factors or disinfectants applied during SARS epidemic (Wang et al., 2020). As per WHO's technical brief, the enveloped coronaviruses SARS-CoV-2 is likely to be less stable in wastewater and should be an easy target for chlorine based disinfectant and any change in pH and temperature (WHO, 2020). Standard treatment and disinfectant processes at conventional WTP are expected to be effective to remove or inactivate the virus. In one of the earliest works by Maris (1990) on coronavirus, authors reported that enveloped nature of coronaviruses make them vulnerable to microbicides as compared to non-enveloped ones. In their study, a parvovirus (non enveloped) required 20 to 500 times higher dose of the microbicides applied than that required by enveloped coronavirus.
In a study byWang et al., 2005, SARS-CoV was shown to survive in hospital wastewater, domestic sewage, and tap water for 2 days at 20 °C and up to 14 days at 4 °C, displaying strong influence of temperature on its survival. Inactivation of coronaviruses is highly dependant on temperature, organic matter, and presence of other bacterial species (Gundy et al., 2009). Corona virus diminishes fast in wastewater (99.9% reduction in 2–3 days) (Gundy et al., 2009). In a recent review by Carducci et al. (2020) authors indicated lower persistence and faster inactivation of enveloped SARS-CoV virus compared with non-enveloped viruses and strong influence of temperature, organic matter and microbial population on its survival. Casanova et al. (2009) evaluated persistence of SARS coronavirus using two potential surrogates viz. transmissible gastroenteritis virus (TGEV) and hepatitis virus (MHV), representing two groups of mammalian coronaviruses. Type of water, incubation period and temperature played major role in the viral inactivation kinetics. Both the virus were found to retain infectivity in settled sewage for substantial period of time showing 99% reduction of TGEV in 9 days and MHV in 7 days. Compared to low temperature (4 °C), infectious virus declined more rapidly at 25 °C. Lai et al. (2005) reported substantial stability of infectious SARS-CoV in faecal and respiratory samples at room temperature. La Rosa et al., 2020 summarized that the virus has low scale water borne infectivity and at present there is no current evidence of the virus transmitting through contaminated water. Previously SARS-CoV in wastewater was successfully inactivated with chlorine dose of 10 mg l−1 in a contact period of 10 min (free residue chlorine 0.4 mg l−1) or chlorine dioxide dose of 40 mg l−1 with a contact period 30 min (free residue chlorine 2.19 mg l−1) (Wang et al., 2005). It was further reported that, SARS-CoV was more sensitive to disinfectants compared to E. coli and f2 phage. In a recent study in China by Wang et al. (2020) hospital wastewater before treatment and after 1st treatment stage with sodium hypochlorite was reported to be positive, but the sample after multi stage disinfection with sodium hypochlorite was negative. Similarly Rimoldi et al. (2020) confirmed raw wastewater as viral RNA positive, while the treated ones were always negative. The authors implied low threat of virus infection from wastewater, based on the observed fact of its absence post conventional wastewater treatment and natural decay of viral vitality after eight days.
5. Possible remedial approaches
5.1. Going ‘decentralized’ for wastewater treatment
During the COVID-19 pandemic, availability of clean water to maintain minimum hygiene as well as treatment of virus contaminated wastewater to ensure minimum public exposure have emerged as the biggest issue in many parts of the world. In absence of a regulated centralized sewer connection, which is a reality in most of the developing countries, decentralised wastewater management strategies can play a significant role (Matto and Singhal, 2020). Being affordable and sustainable low maintenance option both environmentally and economically, decentralized treatment option can deliver the wastewater treatment objectives during this pandemic. Development of small scale treatment infrastructure alternative to centralized sewer system for hotspots including hospitals, clinics, quarantine centres which are generating possible coronavirus contaminated wastewater is one potential research area of great significance at present time. Hospitals or healthcare facilities not connected to a comprehensive WTP may use a decentralized treatment unit and undertake standard disinfection measures of wastewater before releasing it to environment. An in situ decentralized treatment system in these places will help reducing virus load into environment and stop any possible secondary transmission (Naddeo and Liu, 2020).
Incorporation of a relatively robust and simple treatment system such as waste stabilization pond or lagoon may be a better treatment option (www.fao.org 2020). Such technology is particularly efficient in reducing pathogen load under the combined action of relatively long retention time; solar radiation; elevated pH and microbial action. Constructed wetland or treatment pond as tertiary system may also produce significant outcome to achieve additional pathogen reduction. Constructed wetland has been reported to effectively reduce virus (coliphage and enterovirus) load in wastewater by more than 2 log units (Williams et al., 1995). In absence of centralized treatment system, using solar irradiation, UV irradiation and appropriately dosed free chlorine sources or some promising environmental friendly virucidal options such as peracetic acid, performic acid, sodium dichloro isocyanurate etc. (discussed below) seems to be effective in battling any possible contamination through wastewater transmission (WHO, 2020). In this context, a decentralized disinfection system consisting of light emitting diode based UV may be helpful (Naddeo and Liu, 2020). Limited coverage and treatment capacity of centralized treatment facility in many developing countries may be compensated by developing decentralized approaches.
5.2. Potential disinfectants
Survival of viruses in environment depends on several factors and is boosted with viral aggregation and negatively affected with temperature increase, presence of sunlight, presence of indigenous microbial population, whereas the effects of organic matters and humidity are contradictory (Pinon and Vialette, 2018). A virus cell typically contains a genome (single or double stranded RNA or DNA), a protein capsid and may be with or without an envelope. Viral disinfection is primarily targeted to alter one of these components by exerting environmental stress (Pinon and Vialetter, 2018). Compared to other parts, proteins and lipids of viral envelope are relatively vulnerable to disruption, which is why non enveloped virus is less susceptible to adverse conditions and demonstrates the highest resistance to inactivation (Fitzgibbon and Sagripanti, 2008; McDonnell, 2009). In general, secondary wastewater treatment is capable of average removal of 1-log (90%) of viruses, though the level of virus removal is highly variable, ranging from insignificant to more than 2-log removal (99%) (McLellan et al., 2020). Because of this variability, the primary process for the inactivation of viruses in wastewater treatment is chemical or radiation disinfection.
As an immediate response to combat coronavirus in wastewater, in most countries guidelines are issued to wastewater management agencies to continue disinfection of treated wastewater as per prevailing practices. It is stated in the wastewater worker guidance released by OSHA, USA (Occupational Safety and Health Administration, USA) in February 2020 that existing disinfection methods (hypochlorous acid or peracetic acid oxidation and ultraviolet irradiation) employed in water treatment plant should be successful in inactivating coronavirus (OSHA, 2020). In China, in the emergency treatment plan for hospital wastewater during COVID-19, it has been instructed to strictly disinfect the sewage disposal from hospitals and level of residual chlorine must be kept higher than 6.5 ppm (> 6.5 ppm chlorine for a contact period more than 90 min; > 10 ppm chlorine for a contact period less than 90 min) (www.chinacdc.cn, 2020). It has been reported by Ministry of Ecology and Environment, China that, due to the intensive disinfection carried out by all sectors, residual chlorine was detected in drinking water sources, however, the concentrations were lower than that required by the drinking water quality standard (0.3 mg l−1) (www.chinawaterrisk.org, accessed on 19.6.2020). Till now EPA has listed a list of 431 commercial disinfectants in its ‘List N: Disinfectants with claim for affectivity against SARS-CoV-2 as on June 2020 (USEPA 2020). As per the list majority of products that meet EPA's criteria for use against SARS-CoV-2 contain the active ingredient Quaternary ammonium, Sodium hypochlorite, Ethanol, Hydrogen Peroxide, Peroxyacetic Acid, Isopropanol, Phenolic, Triethylene glycol, different organic acid (Lactic Acid, Glycolic Acid, Octanoic acid), Dischloro isocyanurate.
In literature there exists some ambivalence and contradiction in findings about the efficacy of wastewater disinfectants particularly against virus. Hence a close and regular monitoring and guidance on such impact seems to be necessary for proper usage of disinfectant. Moreover, in order to get an actual picture and minimize the probability of infection by the virus, removal efficiency needs to be assessed quantitatively for each inactivation strategy in actual treatment plants or real wastewater scenario.Nonetheless, relatively limited studies are available on virus removal due to complexities in quantifying low concentration of virus in water (Asami et al., 2016). Microbes especially for viruses, another challenge is that, in the harsh environmental state of wastewater to survive, it may remain shielded by physical embedding in organic matter, body cells, suspended particle, occlusion of a biofilm that renders it less vulnerable to inactivation action of disinfectants (Geller et al., 2012). Another concern is that, in most of the countries guidelines or standards related to the microbiological quality of wastewater deal mainly with bacteriological indicators (Zhang et al., 2016). Generally, in unit operations of a wastewater treatment plant, the efficacy of a wastewater disinfection process is monitored or determined based on its activity against indicator bacteria, which does not provide confirmation that other microbial contaminants meet the required level of inactivation (Blatchley et al., 2007; Zhang et al., 2016). Due to its non-enveloped structure most of the studies use MS2 coliphage as indicator organism for evaluating the inactivation of enteric viruses in water, as recommended by the WateReuse Research Foundation (2015).
Recently, apart from the traditional disinfection system, several alternative disinfection methods with reduced application concern as well as environmental impact are being increasingly used. This is due to the issues associated with traditional disinfectants such as reduced efficiency in high organic load wastewater, formation of dangerous, persistent and bio-accumulative by-products, eco toxicity to environment, need of special safety precautions during transport, storage and handling. In the following sections, we will attempt to highlight some of the potential disinfection methods along with some relatively environment friendly emerging disinfectants which might be suitable considerations for use in wastewater for combating against COVID-19 based upon their reported effectiveness against virus as well as environmental friendliness.
5.2.1. Chlorine based disinfectants
Disinfections processes releasing free available chlorine (FAC) i.e., chlorine present as hypochlorous acid (HOCl) and hypochlorite ion (ClO-) remain the most successful way to deal with virus contamination (Abad et al., 1997; Kuznesof, 2004). Mostly used FAC sources are elemental chlorine, sodium hypochlorite, chloramines, calcium hypochlorite chlorine dioxide and chloroisocyanurates. Hypochlorite as strong oxidizing agent is effective for oxidizing organic pollutants, whereas un-dissociated HOCl is primarily the microbiocidal agent (Pinto and Rohrig, 2003). Inactivation by chlorine is attributed to factors such as oxidation of sulfhydryl enzymes and amino acids, ring chlorination of amino acids, loss of intracellular contents, reduced nutrients uptake; inhibited protein synthesis, reduced oxygen uptake and oxidation of respiratory products, decreased ATP production, DNA fragmentation and down-regulation of DNA synthesis (Rutala et al., 2008). Studies have confirmed the effectiveness of chlorine against viruses; however, the relatively higher tolerance of viruses to chlorine disinfectants compared to bacteria may be related to the fact that viruses do not have a metabolic enzyme system, which usually remains the target site of disinfectants in case of bacteria (Chang, 1971). Usually, 30 mg l−1to 50 mg l−1 and 15 mg l−1 to 25 mg l−1 chlorine is added to wastewater after primary and secondary treatment and wastewater, respectively (Wang et al., 2020). Chang (1971), made a supposition that inactivation of viruses by chlorine is likely to be attributed to capsid protein denaturation, which is more resistant to degradation than the breakdown of enzymatic R—S—H bonds by oxidizing agents, resulting in higher levels of Cl consumption to inactivate viruses than bacteria.
Previous work highlighted that a free chlorine residual in the range of 0.2–0.5 mg l−1 for municipal wastewater is sufficient to disinfect the SARS virus readily (Wang et al., 2005). In one of the earliest studies on virus inactivation, Weidenkopf (1958), reported rate of inactivation of poliovirus 1 as a function of FAC and pH at 0 °C. While investigating effectiveness of chlorine solutions (0.1% available chlorine) against six enteric viruses, Engelbrecht et al. (1980) reported broad range of susceptibility of different virus to chlorine disinfection. pH is a critical factor in virus inactivation; the inactivation rate is higher at lower pH (pH 6) than at higher pH (pH 10), yet also with a variation in the relative sensitivity with respect to the different viruses. pH is the regulating factor that controls dissociation of HOCI to the less microbicidal form OCl−. With increasing pH conversion of undissociated HOCI to OCl‑ occurs and disinfecting efficacy of Cl decreases. Hence at pH more than 7 the time required for attaining same degree of inactivation increases by approximately 50% or up to six-fold (Weidenkopf, 1958; Clarke et al., 1956). It is important to understand the speciation of chlorine in wastewater and their relative abundance within the disinfection process, chlorine/chloramines speciation specific to wastewater being treated (Naddeo and Liu, 2020). At a turbidity ≤1 NTU, chlorination can effectively inactivate viruses (LeChevallier et al., 2004). The main concerns in effective chlorination are presence of ammonia, pH and chlorine demand for other co-pollutants. In presence of ammonia Cl binds to it forming combined chlorine (chloramines), which is not as effective against viruses as free chlorine, hence, it is necessary to ensure that Cl is not taken up by other demanding substrate (ammonia, ferrous ion, nitrites, hydrogen sulfide, and organic matter). Chlorine based products are generally neutralized by organic matter posing insignificant or short term environmental risk for soil and plants (Bruins and Dyer, 1995). However, concern has been raised for residual chlorine reaction with organic matter (humic acid and fulvic acid, present in soil) forming primarily trihalomethanes (THM) and haloacetic acids as degradation by-products which are toxic (Bull et al., 1990). THM are potentially carcinogenic chemicals with severe health impact, thereby EPA has regulated its concentration at 80ppb in drinking water. Metal corrosion and odour are the other two concerns of these products.
