Abstract
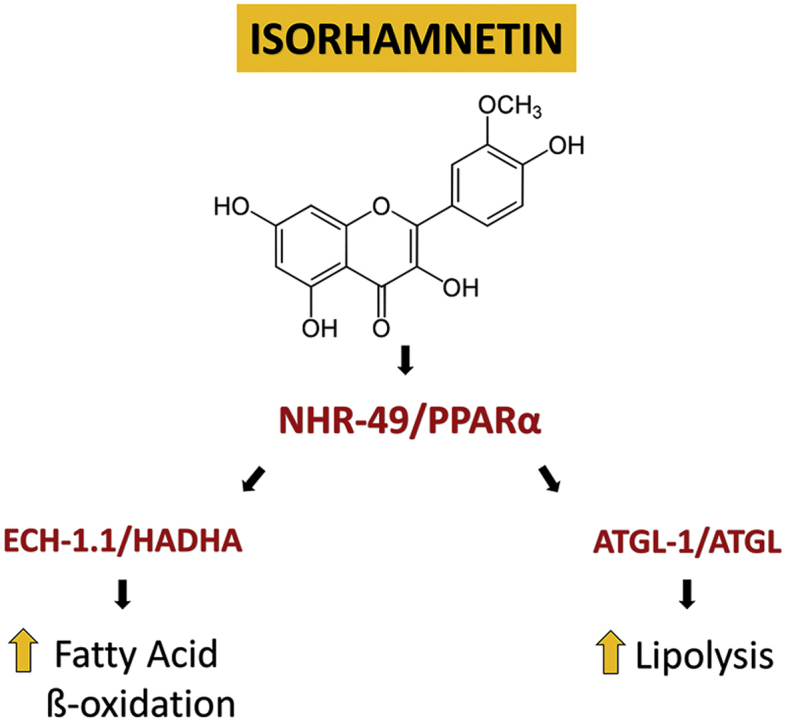
Isorhamnetin (3-O-methylquercetin), a flavonol found in dill weed, sea buckthorn berries, kale and onions, has been suggested to have anti-obesity effects, but there is limited evidence of its mechanisms of action on lipid metabolism. The goal of this study was to investigate the effects of isorhamnetin on lipid metabolism using Caenorhabditis elegans as an animal model. Isorhamnetin reduced fat accumulation without affecting food intake or energy expenditure in C. elegans. The isorhamnetin's fat-lowering effects were dependent on nhr-49, a homolog of the human peroxisome proliferator-activated receptor alpha (PPARα). Isorhamnetin upregulated an enoyl-CoA hydratase (ech-1.1, involved in fatty acid β-oxidation) and adipose triglyceride lipase (atgl-1, involved in lipolysis) via NHR-49-dependent pathway at transcriptional levels. Isorhamnetin also upregulated the C. elegans AMP-activated protein kinase (AMPK) subunits homologs (aak-1 and aak-2), involved in energy homeostasis. These results suggest that isorhamnetin reduces body fat by increasing fat oxidation in part via NHR-49/PPARα-dependent pathway.
Keywords: Flavonoid, Quercetin, Obesity, Diet, PPAR, C. elegans
Graphical abstract
Highlights
-
•
Isorhamnetin reduced fat accumulation in Caenorhabditis elegans.
-
•
Food intake and energy expenditure were not changed by isorhamnetin.
-
•
Isorhamnetin's fat-lowering effects were dependent on nhr-49/PPARα.
-
•
Isorhamnetin upregulated transcriptionally AAK/AMPK, which may activate NHR-49.
-
•
Isorhamnetin increased fat breakdown by upregulating ech-1.1/HADHA and atgl-1/ATGL.
1. Introduction
Isorhamnetin (3-O-methylquercetin), a methylated derivative of the flavonol quercetin, is found in a variety of foods, such as dill weed (43 mg/100 g), sea buckthorn berries (38 mg/100 g), kale (23 mg/100 g) and onions (5 mg/100 g) (Haytowitz et al., 2018). In addition to the potential antioxidant, anti-microbial, anti-inflammatory, anticancer and anti-aging effects, isorhamnetin has been suggested as an anti-obesity agent (Eseberri et al., 2019, Lee et al., 2010, Zhang et al., 2016, Lee and Kim, 2018, Surco-Laos et al., 2011). The suggested mechanisms of action for isorhamnetin's effects on lipid metabolism involve suppressing adipogenesis, especially via peroxisome proliferator-activated receptor gamma (PPARγ)-mediated pathway (Eseberri et al., 2019, Lee et al., 2010, Zhang et al., 2016, Lee and Kim, 2018). However, there are inconsistent reports on the effects of isorhamnetin on PPARγ in vitro (Zhang et al., 2016, Ramachandran et al., 2012).
Caenorhabditis elegans, a multi-organ millimetric transparent worm, is used for food and obesity research with well-established protocols (Shen et al., 2018a, Shen et al., 2018b). Human lipid metabolism pathways are conserved in C. elegans, which help to identify the mechanisms of action of food components prior to vertebrate animals and/or humans (Shen et al., 2018a, Shen et al., 2018b). Previously, studies have examined the anti-obesity effects of plant extracts and food bioactive components in C. elegans, showing the relevance of this animal model for the mechanistic studies of prospective bioactives (Bhattacharya et al., 2013, Farias-Pereira et al., 2018, Farias-Pereira et al., 2020, Lin et al., 2019, Liu et al., 2018, Machado et al., 2018, Shen et al., 2017, Shen et al., 2018a, Shen et al., 2018b, Shen et al., 2018c, Sun et al., 2016, Yue et al., 2019). Thus, the goal of this study was to determine the effects of isorhamnetin on lipid metabolism by using C. elegans as an animal model. In this current study, we showed that isorhamnetin reduced fat accumulation dependent on nhr-49, a functional homolog of proliferator-activated receptor alpha (PPARα), with involvement of enzymes related to fatty acid β-oxidation and lipolysis in C. elegans.
2. Material and methods
2.1. Material
Isorhamnetin was acquired from Frontier Scientific (purity ≥ 98%, from Hippophae rhamnoides L., batch LL60O103, CAS 480-19-3, PubChem CID 5281654, Logan, UT, USA). Chemicals were bought from Fisher Scientific (Pittsburgh, PA, USA) unless stated in the described methods.
