Abstract
Interest in pigment composition of microalgae species is growing as new natural pigments sources are being sought. However, we still have a limited number of species of microalgae exploited to obtain these compounds. Considering these facts, the detailed composition of carotenoids and chlorophylls of two species of green microalgae (Chlorella sorokiniana and Scenedesmus bijuga) were determined for the first time by high-performance liquid chromatography coupled to diode array and mass spectrometry detectors (HPLC-PDA-MS/MS). A total of 17 different carotenoids were separated in all the extracts. Most of the carotenoids present in the two microalgae species are xanthophylls. C. sorokiniana presented 11 carotenoids (1408.46 μg g−1), and S. bijuga showed 16 carotenoids (1195.75 μg g−1). The main carotenoids detected in the two microalgae were all-trans-lutein and all-trans-β-carotene. All-trans-lutein was substantially higher in C. sorokiniana (59.01%), whereas all-trans-β-carotene was detected in higher quantitative values in S. bijuga (13.88%). Seven chlorophyll compounds were identified in both strains with different proportions in each species. Concentrations of chlorophyll representing 7.6% and 10.2% of the composition of the compounds present in the biomass of C. sorokiniana and S. bijuga, respectively. Relevant chlorophyll compounds are reported for the first time in these strains. The data obtained provide significant insights for microalgae pigment composition databases.
Keywords: Microalgal, Chlorella sorokiniana, Scenedesmus bijuga, Chlorophyll, Carotenoid
Graphical abstract
Highlights
-
•
The carotenoids and chlorophylls profile by HPLC-PDA-MS of microalgae is reported.
-
•
Microalgae showed species-specific pigments profiles.
-
•
17 carotenoids and 7 chlorophylls were identified and quantified in details.
-
•
The quantitative profile presented a prevalence of chlorophylls over carotenoids.
-
•
Green microalgae are proposed as an interesting natural source of food pigments.
1. Introduction
Over the last few years, changes in eating habits, and modifications in nutritional requirements have led to considerable alterations in food formulation, shifting consumption trends towards natural products with functional properties (Koyande et al., 2019). Because a consequence of these changes, the natural color of food is estimated as the largest segment of products in the food coloring market, representing more than 80% of the total revenue of this sector. So the global food colors market was set at USD 1.79 billion in 2016, with revenue growth estimated at USD 2.97 billion by 2025 (Grand, 2019). Thus, emerging technologies for obtaining these compounds are necessary to supply this high demand.
In this sense, most of microalgae biotechnology companies concentrate investments and technology in the chemical specialties, as bioactive compounds, which can be successfully allocated in industrial sectors such as the pharmaceutical, nutritional and food industries (Sudhakar et al., 2019, Jacob-Lopes et al., 2018). This is fundamentally supported by the fact that microalgae biomass has become a promising alternative for obtaining natural compounds (Sathasivam et al., 2017). Since its metabolic diversity, coupled with its high biotechnological potential, allows the production of various biocompounds such as fatty acids, amino acids, and pigments that may have beneficial effects on human health. Thus, compounds coming from microalgae can be considered for significant applications in the development of functional food products (Matos, 2017, Khanra et al., 2018). Besides, the production of microalgae biomass has the advantage of high sustainability, as they absorb CO2 from the atmosphere, withstand extreme environmental conditions, have high productivity, and do not compete with terrestrial crops for agricultural land (Draaisma et al., 2013, Khan et al., 2018).
According to Spolaore et al. (2006), the exploration of compounds with bioactive activity has the potential to value up to 100 times the microalgae biomass when compared to the exploitation for energy or animal feed purposes. Associated with this perspective, the possibility of producing these biomolecules from biotechnological processes opens a field of exploitation with high technical-economic potential. Since the productive capacities are some orders of magnitude superior to the conventional systems of production of bioactive compounds currently supported in plant biomass (Yen et al., 2013, Kothari et al., 2017).
In the current state, scientific researches are continually being developed in the academic community with the primary objective of expanding knowledge about the diversity of existing microalgae species for possible commercial applications (Rodrigues et al., 2014, Fernandes et al., 2017, Patias et al., 2017, Fagundes et al., 2019, Maroneze et al., 2019, Nascimento et al., 2019, Vendruscolo et al., 2018). In this context, one of the fundamental parameters to establish the biotechnological capacity of using microalgal biomass as a source of bioproducts is to characterize the chemical composition of the biomass.
However, considering the number of species known in the world (according to some estimates 50,000 species), only 30,000 have been studied (Sathasivam et al., 2017). Among the species that are considered underexploited, the class Chlorophyceae, including species such as Chlorella sorokiniana and Scenedesmus bijuga, have the potential for use in bioprocesses, due to their robustness and simple nutritional requirements, rapid growth, and substantial content of compounds to be exploited (Borowitzka et al., 2018).
As photosynthetic microrganisms, microalgae are one of the most abundant and most varied producers of carotenoids and chlorophylls (Mulders et al., 2014), being the carotenoids the most exploited fraction of microalgae pigments (Gong and Bassi, 2016, Rajesh et al., 2017). In a study carried out by Patias et al. (2017), 23 different carotenoids were identified in biomass extracts of three species of microalgae. Furthermore, the relationship between function and structure was used to explain the antioxidant properties of these carotenoids. Recently, the carotenoid profiles of biomass from five eukaryotic microalgae were evaluated, and it was shown that microalgae showed species-specific carotenoid profiles and some species prevalence of xanthophylls over carotenes (Di Lena et al., 2019). Furthermore, from this promising microorganisms group, carotenoids such as β-carotene, α-carotene, zeaxanthin, lutein, violaxanthin, echinenone, mixoxantophyll, and canthaxanthin can be isolated, being some of these are produced exclusively by microalgae (Takaichi, 2011).
The microalgae represent a successful model in terms of commercial carotenoid production, through the cultivation of Dunaliella salina and Haematococcus pluvialis, with a focus on β-carotene and astaxanthin (up to 7% and 13% dry weight, respectively) (Rammuni et al., 2018). Besides, the global market for carotenoids is projected to reach US $ 1.7 billion by the year 2022. β-carotene, astaxanthin, and lutein have the largest market share. Moreover, world market projections show that in the year 2022, astaxanthin comes to reach the US $ 426.9 million, β-carotene US $ 572.78 million, and lutein US $ 357.7 million (Mcwilliams, 2018).
The main interests associated with these compounds are related to the color and the potential for sequestration of reactive nitrogen species (RNS), oxygen (ROS), especially the singlet oxygen species (1O2) and non-biological radicals, which are associated with antioxidant properties (Rodrigues et al., 2012). Additionally, these pigments have been associated with the provitamin A activity of carotenoids containing β rings, enhanced immune system functions, reduction of the risk of developing chronic diseases such as cancer, age-related macular degeneration, type 2 diabetes, cardiovascular diseases, and adipocyte function, adiposity and obesity (Rodriguez-Concepcion et al., 2018, Meléndez-Martínez, 2019, Khalid et al., 2018). Moreover, In vivo study in mice demonstrated the importance of the microalgae carotenoid fraction in the protective influence against tissue lipid peroxidation (Nascimento et al., 2019).
Chlorophylls are commercially important natural green pigments that constitute a large and diverse family of biomolecules similar to each other, representing the most abundant class of pigments (Roca et al., 2016). They are reported as one of the main fractions of secondary metabolites in the constitution of the microalgae biomass, are mainly present in the species of green microalgae (Borowitzka et al., 2018).
Even if the characterization of chlorophylls has recently been reported in macroalgae (edible seaweeds) (Chen et al., 2015a, Chen et al., 2015b, Chen et al., 2017), the literature lacks information on the qualitative and quantitative profile of these compounds in microalgae species (Chlorophyta) (Fernandes et al., 2017, Garrido and Zapata, 1996, Garrido et al., 2011). This fact may be related to the difficulties of the analysis to characterize these compounds, mainly due to the tremendous chemical instability of these molecules and the need for specific tools, as HPLC coupled with mass spectrometry (MS/MS) for more reliable results.
As well as for carotenoids, these photochemical compounds have also been proved to possess prominent benefits to human health. Chlorophyll is a well-known detoxifying agent and a phytonutrient. It has a positive effect on human reproduction and improves the metabolism of proteins, carbohydrates, and lipids in humans (Koyande et al., 2019, Solymosi and Mysliwa-Kurdziel, 2017). Also, anticarcinogenic, antigenotoxic, antimutagenic properties, anti-inflammatory activity as well as in vitro anti-oxidant activity has been demonstrated for these compounds (Lanfer-Marquez et al., 2005, Pareek et al., 2017, Pérez-Gálvez et al., 2017).
Considering these aspects, we here present the results of a study by HPLC-PDA-MS/MS on the carotenoid and chlorophyll characterization and total content in the biomass from two microalgae species, Chlorella sorokiniana, and Scenedesmus bijuga. Furthermore, the results presented provide extra value for the composition of biomass in these species, since important functional compounds have been appropriately characterized, which may attract attention for the application of these molecules in food and nutraceutical products. As well as, the data provided are valuable in view of industrial exploitation of alternative sources for obtaining natural pigments.
2. Materials and methods
2.1. Chemicals
Standards of all-trans-zeaxanthin, all-trans-lutein, all-trans-β-carotene, all-trans-α-carotene, chlorophyll a, chlorophyll b were purchased from Sigma-Aldrich (Missouri-MO, USA). The pheophytin a standard was obtained in our laboratory through an acid hydrolysis reaction from the standard chlorophyll a, where the Mg2+ ion is replaced by two hydrogen atoms (Fernandes et al., 2017). Methanol, ethanol, acetone, methyl tert-butyl ether (MTBE), ethyl acetate, petroleum ether and diethyl ether were purchassed from Sigma-Aldrich (St. Louis-MO, USA).
2.2. Microorganisms and culture media
Axenic cultures of Chlorella sorokiniana (CPCC138) were obtained from the Canadian Phycological Culture Centre (CPCC) of the University of Toronto, Canada and Scenedesmus bijuga (UTEX2980) was obtained from the Algae Cultures Collection (UTEX) of University of Texas. Stock cultures were propagated and maintained in synthetic BG11 medium (Braun-Grunow medium) (Rippka et al., 1979). The incubation conditions were 25 °C, photon flux density of 150 μmol m−2.s−1 and a photoperiod of 12/12 h day:night with constant agitation were used.
2.3. Microalgal biomass production
The biomass production was carried out in a bubble column photobioreactor operating on batch mode, with a total working volume of 2.0 L synthetic of BG-11 medium (Maroneze et al., 2016). The experimental conditions were as follows: initial concentration of inoculum of 100 mg.L−1, temperature of 25 °C, continuous aeration of 1VVM (volume of air per volume of culture per minute) with the injection of air enriched with 15% carbon dioxide, a photon flux density of 150 μmol m−2.s−1, photoperiod of 12/12 h light:dark, and a residence time of 168 h. The cultivations were performed twice, and in duplicate.
2.4. Biomass concentration
The biomasses were separated from the culture medium by centrifugation. It was subsequently freeze dried (Lyophilizer Liotop L101) for 24 h at −50 °C above −175 μm Hg, and then stored under refrigeration until the time of analysis.
2.5. Dry weight determination
To determine the total dry weight (DW), the freeze-dried biomass was kept in a desiccator and subsequently weighed on an analytical balance.
