Abstract
The chemical composition of Essential Oils Satureja montana and Mentha longifolia was determined, and their activity against important phytopathogenic and post-harvest fungi was studied, to evaluate their potential as natural food preservatives. The major compounds were carvacrol (24.0%), γ-terpinene (15.9%) and p-cymene (14.2%) in S. montana, and piperitenone oxide (52.7%) and piperitone oxide (23.5%) in M. longifolia. EOs were tested in vitro on Alternaria alternata, Botryotinia fuckeliana, Curvularia hawaiiensis, Fusarium equiseti, F. oxysporum lycopersici, Rhizoctonia solani and Verticillium dahliae. S. montana demonstrated excellent results. At 300 μg mL−1 the growth of all fungi was inhibited with 100% mycelial growth inhibition (MGI), except for B. fuckeliana (92%). M. longifolia was less effective, and its best result was against Verticillium dahliae (100% MGI) at 400 and 300 μg mL−1. S. montana EO was selected for in vivo antifungal tests in Cherry tomatoes and kaki “Persimmon” against A. alternata. The S. montana EO biofilm reduced post-harvest fungi development. In tomato, it inhibited up to 90% after 20 days. Necrosis did not occur for 2 months in the persimmon fruits. S. montana EO is an effective non-toxic preservative that can be considered to develop a botanical and enviro-friendly low-risk biofungicide.
Keywords: Essential oils, Antifungal activity, Food preservatives, Phytopatogenic fungi, Post-harvest fungi
Graphical abstract
Highlights
-
•
Satureja montana and Mentha longifolia EOs were tested as food preservatives.
-
•
The in vitro tested Satureja montana EO showed great antifungal activity.
-
•
A natural biodegradable Satureja montana EO biofilm was developed.
-
•
The S. montana EO biofilm controlled Alternaria alternata in tomato and kaki.
-
•
The composition of the EOs was determined by CG and GC/MS.
1. Introduction
Diseases in plants are an important constraint for worldwide crop production, and account for 20–40% of annual harvest loss. Plant pathogenic fungi cause some of the most devastating universal crop diseases (Villa et al., 2017). Postharvest diseases of fruits and vegetables currently provoke a reduction of the total production of 35–55%, with significant differences between geo-economic areas (Sanzani et al., 2016). The greatest losses occur in developing countries.
It is necessary to maximise food production and reduce losses in the present context, with global food demand increasing as the world's population is expected to rise to 9 billion in 2050 (United Nations, 2017). However, this rise in production must be sustainable.
The control of plant pathogens and postharvest diseases relies on the use of synthetic fungicides. Their repeated use has led to the development of fungicide-resistance strains of microorganisms, and has caused environmental pollution and negative side effects on human health (Brent and Hollomon, 2007). Therefore, it is necessary to reduce the use of synthetic fungicides by increasing their efficacy or by developing alternatives (Rahman et al., 2014). Regulations on the use of synthetic pesticides have also become more restrictive in recent years, with less products and applications being allowed. European Union Directive 2009/128/EC promotes the use of integrated pest management (IPM) and alternative techniques to limit pesticide applications in all European Union countries. Society is demanding new solutions to more sustainably manage pathogenic diseases that are environmentally friendly and human safe. One alternative to synthetic fungicides could be to use essential oils (EOs).
EOs are volatile aromatic liquids obtained from plant material, including flowers, roots, bark, leaves, seeds, peel, fruits, wood, and whole plants (Hyldgaard et al., 2012). They offer a good crop protection opportunity thanks to the characteristics they possess. The advantages of using EOs in IPM easily oppose the environmental hazards generated by synthetic pesticides. As metabolic products have resulted from species coevolution, their effects on target species are selective and specific. EOs are enzymatically biodegradable with short half-lives. The association of several compounds can be synergistic, they reduce effective amounts of active ingredients, and they belong to several different chemical families. By increasing the choice of available molecules, they contribute to the diversification of biochemical and molecular targets and, thus, limit or delay the pest resistance phenomenon (Regnault-Roger and Philogène, 2008).
EOs also represent an interesting source of natural antimicrobial products for food preservation, but the food industry primarily uses them as flavouring agents. However, applying EOs as food preservatives requires detailed knowledge about their properties; i.e., the minimum inhibitory concentration (MIC), the range of target organisms, the action mode, the effect of food matrix components on their antimicrobial properties, etc. (Hyldgaard et al., 2012).
The biological activity of EOs depends on their chemical composition, which is determined by the plant genotype and is greatly influenced by several factors, such as geographical origin and environmental and agronomic conditions (Carramiñana et al., 2008).
The genus Satureja belongs to the Lamiaceae family and comprises over 30 species whose centre of distribution is located in the eastern part of the Mediterranean Region. Satureja montana L. (winter savory) is frequently used in local spices and as a traditional medicinal plant. Many research studies have been conducted to determine the chemical profile of S. montana, and the variability in the composition of EOs and extracts correlates with the geographical locality and development stage of S. montana. The major volatile constituents of S. montana are γ-terpinene (4.9–10.6%), carvacrol (13.7–52.4%), p-cymene (3.0–11.8%) and thymol (9.92–45.2%). Due to its pharmacologically relevant chemical composition, S. montana and its extracts possess noteworthy biological activities: antibacterial and fungicidal, antioxidant capacity, anti-inflammatory effect on cultured human erythroleukemic K562 cells and anti-HIV-1 activity (Vladić et al., 2017).
The genus Mentha L. (Lamiaceae) includes approximately 25 species, mainly perennial herbs that grow wild in damp or wet places throughout temperate regions of Eurasia, Australia and South Africa (Lange and Croteau, 1999). This mint species is very important for its medicinal properties and its applications in many industries.
Mentha longifolia L. (wild mint) is a fast-growing perennial herb that grows wild in wet places close to water streams. Besides its traditional applications, mainly against respiratory and digestive diseases and for culinary purposes, recent research has demonstrated that the EOs and methanol extracts from this species possess antimicrobial and antioxidant properties that can be used in food preservation (Hajlaoui et al., 2009, Llorens-Molina et al., 2015).