2.2. Worms culture
C. elegans strains (Supplementary Table S1) provided by Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN, USA), were cultivated on Petri dish with solid nematode growth media (NGM) until eggs were harvested by using bleach solution (Shen et al., 2017). Worm eggs in S-complete (liquid growth media) were incubated overnight at 20 °C or 15 °C (only for daf-2 mutant) on an incubator (model DT2-MP-47, Tritech Research Inc., Los Angeles, CA) (Farias-Pereira et al., 2018). After eggs were hatched, L1 stage worms were cultured in liquid growth media, containing 100 μg/mL ampicillin (Sigma-Aldrich Co., St. Louis, MO, USA), 50 μg/mL carbenicillin (Fisher Bioreagents, Pittsburgh, PA, USA) and live Escherichia coli OP50 for two days. Then, worms at L4 stage/young adult were harvested and placed in 12-well plates containing 120 μM 2′-deoxy-5-fluorouridine (TCI America, Portland, OR, USA) in liquid media, to prevent eggs from becoming larvae (Sun et al., 2016). Dead E. coli (65 °C for 30 min) was used to feed the worms and to prevent isorhamnetin degradation by bacteria (Farias-Pereira et al., 2018, Du et al., 2017). Previously, others have shown the antioxidant and anti-aging properties of isorhamnetin up to 200 μM in C. elegans (Surco-Laos et al., 2011), thus, adult worms were treated with 0.1% dimethyl sulfoxide (DMSO; control) or isorhamnetin (50–200 μM in DMSO) for 2 days.
2.3. Fat measurement
Worm bodies were sonicated with 0.05% Tween 20® for 3 min as previously described (Sun et al., 2016). Triglyceride content was measured by using Infinity™ Triglycerides Reagent (Fisher Diagnostics, Middletown, VA, USA) with glycerol as a standard. Triglyceride levels were normalized by protein levels, measured by using Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Middletown, VA, USA) with bovine serum albumin as a standard. The absorbances were determined by using SpectraMax i3 microplate reader and SoftMax Pro v. 6.5 (Molecular Devices, Sunnyvale, CA, USA).
2.4. Food intake, body size and energy expenditure
Food intake was estimated by pharynx movement (pumping rate), since worm's food intake depends on the pharynx contractions to uptake the food (E. coli) into the gut (Lee et al., 2017, Avery and You, 2012). Worms were placed in low-peptone NGM plates with live E. coli OP50 to attract the worms to the observational sites, as previously described (Farias-Pereira et al., 2018). In order to quantify worms' body size by measuring width (μm) and length (μm), videos of nematodes were recorded and analyzed by WormLab tracking system and software (model MSCOP-002, software v. 3.1.0 64-bit, MBF Bioscience, MicroBrightField Inc., Willinston, VT, USA) (Shen et al., 2018c). Energy expenditure was estimated by the moving speed [μm/s; (forward distance + reverse distance)/time], since the locomotor behavior of C. elegans is related to energy expenditure (Farias-Pereira et al., 2020, Laranjeiro et al., 2017, Shen et al., 2018b).
2.5. Worm viability
Worms viability was determined by motility and fluorescence staining methods as previously described with modifications (Ferreira et al., 2015). Motility of worms was observed in an optical microscope; worms without motility are considered as non-viable worms (Ferreira et al., 2015). SYTOX™ Green Nucleic Acid Stain (Life Technologies Corporation, Eugene, OR, USA), a DNA-binding fluorescence stain that does not penetrate in viable cells, was also used to test worm viability (Ferreira et al., 2015). Worms were placed in a black 96-well microplate in S-complete media, then 1 μM of SYTOX™ Green Nucleic Acid Stain was added to each well, then fluorescence intensities were measured with excitation and emission at 485 and 523 nm, respectively, by using SpectraMax i3 microplate reader and SoftMax Pro v. 6.5 (Molecular Devices, Sunnyvale, CA, USA) (Ferreira et al., 2015). Fluorescence intensity was normalized by the number of worms in a well (60–80 worms/well). As a positive control, 50% methanol treatment, which is known to kill nematodes, was used for both methods (Ferreira et al., 2015), thus higher fluorescence intensity indicates non-viable worms compared to the negative control.
2.6. Real-time PCR
TRIzol® (Thermo Fisher Scientific, Middletown, VA, USA) was used to extract total RNA, then used to synthesize cDNA by using high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific, Inc., Middletown, VA) and thermal cycler (Bio-Rad Laboratories Inc., Hercules, CA) following the respective manufacturer's protocols. TaqMan® gene expression assays (Supplementary Table S2, Thermo Fisher Scientific, Inc., Middletown, VA) were used to perform the relative quantitative RT-PCR, and signal intensity was measured by StepOnePlus™ Real-Time PCR system (Applied Biosystems, Foster City, CA). Comparative threshold cycle (Ct) method was used to show the results as fold change of gene expression ().
2.7. Statistical analysis
One-way (Fig. 1, Fig. 2, Fig. 5) or two-way (Fig. 3, Fig. 4) analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparison test or one-tailed Dunnett's test (Supplementary Fig. S1), performed by SAS Software (version 9.4, SAS Institute, Cay, NC), were used to analyze statistical differences (P < 0.05).
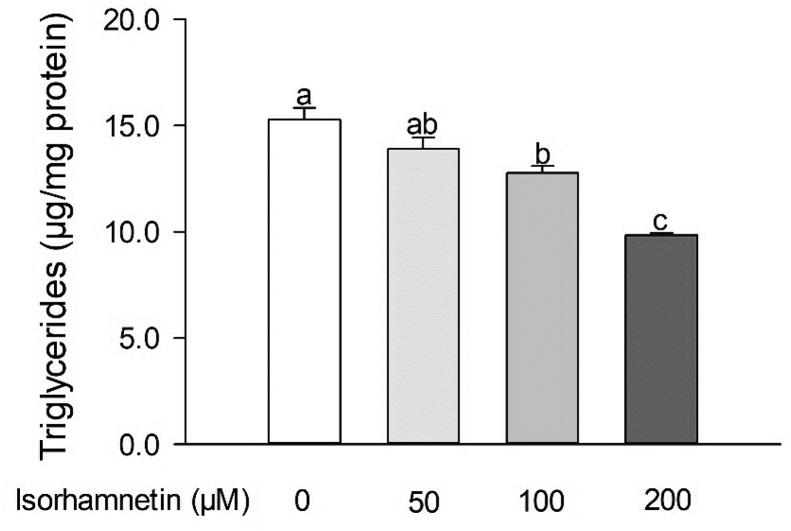
Fig. 1.