2.6. Carotenoid extraction
The carotenoids were exhaustively extracted from the freeze-dried sample (0.2 ± 0.02 g) first with ethyl acetate and then with methanol in a mortar with a pestle followed by centrifugation (Hitachi, Tokyo, Japan) for 7 min at 1500×g (Mandelli et al., 2012). The extraction procedure was repeated until the supernatant becomes colorless, which was reached approximately after 11 extractions with ethyl acetate and 6 with methanol. The homogenized sample suspension was filtered through a 0.22 μm polyethylene membrane, concentrated in a rotary evaporator (T < 30 °C), suspended in a mixture of petroleum ether/diethyl ether [1:1 (v/v)], and saponified overnight (16 h) with 10% (w/v) methanolic KOH at room temperature. The alkali was removed by washing with distilled water, and each extract was once again concentrated in a rotary evaporator, flushed with N2 and kept at −37 °C in the dark until chromatographic analysis. All extractions were performed in triplicate.
2.7. Chlorophyll extraction
The chlorophylls was exhaustively extracted from the freeze-dried samples (0.2 ± 0.02 g) with ethyl acetate and methanol in a mortar with a pestle followed by centrifugation (Hitachi, Tokyo, Japan) for 10 min at 5500 rpm (Fernandes et al., 2017). The extraction procedure was repeated until the supernatant becomes colorless. The homogenized sample suspension was filtered through a 0.22 μm polyethylene membrane, concentrated in a rotary evaporator (T < 30 °C).
In order to separate carotenoids from the chlorophyll, the samples were submitted to preparatory open column chromatography. Separation of the extract was carried out on a 25 × 300 mm glass column packed to a height of about 150 mm with MgO:Hyflosupercel (1:1) activated for 4 h at 110 °C. The carotenoids were eluted with a gradient of petroleum ether with increasing concentrations of acetone (50:20, 50:30, 50:40 and 50:50 v/v) and chlorophyll fraction was obtained in ethanol. To ensure the integrity of the chlorophyll pigments, the separation occurred at room temperature (T < 30 °C), in low light, for approximately 2 h. The separation could be followed visually. The ethanol extract was partitioned in petroleum ether/diethyl ether [1:1 (v/v)] in a separatory funnel, and then washed with water to remove residual ethanol. The petroleum ether phase was collected and concentrated in a rotary evaporator (30 °C), flushed with N2 and kept at −37 °C in the dark until chromatographic analysis.
2.8. HPLC-PDA-MS/MS carotenoids and chlorophylls analysis
The carotenoids and chlorophylls were analyzed by high performance liquid chromatography HPLC (Shimadzu, Kyoto, Japan) equipped with quaternary pumps (model LC-20AD), online degasser, and injection valve with a 20 μL loop (Rheodyne, Rohnert Park, CA, USA). The equipment was connected in series to a PDA detector (model SPD-M20A) and a mass spectrometer with an ion-trap analyzer and atmospheric pressure chemical ionization (APCI) source (model Esquire 4000, Bruker Daltonics, Bremen, Germany). The UV–vis spectra were processed at 450 nm for carotenoids and at 660 nm for chlorophylls. Carotenoid and chlorophylls separation was carried out on a C30 YMC column (5 μm, 250 × 4.6 mm) (Waters, Wilmington, DE, USA). Prior to HPLC-PDA-MS/MS analysis, the carotenoid and chlorophyll extracts were solubilized in MeOH:MTBE (1:1) and filtered through Millipore membranes (0.22 μm). The MS parameters for carotenoids analysis were as follows: positive mode; current corona, 4000 nA; source temperature, 450 °C; dry gas, N2, temperature, 350 °C; flow, 60 L/h; nebulizer, 5 psi; MS/MS fragmentation energy, 1.4 V. The mass spectra were acquired with scan range of m/z from 100 to 700 (de Rosso and Mercadante, 2007). The mobile phase consisted in MeOH (solvent A) and MTBE (solvent B) mixture. A linear gradient was applied from 95:5 to 70:30 in 30 min, to 50:50 in 20 min. The flow rate was 0.9 mL min−1 and the column temperature set to 29 °C. HPLC-PDA parameters for chlorophyll analysis were set as previously described by Murillo et al. (2013) with some minor modifications. The mobile phase consisted in binary solvent mixture system. Solvent A consisted of MeOH:MTBE:H2O (81:15:4) and solvent B MeOH:MTBE:H2O (16:80:4), using a linear gradient program as follows: from 0 to 20 min 0% B; from 20 to 140 min, 0–100% B; from 140 to 141 min, 100 to 0% B, from 141 to 150 min, 0% B. The flow rate was set at 0.8 mL/min, the column temperature was maintained at 35 °C. The MS parameters were the same as described above for carotenoids.
The identification was performed according to the following combined information: elution order on C30 HPLC column, co-chromatography with authentic standards, UV–visible spectrum (λ max, spectral fine structure, peak cis intensity for carotenoids), and mass spectra characteristics (protonated molecule ([M+H]+) and MS/MS fragments), compared with data available in the literature [14, 15, 35 45, 47, 48, 49, 50, 51].
The carotenoids were quantified by HPLC-PDA, using external calibration curves for all-trans-zeaxanthin, all-trans-lutein, all-trans-β-carotene, all-trans-α-carotene of five concentration levels. All other xanthophyll and carotene contents were estimated using the curve of all-trans-lutein and all-trans-β-carotene, respectively. The cis-isomers were estimated using the curve of the corresponding all-trans-carotenoid. Total carotenoid content was calculated as the sum of the contents of each individual carotenoid separated on the C30 column.
The chlorophylls were quantified by HPLC-PDA using external calibration curves for chlorophyll a, chlorophyll b and pheophytin a with a minimum of five concentration levels. Hydroxychlorophyll a, chlorophyll a and chlorophyll a′ where quantified using the curve of chlorophyll a; the hydroxypheophytin a, pheophytin a using the curve of pheophytin a; and chlorophyll b and chlorophyll b′ where quantified using the curve of chlorophyll b. Total chlorophyll content was calculated considering all identified peak areas.
2.9. Statistical analysis
Descriptive statistics, analysis of variance (one-way ANOVA) and Tukey's test (p < 0.05) were applied to experimental data. The analyses were performed with the software GraphPad Prism 5.0 (GraphPad Software Inc., La Jolla-CA, USA).
3. Results and discussion
3.1. Profile carotenoids
Although studies on carotenoids in these microalgae strains have been reported in the literature, most of these present only quantitative values of the total carotenoids profile or some specific compound by PDA without addressing in detail characterization of total carotenoid composition (Chen et al., 2016, Minhas et al., 2016, Azaman et al., 2017). Thus, to the best of our knowledge, this is the first time that this analytical approach has been applied to characterizing, both qualitatively and quantitatively, the composition of carotenoids in C. sorokiniana and S. bijuga by HPLC-PDA-MS/MS.
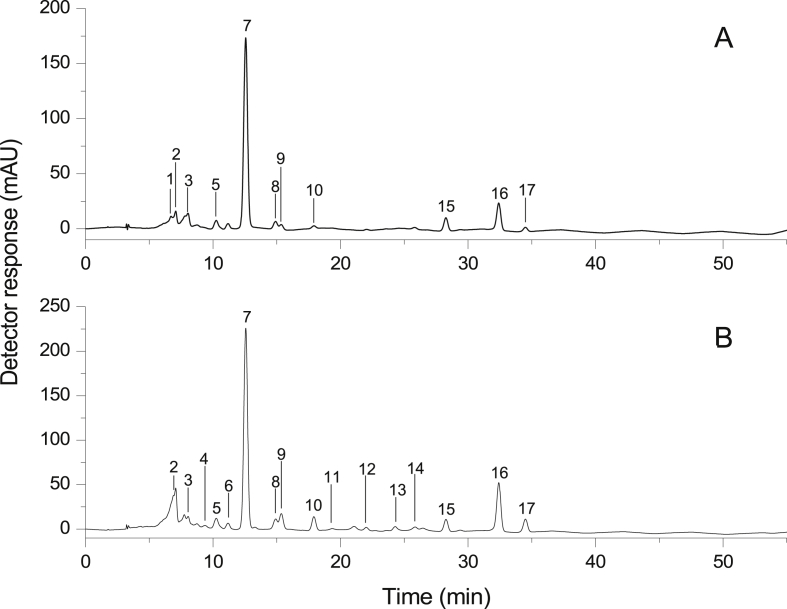
The carotenoids, extracted from biomass of C. sorokiniana and S. bijuga, were chromatographically separated (Fig. 1), which were identified on the based on the combined information obtained from chromatographic elution on a C30 column, co-chromatography with standards, UV–visible, mass spectra characteristics, compared with data available in the literature (Table 1). Since a detailed description of carotenoid identification using the above information was already reported in detail in the literature (Patias et al., 2017, de Rosso and Mercadante, 2007, Rodrigues et al., 2015), only the quantitative observations are discussed below.
Fig. 1.
Chromatogram, obtained by HPLC-PDA, of the carotenoids extract from Chlorella sorokiniana (A) and Scenedesmus bijuga (B). See text for chromatographic conditions. Peak identification and characterization are given in Table 1. Chromatogram was processed at 450 nm.
Table 1.
Chromatographic, UV–vis spectrum and mass characteristics of carotenoids from Chlorella sorokiniana and Scenedesmus bijuga, obtained by HPLC-PDA-MS.
| Peaka | Carotenoid | tR (min)b | UV–Vis characteristics |
Fragment ions (positive mode) (m/z) |
|||
|---|---|---|---|---|---|---|---|
| λmáx (nm)c | III/II (%)d | AB/II (%)e | [M+H]+ | MS/MSi | |||
| 1 | All-trans-neoxanthin | 6.7 | 418, 441, 469 | 67 | 0 | 601 (64.8)h | 583 [M + H-18]+ (100); 565 [M + H-18]+ (78.3);509 [M + H-92]+ (1.0); 491 [M + H-92-18]+ (1.0); 221 (1.8) |
| 2 | 9-cis-neoxanthin | 6.9–7.1 | 421, 445, 469 | 11 | ndf | 601 (50.0) | 583 [M + H-18]+ (100); 565 [M + H-18]+ (42.8); 509 [M + H-92]+ (0.5); 491 [M + H-92-18]+ (1.5); 221(0.4) |
| 3 | 9-cis-violaxanthin | 8.0 | 329, 412, 435, 463 | 77 | 12 | 601 (89.0) | 583 [M + H-18]+ (100); 565 [M + H-18-18]+ (6.2); 547 [M + H-18-18-18]+ (29.6); 509 [M + H-92]+ (23.4);491 [M + H-92-18]+ (0.7); 221 (0.6) |
| 4 | Cis-lutein | 9.3 | 328, 405, 431, 448 | 0 | 31 | ndf | 551 [M + H-18]+ (in source, 100); 533 [M + H-18-18]+ (2.4); 495 [M + H-18-56]+ (0.1) |
| 5 | 15-cis-lutein | 10.2–10.3 | 330, 414, 439, 465 | 25 | 39 | 569 (11.1) | 551 [M + H- (18]+ (in source, 100); 533 [M + H-18-18]+ (3.1); 495 [M + H-18-56]+ (1.0) |
| 6 | 13-cis-lutein | 11.2 | 330, 414, 437, 465 | 38 | 43 | ndf | 551 [M + H-18]+ (in source, 100); 533 [M + H-18-18]+ (3.0); 495 [M + H-18-56]+ (1.6) |
| 7 | All-trans-lutein | 12.6 | 420, 444, 472 | 60 | 0 | 569 (26.3) | 551 [M + H-18]+ (in source, 100); 533 [M + H-18-18]+ (2.5); 495 [M + H-18-56]+ (0.6) |
| 8 | All-trans-zeaxanthin | 14.9 | 425, 450, 476 | 33 | 0 | 569 (100) | 551 [M + H-18]+ (3.3); 533 [M + H-18-18]+ (0.2); 477 [M + H-92]+ (1.8) |
| 9 | 9-cis-lutein | 15.3 | 330, 416, 439, 467 | 63 | 12 | 569 (25.0) | 551 [M + H-18]+ (in source, 100); 533 [M + H-18-18]+ (3.5); 495 [M + H-18-56]+ (1.2) |
| 10 | 9′-cis-lutein | 17.9 | 330,418, 440, 467 | 56 | 19 | 569 (28.5) | 551 [M + H-18]+ (in source, 100); 533 [M + H-18-18]+ (3.4); 495 [M + H-18-56]+ (1.2) |
| 11 | 9-cis-zeaxanthin | 19.3 | 420, 444, 471 | 36 | ncg | 569 (100) | 551 [M + H-18]+ (4.5); 533 [M + H-18-18]+ (2.0); 477[M + H-92]+ (0.8) |
| 12 | β-carotene-5,6-epoxide | 22.0 | 420, 445, 472 | 47 | 0 | 553 (100) | 535 [M + H-18]+ (13.7); 461 [M + H-92]+ (6.8) |
| 13 | All-trans-echinenone | 24.3 | 468 | 0 | ndf | 551 (100) | 533 [M + H-18]+ (1.0); 203 (2.3) |
| 14 | 13-cis-β-carotene | 25.8 | 336, 418, 444, 469 | 7 | 53 | 537 (100) | 481 [M + H-56]+ (1.0); 444 [M-92]+ (54) |
| 15 | All-trans-α-carotene | 28.3 | 420, 445, 473 | 50 | 0 | 537 (100) | 481 [M + H-56]+ (0.5); 444 [M-92]+ (36) |
| 16 | All-trans-β-carotene | 32.4 | 425, 451, 477 | 17 | 0 | 537 (100) | 481 [M + H-56]+ (0.5); 444 [M-92]+ (3.7) |
| 17 | 9-cis-β-carotene | 34.5 | 422, 445, 472 | 18 | ncg | 537 (100) | 444 [M-92]+ (4.6) |
Numbered according to the chromatogram shown in Fig. 1.