In this work, the EOs of S. montana (commercial sample) and M. longifolia (from a population growing at the Polytechnic University of Valencia (UPV), Spain) were evaluated in vitro to determine their possible use as alternatives to synthetic fungicides as part of an IPM strategy against the phytopathogenic and postharvest fungi Alternaria alternata (AA), Botryotinia fuckeliana (BF), Curvularia hawaiiensis (CH), Fusarium equiseti (FE), F. oxysporum lycopersici (FOL), Rhizoctonia solani (RS) and Verticillium dahliae (VD). Many of the studied fungi have been recently considered to be the “Top 10” fungal plant pathogens based on their scientific/economic importance (Dean et al., 2012), and are resistant to control with synthetic fungicides. As S. montana EO was more effective, it was selected for the in vivo assays and was tested against AA, a cosmopolitan fungus present in a wide range of horticultural crops, such as strawberries, tomatoes, kakis, carrots and asparagus. AA affects tomatoes and kakis in the field and under post-harvest conditions (Guerrero-Rodríguez et al., 2007). It causes major economic loss in the Valencian Community (east Spain) and, apart from the damage it provokes, it also produces mycotoxins that can cause chronic diseases in humans (Arcella et al., 2016).
The final target was to obtain organic biofungicides based on EOs that can be used in field crops, as stored products, and for food and grain preservation.
2. Material and methods
2.1. The EOs employed
A commercial sample of the Satureja montana L. EO was supplied by Essential’ arôms (Lleida, Spain) (batch: 772B021005). The Mentha longifolia (L.) Huds EO was obtained by hydrodistillation from plants growing in the experimental plots at the UPV (Valencia, Spain). Plant material of M. longifolia (chemotype piperitone oxide + piperitenone oxide) was obtained from an accession grown in the experimental field of the Universitat Politècnica de València. This accession came from a wild population located in Teruel (Spain) (40° 58′ 48″ N, 1° 18′ 48″ W, 857 m a. s. l.). in which a previous selection of individuals according their EO chemotype was performed. A Voucher specimen belonging to this EO profile was kept at the Herbarium of the Universitat Politècnica de València (VALA No 9576).
Mature leaves of randomly selected individuals were collected during the full flowering stage and air-dried at room temperature in shade conditions. 400 g of dried material were submitted to hydrodistillation by means of a Clevenger type apparatus for 3 h. The essential oil was dehydrated with anhydrous sodium sulphate and kept at 4 °C in a sealed amber vials. 8.18 g of EO were obtained (2.05% w/w).
2.2. Gas chromatography/mass spectrometry analysis of EOs
The analysis of EOs was carried out by gas chromatography coupled to flame ionisation detection (GC-FID) and mass spectrometry (GC-MS), as described in Llorens and Vacas (2015). Each sample was run in triplicate.
2.3. Fungi
The fungi employed in this study were: Alternaria alternata CECT 20943 (AA); Botryotinia fuckeliana CECT 20518 (BF); Curvularia hawaiiensis CECT 20934 (CH); Fusarium equiseti CECT 20925 (FE); F. oxysporum lycopersici CECT 2715 (FOL); Rhizoctonia solani CECT 2819 (RS); Verticillium dahliae CECT 2694 (VD). AA, CH and FE were isolated in the Botany Laboratory of the UPV Department of Agroforestry Ecosystems from rice caryopses of the Bomba variety growing in a coastal lagoon of Valencia. These fungi were identified by morphological and molecular methods, and were deposited in the Spanish Type Culture Collection (CECT). Fungal strains were molecularly determined by the analysis of two different regions of ribosomal DNA genes: the nuclear ribosomal internal transcribed spacer ‘ITS region’ and the D1/D2 domains of 28S rRNA. A third genetic marker, the translation elongation factor 1-alpha (EF-1α) gene region, was used for the species-level identification of the isolate belonging to the genus Fusarium.
The primers used for amplification were ITS1 and ITS4 (White et al., 1990) for the ITS region, NL1 and NL4 (Kurtzman and Robnett, 1998) for the D1/D2 LSU region, and EF1-728F and EF1-986R (Carbone and Kohn, 1999) for the EF-1α gene. BF, FOL, RS and VD were supplied by the CECT.
2.4. Antifungal activity assays
2.4.1. Determination of mycelial growth inhibition (MGI)
The EO was dissolved, mixed and homogenised by shaking in flasks with previously sterilised PDA/Tween 20 (0.1%) growth medium. While still in a liquid form, it was added at the concentrations of 400, 300, 200 or 100 μg mL−1 and distributed in Petri dishes of 90 × 15 mm and 150 × 15 mm. The fungus was sown as discoidal explants, 8 mm in diameter (Ø), taken from a colony after 7 days of development, and placed in the centre of the Petri dishes containing the EO. The experiment was incubated at 25 °C for 7 days. Five repetitions were performed by treatment and fungal species. The control Petri dishes contained only PDA/Tween 20 (0.1%) and the analysed fungus. Mycelial growth was evaluated by measuring perpendicular colony diameters after 7 days.
Mycelial growth inhibition (MGI) was determined by the following formula (Albuquerque et al., 2006):
DC is the average of colonies in the control dishes, DO is the average of the colonies diameter in the dishes with oil.
2.4.2. Determination of the fungicide and/or fungistatic effect
After determining the antifungal activity of both EOs, for those whose results showed 100% inhibition, their fungicide and/or fungistatic effect was checked.
Of those dishes that gave 100% inhibited fungal growth after the 7-day incubation, the same fungal inoculation disc (8 mm) was taken and sown in the centre of the Petri dishes containing only PDA. The experiment was incubated at 25 °C for 7 days. The growth diameter of each fungus was measured after the incubation period.
2.4.3. In vivo antifungal activity tests of the Satureja montana EO against Alternaria alternata on cherry tomato and kaki persimmon
2.4.3.1. Preparation of the EO solution for fruit coating
The S. montana EO solution for coating fruits was prepared at the 600 μg mL−1 concentration. The EO was homogenised by orbital shaking at 170 rpm for 10 min in flasks containing water/Tween 20 (0.1%)/0.25% agar.
2.4.3.2. Preparation of the fungal inoculum
To recovery the fruits with the fungus, a solution containing AA propagules was prepared. To do this, 10 mL of a suspension of 1 × 108 ufc mL−1 of the fungus were added to 90 mL of water/Tween 20 (0.1%)/0.25% agar. The mixture was homogenised by orbital shaking at 170 rpm for 10 min to obtain a homogeneous suspension.
