Abstract
This study investigated the properties of films or bioplastics fabricated using a wet processing method from yellow pea protein isolate (YPI) and protein concentrate (YPC) for potential application in food packaging. The wet processing method included mixing the protein with water and glycerol followed by casting and drying the films in a humidity- and temperature-controlled chamber. Whey protein isolate (WPI) and a film from a blend of equal amounts of YPI and WPI, labelled as YPI + WPI, were also studied. Fourier transform-infra red analysis revealed that films from YPI, YPC, WPI and YPI + WPI were formed by protein polymerisation with the plasticiser, glycerol, via hydrophobic and hydrophilic interactions. The protein films had contact angles of <90° demonstrating that they had a hydrophilic surface, with YPC < YPI < YPI + WPI < WPI. The pattern of ultraviolent light transmission of the films was WPI > YPC > YPI + WPI > YPI, whereas the mechanical and thermal resilience of films formulated from YPI, YPC and the protein blend were comparable to the properties of WPI-based films. The findings demonstrate that yellow pea proteins can be used as biomaterials to develop protein and protein-blend films or bioplastics for food packaging and edible applications.
Keywords: Bioplastics, Protein films, Yellow pea proteins, Whey protein isolate, Food packaging, Sustainability
Graphical abstract
Highlights
-
•
Bioplastics were fabricated from yellow pea protein isolate and concentrate, with glycerol.
-
•
Contact angles of pea protein films indicate more hydrophobic surface than whey protein films.
-
•
Pea protein films had more surface structure homogeneity and limited light transmission.
-
•
Pea + whey protein blend did not produce synergistic effects in film property.
-
•
Film physico-mechanical properties are promising for food packaging application.
1. Introduction
A common phenomenon in the use of conventional plastics is the migration of potentially toxic compounds from the plastic matrix into packaged foods and drugs due to photo-oxidation reactions and exposure to heat (Hahladakis et al., 2018). The shifting global focus to a bioeconomy and the health consciousness of consumers have heightened interest in development of sustainable plastics that are safe, edible, biodegradable, and thermally and mechanically resilient (Lambert and Wagner, 2017, Han et al., 2018). These biodegradable plastics can be used in food and drug packaging and can also be functionalized to act as active and intelligent packaging biomaterials (Han et al., 2018, Roohi et al., 2018, Mlalila et al., 2018). The commonly used biomaterial in the study of biodegradable plastics are whey proteins, soy proteins, and polysaccharides, which are moulded via either wet processing techniques such as casting or dry processing techniques such as thermomoulding (Cao et al., 2018, Garrido et al., 2014, Ramos et al., 2013).
Pulses are sustainable legume crops and their edible dry seeds are rich in proteins and dietary fibres with low fat content. Additionally, pulses contain several bioactive compounds such as polyphenols and saponins (Margier et al., 2018). Pulses present environmental benefits such as nitrogen fixation to the soil, minimal requirement for fertilizers, low carbon and food wastage footprints, water efficiency, and low cost of production (Chan et al., 2019, Lam et al., 2018, Sun et al., 2018). Canada is the world's largest supplier of pulses with yellow peas (Pisum sativum) being one of the major pulse crops (Chan et al., 2019, Abdel-Aal et al., 2019, Pulse Canada, 2019). The presence of split peas and cracked seed coats in pea samples reduces their market value. This necessitates the expansion of utilisation to include the development of novel food and value-added materials, especially due to the high contents of proteins and bioactive compounds in pulses. Thus, use of pulse proteins for the formation of edible films presents a sustainable solution for enhancing pulse utilisation as well as creating environmentally friendly bio-based packaging.
Yellow pea proteins are made up of 70–80% globulins, namely legumin (11S), vicilin (7S), and convicilin, and about 10–20% albumins (Lam et al., 2018, Barac et al., 2010). The legumins consist of two subunits (α-, β-) covalently connected by disulphide bonds while the vicilins are made up of three subunits (α, β, and γ) connected non-covalently by hydrophobic interactions. Convicilin is the least abundant of the pea globulins (Lam et al., 2018). Notably, vicilin and convicilin are potentially allergenic proteins to some consumers (Sanchez-Monge et al., 2004). Nonetheless, allergenic epitopes can be potentially deactivated during heat treatment while forming the protein films and enzymatic digestion of edible protein films in the gastrointestinal tract (Bloom et al., 2014, Huby et al., 2000).
Thermal treatment of proteins, in the presence of a plasticiser (e.g., glycerol), results in their unfolding, irreversible denaturation and interactions to form complex solid networks of proteins and the plasticiser. Factors affecting the denaturing transition process and variation in extent of protein crosslinking include the heating temperature and rate, protein concentration, protein-solvent coupling, solvent pH, structural arrangement of proteins, and presence of thermally resistant amino acids (Wu and Inglett, 1974, Law and Leaver, 2000). Considering the increasing global shift towards sustainable biomaterials, there is a knowledge gap on the properties and utilisation of pulse proteins in fabricating protein films for packaging applications. Recent studies have demonstrated that pea protein isolates are suitable precursors for protein film formation using dry (Carvajal-Piñero et al., 2019, Perez et al., 2016, Perez-Puyana et al., 2016) or wet processing techniques (Kowalczyk et al., 2014). Additionally, some studies have investigated the use of protein blends in the formation of edible and biodegradable plastics (Cao et al., 2007, Song et al., 2014, Ghanbarzadeh and Oromiehi, 2008). Formation of films from protein blends has the potential to produce synergistic properties in the newly formed films. There is also the need to reduce the protein processing steps used in generating the biomaterials, to promote industrial upscaling and reduce processing cost. To the best of our knowledge, there has been no studies comprehensively investigating the formation and properties of yellow pea protein (concentrate and isolate) films and a formulated protein-blend film under the same processing conditions using the wet processing method. Therefore, the objective of this study was to investigate the physicochemical properties of yellow pea proteins, in concentrate and isolate forms, their use in producing edible and biodegradable protein-based films for packaging applications compared to films derived from whey protein isolate.
2. Materials and methods
2.1. Materials
The following reagents were purchased from Sigma-Aldrich: glycerol (≥99.0%), glycine (≥99.0%), sodium dodecyl sulphate bioreagent (SDS) suitable for electrophoresis (≥98.5%), β-mercaptoethanol (≥99.0%), hydrochloric acid (37%), sodium phosphate monobasic (≥98.0%), sodium phosphate dibasic (≥98.5%), bromophenol blue, and 8-anilino-1-naphthalenenesulfonic acid (ANS). Sodium hydroxide (≥98%) and Tris base (≥99.8%) were purchased from Fisher Scientific, and Coomassie Brilliant Blue R-250 staining solution from BioRad Incorporation, Canada. Yellow pea seeds were provided by Pulse Canada (Manitoba, Canada), whereas whey protein isolates (WPI) was purchased from Bulk Barn (Ottawa, Canada).
2.2. Yellow pea protein concentrate (YPC) extraction
To produce the protein concentrate, yellow pea seeds were pulverised into a flour, dispersed in deionised water (1:10, w/v) at 25 °C, acidified to pH 4.5 using 2 M HCl to precipitate the proteins, and stirred for 1 h. The dispersion was then centrifuged at 18,000 g and 4 °C for 30 min. The supernatant was discarded, and the protein concentrate readjusted from pH 4.5 to pH 7.0. The protein concentrate was then frozen at −80 °C for 24 h followed by freeze drying to obtain a protein concentrate (YPC) powder.
