Abstract
In this work, the bioaccessibility of polymethoxyflavones (PMFs) loaded in high internal phase emulsions (HIPE, ϕoil = 0.82) stabilized by whey protein isolate (WPI)-low methoxy pectin (LMP) complexes was evaluated using in vitro lipolysis and dynamic in vitro intestinal digestion studies. PMFs loaded HIPE was prepared by using aqueous dispersion of pre-formed biopolymeric complexes (WPI-LMP, 2:1 ratio) as the external phase and medium chain triglycerides oil (containing PMFs extracted from citrus peel) as the dispersed phase. The in vitro lipolysis study revealed that PMFs in HIPE became bioaccessible much higher than PMFs in medium chain triacylglycerols oil (MCT oil). In addition, by simulating the entire human gastrointestinal (GI) tract, the GI model TIM-1 demonstrated a 5- and 2-fold increase in the total bioaccessibility for two major PMFs encapsulated in HIPE, i.e. tangeretin (TAN) and nobiletin (NOB), respectively, as opposed to PMFs in MCT oil. Together these results from the digestion study showed that the incorporation of a high amount of PMFs into the viscoelastic matrix of HIPE could represent an innovative and effective way to design an oral delivery system. Such a system could be used to control and to improve the delivery of lipophilic bioactive compounds within the different compartments of the digestive tract, especially the human upper GI tract.
Keywords: Polymethoxyflavones, Tangeretin, Nobiletin, High internal phase emulsion, Complexes, Bioaccessibility, TIM-1
Graphical abstract
Highlights
-
•
The amount of loaded PMFs was improved by HIPE containing 80% MCT oil stabilized by WPI-LMP nanocomplexes.
-
•
The HIPE improved the digestion of oil phase compared to MCT oil according to the in vitro lipolysis study.
-
•
The HIPE improved the bioaccessibility of two major PMFs as evaluated by TIM-1 gastrointestinal model.
1. Introduction
In recent decades, there has been a growing interest in flavonoids as dietary bioactive compounds to prevent various diseases without adverse effects (Xiao et al., 2016). Citrus flavonoids, especially polymethoxyflavones (PMFs) have been of particular interest because many of these flavonoids demonstrate a wide spectrum of biological activity, including anti-inflammatory, anti-carcinogenic, anti-bacterial, anti-oxidant, and anti-nociceptive properties (Wang et al., 2014, Kim et al., 2016, Faqueti et al., 2016, Gosslau et al., 2014a). PMFs are one type of flavone compounds with several methoxy groups; more than 20 polymethoxylated flavonoids have been isolated and identified from different tissues of citrus plants (Li et al., 2007).
PMFs are more lipophilic as compared to polyhydroxylated flavonoids, such as quercetin, luteolin, and naringenin, because of the hydrophobic nature of methoxy groups (Li et al., 2008). Therefore, the low aqueous solubility of PMFs may impact their bioaccessibility and bioavailability when they are digested. Since PMFs are highly lipophilic, enhancing their solubility is essential to increase the bioaccessibility (Ting et al., 2015a). Moreover, PMFs tend to easily crystallize in oil medium at a relatively low concentration. The solubility of PMF (5-hydroxytangeretin) in medium chain triacylglycerols (MCT, 6.1 mM) is higher than in long chain triacylglycerols (LCT, 4.2 mM) (Li et al., 2012a). Thus, a high ratio of PMFs to dissolution medium is desirably to prevent the crystallization of PMFs as well as to improve the loading dose of PMFs for a specific therapeutic effect. A stable formulation with a high lipid fraction, i.e. a high internal phase emulsion (HIPE), could be a possible approach to improve the bioaccessibility of PMFs during digestion events including the formation of micelles by bile salts and endogenous phospholipids. The use of HIPE-based delivery systems for flavonoids is still rather limited and needs to be further explored, since they could be an effective strategy to improve the bioaccessibility of flavonoids.
A few works on the impact of biopolymer coating on lipophilic compounds delivery under digestion simulation have been published (Mun et al., 2006, Tokle et al., 2012). The deposition of a layer of non-digestible polysaccharides (i.e. chitosan) onto the surface of oil droplets could modulate the in vitro digestion of the lipid and the fatty acid release. The lipase activity on the emulsified lipid droplets was reduced due to the formation of a relatively thick layer of biopolymer complexes (lecithin-chitosan complexes) around each droplet (Mun et al., 2006). It was also observed by Lesmes and McClements (Tokle et al., 2012), that the conjugated high-molecular weight polysaccharide to the protein provided a more stable emulsion that could resist to the addition of bile by providing electro-steric stabilization. On the other hand, simple emulsifiers could be easily displaced by bile. These studies highlight the potential for protein-polysaccharide coatings to be used to modulate the bioavailability of encapsulated lipids.
The bioaccessibility studies on major PMFs (especially TAN and NOB) are very limited. One study in rats showed that after 1 h of oral administration of 50 mg/kg NOB, a mere 1.78 μg/mL (4.4 μM) of plasma NOB was detected (Singh et al., 2011). There was a study demonstrating that NOB at a concentration of ≤5 μM exhibited anti-proliferative effects against colon cancer cells (Manthey and Guthrie, 2002). Furthermore, oral supplementation of PMFs from orange peel extracts in a mice paw edema model significantly reduced inflammation in a dose-dependent manner. It is reported that a dosage of 250 mg/kg gave an anti-inflammatory effect comparable to ibuprofen (Gosslau et al., 2014b).
PMFs are also rapidly eliminated from the body. Significant adverse effects including toxicity at a relatively high concentration reported after administration of PMFs are rare. In addition to this, the poor solubility of these PMFs limits their bioaccessibility in the human gut system, which eventually leads to a poor bioavailability and reduces the therapeutic dosages they can achieve in the target organs. Some researchers also attempted to improve the bioavailability of PMFs using a chemical modification strategy (Tung et al., 2016). However, this approach is less preferable concerning the safety and acceptance. The use of an encapsulation strategy is more desirable in this context than a chemical modification approach.
Moreover, the encapsulation approach by incorporating a high amount of PMFs in a lipid-rich system stabilized by food-grade complex particles was hypothesized to improve their oral bioaccessibility. An understanding of the bioaccessibility of PMFs becomes even more crucial to assisting in the prediction of bio-efficacy through an in-vitro digestion model that resembles the human digestion system.