2.4.3.3. Tomatoes and kakis coated with the EO and the fungal inoculum
Cherry tomatoes (origin Mazarrón, province of Murcia, Spain) and kaki “Persimmon”, (recently collected; origin Ribera del Xuquer, province of Valencia, Spain), were sterilised superficially with 1% sodium hypochlorite solution for 2 min and then washed twice with sterile distilled water for 4 min. Fruits were distributed into three batches of 50 units each (2 controls and the S. montana-film treatment). They all subjected to a small wound (1 mm depth) on the surface with a sterile needle (punch). In the S. montana-film treatment, fruits were immersed in the solution containing the S. montana EO for 4 min before being placed on racks and dried for 24 h at room temperature. They were then bathed in the fungal inoculum for 2 min. The fruits covered with the suspension of AA were placed on racks. In the assay ‘control 1’ (50 fruits), damaged fruits were only bathed with the fungal inoculum. In the assay ‘control 2’ (50 fruits), damaged fruits were first immersed for 4 min in the coating solution containing only agar and Tween, with no EO, to then be dried for 24 h and later bathed with the fungal inoculum.
In both tests (tomato and kaki), the three lots (control 1, control 2 and the S. montana-film treatment) were placed in the chamber at the same time (85% humidity at 21 °C). The evolution of cherry tomatoes was controlled for 7 and 14 days because they were ready to eat, and for 2 months for kaki Persimmon as they had been recently collected.
2.5. Statistical analysis
The fungal growth results were submitted to an analysis of variance (ANOVA). The HSD Tukey intervals were represented to compare species and treatment, with significant values at p = 0.05. The data analysis was performed by Statgraphics Centurion XVI.
3. Results and discussion
3.1. Determination of the EO components
Thirty-seven compounds were identified in the S. montana EO, which accounted for 98.9% of the composition (Table 1). The main compounds were carvacrol (24.0%), γ-terpinene (15.9%) and p-cymene (14.2%). According to the literature, the composition of the S. montana EO is uniform with a small set of well-defined major compounds. Carvacrol is usually the most abundant, with up to 60–70%. The obtained results agree with previous studies on the composition of this species in different Mediterranean countries (Angelini et al., 2003, Skočibušić and Bezić, 2004, Fraternale et al., 2007, Grosso et al., 2010, García-Rellán et al., 2015, Trifan et al., 2015, Moisa et al., 2017).
Table 1.
Chemical composition of the Satureja montana (commercial sample) and Mentha longifolia (obtained from the population growing at the UPV, Valencia, Spain) essential oils (EOs).
| COMPOUNDa | RI (lineal) exp.b | RI (lineal) ref.c |
S. montana |
M. longifolia |
|---|---|---|---|---|
| Peak area %d | Peak area % | |||
| Tricyclene | 921 | 921 | tre | -f |
| α-thujene | 925 | 924 | 1.1 ± 0.0 | – |
| α-pinene | 930 | 932 | 1.7 ± 0.0 | 1.0 ± 0.0 |
| Camphene | 945 | 946 | 0.5 ± 0.0 | tr |
| Sabinene | 969 | 969 | 0.1 ± 0.0 | 0.8 ± 0.0 |
| β-pinene | 974 | 974 | tr | 1.3 ± 0.0 |
| Octen-2-ol | 980 | 982 | 0.7 ± 0.0 | – |
| Myrcene | 991 | 988 | 3.1 ± 0.0 | 1.2 ± 0.0 |
| 2-octanol | 997 | 994 | – | 0.4 ± 0.0 |
| α-phellandrene | 1004 | 1002 | 0.2 ± 0.0 | tr |
| δ-3-carene | 1010 | 1008 | tr | – |
| α-terpinene | 1016 | 1014 | 1.9 ± 0.0 | – |
| p-cymene | 1019 | 1020 | 14.2 ± 0.1 | – |
| Limonene | 1027 | 1024 | 1.8 ± 0.2 | 1.4 ± 0.0 |
| 1,8-cineole | 1030 | 1026 | 0.7 ± 0.1 | 1.8 ± 0.0 |
| (Z)-β-ocimene | 1038 | 1032 | 0.2 ± 0.0 | 2.2 ± 0.0 |
| (E)-β-ocimene | 1049 | 1044 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| γ-terpinene | 1055 | 1054 | 15.9 ± 0.1 | 2.4 ± 0.0 |
| (Z)-sabinen hydrate | 1067 | 1065 | 0.6 ± 0.0 | – |
| Terpinolene | 1088 | 1086 | 0.3 ± 0.0 | – |
| Isopentyll 2-methyl butanoate | 1099 | 1102 | – | 0.3 ± 0.0 |
| Linalool | 1102 | 1095 | 3.2 ± 0.0 | – |
| 3-octanol acetate | 1120 | 1120 | – | tr |
| Camphor | 1142 | 1141 | 0.1 ± 0.0 | – |
| Neomenthol | 1158 | 1161 | – | tr |
| Borneol | 1166 | 1165 | 3.6 ± 0.0 | – |
| δ-terpineol | 1167 | 1162 | – | 0.2 ± 0.0 |
| terpinen-4-ol | 1177 | 1174 | 2.7 ± 0.0 | tr |
| α-terpineol | 1195 | 1186 | 0.2 ± 0.1 | 0.9 ± 0.0 |
| Thymol, methyl ether | 1235 | 1232 | 0.3 ± 0.0 | – |
| Pulegone | 1236 | 1233 | – | tr |
| Carvone | 1241 | 1239 | – | tr |
| Carvacrol methyl ether | 1246 | 1241 | 7.3 ± 0.1 | – |
| Piperitone oxide | 1248 | 1250 | – | 23.5 ± 0.3 |
| (Z)-carvone oxide | 1267 | 1259 | – | 0.1 ± 0.0 |
| Isopiperitenone | 1272 | 1272 | – | – |
| Neryl formiate | 1275 | 1280 | 0.1 ± 0.0 | – |
| Thymol | 1299 | 1289 | 3.0 ± 0.0 | – |
| 6-hydroxycarvotanacetone | 1300 | 1300 | – | 0.1 ± 0.0 |
| Carvacrol | 1321 | 1298 | 24 ± 0.3 | – |
| Piperitenone | 1333 | 1340 | – | tr |
| Thymol acetate | 1357 | 1349 | tr | – |
| Piperitenone oxide | 1359 | 1366 | – | 52.7 ± 0.2 |
| α-copaene | 1376 | 1374 | tr | – |
| β-bourbonene | 1384 | 1387 | – | tr |
| β-elemene | 1387 | 1389 | – | tr |
| (E)-jasmone | 1400 | 1390 | – | tr |
| (Z)-jasmone | 1407 | 1392 | – | 0.1 ± 0.0 |
| β-caryophyllene | 1420 | 1417 | 9.1 ± 0.1 | 3.0 ± 0.0 |
| (Z)-muurola-3,5-diene | 1447 | 1448 | – | 0.4 ± 0.0 |
| α-humulene | 1452 | 1452 | 0.2 ± 0.0 | – |
| (E)-β-farnesene | 1458 | 1454 | – | – |
| Germacrene-D | 1474 | 1484 | – | 3.8 ± 0.2 |
| Biciclogermacrene | 1487 | 1500 | – | 0.2 ± 0.0 |
| α-muurolene | 1498 | 1500 | – | 0.1 ± 0.0 |
| β- bisabolene | 1508 | 1505 | 1.5 ± 0.0 | – |
| γ-cadinene | 1512 | 1513 | – | 0.1 ± 0.0 |
| δ-cadinene | 1522 | 1522 | tr | – |
| Germacrene-D-4-ol | 1568 | 1574 | – | 0.2 ± 0.0 |
| Caryophyllene oxide | 1580 | 1582 | 0.4 ± 0.0 | tr |
| Muurolol epi-α | 1647 | 1640 | – | 0.1 ± 0.0 |
| α− cadinol | 1655 | 1652 | tr | – |
| TOTAL IDENTIFIED | 98.9 | 98.5 | ||
Compounds listed according to their elution order in a ZB-5 column.