Hypochlorites (aqueous solutions of 5.25%–6.15% NaOCl) are the most predominantly used chlorine disinfectant. Sodium hypochlorite at a minimum free Cl concentration of 5000 ppm could achieve 3 log reductions in one minute on coronavirus 229E as reported by Sattar et al. (1989) in one of the earliest investigation. Hypochlorite was found to be better virucide than chlorine dioxide against SARS-CoV as reported by Wang et al., 2005. They found that chlorine solution (as supplied through hypochlorite) with more than 10 mg l−1 chlorine (FAC >0.4 mg l−1) could completely inactivate SARS-CoV after 30 min of disinfection, while at 20 mg l−1 Cl dose it is 1 min or more. Dellanno et al. (2009) also demonstrated 3 log reduction of surrogate coronavirus MHV by common disinfectant containing 0.21% sodium hypochlorite against in a 30 s contact period. Ansaldi et al. (2004) reported complete inactivation of SARS-CoV after incubating in 0.05% concentration of hypochlorite solution after a contact time of less than 1 min. SARS-CoV could be successfully inactivated by 3 log reduction using 0.05 and 0.1% concentration of sodium hypochlorite within a contact period of 5 min (Lai et al., 2005). 1:100 sodium hypochlorite solution was found to successfully produce 0.35 log reduction for TGEV virus and 0.62 log reduction for MHV in 1 min of contact time, which are recommended surrogate for coronavirus (Hulkower et al., 2011). Only in the very recent work by Zhang et al., 2020), disinfection by sodium hypochlorite (contact period 1.5 h, dose 800–6700 g m−3) was studied to remove RNA of SARS-CoV-2 in septic tanks. The study demonstrated need of revision of the current recommended disinfection strategies by WHO (FAC ≥0.5 mg l−1 after at least 30 min) and China Centers for Disease Control and Prevention (FAC≥ 6.5 mg l−1 after 1.5 h contact) to completely eradicate SARS-CoV-2 in decentralized disinfection system, as significant viral activity was observed after a dose of 800 g m−3. Kampf, 2020 also indicated 0.21% sodium hypochlorite solution as effective to produce 4 log reduction of SARS-CoV-2 within a minute.
Though it has broad spectrum of microbicidal activity, in general, at higher pH levels, as the hypochlorite ion, it is a slower virucide; in the presence of ammonia, as ammonia chloramine, it is further slower; and in the presence of organic nitrogen, as an organic chloramine, it is an even slower virucide. EPA has catagorized hypochlorites as disinfectant with no unreasonable adverse effects to the environment. It can be a suitable virucide even at small scale treatment system than other chlorine disinfectants due to relatively lower residual toxicity, simple equipment, fast activity, stable performance, easier control and lower operation costs (Yu et al., 2014). The performance efficiency remains unaffected by water hardness, but inactivation by organic matters, release of toxic Cl gas upon reaction with ammonia or acid, and less relative stability are main concerns post application (Rutala and Weber, 2015). Another issue with hypochlorite use includes its metal corrosion at high concentrations (>500 ppm) (Emmanuel et al., 2004). Production of the animal carcinogen THM as by-product by haloform reaction with organic substances is a major health concern (Rutala et al., 2008). WHO suggested removal of the organic matter from the wastewater first through pre-filtration before disinfection with chlorine to reduce THM.
Hypochlorous acid (HOCl) is an effective virucidal agent that damages the genome and protein mediated functions (Wigginton and Kohn, 2012). Its virucidal efficiency is more than 50 times higher than that of the chloramines (Kelley and Sanderson, 1958, 1960). Hakim et al. (2015) evaluated hypochlorous solutions for their virucidal ability against avian influenza virus H7N1. They found that 100 and 200 ppm concentration of the disinfectant could inactivate the virus immediately after spraying, while at 50 ppm strength at least 3 min of contact time was required. A contact period of 5–10 min is generally claimed for effective killing of pathogen by hypochlorous acid. Recently, Block and Rowan (2020), indicated that hypochlorous acid can be used with a high predictability for SARS-CoV-2 virus disinfection. Hypochlorous acid can be an excellent disinfectant for non turbid waters that are free of ammonia and organic compounds (Zhang et al., 2016). The main concern during its application is loss of disinfection efficiency for natural loss of available chlorine in long term storage, hence it should preferably be used within three months of manufacturing. Exposure to UV and solar radiation, air contact and a temperature greater than 25 °C decreases its stability (Block and Rowan, 2020). The formation of THM as by-product after reaction with naturally occurring organic matter is also a factor that might restrict its application, which needs to take care (Michael et al., 2020).
Chlorine dioxide (ClO2) as a disinfectant has several advantages over chlorine. It may be a possible alternative to Cl and can be most ideal for virus inactivation (Sanekata et al., 2010). ClO2 can be adsorbed into the capsomeres protein of virus and react with RNA. It appears as an effective microbicide under the pH, temperature and turbidity generally prevailing in WTP (US National Research Council, 1980). In one of the earliest studies by Harakeh et al. (1987), efficacy of ClO2 was investigated against bacteriophage f2, poliovirus 1, echovirus 1, coxsackievirus B5, simian rotavirus (SA11) and human rotavirus. Except a required dose of 17.25 ppm (residual 5 ppm after 1 min) for the most resistant coxsackie virus, all other viruses were inactivated by dose of 15.25 ppm (residual 4 ppm after 1 min) or less. Similarly, more remarkable inactivation effect of ClO2 on viruses such as Coxsackie B3, Poliovirus-1, ECHO-11, Herpes simplex virus 1, Adenovirus-7 and Mumps virus than that of liquid chlorine was reported by Junli et al. (1997). ClO2 was effective under a wider pH range at a dosing of 1.0 mg l−1. Report by White (1999) revealed that ClO2 is even more effective than ozone and chlorine against some particular viruses. However, particularly for SARS-CoV, (Wang et al., 2005) reported less efficacy of chlorine dioxide than chlorine. ClO2 could successfully inactivate SARS-CoV only after 30 min of disinfection at a dose of 40 mg l−1 (FAC 2.19 mg l−1). However, (Kim et al., 2016) reported successful inactivation of murine coronavirus after direct exposure to ClO2 gas at a concentration 0.16 ppmv min−1. The authors observed 3.5 times reduction after an exposure of 6 h and detected no viable virus after 12 h of exposure.
The advantages of using ClO2 include no formation of potentially toxic by-products like THM, no reaction with ammonia, its powerful oxidation over a broad pH range (pH 3–7, higher at alkaline pH), capacity to decolourize, deodorize; while the concerns are; reaction with oxidizable material, unstability (must be generated at the point of use), potential explosiveness production of halogenated organic compounds (Harakeh et al., 1987). ClO2 is 700 times more volatile than HOCl and may escape during treatment, especially over cooling towers. The predominant end product is chlorite (ClO2 -), a regulated drinking water pollutant with a maximum permissible level of 1.0 mg l−1 (USEPA, 2003a).
Sodium dichloro isocyanurate (C3Cl2N3NaO3) (NaDCC) is the sodium salt of a chlorinated hydroxytriazine. It is a colourless, water-soluble solid requiring a treatment time of 5–10 min for disinfection. It is and easy-to-use source of free chlorine increasingly used as drinking water disinfectant and for household point-of-use water treatment. For disinfection of public water systems, a quantity of NaDCC necessary to produce at least 0.2 ppm of FAC is suggested. The FAC for anhydrous NaDCC is 63% and the dihydrate is 56%. Therefore, to develop 1 mg l−1 FAC typical for drinking water treatment, 1.6 mg l−1 of anhydrous NaDCC and 1.8 mg l−1 for the dehydrate are required (Kuznesof, 2004). NaDCC tablets are stable over hypochlorites and retain Cl longer thus produce a more prolonged microbicidal effect. Microbicidal activity of NaDCC solutions appears to be greater than that of NaOCl containing same total available Cl because, in NaDCC only 50% of the chlorine is FAC (62% of it is available Cl), leaving “reservoir Cl” (monochloroisocyanurate or dichloroisocyanurate) that is released once the original FAC is used up to restore the equilibrium (Clasen and Edmondson, 2006). Second, NaDCC solutions are acidic where microbicidal HOCl−is prevalent, unlike sodium hypochlorite solutions. Dissolution of NaDCC in water produces complex equilibria among chlorinated and non-chlorinated isocyanurates and FAC in the form of hypochlorous acid. Findings reported by Clasen and Edmondson (2006) and Jain et al. (2010) indicated suitability of NaDCC as a feasible alternative water disinfection method for routine water treatment due to no serious health concern and its adherence to water treatment recommendations.
This is an EPA registered disinfectant, which has been reported effective against several Norovirus. Toxicity study indicated no toxicity of this compound, it is not carcinogenic, fetotoxic, teratogenic or mutagenic (Clasen and Edmondson, 2006). Any residue of NaDCC on contact with saliva is immediately converted to cyanuric acid. Chlorinated isocyanurates are not bioaccumulative and are not metabolized in body. In 2003, JECFA (Joint Food and Agriculture Organization/WHO Expert Committee on Food Additives recommended 0–2.0 mg NaDCC kg−1 of body weight per day as the tolerable daily intake of anhydrous NaDCC in treated drinking water (WHO, 2008). Unlike Cl, NaDCC remains effective by releasing free Cl over a wide pH range (at high pH also). At high concentration (>40 mg l−1) it might increase COD, not much effect is found up to 10 mg l−1. As per Sodium Dichloroisocyanurate market (2019–2027) report, rising demand for chlorinating agents in the water treatment industry is projected to significantly drive significant growth and expansion opportunity in the global NaDCC market in the upcoming years. EPA's effective chemical list (List N) against SARS Cov-2 also includes NaDCC as one of the active ingredients to destroy the virus. Though no report could be found on its application for wastewater treatment against SARSCoV-2 it may be further explored considering its virucidal property as well as environmental compatibility.
Chloramaine is another class of combined chlorinated compounds which utilization has recently increased. In spite of being a weaker oxidizing and disinfecting agent as compared to HOCl acid and ClO− ion and with slower viral inactivation rate, they possess advantages such as better stability, releasing chlorine over long period of time (US National Research Council, 1980; WHO, 2004). Kelly and Sanderson (1958) reported that under the conditions of pH 7, temperature range 25 °C-28 °C, 0.2- 0.3 mg l−1 free Cl inactivated 99.9% of enteric viruses (Polioviruses and Coxsackie virus) in 8 min, whereas combined chlorine resulted in 99.7% inactivation of the viruses at a dose of 0.7 mg l−1 and contact period of minimum 4 h. A pH in the range of 6–7 is better for better performance by the disinfectant (Kelly and Sanderson, 1958, 1960) indicating more virucidal activity of dichloramine (predominant at that pH 6–7). Sattar et al. (1989) reported successful 3 log reduction of coronavirus 229E at a minimum free Cl level of 3000 ppm at a contact time 1 min. Chloramine tablets are frequently used by the military for emergency purification of water. US EPA recommended 4.0 mg l−1 as the Maximum Residual Disinfectant Level for public water systems as the enforceable maximum safety level for chloramines (measured as chlorine, Cl2) (WQA, 2013). In USA, number of drinking water facilities now use monochloramine as a secondary disinfectant to reduce by-product formation as well as biofilm development (Cromeans et al., 2010). Since cholaramines react slowly, they can penetrate into biofilm eventually inactivating embedded virus, however; consequently, they might produce long lasting residual (Symons et al., 1977). Chloramines are not preferred as primary disinfectant due to weak action, but it may be good choice for secondary disinfection because of low by-product formation and its stability and persistence which is beneficial to generate residual protection in distribution network (Earth Tech, 2005). In systems using monochloramine, free chlorine, ozone or chlorine dioxide is usually applied as primary disinfectant to meet the necessary biocidal efficiency, before addition of ammonia (WQA, 2013). Ammonia addition results in monochloramine as the residual chemical, with longer persistence and reduced risk of THM formation. Using chloramines along with Cl for will produce a persistent disinfection effect with less by-product formation thereby incresing effectiveness of monochloramine as an ideal alternative for virus abatement.
The above discussion implies that standard chlorination based disinfection system of wastewater treatment unit and hyper chlorination should be sufficient to inactivate the virus, provided sufficient availability and persistence of FAC during and post treatment is ensured (WEF, 2020a). Further, removal of the organic matter from the wastewater through pre-filtration before disinfection with chlorine to reduce possibility of THM formation may be an effective strategy to achieve desirable disinfection level as well environmental and health safety. Table 1 shows the different Cl based disinfectants in terms of their viral abatement and applied doses. Since limited works were reported about their efficacy against coronavirus and in water environment, we have compiled data that used surrogate coronavirus and other related virus and also from surface disinfection study.
Table 1.