2.3. Pea protein isolation
For the protein isolate, yellow pea flours were dispersed in 2 M NaOH (1:10 w/v) at 25 °C for 4 h, followed by centrifugation at 18,000 g and 4 °C for 30 min. The supernatant was then acidified to pH 4.5 using 2 M HCl for 4 h to precipitate the proteins, followed by centrifugation at 18,000 g and 4 °C for 30 min. Protein precipitates were neutralised to pH 7.0 using 2 M NaOH. The protein isolate was frozen at −80 °C for 24 h followed by freeze drying to obtain the yellow pea protein isolate (YPI) powder.
2.4. Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS–PAGE)
Protein profiles of WPI, YPI, YPI + WPI and YPC, prepared at 4% (w/v) at pH 8.0, were analysed using SDS-PAGE. A gradient resolving polyacrylamide gel (6%, 9%, 12%, 15%, and 18%) with 3% w/v stacking gel was cast by adapting the method of Laemmli (1970). Sample buffer was prepared by using 20% glycerol, 2% SDS, 0.5% bromophenol blue (dissolved in 62.5 mM Tris-HCl buffer, pH 6.8, 1 M β-mercaptoethanol). Protein samples were mixed with the sample buffer at 1:1 (v/v), followed by heat denaturing at 90 °C for 5 min. The treated samples (20 μL) were loaded into each well and electrophoresis was conducted at 120 V for 2 h. A standard molecular weight marker (10 μL) ranging from 10 to 250 kDa was loaded into a separate well in the gel. SDS-PAGE was conducted with a BioRad Mini-PROTEAN Tetra Cell electrophoresis unit. Thereafter, gels were gently washed with Milli-Q water followed by overnight staining in Coomassie brilliant blue solution. Destaining was done with Milli-Q water under orbital shaking for about 4 h. Image scanning of gels was done with a ChemiDoc Imaging System (BioRad Inc., Canada).
2.5. Determination of protein surface hydrophobicity
A solution of 8 mM ANS was prepared in 0.1 M phosphate buffer (pH 7.4) in a Falcon tube wrapped with aluminium foil. Five concentrations (0.25, 0.5, 1, 1.5 and 2 mg/mL) of each sample (200 μL) was mixed with 1 μL of ANS solution in a Grenier UV-Star (96-well) microplate. The mixture was kept in the dark for 5 min. Fluorescence readings were measured at excitation and emission wavelengths of 390 and 470 nm, respectively, using a Spark multimode microplate reader (Tecan, Switzerland). Slope of the fluorescence vs. concentration plots was taken to be the surface hydrophobicity of the proteins.
2.6. Particle size and polydispersity index measurement
Prior to casting the films, the particle size and polydispersity index of the protein powders were measured by dynamic light scattering using a Zetasizer Nano ZS with non-invasive backscatter optics (Malvern Panalytical Ltd, UK) at 25 °C. The optical parameters used for the measurement include refractive indices of 1.450 and 1.330 for protein and water dispersant, respectively, and an absorbance value of 0.001.
2.7. Casting of the protein films
Casting of the protein films was done following published methods (Seydim and Sarikus, 2006, Sharma et al., 2017, Oliveira et al., 2017), with some modifications. WPI, YPI, YPC and a blend of YPI and WPI (1:1 v/v; YPI + WPI) at 4% (w/v) were each dispersed in deionised water, followed by agitation using a magnetic bead stirrer for 10 min. The solution was adjusted to pH 8.0 with 0.1 M NaOH, followed by heating at 75 °C for 30 min in an isothermal water bath shaking at 30 rpm. Glycerol (50%, w/w) was added to the mixture based on the mass of the starting protein powders. Thereafter, the solution was kept at room temperature for 30 min on a magnetic bead stirrer. Formulated solutions (30 mL) were spread on sterile plastic petri dishes (diameter, 90 mm) followed by drying for 72 h at 25 °C. Dried films were manually removed from the petri dishes and conditioned in a humidity chamber at 25 ± 2 °C, and 50 ± 2% relative humidity before further analysis (ASTM and D618, 2000).
2.8. Thickness, topography and Fourier Transform Infrared spectroscopic analyses
The thickness of protein-based films was measured with digital Vernier calipers of resolution 0.01 mm at 10 different locations, and average thickness values were obtained. Surface morphology of the protein films were imaged using a field emission scanning electron microscope (SEM) (model JSM-7500F, JEOL, USA). Prior to imaging, samples were coated with gold to prevent charge formation and increase the signal-to-noise ratio. Functional groups present in the protein powders and films were determined using a Cary 630 Fourier Transform Infrared (FT-IR) spectroscopy with an Attenuated Total Reflectance (ATR) diamond accessory (Agilent Technologies, Canada) at wavenumbers of 500–4000 cm−1.
2.9. Contact angle measurement and colour estimation
Surface hydrophobicity of the protein films was measured using the sessile drop method with a Video Contact Angle System (VCA optima, AST, USA) by adapting the method of Ramos et al. (2013). Ultrapure water (2-μL droplet) was released onto the surface of each film using a 1 mL precision syringe. The experiment was done in 10 replicates for each sample. The contact angle was recorded using the VCA OPtimaXE software within 10 s upon the release of each droplet and profile resolved. The colours of the protein films were determined with an Image Colour Summarizer tool available online http://mkweb.bcgsc.ca/color-summarizer/(Krzywinski, 2019).
2.10. Moisture content and solubility
Moisture content was estimated by measuring the differential mass of the protein films with a halogen analyser. Solubility was determined by immersing ~0.5 g of the films in 25 mL of distilled water for 24 h. Thereafter, the films were removed from the water and dried. Percentage of the dry films was calculated as shown in equation (1):
| (1) |
where % S is the percent solubility, mi is the initial dry mass, and mf is the final dry mass after filtering and drying of the protein-based films.
2.11. Thermal analysis
Thermal behaviour of the protein films was determined by differential scanning calorimetry (Q1000, TA Instruments, USA). Each sample (~10 mg) was heated on an aluminium pan from −40 °C to 300 °C at 10 °C/min under inert atmospheric condition (100 mL/min of N2). Star thermal analysis software was used to obtain the glass transition temperature (Tg), enthalpy of melting (ΔHm), and estimated percentage of crystallinity relative to pure polyethylene (ΔHm = 293 J/g). The experiment was done in triplicate and an empty aluminium pan was used as the reference.
2.12. Mechanical properties
The mechanical properties of the protein films, including Young's modulus (E), elongation at break (EB), and tensile strength (TS), were determined using an Instron 3000 Universal Tester, with Bluehill 2 Materials Testing Software, equipped with a 250 N load cell at a crosshead speed of 5 mm/min. Measurements were done at a temperature- and humidity-controlled room (23 ± 1 °C and 50% ± 5% RH) for five replicate of each protein film with effective length and width of 50 mm and 10 mm, respectively.
2.13. Light transmission and film transparency
The ultraviolet (UV) and visible light barrier properties of the protein films were measured at wavelengths of 300–800 nm at 5 nm intervals using a Spark multimode microplate reader (Tecan, Switzerland). Protein film samples were cut into square strips (0.5 × 0.5 cm) and placed on the bottom of a UV-Star 96 well microplate. Average 100% transmittance values for each sample were plotted against wavelength. Relative transparency (Tr) of the protein films was calculated using equation (2):
| (2) |
where A600 is the absorbance at 600 nm and X is the film thickness (mm). Three strips of each protein film type were tested.
2.14. Statistical analysis
Experiments were done in triplicate and results expressed as mean values ± standard deviation. Statistical analysis was done using one-way analysis of variance and significant difference of means was determined using Tukey's test (Origin 8, OriginLab Corporation, Massachusetts, USA). The level of significance was set at P < 0.05.