Isorhamnetin reduced fat accumulation in C. elegans. Adult worms (wild type) were treated with isorhamnetin for 2 days. Triglyceride levels were normalized by protein content. Data are means ± S.E. (n = 3–6). Means with different letters are significantly different at P < 0.05.
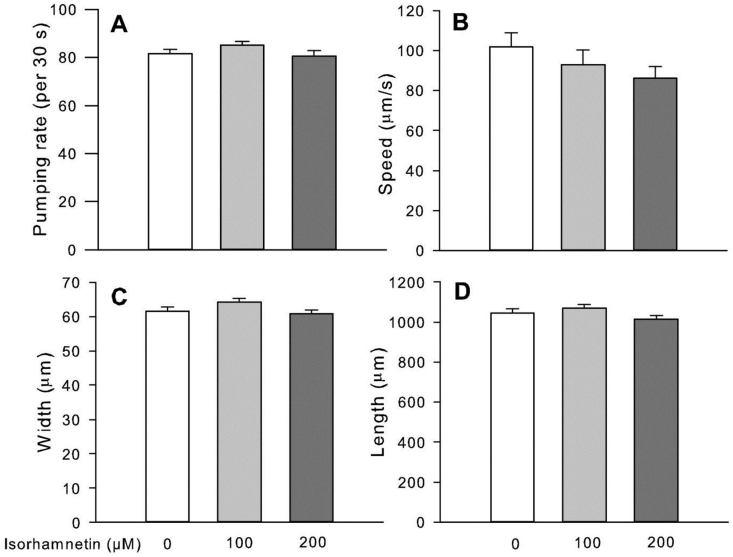
Fig. 2.
Isorhamnetin did not change feeding or locomotor behavior of wild-type C. elegans. Adult worms were treated with isorhamnetin for 2 days, then transferred to a low-peptone NGM plates before observations. Data are mean ± S.E. A - Number of pharyngeal contractions of randomly selected worms was counted for 30 s (n = 20). An automatic tracking system measured worm locomotor behavior and body size (n = 62–79). B - Moving speed. C - Width. D - Length.
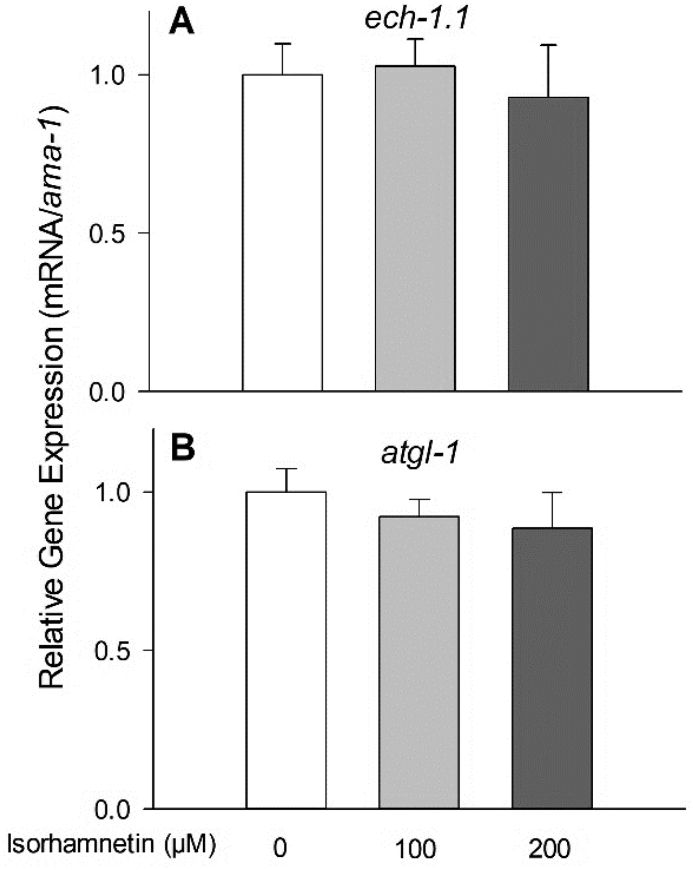
Fig. 5.
The upregulated ech-1.1 (A) and atgl-1 (B) expression by isorhamnetin was abolished on nhr-49 mutant, measured by real time-PCR. Data are means ± S.E. (n = 3–4).
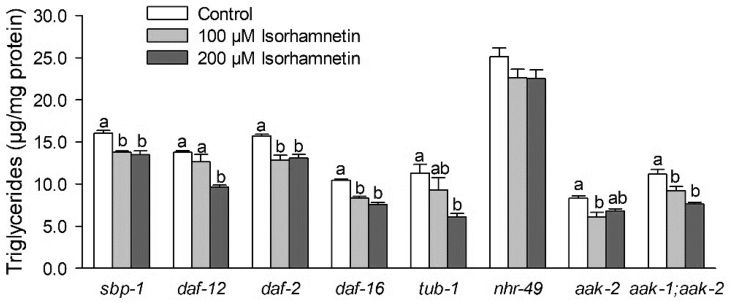
Fig. 3.
Effects of isorhamnetin on fat accumulation in C. elegans knockout mutants. Adult worms were treated with isorhamnetin for 2 days. Triglyceride levels were normalized by protein content. Data are means ± S.E. (n = 3–6, from 1 to 2 independent experiments). Means with different letters at each variable are significantly different at P < 0.05.
Fig. 4.
Effects of isorhamnetin on lipid metabolism-related genes expression on wild-type worms measured by real time-PCR (A & B). Data are means ± S.E. (n = 3–8, from 1 to 2 independent experiments). Means with different letters at each variable are significantly different at P < 0.05.
3. Results
Isorhamnetin at 100 and 200 μM decreased triglyceride content in wild-type C. elegans by 17% (P = 0.0105) and 36% (P<0.0001) compared to the control, respectively (Fig. 1). In order to assess whether isorhamnetin reduced fat accumulation by decreasing food intake and/or increasing energy expenditure, we measured the pharynx pumping rate and moving speed, as an indicator of food intake and energy expenditure, respectively (Shen et al., 2018c). Our results showed that isorhamnetin did not change pharynx pumping rate or moving speed of worms (Fig. 2A, B). Moreover, isorhamnetin did not change worm body size (Fig. 2C, D) or viability (Suppl. Fig S1). Overall, these suggest that the reduced body fat by isorhamnetin is unlikely to be due to modifications in energy intake or expenditure.