Viral abatement efficacy of Cl based disinfectants (with particular focus on coronavirus or surrogate coronavirus).
| Cl based disinfectants | Viral abatement | Experimental medium | Dose and effect | Reference |
|---|---|---|---|---|
| Hypochlorite | Canine coronavirus, mouse hepatitis virus | Suspension | Effective at 10 and 100 ppm concentration, contact period: 10 min | Saknimit et al., 1988 |
| SARS-CoV | Wastewater | Complete inactivation At 10 mg l − 1 Cl (FAC >0.4 mg l − 1), contact period: 30 min At 20 mg l − 1 Cl, contact period: 1 min |
||
| SARS-CoV-2 | Septic tank | At 800–6700 g m − 3, contact period 1.5 h | Zhang et al., 2020 | |
| SARS-CoV surrogate (mouse hepatitis virus (MHV) and transmissible gastroenteritis virus (TGEV) | Surface | At 1:100 solution 0.35 log reduction in 1 min for TGEV 0.62 log reduction in 1 min for MHV |
Hulkower et al., 2011 | |
| Human coronavirus 229E | Surface | 3 log reduction at a minimum free Cl level of 5000 ppm, contact time: 1 min | Sattar et al., 1989 | |
| SARS-CoV | Incubation in Disinfectant solution | 3 log reduction, at a concentration of 0.05 and 0.1% contact period:5 min | Lai et al., 2005 | |
| SARS-CoV | Incubation in Disinfectant solution | Complete inactivation at 0.05%, contact time: less than 1 min | Ansaldi et al., 2004 | |
| SARS-CoV-2 | Suspension | For 4 log reduction, at 0.21%, contact period: 1 min | Kampf, 2020 | |
| Surrogates of norovirus (feline calicivirus (FCV), murine norovirus (MNV), and coliphage (MS2)) | Surface | 3 log reduction at 5000 ppm contact time: 1.9 min (FCV), 3.2 min (MNV), 4.5 min (MS2) | Park and Sobsey, 2011 | |
| Surrogates of norovirus (murine norovirus, feline calicivirus) | Surface | 5 log reduction At 2700 ppm, contact period: 1 min | Chiu et al., 2015 | |
| coxsackie virus A16 (CAV16) and enterovirus 71 (EV71). | Surface | Complete inactivation At 3120 ppm, contact period: 5 min | Kadurugamuwa and Shaheen, 2011 | |
| Hypochlorous acid | Poliovirus | Wastewater | 99% inactivation by Cl concentration 0.4–0.8 mg l−1 within 22–46 s | Kott et al., 1975 |
| Murine Norovirus | Surface | ≥99.9% (≥3 log10) reductions at 20 to 200 ppm of HOCl solution within 10 min | Park and Sobsey., 2011 | |
| Avian influenza virus H7N1 | Surface | Complete inactivation At 100 & 200 ppm immediately after spraying At 50 ppm, contact time 3 min | Hakim et al., 2015 | |
| Chloramine | Enteric virus | Sewage | 99.7% inactivation At of 0.7 mg l − 1, contact period: minimum 4 hr | Kelly and Sanderson, 1958 |
| coronavirus 229E | Surface | 3 log reduction at a minimum free Cl level of 3000 ppm, contact time: 1 min | Sattar et al., 1989 | |
| Chlorine dioxide | bacteriophage f2, polio 1, echo 1, coxsackie B5, simian and human rotavirus | Sewage effluent | Complete inactivation At 15.25 ppm, contact period: 1 min | Harakeh et al., 1987 |
| SARS-CoV | Wastewater | Complete inactivation At 40 mg l−1 (FAC 2.19 mg l−1), contact period: 30 min | ||
| Feline calicivirus (F9 strain), HAV (strain HM-175), Coxsackie B5 | Water | Complete inactivation FCV: at 0.8 mg l−1: 2 min, at 0.2 mg l−1:30 min Coxsackie: at 0.4 mg l−1: 4 min, at 0.2 mg l−1: 30 min HAV: at 0.8 mg l−: 30 s, at 0.4 mg l−1:5 min |
Zoni et al., 2007 | |
| Influenza A virus | Surface | ≥5log10 reduction by a concentration of 0.14 mg m−3 within 5 h | Morino et al., 2011 | |
| Murine coronavirus A59 | Direct exposure to gas | Concentration 0.16 ppmv/min, 3.5 times reduction after 6 h, No viable virus after 12 h | Kim et al., 2016 | |
| Human influenza virus | Suspension | 99.99% inactivation at 10 ppm, contact period: 15 s | Sanekata et al., 2010 | |
| Free Chlorine | Coxsackievirus B5 (CVB5), echovirus 1 (E1), murine norovirus (MNV), and human adenovirus 2 | Ground water, Surface water | At 0.2 and 1 mg l−1 Most effective for MNV (3-log10 CT values ≤0.020 to 0.034) Least effective for CVB5 (3-log10 CT values 2.3 to 7.9) |
Kahler et al., 2010 |
5.2.2. Hydrogen peroxide
In many biological treatment methods H2O2 is added as a source of dissolved oxygen in pretreatment of high strength wastewater where bio treatment may not be practical and in pre-digestion of wastewaters which contain varying levels of toxic compounds. Liquid chemical disinfection might be alternatively used due to advantages of simple operation and rapid start-up, though use of H2O2 for full-scale wastewater disinfection is limited (Wagner et al., 2002). It is safer, healthier oxidizing option typically available at a concentration of 3% which is effective at fighting against bacteria, fungi, yeasts, viruses and spores. Excessive damage to viral nucleic acids, membrane lipids and other cell components (for which viruses do not have repair mechanisms); by OH−radicals generated by H2O2 is the primary mechanism of virucidal effects of H2O2 (McDonnell, 2009). Mental and Schmidt (1973) reported inactivation of three serotypes of rhinovirus using a 3% H2O2 solution in 6–8 min, a time which rose to 18–20 min at 1.5% and 50–60 min at 0.75%. Under a quantitative carrier test, a 7% H2O2 proved to be virucidal (Poliovirus type 1) at 5 min and bactericidal at 3 min at a 1:16 dilution as reported by Sattar et al. (1998). Omidbakhsh and Sattar (2006) evaluated activity of 0.5% accelerated H2O2 based disinfectant against several enveloped and non-enveloped viruses (Poliovirus type 1, HIV-1, Feline calicivirus, Human coronavirus, Herpes virus type 1 and 2, Human rhino virus, Human rotavirus, Influenza virus, Bovine viral diarrhoea virus) at 20 °C. This virucidal activity carried out under the presence of 5% of serum (mixture of proteins) is quantified as more than 4 Log10 reduction of infectivity within a minute. Exposure of H2O2 vapour (20 μl) to TGEV, a coronavirus surrogate on stainless steel for 2–3 h was found to result in approximately a 5 log10 (TCID 50 ml−1) reduction (Goyal et al., 2014). Apart from microbicidal effect, H2O2 oxidizes both organic and inorganic pollutants contributing to reduction in BOD and COD (by up to 85%) (Ksibi, 2006). Bruins and Dyer (1995) suggested a concentration of 50–250 mg l−1 of H2O2 to disinfect treated wastewater.
In terms of health and environmental impacts, H2O2 is extremely safe as it yields water and oxygen upon dissolution and thus non-pollutant. It is suitable for application at high temperature and concentrations often with immediate effect. Under normal conditions, H2O2 is substantially stable provided it is stored properly. However, high operational cost due to chemical cost associated with peroxide is likely to prohibit its consideration as a primary disinfectant in wastewater applications. H2O2 being a strong oxidizing agent, the type and degree of treatment provided at treatment facility and quality of treated wastewater are important to be assessed if it causes any effluent toxicity.
5.2.3. Quaternary ammonium compounds
Quaternary ammonium compounds (positively charged derivatives of ammonium compounds, NR4 +) are environmental friendly option for wastewater treatment. A common quaternary ammonium compound is Benzalkonium Chloride (BKC). The hydrophilic cationic region of BKC establishes electrostatic interactions with negatively charged components of pathogen's surface and thus destabilizes it (McDonnell and Russel, 1999). Through membrane destruction, it is efficient to act against bacteria, some enveloped viruses, fungi, yeasts and protozoa (Fazlara and Ekhtelat, 2012). Therefore, enveloped viruses such as HIV, hepatitis B virus, influenza virus are all susceptible to BKC (McDonnell and Russel, 1999).
BKC was found to inactivate influenza, measles, vaccinia, canine distemper, meningopneumonitis, rabies, fowl laryngotracheitis, Semliki Forest, feline pneumonitis and herpes simplex viruses after 10 min of exposure at 30 °C or at room temperature (Armstrong and Froelich, 1964). Saknimit et al. (1988) investigated virucidal activity of BKC against the canine coronavirus and mouse hepatitis virus, Kilham rat virus and canine parvovirus. BKC showed sufficient efficacy and could readily inactivate coronaviruses, whereas the two parvoviruses were relatively less susceptible. The antiviral action of BKC was assessed against a number of enveloped and non-enveloped human viruses (herpesvirus type 1, HIV-1 and a human coronavirus) using a suspension test method, in which coronavirus showed higher resistance than enveloped viruses (Wood and Payne, 1998). The use of 1% (1000 ppm) BKC against SARS-CoV resulted in a loss of virus viability, though PCR detection of viral RNA occurred 30 min after exposure (Ansaldi et al., 2004). Rabenau et al. (2005) showed BKC based surface disinfectant to inactivate SARS-CoV below the limit of detection with a reduction factor >4. A BKC concentration of 0.1% was found virucidal for Adenovirus Ad19, Ad3, Ad7a, Ad5 and Ad37 (Romanowski et al., 2019). In general, quaternary compounds are reported to be effective against influenza viruses (Schrank et al., 2020). Hence, potential efficacy of such compounds against SARS-CoV-2 is postulated based on the comparable outer membranes structure (relatively similar phospholipid bilayers) between influenza and SARS-CoV-2 virus by Schrank et al. (2020). However, earlier studies also reported that due to limited activity of quaternary ammonium compounds against virus, it might be necessary to combine it with other disinfectants for better results (Bruins and Dyer, 1995).
The concern of use of such compound is that, their biocidal activity may be neutralized quickly if used in hard water situations, by soap residues and organic matters (Bruins and Dyer, 1995). They have low toxicity, degrade rapidly in the environment and may be used under broad pH conditions and are non-corrosive to metals.
5.2.4. Organic peroxides
As chorine based disinfectants are having environmental concerns, peracids or peroxyacids are increasingly considered as one of the most promising and widespread disinfectant alternatives in wastewater, sewage and effluent treatment. Due to their broad spectrum microbicidalproperty, absence of dangerous disinfection byproducts and high oxidizing power, the use of peracids as disinfectant for wastewater is drawing increased attention in recent times (Kitis, 2004; Rossi et al., 2007; Luukkonen et al., 2015; Luukkonen et al., 2014).
Peracetic acid (PAA) is a reliable, proven disinfectant with a wide range of microbicidal activity (Wagner et al., 2002; Antonelli et al., 2013). In general, the disinfection efficiency of PAA towards different microbes can be ranked as follows; bacteria> viruses>bacterial spores>protozoan cysts (Kitis, 2004). The biocidal form is thought to be the undissociated acid (i.e. CH3CO3H) predominant at pH ≤4.7 (Liberti et al., 1999). Disinfectant property is attributed to the active oxygen released that disrupts sulfhydryl (–SH) and sulfur (S–S) bonds of enzymes present inside cell membrane of pathogen (Liberti et al., 1999). According to the EPA, PAA commercially available at 5% and 15% concentration generally is a stronger oxidizing agent than hypochlorite or ClO2, but not as strong as O3. Wastewater characteristics, concentration of PAA, time of exposure, reactor configuration define its effectiveness (EPA fact sheet, 2012). WHO has also included PAA among recommended virucide of SARS-CoV-2.
For viruses, the PAA dosage range is broad (12–2250 ppm) and relatively high concentrations is required to attain significant virus inactivation in sewage effluent (20–140 ppm) (Lazarova et al., 1998; CDC, 2008). Although coronaviruses have not been tested in water environment, PAA is shown to have some efficacy against some other non-enveloped viruses (e.g., norovirus) which is supposed to have higher resistance than enveloped viruses (WEF, 2020b). Ansaldi et al. (2004) reported that 35 ppm solution of PAA could disrupt SARS-CoV-1 replication in cell culture with <2 min of contact period, whereas the same concentration was not found to produce any affect after 30 min of exposure; needing further research investigation. While studying inactivation of rotaviruses, enteroviruses and bacteriophages by PAA in sewage effluent, Harakeh (1984) reported that relatively high concentrations of acid were required to achieve significant inactivation. In this study, the most resistant Human rotavirus required 140 ppm to achieve 99.99% inactivation, while 20 ppm was sufficient to get same level of disinfection with the least resistant simian rotavirus. Earlier laboratory studies found it effective against viruses (Echovirus, Coxsackievirus and poliovirus; polio virus being the most resistant) typically found in sewage (Baldry et al., 1991). This study reported retention of PAA's activity even under high organic load condition in wastewater. Viral activity studies by Lazarova et al. (1998) showed that different bacteriophages showed different sensitivities to PAA, and dose required for virus is higher than that required for bacteria. At 120 min of contact time, 10 mg l−1 PAA could achieve 7.5 log reduction of the bacteriophage Ø X174 which rose to 500 mg l−1 PAA achieving 3.5 log reduction of more resistant bacteriophage MS2.
Several studies reported number of advantages of PAA, for which PAA may be considered as an alternative for other conventional disinfectants having far greater adverse environmental impact. Most importantly PAA is receiving attention because of no or reduced harmful byproducts formation (Martin et al., 2013; Martin, 2014). Its environmental impact appears to be small, as it breaks down into water, oxygen and acetic acid. These degradation products are not toxic, carcinogenic or mutagenic and are not required to be removed or neutralized from treated water (Monarca et al., 2001; Stampi et al., 2002). PAA possesses a wide spectrum of microbicidal activity, even in the presence of heterogeneous organic substance, which appears to be quite promising over other traditional disinfectants (Kitis, 2004). PAA's disinfection action is reported to be negligibly affected by suspended solids concentration in the range 10–40 mg l−1, often yielding satisfactory inactivation up to 100 mg l−1 of suspended solid (Lefevre et al., 1992; Stampi et al., 2001). Further, adopting PAA disinfection requires minimum retrofit in WTP, as existing Cl contact tanks can be used (EPA fact sheet, 2012). Other desirable attributes of PAA are ease of implementation, higher stability than bleach or chlorine, lower freezing point, no formation of chlorinated disinfection byproduct (THM), quick reaction time, satisfactory disinfection performance in the presence of organics, no quenching requirement, requires lower concentration or contact time to achieve the target microbial kill, less influence of wastewater quality, such as pH, suspended solid, nitrate and ammonia, and effectiveness for both primary and secondary effluents.
Major concern during usage of PAA is increase in organic content (BOD) of wastewater under treatment due to addition of acetic acid and potential microbial regrowth (Kitis, 2004; Rossi et al., 2007). However, such addition is not likely to be significant, as the BOD is partially offset by the dissolved oxygen generated from the decomposition of the PAA and H2O2 components of the PAA solution (www. peroxychem.com, 2016, accessed on 5.7.2020). PAA is very reactive with brass, copper, iron and zinc and might ruin such finishing. Another drawback is the chemical cost of PAA due to limited worldwide production however; as per the recent study by Bettenhausen (2020), its cost is expected to decrease, as more and more numbers of plants are adopting PAA based disinfection.
Performic acid (CH2O3) (PFA), a well-known oxidizing agent and disinfectant is a mixture of hydrogen peroxide (35%) and formic acid (10 to 20%) with stabilizing substances, mixed in the ratio 1:1 (Lasik et al., 2013). In commercial wastewater disinfection, it is relatively a newer addition with significant environmental and financial promise, which has been shown to be successful with easy installation and steady state service (Gehr et al., 2009; Lasik et al., 2013; Ragazzo et al., 2013; Chhetri et al., 2014; Chhetri et al., 2015). It is a wide spectrum disinfectant showing efficiency against viruses, bacteria and bacterial spores, bacilli and fungus and exhibit high antibacterial activity also in low temperatures (Heinonen-Tanski and Miettinen 2010). Gehr et al. (2009) reported successful application of PFA to an advanced primary effluent recalcitrant to disinfection by UV and peracetic acid.