tR: Retention time on the C30 column.
Linear gradient Methanol:MTBE.
Spectral fine structure: Ratio of the height of the longest wavelength absorption peak (III) and that of the middle absorption peak (II).
Ratio of the cis peak (AB) and the middle absorption peak (II).
Not detected.
Not calculated.
Relatives intensities for each m/z value appear in parentheses and are expressed as a percentage of the most abundant fragment ion.
Detailed data about mass fragmentation were reported in detail in the literature (Patias et al., 2017, de Rosso and Mercadante, 2007, Van Breemen et al., 2012).
A total of seventeen carotenoids were separated in the extracts of C. sorokiniana and S. bijuga, all of which are structure derived from α or β-carotene synthesized through hydroxylation, epoxidation, isomerization (cis) or ketolation reactions. Of the identified compounds, the microalgae strains show ten carotenoids in common.
Considering the quantitative profile, the highest total carotenoid content was determined in the extract of C. sorokiniana (1408.46 μg g−1) and S. bijuga exhibited the lowest content (1195.75 μg g−1) (Table 2).
Table 2.
Quantitative characterization of carotenoids in microalgae extracts (μg.g-1 dry weight).
| Peak | Carotenoid | Chlorella sorokiniana | Scenedesmus bijuga |
|---|---|---|---|
| 1 | All-trans-neoxanthin | 63.39 ± 0.09 | nd |
| 2 | 9-cis-neoxanthin | 68.04a ± 0.08 | 151.52b ± 1.26 |
| 3 | 9-cis-violaxanthin | 53.79a ± 0.07 | 32.99b ± 2.76 |
| 4 | Cis-lutein | nd | 13.66 ± 1.14 |
| 5 | 15-cis-lutein | 36.65a ± 0.05 | 35.16a ± 2.94 |
| 6 | 13-cis-lutein | nd | 14.75 ± 1.23 |
| 7 | All-trans-lutein | 831.18a ± 1.18 | 526.40b ± 4.40 |
| 8 | All-trans-zeaxanthin | 44.81a ± 0.06 | 27.59b ± 2.30 |
| 9 | 9-cis-lutein | 24.13a ± 0.03 | 40.74b ± 3.41 |
| 10 | 9′-cis-lutein | 16.52a ± 0.02 | 40.18b ± 3.36 |
| 11 | 9-cis-zeaxanthin | nd | 7.52 ± 0.62 |
| 12 | β-carotene-5,6-epoxide | nd | 13.39 ± 1.12 |
| 13 | All-trans-echinenone | nd | 15.46 ± 1.29 |
| 14 | 13-cis-β-carotene | nd | 15.95 ± 1.33 |
| 15 | All-trans-α-carotene | 71.47a ± 0.10 | 40.51b ± 3.39 |
| 16 | All-trans-β-carotene | 156.21a ± 0.22 | 165.95a ± 1.38 |
| 17 | 9-cis-β-carotene | 42.27a ± 0.06 | 53.98b ± 4.51 |
| Total carotenoids | 1408.46a | 1195.75b |
Values are average and standard deviation of triplicates.
nd: not detected.
Different letters in the same line differ significantly by Student's t-test (α = 0.05).
Eleven carotenoids were identified in C. sorokiniana (Fig. 1A), being three epoxycarotenoid (peak 1, 2 and peak 3), five hydroxycarotenoids (peak 5, 7, 8, 9 and peak 10) and three carotenes (peak 15, 16 and peak 17) (Fig. 2). All-trans-lutein (831.18 μg g−1) and all-trans-β-carotene (156.21 μg g−1) were the major, as shown in Table 2, which represented 70.01% of the total carotenoid content followed by all-trans-α-carotene (5.07%) and 9-cis-neoxanthin (4.83%) as major carotenoids in this biomass. All-trans-neoxanthin (4.51%), all-trans-zeaxanthin (3.18%) have also been identified in this microalgae species, as well as the cis isomers 9-cis-violaxanthin (3.82%), 9-cis-β-carotene (3.01%), 15-cis-lutein (2.60%), 9-cis-lutein (1.71%) and 9′-cis-lutein (1.17%).
Fig. 2.
Composition of the carotenoid fraction in the extracts of Chlorella sorokiniana and Scenedesmus bijuga.
The S. bijuga specie exhibited the most complete profile constituted by sixteen carotenoids (Fig. 1B). The major carotenoids were the same detected in C. sorokiniana, all-trans-lutein (526.40 μg g−1), and all-trans-β-carotene (165.95 μg g−1), which corresponds to 57.91% of the fraction of carotenoids in the extract. Other major peaks were identified as 9-cis-neoxanthin (12.67%) and 9-cis-β-carotene (4.51%). In addition, 9-cis-lutein (3.41%), all-trans-α-carotene (3.38%), 9′-cis-lutein (3.36%), 15-cis-lutein (2.94%), 9-cis-violaxanthin (2.76%), all-trans-zeaxanthin (2.31%), 13-cis-β-carotene (1.34%), all-trans-echinenone (1.29%), 13-cis-lutein (1.24%), cis-lutein (1.14%), β-carotene-5,6-epoxide (1.12%) and 9-cis-zeaxanthin (0.62%) were detected as minor carotenoids. Different from the results described in C. sorokiniana, S. bijuga presented one ketocarotenoid (peak 13), three epoxycarotenoid (peak 2, 3 and 4), eight hydroxycarotenoids (peak 4, 5, 6, 7, 8, 9, 10 and peak 11) and four carotene (peak 14, 15, 16 and 17) (Fig. 2). In contrast, as far as we know, the literature lacks information on the profile of carotenoids in S. bijuga. Only one report identified two carotenoids (lutein and astaxanthin) present in this microalgae (Minhas et al., 2016).
As well as described above, the total carotenoid content was notably higher in C. sorokiniana. On the other hand, S. bijuga presented a carotenoid profile with six different compounds than C. sorokiniana. Of these compounds, two were mono-cis isomers of all-trans-lutein (peak 4, peak 6), mono-cis isomer of all-trans-zeaxanthin (peak 11), β-carotene-5,6-epoxy (peak12), all-trans-equinenone (peak 13) and 13-cis-β-carotene (peak 14). In contrast, all-trans-neoxanthin (peak 1) was only detected in the carotenoid extract of C. sorokiniana.
Although MS fragments have been mapped, carotenoids intermediate of the synthesis of lutein as zeinoxanthin (identified in Rodrigues et al. (2015)) and α-cryptoxanthin (identified in Di Lena et al. (Di Lena et al., 2019)) were not identified in our study. This fact may result from the high enzymatic activity of carotene β-hydroxylase (CYP97A) and carotene ε-hydroxylase (CYP97C), enzymes responsible for catalyzing the synthesis of these compounds in lutein in the α-carotene pathway (Rodriguez-Concepcion et al., 2018).
Taking as reference the same genus Scenedesmus and Chlorella, all-trans-lutein and β-carotene also exhibited a major profile in Scenedesmus obliquus presenting higher quantitative values when compared to S. bijuga; whereas, Chlorella vulgaris showed lower values for all-trans-lutein and higher for β-carotene when compared to C. sorokiniana (Patias et al., 2017). It is interesting to note that although the green microalgae compared belong to the same genus, the quantitative profile of carotenoids varies depending on species. These differences could be attributed to factors such as type of cultivation, source of nutrients, phylogenetic diversity, morphological and cytological characteristics, and the composition of genes and enzymes specific in each species of microalgae (Borowitzka et al., 2016, Begum et al., 2016).
Regarding the content of lutein, our results are in agreement with those obtained by Paliwal et al. (2016) who, after analyzing 57 strains of microalgae of different phylum, concluded that green algae (Chlorophyta) are a potential source this xanthophyll. Also, according to our results, Chen et al. (2016) showed that with distinct extraction methods and different culture media, such as cultures in heterotrophic systems (Chen et al., 2018) or mixotrophic (Chen and Liu, 2018, Chen et al., 2019), the production of lutein by C. sorokiniana is substantially significant, and may be considered as a potential source of commercial output this pigment. In addition, the obtained data by Přibyl et al. (2016) show that Scenedesmus sp. strain can produce high levels of carotenoids, mainly lutein, whit levels of 0.75 at 1% of dry weight biomass.
Our results are in line with those found by Cordero et al. (2011), where lutein and β-carotene predominated in biomass of the C. sorokiniana cultivated under mixotrophic conditions, as well as α-carotene, violaxanthin and zeaxanthin were also detected in smaller quantities. Additionally, Miazek et al. (2017) obtained a carotenoids concentration of 0.86% in dry weight for C. sorokiniana, when grown in a beech wood dilute-acid hydrolysate. In our study, we obtained a concentration of 0.70% in dry mass. By contrast, in a recent study, C. sorokiniana was cultured in photoautotrophic conditions and analyzed the profile carotenoids by spectrophotometer, showing a quantitative value of 3.8 μg mg−1, higher to that found in our study (Azaman et al., 2017). In addition to these results, Matsukawa and co-workers (Matsukawa et al., 2000) submitted C. sorokiniana to cultivation with 10% CO2 incorporation, in which the total carotenoids (determined spectrophotometrically) contained 0.69% dry weight, similar to the value found in our study. The lutein and β-carotene contents were 4300 and 600 μg g−1 dry weight, respectively. Zeaxanthin, β-cryptoxanthin, α-carotene were identified in minor amounts.
Safafar et al. (2015) identified by HPLC-PDA a profile of carotenoids similar to that found in our study, with lutein and β-carotene being the main carotenoids, followed by neoxanthin, zeaxanthin, fucoxanthin and dihydro from C. sorokiniana, but the methodology used did not allow the identification of the fraction of cis isomers of these compounds. Interestingly, in this study, we did not detect the presence of fucoxanthin and dihydro lutein. Whereas in the study in Van Wagenen et al. (Van Wagenen et al., 2015), using UHPLC obtained a qualitative profile of carotenoids in C. sorokiniana constituted by lutein, violaxanthin, astaxanthin, fucoxanthin, zeaxanthin, and α+β-carotene. The xanthophylls astaxanthin and fucoxanthin were not identified in our study.