3. Results and discussion
3.1. Protein film preparation from food proteins
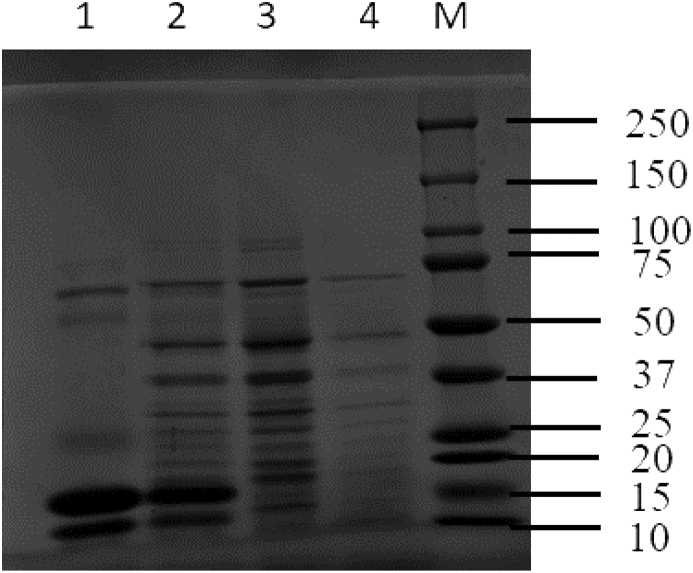
The profile of proteins in the powders used for casting of the films is shown in Fig. 1. The SDS-PAGE profiles are similar to published results for whey and pea proteins, with the presence of bands corresponding to molecular weights of ~12–100 kDa (Fig. 1). Specifically, the pea protein SDS-PAGE profile in this study showed the previously reported bands for legumin (α-β) subunits, acidic (38–40 kDa) and basic (19–22 kDa); three vicilin subunits (47–50 kDa); and convicilin subunit (~72 kDa) (Lam et al., 2018, Shand et al., 2007). SDS-PAGE of WPI showed three major bands at ~13, 18 and 66 kDa, which correspond to α-lactalbumin, β-lactoglobulin and bovine serum albumin, respectively. Moreover, the YPI + WPI blend had a combination of protein bands from both WPI and YPI, while YPC had similar profiles as YPI but with lower band intensities.
Fig. 1.
Electrophoretic band profiles for 4% (w/v) protein powders; lane 1, WPI; lane 2, YPI + WPI; lane 3, YPI; lane 4, YPC; lane M, standard molecular weight (10–250 kDa) marker.
Thermal treatment of all the protein samples resulted in a reduction in their particle size distribution and decrease in polydispersity index when suspended in water (Table 1). Size reduction and lower polydispersity index of protein suspensions contribute to increased dissolution and formation of homogenous films from the proteins.
Table 1.
Effect of thermal treatment on particle size of aqueous solutions of the yellow pea protein and whey protein samples.
| Sample | Size before heating (nm) | PDI before heating | Size after heating (nm) | PDI after heating |
|---|---|---|---|---|
| WPI | 292.7 ± 3.47b | 0.456 ± 0.061a | 259.8 ± 1.13b | 0.343 ± 0.005a |
| YPI + WPI | 408.6 ± 11.02c | 0.839 ± 0.07b | 347.7 ± 12.46d | 0.668 ± 0.125b |
| YPI | 719.6 ± 24.66d | 1 ± 0.00c | 339.5 ± 4.10c | 0.713 ± 0.038b |
| YPC | 293.1 ± 1.03a | 0.757 ± 0.007b | 217 ± 2.70a | 0.669 ± 0.07b |
Abbreviations: WPI, Whey protein isolate; YPI, Yellow pea isolate; YPC, Yellow pea concentrate. Statistical analysis was performed for each column and same superscript letter indicate no significant difference (P > 0.05).
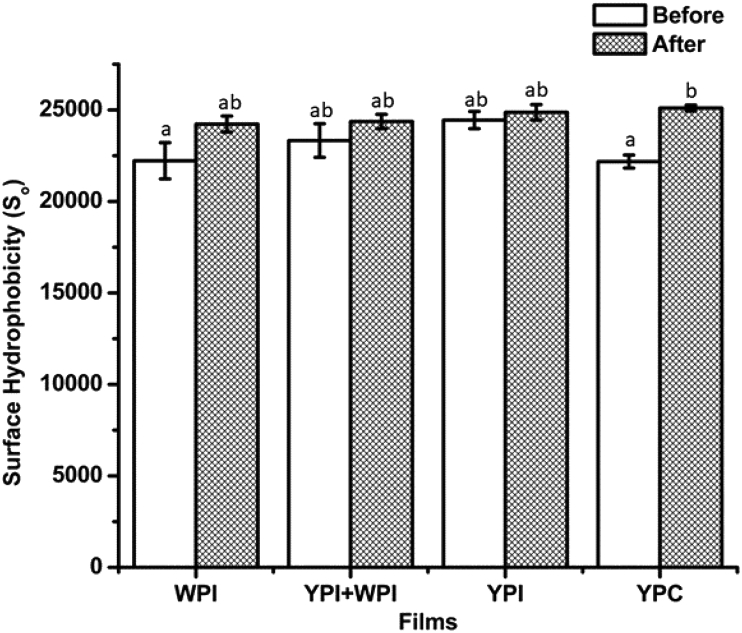
Thermal treatment of food proteins causes physical and chemical changes to their structures. These changes may be reversible at 60 °C or lower temperatures, whereas denaturation transitions and conformational changes may occur above 60 °C (Law and Leaver, 2000, Wijayanti et al., 2014). The structural changes are irreversible once the denaturation temperature of the protein is exceeded. Protein solutions in this study were thermally treated at 75 °C to unfold the proteins and trigger the formation of intermolecular bonds in order to form polymeric films. In addition, thermally treated proteins undergo structural rearrangement to cause their secondary (α-helix and β-sheets) and tertiary structures to unfold due to the disruption of hydrogen bonds. The structural rearrangement leads to an increased exposure of hydrophobic amino acid residues hidden in the interior part of the protein structure. In this study, the increase in surface hydrophobicity after heating, measured using ANS probe, was significant for YPC only (Fig. 2). The thermally unfolded proteins are expected to associate with each other by intermolecular (hydrophobic and disulphide) bonds leading to aggregation and formation of intact protein films. According to previously reported amino acid compositions, whey proteins contain a higher proportion of cysteine residues than yellow pea proteins (Gorissen et al., 2018, Pownall et al., 2010). As a result, unfolding of proteins is expected to result in the formation of more covalent disulphide (S–S) bonds in whey protein-based films than in pea protein-based films.
Fig. 2.
Surface hydrophobicity values for protein samples (4%, w/v solution), before and after thermal treatment, used for casting the films; WPI, whey protein isolate; YPI, yellow pea protein isolate; YPC, yellow pea protein concentrate. Mean values in each column with identical superscript letters are not significantly different at P = 0.05.