Just like PAA, PFA possesses several advantages over chlorine while being more effective against viruses. Disinfection tests revealed PFA to be a more potent disinfectant than PAA and perpropionic acid (Luukkonen et al., 2015). In one of the earlier studies by Mĕrka and Horácek (1979), it was demonstrated that the antiviral activity of PFA against Coxsackie virus B 1 is greater than that of PAA under the given set of experimental conditions. Bydzovska and Merka (1981) reported over 5 log reductions of bacteriophage Ø x 174 in wastewater at PFA doses of 0.025 ml l−1 (25 mg l−1) and contact time of 5 min. Karpova et al. (2013) reported efficiency of PFA in inactivating viruses (MS2-coliphages, DNA coliphages) even at low doses, resistance being MS2-coliphages > DNA-coliphages > enterococci. However, the efficiency was shown to depend upon effluent quality as background organics might also account for some PFA demand. Only a low dose of 0.5 mg l−1 for 10 min was sufficient to disinfect the effluent and to stop microbial re-growth over 24 h.
The by-products of PFA dissolution are H2O2 and formic acid, none of which has any eco-toxicological effect (Gehr et al., 2009). Though PFA tends to form by-product under high bromide concentrations, Ragazzo et al. (2013) confirmed no observation of such by-product formation in real operational conditions. This was further supported by Karpova et al. (2013) who reported formation of significantly lower level of organically bound halogens compared to that of Cl disinfection. Since PFA has less stability than PAA and tends to decompose faster, its degradation leads to greater amount of reactive oxygen formation, making disinfection faster and powerful than PAA (Ripin et al., 2007). PFA also works at low temperature condition (below 25 °C) and can be applied in cold region or during winter (Heinonen-Tanski and Miettinen, 2010). Luukkonen et al. (2015) estimated lower operational costs of PFA based disinfection (0.0114 € m−3) as compared to PAA (0.0261 € m−3). The authors reported thatfor small scale WTP, the investment cost of PAA could be lower than for PFA, and PFA would become more economical for larger plants. The main user concern for PFA is its instability, making it necessary to prepare afresh prior to use, storage at below 20 °C and can release a large amount of energy if not prepared and controlled carefully (Ripin et al., 2007; Gehr et al., 2009). In terms of shelf life and decomposition of residual concentration, PFA was more unstable than PAA (Luukkonen et al., 2015). PFA is also expected to have very high mobility in soil. Considering the overall broad scale application prospects, compliance with microbiological criteria for various water reuse applications and environmental advantages, increased research is necessary to establish when contemplating PFA based full-scale applications.
5.2.5. Ozonation
Ozone is an effective, clean oxidizing agent possessing strong microbicidal effect against bacteria, viruses, and protozoan (Hudson et al., 2009; Tizaoui, 2020). Ozone is effective in destroying viruses by attacking the viral protein (Wigginton and Kohn 2012). Microbes get inactivated through O3 acting on the cytoplasmic membrane by breaking apart lipid molecules at sites of multiple bond configuration. Further, when ozone comes in contact with virus capsid proteins, protein hydroxides and protein hydroperoxides are formed creating oxidative stress, against which viruses do not possess any protective strategy (Sunnen, 1997).
Currently no reports could be found on ozone disinfection in wastewater environment against SARS-CoV-2, however, it is expected to be effective against the virus as ozonation was successfully used against similar corona virus SARS-CoV-1 (Schwartz et al., 2020). In a recent analysis, Tizaoui (2020) suggested use of ozone as effective oxidant against SARS-CoV-2 as it can disrupt proteins and lipids of virus's spikes and envelope, particularly tryptophan, methionine cysteine, and the fatty acids, arachidonic, linoleic and oleic acid and N-glycopeptides of the spike protein subunits 1 and 2. Viruses are generally more resistant to O3 than bacteria, although phages seem to be more susceptible than human viruses (Langlais et al., 1991). In general, a typical initial O3 dose of 3–10 mg l−1 and contact time of 10 min, which results in Ct (product of the concentration of a disinfectant and the contact time with the water being disinfected) values between 30 and 100 mg min l−1, much lower than chlorination is reported successful for ozonation (Paraskeva and Graham 2002). By ozonation Ct value as low as 0.5 mg min l−1 and 1 mg min l−1 were also found to achieve 6 and 4 log inactivation of studied virus, respectively (Burns et al., 2007; Sigmon et al., 2015). Gehr et al. (2003) suggested ozone as highly effective against MS-2 coliphage, achieving over 3 logs inactivation for a dose of 17 mg l−1, making it a suitable consideration to target virus. Hudson et al., 2009 showed susceptibility of range of virus (corona, adeno, herpes, vaccinia, yellow fever, sindbis, influenza, rhino, stomatitis, polio) against O3 exposure on different surfaces by at least 3 log10 reduction.
Ozone is a powerful disinfectant that can improve the biological water quality in lower contact time, concentration and with higher efficiency. Its short half-life may allow treated water to be released without any environmental concern. The issue related to ozonation is increase in the water acidity level (Zaied et al., 2020). Due to its instability in water, it is often unable to provide a stable disinfectant residual with no continuous disinfection effect.. The operation cost of ozone preparation is still high (Arslan et al., 2017). Ozone is highly toxic, reactive and has several health impacts even at nominal concentration and is one of six common contaminants limited by the USEPA. Because of its short half-life, ozone it is generally suggested as a primary disinfectant as it is unable to maintain persistent residuals in treatment network and therefore has to be applied along with secondary disinfectant such as Cl, chloramines or ClO2 for a complete disinfection effect (Earth Tech, 2005). Further, the treatment sometimes may not be adequate due to reduced contact time caused by its shorter half-life. For an effective disinfection by ozonation, potential ozone demand by certain inorganics, organics, and suspended solids needs to be evaluated (Gehr and Nicell, 1996). Because of its instability, it is required to be generated at the point of application.
5.2.6. Ultraviolet irradiation
UV disinfection technology is gaining increased interest in water purification due to its efficacy against almost all waterborne pathogens including some relatively resistant microbial contaminants (Hijnen et al., 2006). Under UV light virus loses the capacity to replicate and infect due to damage caused to genome and protein (disruption of phosphodiester bond, cross-links to other molecules) (Wigginton and Kohn 2012). Virus becomes sterile as Thymine bases on viral nucleic acid react with UV light to form dimers (thymine–thymine double bonds) that inhibit transcription and replication of nucleic acids (WHO International, 2020). UV lamps emit significant radiation in the range in which nucleic acids absorb energy (240–260 nm).
Viruses are considered to be among the relatively resistant microbes against UV disinfection (Earth Tech, 2005). Chevrefils et al. (2006) compiled UV dose required to achieve incremental log inactivation of viruses based on reported values published. Based on previous literature the authors reported a range of 2–21, 3.5–105 and 10–210 mJ cm−2 for 1, 2 and 5 log reduction of virus, respectively, where Adeno virus was seen to be the most resistant and Ø X 174 phage as the most susceptible. It has been shown to successfully inactivate MS2 phage, Ø X 174 phage, Canine calcivirus, Adenovirus, Polio virus, Coxsackievirus, Reo virus, Rota virus, Hepatitis virus, Echo virus and coronavirus. Saknimit et al. (1988) initially showed virucidal efficacy of UV radiation within 15 min of exposure against mouse canine coronavirus, hepatitis virus, Kilham rat virus and canine parvovirus. Based on observation of virus (phage) diversity and concentration, Blatchley et al. (2007) found better performance of UV as a virucide than chlorination under given set of conditions. SARS-CoV could be inactivated by an exposure to UV as shown by Darnell et al. (2004). In their study, an exposure of UV-C (254 nm, dose 4016 µW cm−2) light resulted in partial inactivation (400-fold decrease in infectious virus) at 1 min with increasing efficiency up to 6 min. Virus became completely inactivated after 15 min below detection limit, whereas, no significant effects of UV-A ((254 nm, dose 2133 µW cm−2) exposure was observed on virus over a 15 min exposure period. UV light irradiation at 134 µW cm−2 for 15 min decreased the infectivity from 3.8 × 107 to 180 TCID50 ml−1; however, no further removal of residual virus was observed following prolonged irradiation (60 min), leaving 18.8 TCID50 ml−1 (Kariwa et al., 2006). As compared to relatively resistant MS2 and adenovirus aerosols, susceptibility of coronavirus aerosols was 7–10 times higher to an UV exposure (254 nm) dose of 2608 µW s cm−2 (Walker and Co, 2007). Bedel et al. (2016) investigated efficacy of an automated triple-emitter whole room UV-C disinfection system to inactivate MERS-CoV viruses on surfaces. The study reported undetectable virus levels with a >5 log10 reduction with an exposure time of 5 min, that remained undetectable following 30 min of total exposure. With the development of UV based advanced oxidation technology, such as UV–H2O2,UV–Cl2, UV–O3 and UV–TiO2, the possibilities of using reactive photolysis radicals to inactivate viruses is being increasingly explored (Zhang et al., 2016). Using a SARS coronavirus strain CoV-P9, Duan et al., 2003 found that irradiation of UV for 60 min was sufficient to destroy the viral infectivity at an undetectable level.
UV irradiation is a clean and effective disinfection technology because of its high viral inactivation efficiency and no generation of disinfection or oxidation by-product (Zhang et al., 2016). The technology has other merits such as no need of external chemical addition, easy installation and operation and non-corrosive. Further UV disinfection being a physical process, water quality parameters such alkalinity, temperature, pH do not have notable impact on the disinfection performance (USEPA, 1999). In some cases, the method is still considered energy intensive and expensive, however, possibility of solar or wind powered UV at relatively low cost is also on its way (WHO International, 2020).
Securing optimal efficiency by UV lamps in virus inactivation is dependant on the penetration of the radiation (typically at 254 nm) through water, which sometimes is challenging. Disinfection effect may be significantly obstructed by biofouling of lamp by algae, turbid and coloured substances shielding microorganisms (Malley and Burris, 2001; Salgot et al., 2002). UV radiation cannot provide residual disinfection functions in water post treatment. Therefore, the use of a secondary disinfectant followed by UV is recommended to provide residual protection and to ensure redundancy of microbial protection (USEPA, 2003b).
5.2.7. Sunlight mediated wastewater disinfection
The sunlight mediated wastewater disinfection is an applicable and feasible option in many types of aquatic environments (Nelson et al., 2018). Solar disinfection of drinking water also known as SODIS is being globally promoted and implemented as a low-cost water treatment method at household level. Solar radiation has been shown advantageous in terms of cleanliness, minimum operation and maintenance cost with satisfactory inhibitory effect of virus inactivation for small scale treatments.
Sunlight mediated virus inactivation depends on radiation strength, season, optical and physicochemical properties of wastewater and the type of virus (Verbyla and Mihelcic, 2015). Mechanism of virus inactivation by sunlight occurs through three processes viz. i. direct mechanism that requires absorption of photons directly by virus or a endogenous component such as nucleic acids, proteins, other biomolecules (initiated by absorption of UV-B fraction of solar light) resulting in structural change; ii. Indirect disinfection occurs when an endogenous or exogenous components absorbs a photon and directs the production of photo produced reactive intermediates that, in turn, damage virus or cell components (Bosshard et al., 2013; Nelson et al., 2018).
Fisher et al. (2011) investigated effect of simulated sunlight for inactivating a double stranded DNA bacteriophage PRD1 and a single-stranded RNA bacteriophage MS2 in clear water. After an exposure of 22 h to simulated sunlight (directly or through filter with 50% cutoff, wavelengths range from 280 to 350 nm), both UVA (320–400 nm) and UV-B (280–320 nm) light could inactivate PRD1, while only UV-B could inactivate MS2. Somatic phage, bacteriophage and bovine rotavirus were all found to get inactivated completely (3 log unit reduction) in less than 3 h of full sunshine (McGuigan et al., 2012). Destruction of nucleic acids through the formation of pyrimidine dimers or other products has been shown as the primary effect by sunlight against viruses such as norovirus and bacteriophage GA (Flannery et al., 2013). In another study sunlight was successful in inactivating human viruses (adenovirus type 2, poliovirus type 3) and bacteriophages (MS2, Q-Beta SP, Fi, M13, PRD1, Ø X174, and coliphage), where adenovirus type 2 and bacteriophage MS2 was relatively resistant (Love et al., 2010). Silverman et al. (2013) studied how sunlight inactivation of adenovirus type 2, poliovirus type 3, and bacteriophage (MS2 and PRD1) varies with respect to natural water constituents in coastal waters. The study reported influence of water quality on absolute and relative inactivation rates of viruses, which is significant for developing natural sunlight-based treatment unit.
A recent research from National Biodefense Analysis and Counter measures centre, U.S. Department of Homeland Security provides the first data on the influence of simulated sunlight on the survival of SARS-CoV-2 suspended in simulated saliva or culture media and suggests that sunlight may have direct impact on survival of the virus (Ratnesar-Shumate et al., 2020). The study showed that under simulated sunlight (representative of midday on summer solstice at 40°N latitude), 90% of infectious virus gets inactivated in every 6.8 min in simulated saliva and in every 14.3 min in culture media. The inactivation was still significant under lower simulated light levels, but at a slower rate (Ratnesar-Shumate et al., 2020). Same research group also reported effect of simulated sunlight on SARS-CoV-2 in aerosols. 90% of the viral loss was observed in 19 min and 8 min under simulated sunlight typical of late winter/early fall and summer were, respectively, in absence of which it took 286 min to achieve 90% loss (Schuit et al., 2020). In another latest work by Sagripanti and Lytle (2020), the authors predicted inactivation of the virus by the UV-B in sunlight in different populated cities of the world. The study indicated relatively faster (faster than influenza A) inactivation of SARS-CoV-2 during summer, indicating potential significant role of sunlight on its occurrence and transmission. They reported 90% or more of SARS-CoV-2 virus will be inactivated after 11‐34 min exposure of midday sunlight in most of the US cities during summer. Table 2 compiled various potential wastewater disinfectants against SARS-CoV-2, their comparative advantages, virucidal effect, concerns during usage and environmental concerns.