We further assessed the mechanisms of action for the isorhamnetin's effects on lipid metabolism by a targeted approach using C. elegans mutants and gene expression. First, we evaluated the effects of isorhamnetin on fat accumulation in the C. elegans mutant lacking sbp-1, a homolog of the human sterol regulatory element-binding protein (SREBP), one of the major players for lipogenesis (Farias-Pereira et al., 2018). Isorhamnetin at 100 and 200 μM reduced fat accumulation over the control in sbp-1 mutant (Fig. 3, P = 0.0032 and 0.0009, respectively), suggesting that the reduced fat accumulation by isorhamnetin is independent on sbp-1 in C. elegans.
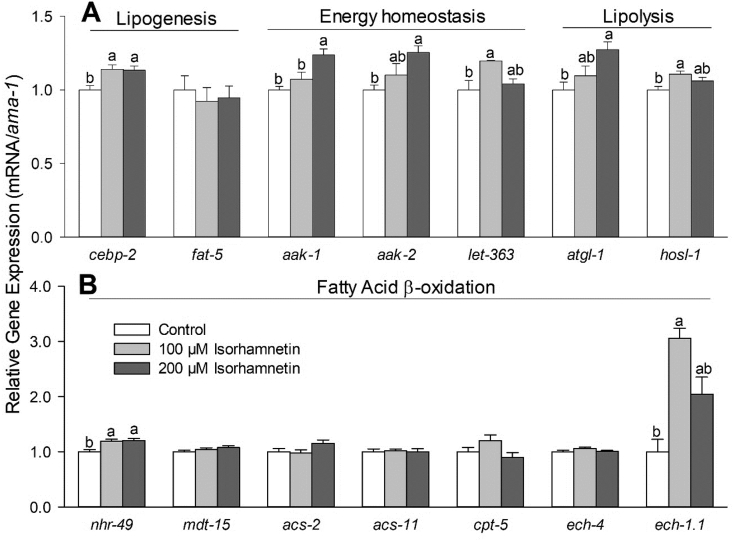
The C. elegans CEBP-2, a homolog of the adipocyte differentiation-related CCAAT/enhancer-binding proteins (C/EBPs), also regulates the expression of lipogenesis-related genes, including fat-5/SCD, an ortholog of the human stearoyl-CoA desaturase (Xu et al., 2015). Treatment of isorhamnetin at 100 and 200 μM upregulated cebp-2 expression by 13% on wild-type worms compared to the control (Fig. 4A, P = 0.0097 and 0.0136, respectively). However, the isorhamnetin's fat-lowering effects were not associated with changes on fat-5 in C. elegans (Fig. 4A). These suggest that isorhamnetin's effects on CEBP-2 may not involve the lipogenesis regulation in C. elegans.
DAF-12, a homolog of the human farnesoid X receptors (FXR), is another transcription factor that regulates lipid metabolism in C. elegans (Dowell et al., 2003). Thus, we evaluated the effects of isorhamnetin on fat accumulation in the C. elegans mutant lacking daf-12. The fat-lowering effects of 200 μM isorhamnetin remained in daf-12 mutant (Fig. 3, P = 0.0012 compared to the control), suggesting that daf-12 is not a genetic requirement for isorhamnetin's fat-lowering effects in C. elegans.
The insulin/insulin-like growth factor signaling pathway, also known for its effects on lipid metabolism in C. elegans, is regulated by DAF-2, a homolog of the insulin/insulin-like growth factor receptor, and its downstream target DAF-16, an ortholog of the mammalian Forkhead box O transcription factor (Farias-Pereira et al., 2018). Isorhamnetin at 100 and 200 μM reduced fat accumulation in daf-2 mutant (P = 0.0036 and 0.0061, respectively) and daf-16 mutant (P = 0.0002 and < 0.0001, respectively). These suggest that isorhamnetin's fat-lowering effects were independent on daf-2 and daf-16.
TUB-1, an ortholog of the mammalian TUBBY, is associated with the regulation of lipid metabolism via a neuroendocrine pathway in C. elegans (Yue et al., 2019). Isorhamnetin at 200 μM decreased triglyceride content in tub-1 mutant by 46%, compared to the control (Fig. 3, P = 0.0187), suggesting that the effects of isorhamnetin on fat accumulation were independent on tub-1 in C. elegans.
In C. elegans, the nuclear hormone receptor NHR-49, a homolog of the peroxisome proliferator-activated receptor α (PPARα), regulates fatty acid β-oxidation (Pathare et al., 2012, Ratnappan et al., 2014, Van Gilst et al., 2005a, Van Gilst et al., 2005b). The reduced fat accumulation by isorhamnetin was abolished in nhr-49 mutant, suggesting that isorhamnetin's fat-lowering effects were dependent on nhr-49 (Fig. 3). Consistently, isorhamnetin at 100 μM and 200 μM upregulated transcription of nhr-49 by 19% (P = 0.0065) and 20% (P = 0.0038), respectively, compared to the control (Fig. 4B). Isorhamnetin did not change the expression of a mediator complex that interacts with NHR-49 protein, mdt-15 (a homolog of the human mediator complex subunit 15, MED15), neither the NHR-49-downstream genes: acs-2 (a homolog of the human acyl-coA synthetase 2), acs-11 (an ortholog of the human acyl-CoA synthetase 3), cpt-5 (an ortholog of the human carnitine palmitoyl transferase 1, CPT1) and ech-4 (an ortholog of the human enoyl-CoA hydratase 2) (Pathare et al., 2012, Ratnappan et al., 2014, Van Gilst et al., 2005a, Van Gilst et al., 2005b). However, 100 μM isorhamnetin upregulated the expression of ech-1.1 (an enoyl-CoA hydratase, an ortholog of the human hydroxyacyl-CoA dehydrogenase trifunctional multienzyme complex, HADHA) by 200% (P = 0.0025) compared to the control (Fig. 4B). Thus, we further determined if the effects of isorhamnetin on ech-1.1 expression were dependent to nhr-49 by using nhr-49 mutant. The upregulation of ech-1.1 by isorhamnetin was abolished in nhr-49 mutant (Fig. 5A), suggesting that isorhamnetin increases expression of ech-1.1 via NHR-49-dependent pathway.