An advanced in vitro digestion model using a computer programming system to control relevant physiological parameters such as body temperature, gastric and intestinal pH curves, peristaltic movements, physiological intestinal volumes, and appropriate secretion of gastrointestinal (GI) fluids, may closely resemble the in vivo digestion system (Minekus et al., 2015). These in vivo parameters are essential to predict the behavior under in vivo exposure and to quantify the compound that becomes bioavailable for absorption or bioaccessible (Lyng et al., 2016, Naylor et al., 2006).
In this work, PMFs isolated from citrus peel powder were loaded in HIPE stabilized by WPI-LMP complexes (HIPE-complexes) and the PMFs bioaccessibility was evaluated. In our previous study, we have successfully formed ultra-stable HIPEs by optimizing the formation of biopolymeric complexes from WPI and LMP (Wijaya et al., 2017a). To our knowledge, little is known about the impact of the HIPE-complexes system on the intestinal absorption of PMFs. The bioaccessibility of PMFs may be improved when delivered within systems rich in lipids. The responsiveness of HIPE-complexes loaded with PMFs to simulated digestion was investigated using in vitro pH-stat lipolysis and an in vitro dynamic digestion model (TIM-1). The methodology used in this study could provide more insight before performing in vivo studies to further understand the impact of a stable HIPE-complexes system on the oral bioavailability of PMFs. Such systems could be used to deliver bioactive compounds in HIPE-based food products such as mayonnaise which could then be used as functional foods.
2. Materials and methods
2.1. Materials
A minimally heat-treated whey protein isolate (WPI), enriched in β-lactoglobulin (approx. 85% of total protein), was obtained from Davisco Foods International, Inc. (Agropur, CA). According to the manufacturer, the WPI sample contained ≥92% protein, ≤ 6% moisture, ≤ 4.5% minerals, ≤ 0.2% fat, and ≤0.2% lactose. Unipectine OB700 - a low methoxy apple pectin with a DE between 33 and 38% was received from Cargill R&D (Vilvoorde, Belgium). Neobee 895 Medium Chain Triglyceride (MCT), 97% caprylic triglycerides, was received from Stepan Company (Northfield, IL). Citrus peel powder was obtained from Dr. Qingrong Huang's laboratory (Rutgers University, NJ). Tangeretin (TAN) and nobiletin (NOB) standard (98% purity) were obtained from Herb-key Botanical Development Co. (Shaanxi). Tris maleate, silica gel, sodium chloride, calcium chloride dihydrate, potassium dihydrogen phosphate, sodium azide, hydrochloric acid, and sodium hydroxide were purchased from Sigma Aldrich (St. Louis, MO). Sodium taurodeoxycholate (NaTDC) was purchased from CalBiochem (La Jolla, CA). Phosphatidylcholine (PC75 rapeseed lecithin) was a gift from the American Lecithin Co. (Oxford, CT). Pancreatin of 8 × USP specification, pepsin A from porcine stomach mucosa (2500–3500 units/mg, P-7012), trypsin from bovine pancreas (7500 N-a-benzoyl-L-arginine ethyl ester (BAEE) units/mg, T9201), and α-amylase Type II-A from Bacillus species (1333 units/mg A-6380) were obtained from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO). Fresh pig bile was obtained from TNO Zeist, Netherlands. Rhizopus lipase (150,000 units/mg F-AP-15) was obtained from Amano Enzyme Inc. (Nagoya). HPLC-grade acetonitrile (ACN) and HPLC-grade water were purchased from J.T. Baker (Phillipsburg, NJ). Sterile filtered, cell culture compatible dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO) was used as HPLC sample solvent. Distilled water was used in this study. All reagents were used without further purification or modification, and all samples were formulated and reported on a weight-by-weight basis (g/g).
2.2. Isolation and characterization of PMFs
PMFs were extracted using ethyl acetate at a 5:1 ratio of solvent to citrus peel powder. The mixture was stirred for 2 h and sonicated 1 h. Afterwards, the extract was filtered using a filter paper (0.45 μm) to separate the supernatant from the precipitate. The precipitate was then subjected to another extraction process by adding the same volume of ethyl acetate. The supernatants obtained were mixed together followed by evaporation of solvent to obtain a concentrated extract. Subsequently, the concentrated extract was slowly poured down into a silica gel column wetted by hexane. The silica gel column (2-inch diameter and 24-inch length) was filled by 440 g of silica gel with particle size of 100–200 mesh. The ethyl acetate extract was poured onto the top of the column. The extract passed through the column due to the gravitational force, resulting in a brown and yellow band. The brown band was retained in the column and the yellow band dripped out of the column. The insoluble yellow precipitates were collected, while the mixture of mobile phase solvents was evaporated. After the evaporation, some essential oils still remained in the sample because of their high solubility in ethyl acetate. The essential oils were removed by adding hexane at a ratio 1:5 followed by sonicating for 2 h, because PMFs are insoluble in hexane at room temperature. The PMFs were then recovered by filtration followed by recrystallization in hexane to remove other impurities including the remaining essential oils. Briefly, recovered PMFs powder (50 mg) was added into 10 mL of hexane followed by boiling at 130 °C for 1–2 min to completely dissolve the PMFs. Hot filtration was performed. The filtrate was allowed to slowly cool down at room temperature to promote crystal formation. Eventually, the yellow bright crystals of PMFs were recovered using a filter paper. The microscopy images of PMFs powder before and after recrystallization in hexane are shown in Fig. S1 (Supplementary Information).
2.3. HPLC analysis
PMFs were identified by using an UltiMate 3000 HPLC system equipped with a 25D UV-VIS absorption detector (Dionex, CA) and a Supelco RP-amide column, 15 cm × 4.6 mm I.D., 3 μm particles (Bellefonte, PA). The elution of PMFs was performed using a gradient of water (solvent A) and ACN (solvent B). The optimized conditions were modified from previous literature (Gosslau et al., 2014b). The total elution time was 22 min, where the mobile phase started from 40% of ACN, then linearly increased to 55% over 10 min, then increased to 70% in 5 min, and then to 80% in 5 min, and was then linearly reduced back to 40% at 21 min and held isocratically for the final minute. The flow rate was held constant at 1.0 mL/min, injection volume was 10 μL and the detection wavelength was 320 nm. The PMFs standards, i.e. TAN and NOB (98% purity), were injected into the column using the same elution scheme as a reference. The concentration of TAN and NOB in the sample was calculated from their standard curve at a concentration ranging from 1 to 150 μg/mL.