Table 2.
Suggested wastewater disinfection method during Covid-19, their advantages, concerns during usage and environmental concerns.
| Method | Effectiveness | Advantage | Concern | Environmental concerns |
|---|---|---|---|---|
| Peracetic acid | Stronger oxidant than HOCl or ClO2, less than O3 Broad spectrum of activity against microbes |
|
|
|
| Performic acid | More effective against viruses than peracetic acid, Wide-spectrum disinfectant. |
|
|
|
| UV | High viral inactivation efficiency |
|
|
|
| Ozonation | Higher microbicidal efficacy than chlorine, Very strong oxidant |
|
|
|
| Sodium dichloro isocyanurate | Broad germicidal action |
|
|
|
| Hypochlorite | Broad spectrum of antimicrobial activity |
|
|
|
| Chloramine | Viral inactivation is slower than free chlorine, weaker |
|
|
|
| Hypochlorous acid | Virucidal efficiency more than 50 times greater than chloramines |
|
|
|
| Chlorine dioxide | Effective against a wide range of pathogens |
|
|
|
| Benzalconium chloride | Relatively limited spectrum of activity |
|
|
|
| Hydrogen peroxide | Effective against virus |
|
|
|
| Solar radiation | Sufficient effect of virus inactivation |
|
|
|
5.3. A multiple disinfection system barrier
The use of multiple disinfection barriers is receiving attention to ensure and maximize the efficiency of current disinfectants. As removal of SARS-CoV- 2 by the current conventional water treatment procedures is yet to be confirmed, it is imperative to take extra precaution by combining different compatible disinfection strategies to ensure complete eradication of the viruses from water (Venugopal et al., 2020). A system with multiple disinfection steps provides synergistic benefits, enhanced reliability, robustness and flexibility for water disinfection (IWA, 2020). The main advantage of combined disinfection system is less influence of the influent flow and water quality on its efficacy and broad spectrum of activity against multiple biological contaminants and residual protection in water system (IWA, 2020).
Effectiveness of combined disinfection during COVID-19 outbreak was shown by research team from Tsinghua University, China (IWA-Network, 2020). The combined treatment processes consisting of ozonation, UV treatment, and chlorination (sodium hypochlorite) achieved 99.99% inactivation of faecal coliform based on influent water quality and no SARS-CoV-2 was detected post disinfection. The findings indicated that employing ozonation improved UV transmittance by 20–30% in the water, consequently UV dose could be reduced.
Koivunen and Heinonen-Tanski (2005) evaluated the efficiency of combined PAA/UV and H2O2/UV treatments to see any synergistic microbial inactivation on coliphage MS2 virus. As compared to enteric bacteria, the combined PAA/UV treatment resulted in lower disinfection efficiency and synergistic benefit for coliphage MS2. In another similar work, as compared to disinfection by PAA and UV radiation separately, their combined treatment showed superior efficacy in inactivation of coliphages under doses of 2, 3, and 4 mg l−1 of PAA and contact time of 10 min and 60 and 90 s of UV exposure (Beber de Souza et al., 2015). Combined PAA/UV disinfection allows reductions in lamp intensity and frequency of lamp cleaning (Martin, 2014). The combination of ozone with UV irradiation provides a robust energy efficient disinfection option without the need of additional secondary disinfection process (Gassie and Englehardt, 2017). It was reported by Fang et al. (2014) that only ozone could produce 0.3 log removal of MS2 coliphage at a dose of 0.1 mg l−1 in tap water, which increased to 4 log reductions under combined O3/UV treatment, with a UV dose of 35 mJ cm−2. Fang et al. (2014) found that the synergistic effect on MS2 inactivation was less prominent after the combined UV/O3 co-exposure (0.2 log inactivation), and more prominent after the sequential O3/UV and UV/O3 exposures (0.8 log inactivation) at ozone dose of 0.1 mg l−1 and UV dose of 8.55 mJ cm−2 in ultrapure water. Generally, if UV is the first disinfection step, followed by ozone, inclusion of a fine filtration system may be appropriate to make the UV step more effective (IWA, 2020).
Advanced oxidation processes (AOP) are relatively new development in water treatment which may be hydroxyl radical based, ozone based, Fenton related, Sulphate radical based and UV based AOP (Deng and Zhao, 2015). These methods are used worldwide for sewage water, groundwater, drinking water, industrial wastewater for recalcitrant organic matter removal and disinfection. The methods have significantly higher oxidation potential to degrade relatively difficult organic matter, without the production of residues or sludge. H2O2 can be applied alone or along with catalysts such as UV, O3, iron (Fe2+ or Fe3+) and alkali. The ozone & hydrogen peroxide (peroxone) AOP is one of the most potent, energy efficient and effective microbicide used in water treatment (USEPA, 1999). H2O2 in combination with UV radiation is reported to be more efficient as germicide and degrading organic matter in water (Amin et al., 2008). Bounty et al. (2012) investigated inactivation of adenovirus under UV-H2O2 AOP process. While 4 log reduction of adenovirus was achieved at an UV dose of about 200 mJ cm−2, addition of 10 mg l−1 H2O2 could help achieve 4 log inactivation at a lower UV dose of 120 mJ cm−2. The use of iron as a catalyst along with H2O2 (referred to as Fenton process) is a common wastewater treatment approach, which is suitable for treatment of low COD wastewater (Stasinakis, 2008).
Though use of multiple disinfection barriers has been shown successful against bacterial contaminants, not much work has been done on virus removal. Hence disinfection efficiency of such advanced processes may further be explored against virus contaminated water. For combined disinfection in wastewater, different combinations of disinfectants (primary and secondary) can be considered. However, the workable combinations and sequence of the disinfection processes should be carefully determined, as different treatment influences water quality in different way, which in turn can affect the performance of disinfectant used in sequence (Earth Tech, 2005). Different wastewater treatment strategies and concerns that should be taken in to account in wastewater treatment sector during Covid-19 is presented in Fig. 2 .
Fig. 2.
Wastewater treatment strategies and concerns during Covid-19.
Based on the discussion on strategies for viral contaminated wastewater disinfection, present study summarized following points that need immediate attention: 1) Need of additional research to reassure the effectiveness, adequacy and proper usage of traditional wastewater disinfectants; 2) Need of generation of data from water quality experiments through close and regular monitoring of disinfection performance (in terms of contact time, disinfectant dose, residual persistance, pH, temperature); 3) Viral populations being mostly resistant; it is necessary to consider all factors influencing virus survival; 4) Need of augmenting the disinfection dosages or employing additional (multiple) disinfection barrier as a core step in protection and prevention; 5) Need of evaluation of individual inactivation strategy in actual water treatment plants to select the most appropriate disinfection technology; 6) Environmental impacts must also be taken into consideration while considering the advantages provided by disinfectants; 7) Need to establish coronaviruses specific quantitative disinfection kinetics using appropriate virus surrogate; 8) Need of more coronavirus specific research for emerging disinfectants such as peracetic acid performic acid, sodium dichloro isocyanurate, chloramines, chlorine dioxide, benzalconium chloride; 9) Need of making ongoing research data (details of experimental conditions) available in public domain so that major conclusions can be drawn on their fate in water environment through a global approach.
6. Conclusions
The current assessment shows that SARS-CoV-2 transmission can also be potentially linked to water and wastewater and the risks associated with wastewater sector in COVID-19 pandemic need to be rapidly assessed to put in place appropriate control measures. The assessment shows that till now though the presence of the virus is confirmed at sewage plants, no data could be found on usage of existing disinfection methods in real wastewater condition in treatment plants. Presently, the immediate challenges to account in wastewater management are fate about this new biothreat in water environment, relying on prediction based treatments options, increased risk of passing out of the virus to sewage network by asymptomatic carrier, inadequacy of wastewater treatment facility particularly in populated developing countries and the increased use and generation of wastewater due to increased cleanliness concern of people. To ensure minimum human exposure to this new biothreat through water transmission pathway, contaminated water from hospitals, clinics, testing facilities, isolation wards, quarantine centres must be treated and disinfected properly before being discharged; particularly in areas where wastewater treatment is not adequate and regulated and high natural precipitation and local inundation are common. It seems to be very urgent for such facilities to connect to central wastewater treatment plant or to setup decentralized treatment system with single or multiple disinfection barriers for minimizing any potential risk of public exposure or wastewater transmission of the virus. In absence of confirmation of current water treatment routine to effectively eliminate SARS-CoV-2, it is imperative to take extra precaution through multiple treatment barriers to ascertain complete removal of the viruses from water. The use of certain emerging disinfectants (peracetic acid, performic acid, sodium dichloro isocyanurate, chloramines, chlorine dioxide, benzalconium chloride) shows prospects in terms of virucidal properties. Nonetheless, additional scientific information is required on various fields on viral disinfection strategies in wastewater. To fight against the kind of viral pandemic, investigation strategy on wastewater treatment may include regular testing of efficiency and dosage of selected disinfectants taking into account all factors influencing viral survival, multiple performance evaluation in fields, with different environmental conditions, effect of contaminated wastewater having varied qualitative and quantitative components and environmental implications of the disinfection technology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors sincerely acknowledge the Director, Defence Research Laboratory, Tezpur. SK also acknowledges DRDO fellowship. The authors apologize for the many colleagues who are not referenced in this work due to space limitations.
References
- Abad F.X., Pintó R.M., Bosch A. Disinfection of human enteric viruses on fomites. FEMS Microbiol. Lett. 1997;156(1):107–111. doi: 10.1111/j.1574-6968.1997.tb12713.x. [DOI] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. The Tot. Environ. 2020 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin H., Amer A., Elfecky A., Ibrahim I. Treatment of textile waste water using H2O2/UV system. Physicochem. Problems Miner. Process. 2008;42:17–28. [Google Scholar]
- Amirian E.S. Potential fecal transmission of SARS-CoV-2: current evidence and implications for public health. Inter. J. Infect. Dis. 2020;95:363–370. doi: 10.1016/j.ijid.2020.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansaldi F., Banfi F., Morelli P., Valle L., Durando P., Sticchi L., Contos S., Gasparin R., Crovari P. SARS CoV, influenza A and syncitial respiratory virus resistance against common disinfectants and ultraviolet irradiation. J. Prev. Med. Hyg. 2004;45:5–8. [Google Scholar]
- Antonelli M., Turolla A., Mezzanotte V., Nurizzo C. Peracetic acid for secondary effluent disinfection: a comprehensive performance assessment. Water. Sci. Technol. 2013;68(12):2638–2644. doi: 10.2166/wst.2013.542. [DOI] [PubMed] [Google Scholar]
- Armstrong J.A., Froelich E.J. Inactivation of viruses by benzalkonium chloride. Appl. Environ. Microbiol. 1964;12(2):132–137. doi: 10.1128/am.12.2.132-137.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan A., Topkaya E., Özbay B., Özbay I., Veli S. Application of O3/UV/H2O2 oxidation and process optimization for treatment of potato chips manufacturing wastewater. Water Environ. J. 2017;31:64–71. [Google Scholar]
- Asami T., Katayama H., Torrey J.R., Visvanathan C., Furumai H. Evaluation of virus removal efficiency of coagulation-sedimentation and rapid sand filtration processes in a drinking water treatment plant in Bangkok, Thailand. Water Res. 2016;101:84–94. doi: 10.1016/j.watres.2016.05.012. [DOI] [PubMed] [Google Scholar]
- ASTM, 2020, Available at: https://www.astm.org/standardization-news/?q=features/surrogates-and-fight-against-covid-19-.html.
- AWWA, 2020, Utility Actions to Sustain Operations During COVID-19, Available: https://www.awwa.org/Portals/0/AWWA/Education/Webinars/2020PDFs/W200320_COVID-19_Handouts.pdf (accessed on 10.7.2020).
- Baldry M.G.C., French M.S., Slater D. The activity of peracetic acid on sewage indicator bacteria and viruses. Water Sci. Technol. 1991;24(2):353–357. [Google Scholar]
- Barcelo D. An environmental and health perspective for COVID-19 outbreak: meteorology and air quality influence, sewage epidemiology indicator, hospitals disinfection, drug therapies and recommendations. J. Environ. Chem. Eng. 2020;8(4) doi: 10.1016/j.jece.2020.104006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basel International, 2020. Available at:http://www.basel.int/Default.aspx?tabid=8376, Accessed on 27.6.2020.