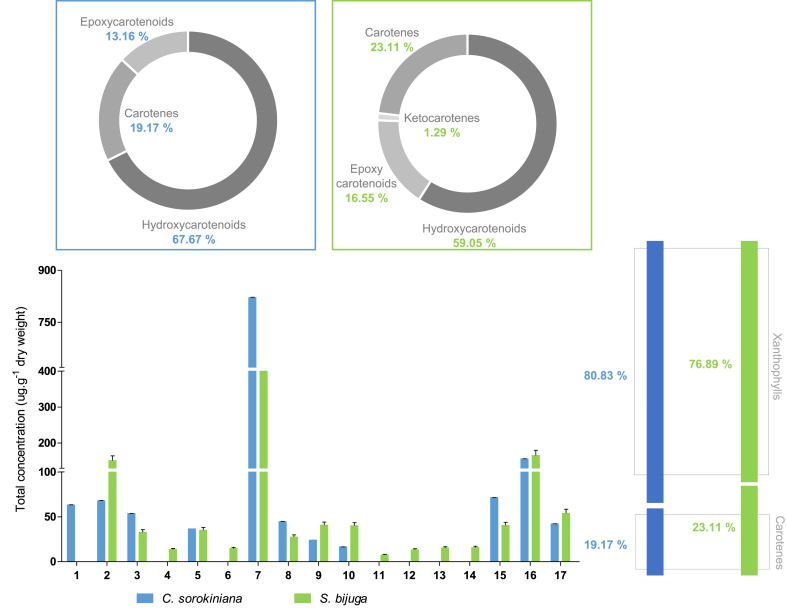
As can be seen in Fig. 2, most of the carotenoids present in the two microalgae species are xanthophylls, which represent a fraction of 80.83% in C. sorokiniana and 76.89% in S. bijuga, being the percentage of epoxycarotenoids more significant in S. bijuga and hydroxycarotenoids in C. sorokiniana. Thus, the remaining value of carotenoids detected corresponds to the fraction of carotenes 19.17% and 23.11%, respectively.
Among the identified xanthophylls, the epoxycarotenoid all-trans-neoxanthin (peak 1), detected only in C. sorokiniana, and its isomer 9-cis-neoxanthin (peak 2), detected in both microalgae, showed values which represent a fraction of 9.34% (peak 1 and peak 2) and 12.67% (peak 2), of the total xanthophyll content in C. sorokiniana and S. bijuga, respectively. The value here found is in substantial accordance with the literature, reporting low concentration of neoxanthin in C. sorokiniana at values of 48.29 μg.g−1and 20.0 μg g−1 when cultivated in reactors with light intensities, 2000 μmol photon m−2.s−1, and 200 μmol photon m−2.s−1, respectively (Safafar et al., 2015).
Violaxanthin, with the 5,6-epoxide group in its structure, is a xanthophyll present in microalgae belonging to the class of Chlorophyceae (Takaichi, 2011). In our study, we identified only its 9-cis-violaxanthin isomer (peak 3), although we have mapped the MS fragments of the compound in their all-trans form. In the literature, no reports on the presence of this compound were found in the studied species, is this the first report. It is important to emphasize that anti-inflammatory properties have already been associated with violaxanthin obtained from microalgae (Soontornchaiboon et al., 2012).
Moreover, within the class of xanthophylls, the polyhydroxylated carotenoids as lutein and its structural isomer zeaxanthin are the most studied ones. This is because they owned innumerable attributions in health promotion and, although they have no provitamin A activity in humans, both display other biological activities as antioxidant property, anti-inflammatory action, and mainly they are associated prevention of macular degeneration (Nwachukwu et al., 2016). Likewise, studies strongly report the significant potential of lutein as an antioxidant agent, which has values higher than β-carotene (Sun et al., 2015). In addition, there has been ever-increasing evidence supporting protective effects in preventing or delaying chronic diseases (Dufossé, 2006, Zhang et al., 2018). These compounds are formed by the hydroxylation of the 3 and 3’ carbon atoms of β, ε-carotene or β, β-carotene, respectively, by separate hydroxylases specific for the β and ε rings (Morais et al., 2006).
At the level of industrial production, marigold flowers are the most abundant source of commercial lutein. However, its production is limited by seasons, planting areas, and the high manpower cost. The lutein production from dried flowers of marigold Calendula officinalis varies from 0.04 to 0.301 mg g−1 (Lin et al., 2015). The lutein production rate of microalgae in study is 13–20 times higher than marigold flowers. Thus, green microalgae are considered an excellent alternative for obtaining this compound (Sun et al., 2015). One of the most studied green microalgae for lutein production is Muriellopsis sp. The content of carotenoid can vary from 0.4% to 0.6% per dry biomass (D'Alessandro and Antoniosi Filho, 2016). In contrast, in our study, we found values de 0.45% and 0.33% for C. sorokiniana and S. bijuga, respectively. Considering only the lutein production based on the weight of biomass, Minhas et al. (2016) mentioned that lutein productivity per day of C. sorokiniana and S. bijugus are similar under photoautotrophic conditions, with values of 0.5 and 0.47 mg L−1.d−1, respectively.
On the basis of published data, C. sorokiniana and S. bijuga, is often described as a species that has the ability to accumulate large amounts of all-trans-lutein (Chen et al., 2016, Chen et al., 2018, Minhas et al., 2016, Cordero et al., 2011, Safafar et al., 2015). This fact is in agreement with our study in which all-trans-lutein and their isomers cis represent values of 64.51% (908.48 μg g−1) and 56.14% (670.89 μg g−1) of the total biomass carotenoids of C. sorokiniana and S. bijuga, respectively.
In this study, we detected the presence of a carotenoid epoxide (β-carotene-5,6-epoxide) only in the S. bijuga extract. However, the quantitative value was relatively low, representing a fraction of 1.45% of the total xanthophylls. Exceptionally, in this microalga, all-trans-echinenone (peak 13) was found constituting the profile of carotenoids with the ketonic group. In a previous study, these compounds were also detected in species of green microalgae (Patias et al., 2017). However, as far as we know, β-carotene-5,6-epoxide and all-trans-echinenone had not yet been reported in the S. bijuga biomass.
Although the most significant fraction of carotenoids found consisted of xanthophylls, important carotenes were detected in this study. β-carotene, a second compound more abundant in both microalgae, is one of the most important carotenoids since it has innumerable physiological functions related to its structure, which includes the activities of pro-vitamin A and antioxidant, acting as a potent chemo-preventive agent in various disorders. Also, is a first-choice natural pigment how bioactive compound in functional food (Rodriguez-Concepcion et al., 2018, Meléndez-Martínez, 2019, Paliwal et al., 2016, Cicero and Colletti, 2017). The quantitative values of this compound were from 156.21 (C. sorokiniana) and 165.95 μg g−1 (S. bijuga), showing no significant difference between the two species. In the same way as β-carotene in its all-trans configuration, its cis isomers are also associated with beneficial functions for health. Previous reports have highlighted the importance of 9-cis isomer, which plays an important role in the suppression of oxygen free radicals, protecting human body cells from oxidative stress. 9-cis-β-carotene was detected in the two microalgae species studied, representing values of 3.01% and 4.52% (C. sorokiniana and S. bijuga, respectively). Besides, the isomer 13-cis-β-carotene, present only in S. bijuga, represents a fraction of 1.34% of the total carotenoids. All-trans-α-carotene was present at lower but significant concentrations in the two strains (71.47 and 40.51 μg g−1, respectively).
3.2. Profile chlorophylls
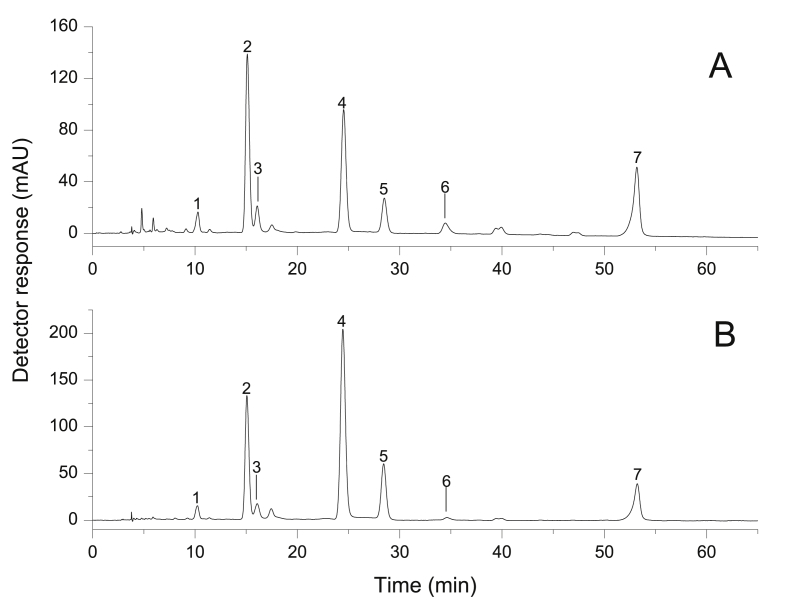
The chromatogram obtained by HPLC-PDA of chlorophylls profile in the microalgae C. sorokiniana and S. bijuga is demonstrated in Fig. 3, while the chlorophylls compounds composition of the two species of microalgae is shown in Table 3.
Fig. 3.
Chromatogram, obtained by HPLC-PDA, of the chlorophylls extract from Chlorella sorokiniana (A) and Scenedesmus bijuga (B). See text for chromatographic conditions. Peak identification and characterization are given in Table 3. Chromatogram was processed at 660 nm.
Table 3.
Characterization by HPLC-PDA-MS/MS of profile of chlorophyll compounds present in biomass of Chlorella sorokiniana and Scenedesmus bijuga.
| Peaka | Chlorophyll | tR (min)b | λmáx (nm)c | [M+H]+ | MS/MS fragment ions (m/z)e |
|---|---|---|---|---|---|
| 1 | Chlorophyll b | 10.2 | 464, 648 | 907 (100)d | 875[M + H-32]+ (10.9); 629[M + H-278]+ (98.1); 597[M + H-278-32]+ (52.7); 569[M + H-278-60]+ (36.3) |
| 2 | Hydroxychlorophyll a | 15.1 | 431, 662 | 909 (23.0) | 891[M + H-18]+ (30.7); 631[M + H-278]+ (34.6); 613[M + H-278-18]+ (42.3); 553[M + H-278-18-60]+ (100); 555[M + H-278-60]+ (24) |
| 3 | Chlorophyll b' | 16.0 | 462, 654 | 907 (1.9) | 875[M + H-32]+ (7.6); 629[M + H-278]+ (100); 597[M + H-278-32]+ (13.4); 569[M + H-278-60]+ (15.3) |
| 4 | Chlorophyll a | 24.4–25.4 | 431, 664 | 893 (100) | 615[M + H-278]+ (44.8); 583[M + H-278-32]+ (24.1); 555[M + H-278-60]+ (27.5) |
| 5 | Chlorophyll a' | 28.4 | 429, 665 | 893 (90.9) | 615[M + H-278]+ (100); 583[M + H-278-32]+ (31.8); 555[M + H-278-60]+ (59.0) |
| 6 | Hydroxypheophytin a | 34.4–35.4 | 408, 668 | 887 (7.1) | 869[M + H-18]+ (10.7); 803[M + H-63]+ (3.5); 609[M + H-278]+ (100); 591[M + H-278-18]+ (7.1); 533[M + H-278-60]+ (18); 531[M + H-278-18-60]+ (6.4) |
| 7 | Pheophytin a | 53.1–53.2 | 408, 666 | 871 (40) | 593[M + H-278]+ (100); 533[M + H-278-60]+ (35) |
Numbered according to the chromatogram shown in Fig. 3.
tR: Retention time on the C30 column.