2.4. Loading of PMFs into HIPE stabilized by WPI-LMP complexes
WPI and LMP dispersion were mixed together to achieve 2.5% and 1.25% final concentration in HIPE. Hence, WPI-LMP complexes were prepared by dissolving WPI and LMP powder in an aqueous phase containing 0.02% sodium azide at a concentration of 10% and 5%, respectively. This complex dispersion was adjusted to pH 5.25 by 1 and 0.1 N hydrochloric acid for promoting electrostatic complexation. The aqueous phase of the HIPE was kept constant at 20%, and 80% oil phase. To evaluate the effect of the PMFs loading concentration on the bioaccessibility in the emulsion, 2% PMFs were added to the carrier oil, and dissolved at 130 °C with continuous stirring until they were completely dissolved (for 5 min). After the PMFs loaded oil reached a temperature of about 30 °C, the aqueous phase containing WPI-LMP complexes was added to the oil phase. HIPEs were homogenized for 3 min at 6000 rpm using a T25 digital ULTRA-TURRAX® (IKA-Werke GmbH & Co. KG, Germany) equipped with a dispersing rotor S25N-10G. The resulting self-standing emulsion gels were then stored at 5 °C prior to analysis on the following day.
2.5. Particle size analysis of WPI-LMP complexes
The mean particle size and polydispersity index (PDI) of the WPI-LMP complexes were determined using a Zeta-sizer Nano-ZS90 instrument (Malvern Instruments Worcestershire, UK), and the mean particle size was calculated from the z-average diffusion coefficient by the Stokes-Einstein equation. The WPI-LMP dispersion at pH 5.25 was diluted 20 times to avoid multiple light scattering effects.
2.6. Microstructure of PMFs-loaded HIPE-complexes
The microstructure of PMFs-loaded HIPE-complexes was analyzed by using a Nikon Eclipse TE2000-U (Nikon Corporation, Japan) microscope equipped with a charge-coupled device (CCD) camera (Retiga EXi, QImaging). The sample was placed on a glass microscopic slide with a cover glass.
2.7. In vitro lipolysis study
In vitro lipolysis was carried out according to our previously published method (Yu et al., 2012). In short, a lipolysis buffer was prepared with Tris maleate, sodium chloride, calcium chloride dihydrate, sodium taurodeoxycholate, and phosphatidylcholine in concentrations of 50, 150, 5, 20, and 5 mM, respectively. Pancreatin was freshly prepared for each study by mixing 1 g of pancreatin powder with 5 mL lipolysis buffer and centrifuging at 2000 rpm for 10 min; the supernatant was collected and stored on ice. To begin the lipolysis study, a sample containing 250 mg oil phase was mixed with 9 mL of fed state buffer in the glass reaction vessel, which was put in an oil bath with temperature maintained at 37 ± 1 °C. The mixture was stirred at 200 rpm for 10 min and its pH was monitored by a pH meter. Before 1 mL of ice-chilled pancreatin was added to initiate the digestion, the pH of the mixture was adjusted to 7.50 ± 0.02 by adding 0.2 M sodium hydroxide solution. This temperature and pH were maintained during the lipolysis study to neutralize the free fatty acids (FFA) released from the lipid digestion. The volume of sodium hydroxide added at each time point was recorded for later analysis. The percentage of FFA released was calculated using eq. (1):
| (1) |
VNaOH is the volume of titrant in liters, mNaOH is the molarity of sodium hydroxide, Mwlipid is the apparent molecular weight of MCT oil obtained from eq. (2), and Wlipid is the weight of oil in the digestion system in grams. Molecular weight of the triglycerides was estimated using the saponification value (SV) of the MCT oil (97% caprylic triglycerides), i.e. 384.
| (2) |
Immediately upon completion of the lipolysis study, the resulting lipolysis solutions were subjected to ultracentrifugation (Type 60 Ti rotor, Beckman Coulter) for 40 min at 40,000 rpm. After ultracentrifugation, the digestion medium was separated into an opaque sediment phase, an aqueous phase containing formulated PMFs micelles in the middle, and an oil phase at the top. The micelle phase was collected using a syringe and the volume was recorded. For HPLC analysis, 200 μL of lipolysis supernatant sample (0.22 μm filtered) was mixed with 400 μL of DMSO. The concentration of PMFs in the micelles was analyzed using HPLC. The bioaccessibility (%) of PMFs was calculated using eq. (3):
| (3) |
2.8. Dynamic in vitro GI model
To mimic physiological states, the secretion of digestive juices and adjustment of pH conditions were controlled by computer programs according to physiological data described in previous literatures (Ting et al., 2015b, Ribnicky et al., 2014). The half-life of gastric emptying was set at 70 min. The temperature during the digestion simulation was maintained at 37 °C. The pH was regulated by secretion of hydrochloric acid in the stomach and sodium bicarbonate in intestinal compartments, i.e. stomach (pH 1.5), duodenum (pH 6.4), jejunum (pH 6.9) and ileum (pH 7.2). Lipase and pepsin were used for the gastric phase (reducing the pH from 5.5 to 1.5 with a half time of 40 min). The pancreatin and bile extract (fresh pig bile) were secreted in the intestinal digestions. Hollow fiber filtration devices, composed of semi-permeable membranes (0.05 μm pore size, Spectrum Milikros modules M80S-300-01P), were connected to the jejunal and ileal compartments to simulate absorption of released/digested water or fat-soluble compounds less than 50 nm in size.
TIM-1 was used to evaluate the bioaccessibility of PMFs in HIPE-complexes and MCT oil (2% PMFs with respect to the oil content) in simulated fed-state digestion. The formulation ‘meals’ (300 g) were a mixture of sample (7.7 g) containing PMFs (124 mg), 95 g gastric electrolyte solution, 0.011 g amylase, 5 g gastric enzyme solution and 180 g water. Each compartment was first filled with defined start residues that resemble the actual physiological GI conditions. All system conditions used are summarized in Table 1.
Table 1.