Adenosine monophosphate-activated protein kinase (AMPK), encoded by two subunits homologs aak-1 and aak-2 in C. elegans, regulates multiple cellular pathways by sensing the energy balance (Shen et al., 2018c). Although aak-1 and aak-2 were not a genetic requirement for fat-lowering effects of isorhamnetin (Fig. 3), isorhamnetin at 200 μM upregulated the expression of aak-1 (P = 0.0048) and aak-2 (P = 0.0236) by 24% and 25%, respectively, compared to the control (Fig. 4A). These suggest that AMPK activation may have contributed to isorhamnetin's fat-lowering effects in C. elegans.
The C. elegans let-363 (a homolog of the human mechanistic target of rapamycin, mTOR) is another energy sensor that regulates lipid metabolism (Farias-Pereira et al., 2018). Isorhamnetin at 100 μM upregulated let-363 expression by 20%, compared to the control (Fig. 4A, P = 0.0216), however, 200 μM isorhamnetin did not regulate let-363 expression (Fig. 4A) these results suggest that let-363/mTOR may not be involved in the effects of isorhamnetin in C. elegans.
The lipases, atgl-1 (a homolog of the human adipose triglyceride lipase or patatin-like phospholipase domain-containing protein 2, PNPLA2) and hosl-1 (a homolog of the human hormone-sensitive lipase E, LIPE), are targets for anti-obesity agents due to their effects on lipolysis (Farias-Pereira et al., 2018, Liu et al., 2018, Machado et al., 2018). Treatment of 200 μM isorhamnetin upregulated atgl-1 expression by 27% (P = 0.0211), while hosl-1 expression was upregulated only by 6% in the 100 μM isorhamnetin-treated worms (P = 0.0106) compared to the control (Fig. 4A). We further determined the effects of isorhamnetin on atgl-1 expression in nhr-49 mutant and we observed that the upregulation of atgl-1 by isorhamnetin was abolished (Fig. 5B). These suggest that the fat-lowering effects of isorhamnetin involve an increased lipolysis by atgl-1 upregulation via NHR-49-dependent pathway in C. elegans.
4. Discussion
Isorhamnetin is a flavonol found in sea buckthorn berries, kale and onions with potential use as anti-obesity agent. The fat-lowering effects of isorhamnetin reported in this study are consistent with other reports showing that flavonols, including isorhamnetin, reduced fat accumulation in C. elegans, Drosophila melanogaster and/or rodent models (Zhang et al., 2016, Bhattacharya et al., 2013, Azuma et al., 2019). Furthermore, we showed that isorhamnetin reduced fat accumulation dependent on nhr-49, a functional homolog of PPARα, by upregulating an NHR-49 downstream target (ech-1.1) involved in fatty acid β-oxidation in C. elegans. In addition, the current study suggests that the energy sensor AAK/AMPK and lipase ATGL-1 may contribute to the reduced body fat by isorhamnetin in C. elegans.
The PPARs (PPARα, PPARβ/δ and PPARγ), which are structurally similar (i.e. ligand biding site), are nuclear receptors that regulate lipid metabolism by tissue-specific mechanisms (Chung et al., 2016, Mirza et al., 2019). Previously, it was reported that isorhamnetin suppresses adipocyte differentiation by antagonizing PPARγ (Eseberri et al., 2019, Lee et al., 2010, Zhang et al., 2016, Lee and Kim, 2018); however, others reported that isorhamnetin acts as a PPARγ agonist in gastric AGS cells (Ramachandran et al., 2012). In C. elegans, there is evidence that the functions of PPARs on lipid metabolism are led by the nuclear hormone receptors, such as NHR-49 and DAF-12 (Dowell et al., 2003, Van Gilst et al., 2005a). The current results showed that isorhamnetin reduced body fat via NHR-49/PPARα-dependent only, but not via DAF-12-dependent pathway.
Isorhamnetin may regulate nhr-49 by its effects on upstream regulators, such as AAK/AMPK, as previously reported that AAK upregulates transcription of nhr-49 in C. elegans (Moreno-Arriola et al., 2016). Current observation of increased aak-1 and aak-2 expressions by isorhamnetin is consistent to the previous report of activation of AMPK by isorhamnetin in HepG2 and 3T3-L1 cells (Lee and Kim, 2018, Dong et al., 2014). Thus, it is possible that increased expression of aak-1 and aak-2 by isorhamnetin may lead to an increased nhr-49 expression; however, it is yet not known if the isorhamnetin post-translationally regulates AAK in C. elegans. Others have suggested that isorhamnetin may regulate transcription of PPARα by inhibiting p38 mitogen-activated protein kinase (MAPK) in murine hepatocytes (Lu et al., 2018). However, it is known that the p38 MAPK homolog (pmk-1) pathway has little influence on the NHR-49-mediated pathway in C. elegans (Goh et al., 2018). Although AAK/AMPK is not a genetic requirement for the effects of isorhamnetin on body fat, these suggest that the AAK/AMPK activation, transcriptionally and/or post-translationally, may play a part in the fat-lowering effects of isorhamnetin via NHR-49/PPARα pathway.
Isorhamnetin may activate NHR-49 directly by binding its ligand-binding domain in C. elegans. An isorhamnetin sulfate (isorhamnetin-7-O-sulfate), along with other flavonol sulfates, were identified as a NHR-49-ligand in silico by using LibDock, a docking algorithm that analyses the ligand-protein conformational interactions (Lee et al., 2016). Whether only sulfate forms of flavonols, originated by metabolization of flavonols, but not flavonols themselves, are able to directly activate NHR-49/PPARα is still not clear (Chung et al., 2016, Yeh et al., 2016).
The PPAR may have different target genes dependent to co-factors, such as C/EBPs and PPAR coactivator 1α (PGC1α) (Chung et al., 2016); PGC1α and C/EBPβ were both involved in the increased fatty acid oxidation by CPT1 in human nasopharyngeal cancer cells (Du et al., 2019). Homologs of these co-factors are MDT-15/PGC1α and CEBP-2/C/EBP in C. elegans, respectively (Lin et al., 2019, Xu et al., 2015). However, neither MDT-15 nor CEBP-2 was identified as co-factors for the isorhamnetin's fat-lowering effects via NHR-49-mediated pathway in the current study, which also explain the null effect of isorhamnetin on cpt-5/CPT1 expression.