Linear gradient MeOH:MTBE:H2O (81:15:4) and MeOH:MTBE:H2O (16:80:4).
Relatives intensities for each m/z value appear in parentheses and are expressed as a percentage of the most abundant fragment ion.
Detailed data about mass fragmentation was reported in detail in the literature (Fernandes et al., 2017).
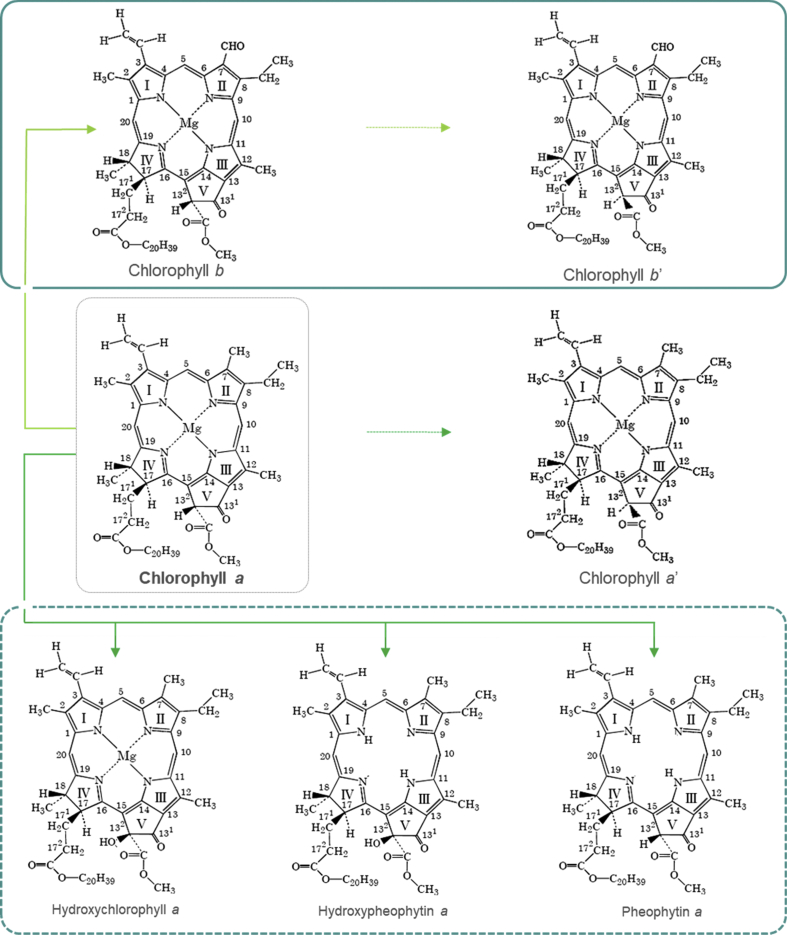
As is characteristic of the two species of microalgae belonging to phylum Chlorophyta, only species of chlorophyll a and b (Borowitzka et al., 2018), and its derivative compounds, which are formed by hydroxylation, pheophytinization and decarboxymethylation reactions, were detected. Furthermore, the characterization of chlorophylls derivatives compounds was only possible through the mass spectral characteristics of the molecules (protonated molecule [M+H]+ and fragments m/z).
A detailed description of chlorophylls identification using chromatographic information HPLC-PDA-MS/MS has already been reported in recent works (Fernandes et al., 2017, Chen et al., 2017, Kao et al., 2011). However, as far as we know, this is the first report of the detailed characterization of the quantitative and qualitative profile of chlorophylls in microalgae species C. sorokiniana and S. bijuga.
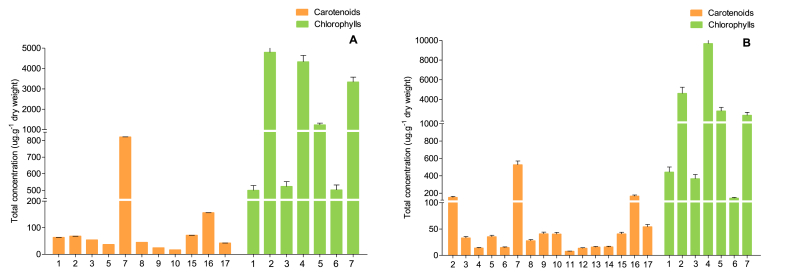
As expected, the presence of chlorophylls in the extracts of C. sorokiniana and S. bijuga was significant, being 10–17 times more in comparison to the fraction of carotenoids. As shown in Table 4 and Fig. 4, pigment analyses provided identical chlorophylls compounds qualitative composition for the two strains tested (seven compounds). However, it showed a species-specific chlorophyll quantitative profile. The chemical structures of these compounds are shown in Fig. 5.
Table 4.
Quantitative characterization of chlorophyll compounds in microalgae extracts (mg.g−1 dry weight).
| Peak | Chlorophyll | Chlorella sorokiniana | Scenedesmus bijuga |
|---|---|---|---|
| 1 | Chlorophyll b | 0.49a ± 0.03 | 0.44a ± 0.06 |
| 2 | Hydroxychlorophyll a | 4.79a ± 0.35 | 4.61a ± 0.63 |
| 3 | Chlorophyll b' | 0.52a ± 0.03 | 0.36b ± 0.05 |
| 4 | Chlorophyll a | 4.32a ± 0.32 | 9.67b ± 1.32 |
| 5 | Chlorophyll a' | 1.22a ± 0.09 | 2.82b ± 0.38 |
| 6 | Hydroxypheophytin a | 0.50a ± 0.03 | 0.14b ± 0.01 |
| 7 | Pheophytin a | 3.33a± 0.24 | 2.38b ± 0.32 |
| Total chlorophyll | 15.20a ± 1.13 | 20.44b ± 2.8 |
Values are average and standard deviation of triplicates.
Different letters in the same line differ significantly by Tukey test (α = 0.05).
Fig. 4.
The total carotenoids and chlorophyll compounds content in the extracts of Chlorella sorokiniana (A) and Scenedesmus bijuga (B).
Fig. 5.
Structure of the chlorophyll compounds present in extracts of Chlorella sorokiniana and Scenedesmus bijuga. Solid line: species of chlorophyll; Dotted line: derivatives compounds.
The contents of chlorophyll and their derivatives in chlorophyll extract from C. sorokiniana was 15.20 mg g−1. Hydroxychlorophyll a was significantly higher in this microalgae strain, representing 31.47% (4.79 mg.g-1) of the total chlorophyll content, followed by chlorophyll a 28.43% (4.32 mg g−1), pheophytin a 21.91% (3.33 mg g−1), and chlorophyll a' 8.15% (1.22 mg g−1). The other minor chlorophylls, each <4%, summed to 10.05% of the total content. Between these compounds, chlorophyll b (0.49 mg g−1) and chlorophyll b' (0.52 mg g−1) contributing 6.67% to the total chlorophyll content. According to Petruk et al. (2018), molecules of chlorophyll a and chlorophyll b was also found in C. sorokiniana biomass at a concentration of 0.79 ± 0.11 mg and 0.66 ± 0.09 mg, respectively.
The fact that hydroxychlorophyll a is the most abundant chlorophyll compound in C. sorokiniana, also described by the first time in this microalgae, is a differentiated characteristic and which may be attributed to the high concentration of peroxidase in these strains (Loh et al., 2012). Previous work postulated that peroxidase might catalyze the oxidation of chlorophyll a to produce hydroxychlorophyll a through the intermediate phenoxy radical, formed between phenolic compounds at the para-position like p-coumaric acid and peroxide in the presence of peroxidase (Yamauchi et al., 2004). Nevertheless, the chlorophyll oxidation may also occur through several other mechanistic pathways as well, as elaborated by Hynninen and Sievers (1981).
Hydroxy derivative compounds have also been detected in green seaweeds. Chen et al. (2017) identified the presence of hydroxypheophytin a and hydroxychlorophyll a in Ulva spp. and Enteromorpha spp. in significant concentrations. Besides, previous studies by Steele et al. (2015), it was observed that the occurrence of hydroxychlorophyll a in phytoplankton was directly associated with two diatom species, the prymnesiophyte Phaeocystis spp. and the coccolithophorid Emiliania huxleyi. Also, Hussein et al. (2019) showed that hydroxypheophytin a can also be isolated from Microcystis aeruginosa cyanobacteria (21.80% of extract), demonstrating the possible marked antioxidant and anti-inflammatory activities at 500 mg/kg dose level.
The microalgae S. bijuga demonstrated to have the higher concentrations of the total chlorophylls contents from biomass with a value of 20.44 mg g−1. The chlorophyll a (9.67 mg g−1) (47.38%) was quantitatively dominant in chlorophyll profile of microalgae, followed by hydroxychlorophyll a (4.61 mg g−1) (22.33%), chlorophyll a' (2.82 mg g−1) (13.88%), pheophytin a (2.38 mg g−1) (11.68%), chlorophyll b (0.44 mg g−1) (2.19%), chlorophyll b' (0.36 mg g−1) (1.82%), and hydroxypheophytin a (0.14 mg g−1) (0.72%). Chlorophyll b and hydroxychlorophyll a (peak 1, 2) showed no significant difference between the two species of microalgae evaluated (Table 4 and Fig. 4).
The content of chlorophyll a is in agreement with those found by Santhakumaran et al. (2018) that demonstrated that the microalgae strain Scenedesmus bijuga, cultured in Bold's Basal Medium (BBM) under controlled conditions of light (8000 Lx), temperature (24 °C) and pH (7.3), can synthesize approximately 9 mg g−1 of chlorophyll a and 3.5 mg g−1 chlorophyll b (higher value than found in our study). Other valuable compounds such as carbohydrate (9.7% DW), protein (20.43%), lipid (21.08%) and carotenoids (4 mg g−1) also were detected. Bhatnagar et al. (2011) analyzed the concentration of content chlorophyll (a + b) in biomass of S. bijuga cultivated in phototrophic, heterotrophic, and mixotrophic systems and found that the chlorophyll concentration was substantially higher under mixotrophic conditions, followed by standard phototrophic conditions. Values of 25.83 and 10.65 mg.L−1 were found for total chlorophyll content in mixotrophic and phototrophic conditions, respectively.
As established above, the total chlorophyll content investigated in S. bijuga, mainly by spectrophotometric methods, concentrates only on species of chlorophyll a and b, without an evaluation of its derivative compounds, such as those identified in this work including the epimers chlorophyll a', chlorophyll b', hydroxychlorophyll a, hydroxypheophytin a and pheophytin a. These derivatives exist in very low concentrations in fruits and vegetables (Hosikian et al., 2010).
Sassi and co-workers (Sassi et al., 2019) reported for dried microalgae of the genus Chlorella and Scenedesmus, a chlorophyll a and chlorophyll b concentration of 58.42 and 132.32 mg g−1, respectively, using controlled cultivation conditions. Additionally, in a study by Paliwal et al. (2016) the total chlorophyll content (a and b) ranged from 2.16 to 18.59 mg g−1 dry cell weight in fourteen microalgae strains of the genus Chlorella. In contrast, three microalgae strains of the genus Scenedesmus showed concentrations of chlorophyll in the range of 0.92–18.53 mg g−1 dry cell weight. Thus, it is possible determined that the concentration of chlorophylls in microalgae varies significantly in each species although they are of the same genus.