Experimental linear retention index.
Linear retention index according to Adams (2007).
Normalised peak area from the FID chromatogram (mean ± SD from triplicate runs).
tr = traces (% < 0.05).
Not detected.
In the M. longifolia EO (Table 1), 38 compounds were determined (98.5% of the EO's composition). Two compounds were the most abundant, piperitenone oxide (52.7%) and piperitone oxide (23.5%). Other noteworthy components were Germacrene-D (3.8%) and β-caryophyllene (3%). The obtained results are consistent with research on the M. longifolia EO composition conducted in different countries. Italian authors (Maffei, 1988) report that piperitenone oxide (77.4%), germacrene D (3.7%) and 1,8-cineol (1.6%) are the major components. Works carried out in India indicate piperitenone oxide (54.2%), trans- and cis-piperitone oxide (24.1% and 7%), β-caryophyllene (3%), D-limonene (1.4%) and eucalyptol (1.2%) (Singh et al., 2008). Finally, a study conducted in Pakistan (Hussain et al., 2010) states that piperitenone oxide (40.1–64.6%), piperitenone (2–16.4%), borneol (4.4–13.3%), germacrene D (5.1–6%), β-caryophyllene (2.5–4.2%) and pulegone (2.1–3.9%) are the main components.
3.2. Antifungal activity
3.2.1. Calculation of mycelial growth inhibition (MGI)
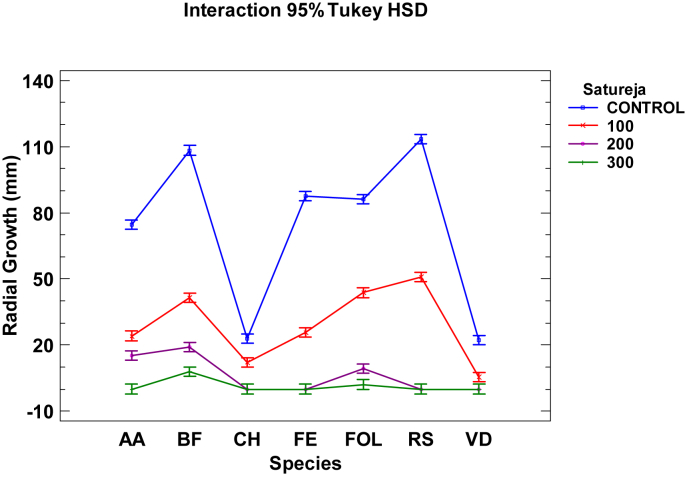
The S. montana EO gave excellent results (Table 2, Table 3 and Fig. 1) as the 300 μg mL−1 dose inhibited the growth of all fungi under study, with values of 100% MGI, except BF and FOL with 92.8% and 97.6%, respectively. The 200 μg mL−1 dose totally inhibited the growth of four of the seven tested fungi, and antifungal activity was observed at 100 μg mL−1, with results ranging from 50% to 80% inhibition of the tested fungi. The effectiveness of the S. montana EO on VD even at the 100 μg mL−1 dose was quite remarkable. With the control Petri dishes (PDA), fungi AA, BF, FE, FOL and RS showed characteristic rapid growth after 7 days of inoculation as they covered all or most of the dishes. Fungi CH and VD grew at a slower rate, with little development observed 7 days after inoculation. The addition of the S. montana EO to the culture medium caused morphological changes to those fungi, intensity of tone diminished, their appearance varied in texture terms and their growth pattern altered.
Table 2.
Mean growth (mm) and standard deviation values for each fungus grown on PDA (control), PDA-Satureja montana EO and PDA-Mentha longifolia EO at different concentrations. AA (Alternaria alternata), BF (Botryotinia fuckeliana), CH (Curvularia hawaiiensi), FE (Fusarium equiseti), FOL (Fusarium oxysporum lycopersici), RS (Rhizoctonia solani) and VD (Verticillium dahliae).