- Beber de Souza J., Queiroz Valdez F., Jeranoski R.F., Vidal C.M.D.S., Cavallini G.S. Water and wastewater disinfection with peracetic acid and UV radiation and using advanced oxidative process PAA/UV. Inter. J. Photoenergy. 2015;2015 [Google Scholar]
- Bedell K., Buchaklian A.H., Perlman S. Efficacy of an automated multiple emitter whole-room ultraviolet-C disinfection system against coronaviruses MHV and MERS-CoV. Infec. Cont. Hos. Epidemiol. 2016;37(5):598–599. doi: 10.1017/ice.2015.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatchley E.R., III, Gong W.L., Alleman J.E., Rose J.B., Huffman D.E., Otaki M., Lisle J.T. Effects of wastewater disinfection on waterborne bacteria and viruses. Water Environ. Res. 2007;79(1):81–92. doi: 10.2175/106143006x102024. [DOI] [PubMed] [Google Scholar]
- Block M.S., Rowan B.G. Hypochlorous acid–a review. J. Oral Maxillofacial Surgery. 2020 doi: 10.1016/j.joms.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshard F., Armand F., Hamelin R., Kohn T. Mechanisms of human adenovirus inactivation by sunlight and UVC light as examined by quantitative PCR and quantitative proteomics. Appl. Environ. Microbiol. 2013;79(4):1325–1332. doi: 10.1128/AEM.03457-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bounty S., Rodriguez R.A., Linden K.G. Inactivation of adenovirus using low-dose UV/H2O2advanced oxidation. Water Res. 2012;46(19):6273–6278. doi: 10.1016/j.watres.2012.08.036. [DOI] [PubMed] [Google Scholar]
- Bruins G., Dyer J.A. Environmental considerations of disinfectants used in agriculture. Revue Scientifique et Technique-Office International des Epizooties. 1995;14 doi: 10.20506/rst.14.1.826. 81-81. [DOI] [PubMed] [Google Scholar]
- BullR J., Gerba C., Trussel R.R. Evaluation of the health risks associated with disinfection. Crit. Rev. Env. Control. 1990;20(2):77–113. [Google Scholar]
- Burns N., Hunter G., Jackman A., Hulsey R., Coughenour J., Walz T. The return of ozone and the hydroxyl radical to wastewater disinfection. Ozone Sci. Eng. 2007;29:303–306. [Google Scholar]
- Bydzovska O., Merka V. Disinfecting properties of performic acid against bacteriophage (X 174 as a model of small envelope-free viruses. J. Hygiene Epidemiol. Microbiol. Immunol. 1981;25(4):414–423. [PubMed] [Google Scholar]
- Carducci A., Federigi I., Liu D., Thompson J.R., Verani M. Making waves: coronavirus detection, presence and persistence in the water environment: state of the art and knowledge needs for public health. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43:1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. Statpearls [internet] StatPearls Publishing; 2020. Features, evaluation and treatment coronavirus (COVID-19) [PubMed] [Google Scholar]
- Chan J.F.W., Yuan S., Kok K.H., To K.K.W., Chu H., Yang J., Xing F., Liu J., Yip C.C.Y., Poon R.W.S., Tsoi H.W. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.L. Modern concepts of disinfection. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1971;97:689–707. [Google Scholar]
- Chattopadhyay S., Taft S. Exposure Pathways to High-Consequence Pathogens in the Wastewater Collection and Treatment Systems. US Environmental Protection Agency, Cincinnati, OH. EPA/600/R-18/221. 2018 [Google Scholar]
- Chevrefils G., Caron É., Wright H., Sakamoto G., Payment P., Barbeau B., Cairns B. UV dose required to achieve incremental log inactivation of bacteria, protozoa and viruses. IUVA News. 2006;8(1):38–45. [Google Scholar]
- Chhetri R.K., Flagstad R., Munch E.S., Hørning C., Berner J., Kolte-Olsen A., Thornberg D., Andersen H.R. Full scale evaluation of combined sewer overflows disinfection using performic acid in a sea-outfall pipe. Chem. Eng. J. 2015;270:133–139. [Google Scholar]
- Chhetri R.K., Thornberg D., Berner J., Gramstad R., Ojstedt U., Sharma A.K., € Andersen H.R. Chemical disinfection of combined sewer overflow waters using performic acid or peracetic acids. Sci. Total Environ. 2014;490:1065–1072. doi: 10.1016/j.scitotenv.2014.05.079. [DOI] [PubMed] [Google Scholar]
- Chinawaterrisk.org, Available at:http://www.chinawaterrisk.org/resources/analysis-reviews/medical-wastewater-treatment-in-covid-times/, accessed on 7.72020.
- Chiu S., Skura B., Petric M., McIntyre L., Gamage B., Isaac-Renton J. Efficacy of common disinfectant/cleaning agents in inactivating murine norovirus and feline calicivirus as surrogate viruses for human norovirus. Amer. J. Infec. Con. 2015;43(11):1208–1212. doi: 10.1016/j.ajic.2015.06.021. [DOI] [PubMed] [Google Scholar]
- Clarke N.A., Stevenson R.E., Kabler P.W. The inactivation of purified type 3 adenovirus in water by chlorine. Am. J. Hyg. 1956;64:314–319. doi: 10.1093/oxfordjournals.aje.a119844. [DOI] [PubMed] [Google Scholar]
- Clasen T., Edmondson P. Sodium dichloroisocyanurate (NaDCC) tablets as an alternative to sodium hypochlorite for the routine treatment of drinking water at the household level. Int. J. Hyg. Environ. Health. 2006;209:173–181. doi: 10.1016/j.ijheh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Cromeans T.L., Kahler A.M., Hill V.R. Inactivation of adenoviruses, enteroviruses, and murine norovirus in water by free chlorine and monochloramine. Appl. Environ. Microbiol. 2010;76(4):1028–1033. doi: 10.1128/AEM.01342-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell M.E., Subbarao K., Feinstone S.M., Taylor D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Method. 2004;121(1):85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Zhao R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollution Rep. 2015;1(3):167–176. [Google Scholar]
- Dellanno C., Vega Q., Boesenberg D. The antiviral action of common household disinfectants and antiseptics against murine hepatitis virus, a potential surrogate for SARS coronavirus. Americ. J. infec. Con. 2009;37(8):649–652. doi: 10.1016/j.ajic.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterle M.E., Haslwanter D., Bortz R.H., III, Wirchnianski A.S., Lasso G., Vergnolle O., Abbasi S.A., Fels J.M., Laudermilch E., Florez C., Mengotto A. A replication-competent vesicular stomatitis virus for studies of SARS-CoV-2 spike-mediated cell entry and its inhibition. bioRxiv. 2020 doi: 10.1016/j.chom.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S.M., Zhao X.S., Wen R.F., Huang J.J., Pi G.H., Zhang S.X., Han J., Bi S.L., Ruan L., Dong X.P. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed. Environ. Sci. 2003;16:246–255. [PubMed] [Google Scholar]
- Earth Tech, 2005. Available at:https://www.gov.mb.ca/sd/waterstewardship/odw/reginfo/approvals/odw_chlorine_and_alternative_disinfectants.pdf. accessed on 26.6.2020.
- Economictimes, India, 2020. Available at:https://economictimes.indiatimes.com/news/politics-and-nation/could-wastewater-spread-the-novel-coronavirus/structural-makeup/slideshow/75619233. accessed on 25.6.2020.
- El Baz S., Imziln B. Can aerosols and wastewater be considered as potential transmissional sources of COVID-19 to humans? Euro. J. Environ. Public Health. 2020;4(2):em0047. [Google Scholar]
- Emmanuel E., Keck G., Blanchard J.M., Vermande P., Perrodin Y. Toxicological effects of disinfections using sodium hypochlorite on aquatic organisms and its contribution to AOX formation in hospital wastewater. Environ. Inter. 2004;30:891–900. doi: 10.1016/j.envint.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Engelbrecht R.S., Weber M.J., Salter B.L., Schmidt C.A. Comparative inactivation of viruses by chlorine. Appl. Environ. Microbiol. 1980;40(2):249–256. doi: 10.1128/aem.40.2.249-256.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA factsheet, 2012. Alternative Disinfection Methods Fact Sheet: peracetic Acid. Available at:https://www.epa.gov/sites/production/files/2019-08/documents/disinfection_-_paa_fact_sheet_-_2012.pdf.
- Fang J., Liu H., Shang C., Zeng M., Ni M., Liu W. E. coli and bacteriophage MS2 disinfection by UV, ozone and the combined UV and ozone processes. Front. Environ. Sci. Eng. 2014;8(4):547–552. [Google Scholar]
- Fazlara A., Ekhtelat M. The disinfectant effects of benzalkonium chloride on some important foodborne pathogens. American-Eurasian J. Agric. Environ. Sci. 2012;12(1):23–29. [Google Scholar]
- Fears A.C., Klimstra W.B., Duprex P., Hartman A., Weaver S.C., Plante K.C., Mirchandani D., Plante J.A., Aguilar P.V., Fernández D., Nalca A., Totura A., Dyer D., Kearney B., Lackemeyer M., Bohannon J.K., Johnson R., Garry R.F., Reed D.S., Roy C.J. Comparative dynamic aerosol efficiencies of three emergent coronaviruses and the unusual persistence of SARS-CoV-2 in aerosol suspensions. medRxiv. 2020 [Google Scholar]
- Fisher M.B., Love D.C., Schuech R., Nelson K.L. Simulated sunlight action spectra for inactivation of MS2 and PRD1 bacteriophages in clear water. Environ. Sci. Technol. 2011;45(21):9249–9255. doi: 10.1021/es201875x. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon J.E., Sagripanti J.L. Analysis of the survival of Venezuelan equine encephalomyelitis virus and possible viral simulants in liquid suspensions. J. Appl. Microbiol. 2008;105:1477–1483. doi: 10.1111/j.1365-2672.2008.03919.x. [DOI] [PubMed] [Google Scholar]
- Flannery J., Rajko-Nenow P., Keaveney S., O'Flaherty V., Doré W. Simulated sunlight inactivation of norovirus and FRNA bacteriophage in seawater. J. Appl. Microbiol. 2013;115:915–922. doi: 10.1111/jam.12279. [DOI] [PubMed] [Google Scholar]
- Gassie L.W., Englehardt J.D. Advanced oxidation and disinfection processes for onsite net-zero greywater reuse: a review. Water Res. 2017;125:384–399. doi: 10.1016/j.watres.2017.08.062. [DOI] [PubMed] [Google Scholar]
- Gehr R., Chen D., Moreau M. Performic acid (PFA): Tests on an advanced primary effluent show promising disinfection performance. Water Sci. Technol. 2009;59(1):89–96. doi: 10.2166/wst.2009.761. [DOI] [PubMed] [Google Scholar]
- Gehr R., Nicell J. Pilot studies and assessment of downstream effects of UV and ozone disinfection of a physicochemical wastewater. Water Qual. Res. J. Can. 1996;31(2):263–281. [Google Scholar]
- Geller C., Varbanov M., Duval R.E. Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses. 2012;4:3044–3068. doi: 10.3390/v4113044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehr R., Wagner M., Veerasubramanian P., Payment P. Disinfection efficiency of peracetic acid, UV and ozone after enhanced primary treatment of municipal wastewater. Water Res. 2003;37(19):4573–4586. doi: 10.1016/S0043-1354(03)00394-4. [DOI] [PubMed] [Google Scholar]
- Goyal S.M., Chander Y., Yezli S., Otter J. Evaluating the virucidal efficacy of hydrogen peroxide vapour. J. Hosp. Infect. 2014;86:255–259. doi: 10.1016/j.jhin.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2009;1:10. [Google Scholar]
- Hakim H., Thammakarn C., Suguro A., Ishida Y., Kawamura A., Tamura M., Satoh K., Tsujimura M., Hasegawa T., Takehara K. Evaluation of sprayed hypochlorous acid solutions for their virucidal activity against avian influenza virus through in vitro experiments. J. Vet. Medical Sci. 2015 doi: 10.1292/jvms.14-0413. 14-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harakeh M.S. Inactivation of enteroviruses, rotaviruses and bacteriophages by peracetic acid in a municipal sewage effluent. FEMS Microbiol. Lett. 1984;23(1):27–30. [Google Scholar]
- Harakeh S. The behavior of viruses on disinfection by chlorine dioxide and other disinfectants in effluent. FEMS Microbiol. Lett. 1987;44(3):335–341. [Google Scholar]
- Hasöksüz M., Kiliç S., Saraç F. Coronaviruses and SARS-COV-2. Turk. J. Med. Sci. 2020;50(SI–1):549‐556. doi: 10.3906/sag-2004-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen-Tanski H., Miettinen H. Performic acid as a potential disinfectant at low temperature. J. Food Proc. Eng. 2010;33:1159–1172. [Google Scholar]
- Hijnen W.A.M., Beerendonk E.F., Medema G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo) cysts in water: a review. Water Res. 2006;40(1):3–22. doi: 10.1016/j.watres.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Hudson J.B., Sharma M., Vimalanathan S. Development of a practical method for using ozone gas as a virus decontaminating agent. Ozone Sci. Eng. 2009;31:216–223. [Google Scholar]
- Hulkower R.L., Casanova L.M., Rutala W.A., Weber D.J., Sobsey M.D. Inactivation of surrogate coronaviruses on hard surfaces by health care germicides. Americ. J. Infect. Control. 2011;39(5):401–407. doi: 10.1016/j.ajic.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IWA . Vol. 2020. International Water Association; 2020. (COVID-19 and Safe Water Treatment Utilities Are in the Focus). (Accessed on: 3.7.2020) [Google Scholar]
- CDC, 2008. Peracetic acid sterilization guideline for disinfection and sterilization in healthcare facilities, Centre for Disease Control and Prevention(Accessed on 5.7.2020).
- Singhal, 2020. Every drop counts in these times of COVID-19. Available at:https://www.downtoearth.org.in/blog/water/every-drop-counts-in-these-times-of-covid-19-70881(Accessed on 4.7.2020).
- McLellan, N., Pernitsky, D., Umble, A., 2020. Coronavirus and the water cycle — here is what treatment professionals need to know, Available at:https://www.wateronline.com/doc/coronavirus-and-the-water-cycle-here-is-what-treatment-professionals-need-to-know-0001(Accessed on 1.7.2020).