Established parameters of GI digestion in the TIM-1 for simulating digestive conditions of a healthy adult after meal intake.
| Gastric phase | Small intestinal phase |
|||
|---|---|---|---|---|
| Duodenum | Jejunum | Ileum | ||
| Start residue | Gastric enzyme solution: 5 g | Small intestinal electrolyte solution (SIES): 15 g Pancreatin solution: 15 g Bile: 30 g Trypsin solution (2 mg/cup): 1 cup |
SIES: 40 g Pancreatin solution: 40 g Bile: 80 g |
SIES: 160 g |
| pH/time (min) | 6/0, 5.7/15, 4.5/45, 2.9/90, 2.3/120, 1.8/240 | maintained at 6.4 | maintained at 6.9 | maintained at 7.2 |
| Enzyme andfluid secretion | 0.25 ml/min pepsin 0.25 ml/min lipase 0.25 ml/min of 1.5 M HCl if necessary |
0.5 ml/min bile salts (4% during the first 30 min of digestion then 2%) 0.25 ml/min pancreatic solution 0.25 ml/min SIES 0.25 ml/min 1 M NaHCO3 if necessary |
10 ml/min jejunal fluid solution 0.25 ml/min 1 M NaHCO3 if necessary |
10 mL/min ileal fluid solution 0.25 ml/min 1 M NaHCO3 if necessary |
The final mixture was introduced in the gastric compartment of TIM-1 and digestion was initiated. The physiological conditions of upper GI digestion were mimicked and programmed according to the parameters in Table 1; enzymes and GI fluids were continually secreted into the stomach and duodenum compartment, based on the TIM-1 computer-controlled settings. Each TIM-1 digestion experiment was terminated at 4 h when approximately 80% of the stomach contents had passed the ileocaecal valve of the model and become the ileal efflux. Bioaccessibility of PMFs was evaluated by collecting 40 mL of jejunum, ileum filtrate and ileal efflux sample in 1 h intervals for 4 h after initiation of digestion. Collected samples were stored on ice until subsequent HPLC analysis.
For HPLC analysis, 5 mL of sample was then extracted by mixing with 5 mL of ethyl acetate and centrifuged at 15,000 g for 30 min at ambient temperature. After being centrifuged, the extract was purged using nitrogen to evaporate the solvent. The dried extract was mixed with 500 μL of methanol for use in HPLC analysis (section 2.3). The amount of two major PMFs in the samples, i.e. TAN and NOB, which was available for absorption was calculated based on its concentration in the dialysis fluids. In addition, the bioaccessibility of TAN and NOB (defined as % of input) was calculated as a percent relative to the initial concentration of TAN/NOB in HIPE-complexes and MCT oil samples.
2.9. Statistical analysis
All experiments were conducted in triplicate, while the whole TIM-1 study was conducted in duplicate, in which each sample was analyzed in three repetitions. Results were expressed as the mean ± standard deviation (SD). Error bars on figures represent standard deviations.
3. Results and discussion
3.1. Isolation and characterization of PMFs
PMFs were extracted from citrus peel powder using ethyl acetate as solvent, followed by isolation and recrystallization of PMFs to remove essential oils and other impurities. The isolation process of PMFs yielded about 3% from the citrus peel powder. PMFs were identified by HPLC conducted using step gradient elution that enabled a clear separation of individual PMFs. The HPLC chromatogram of the isolated PMFs is presented in Fig. S2 (supplementary information). The major PMFs in citrus peel extract, i.e. TAN and NOB, were identified using a 98% purity standard of TAN and NOB (Fig. S3, S4, supplementary information). The other minor PMFs, i.e. 5-DTAN (5-hydroxy-6, 7, 8, 4′-tetramethoxyflavone) and 5-DNOB were identified by their retention time, i.e. 15.49 and 13.31 min, respectively, according to our previous works, (Gosslau et al., 2014b). The relative peak areas (%) of TAN, NOB, 5-DTAN and 5-DNOB are 65.88 ± 3.14, 23.19 ± 1.73, 4.70 ± 0.01, 5.94 ± 0.81, respectively.
3.2. Characterization of PMFs-loaded HIPE stabilized by WPI-LMP complexes
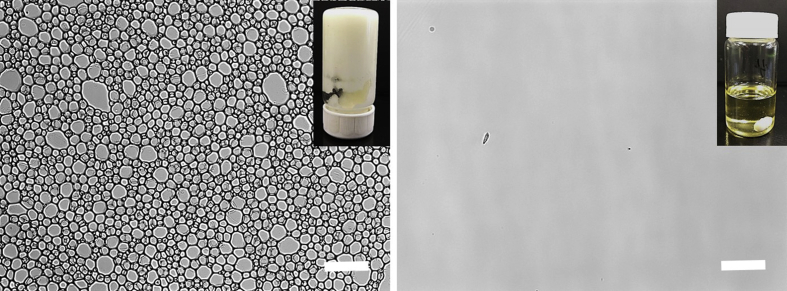
HIPE stabilized by WPI-LMP complexes have been prepared according to our previous research (Wijaya et al., 2017a). In this work, the WPI-LMP complexes which were pre-formed at pH 5.25 had a Z-average diameter of 350 nm. The aqueous phase containing pre-formed complexes (20%) was homogenized with the oil phase (80%) containing PMFs (2%). The final concentration of WPI, LMP, and PMFs in the HIPE is 2.5, 1.25, and 1.6%, respectively. The visual appearance and microscopic images of PMFs encapsulated in HIPE stabilized by WPI-LMP complexes and PMFs in MCT oil are shown in Fig. 1. The microscopic image of the emulsion showed that there were no crystals of PMFs detected in the emulsion at a concentration of 2% with respect to the oil content, which indicated that the PMFs remained soluble after being encapsulated in the HIPE. A relatively low ratio of PMFs to MCT oil as the dissolution medium was important to prevent the crystallization of PMFs which may affect their rate and extent of absorption. It is notable that when a high content of PMFs is dissolved in bulk oil at high temperature, some PMFs will recrystallize rapidly if the temperature returns to room temperature (Li et al., 2012a, Yang et al., 2017). It has also been previously reported that the absorption of crystalline compounds in the GI tract is typically dissolution rate-limited (Wang et al., 2014, Xia et al., 2015). The use of high oil fraction in this study improved the loaded amount of the isolated PMFs without any evidence of PMFs crystals two days after preparation (data not shown).