3.2. Intermolecular interactions of proteins in the films
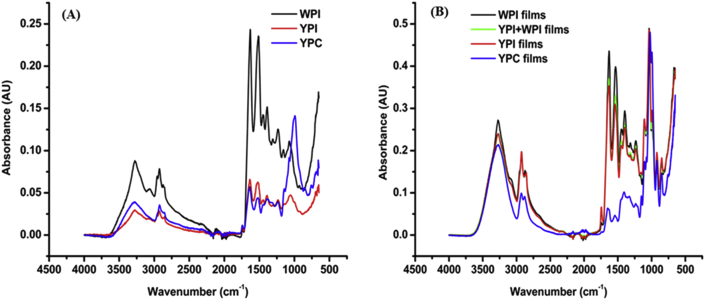
Intermolecular interactions of proteins in the films and their precursor proteins were determined with FTIR spectroscopy. As shown in the FTIR spectra (Fig. 3), maximum absorbance for the protein isolates (WPI and YPI) occurred at 1600-1700 cm−1, which corresponds to the C O stretching vibration and the backbone conformation of proteins. YPC had a maximum absorbance at 991.76 cm−1, which was absent in the protein isolates, corresponding to a strong monosubstituted C C bending likely from aromatic rings present in phenolic compounds (Abbas et al., 2017). All the protein films had a maximum absorbance at ~1034.6 cm−1 corresponding to the absorption bands of glycerol, which was absent in the precursor proteins.
Fig. 3.
(A) FTIR spectra of the protein powders and (B) protein films; WPI, whey protein isolate; YPI, yellow pea isolate; YPC, yellow pea protein concentrate.
Five absorption peaks were observed from 800 to 1100 cm−1 associated with C–C and C–O bonds in the protein films. These characteristic peaks appeared because of the addition of glycerol to WPI, YPI and YPC solutions to plasticise the proteins.
The amide A spectral region covers the wavelength range from 3000 to 3600 cm−1. A maximum absorption band for the protein samples occurred at ~3277 cm−1 for the protein powders. Plasticisation of the proteins with the same amount of glycerol resulted in a shift in peaks as follows: 3277 cm−1 to 3275 cm−1 (WPI), 3269 cm−1 (YPI + WPI), 3277 cm−1 to 3275 cm−1 (YPI), and 3277 cm−1 to 3267 cm−1 (YPC). This phenomenon is linked to the occurrence of a fermi resonance between the free and bound O–H and N–H stretching vibrations (Kananenka and Skinner, 2018). Additionally, absorption bands between 2500 and 3000 cm−1 arise because of the C–H stretching originating from the protein samples.
Amide I and II are two significant bands from infrared spectra associated with the secondary structure of proteins and polypeptides due to their involvement in hydrogen bonding. However, the amide I region, which occurs from 1600 to 1700 cm−1, is highly sensitive to protein conformational changes and linked to C O and C–N stretching vibrations. The C O bonds account for about 70–85% of this band and are linked to the backbone conformation of protein structures. There were notable differences in signal intensity in FTIR spectra of the protein samples at the amide region (WPI > YPI > YPC), which could be due to the protein sample purity. After casting of the protein films, intensity of the FTIR peaks for YPI significantly improved relative to the peak intensity of YPC. From the spectra, WPI, YPI and YPC are approximated to contain mainly β-sheet structures with YPC having the least intensity. The addition of glycerol caused different shifts in amide I peaks from 1636.8 cm−1 to 1629.3 cm−1 for YPI, whereas YPI + WPI had an absorption peak at 1631.1 cm−1 and WPI remained at 1629.3 cm−1. A shift towards lower wavenumbers is indicative of a stronger protein network through hydrogen bonding and possibly a higher content of ordered β-sheets (Ramos et al., 2013, Ullah et al., 2011). On the other hand, plasticisation of YPC shifted the peak from 1636.7 cm−1 to 1653.5 cm−1, which is indicative of a reduction in β-sheet interaction with the plasticiser and an enhancement of disordered structures.
Amide II bands occur between 1500 cm−1 and 1600 cm−1 with the N–H, C–N and C O groups of the protein conformationally sensitive towards the microenvironment of the solution (Ullah et al., 2011, Zhao and Wang, 2016). Plasticisation of proteins caused a shift in peaks towards higher wavenumbers in this band for all samples, from 1513.7 cm−1 to 1534.25 cm−1 (WPI); 1523.06 cm−1 to 1541.7 cm−1 (YPI); and 1528.6 cm−1 to 1541.7 cm−1 (YPC). The peak of YPI + WPI films spectrum were identical to those of the YPI films spectrum. Comparison of peak intensities in this region among the protein films showed that YPC < YPI < YPI + WPI < WPI, which demonstrates the occurrence of more robust hydrogen bonding from hydroxyl groups of the plasticiser with polypeptide chains.
3.3. Thickness, colour and surface morphology of the protein films
Results of the colour estimate and thickness of the protein films are presented in Table 2. YPC films had a lower thickness due to the lower protein content per unit volume when compared to the protein isolate (WPI, YPI + WPI and YPI) films. Measurements across the surface of each film macroscopically showed homogeneity in film thickness. Furthermore, the source of protein and pH of the protein solution prior to plasticisation may have influenced the film colour. These properties are crucial when using the protein films as packaging materials as their appearance must be acceptable to consumers (Chrysochou and Festila, 2019).
Table 2.
Thickness and colour estimation of films fabricated with yellow pea and whey proteins.
| Sample | Thickness of films (mm) | Colour |
Colour difference (ΔE) | ||
|---|---|---|---|---|---|
| R | G | B | |||
| WPI | 0.23 ± 7.2 × 10−3a | 178 | 169 | 157 | 1.4 |
| YPI + WPI | 0.23 ± 3.3 × 10−3a | 193 | 175 | 147 | 1.2 |
| YPI | 0.23 ± 6.6 × 10−3a | 183 | 160 | 119 | 1.6 |
| YPC | 0.20 ± 6.8 × 10−3b | 153 | 143 | 128 | 1.0 |
Abbreviations: WPI, Whey protein isolate; YPI, yellow pea protein isolate; YPC, yellow pea protein concentrate. Data with the same superscript letter in a column indicate no significant difference (P > 0.05).
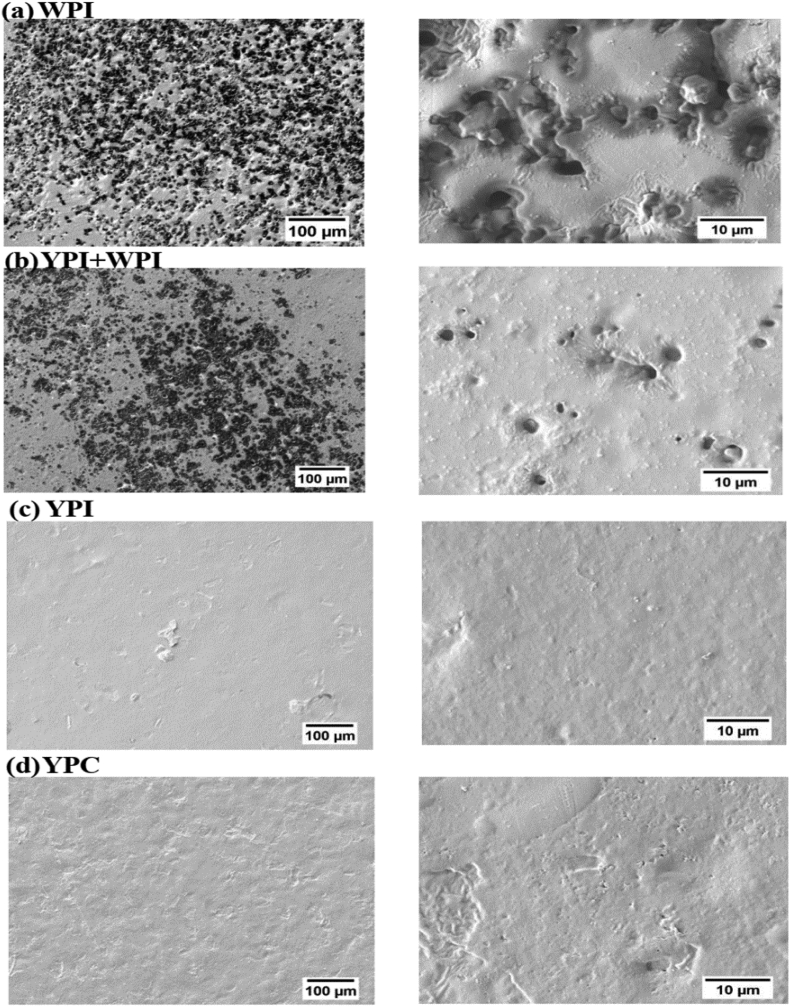
The surface morphology of the protein films is shown in Fig. 4 (A-D). Notably, visualisation of WPI, YPI + WPI, YPI and YPC films using a scanning electron microscope revealed the presence of particles on the surface of the polymers. This could be attributed to the high polydispersity index of protein particles. Dehydration patterns were observed on the surface of the two films formed with WPI. This could be due to the hydrophilicity of whey proteins, during phase separation in the mould. Furthermore, YPI + WPI film was observed to be homocomposite and formed a continuous structure, which was in agreement with previously reported bioplastics formed from protein blends (Liang and Chen, 2018, Gounga et al., 2007).