|
Satureja montana EO | |||||||
|---|---|---|---|---|---|---|---|
| concentration (μg mL−1) | AA | BF | CH | FE | FOL | RS | VD |
| Control | 85.50 ± 0.40 | 108.30 ± 0.38 | 26.00 ± 0.24 | 89.50 ± 0.45 | 86.10 ± 0.51 | 113.40 ± 0.30 | 28.60 ± 0.09 |
| 300 | 0.00 ± 0.00 | 7.80 ± 0.19 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.10 ± 0.03 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 200 | 15.10 ± 0.21 | 19.10 ± 0.31 | 0.00 ± 0.00 | 0.00 ± 0.00 | 9.60 ± 0.23 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 100 |
24.20 ± 0.18 |
41.30 ± 0.54 |
12.10 ± 0.12 |
25.70 ± 0.32 |
43.40 ± 0.22 |
51.30 ± 0.44 |
5.40 ± 0.11 |
| Mentha longifolia EO | |||||||
| concentration (μg mL−1) |
AA |
BF |
CH |
FE |
FOL |
RS |
VD |
| Control | 85.50 ± 0.40 | 108.30 ± 0.38 | 26.00 ± 0.24 | 89.50 ± 0.45 | 86.10 ± 0.51 | 113.40 ± 0.30 | 28.60 ± 0.09 |
| 400 | 32.30 ± 1.83 | 33.20 ± 1.29 | 9.40 ± 3.44 | 18.00 ± 3.65 | 4.90 ± 0.99 | 59.40 ± 2.99 | 0.00 ± 0.00 |
| 300 | 47.50 ± 2.12 | 37.50 ± 2.05 | 12.10 ± 1.10 | 34.80 ± 1.99 | 17.10 ± 0.99 | 49.90 ± 2.85 | 0.00 ± 0.00 |
| 200 | 63.00 ± 0.82 | 45.60 ± 2.46 | 15.00 ± 1.15 | 48.70 ± 1.25 | 45.20 ± 0.92 | 74.10 ± 1.52 | 8.30 ± 2.63 |
| 100 | 72.60 ± 1.43 | 50.50 ± 1.17 | 18.10 ± 2.69 | 54.70 ± 2.87 | 64.40 ± 1.71 | 82.80 ± 2.10 | 17.30 ± 0.82 |
Table 3.
Mycelial Growth Inhibition (MGI) percentage for each fungus grown on the PDA-Satureja montana EO and PDA-Mentha longifolia EO at different doses. AA (Alternaria alternata), BF (Botryotinia fuckeliana), CH (Curvularia hawaiiensi), FE (Fusarium equiseti), FOL (Fusarium oxysporum lycopersici), RS (Rhizoctonia solani) and VD (Verticillium dahliae).
|
Satureja montana EO | |||||||
|---|---|---|---|---|---|---|---|
| concentration (μg mL−1) | AA | BF | CH | FE | FOL | RS | VD |
| 300 | 100 | 92.80 | 100 | 100 | 97.56 | 100 | 100 |
| 200 | 80 | 82.36 | 100 | 100 | 88.85 | 100 | 100 |
| 100 |
67.95 |
61.87 |
49.58 |
70.66 |
49.59 |
54.74 |
79.23 |
| Mentha longifolia EO | |||||||
| concentration (μg mL−1) |
AA |
BF |
CH |
FE |
FOL |
RS |
VD |
| 400 | 62.22 | 69.34 | 60.38 | 79.89 | 93.41 | 56 | 100 |
| 300 | 44.44 | 65.37 | 53.46 | 61.12 | 76.99 | 47.62 | 100 |
| 200 | 26.32 | 58 | 42.31 | 45.59 | 38.93 | 34.74 | 70.98 |
| 100 | 15.09 | 53.37 | 30.77 | 38.69 | 13.02 | 26.98 | 62.24 |
Fig. 1.
Interaction plot, mean growth, species, concentration 100, 200, and 300 μg mL−1 of Satureja essential oil against Alternaria alternata (AA), Botryotinia fuckeliana (BF), Curvularia hawaiiensis (CH), Fusarium equiseti (FE), Fusarium oxysporumlycopersici (FOL), Rhizoctonia solani (RS) and Verticillium dahliae (VD).
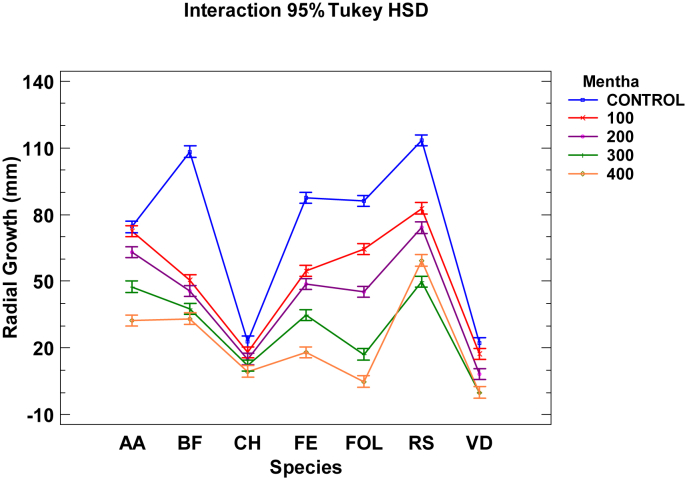
The M. longifolia EO (Table 2, Table 3, and Fig. 2) obtained quite lower MGI results. This oil was applied at 400, 300, 200 and 100 μg mL−1. The best results were obtained in fungus VD, with an MGI value of 100% at the 400 μg mL−1 and 300 μg mL−1 doses, and inhibition of 70.98% and 62.24% at the 200 μg mL−1 and 100 μg mL−1 doses, respectively. The inhibition of FOL was 93.41% at the 400 μg mL−1 dose. Finally at the 300 μg mL−1 dose, inhibition of 60–80% of mycelial growth was observed in BF, FE and FOL.
Fig. 2.
Interaction plot, mean growth, species, concentration 100, 200, 300 and 400 μg mL−1 of Mentha essential oil against Alternaria alternata (AA), Botryotinia fuckeliana (BF), Curvularia hawaiiensis (CH), Fusarium equiseti (FE), F. oxysporum lycopersici (FOL), Rhizoctonia solani (RS) and Verticillium dahliae (VD).
Based on these results, the S. montana EO was selected for the in vivo studies.
3.2.2. Determination of the fungistatic and/or fungicidal effect
The S. montana EO behaved as an excellent fungicide at the 300 μg mL−1 concentration on RS, while its fungistatic effect on the tested fungi was confirmed at 200 μg mL−1. The M. longifolia EO had a fungistatic effect on VD at both 400 and 300 μg mL−1.
The study of the antifungal activity of the EOs indicated that activity could not be attributed to a single major component because they are very complex mixtures, and their antimicrobial activity is due to a synergy between their compounds. Some authors have suggested that EOs are significantly more effective than their major components (Santamarina et al., 2015).