Fig. 1.
HIPE-complexes (left) and MCT oil (right) containing 2% of PMF with respect to the oil content. The microscopic images were taken immediately after the preparation of the samples. Bar scale is 20 μm. Inset: Photographs of respective samples.
3.3. Bioaccessibility of PMFs using the pH-stat lipolysis digestion model
pH stat-lipolysis has been previously used to study the lipid digestion affecting the bioaccessibility of lipophilic compounds (Li et al., 2011, Chen et al., 2017, Calligaris et al., 2015). During the lipid digestion, fatty acids are continuously released, causing a pH decrease. The optimum pH of the digestion was maintained by the addition of sodium hydroxide, so that the kinetics of lipid digestion could be monitored. Free fatty acids bound with the lipophilic compounds were further processed into lipid micelles by bile salts and phospholipids. In theory, these micelles, acting as a container for lipids and the bioactive compounds, will be collectively transported to epithelium cells for absorption. The amount of bioactive compounds incorporated into the micelles was quantified to determine their bioaccessibility.
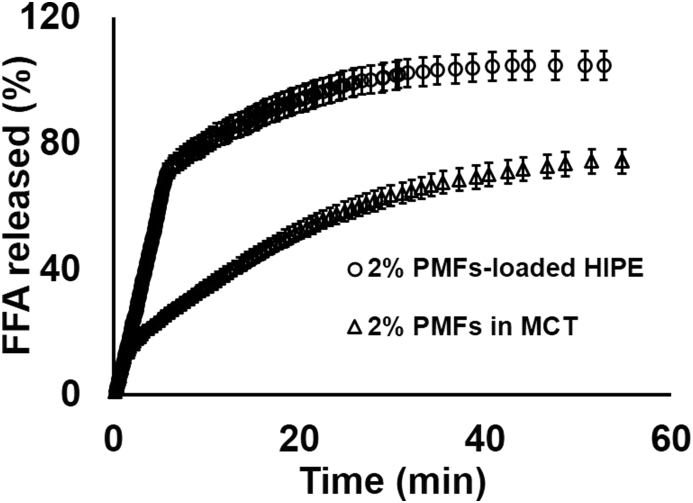
In this study, the titration kinetics of the HIPE sample proceeded at a much faster rate than the bulk oil sample (Fig. 2). According to the % FFA volume versus time curve, the majority of the lipid in the HIPE system was digested within 10 min from the onset of the study and reached a plateau after 40 min of digestion indicating that almost all lipid in the HIPE-complexes has been digested. A higher final amount of FFAs (105%) were released in the HIPE-complexes which this might be attributed by a relatively high level of proteins in the sample. According to previous work by Mun et al. (2015), the overestimation of % FFA was found due to a relatively high content of mung bean which was presence in the system. The digested protein may release peptides, amino acids, and some protons (H+), thereby leading to an over-estimate of the actual value of FFA released due to the neutralization of excessive protons by sodium hydroxide.
Fig. 2.
Comparison of lipid digestion kinetics from PMFs-loaded HIPE-complexes and PMFs in MCT oil expressed as the percentage of FFA as a function of time.
On the other hand, the percentage of FFA released in bulk oil was much lower (about 76% FFA released). The faster digestion kinetics of the oil phase after being emulsified by WPI-LMP complexes was attributed to the greater surface area of emulsion droplets compared to the bulk oil phase. It is also reported that the rate and extent of lipid digestion plays an important role in the dissolution and bioaccessibility of lipophilic bioactives (Ozturk et al., 2015, Verwei et al., 2016). In agreement with these studies, the improvement of lipid digestion in HIPE-complexes resulted in an increase of the bioaccessibility of two major PMFs identified in the citrus peel extract, i.e. TAN and NOB.
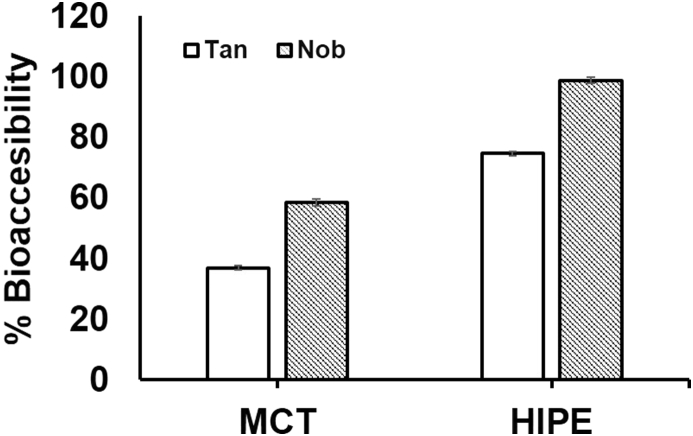
The dissolution state of bioactive molecules in the lipid phase and during the micellization is very important to ensure the availability of the compounds for absorption in the epithelial cells of the small intestines. In this lipolysis study, 2% PMFs were well dissolved which was a prerequisite to have a good incorporation of PMFs in the formed micelles. The presence of bioactive crystals might reduce the bioavailability of the selected component due to the fact that they might not be absorbed in this form into the GI tract. Besides, the crystals could also affect the physical stability of the emulsion system (McClements, 2018). Fig. 3 reveals that the HIPE-complexes system improved the bioaccessibility of two major PMFs (i.e. TAN and NOB) compared to the bioaccessibility of those compounds in the bulk oil. The bioaccessibility of TAN and NOB as the major PMFs in HIPE-complexes was approximately 2 and 1.5 fold from this in the bulk oil, respectively. Ting et al. (Li et al., 2012a) reported that the bioaccessibility of TAN in MCT oil was poor compared to that in a 50% O/W emulsion system during a similar lipolysis study at fasted state. In addition, NOB was observed to have a better solubility in the oil phase compared to TAN at the original input concentration. According to Raman et al. (2005), NOB has more apolar characteristics due to more methoxylated groups compared to TAN, which makes it more soluble in the oil medium.
Fig. 3.
Bioaccessibility of PMFs (i.e. TAN and NOB) from HIPE-complexes and MCT oil during 1 h pH-stat lipolysis.