Fig. 4.
Surface morphology of films formulated with 4% (w/v) protein powders at × 150 and × 2000 for (A) WPI, (B) YPI + WPI, (C) YPI and (D) YPC; WPI, whey protein isolate; YPI, yellow pea protein isolate; YPC, yellow pea protein concentrate.
3.4. Moisture content and solubility
Moisture content and solubility of the protein films are presented in Table 3. Due to the presence of carboxyl and hydroxyl groups in proteins, protein films inherently have high water absorption capacity, which remains a challenge for some food packaging applications (Yue et al., 2012). Application of bioplastics in food packaging varies depending on the type of food. Food samples with high moisture content require packaging materials with high water resistance (Stuchell and Krochta, 1994). Packaging materials with a high tendency of absorbing moisture may alter the consistency, appearance, taste and shelf-life of packaged foods (Dey and Neogi, 2019). In contrast, low solubility indicates that the edible packaging materials would have low digestibility (Stuchell and Krochta, 1994). Thus, a degree of water resistance and solubility is expected for edible packaging materials. Substantial amount of the film matter dissolved in water after immersion, possibly due to the non-protein components or proteins that were not incorporated into the films. Orliac et al. (2003) demonstrated that the dissolved components of films derived from sunflower proteins were mainly plasticisers that were weakly bound to macromolecules in the film network. Prior to immersion, YPI and YPI + WPI films had similar moisture content to the control WPI, whereas YPC had the highest amount of water absorbed in the protein film after casting (Table 3). The moisture content values of the protein films were lower than previously reported values (Ramos et al., 2013, Gounga et al., 2007), possibly due to the lower protein density used for film formulation as proteins are hygroscopic. To further understand the behaviour of protein films under humid conditions and in contact with moist foods, films were immersed in water for 24 h. It was observed that the moisture content in protein films increased by ~42 folds for WPI, ~27 folds for YPI and YPI + WPI and ~16 folds for YPC. Furthermore, it was expected that the YPI and YPC films would absorb less water than the WPI film due to the relatively high hydrophobicity of the yellow pea protein films. In a previous water absorption kinetic study, water absorption of 30%–40% was observed for different cottonseed protein blends after 1000 min (~17 h) (Yue et al., 2012). Nonetheless, no difference was observed (P > 0.05) for dissolved matter and moisture content, and the structural integrity of all the protein films was intact after removal of the films from water, as previously reported for other protein films (Ramos et al., 2013, Yue et al., 2012, Kocakulak et al., 2019).
Table 3.
Moisture content and solubility of films fabricated with yellow pea and whey proteins.
| Sample | Solubility (%) | Moisture content before immersion, (%) | Moisture content after immersion, (%) |
|---|---|---|---|
| WPI | 49.3 ± 1.89a | 2.03 ± 0.75a | 84.86 ± 2.33a |
| YPI + WPI | 50.4 ± 5.01a | 3.03 ± 0.47a | 81.31 ± 0.35a |
| YPI | 36.5 ± 5.22a | 3.21 ± 0.42ab | 82.78 ± 1.45a |
| YPC | 51.6 ± 4.84a | 5.69 ± 0.52b | 82.90 ± 0.42a |
Abbreviations: WPI, Whey protein isolate; YPI, Yellow pea isolate; YPC, Yellow pea concentrate. Data with the same superscript letter in a column indicate no significant difference (P > 0.05).
3.5. Surface analysis of the protein films
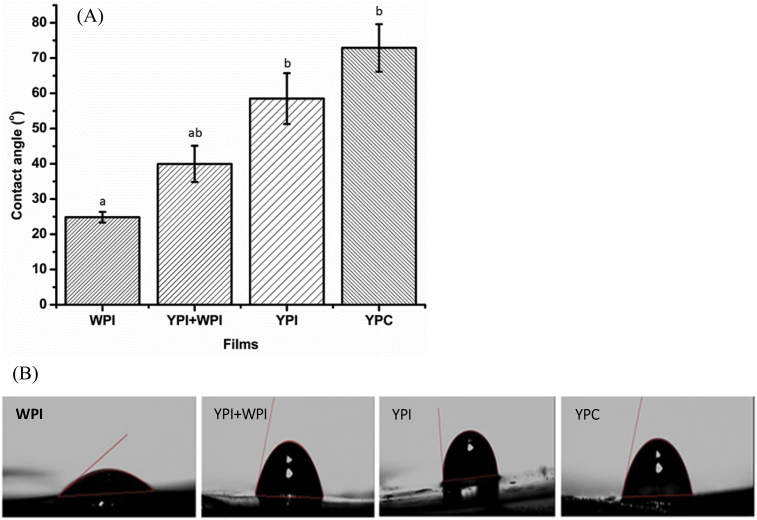
Measurement of the static contact angle provides an indication of the extent of hydrophobicity and hydrophilicity of the surface of materials, by estimating the final state of water droplets released onto the films. Polymeric surfaces with lower contact angles have lower hydrophobicity (higher hydrophilicity), higher wettability, and higher surface energy. Materials with a water contact angle <90° are taken to be hydrophilic whereas materials with a contact angle >90° are hydrophobic (Marichelvam et al., 2019). As shown in Fig. 5, the protein films in this study had contact angles <90° and this could be due to the presence of active polar groups in the protein structure. Heat treatment of proteins before film casting causes proteins to unfold. As proteins consist of amino acid residues with different chemical properties, the heat treatment could result in different protein interactions in a microenvironment and different surface energy of the resulting films. The pattern of surface hydrophilicity of the protein films was YPC < YPI < YPI + WPI < WPI. This difference could be due to factors such as the protein types, purity and amino acid composition of the protein samples. YPC comprises of a complex mixture of proteins, fibres and possibly phytochemicals, which reduce the protein content and active polar molecules responsible for enhancing the disruption of the surface energy of the films. Protein films have high surface hydrophilicity and are suitable for applications requiring high wettability and visibility (de Oliveira Gama et al., 2018). Nevertheless, hydrophobicity of the films can be enhanced by chemical modifications, such as acetylation of the proteins, and by incorporation of nanomaterials to form composite films (de Oliveira Gama et al., 2018, He et al., 2019).
Fig. 5.
(A) Water contact angle of protein films (n = 10) and (B) characteristic images of water droplets on protein films; WPI, whey protein isolate; YPI, yellow pea protein isolate; YPC, yellow pea protein concentrate. Mean values in each column with identical superscript letters are not significantly different at P = 0.05.