Many works have corroborated the antifungal activity of the S. montana EO on different fungi. Bezić, Skočibušić, and Dunkić (2005) proved its antifungal activity (oil containing 45.7% carvacrol) on Candida albicans, Saccharomyces cerevisiae, and Aspergillus fumigatus (responsible for pulmonary aspergillosis) in immunocompromised patients with very satisfactory results. Fraternale et al. (2007) tested the S. montana EO (with carvacrol as the major component, 18.0%) on nine phytopathogenic fungi: Fusarium poae, F. equiseti, F. graminearum, F. sporotrichoides, F. culmorum, Alternaria solani, Rhizoctonia solani, Phytophthora cryptogea and Botrytis cinerea (syn. Botryotinia fuckeliana), and revealed the antifungal potential of this EO. All these works obtained very satisfactory results, but used much higher concentrations than those reported herein.
Vladić et al. (2017) highlighted the antioxidant, antibacterial and antifungal, anti-inflammatory and anti-HIV-1 activities of S. montana and its EO. Moreover, one of its components, terpinen-4-ol (2.7%), was considered important.
The S. montana EO (p-cymene 40.59% and carvacrol 36.19%) has been applied as an antifungal agent to control two postharvest diseases caused by Penicillium expansum and Botrytis cinerea with good results (Morales et al., 2010).
In the present study, the S. montana EO showed very strong antifungal activity. Considering its chemical composition, a relation exists between its marked fungicidal activity and the presence of phenolic compounds, such as carvacrol and their biogenic precursors p-cymene and γ-terpinene. Santamarina et al., 2015, Santamarina et al., 2017 studied antifungal activity to control the same fungi species with other EOs. The Origanum compactum EO (carvacrol 43.2%, thymol 21.6%, p-cymene 13.95% and γ-terpinene 11.28%) and the Thymus zygis EO (carvacrol 37%, thymol 52%, p-cymene 32% and γ-terpinene 3.3%) gave similar results to those obtained by S. montana EO. The composition of the S. montana EO contained lower polyphenol concentrations, and the doses used in this study were also lower.
3.2.3. In vivo tests of the antifungal activity of the S. montana EO against Alternaria alternata on cherry tomatoes and kaki “persimmon”
3.2.3.1. In vivo tests on cherry tomatoes
During this trial, two measurements were taken after 7 and 14 days (Fig. 3). The percentage of stained fruits without the S. montana EO was much higher than that of the fruits bathed in the EO. The results obtained on days 7 and 14 are found in Table 4. In control 1 (tomatoes bathed only with the fungal inoculum), 60% of the tomatoes showed spots caused by AA after 7 and 14 days. In control 2 (tomatoes in the coating solution with no EO, and then bathed in the fungal solution), 40% of the tomatoes were tainted on 7 days, which rose to 50% after 14 days. Only 10% of the tomatoes with the coating containing EO and subsequent dipped in the fungal inoculum presented damage by AA after 14 days, and this result lasted 20 days.
Fig. 3.
The Satureja montana EO applied to cherry tomatoes against Alternaria alternata at 14 days. (A) Control 1: cherry tomatoes without film and no EO. (B) Control 2: cherry tomatoes with film and no EO. (C) Cherry tomatoes with film and EO. Arrows indicate the inoculation point (i).
Table 4.
Efficacy of the treatment with Satureja montana EO biofilm at 600 μg mL−1 against fungal development of Alternaria alternata on cherry tomatoes.
| Efficacy on cherry tomatoes | ||||
|---|---|---|---|---|
| test | 7 days |
14 days |
||
| healthy (%) | stained (%) | healthy (%) | stained (%) | |
| control 1 | 40 a | 60 a | 40 a | 60 a |
| control 2 | 60 b | 40 b | 50 a | 50 a |
| S. montana film | 90 c | 10 c | 90 b | 10 b |
Control 1. Fruits without film and without EO, control 2. Fruits with film and no EO.
Different letters in the same column indicate significant difference at 95% level probability using HSDTukey.
3.2.3.2. In vivo tests on kaki ‘persimmon’
After 2 months, the percentage of fruits not bathed in the S. montana EO stained by AA was 100%, but those bathed in the EO only presented 40% of damaged fruits (Table 5, Fig. 4, Fig. 5). In control 1 (the fruits bathed only with the fungal inoculum), 100% were stained by AA and displayed spots that were 3–4 mm in diameter and 2–4 mm deep, while 40% of the fruits presented soft outer necrosis (approx. 250 mm in diameter).
Table 5.
Efficacy of the treatment with Satureja montana EO biofilm at 600 μg mL-1 against fungal development of Alternaria alternata on kaki “Persimon” fruits”.
| Efficacy on kaki “Persimon” | |||||||
|---|---|---|---|---|---|---|---|
| test | healthy |
stained |
stain ø |
stain depth |
sof external necrosis |
basal peduncle | |
| % | % | mm | mm | % | ø mm | ||
| control 1 | 0 a | 100 a | 3-4 a | 2-4 a | 40 a | 250 a | healthy |
| control 2 | 0 a | 100 a | 2-3 ab | 1-4 ab | 20 b | 10 b | healthy |
| S. montana film | 60 b | 40 b | 1.5 b | 1–1.5 b | 0 c | 0 c | healthy |
Control 1. Fruits without film and without EO, control 2. Fruits with film and no EO.
Different letters in the same column indicate significant difference at 95% level probability using HSDTukey.
Fig. 4.
The Satureja montana EO applied to persimmon against Alternaria alternata at 1 month. (A) Control 1: persimmon without film and wit no EO. (B) Control 2: persimmon with film and no EO. (C) persimmon with film and EO. i, inoculation point. s, spotted from the field.
Fig. 5.
The Satureja montana EO applied to persimmon against Alternaria alternata at 2 months. (A) Control 1: persimmon without film and no EO. (B) Control 2: persimmon with film and no EO. (C) persimmon with film and EO. i, inoculation point. s, spotted from the field.
In control 2 (the fruits dipped in coating solution, but with no EO, and then bathed in the fungal solution), 100% of the fruits also developed AA and spots that were 2–3 mm in diameter and 1–4 mm deep, while 20% of the fruits displayed external soft necrosis (only 4 mm in diameter).
In the experiment, in the fruits with the coating containing the S. montana EO and subsequently dipped into the fungal inoculum, AA poorly developed and showed very small spots (1–1.5 mm in diameter and depth), which almost matched the initial inoculum. In any case, soft outer necrosis developed.