As a simplified digestion model, the pH-stat lipolysis model only observed the lipid digestion based on sodium hydroxide titration into the digestion buffer. This model may also control the pH, temperature, initial endogenous bile salts and pancreatin concentrations for digestion. However, some important factors in intestinal digestion events were neglected such as intestinal transit, dynamic bile salts and enzymes secretion, peristaltic movements, and absorption of digestion fluids. In the next discussion, the bioaccessibility of the PMFs was evaluated using TNO's GI model, a dynamic in-vitro digestion simulation, in which the other important factors in human digestion events are well considered.
3.4. Bioaccessibility of PMFs using TNO's GI model
In this study, HIPE stabilized by WPI-LMP complexes and MCT oil samples containing PMFs were fed into a computer programmed in vitro digestion simulation. These samples underwent digestion events in the upper GI tract in which pre-absorption factors, such as temperature, pH, gastric emptying time, enzymatic and bile salts concentrations, as well as peristaltic movements, are controlled. These parameters should be considered to significantly influence the amount of orally ingested nutrients or nutraceuticals that becomes bioavailable [33]. The samples were collected after passing through semi-permeable capillary membranes connected to the jejunum and ileum compartment to determine the amount of PMFs that became available for absorption. From the ileum, the digested samples were secreted through the ileal valve. The samples were also collected to determine the amount of PMFs that was theoretically delivered to the colon for further biotransformation or metabolism.
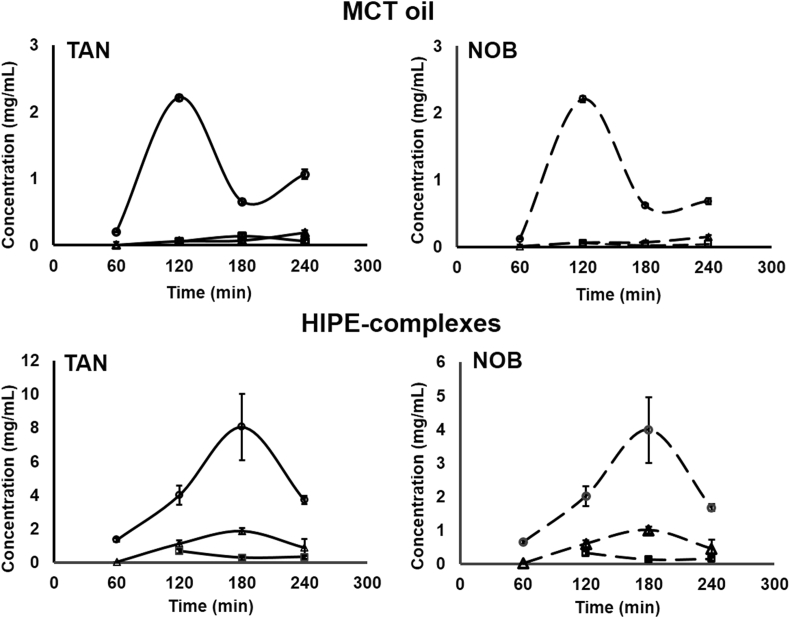
The concentration of PMFs released from HIPE-complexes and MCT oil at each intestinal section as a function of time is shown in Fig. 4. Fig. 4A and C reveals that the concentration of TAN and NOB in HIPE systems, gradually increased from 1 to 3 h at major sites of absorption, i.e. jejunum and ileum. On the other hand, the bulk oil system showed a quite low concentration of the two major PMFs in jejunum and ileum (Fig. 4B and D). The concentration of PMFs for all samples in the ileal efflux was also lower than that in previous absorption sites because most of the PMFs had been absorbed in the jejunum and ileum. Overall, the PMF concentrations in HIPE-complexes were higher than those in bulk oil, suggesting that the HIPE-complexes system could protect PMFs from the digestion by enzymes and severe acidic conditions of the gastric compartment. Moreover, HIPE-complexes could also release PMFs gradually from the emulsion in the intestinal compartments after oral administration. The highest level of TAN and NOB was found in the jejunum, indicating that this section was the main part for the absorption in the small intestines, regardless of the type of system.
Fig. 4.
Concentration of TAN (line) and NOB (dash line) absorbed at jejunum (○), ileum (Δ) and as an ileal efflux (□), as a function of time after feeding MCT oil and HIPE-complexes samples.
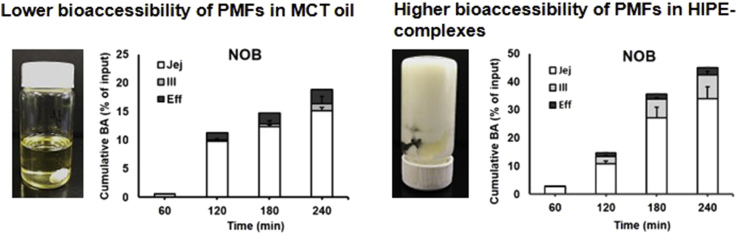
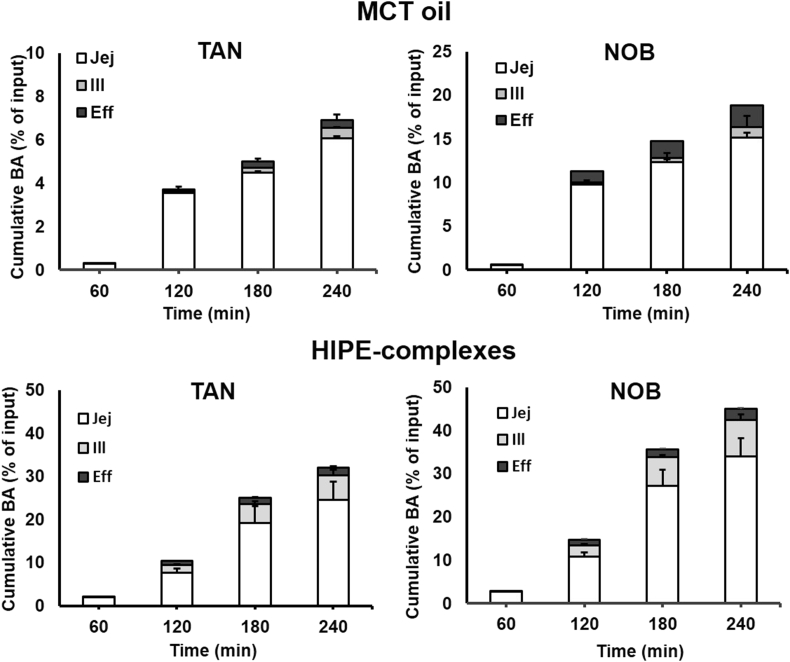
The cumulative bioaccessibility of TAN and NOB was expressed as the amount of TAN and NOB recovered from each absorption site relative to the original input weight. Fig. 5 shows that the cumulative bioaccessibility of TAN and NOB increased remarkably when they were incorporated into the HIPE system as compared to the bulk oil. As shown in Fig. 5, the mechanistic examination of TIM-1 suggested that the absorption of TAN and NOB majorly happened in the jejunum section and was continuously eliminated from the system through efflux. The results from the TIM-1 GI study indicated that the faster dissolution rate of samples in the intestinal lumen could be greatly beneficial for achieving a higher oral bioavailability, which can be achieved by incorporating PMFs into a HIPE system. This observation was again confirmed as the total cumulative bioaccessibility at two major absorption sites of TAN and NOB was 5- and 2-fold higher in the HIPE than in the MCT oil sample, indicating that the HIPE-complexes that consisted of 80% oil could efficiently be loaded by a relatively high amount of PMFs. The low ratio of PMFs to MCT oil as dissolution medium could also prevent crystallization of PMFs since PMFs have a limited solubility in MCT oil (Li et al., 2012a), suggesting the impact of solubility on the rapid absorption and the higher bioaccessibility of PMFs.