3.6. Thermal analysis of the protein films
Thermally resilient films are essential to tolerate temperature fluctuations from the outside environment and from inside the packaged food (Youssef and El-Sayed, 2018). The thermal behaviour of films formulated from YPI and YPC was compared to that of WPI using differential scanning calorimetry. DSC thermograms showed two endothermic thermal transitions, viz. glass transition and melting transition, within the thermal scan range of −40 °C–300 °C. Thus, the protein films had properties of a partially amorphous polymer. Additionally, the absence of multiple Tg values indicate that the protein solutions reacted with glycerol to form a co-polymer (Kurt and Kahyaoglu, 2014, Hamdi et al., 2019). The thermal transition pattern is similar to data from a previous work of Ramos et al. (2013) on whey proteins. As shown in Table 4, YPI + WPI films had the lowest glass transition temperature, percent crystallinity, and energy required to break the intermolecular chains. There was no difference in Tg values for protein films formulated with WPI, YPI and YPC (P > 0.05). Nevertheless, WPI required higher energy to break the intermolecular chains to melt the protein films, indicating higher resilience to heat effect. The lower Tg and estimated crystallinity value observed for YPI + WPI film, compared to WPI and YPI films, could be due to the introduction of weaker intermolecular interactions and increased protein chain mobility between WPI and YPI in the blend matrix.
Table 4.
Thermal and mechanical properties of protein films.
| Films | Tg (°C) | ΔHm (J/g) | %C | E (MPa) | T.S (MPa) | P.E (%) |
|---|---|---|---|---|---|---|
| WPI | 94.6 ± 0.34a | 103.46 ± 15.25b | 35.24 ± 5.19b | 66.63 ± 23.56a | 1.72 ± 0.61a | 39.26 ± 13.88a |
| YPI + WPI | 85.3 ± 4.95a | 22.54.5 ± 2.97a | 7.67 ± 1.01a | 28.64 ± 12.81b | 0.43 ± 0.22b | 35.57 ± 17.79a |
| YPI | 95.5 ± 0.30a | 73.99.1 ± 0.18b | 25.2 ± 0.06b | 6.65 ± 2.97c | 0.65 ± 0.29b | 65.64 ± 29.35a |
| YPC | 95.8 ± 1.35a | 42.41 ± 2.54ab | 14.45 ± 0.87ab | 0.026 ± 0.013d | 0.18 ± 0.092c | 9.69 ± 4.844b |
Abbreviations: Tg, glass transition; ΔHm, enthalpy of melting; %C, percentage of crystallinity; E, Young's modulus; T.S., tensile strength; P.E., percent elongation. WPI, whey protein isolate; YPI, yellow pea protein isolate; YPC, yellow pea protein concentrate. Data with the same superscript letter in a column indicate no significant difference (P > 0.05).
3.7. Mechanical analysis of the protein films
Food packaging materials with good mechanical properties preserve the integrity of the packaged food matrix by providing a physical barrier during storage and distribution. The mechanical properties are characterised based on mechanical resistance, stiffness and extensibility of the materials, which are measured as E, T.S., and P.E. at break, respectively (Ramos et al., 2013, Kowalczyk et al., 2014). WPI films showed better mechanical resistance and stiffness, which corroborated the infrared spectral peak patterns, followed by protein films formed from YPI + WPI, whereas YPI film showed better performance in extensibility (Table 4). It is evident that, given the same condition, the proteins interacted differently with the –OH moieties of glycerol. Notably, a low number of hydrogen bonds between protein molecules increases the intermolecular space and protein mobility thereby reducing the mechanical properties of the polymeric material (Ramos et al., 2013). This was evident in the mechanical properties of YPC protein films, which also had the lowest protein density. The presence of other non-protein constituents in the matrix of YPC protein film also made it brittle compared to the YPI, YPI + WPI and WPI protein films.
3.8. Light transmission and film transparency
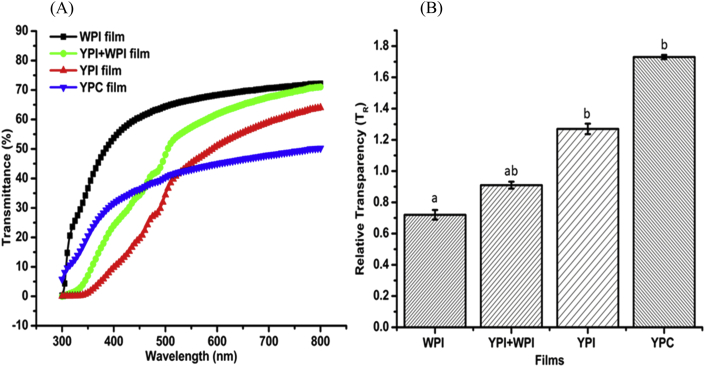
Exposure of food to light may alter the sensory and nutritional qualities of food due to photocatalytic reactions that produce activated free radicals. This phenomenon is heightened in the lower regions of the UV–visible light spectrum. Some of the damaging effects of this process include oxidation of fats and oils, discoloration of pigments, formation of off-tastes, and loss of vitamins A, B and C (Han et al., 2018, Nerín et al., 2008). Nonetheless, controlled UV is utilised actively in food processing to inactivate food spoilage microbes. Films formulated from YPI and YPI + WPI did not allow light transmission in the UV-C spectrum (200–280 nm) (data not shown), making them suitable packaging materials in preventing food spoilage or contamination due to photodegradation. As a result, measurement of light transmission of the protein films commenced from 300 to 800 nm. As shown in Fig. 6, the pattern of light transmission in the UV region was WPI > YPC > YPI + WPI > YPI. The penetrative power of UV light through a barrier is proportional to the absorption coefficient and can be reduced through increase in film thickness and decrease in transparency. The films from YPC had the lowest transparency (WPI > YPI + WPI > YPI > YPC), which could be attributed to the presence of phenolic compounds in the sample. However, a higher UV transmission was observed for YPC films due to their lower thickness value.
Fig. 6.
(A) Light transmittance in the near UV–visible spectrum and (B) transparency of the protein films; WPI, whey protein isolate; YPI, yellow pea protein isolate; YPC, yellow pea protein concentrate. Mean values in each column with identical superscript letters are not significantly different at P = 0.05.
4. Conclusion
This study explored the use of yellow pea proteins as a biomaterial for developing films for use in food packaging. Using glycerol as plasticiser and water as solvent, YPI and YPC formed films with promising physico-mechanical properties comparable to the properties of WPI films formed under the same condition. It is likely that the protein films were formed due in part to hydrogen bonding between hydroxyl groups of the plasticiser and polypeptide chains. WPI films had better thermal and mechanical resilience and degree of hydrophilicity, whereas YPI and YPC films showed better protection in limiting light transmission. Thus, a blend of the proteins was expected to result in films with the combined beneficial features. However, YPI + WPI films had properties that were not indicative of a synergistic effect of the protein mixtures, possibly because of an increase in chain mobility of the proteins in the blend matrix. In addition, the protein films formed in this study had high water absorption capacity and solubility index due to the existence of carboxyl and hydroxyl groups in proteins. Future studies will focus on generally regarded as safe protein modification and cross-linking processes to minimise the water absorption and solubility index as well as evaluating the performance of films fabricated with the yellow pea proteins as a bio-based packaging material for food and biomaterial applications.
Declaration of Competing Interest
The authors declare no conflict of interest in this study.
Acknowledgements
We acknowledge the support of the Natural Sciences and Engineering Research Council of Canada (NSERC), reference numbers RGPIN-2018-06839 (C.C. Udenigwe) and RGPIN-2014-05892 (M.A. Dubé). Cette recherche a été financée par le Conseil de recherches en sciences naturelles et en génie du Canada (CRSNG), numéros de référence RGPIN-2018-06839 (C.C. Udenigwe) et RGPIN-2014-05892 (M.A. Dubé). We thank Pulse Canada (Winnipeg, MB, Canada) for providing the yellow pea seeds used in this study.