HADHA, an enzyme that has the enoyl-CoA hydratase and hydroxyacyl-CoA dehydrogenase activities as ECH-1.1 in C. elegans, is responsible for the last three steps of mitochondrial β-oxidation of long chain fatty acids (Fould et al., 2010, de la Rosa Rodriguez et al., 2018). Consistently, it was reported that daily intake of a PPARα agonist (fenofibrate) upregulated the gene expression of HADHA in humanized liver of chimeric mice after 4 days (de la Rosa Rodriguez et al., 2018). Thus, the upregulation of ech-1.1 by isorhamnetin via NHR-49-dependent pathway observed in the current study suggests that isorhamnetin increases fatty acid β-oxidation that can contribute to reduced fat accumulation in C. elegans.
The current study also showed that the upregulation of atgl-1 by isorhamnetin was dependent on nhr-49, suggesting that isorhamnetin increases lipolysis via NHR-49-dependent pathway. Previously, it was reported that fenofibrate had lipolytic effects via PPARα-ATGL pathway in muscle tissue in rats (Biswas et al., 2016). Consistently, yerba mate tea extract reduced fat accumulation via NHR-49-dependent pathway with the upregulation of atgl-1 in C. elegans (Machado et al., 2018). Therefore, the lipolytic effects of isorhamnetin, particularly via atgl-1 upregulation, may contribute for its fat-lowering effects in C. elegans.
After supplementation with St. John's wort and onion extracts containing 97.2–163 mg of flavonols, maximum plasma concentrations (Cmax) of isorhamnetin reached 40–50 nM and time to achieve the maximum concentration (tmax) of isorhamnetin was 84–268 min in humans (Burak et al., 2017, Schulz et al., 2005). These relatively low plasma concentrations of isorhamnetin may be related to the metabolism of flavonols by gut microbiota and/or detoxification pathways in vivo (Du et al., 2017, Yeh et al., 2016). Even though, others have reported that 200 μM isorhamnetin treatment is absorbed in the worms body up to 50 μg isorhamnetin/mg protein after 4–6 days, the doses here used are not yet translatable from C. elegans to humans (Shen et al., 2018a, Surco-Laos et al., 2011).
The anti-obesity mechanisms of action of dietary bioactives and plant extracts on lipid metabolism have various molecular targets in C. elegans (Farias-Pereira et al., 2018, Lin et al., 2019, Liu et al., 2018, Machado et al., 2018, Shen et al., 2017, Shen et al., 2018c, Sun et al., 2016, Yue et al., 2019). It was reported that the inhibitory effects of NHR-49 on fatty acid desaturases (lipogenesis) were related to the fat-lowering effects of a saponin extract in C. elegans (Lin et al., 2019). AAK was found to be regulated for isorhamnetin and methylated resveratrol associated with fatty acid oxidation and lipogenesis, respectively (Yue et al., 2019). These suggest that food can be used to target lipid metabolism at various mechanisms to effectively control body fat. For this, studies using C. elegans on lipid metabolic pathways can be useful to identify potential food bioactive to test these in vertebrate animals and/or humans.
The current study investigated the mechanisms for the effects of isorhamnetin on lipid metabolism in C. elegans by a targeted approach. Additional studies would be necessary to confirm our observation that isorhamnetin has any effects on energy expenditure by using direct measurements, such as microcalorimetry, oxygen consumption and mitochondria function assays (Farias-Pereira et al., 2020, Laranjeiro et al., 2017, Shen et al., 2018b). In addition, results from the current study do not exclude the possibility that isorhamnetin reduced body fat via an alternative pathway and/or post-transcriptional regulation of factors involved in lipid metabolism.
In conclusion, isorhamnetin reduces fat accumulation in C. elegans dependent on nhr-49, which are involved in fatty acid β-oxidation and lipolysis. The current study is consistent with others that isorhamnetin is a potential anti-obesity bioactive.
CRediT author statement
Renalison Farias-Pereira: Methodology, Investigation, Formal Analysis, Writing – Original Draft, Funding Acquisition, Project Administration. Jessica Savarese: Investigation, Formal Analysis. Yiren Yue: Methodology. Seong-Ho Lee: Conceptualization. Yeonhwa Park: Conceptualization, Resources, Validation, Supervision, Writing – Reviewing and Editing, Funding Acquisition.
Research data for this article
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of Competing Interest
We have no conflict of interest to disclose.
Acknowledgements
The National Counsel of Technological and Scientific Development [CNPq, Conselho Nacional de Desenvolvimento Científico e Tecnológico] from Brazil funded this study in part and Mr. R. Farias-Pereira. Ms. Jessica Savarese was supported by The Center for Agriculture Food and the Environment (CAFE) sponsored by Massachusetts Agricultural Experiment Station. C. elegans was obtained from Caenorhabditis Genetics Center supported by NIH Office of Research Infrastructure Programs (P40 OD010440). International C. elegans Gene Knockout Consortium funded the construction of some worm mutants used in this study. The authors thank Mr. Joshua Barsczewski for his assistance in editing this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2019.11.002.
Contributor Information
Renalison Farias-Pereira, Email: rfpereira@umass.edu.
Jessica Savarese, Email: jsavarese@umass.edu.
Yiren Yue, Email: yirenyue@umass.edu.
Seong-Ho Lee, Email: slee2000@umd.edu.