Recent research has revealed that EOs are a powerful alternative to conserve seeds and fruits. Thus Acosta-Dávila et al. (2016) demonstrated the antifungal activity of a starch-gelatin film (starch-gelatin blend) containing oregano, clove and cinnamon EOs against Fusarium oxysporum and Colletotrichum gloesporoides. Roselló, Giménez, Ibáñez, Blázquez, and Santamarina (2018) managed to control Alternaria alternata, Curvularia Hawaiiensis, Fusarium proliferatum and Fusarium oxysporum in the caryopsis of rice stored up to 30 days with clove oil, where eugenol was the main component (89%).
4. Conclusions
Satureja montana, widely used in Mediterranean diets as a culinary herb whose EO is employed as a natural ingredient in foods, medicines and beverages with the amounts carvacrol (24.0%), γ-terpinene (15.9%) and p-cymene (14.2), showed significant in vitro antifungal activity against seven phytopatogenic and post-harvest fungi. In vivo studies demonstrated that the S. montana EO used together with a polysaccharide like agar-agar forms a fine coat that wraps the fruits inoculated with Alternaria alternata and creates a natural biofilm that reduces post-harvest fungi development in the tomato controlled up to 90% after 20 days. In kaki, A. alternata displayed very weak development in the initial inoculum area, and no soft outer necrosis occurred for 2 months. This work offers an alternative to reduce the use of molecules with high environmental impact. The S. montana EO is an effective non-toxic preservative in stored tomatoes and kaki fruits against Alternaria alternata. A natural film was obtained, with a very low concentration of Satureja EO, an edible film, not harmful not only to human health but also to the environment.
Funding
This study has been financed by Generalitat Valenciana, Agencia Estatal de Investigación “materiales biodegradables multicapa de alta barrera para el envasado activo de alimentos”. (AGL2016-76699-R).
References
- Acosta-Dávila S.C., Chiralt A., Santamarina M. P.s, Roselló J., González M.C., Cháfer M.T. Antifungal films based on starch-gelatin blend, containing essential oils. Food Hydrocolloids. 2016;61:233–240. doi: 10.1016/j.foodhyd.2016.05.008. [DOI] [Google Scholar]
- Adams R.P. 4 th. Allured Publishing Corporation; Illinois: 2007. In identification of essential oil components by Gas Chromatography/Mass Spectrometry. [Google Scholar]
- Albuquerque C.C., Camara T.R., Mariano R.R., Willadino L., Júnior C.M., Ulisses C. Antimicrobial action of the essential oil of Lippia gracilis Schauer. Braz. Arch. Biol. Technol. 2006;49:527–535. doi: 10.1590/S1516-89132006000500001. [DOI] [Google Scholar]
- Angelini L.G., Carpanese G., Cioni P.L., Morelli I., Macchia M., Flamini G. Essential oils from Mediterranean Lamiaceae as weed germination inhibitors. J. Agric. Food Chem. 2003;51:6158–6164. doi: 10.1021/jf0210728. [DOI] [PubMed] [Google Scholar]
- Arcella D., Eskola M., Gómez Ruiz J.A. Dietary exposure assessment to Alternaria toxins in the European population. EFSA Journal. 2016;14:4654. doi: 10.2903/j.efsa.2016.4654. [DOI] [Google Scholar]
- Bezić N., Skočibušić M., Dunkić V. Phytochemical composition and antimicrobial activity of Satureja Montana L. and Satureja cuneifolia Ten. essential oils. Acta Bot. Croat. 2005;64:313–322. [Google Scholar]
- Brent K.J., Hollomon D.W. 2nd revised ed. Fungicide Resistance Action Committee (FRAC); Brussels: 2007. Fungicide Resistance: the Assessment of Risk; p. 35. (monograph No. 2) [Google Scholar]
- Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in fifilamentous ascomycetes. Mycologia. 1999;91:553–556. [Google Scholar]
- Carramiñana J.J., Rota C., Burillo J., Herrera A. Antibacterial efficiency of Spanish Satureja montana essential oil against Listeria monocytogenes among natural flora in minced pork. J. Food Prot. 2008;71(3):502–508. doi: 10.4315/0362-028X-71.3.502. [DOI] [PubMed] [Google Scholar]
- Dean R., Van Kan J.A., Pretorius Z.A., Hammond-Kosack K.E., Di Pietro A., Spanu P.D., Rudd J.J., Dickman M., Kahmann R., Ellis J., Foster G.D. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012;13:414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraternale D., Giampieri L., Bucchini A., Ricci D., Epifano F., Genovese S., Curini M. Chemical composition and antifungal activity of the essential oil of Satureja montana from central Italy. Chem. Nat. Comp. 2007;43(5):622–624. doi: 10.1007/s10600-007-0210-2. [DOI] [Google Scholar]
- García-Rellán D., Blázquez M.A., Boira H. Differential essential oil composition and morphology between perennial Satureja species growing in Spain. Rec. Nat. Prod. 2015;9(4):623–627. doi: 10.13140/RG.2.1.1356.6565. [DOI] [Google Scholar]
- Grosso C., Coelho J.A., Urieta J.S., Palavra A.M.F., Barroso J.G. Herbicidal activity of volatiles from coriander, winter savory, cotton lavender, and thyme isolated by hydrodistillation and supercritical fluid extraction. J. Agric. Food Chem. 2010;58:11007–11013. doi: 10.1021/jf102378d. [DOI] [PubMed] [Google Scholar]
- Guerrero-Rodríguez E., Solís-Gaona S., Hernández-Castillo F.D., Flores-Olivas A., Sandoval-López V., Jasso-Cantú D. Actividad biológica in vitro de extractos de Flourensia cernua DC en patógenos de postcosecha: Alternaria alternata (Fr.: Fr.) Keissl., Colletotrichum gloeosporioides (Penz.) Penz. y Sacc. y Penicillium digitatum (Pers.: Fr.) Sacc. Rev. Mex. Fitopatol. 2007;25(1):48–53. [Google Scholar]