References
- Abbas O., Compère G., Larondelle Y., Pompeu D., Rogez H., Baeten V. Phenolic compound explorer: a mid-infrared spectroscopy database. Vib. Spectrosc. 2017;92:111–118. doi: 10.1016/J.VIBSPEC.2017.05.008. [DOI] [Google Scholar]
- Abdel-Aal E.-S.M., Ragaee S., Rabalski I., Warkentin T., Vandenberg A. Nutrient content and viscosity of Saskatchewan-grown pulses in relation to their cooking quality. Can. J. Plant Sci. 2019;99:67–77. doi: 10.1139/cjps-2018-0140. [DOI] [Google Scholar]
- A.S. ASTM, D618 . ASTM International; West Coshoshocken, PA: 2000. Standard Practice for Conditioning Plastics for Testing.www.astm.org West Coshoshocken. [DOI] [Google Scholar]
- Barac M., Cabrilo S., Pesic M., Stanojevic S., Zilic S., Macej O., Ristic N. Profile and functional properties of seed proteins from six pea (Pisum sativum) genotypes. Int. J. Mol. Sci. 2010;11:4973. doi: 10.3390/IJMS11124973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom K.A., Huang F.R., Bencharitiwong R., Bardina L., Ross A., Sampson H.A., Nowak-Wegrzyn A. Effect of heat treatment on milk and egg proteins allergenicity. Pediatr. Allergy Immunol. 2014;25:740–746. doi: 10.1111/pai.12283. [DOI] [PubMed] [Google Scholar]
- Cao N., Fu Y., He J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocolloids. 2007;21:1153–1162. doi: 10.1016/j.foodhyd.2006.09.001. [DOI] [Google Scholar]
- Cao L., Liu W., Wang L. Developing a green and edible film from Cassia gum: the effects of glycerol and sorbitol. J. Clean. Prod. 2018;175:276–282. doi: 10.1016/j.jclepro.2017.12.064. [DOI] [Google Scholar]
- Carvajal-Piñero J.M., Ramos M., Jiménez-Rosado M., Perez-Puyana V., Romero A. Development of pea protein bioplastics by a thermomoulding process: effect of the mixing stage. J. Polym. Environ. 2019;27:968–978. doi: 10.1007/s10924-019-01404-3. [DOI] [Google Scholar]
- Chan E., Masatcioglu T.M., Koksel F. Effects of different blowing agents on physical properties of extruded puffed snacks made from yellow pea and red lentil flours. J. Food Process. Eng. 2019;42 doi: 10.1111/jfpe.12989. [DOI] [Google Scholar]
- Chrysochou P., Festila A. A content analysis of organic product package designs. J. Consum. Mark. 2019:1–25. doi: 10.1108/JCM-06-2018-2720. [DOI] [Google Scholar]
- de Oliveira Gama R., Bretas R.E.S., Oréfice R.L. Control of the hydrophilic/hydrophobic behavior of biodegradable natural polymers by decorating surfaces with nano- and micro-components. Adv. Polym. Technol. 2018;37:654–661. doi: 10.1002/adv.21706. [DOI] [Google Scholar]
- Dey A., Neogi S. Oxygen scavengers for food packaging applications: a review. Trends Food Sci. Technol. 2019;90:26–34. doi: 10.1016/J.TIFS.2019.05.013. [DOI] [Google Scholar]
- Garrido T., Etxabide A., Leceta I., Cabezudo S., de la Caba K., Guerrero P. Valorization of soya by-products for sustainable packaging. J. Clean. Prod. 2014;64:228–233. doi: 10.1016/J.JCLEPRO.2013.07.027. [DOI] [Google Scholar]
- Ghanbarzadeh B., Oromiehi A.R. Biodegradable biocomposite films based on whey protein and zein: barrier, mechanical properties and AFM analysis. Int. J. Biol. Macromol. 2008;43:209–215. doi: 10.1016/j.ijbiomac.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Gorissen S.H.M., Crombag J.J.R., Senden J.M.G., Waterval W.A.H., Bierau J., Verdijk L.B., van Loon L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids. 2018;50:1685–1695. doi: 10.1007/s00726-018-2640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounga M.E., Xu S.Y., Wang Z. Whey protein isolate-based edible films as affected by protein concentration, glycerol ratio and pullulan addition in film formation. J. Food Eng. 2007;83:521–530. doi: 10.1016/j.jfoodeng.2007.04.008. [DOI] [Google Scholar]
- Hahladakis J.N., Velis C.A., Weber R., Iacovidou E., Purnell P. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard Mater. 2018;344:179–199. doi: 10.1016/J.JHAZMAT.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Hamdi M., Nasri R., Li S., Nasri M. Bioactive composite films with chitosan and carotenoproteins extract from blue crab shells: biological potential and structural, thermal, and mechanical characterization. Food Hydrocolloids. 2019;89:802–812. doi: 10.1016/J.FOODHYD.2018.11.062. [DOI] [Google Scholar]
- Han J.-W., Ruiz-Garcia L., Qian J.-P., Yang X.-T. Food packaging: a comprehensive review and future trends. Compr. Rev. Food Sci. Food Saf. 2018;17:860–877. doi: 10.1111/1541-4337.12343. [DOI] [PubMed] [Google Scholar]
- He R., Dai C., Li Y., Wang Z., Li Q., Zhang C., Ju X., Yuan J. Effects of succinylation on the physicochemical properties and structural characteristics of edible rapeseed protein isolate films. J. Am. Oil Chem. Soc. 2019 doi: 10.1002/aocs.12264. aocs.12264. [DOI] [Google Scholar]
- Huby R.D., Dearman R.J., Kimber I. Why are some proteins allergens? Toxicol. Sci. 2000;55:235–246. doi: 10.1093/toxsci/55.2.235. [DOI] [PubMed] [Google Scholar]
- Kananenka A.A., Skinner J.L. Fermi resonance in OH-stretch vibrational spectroscopy of liquid water and the water hexamer. J. Chem. Phys. 2018;148:244107. doi: 10.1063/1.5037113. [DOI] [PubMed] [Google Scholar]
- Kocakulak S., Sumnu G., Sahin S. Chickpea flour-based biofilms containing gallic acid to be used as active edible films. J. Appl. Polym. Sci. 2019;136 doi: 10.1002/app.47704. [DOI] [Google Scholar]
- Kowalczyk D., Gustaw W., Świeca M., Baraniak B. A study on the mechanical properties of pea protein isolate films. J. Food Process. Preserv. 2014;38:1726–1736. doi: 10.1111/jfpp.12135. [DOI] [Google Scholar]
- Krzywinski M. 2019. Image Color Summarizer.http://mkweb.bcgsc.ca/color-summarizer/? [Google Scholar]
- Kurt A., Kahyaoglu T. Characterization of a new biodegradable edible film made from salep glucomannan. Carbohydr. Polym. 2014;104:50–58. doi: 10.1016/j.carbpol.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam A.C.Y., Can Karaca A., Tyler R.T., Nickerson M.T. Pea protein isolates: structure, extraction, and functionality. Food Rev. Int. 2018;34:126–147. doi: 10.1080/87559129.2016.1242135. [DOI] [Google Scholar]
- Lambert S., Wagner M. Environmental performance of bio-based and biodegradable plastics: the road ahead. Chem. Soc. Rev. 2017;46:6855–6871. doi: 10.1039/C7CS00149E. [DOI] [PubMed] [Google Scholar]