Fig. 5.
Cumulative in-vitro bioaccessibility of TAN and NOB in MCT oil was determined in jejunum, ileum and ileal efflux samples expressed as mean cumulative BA of PMFs (% of input) for each compartment and standard deviation (SD) are presented as stacked bar graphs for each time point.
During 3 h digestion of HIPE-complexes, the cumulative bioaccessibility of PMFs increased significantly to about 24% and 34% for TAN and NOB, respectively. As shown in Fig. 4, the concentration of TAN and NOB in HIPE-complexes reached a peak followed by a decrease of the concentration of both PMFs after 3 h digestion. Ribnicky et al. (2014) studied the bioaccessibility of polyphenols during 4 h digestion: the bioaccessibility reached a peak during 3 h digestion and decreased after 3 h. According to Lu et al. (2019), the bioaccessibility of curcumin in a Pickering emulsion stabilized by starch particles increased significantly to around 40% and leveled off after 4 h. Moreover, the release of over 30% of curcumin exhibited near-zero-order kinetics in the simulated conditions, suggesting that the emulsion sample gives sustained release of the encapsulated compound over a period of 4 h. In the HIPE-complexes system, the oil droplets were coated by protein-polysaccharide complexes (Wijaya et al., 2017a, Wijaya et al., 2017b). The bile salts might have to replace the complexes before the digestive enzymes could access the surface of the droplets. This restriction in the lipid hydrolysis would sustain the release of PMFs from the oil phase. Clearly, the simulated conditions using TIM-1 were quite different from the in vitro lipolysis study, where some essential parameters related to in vivo lipid digestion were not considered resulting in a significant difference in the evaluation of the PMFs’ bioaccessibility.
The higher bioaccessibility of TAN and NOB in the HIPE-complexes system compared to that of both PMFs in the bulk oil sample was mainly due to the accessibility of digestion enzymes because of the greater surface area to volume ratio. Due to the greater surface area available for digestion enzymes, the rate of mixed micelle formation from the digestion products was greater, as it was proven from the pH-stat lipolysis study. Generally, the oil phase of formulations with medium-chain TAGs can be completely digested during 1–2 h of pH-stat lipolysis (Zhang et al., 2014). According to Sarkar et al. (2018) and Pilosof (2017), the role of protein-polysaccharide complexes as emulsifier could facilitate and modulate lipolysis as well as lipid absorption. In addition, protein-polysaccharide complexes could also improve the lipophilic nutraceutical bioaccessibility compared to protein-stabilized emulsions through the irreversible adsorption of the particles to the oil/water interface, which are not easily displaced by bile salts as a bio-surfactant (Sah et al., 2016). The interpolymeric gel network around the emulsion droplets formed by electrostatic interactions could serve as a strong mechanical barrier at the oil/water interface (Wijaya et al., 2018a, Wijaya et al., 2018b, Wijaya et al., 2019). In addition, the complexation of proteins with non-digested polysaccharides like pectin might also hinder the disruption of the droplet's surface by pepsin in the stomach (Qin et al., 2016). Hence, intact droplets remained after gastric digestion, proven by the evidence of efficient lipid digestion in HIPE-complexes compared to MCT oil due to a larger surface area to volume ratio. According to Xu et al. (2014), the stability against droplet flocculation and coalescence during gastric and small intestine digestion was increased for the WPI–beet pectin complexes and conjugate stabilized emulsion. Interestingly, this interpolymeric network showed a pH-responsive behavior, where it dissociates at a high pH (Wijaya et al., 2017c). This behavior makes the emulsion droplets physically stable towards coalescence and lipolysis under low pH (around or below the isoelectric point of the protein) and releases the oil droplets at pH conditions above the isoelectric point of the protein during exposure at neutral pH of intestines.
WPI which formed a complex with LMP might be more resistant to enzymatic digestion, especially to pepsinolysis at gastric pH. This hypothesis is in close agreement to that of Li et al. (2012b) who reported that chia protein-gum complexes and lactoferrin-pectin complexes were able to protect the proteins from proteolysis. Furthermore, at the pH of the small intestines, the pectin might (partly) become dissociated from the complexes, which would made the lipid droplets more accessible to proteolysis. The remaining complexes, free protein and pectin would be further digested in the large intestines by microbiota and relevant enzymes. These protein and pectin metabolites are largely absorbed through the large intestinal epithelial cells, while others are released in feces in large amounts (Davila et al., 2013, Holloway et al., 1983).