The proportion of derived compounds exceeds chlorophyll a and chlorophyll b species in the two microalgae strains. In C. sorokiniana represent 68.32% of total chlorophylls, while in S. bijuga it was 50.44%. These values represent concentrations of 10.36 and 10.31 mg.g-1, respectively and not presented a significant difference between the two species. Besides, the presence of chlorophyll derivatives only of the chlorophyll a species in the two green microalgae species analyzed can be attributed to the higher chemical stability of the chlorophyll b molecule, or also due to the low concentration of this compound. Similarly, Chen et al. (2017) analyzed the chlorophylls composition in the red, green and brown seaweeds, and detected maximum content in chlorophyll derivatives (higher than 9 mg.g-1 dw.w.) in green seaweeds extracts (Enteromorpha spp. and Ulva spp.). Curiously, this concentration is in agreement with the values found for chlorophyll derivatives in our study. In this sense, results suggest that the synthesis of compounds derivatives from chlorophyll may be attributed to a characteristic of green microalgae species included in the phylum Chlorophyta.
Furthermore, a striking difference between the two species of microalgae under study is the concentration of chlorophyll a, which is significantly higher in S. bijuga (18.95% more). This may be positively related to the lowest levels of derivative compounds present in this strain, as chlorophyll a is the precursor of all other compounds identified in this study, as presented in Fig. 5. Zepka et al. (2019) provide an updated outline of the most recent advances that have occurred in the of chlorophyll catabolism, showing that enzymatic reactions are not involved in epimeric chlorophyll derivatives (chlorophyll a' and chlorophyll b') In contrast, oxidized derivatives such as hydroxychlorophylls, hydroxypheophytins and pheophytins can have both chemical and enzymatic origin.
Green microalgae Chlorella sp. is popular as a primary source of chlorophyll production and called ‘Emerald food’ because of its high chlorophyll content (Bewicke and Potter, 2009, Safi et al., 2014). The chlorophyll content in Chlorella is about 7% of the biomass, which is five times than the chlorophyll content of Spirulina, microalgae explored for commercial production of this compound (Khanra et al., 2018). Thus, the results found in our study contain an above-average ratio of chlorophylls per dry mass when compared those reported in the literature, since we obtained concentrations of chlorophyll representing 7.6% and 10.2% of the composition of the compounds present in the biomass of C. sorokiniana and S. bijuga, respectively. This is strongly evidenced by the fact that some authors consider that the total chlorophyll content in algae is in the range of 0.5–1.5% of dry weight (Becker and Richmond, 2004). Also, as per a recent observation by Basu et al. (2013) the chlorophyll a and b content of different microalgae strains (Scenedesmus obliquus and Chlorella sp.) varies within 1–6% of dry biomass. In this regard, factors such as the cultivation mode and choice of species-specific are determinants to chlorophyll accumulation in microalgae biomass, being generally assumed that sub saturating light intensities induce higher these compounds synthesis (da Silva Ferreira and Sant’Anna, 2017). Thus, a justification for the high concentration of chlorophylls found in this study may be directly related to our experimental conditions and the microalgae species explored, which have shown to be promising alternative sources for the production of these compounds.
Görs et al. (2010) showed that chlorophyll a can also be isolated from commercial Chlorella products, demonstrating concentrations ranging from 2.5 to 17.5 mg g−1 dry weight. In this study, the authors also reported the significant presence of pheophytin a in all samples. Furthermore, the axenic strain C. vulgaris used as reference presented percentage of pheophytin a of 30%.
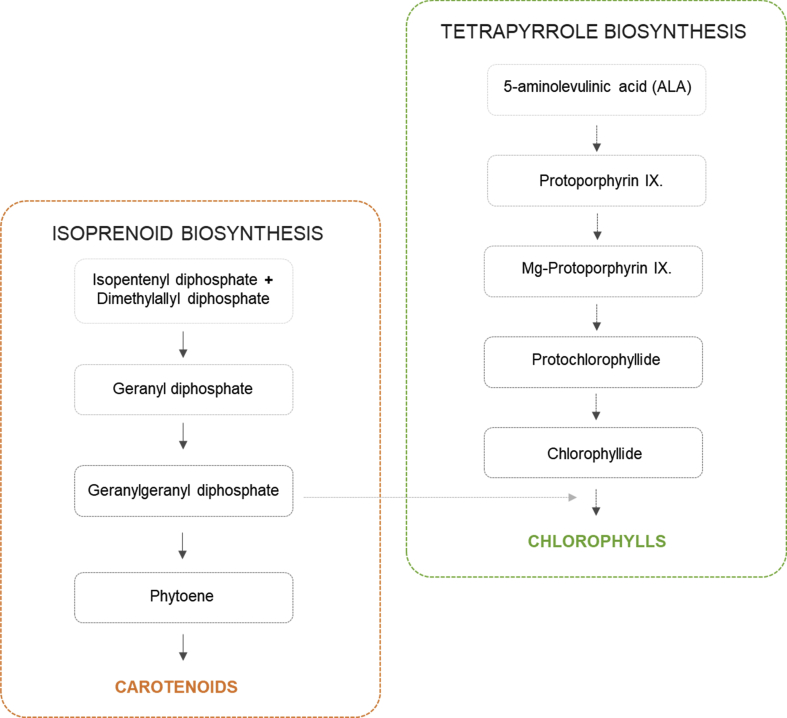
Curiously, the biosynthesis of chlorophylls involves the synthesis of two moieties: (1) a chlorin ring, which is synthesized by a specific branch of tetrapyrrole biosynthesis, and (2) a phytol chain produced by the isoprenoid (terpenoid) biosynthesis pathway (Fig. 6) (Solymosi and Mysliwa-Kurdziel, 2017). In this sense, the fact that the chlorophyll composition is higher in the S. bijuga species can be associated with the lower quantitative profile of carotenoid present in this strain, since carotenoid precursor compounds can be targeted to the biosynthetic pathway of chlorophyll. The opposite was observed in C. sorokiniana, where the values were lower for the total chlorophyll content and higher values for the carotenoid class (Fig. 4).
Fig. 6.
Simplified scheme of the tetrapyrrole biosynthesis and its relation to isoprenoid biosynthetic pathway providing the phytol side chain for chlorophylls.
In addition to its primary function in the photosynthesis, chlorophylls are in demand due to their bioactive properties. Several researchers evaluated the biological properties of chlorophyll molecules and demonstrated that chlorophyll a, chlorophyll b and pheophytin a (components of the photosynthetic chain identified in this study) were previously shown to exert antimutagenic, chemopreventive and anti-inflammatory activity as well as in vitro anti-oxidant activity (Hoshina et al., 1998, Ferruzzi and Blakeslee, 2007, Szczygieł et al., 2008, Subramoniam et al., 2012, Islam et al., 2013). Specifically, the pheophytin a from microalgae species macroalgae species has been identified as a potent suppressor against genotoxininduced umu C gene expression in S. typhimurium (TA 1535/pSK1002) probably associated with carcinogenesis. According to this, pheophytin a derivatives have been proposed to display a potent suppressive activity against chemically induced mouse skin tumorigenesis (Okai et al., 1996).
Besides, compounds chlorophylls derivatives as pheophorbide b and pheophytin b) demonstrated antioxidant activities superior to butylated hydroxytoluene (BHT). This fact demonstrated the importance of the aldehyde group and Mg-free derivatives for the functionality of these molecules (Lanfer-Marquez et al., 2005). Therefore, the presence of a mixture of chlorophyll a and chlorophyll b and their derivatives such as hydroxychlorophyll a, chlorophyll b', chlorophyll a', hydroxypheophytin a, and pheophytin a could represent an antioxidant defence system working with synergistic effect. Thus, considering the significant profile of chlorophyll derivatives compounds in C. sorokiniana and S. bijuga, further investigations on the actual antioxidant and biological activity of these compounds, together with their bioavailability remains the next primary task in our research.
4. Conclusion
The novelty of this study was a complete characterization of the profile of carotenoids and chlorophylls and their derivatives compound by HPLC-PDA-MS/MS from biomass extracts of C. sorokiniana and S. bijuga. Specifically, the analytical characterization revealed the presence of eleven and sixteen carotenoids in C. sorokiniana and S. bijuga, as well as seven chlorophyll compounds, were characterized in both species of microalgae.
Considering the quantitative profile, the highest total carotenoid content was determined in the extract of C. sorokiniana (1408.46 μg g−1), and S. bijuga exhibited the lowest content (1195.75 μg g−1). Additionally, the results showed that green microalgae synthesize carotenoids with 5,6-epoxy-groups and ketocarotenes in low concentrations and that the α-branch of the carotenoid biosynthetic pathway is active preferably. In contrast to chlorophyll compounds, the highest quantitative profile was demonstrated in S. bijuga (20.44 mg g−1); C. sorokiniana presented substantially lower values (15.20 mg g−1). Also, the quantification of each compound was species-specific. Among the compounds identified, relevant compounds such as all-trans-lutein, β-carotene, and chlorophyll a were major compounds in both strains, with values significantly higher than the conventional sources.
When considering the profile of carotenoids/chlorophylls, S. bijuga stands out with 10.79% of the composition of the compounds present in the biomass, together with its diversified profile, totaling twenty-three compounds among the two class studied.
Although our study does not highlight any specific molecules, we emphasize that it is possible to increase the potential of these studied species as potential alternative sources of natural carotenoids and chlorophylls, generating new possibilities mainly for the food industry.
Credit author statement
Andrêssa S. Fernandes: Conceptualization, Investigation, Writing - original draft, Writing – review & editing, Data curation, Formal analysis.
Fabiane C. Petry: Investigation.
Adriana Z. Mercadante: Investigation, Resources.
Eduardo Jacob-Lopes: Data curation, Formal analysis, Supervision, Writing - original draft, Writing - review & editing.
Leila Q. Zepka: Conceptualization, Resources, Formal analysis, Writing - original draft, Writing – review & editing, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors are grateful to the National Academic Cooperation Program PROCAD/CAPES and National Counsel of Technological and Scientific Development (CNPq) for the financial support.