- Law A.J.R., Leaver J. 2000. Effect of pH on the Thermal Denaturation of Whey Proteins in Milk. [DOI] [PubMed] [Google Scholar]
- Liang J., Chen R. Impact of cross-linking mode on the physical properties of zein/PVA composite films. Food Packag. Shelf Life. 2018;18:101–106. doi: 10.1016/j.fpsl.2018.10.003. [DOI] [Google Scholar]
- Margier M., Georgé S., Hafnaoui N., Remond D., Nowicki M., Du Chaffaut L., Amiot M.-J., Reboul E. Nutritional composition and bioactive content of legumes: characterization of pulses frequently consumed in France and effect of the cooking method. Nutrients. 2018;10:1668. doi: 10.3390/nu10111668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marichelvam M.K., Jawaid M., Asim M., Marichelvam M.K., Jawaid M., Asim M. Corn and rice starch-based bio-plastics as alternative packaging materials. Fibers. 2019;7:32. doi: 10.3390/fib7040032. [DOI] [Google Scholar]
- Mlalila N., Hilonga A., Swai H., Devlieghere F., Ragaert P. Antimicrobial packaging based on starch, poly(3-hydroxybutyrate) and poly(lactic-co-glycolide) materials and application challenges. Trends Food Sci. Technol. 2018;74:1–11. doi: 10.1016/J.TIFS.2018.01.015. [DOI] [Google Scholar]
- Nerín C., Tovar L., Salafranca J. Behaviour of a new antioxidant active film versus oxidizable model compounds. J. Food Eng. 2008;84:313–320. doi: 10.1016/J.JFOODENG.2007.05.027. [DOI] [Google Scholar]
- Oliveira S.P.L.F., Bertan L.C., De Rensis C.M.V.B., Bilck A.P., Vianna P.C.B., Oliveira S.P.L.F., Bertan L.C., De Rensis C.M.V.B., Bilck A.P., Vianna P.C.B. Whey protein-based films incorporated with oregano essential oil. Polímeros. 2017;27:158–164. doi: 10.1590/0104-1428.02016. [DOI] [Google Scholar]
- Orliac O., Rouilly A., Silvestre F., Rigal L. Effects of various plasticizers on the mechanical properties, water resistance and aging of thermo-moulded films made from sunflower proteins. Ind. Crops Prod. 2003;18:91–100. doi: 10.1016/S0926-6690(03)00015-3. [DOI] [Google Scholar]
- Perez V., Felix M., Romero A., Guerrero A. Characterization of pea protein-based bioplastics processed by injection moulding. Food Bioprod. Process. 2016;97:100–108. doi: 10.1016/j.fbp.2015.12.004. [DOI] [Google Scholar]
- Perez-Puyana V., Felix M., Romero A., Guerrero A. Effect of the injection moulding processing conditions on the development of pea protein-based bioplastics. J. Appl. Polym. Sci. 2016;133 doi: 10.1002/app.43306. [DOI] [Google Scholar]
- Pownall T.L., Udenigwe C.C., Aluko R.E. Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. J. Agric. Food Chem. 2010;58:4712–4718. doi: 10.1021/jf904456r. [DOI] [PubMed] [Google Scholar]
- Pulse Canada Producers and Industry. 2019. http://www.pulsecanada.com/producers-industry/
- Ramos Ó.L., Reinas I., Silva S.I., Fernandes J.C., Cerqueira M.A., Pereira R.N., Vicente A.A., Poças M.F., Pintado M.E., Malcata F.X. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocolloids. 2013;30:110–122. doi: 10.1016/J.FOODHYD.2012.05.001. [DOI] [Google Scholar]
- Roohi P., Srivastava K., Bano M.R., Zaheer M., Kuddus . Bio-based Mater. Food Packag. Springer Singapore; Singapore: 2018. Biodegradable smart biopolymers for food packaging: sustainable approach toward green environment; pp. 197–216. [DOI] [Google Scholar]
- Sanchez-Monge R., Lopez-Torrejón G., Pascual C.Y., Varela J., Martin-Esteban M., Salcedo G. Vicilin and convicilin are potential major allergens from pea. Clin. Exp. Allergy. 2004;34:1747–1753. doi: 10.1111/j.1365-2222.2004.02085.x. [DOI] [PubMed] [Google Scholar]
- Seydim A.C., Sarikus G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006;39:639–644. doi: 10.1016/J.FOODRES.2006.01.013. [DOI] [Google Scholar]
- Shand P.J., Ya H., Pietrasik Z., Wanasundara P.K.J.P.D. Physicochemical and textural properties of heat-induced pea protein isolate gels. Food Chem. 2007;102:1119–1130. doi: 10.1016/J.FOODCHEM.2006.06.060. [DOI] [Google Scholar]
- Sharma D., Dhanjal D.S., Mittal B. Development of edible biofilm containing cinnamon to control food-borne pathogen. J. Appl. Pharm. Sci. 2017;7:160–164. doi: 10.7324/JAPS.2017.70122. [DOI] [Google Scholar]
- Song N.B., Lee J.H., Al Mijan M., Bin Song K. Development of a chicken feather protein film containing clove oil and its application in smoked salmon packaging. LWT - Food Sci. Technol. 2014;57:453–460. doi: 10.1016/j.lwt.2014.02.009. [DOI] [Google Scholar]
- Stuchell Y.M., Krochta J.M. Enzymatic treatments and thermal effects on edible soy protein films. J. Food Sci. 1994;59:1332–1337. doi: 10.1111/j.1365-2621.1994.tb14709.x. [DOI] [Google Scholar]
- Sun Z., Wang M., Han S., Ma S., Zou Z., Ding F., Li X., Li L., Tang B., Wang H., Li N., Che H., Dai Y. Production of hypoallergenic milk from DNA-free beta-lactoglobulin (BLG) gene knockout cow using zinc-finger nucleases mRNA. Sci. Rep. 2018;8:15430. doi: 10.1038/s41598-018-32024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah A., Vasanthan T., Bressler D., Elias A.L., Wu J. Bioplastics from feather quill. Biomacromolecules. 2011;12:3826–3832. doi: 10.1021/bm201112n. [DOI] [PubMed] [Google Scholar]
- Wijayanti H.B., Bansal N., Deeth H.C. Stability of whey proteins during thermal processing: a review. Compr. Rev. Food Sci. Food Saf. 2014;13:1235–1251. doi: 10.1111/1541-4337.12105. [DOI] [Google Scholar]
- Wu Y.V., Inglett G.E. Denaturation of plant proteins related to functionality and food applications. A Review. J. Food Sci. 1974;39:218–225. doi: 10.1111/j.1365-2621.1974.tb02861.x. [DOI] [Google Scholar]
- Youssef A.M., El-Sayed S.M. Bionanocomposites materials for food packaging applications: concepts and future outlook. Carbohydr. Polym. 2018;193:19–27. doi: 10.1016/J.CARBPOL.2018.03.088. [DOI] [PubMed] [Google Scholar]
- Yue H.B., Cui Y.D., Shuttleworth P.S., Clark J.H. Preparation and characterisation of bioplastics made from cottonseed protein. Green Chem. 2012;14:2009–2016. doi: 10.1039/c2gc35509d. [DOI] [Google Scholar]
- Zhao J., Wang J. Uncovering the sensitivity of Amide-II vibration to peptide–ion interactions. J. Phys. Chem. B. 2016;120:9590–9598. doi: 10.1021/acs.jpcb.6b05889. [DOI] [PubMed] [Google Scholar]