- Jain S., Sahanoon O.K., Blanton E., Schmitz A., Wannemuehler K.A., Hoekstra R.M., Quick R.E. Sodium dichloroisocyanurate tablets for routine treatment of household drinking water in periurban Ghana: a randomized controlled trial. Americ. J. Tropic. Med. Hygiene. 2010;82(1):16–22. doi: 10.4269/ajtmh.2010.08-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junli H., Li W., Nenqi R., Li L.X., Fun S.R., Guanle Y. Disinfection effect of chlorine dioxide on viruses, algae and animal planktons in water. Water Res. 1997;31(3):455–460. [Google Scholar]
- Kadurugamuwa J.L., Shaheen E. Inactivation of human enterovirus 71 and coxsackie virus A16 and hand, foot, and mouth disease. Amer. J. Infec. Cont. 2011;39(9):788–789. doi: 10.1016/j.ajic.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Kam K, Yung C.F., Cui L., Lin R.T.P., Mak T.K., Maiwald M., Li J., Chong C.W., Nadua K., Tan N.W.H., Thoon K.C. A Well Infant With Coronavirus Disease 2019 With High Viral Load. Clin. Infect. Dis. 2020;71(15):847–849. doi: 10.1093/cid/ciaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G. Potential role of inanimate surfaces for the spread of coronaviruses and their inactivation with disinfectant agents. Infect. Prev. Pract. 2020;2 doi: 10.1016/j.infpip.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler A.M., Cromeans T.L., Roberts J.M., Hill V.R. Effects of source water quality on chlorine inactivation of adenovirus, coxsackievirus, echovirus, and murine norovirus. Appl. Environ. Microbiol. 2010;76(15):5159–5164. doi: 10.1128/AEM.00869-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariwa H., Fujii N., Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatol. 2006;212:119–123. doi: 10.1159/000089211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova T., Pekonen P., Gramstad R., Öjstedt U., Laborda S., Heinonen-Tanski H., Chávez A., Jiménez B. Performic acid for advanced wastewater disinfection. Water Sci. Technol. 2013;68(9):2090–2096. doi: 10.2166/wst.2013.468. [DOI] [PubMed] [Google Scholar]
- Kelly S., Sanderson W.W. The effect of chlorine in water on enteric viruses. Am. J. Public Health. 1958;48:1323–1334. doi: 10.2105/ajph.48.10.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S.M., Sanderson W.W. The effect of chlorine in water on enteric viruses. II. The effect of combined chlorine on poliomyelitis and coxsackie viruses. Am. J. Public Health. 1960;50:14–20. doi: 10.2105/ajph.50.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Shin B.H., Song K.J., Kim J.R., Kim K. Virucidal Effect of Gaseous Chlorine Dioxide on Murine Coronavirus A59. Available at: http://repository.ajou.ac.kr/handle/201003/13342?mode=full. 2016 [Google Scholar]
- Kitis M. Disinfection of Wastewater with Peracetic Acid: a Review. Environ. Int. 2004;30(1):47–55. doi: 10.1016/S0160-4120(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Koivunen J., Heinonen-Tanski H. Peracetic Acid (PAA) disinfection of primary, secondary and tertiary treated municipal wastewaters. Water Res. 2005;39:4445–4453. doi: 10.1016/j.watres.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Kott Y., Nupen E., Ross W. The effect of pH on the efficiency of chlorine disinfection and virus enumeration. Water Res. 1975;9:869–872. [Google Scholar]
- Ksibi M. Chemical oxidation with hydrogen peroxide for domestic wastewater treatment. Chem. Eng. J. 2006;119(2–3):161–165. [Google Scholar]
- Kuznesof, P.M., 2004. Sodium Dichloroisocyanurate (NaDCC–anhydrous and dihydrate). Chemical and Technical Assessment 61st JECFA FAO.
- La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: Occurrence, persistence and concentration methods-A scoping review. Water Res. 2020;115899 doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.Y., Cheng P.K., Lim W.W. Survival of severe acute respiratory syndrome coronavirus. Clin. Infect. Dis. 2005;41(7):67–71. doi: 10.1086/433186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlais B., Reckhow D.A., Brink D.R., editors. Ozone in Water Treatment: Application and Engineering. Routledge; 1991. [Google Scholar]
- Lasik M., Dobrucka R., Konieczny P. Impedimetric test for rapid determination of performic acid (PFA) biocidal activity toward Echerichia coli. Acta Scientiarum Polonorum Technologia Alimentaria. 2013;12(4):385–394. [Google Scholar]
- Lazarova V., Janex M.L., Fiksdal L., Oberg C., Barcina I., Pommepuy M. Advanced wastewater disinfection technologies: short and long term efficiency. Water Sci. Technol. 1998;38(12):109–117. [Google Scholar]
- LeChevallier M.W., Au K.K. IWA Publishing; 2004. Water Treatment and Pathogen Control. [Google Scholar]
- Lefevre F., Audic J.M., Ferrand F. Peracetic acid disinfection of secondary effluents discharged off coastal seawater. Water Sci. Technol. 1992;25(12):155–164. [Google Scholar]
- Liberti L., Lopez A., Notarnicola M. Disinfection with peracetic acid for domestic sewage reuse in agriculture. CIWEM J. 1999;13:262–269. [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol. Hepatol. 2020;5(6):533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love D.C., Silverman A., Nelson K.L. Human virus and bacteriophage inactivation in clear water by simulated sunlight compared to bacteriophage inactivation at a southern California beach. Environ. Sci. Technol. 2010;44(18):6965–6970. doi: 10.1021/es1001924. [DOI] [PubMed] [Google Scholar]
- Luukkonen T., Heyninck T., Rämö J., Lassi U. Comparison of organic peracids in wastewater treatment: disinfection, oxidation and corrosion. Water Res. 2015;85:275–285. doi: 10.1016/j.watres.2015.08.037. [DOI] [PubMed] [Google Scholar]
- Luukkonen T., Teeriniemi J., Prokkola H., Rämö J., Lassi U. Chemical aspects of peracetic acid based wastewater disinfection. Water SA. 2014;40(1):73–80. [Google Scholar]
- Maris P. Virucidal efficacy of eight disinfectants against pneumovirus, coronavirus and parvovirus. Ann. Rech. Vet. 1990;21:275–279. [PubMed] [Google Scholar]
- Martin H., Christophe S., Rachelle F.T., Morin S., Lamaudière A.L., Le S., Karine D., Pierre M. Comparison of the virucidal efficiency of peracetic acid, potassium monopersulfate and sodium hypochlorite on hepatitis A and enteric cytopathogenic bovine orphan virus. J. Appl. Microbiol. 2013;115:955–968. doi: 10.1111/jam.12297. [DOI] [PubMed] [Google Scholar]
- Martin, L., 2014. EPA Investigates Chlorine Alternative. Available at:https://www.wateronline.com/doc/epa-investigates-peracetic-acid-as-a-green-alternative-to-chlorine-0001(Accessed on 28.6.2020).
- Matto, M., Singhal, S., 2020. COVID-19: the need is to decentralise how we manage wastewater. Available at:https://www.downtoearth.org.in/blog/water/covid-19-the-need-is-to-decentralise-how-we-manage-wastewater-70991. Accessed on 5.7.2020.
- McDonnell G. The use of hydrogen peroxide for disinfection and sterilization applications. PATAI’S Chem. Funct. Groups. 2009:1–34. [Google Scholar]
- McDonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clinic. Microbiol. Rev. 1999;12(1):147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuigan K.G., Conroy R.M., Mosler H.J., du Preez M., Ubomba-Jaswa E., Fernandez-Ibanez P. Solar water disinfection (SODIS): a review from bench-top to roof-top. J. Hazard. Mater. 2012;235:29–46. doi: 10.1016/j.jhazmat.2012.07.053. [DOI] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 in sewage. MedRxiv. 2020 doi: 10.1101/2020.03.29.20045880. [DOI] [PubMed] [Google Scholar]
- Malley J.P., Jr., Burris B. In: Bridging the Gap: Meeting the World's Water and Environmental Resources Challenges, Proceedings from the World Water and Environmental Resources Congress 2001; May 20e24, 2001, Orlando, FL, USA. Phelps D., Shelke G., editors. American Society of Civil Engineers (ASCE); 2001. Ultraviolet disinfection; pp. 1–13. [Google Scholar]
- Mentel R., Schmidt J. Investigations on rhinovirus inactivation by hydrogen peroxide. Acta Virol. 1973;17:351–354. [PubMed] [Google Scholar]
- Mĕrka V., Horácek J. Die antivirale Wirkung der Perameisensäure [The antiviral activity of performic acid. Pharmazie. 1979;34(3):182–183. [PubMed] [Google Scholar]
- Michael S., Block D.M.D., Brian G., Rowan D.M.D.M.D. Hypochlorous acid – a review. J. Oral Maxillofacial Surgery. 2020 doi: 10.1016/j.joms.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monarca S., Feretti D., Zerbini I., Zani C., Alberti A., Richardson S.D. Studies of mutagenicity and disinfection by-products in river drinking water disinfected with peracetic acid or sodium hypochlorite. Proceedings of the IWA World Conference, Berlin, Germany, Oct. 15–19; London; International Water Association; 2001. [Google Scholar]
- Morino H., Fukuda T., Miura T., Shibata T. Effect of low-concentration chlorine dioxide gas against bacteria and viruses on a glass surface in wet environments. Lett. Appl. Microbiol. 2011;53:628–634. doi: 10.1111/j.1472-765X.2011.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naddeo V., Liu H. Editorial Perspectives: 2019 novel coronavirus (SARS-CoV-2): what is its fate in urban water cycle and how can the water research community respond? Water Res. Technol. Environ. Sci. 2020;2020 doi: 10.1039/d0ew90015j. [DOI] [Google Scholar]
- National Health Commission, China Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 7). Editor(s): wei, Pei-Fang (Released by National Health Commission & National Administration of Traditional Chinese Medicine on March 3, 2020) Chin. Med. J. 2020;133(9):1087–1095. doi: 10.1097/CM9.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Statistical Office, 2019, Drinking water, sanitation, hygiene and housing conditions in India.
- Nelson K.L., Boehm A.B., Davies-Colley R.J., Dodd M.C., Kohn T., Linden K.G., …, Nguyen T.H., Parker K.M., Rodriguez R.A., Sassoubre L.M., Silverman A.I., Wigginton K.R., Zepp R.G. Sunlight-mediated inactivation of health-relevant microorganisms in water: a review of mechanisms and modeling approaches. Environ. Sci. Processes Impacts. 2018;20(8):1089–1122. doi: 10.1039/c8em00047f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Surya K., Wiegand T., Buyukyoruk M., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. medRxiv. 2020 doi: 10.1101/2020.04.15.20066746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem L.D., Morgan B., Donner E., Short M.D. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Studies in Chem. Environ. Eng. 2020 doi: 10.1016/j.cscee.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omidbakhsh N., Sattar S.A. Broad-spectrum microbicidal activity, toxicologic assessment, and materials compatibility of a new generation of accelerated hydrogen peroxide-based environmental surface disinfectant. Americ. J. Infection Control. 2006;34(5):251–257. doi: 10.1016/j.ajic.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSHA OSHA, Standards and directives for COVID-19, United States of America Occupational Safety and Health Administration. Available at: https://www.osha.gov/ SLTC/covid-19/standards.html. 2020 [Google Scholar]
- Packman, A., 2020; https://news.northwestern.edu/stories/2020/05/risk-of-covid-19-transmission-through-wastewater/&fj=1.
- Paraskeva P., Graham N.J. Ozonation of municipal wastewater effluents. Water environ. Res. 2002;74(6):569–581. doi: 10.2175/106143002x140387. [DOI] [PubMed] [Google Scholar]
- Park G.W., Sobsey M.D. Simultaneous comparison of murine norovirus, feline calicivirus, coliphage MS2, and GII. 4 norovirus to evaluate the efficacy of sodium hypochlorite against human norovirus on a fecally soiled stainless steel surface. Foodborne Pathogens Dis. 2011;8(9):1005–1010. doi: 10.1089/fpd.2010.0782. [DOI] [PubMed] [Google Scholar]
- Pinon A., Vialette M. Survival of viruses in water. Intervirol. 2018;61(5):214–222. doi: 10.1159/000484899. [DOI] [PubMed] [Google Scholar]
- Pinto B., Rohrig B. Use of Chloroisocyanurates for Disinfection of Water. J. Chem. Educ. 2003;80:41–44. [Google Scholar]
- Quilliam R.S., Weidmann M., Moresco V., Purshouse H., O’Hara Z., Oliver D.M. COVID-19: the environmental implications of shedding SARS-CoV-2 in human faeces. Environ. Inter. 2020;140 doi: 10.1016/j.envint.2020.105790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenau H., Kampf G., Cinatl J., Doerr H. Efficacy of various disinfectants against SARS coronavirus. J. Hosp. Infect. 2005;61:107–111. doi: 10.1016/j.jhin.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragazzo P., Chiucchini N., Piccolo V., Ostoich M. A new disinfection system for wastewater treatment: performic acid full-scale trial evaluations. Water Sci. Technol. 2013;67(11):2476–2487. doi: 10.2166/wst.2013.137. [DOI] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnesar-Shumate S., Williams G., Green B., Krause M., Holland B., Wood S., Bohannon J., Boydston J., Freeburger D., Hooper I., Beck K. Simulated sunlight rapidly inactivates SARS-CoV-2 on surfaces. J. Infect. Dis. 2020;222(2):214–222. doi: 10.1093/infdis/jiaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C. Presence and vitality of SARS-CoV-2 virus in wastewaters and rivers. medRxiv. 2020 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripin D.H.B., Weisenburger G.A., am Ende D.J., Bill D.R., Clifford P.J., Meltz C.N., Phillips J.E. Execution of a performic acid oxidation on multikilogram scale. Org. Process. Res. Dev. 2007;11:762–765. [Google Scholar]
- Rohila S.K. COVID-19 outbreak: More hand washing can increase India’s water woes. Available at: https://www.downtoearth.org.in/blog/water/covid-19-outbreak-more-hand-washing-can-increase-india-s-water-woes-69900. 2020 [Google Scholar]
- Romanowski E.G., Yates K.A., Shanks R.M., Kowalski R.P. Benzalkonium chloride demonstrates concentration-dependent antiviral activity against adenovirus in vitro. J. Ocu. Pharmacol. Therap. 2019;35(5):311–314. doi: 10.1089/jop.2018.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S., Antonelli M., Mezzanotte V., Nurizzo C. Peracetic acid disinfection: a feasible alternative to wastewater chlorination. Water Environ. Res. 2007;79(4):341–350. doi: 10.2175/106143006x101953. [DOI] [PubMed] [Google Scholar]
- Rutala, W.A., Weber, D.J., 2008. Guideline for disinfection and sterilization in healthcare facilities, 2008.