References
- Azaman S.N.A., Nagao N., Yusoff F.M., Tan S.W., Yeap S.K. A comparison of the morphological and biochemical characteristics of Chlorella sorokiniana and Chlorella zofingiensis cultured under photoautotrophic and mixotrophic conditions. PeerJ. 2017;5:e3473. doi: 10.7717/peerj.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S., Roy A.S., Mohanty K., Ghoshal A.K. Enhanced CO2 sequestration by a novel microalga: Scenedesmus obliquus SA1 isolated from bio-diversity hotspot region of Assam, India. Bioresour. Technol. 2013;143:369–377. doi: 10.1016/j.biortech.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Becker E.W. Microalgae in human and animal nutrition. In: Richmond A., editor. Handbook of Microalgal Culture. Blackwell; Oxford: 2004. pp. 312–351. [Google Scholar]
- Begum H., Yusoff F.M., Banerjee S., Khatoon H., Shariff M. Availability and utilization of pigments from microalgae. Crit. Rev. Food Sci. Nutr. 2016;56(13):2209–2222. doi: 10.1080/10408398.2013.764841. [DOI] [PubMed] [Google Scholar]
- Bewicke D., Potter B.A. Ronin Publishing, Ronin Publishing; 2009. Chlorella: the Emerald Food. [Google Scholar]
- Bhatnagar A., Chinnasamy S., Singh M., Das K.C. Renewable biomass production by mixotrophic algae in the presence of various carbon sources and wastewaters. Appl. Energy. 2011;88(10):3425–3431. [Google Scholar]
- Borowitzka M.A., Beardall J., Raven J.A., editors. vol. 6. Springer; Cham: 2016. (The Physiology of Microalgae). [Google Scholar]
- Borowitzka M.A. Biology of microalgae. In: Levine I.A., Fleurence J., editors. Microalgae in Health and Disease Prevention. Academic Press; 2018. pp. 23–72. [Google Scholar]
- Chen C.Y., Liu C.C. Optimization of lutein production with a two-stage mixotrophic cultivation system with Chlorella sorokiniana MB-1. Bioresour. Technol. 2018;262:74–79. doi: 10.1016/j.biortech.2018.04.024. [DOI] [PubMed] [Google Scholar]
- Chen K., Ríos J.J., Pérez-Gálvez A., Roca M. Development of an accurate and high-throughput methodology for structural comprehension of chlorophylls derivatives.(I) Phytylated derivatives. J. Chromatogr. A. 2015;1406:99–108. doi: 10.1016/j.chroma.2015.05.072. [DOI] [PubMed] [Google Scholar]
- Chen K., Ríos J.J., Roca M., Pérez-Gálvez A. Development of an accurate and high-throughput methodology for structural comprehension of chlorophylls derivatives.(II) Dephytylated derivatives. J. Chromatogr. A. 2015;1412:90–99. doi: 10.1016/j.chroma.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Chen C.Y., Hsieh C., Lee D.J., Chang C.H., Chang J.S. Production, extraction and stabilization of lutein from microalga Chlorella sorokiniana MB-1. Bioresour. Technol. 2016;200:500–505. doi: 10.1016/j.biortech.2015.10.071. [DOI] [PubMed] [Google Scholar]
- Chen K., Ríos J.J., Pérez-Gálvez A., Roca M. Comprehensive chlorophyll composition in the main edible seaweeds. Food Chem. 2017;228:625–633. doi: 10.1016/j.foodchem.2017.02.036. [DOI] [PubMed] [Google Scholar]
- Chen C.Y., Lu I.C., Nagarajan D., Chang C.H., Ng I.S., Lee D.J., Chang J.S. A highly efficient two-stage cultivation strategy for lutein production using heterotrophic culture of Chlorella sorokiniana MB-1-M12. Bioresour. Technol. 2018;253:141–147. doi: 10.1016/j.biortech.2018.01.027. [DOI] [PubMed] [Google Scholar]
- Chen J.H., Kato Y., Matsuda M., Chen C.Y., Nagarajan D., Hasunuma T. Bioresource technology; 2019. A Novel Process for the Mixotrophic Production of Lutein with Chlorella Sorokiniana MB-1-M12 Using Aquaculture Wastewater; p. 121786. [DOI] [PubMed] [Google Scholar]
- Cicero F.G., Colletti A. Effects of carotenoids on health: are all the same? Results from clinical trials. Curr. Pharmaceut. Des. 2017;23:2422–2427. doi: 10.2174/1381612823666170207095459. [DOI] [PubMed] [Google Scholar]
- Cordero B.F., Obraztsova I., Couso I., Leon R., Vargas M.A., Rodriguez H. Enhancement of lutein production in Chlorella sorokiniana (Chorophyta) by improvement of culture conditions and random mutagenesis. Mar. Drugs. 2011;9(9):1607–1624. doi: 10.3390/md9091607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Ferreira V., Sant'Anna C. Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J. Microbiol. Biotechnol. 2017;33(1):20. doi: 10.1007/s11274-016-2181-6. [DOI] [PubMed] [Google Scholar]
- de Rosso V.V., Mercadante A.Z. Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. J. Agric. Food Chem. 2007;55(13):5062–5072. doi: 10.1021/jf0705421. [DOI] [PubMed] [Google Scholar]
- Di Lena G., Casini I., Lucarini M., Lombardi-Boccia G. Carotenoid profiling of five microalgae species from large-scale production. Food Res. Int. 2019;120:810–818. doi: 10.1016/j.foodres.2018.11.043. [DOI] [PubMed] [Google Scholar]
- Draaisma R.B., Wijffels R.H., Slegers P.E., Brentner L.B., Roy A., Barbosa M.J. Food commodities from microalgae. Curr. Opin. Biotechnol. 2013;24(2):169–177. doi: 10.1016/j.copbio.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Dufossé L. Microbial production of food grade pigments. Food Technol. Biotechnol. 2006;44(3):313–323. [Google Scholar]
- D'Alessandro E.B., Antoniosi Filho N.R. Concepts and studies on lipid and pigments of microalgae: a review. Renew. Sustain. Energy Rev. 2016;58:832–841. [Google Scholar]
- Fagundes M.B., Falk R.B., Facchi M.M.X., Vendruscolo R.G., Maroneze M.M., Zepka L.Q., Wagner R. Insights in cyanobacteria lipidomics: a sterols characterization from Phormidium autumnale biomass in heterotrophic cultivation. Food Res. Int. 2019;119:777–784. doi: 10.1016/j.foodres.2018.10.060. [DOI] [PubMed] [Google Scholar]
- Fernandes A.S., Nogara G.P., Menezes C.R., Cichoski A.J., Mercadante A.Z., Jacob-Lopes E., Zepka L.Q. Identification of chlorophyll molecules with peroxyl radical scavenger capacity in microalgae Phormidium autumnale using ultrasound-assisted extraction. Food Res. Int. 2017;99:1036–1041. doi: 10.1016/j.foodres.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Ferruzzi M.G., Blakeslee J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. (N.Y.) 2007;27(1):1–12. [Google Scholar]
- Garrido J.L., Zapata M. Ion-pair reversed phase high performance liquid chromatography of algal chlorophylls. J. Chromatogr. A. 1996;738:285–289. [Google Scholar]
- Garrido J.L., Airs R., Rodríguez F., Van Heukelem L., Zapata M. New HPLC separation techniques. In: Roy S., Egeland E.S., Johnsen G., Llewellyn C.A., editors. Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography. Cambridge University Press; 2011. pp. 165–194. [Google Scholar]
- Gong M., Bassi A. Carotenoids from microalgae: a review of recent developments. Biotechnol. Adv. 2016;34(8):1396–1412. doi: 10.1016/j.biotechadv.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Görs M., Schumann R., Hepperle D., Karsten U. Quality analysis of commercial Chlorella products used as dietary supplement in human nutrition. J. Appl. Phycol. 2010;22(3):265–276. [Google Scholar]
- Grand View. 2019. Natural Food Colors Market Estimates & Trend Analysis by Product (Curcumin, Carotenoids, Anthocyanin, Carmine, Chlorophyllin), by Application (Bakery & Confectionery, Beverages, Dairy & Frozen Products, Meat Products), and Segment Forecasts, 2014-2025”.http://www.grandviewresearch.com Accessed in 21.05.19. [Google Scholar]
- Hoshina C., Tomita K., Shioi Y. Photosynthesis: Mechanisms and Effects. Springer; Dordrecht: 1998. Antioxidant activity of chlorophylls: its structure-activity relationship; pp. 3281–3284. [Google Scholar]
- Hosikian A., Lim S., Halim R., Danquah M.K. Chlorophyll extraction from microalgae: a review on the process engineering aspects. Int. J. Chem. Eng. 2010;2010:1–11. [Google Scholar]
- Hussein R.A., Salama A.A., El Naggar M.E., Ali G.H. Medicinal impact of microalgae collected from high rate algal ponds; phytochemical and pharmacological studies of microalgae and its application in medicated bandages. Bio. Agri. Boit. 2019:101237. [Google Scholar]
- Hynninen P.H., Sievers G. Conformations of chlorophylls a and a′ and their magnesium-free derivatives as revealed by circular dichroism and proton magnetic resonance. Z. Naturforsch. B Chem. Sci. 1981;36(8):1000–1009. [Google Scholar]
- Islam M.N., Ishita I.J., Jin S.E., Choi R.J., Lee C.M., Kim Y.S. Anti-inflammatory activity of edible brown alga Saccharina japonica and its constituents pheophorbide a and pheophytin a in LPS-stimulated RAW 264.7 macrophage cells. Food Chem. Toxicol. 2013;55:541–548. doi: 10.1016/j.fct.2013.01.054. [DOI] [PubMed] [Google Scholar]
- Jacob-Lopes E., Maroneze M.M., Deprá M.C., Sartori R.B., Dias R.R., Zepka L.Q. Bioactive food compounds from microalgae: an innovative framework on industrial biorefineries. Curr. Opi. Food. Sci. 2018;25:1–7. [Google Scholar]
- Kao T.H., Chen C.J., Chen B.H. An improved high performance liquid chromatography–photodiode array detection–atmospheric pressure chemical ionization–mass spectrometry method for determination of chlorophylls and their derivatives in freeze-dried and hot-air-dried Rhinacanthus nasutus (L.) Kurz. Talanta. 2011;86:349–355. doi: 10.1016/j.talanta.2011.09.027. [DOI] [PubMed] [Google Scholar]
- Khalid M., Bilal M., Iqbal H.M., Huang D. Biosynthesis and biomedical perspectives of carotenoids with special reference to human health-related applications. Bio. Agri. Boit. 2018;17:399–407. [Google Scholar]
- Khan M.I., Shin J.H., Kim J.D. The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories. 2018;17(1):36. doi: 10.1186/s12934-018-0879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanra S., Mondal M., Halder G., Tiwari O.N., Gayen K., Bhowmick T.K. Downstream processing of microalgae for pigments, protein and carbohydrate in industrial application: a review. Food Bioprod. Process. 2018;110:60–84. [Google Scholar]
- Kothari R., Pandey A., Ahmad S., Kumar A., Pathak V.V., Tyagi V.V. Microalgal cultivation for value-added products: a critical enviro-economical assessment. 3 Biotech. 2017;7(4):243. doi: 10.1007/s13205-017-0812-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyande A.K., Chew K.W., Rambabu K., Tao Y., Chu D.T., Show P.L. Microalgae: a potential alternative to health supplementation for humans. Food. Sci. Hum. Wellness. 2019;8:16–24. [Google Scholar]
- Lanfer-Marquez U.M., Barros R.M., Sinnecker P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005;38(8–9):885–891. [Google Scholar]
- Lin J.H., Lee D.J., Chang J.S. Lutein production from biomass: marigold flowers versus microalgae. Bioresour. Technol. 2015;184:421–428. doi: 10.1016/j.biortech.2014.09.099. [DOI] [PubMed] [Google Scholar]
- Loh C.H., Inbaraj B.S., Liu M.H., Chen B.H. Determination of chlorophylls in Taraxacum formosanum by high-performance liquid chromatography–diode array detection–mass spectrometry and preparation by column chromatography. J. Agric. Food Chem. 2012;60(24):6108–6115. doi: 10.1021/jf301422m. [DOI] [PubMed] [Google Scholar]
- Mandelli F., Miranda V.S., Rodrigues E., Mercadante A.Z. Identification of carotenoids with high antioxidant capacity produced by extremophile microorganisms. World J. Microbiol. Biotechnol. 2012;28(4):1781–1790. doi: 10.1007/s11274-011-0993-y. [DOI] [PubMed] [Google Scholar]