- Hajlaoui H., Trabelsi N., Noumi E., Snoussi M., Fallah H., Ksouri R., Bakhrouf A. Biological activities of the essential oils and methanol extract of tow cultivated mint species (Mentha longifolia and Mentha pulegium) used in the Tunisian folkloric medicine. World J. Microbiol. Biotechnol. 2009;25(12):2227–2238. doi: 10.1007/s11274-009-0130-3. [DOI] [Google Scholar]
- Hussain A.I., Anwar F., S.Nigam P., Ashraf M., Gilani. A.H. Seasonal variation in content, chemical composition and antimicro¬bial and cytotoxic activities of essential oils from four Mentha species. J. Sci. Food Agric. 2010;90:1827–1836. doi: 10.1002/jsfa.4021. [DOI] [PubMed] [Google Scholar]
- Hyldgaard M., Mygind T., Meyer R.L. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012;3(12):1–24. doi: 10.3389/fmicb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzman C.P., Robnett C.J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ri-bosomal DNA partial sequences. Antonie Leeuwenhoek. 1998;73:331–371. doi: 10.1023/a:1001761008817. [DOI] [PubMed] [Google Scholar]
- Lange B.M., Croteau R. Genetic engineering of essential oil production in mint. Curr. Opin. Plant Biol. 1999;2:139–144. doi: 10.1016/s1369-5266(99)80028-4. [DOI] [PubMed] [Google Scholar]
- Llorens J.A., Vacas S. Seasonal variations in essential oil of aerial parts and roots of an Artemisia absinthium L. population from a Spanish area with supramediterranean climate (Teruel, Spain) J. Essent. Oil Res. 2015;27:395–405. doi: 10.1080/10412905.2015.1043400. [DOI] [Google Scholar]
- Llorens-Molina J.A., García-Rellán D., Vacas S., Bonet A. Individual sampling approach to study the chemodiversity of volatile and semivolatile compounds of Mentha longifolia L. growing wild in Jiloca basin, Spain. Int. J. Biosci. 2015;7(4):166–176. doi: 10.12692/ijb/7.4.166-176. [DOI] [Google Scholar]
- Maffei M. Environmental factors affecting the oil composition of some Mentha species grown in North West Italy. Flavour Fragrance J. 1988;3(2):79–84. doi: 10.1002/ffj.2730030206. [DOI] [Google Scholar]
- Moisa C., Copolovici L., Pop G., Copolovici D. Dry and fresh herba of Satureja montana L.: a comparative study regarding chemical composition and antioxidant capacity of volatile oils. Sci. Bull. Ser. F, Biotechnol. 2017;21:349–352. [Google Scholar]
- Morales R., López G., Sánchez P. Satureja L. In: Castroviejo S., Aedo C., Laínz M., Muñoz F., Nieto G., Paiva J., Benedí C., editors. Flora Iberica Real Jardín Botánico. C.S.I.C; Madrid, España: 2010. pp. 414–421. [Google Scholar]
- Rahman M.H., Shovan L.R., Hjeljord L.G., Aam B.B., Eijsink V.G.H., Sorlie M., Tronsmo A. Inhibition of fungal plant pathogens by synergistic action of chito-oligosaccharides and commercially available fungicides. PLoS One. 2014;9(4):1–10. doi: 10.1371/journal.pone.0093192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault-Roger C., Philogène B.J.R. Past and current prospects for the use of botanicals and plant allelochemicals in integrated pest management. Pharm. Biol. 2008;46:41–52. doi: 10.1080/13880200701729794. [DOI] [Google Scholar]
- Roselló J., Giménez S., Ibáñez M.D., Blázquez M.A., Santamarina M.P. ‘Bomba’ rice conservation with a natural biofilm. ACS Omega. 2018;3(3):2518–2526. doi: 10.1021/acsomega.7b01804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamarina M.P., Roselló J., Sempere F., Giménez S., Blázquez M.A. Commercial Origamum compactum Benth and Cinnamomum zeylanicum Blume essential oils against natural mycoflora in Valencia rice. J. Nat. Prod. 2015;29:2215–2218. doi: 10.1080/14786419.2014.1002406. [DOI] [PubMed] [Google Scholar]
- Santamarina M.P., Ibáñez M.D., Marqués M., Roselló J., Giménez S., Blázquez M.A. Bioactivity of essential oils in phytopathogenic and post-harvest fungi control. Nat. Prod. Res. 2017;31(22):2675–2679. doi: 10.1080/14786419.2017.1286479. [DOI] [PubMed] [Google Scholar]
- Sanzani S.M., Reverberi M., Geisen R. Mycotoxins in harvested fruits and vegetables: insights in producing fungi, biological role, conducive conditions, and tools to manage postharvest contamination. Postharvest Biol. Technol. 2016;122:95–105. doi: 10.1016/j.postharvbio.2016.07.003. [DOI] [Google Scholar]
- Singh H.P., Batish D.R., Mittal S., Dogra K.S., Yadav S., Kohli R.K. Constituents of leaf essential oil of Mentha longifolia from India. Chem. Nat. Comp. 2008;44(4):528–529. doi: 10.1007/s10600-008-9124-x. [DOI] [Google Scholar]
- Skočibušić M., Bezić N. Phytochemical analysis and in vitro antimicrobial activity of two Satureja species essential oils. Phytother Res. 2004;18:967–970. doi: 10.1002/ptr.1489. [DOI] [PubMed] [Google Scholar]
- Trifan A., Aprotosoaie A.C., Brebu M., Cioancă O., Gille E., Hăncianu M., Miron A. Chemical composition and antioxidant activity of essential oil from Romanian Satureja montana L. Farmacia. 2015;63(3):413–416. [Google Scholar]
- United Nations (UN) UN Department of Economic and Social Affairs, Population Division; New York: 2017. The World Population Prospects: the 2017 Revision. [Google Scholar]
- Villa F., Cappitelli F., Cortesi P., Kunova A. Fungal biofilms: targets for the development of novel strategies in plant disease management. Front. Microbiol. 2017;8(654):1–9. doi: 10.3389/fmicb.2017.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladić J., Canli O., Pavlić B., Zeković Z., Vidović S., Kaplan M. Optimization of Satureja montana subcritical water extraction process and chemical characterization of volatile fraction of extracts. J. Supercrit. Fluids. 2017;120:86–94. doi: 10.1016/j.supflu.2016.10.016. [DOI] [Google Scholar]
- White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols. A Guide to Methods and Applications. Academic Press; San Diego: 1990. pp. 315–322. [Google Scholar]