- Rutala W.A., Weber D.J. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 2015. Disinfection, sterilization, and control of hospital waste; p. 3294. [Google Scholar]
- Sagripanti J.L., Lytle C.D. Estimated Inactivation of Coronaviruses by Solar Radiation With Special Reference to COVID‐19. Photochem. Photobiol. 2020 doi: 10.1111/php.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin A.H., Aysegul E., Pelin M.A., Yeliz D., Ahmet Y.C., Mahmut E.S., Ramazan A.O., Ali Muhittin T. 2019 novel coronavirus (COVID-19) outbreak: a review of the current literature. EJMO. 2020;4(1):1–7. [Google Scholar]
- Saknimit M., Inatsuki I., Sugiyama Y., Yagami K. Virucidal efficacy of physico-chemical treatments against coronaviruses and parvoviruses of laboratory animals. Jikken Dobutsu. 1988;37:341–345. doi: 10.1538/expanim1978.37.3_341. [DOI] [PubMed] [Google Scholar]
- Sanekata T., Fukuda T., Miura T., Morino H., Lee C., Maeda K.E.N., Araki K., Otake T., Kawahata T., Shibata T. Evaluation of the antiviral activity of chlorine dioxide and sodium hypochlorite against feline calicivirus, human influenza virus, measles virus, canine distemper virus, human herpesvirus, human adenovirus, canine adenovirus and canine parvovirus. Biocon. Sci. 2010;15(2):45–49. doi: 10.4265/bio.15.45. [DOI] [PubMed] [Google Scholar]
- Sattar S.A., Springthorpe V.S., Rochon M. A product based on accelerated and stabilized hydrogen peroxide: evidence for broad-spectrum germicidal activity. Can. J. Infect. Control. 1998;13:123–130. [Google Scholar]
- Sattar S.A., Springthorpe V.S., Karim Y., Loro P. Chemical disinfection of non-porous inanimate surfaces experimentally contaminated with four human pathogenic viruses. Epidemiol. Infect. 1989;102(3):493–505. doi: 10.1017/s0950268800030211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrank C.L., Minbiole K.P., Wuest W.M. Are quaternary ammonium compounds, the workhorse disinfectants, effective against severe acute respiratory syndrome-Coronavirus-2? ACS Infect. Dis. 2020;6(7):1553–1557. doi: 10.1021/acsinfecdis.0c00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit M., Ratnesar-Shumate S., Yolitz J., Williams G., Weaver W., Green B., Miller D., Krause M., Beck K., Wood S., Holland B., Bohannon J., Freeburger D., Hooper I., Biryukov J., Altamura L.A., Wahl V., Hevey M., Dabisch P. Airborne SARS-CoV-2 is rapidly inactivated by simulated sunlight. J. Infect. Dis. 2020;222(4):564–571. doi: 10.1093/infdis/jiaa334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A., Martínez-Sánchez, G., 2020, Potential use of ozone in SARS-CoV-2 / COVID-19, SOP: ISCO3/ EPI /00/04. Available:https://aepromo.org/coronavirus/pdfs_doc_ISCO3/Covid19_en.pdf(accessed on 15.82020).
- Sigmon C., Shin G.A., Mieog J., Linden K.G. Establishing surrogate–virus relationships for ozone disinfection of wastewater. Environ. Eng. Sci. 2015;32(6):451–460. [Google Scholar]
- Silverman A.I., Peterson B.M., Boehm A.B., McNeill K., Nelson K.L. Sunlight inactivation of human viruses and bacteriophages in coastal waters containing natural photosensitizers. Environ. Sci. Technol. 2013;47(4):1870–1878. doi: 10.1021/es3036913. 2013. [DOI] [PubMed] [Google Scholar]
- Stampi S., De Luca G., Onorato M., Ambrogiani E., Zanetti F. Peracetic acid as an alternative wastewater disinfectant to chlorine dioxide. J. Appl. Microbiol. 2002;93(5):725–731. doi: 10.1046/j.1365-2672.2002.01732.x. [DOI] [PubMed] [Google Scholar]
- Stampi S., De Luca G., Zanetti F. Evaluation of the Efficiency of Peracetic Acid in the Disinfection of Sewage Effluents. J. Appl. Microbiol. 2001;91(5):833–838. doi: 10.1046/j.1365-2672.2001.01451.x. [DOI] [PubMed] [Google Scholar]
- Stasinakis A.S. Use of selected advanced oxidation processes (AOPs) for wastewater treatment - a mini review. Global NEST J. 2008;10:376–385. [Google Scholar]
- Statista.com, 2020. Available at:https://www.statista.com/statistics/1104075/india-coronavirus-covid-19-public-private-testing-centers-by-state/. Accessed on 28.6.2020.
- Steinmann J. Surrogate viruses for testing virucidal efficacy of chemical disinfectants. J. Hospital Infect. 2004;56:49–54. doi: 10.1016/j.jhin.2003.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettenhausen, C.A., 2020. https://cen.acs.org/environment/water/peracetic-acid-changing-wastewater-treatment/98/i15.
- Sulabhenvis, 2020. Available at: sulabhenvis.nic.in, http://www.sulabhenvis.nic.in/Database/STST_wastewater_2090.aspx. accessed on 3.7.2020.
- Sunnen, G.V.A., 1997. Virology Primer: with Special Reference to Ozone.Available at:http://ozoneinmedicine.com/articles_virol.html.
- Symons J.M., Carswell J.K., Clarke R.M., Dorsey P., Geldreich E.E., Heffernam W.P., Hoff J.C., Love O.T., McCabe L.J., Stevens. A.A. Water Supply Research Division, U.S. Environmental Protection Agency; Cincinnati, Ohio: 1977. Ozone, Chlorine Dioxide, and Chloramines as Alternatives to Chlorine for Disinfection of Drinking Water: State of the Art; p. 84. [Google Scholar]
- Technavio Water and Wastewater Treatment Chemicals Market by Type, Application, and Geography - Forecast and Analysis 2020-2024. Research report. IRTNTR43392. 2020 [Google Scholar]
- Tizaoui C. Ozone: a Potential Oxidant for COVID-19 Virus (SARS-CoV-2) Ozone. 2020:1–8. [Google Scholar]
- UNICEF, 2018; https://www.unicef.org/wash/files/UNICEF_Game_plan_to_end_open_defecation_2018.pdf. accessed on 22.6.2020.
- USEPA, 1999. Wastewater Technology Factsheet, Ultraviolet Disinfection, United States Environment Protection Agency, EPA 832-F-99-064.
- USEPA, 2020. List N: disinfectants for Use Against SARS-CoV. Available at:https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2.
- USEPA Alternative disinfectants and oxidants guidance manual. United States. Environmental Protection Agency. Office of Water Programs Operations. 1999 [Google Scholar]
- USEPA . United States Environmental Protection Agency; Cincinnati, OH: 2003. Long Term 2 Enhanced Surface Water Treatment Rule – Toolbox Guidance Manual. [Google Scholar]
- USEPA . United States Environmental Protection Agency; Cincinnati, OH: 2003. Ultraviolet Disinfection Guidance Manual. [Google Scholar]
- Usman M., Muhammad F., Khalil H. Existence of SARS-CoV-2 in wastewater: implications for its environmental transmission in developing communities. Environ. Sci. Technol. 2020;13:7758–7759. doi: 10.1021/acs.est.0c02777. [DOI] [PubMed] [Google Scholar]
- US National Research Council . Vol. 2. National Academy Press; Washington, DC: 1980. (Drinking Water and Health). [Google Scholar]
- Venugopal A., Ganesan H., Raja S.S.S., Govindasamy V., Arunachalam M., Narayanasamy A., Sivaprakash P., Rahman P.K., Gopalakrishnan A.V., Siama Z., Vellingiri B. Novel wastewater surveillance strategy for early detection of COVID–19 hotspots. Curr. Opinion Environ. Sci. Health. 2020;17:8–13. doi: 10.1016/j.coesh.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbyla M.E., Mihelcic J.R. A review of virus removal in wastewater treatment pond systems. Water Res. 2015;71:107–124. doi: 10.1016/j.watres.2014.12.031. [DOI] [PubMed] [Google Scholar]
- Wagner M., Brumelis D., Gehr R. Disinfection of wastewater by hydrogen peroxide or peracetic acid: development of procedures for measurement of residual disinfectant and application to a physicochemically treated municipal effluent. Water Environ. Res. 2002;74(1):33–50. doi: 10.2175/106143002x139730. [DOI] [PubMed] [Google Scholar]
- Walker C.M., Ko G. Effect of ultraviolet germicidal irradiation on viral aerosols. Environ. Sci. Technol. 2007;41(15):5460–5465. doi: 10.1021/es070056u. [DOI] [PubMed] [Google Scholar]
- Wang X.W., Li J.S., Jin M., Zhen B., Kong Q.X., Song N., Xiao W.J., Yin J., Wei W., Wang G.J., Si B.Y. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126(1–2):171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Shen J., Ye D., Yan X., Zhang Y., Yang W., Li X., Wang J., Zhang L., Pan L. Disinfection technology of hospital wastes and wastewater: suggestions for disinfection strategy during coronavirus disease 2019 (COVID-19) pandemic in China. Environ. Poll. 2020 doi: 10.1016/j.envpol.2020.114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WateReuse Research Foundation . WateReuse Research Foundation; Alexandria: 2015. American Water Works Association, Water Environment Federation, National Water Research Institute, Tchobanoglous, G., Cotruvo, J., Crook, J., McDonald, E., Olivieri, A., Salveson, A., Shane Trussell, R. Framework for Direct Potable Reuse. [Google Scholar]
- WQA, 2013. Chloramine Fact Sheet., https://www.wqa.org/Portals/0/Technical/Technical%20Fact%20Sheets/2014_Chloramine.pdf.
- WEF, 2020. Available at:https://www.weforum.org/agenda/2020/05/statistics-are-key-to-understanding-covid-19/.
- WEF, 2020. Available at:https://www.weforum.org/agenda/2020/05/covid-19-will-hit-the-developing-worlds-cities-hardest-heres-why/.
- Weidenkopf S.J. Inactivation of type I poliomyelites virus with chlorine. Virol. 1958;5:56–67. doi: 10.1016/0042-6822(58)90005-9. [DOI] [PubMed] [Google Scholar]
- White G.C. Fourth Edition. John Wiley & Sons Inc.; USA: 1999. Handbook of Chlorination and Alternative Disinfectants. [Google Scholar]
- WHO . World Health Organization (WHO/SDE/WSH/03.04/83); Geneva: 2004. Monochloramine in Drinking-water. Background document For Preparation of WHO Guidelines for Drinking-Water Quality.https://www.who.int/water_sanitation_health/water-quality/guidelines/chemicals/chloramine-background.pdf Available at: [Google Scholar]
- WHO . World Health Organization (WHO/HSE/AMR/08.03/3); Geneva: 2008. Sodium Dichloroisocyanurate in drinking-water. Background document For Preparation of WHO Guidelines for Drinking-Water Quality.https://www.who.int/water_sanitation_health/dwq/chemicals/second_addendum_sodium_dichloroisocyanurate.pdf Available at: [Google Scholar]
- WHO Covid dashboard, 2020. Available at:https://covid19.who.int/, accessed on 12.7.2020.
- WHO, 2020. Water, sanitation, hygiene and waste management for COVID-19.
- Wigginton K.R., Kohn T. Virus disinfection mechanisms: the role of virus composition, structure, and function. Curr. Opinion Virol. 2012;2(1):84–89. doi: 10.1016/j.coviro.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J., Bahgat M., May E., Ford M., Butler J. Mineralisation and pathogen removal in gravel bed hydroponic constructed wetlands for wastewater treatment. Water Sci. Technol. 1995;32(3):49–58. [Google Scholar]
- Wood A., Payne D. The action of three antiseptics/disinfectants against enveloped and non-enveloped viruses. J. Hosp. Infect. 1998;38:283–295. doi: 10.1016/S0195-6701(98)90077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2020. Water, Sanitation, Hygiene, and Waste Management For the COVID-19 virus: Interim guidance, 23 April 2020 (No. WHO/2019-nCoV/IPC_WASH/2020.3) [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.M., Maday Y., Teyssou R., Richard E., Almayrac J.L., Moulin L. Evaluation of lockdown impact on SARS-CoV-2 dynamics through viral genome quantification in Paris wastewaters. medRxiv. 2020 doi: 10.2807/1560-7917.ES.2020.25.50.2000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- www.chinacdc.cn, Technical solution for emergency treatment of medical sewage contaminated by novel coronavirus, 2020.
- www.fao.org. Available at:http://www.fao.org/land-water/overview/covid19/circular/fr/. Accessed on 1.7.2020.
- www.hindu.com, 2020, https://www.thehindu.com/news/cities/chennai/coronavirus-metrowater-tests-show-prevalence-of-viral-rna-in-sewage-collected-from-chennai/article31485182.ece.
- www.peroxychem.com., 2016. Available at:https://www.peroxychem.com/media/164735/january-2016-paa-and-bod-and-do.pdf.
- Xu Y. Unveiling the Origin and Transmission of 2019-nCoV. Trends. Microbiol. 2020;28:239–240. doi: 10.1016/j.tim.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Shin W.I., Pang Y.X., Meng Y., Lai J., You C., Zhao H., Lester E., Wu T., Pang C.H. The first 75 Days of novel coronavirus (SARS-CoV-2) outbreak: recent advances, prevention, and treatment. Inter. Environ. Res. Public Health. 2020;17(7):2323. doi: 10.3390/ijerph17072323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Zhou Y., Huang Z.W. Research on simple disinfection system for medical wastewater of township hospital. Asian J. Chem. 2014;26:3243–3245. [Google Scholar]
- Zaied B.K., Rashid M., Nasrullah M., Zularisam A.W., Pant D., Singh L. A comprehensive review on contaminants removal from pharmaceutical wastewater by electrocoagulation process. Sci. The Tot. Environ. 2020 doi: 10.1016/j.scitotenv.2020.138095. [DOI] [PubMed] [Google Scholar]
- Zhang C., Xu L., Xu P., Wang X.C. Elimination of viruses from domestic wastewater: requirements and technologies. World J. Microbiol. Biotechnol. 2016;32:69. doi: 10.1007/s11274-016-2018-3. [DOI] [PubMed] [Google Scholar]
- Zhang T., Cui X, Zhao X., Wang J., Zheng J., Zheng G., Guo W., Cai C., He S., Xu Y. Detectable SARS‐CoV‐2 viral RNA in feces of three children during recovery period of COVID‐19 pneumonia. J. Med. Virol. 2020;92(7):909–914. doi: 10.1002/jmv.25795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Ling H., Huang X., Li J., Li W., Yi C., Zhang T., Jiang Y., He Y., Deng S., Zhang X. Potential spreading risks and disinfection challenges of medical wastewater by the presence of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of fangcang hospital. Sci. The Tot. Environ. 2020 doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoni R., Zanelli R., Riboldi E., Bigliardi L., Sansebastiano G. Investigation on virucidal activity of chlorine dioxide. experimental data on feline calicivirus, HAV and Coxsackie B5. J. Preven. Med. Hygiene. 2007;48(3) [PubMed] [Google Scholar]