- Maroneze M.M., Siqueira S.F., Vendruscolo R.G., Wagner R., de Menezes C.R., Zepka L.Q., Jacob-Lopes E. The role of photoperiods on photobioreactors–A potential strategy to reduce costs. Bioresour. Technol. 2016;219:493–499. doi: 10.1016/j.biortech.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Maroneze M.M., Jacob-Lopes E., Zepka L.Q., Roca M., Pérez-Gálvez A. Esterified carotenoids as new food components in cyanobacteria. Food Chem. 2019;287:295–302. doi: 10.1016/j.foodchem.2019.02.102. [DOI] [PubMed] [Google Scholar]
- Matos Â.P. The impact of microalgae in food science and technology. J. Am. Oil Chem. Soc. 2017;94(11):1333–1350. [Google Scholar]
- Matsukawa R., Hotta M., Masuda Y., Chihara M., Karube I. Antioxidants from carbon dioxide fixing Chlorella sorokiniana. J. Appl. Phycol. 2000;12(3–5):263–267. [Google Scholar]
- Mcwilliams A. 2018. The Global Market for Carotenoids. BCC Research Report Overview; pp. 1–9.https://www.bccresearch.com/market-research/food-and-beverage/the-global-market-for-carotenoids.html Accessed 12.05.19. [Google Scholar]
- Meléndez-Martínez A.J. An overview of carotenoids, apocarotenoids and vitamin A in agro-food, nutrition. Heal. Dis. Molecular nutrition & food research. 2019;63(15) doi: 10.1002/mnfr.201801045. [DOI] [PubMed] [Google Scholar]
- Miazek K., Kratky L., Sulc R., Jirout T., Aguedo M., Richel A., Goffin D. Effect of organic solvents on microalgae growth, metabolism and industrial bioproduct extraction: a review. Int. J. Mol. Sci. 2017;18(7):1429. doi: 10.3390/ijms18071429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minhas A.K., Hodgson P., Barrow C.J., Sashidhar B., Adholeya A. The isolation and identification of new microalgal strains producing oil and carotenoid simultaneously with biofuel potential. Bioresour. Technol. 2016;211:556–565. doi: 10.1016/j.biortech.2016.03.121. [DOI] [PubMed] [Google Scholar]
- Morais H., Abram A., Ferreira F. Carotenoids biosynthesis-a review. Revista Lusófona de Humanidades e Tecnologias. 2006;1(10) [Google Scholar]
- Mulders K.J., Lamers P.P., Martens D.E., Wijffels R.H. Phototrophic pigment production with microalgae: biological constraints and opportunities. J. Phycol. 2014;50(2):229–242. doi: 10.1111/jpy.12173. [DOI] [PubMed] [Google Scholar]
- Murillo E., Giuffrida D., Menchaca D., Dugo P., Torre G., Meléndez-Martinez A.J., Mondello L. Native carotenoids composition of some tropical fruits. Food Chem. 2013;140(4):825–836. doi: 10.1016/j.foodchem.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Nascimento T.C., Cazarin C.B., Maróstica M.R., Jr., Risso É.M., Amaya-Farfan J., Grimaldi R., Zepka L.Q. Microalgae biomass intake positively modulates serum lipid profile and antioxidant status. J. Func. Foods. 2019;58:11–20. [Google Scholar]
- Nwachukwu I.D., Udenigwe C.C., Aluko R.E. Lutein and zeaxanthin: production technology, bioavailability, mechanisms of action, visual function, and health claim status. Trends Food Sci. Technol. 2016;49:74–84. [Google Scholar]
- Okai Y., Higashi-Okai K., Yano Y., Otani S. Identification of antimutagenic substances in an extract of edible red alga, Porphyra tenera (Asadusa-nori) Canc. Lett. 1996;100(1–2):235–240. doi: 10.1016/0304-3835(95)04101-x. [DOI] [PubMed] [Google Scholar]
- Paliwal C., Ghosh T., George B., Pancha I., Maurya R., Chokshi K. Microalgal carotenoids: potential nutraceutical compounds with chemotaxonomic importance. Algal Research. 2016;15:24–31. [Google Scholar]
- Pareek S., Sagar N.A., Sharma S., Kumar V., Agarwal T., González-Aguilar G.A., Yahia E.M. Chlorophylls: chemistry and biological functions. Fruit. Vegs. Phyto: Chem. Hum. Health. 2017;2(1):269. [Google Scholar]
- Patias L.D., Fernandes A.S., Petry F.C., Mercadante A.Z., Jacob-Lopes E., Zepka L.Q. Carotenoid profile of three microalgae/cyanobacteria species with peroxyl radical scavenger capacity. Food Res. Int. 2017;100:260–266. doi: 10.1016/j.foodres.2017.06.069. [DOI] [PubMed] [Google Scholar]
- Pérez-Gálvez A., Viera I., Roca M. Chemistry in the bioactivity of chlorophylls: an overview. Curr. Med. Chem. 2017;24(40):4515–4536. doi: 10.2174/0929867324666170714102619. [DOI] [PubMed] [Google Scholar]
- Petruk G., Gifuni I., Illiano A., Roxo M., Pinto G., Amoresano A. Simultaneous production of antioxidants and starch from the microalga Chlorella sorokiniana. Algal research. 2018;34:164–174. [Google Scholar]
- Přibyl P., Pilný J., Cepák V., Kaštánek P. The role of light and nitrogen in growth and carotenoid accumulation in Scenedesmus sp. Algal research. 2016;16:69–75. [Google Scholar]
- Rajesh K., Rohit M.V., Mohan S.V. Algal Green Chemistry. Elsevier; 2017. Microalgae-based carotenoids production; pp. 139–147. [Google Scholar]
- Rammuni M.N., Ariyadasa T.U., Nimarshana P.H.V., Attalage R.A. Comparative assessment on the extraction of carotenoids from microalgal sources: astaxanthin from H. pluvialis and β-carotene from D. salina. Food Chem. 2018;277:128–134. doi: 10.1016/j.foodchem.2018.10.066. [DOI] [PubMed] [Google Scholar]
- Rippka R., Deruelles J., Waterbury J.B., Herdman M., Stanier R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979;111:1–61. [Google Scholar]
- Roca M., Chen K., Pérez-Gálvez A. Chlorophylls. In: Carle R., Schweiggert R., editors. Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color. Woodhead Publishing; Cambridge, UK: 2016. pp. 125–158. [Google Scholar]
- Rodrigues E., Mariutti L.R., Chisté R.C., Mercadante A.Z. Development of a novel micro-assay for evaluation of peroxyl radical scavenger capacity: application to carotenoids and structure–activity relationship. Food Chem. 2012;135(3):2103–2111. doi: 10.1016/j.foodchem.2012.06.074. [DOI] [PubMed] [Google Scholar]
- Rodrigues D.B., Flores É.M., Barin J.S., Mercadante A.Z., Jacob-Lopes E., Zepka L.Q. Production of carotenoids from microalgae cultivated using agroindustrial wastes. Food Res. Int. 2014;65:144–148. [Google Scholar]
- Rodrigues D.B., Menezes C.R., Mercadante A.Z., Jacob-Lopes E., Zepka L.Q. Bioactive pigments from microalgae Phormidium autumnale. Food Res. Int. 2015;77:273–279. [Google Scholar]
- Rodriguez-Concepcion M., Avalos J., Bonet M.L., Boronat A., Gomez-Gomez L., Hornero-Mendez D. A global perspective on carotenoids: metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018;70:62–93. doi: 10.1016/j.plipres.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Safafar H., Van Wagenen J., Møller P., Jacobsen C. Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar. Drugs. 2015;13(12):7339–7356. doi: 10.3390/md13127069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safi C., Zebib B., Merah O., Pontalier P.Y., Vaca-Garcia C. Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew. Sustain. Energy Rev. 2014;35:265–278. [Google Scholar]
- Santhakumaran P., Kookal S.K., Ray J.G. Biomass yield and biochemical profile of fourteen species of fast-growing green algae from eutrophic bloomed freshwaters of Kerala, South India. Biomass Bioenergy. 2018;119:155–165. [Google Scholar]
- Sassi K.K.B., Silva J.A.D., Calixto C.D., Sassi R., Sassi C.F.D.C. Metabolites of interest for food technology produced by microalgae from the Northeast Brazil. Rev. Cienc. Agron. 2019;50(1):54–65. [Google Scholar]
- Sathasivam R., Radhakrishnan R., Hashem A., Abd_Allah E.F. Microalgae metabolites: a rich source for food and medicine. Saudi J. Biol. Sci. 2017;26:709–722. doi: 10.1016/j.sjbs.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solymosi K., Mysliwa-Kurdziel B. Chlorophylls and their derivatives used in food industry and medicine. Mini Rev. Med. Chem. 2017;17(13):1194–1222. doi: 10.2174/1389557516666161004161411. [DOI] [PubMed] [Google Scholar]
- Soontornchaiboon W., Joo S.S., Kim S.M. Anti-inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoidea in RAW 264.7 macrophages. Biol. Pharm. Bull. 2012;35(7):1137–1144. doi: 10.1248/bpb.b12-00187. [DOI] [PubMed] [Google Scholar]
- Spolaore P., Joannis-Cassan C., Duran E., Isambert A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006;101(2):87–96. doi: 10.1263/jbb.101.87. [DOI] [PubMed] [Google Scholar]
- Steele D.J., Tarran G.A., Widdicombe C.E., Woodward E.M.S., Kimmance S.A., Franklin D.J., Airs R.L. Abundance of a chlorophyll a precursor and the oxidation product hydroxychlorophyll a during seasonal phytoplankton community progression in the Western English Channel. Prog. Oceanogr. 2015;137:434–445. [Google Scholar]
- Subramoniam A., Asha V.V., Nair S.A., Sasidharan S.P., Sureshkumar P.K., Rajendran K.N. Chlorophyll revisited: anti-inflammatory activities of chlorophyll a and inhibition of expression of TNF-α gene by the same. Inflammation. 2012;35(3):959–966. doi: 10.1007/s10753-011-9399-0. [DOI] [PubMed] [Google Scholar]
- Sudhakar M.P., Kumar B.R., Mathimani T., Arunkumar K. A review on bioenergy and bioactive compounds from microalgae and macroalgae-sustainable energy perspective. J. Clean. Prod. 2019;228:1320–1333. [Google Scholar]
- Sun Z., Li T., Zhou Z.G., Jiang Y. Microalgae Biotechnology. Springer; Cham: 2015. Microalgae as a source of lutein: chemistry, biosynthesis, and carotenogenesis; pp. 37–58. [DOI] [PubMed] [Google Scholar]
- Szczygieł M., Urbanska K., Jurecka P., Stawoska I., Stochel G., Fiedor L. Central metal determines pharmacokinetics of chlorophyll-derived xenobiotics. J. Med. Chem. 2008;51(15):4412–4418. doi: 10.1021/jm7016368. [DOI] [PubMed] [Google Scholar]
- Takaichi S. Carotenoids in algae: distributions, biosyntheses and functions. Mar. Drugs. 2011;9(6):1101–1118. doi: 10.3390/md9061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen R.B., Dong L., Pajkovic N.D. Atmospheric pressure chemical ionization tandem mass spectrometry of carotenoids. Int. J. Mass Spectrom. 2012;312:163–172. doi: 10.1016/j.ijms.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wagenen J., De Francisci D., Angelidaki I. Comparison of mixotrophic to cyclic autotrophic/heterotrophic growth strategies to optimize productivity of Chlorella sorokiniana. J. Appl. Phycol. 2015;27(5):1775–1782. [Google Scholar]
- Vendruscolo R.G., Facchi M.M.X., Maroneze M.M., Fagundes M.B., Cichoski A.J., Zepka L.Q., Wagner R. Polar and non-polar intracellular compounds from microalgae: methods of simultaneous extraction, gas chromatography determination and comparative analysis. Food Res. Int. 2018;109:204–212. doi: 10.1016/j.foodres.2018.04.017. [DOI] [PubMed] [Google Scholar]
- Yamauchi N., Funamoto Y., Shigyo M. Peroxidase-mediated chlorophyll degradation in horticultural crops. Phytochemistry Rev. 2004;3(1–2):221–228. [Google Scholar]
- Yen H.W., Hu I.C., Chen C.Y., Ho S.H., Lee D.J., Chang J.S. Microalgae-based biorefinery–from biofuels to natural products. Bioresour. Technol. 2013;135:166–174. doi: 10.1016/j.biortech.2012.10.099. [DOI] [PubMed] [Google Scholar]
- Zepka L.Q., Jacob-Lopes E., Roca M. Catabolism and bioactive properties of chlorophylls. Curr. Opi. Food. Sci. 2019;26:94–100. [Google Scholar]
- Zhang Y., Liu Z., Sun J., Xue C., Mao X. Biotechnological production of zeaxanthin by microorganisms. Trends Food Sci. Technol. 2018;71:225–234. [Google Scholar]