Yeonhwa Park, Email: ypark@foodsci.umass.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Avery L., You Y.J. C. elegans feeding. In: Research Community, editor. WormBook : the Online Review of C. elegans Biology. 2012. pp. 1–23. Wormbook. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma M., Dat Le T., Yoshimoto Y., Hiraki N., Yamanaka M., Omura F., Inoue Y.H. RNA-seq analysis of diet-driven obesity and anti-obesity effects of quercetin glucoside or epigallocatechin gallate in Drosophila adults. Eur. Rev. Med. Pharmacol. Sci. 2019;23:857–876. doi: 10.26355/eurrev_201901_16901. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S., Christensen K.B., Olsen L.C.B., Christensen L.P., Grevsen K., Faergeman N.J., Kristiansen K., Young J.F., Oksbjerg N. Bioactive components from flowers of Sambucus nigra L. increase glucose uptake in primary porcine myotube cultures and reduce fat accumulation in Caenorhabditis elegans. J. Agric. Food Chem. 2013;61:11033–11040. doi: 10.1021/jf402838a. [DOI] [PubMed] [Google Scholar]
- Biswas D., Ghosh M., Kumar S., Chakrabarti P. PPARα-ATGL pathway improves muscle mitochondrial metabolism: implication in aging. FASEB J. 2016;30:3822–3834. doi: 10.1096/fj.201600571RR. [DOI] [PubMed] [Google Scholar]
- Burak C., Brüll V., Langguth P., Zimmermann B.F., Stoffel-Wagner B., Sausen U., Stehle P., Wolffram S., Egert S. Higher plasma quercetin levels following oral administration of an onion skin extract compared with pure quercetin dihydrate in humans. Eur. J. Nutr. 2017;56:343–353. doi: 10.1007/s00394-015-1084-x. [DOI] [PubMed] [Google Scholar]
- Chung S., Kim Y.J., Yang S.J., Lee Y., Lee M. Nutrigenomic functions of PPARs in obesogenic environments. PPAR Res. 2016;2016:4794576. doi: 10.1155/2016/4794576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rosa Rodriguez M.A., Sugahara G., Hooiveld G.J.E.J., Ishida Y., Tateno C., Kersten S. The whole transcriptome effects of the PPARalpha agonist fenofibrate on livers of hepatocyte humanized mice. BMC Genomics. 2018;19:443. doi: 10.1186/s12864-018-4834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G.-Z., Lee J.-H., Ki S.H., Yang J.H., Cho I.J., Kang S.H., Zhao R.J., Kim S.C., Kim Y.W. AMPK activation by isorhamnetin protects hepatocytes against oxidative stress and mitochondrial dysfunction. Eur. J. Pharmacol. 2014;740:634–640. doi: 10.1016/j.ejphar.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Dowell P., Otto T.C., Adi S., Lane M.D. Convergence of peroxisome proliferator-activated receptor gamma and Foxo1 signaling pathways. J. Biol. Chem. 2003;278:45485–45491. doi: 10.1074/jbc.M309069200. [DOI] [PubMed] [Google Scholar]
- Du L.-Y., Zhao M., Tao J.-H., Qian D.-W., Jiang S., Shang E.-X., Guo J.-M., Liu P., Su S.-L., Duan J.-A. The metabolic profiling of isorhamnetin-3-O-neohesperidoside produced by human intestinal flora employing UPLC-Q-TOF/MS. J. Chromatogr. Sci. 2017;55:243–250. doi: 10.1093/chromsci/bmw176. [DOI] [PubMed] [Google Scholar]
- Du Q., Tan Z., Shi F., Tang M., Xie L., Zhao L., Li Y., Hu J., Zhou M., Bode A., Luo X., Cao Y. PGC1α/CEBPB/CPT1A axis promotes radiation resistance of nasopharyngeal carcinoma through activating fatty acid oxidation. Cancer Sci. 2019;110:2050–2062. doi: 10.1111/cas.14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eseberri I., Miranda J., Lasa A., Mosqueda-Solis A., Gonzalez-Manzano S., Santos-Buelga C., Portillo M.P. Effects of quercetin metabolites on triglyceride metabolism of 3T3-L1 preadipocytes and mature adipocytes. Int. J. Mol. Sci. 2019;20:264. doi: 10.3390/ijms20020264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias-Pereira R., Oshiro J., Kim K.-H., Park Y. Green coffee bean extract and 5-O-caffeoylquinic acid regulate fat metabolism in Caenorhabditis elegans. J. Funct. Foods. 2018;48:586–593. doi: 10.1016/j.jff.2018.07.049. [DOI] [Google Scholar]
- Farias-Pereira R., Kim E., Park Y. Cafestol increases fat oxidation and energy expenditure in Caenorhabditis elegans via DAF-12-dependent pathway. Food Chem. 2020;307:125537. doi: 10.1016/j.foodchem.2019.125537. [DOI] [PubMed] [Google Scholar]
- Ferreira S.R., Mendes T.A.O., Bueno L.L., de Araujo J.V., Bartholomeu D.C., Fujiwara R.T. A new methodology for evaluation of nematode viability. Biomed Res. Int. 2015;2015:879263. doi: 10.1155/2015/879263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fould B., Garlatti V., Neumann E., Fenel D., Gaboriaud C., Arlaud G.J. Structural and functional characterization of the recombinant human mitochondrial trifunctional protein. Biochemistry. 2010;49:8608–8617. doi: 10.1021/bi100742w. [DOI] [PubMed] [Google Scholar]
- Goh G.Y.S., Winter J.J., Bhanshali F., Doering K.R.S., Lai R., Lee K., Veal E.A., Taubert S. NHR-49/HNF4 integrates regulation of fatty acid metabolism with a protective transcriptional response to oxidative stress and fasting. Aging Cell. 2018;17 doi: 10.1111/acel.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haytowitz D.B., Wu X., Bhagwat S. U.S. Department of Agriculture, Agricultural Research Service; 2018. USDA Database for the Flavonoid Content of Selected Foods, Release 3.3.http://www.ars.usda.gov/nutrientdata/flav [Google Scholar]
- Laranjeiro R., Harinath G., Burke D., Braeckman B.P., Driscoll M. Single swim sessions in C. elegans induce key features of mammalian exercise. BMC Biol. 2017;15:30. doi: 10.1186/s12915-017-0368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.-S., Kim Y. Effects of isorhamnetin on adipocyte mitochondrial biogenesis and AMPK activation. Molecules. 2018;23:1853. doi: 10.3390/molecules23081853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Lee J., Jung E., Hwang W., Kim Y.-S., Park D. Isorhamnetin-induced anti-adipogenesis is mediated by stabilization of beta-catenin protein. Life Sci. 2010;86:416–423. doi: 10.1016/j.lfs.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Lee K., Goh G.Y.S., Wong M.A., Klassen T.L., Taubert S. Gain-of-function alleles in Caenorhabditis elegans nuclear hormone receptor nhr-49 are functionally distinct. PLoS One. 2016;11 doi: 10.1371/journal.pone.0162708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.S., Iwanir S., Kopito R.B., Scholz M., Calarco J.A., Biron D., Levine E. Serotonin-dependent kinetics of feeding bursts underlie a graded response to food availability in C. elegans. Nat. Commun. 2017;8:14221. doi: 10.1038/ncomms14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Lin Y., Chen Y., Xu J., Li J., Cao Y., Su Z., Chen Y. Effects of Momordica saponin extract on alleviating fat accumulation in Caenorhabditis elegans. Food Funct. 2019;10:3237–3251. doi: 10.1039/c9fo00254e. [DOI] [PubMed] [Google Scholar]
- Liu J., Peng Y., Yue Y., Shen P., Park Y. Epigallocatechin-3-gallate reduces fat accumulation in Caenorhabditis elegans. Prev. Nutr. food Sci. 2018;23:214–219. doi: 10.3746/pnf.2018.23.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Liu T., Chen K., Xia Y., Dai W., Xu S., Xu L., Wang F., Wu L., Li J., Li S., Wang W., Yu Q., Feng J., Fan X., Zhou Y., Niu P., Guo C. Isorhamnetin: a hepatoprotective flavonoid inhibits apoptosis and autophagy via P38/PPAR-α pathway in mice. Biomed. Pharmacother. 2018;103:800–811. doi: 10.1016/j.biopha.2018.04.016. [DOI] [PubMed] [Google Scholar]
- Machado M.L., Arantes L.P., Gubert P., Zamberlan D.C., da Silva T.C., da Silveira T.L., Boligon A., Soares F.A.A. Ilex paraguariensis modulates fat metabolism in Caenorhabditis elegans through purinergic system (ADOR-1) and nuclear hormone receptor (NHR-49) pathways. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza A.Z., Althagafi I.I., Shamshad H. Role of PPAR receptor in different diseases and their ligands: physiological importance and clinical implications. Eur. J. Med. Chem. 2019;166:502–513. doi: 10.1016/j.ejmech.2019.01.067. [DOI] [PubMed] [Google Scholar]
- Moreno-Arriola E., El Hafidi M., Ortega-Cuellar D., Carvajal K. AMP-activated protein kinase regulates oxidative metabolism in Caenorhabditis elegans through the NHR-49 and MDT-15 transcriptional regulators. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathare P.P., Lin A., Bornfeldt K.E., Taubert S., Van Gilst M.R. Coordinate regulation of lipid metabolism by novel nuclear receptor partnerships. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran L., Manu K.A., Shanmugam M.K., Li F., Siveen K.S., Vali S., Kapoor S., Abbasi T., Surana R., Smoot D.T., Ashktorab H., Tan P., Ahn K.S., Yap C.W., Kumar A.P., Sethi G. Isorhamnetin inhibits proliferation and invasion and induces apoptosis through the modulation of peroxisome proliferator-activated receptor gamma activation pathway in gastric cancer. J. Biol. Chem. 2012;287:38028–38040. doi: 10.1074/jbc.M112.388702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnappan R., Amrit F.R.G., Chen S.-W., Gill H., Holden K., Ward J., Yamamoto K.R., Olsen C.P., Ghazi A. Germline signals deploy NHR-49 to modulate fatty-acid β-oxidation and desaturation in somatic tissues of C. elegans. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004829. e1004829–e1004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz H.-U., Schurer M., Bassler D., Weiser D. Investigation of pharmacokinetic data of hypericin, pseudohypericin, hyperforin and the flavonoids quercetin and isorhamnetin revealed from single and multiple oral dose studies with a Hypericum extract containing tablet in healthy male volunteers. Arzneimittelforschung. 2005;55:561–568. doi: 10.1055/s-0031-1296905. [DOI] [PubMed] [Google Scholar]
- Shen P., Yue Y., Kim K.-H., Park Y. Piceatannol reduces fat accumulation in Caenorhabditis elegans. J. Med. Food. 2017;20:887–894. doi: 10.1089/jmf.2016.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P., Yue Y., Park Y. A living model for obesity and aging research: Caenorhabditis elegans. Crit. Rev. Food Sci. Nutr. 2018;58:741–754. doi: 10.1080/10408398.2016.1220914. [DOI] [PubMed] [Google Scholar]
- Shen P., Yue Y., Zheng J., Park Y. Caenorhabditis elegans: a convenient in vivo model for assessing the impact of food bioactive compounds on obesity, aging, and Alzheimer’s disease. Annu. Rev. Food Sci. Technol. 2018;9:1–22. doi: 10.1146/annurev-food-030117-012709. [DOI] [PubMed] [Google Scholar]
- Shen P., Kershaw J.C., Yue Y., Wang O., Kim K.-H., McClements D.J., Park Y. Effects of conjugated linoleic acid (CLA) on fat accumulation, activity, and proteomics analysis in Caenorhabditis elegans. Food Chem. 2018;249:193–201. doi: 10.1016/j.foodchem.2018.01.017. [DOI] [PubMed] [Google Scholar]
- Sun Q., Yue Y., Shen P., Yang J.J., Park Y. Cranberry product decreases fat accumulation in Caenorhabditis elegans. J. Med. Food. 2016;19:427–433. doi: 10.1089/jmf.2015.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surco-Laos F., Cabello J., Gomez-Orte E., Gonzalez-Manzano S., Gonzalez-Paramas A.M., Santos-Buelga C., Duenas M. Effects of O-methylated metabolites of quercetin on oxidative stress, thermotolerance, lifespan and bioavailability on Caenorhabditis elegans. Food Funct. 2011;2:445–456. doi: 10.1039/c1fo10049a. [DOI] [PubMed] [Google Scholar]
- Van Gilst M.R., Hadjivassiliou H., Jolly A., Yamamoto K.R. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol. 2005;3 doi: 10.1371/journal.pbio.0030053. e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gilst M.R., Hadjivassiliou H., Yamamoto K.R. A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR-49. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13496–13501. doi: 10.1073/pnas.0506234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.-Y., Hu J.-P., Wu M.-M., Wang L.-S., Fang N.-Y. CCAAT/enhancer-binding protein CEBP-2 controls fat consumption and fatty acid desaturation in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2015;468:312–318. doi: 10.1016/j.bbrc.2015.10.106. [DOI] [PubMed] [Google Scholar]
- Yeh S.-L., Lin Y.-C., Lin Y.-L., Li C.-C., Chuang C.-H. Comparing the metabolism of quercetin in rats, mice and gerbils. Eur. J. Nutr. 2016;55:413–422. doi: 10.1007/s00394-015-0862-9. [DOI] [PubMed] [Google Scholar]
- Yue Y., Shen P., Chang A.L., Qi W., Kim K.-H., Kim D., Park Y. trans-Trismethoxy resveratrol decreased fat accumulation dependent on fat-6 and fat-7 in Caenorhabditis elegans. Food Funct. 2019;10:4966–4974. doi: 10.1039/c9fo00778d. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Gu M., Cai W., Yu L., Feng L., Zhang L., Zang Q., Wang Y., Wang D., Chen H., Tong Q., Ji G., Huang C. Dietary component isorhamnetin is a PPARgamma antagonist and ameliorates metabolic disorders induced by diet or leptin deficiency. Sci. Rep. 2016;6:19288. doi: 10.1038/srep19288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.