4. Conclusions
In this work, we isolated PMFs from citrus peel powder and incorporated them into HIPE stabilized by WPI-LMP complexes. The WPI-LMP complexes which had a Z-average diameter of 350 nm were used to stabilize the HIPE (φoil = 0.82) resulting in a stable emulsion without any evidence of coalescence as microstructurally observed. The bioaccessibility of two major PMFs (i.e. TAN and NOB) in HIPE-complexes and MCT oil (as a reference) was evaluated using an in vitro lipolysis and a dynamic in-vitro digestion model. The in vitro lipolysis study showed that the lipid in HIPE-complexes was digested faster than in MCT oil, which resulted in a higher bioaccessibility of TAN and NOB in HIPE-complexes than in MCT oil. However, a more adequate digestion simulation which better represented the complexity of the human digestion system was needed to provide a reliable and meaningful result for a crystalline bioactive using a high fraction of the lipid-based delivery formulation. According to the TIM-1 results, PMFs tended towards being more bioaccessible when incorporated into the HIPE-complexes compared to PMFs in MCT oil calculated from two major absorption sites, i.e. jejunum and ileum. The improved digestion of the oil phase greatly contributed to the release of both PMFs, which might explain the higher bioaccessibility of the PMFs in HIPE-complexes. In vivo assessment may still need to be conducted to further confirm the in vitro-in vivo correlations since the advanced TIM-1 model also has some limitations: it does not fully capture complex endothelial absorption events nor the presence of microorganisms in the upper GI tract (Minekus et al., 2015). Nevertheless, TIM-1 enabled the assessment of the impact of HIPE stabilized by protein-polysaccharide complexes on the PMFs bioaccessibility from gastric ingestion to pre-colonic delivery, whereby this assessment could contribute to a more relevant comparison for the in vivo studies. Moreover, this concentrated lipid-based delivery system could be used as a strategy to ensure an optimal therapeutic bioactivity via a high loading of lipophilic crystalline compounds.
Author Contributions
Wahyu Wijaya: Conceptualization, Methodology, Validation, Formal Analysis, Investigation, Writing – Original Draft, Writing – Reviewing & Editing, Visualization, Funding Acquisition.
Huijuan Zhang: Methodology, Investigation.
Ting Zheng and Shiwei Su: Investigation.
Ashok R. Patel: Supervision, Writing – Reviewing & Editing, Funding Acquisition.
Paul Van der Meeren: Supervision, Writing – Reviewing & Editing, Project Administration, Funding Acquisition.
Qingrong Huang: Supervision, Conceptualization, Writing – Reviewing & Editing, Resources, Project Administration, Funding Acquisition.
Declaration of Competing Interest
We have no conflict of interest in this research.
Acknowledgment
The first author would like to thank the BOF (Special Research Fund) and CWO (Research Mobility Fund) of Ghent University for providing financial support. This work was also supported by United State Department of Agriculture, National Institute of Food and Agriculture (grant No. 2019-67017-29176). Man Zhang is acknowledged for providing useful scientific discussion in the isolation and identification of PMFs.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2019.11.007.
Contributor Information
Wahyu Wijaya, Email: wahwi@dtu.dk.
Ashok R. Patel, Email: ashok.patel@gtiit.edu.cn.
Paul Van der Meeren, Email: paul.vandermeeren@ugent.be.
Qingrong Huang, Email: qhuang@sebs.rutgers.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Calligaris S., Comuzzo P., Bot F., Lippe G., Zironi R., Anese M., Nicoli M.C. Nanoemulsions as delivery systems of hydrophobic silybin from silymarin extract: effect of oil type on silybin solubility, in vitro bioaccessibility and stability. LWT - Food Sci. Technol. 2015;63(1):77–84. doi: 10.1016/j.lwt.2015.03.091. [DOI] [Google Scholar]
- Chen E., Wu S., McClements D.J., Li B., Li Y. Influence of pH and cinnamaldehyde on the physical stability and lipolysis of whey protein isolate-stabilized emulsions. Food Hydrocoll. 2017;69:103–110. doi: 10.1016/j.foodhyd.2017.01.028. [DOI] [Google Scholar]
- Davila A.M., Blachier F., Gotteland M., Andriamihaja M., Benetti P.H., Sanz Y., Tomé D. Re-print of intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host. Pharmacol. Res. 2013;69(1):114–126. doi: 10.1016/j.phrs.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Faqueti L.G., Brieudes V., Halabalaki M., Skaltsounis A.L., Nascimento L.F., Barros W.M., Santos A.R.S., Biavatti M.W. Antinociceptive and anti-inflammatory activities of standardized extract of polymethoxyflavones from Ageratum conyzoides. J. Ethnopharmacol. 2016;194:369–377. doi: 10.1016/j.jep.2016.09.025. [DOI] [PubMed] [Google Scholar]
- Gosslau A., Kuang Y.C., Ho C.T., Shiming L. Anti-inflammatory effects of characterized orange peel extracts enriched with bioactive polymethoxyflavones. Food Sci. Hum. Wellness. 2014:26–35. doi: 10.1016/j.fshw.2014.02.002. [DOI] [Google Scholar]
- Gosslau A., Chen K.Y., Ho C.T., Li S. Anti-inflammatory effects of characterized orange peel extracts enriched with bioactive polymethoxyflavones. Food Sci. Hum. Wellness. 2014;3(1):26–35. [Google Scholar]
- Holloway W.D., Tasman-Jones C., Maher K. Pectin digestion in humans. Am. J. Clin. Nutr. 1983;37(2):253–255. doi: 10.1093/ajcn/37.2.253. [DOI] [PubMed] [Google Scholar]
- Kim T.W., Lee D.R., Choi B.K., Kang H.K., Jung J.Y., Lim S.W., Yang S.H., Suh J.W. Hepatoprotective effects of polymethoxyflavones against acute and chronic carbon tetrachloride intoxication. Food Chem. Toxicol. 2016;91:91–99. doi: 10.1016/j.fct.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Li S., Lambros T., Wang Z., Goodnow R., Ho C.T. Efficient and scalable method in isolation of polymethoxyflavones from orange peel extract by supercritical fluid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007;1–2:291–297. doi: 10.1016/j.jchromb.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Li S., Lo C.Y., Dushenkov S., Ho C.T. Polymethoxyflavones: chemistry, biological activity, and occurrence in orange peel. Diet. Suppl. 2008:191–210. doi: 10.1021/bk-2008-0987.ch013. [DOI] [Google Scholar]
- Li Y., Hu M., McClements D.J. Factors affecting lipase digestibility of emulsified lipids using an in vitro digestion model: proposal for a standardised pH-stat method. Food Chem. 2011;126(2):498–505. doi: 10.1016/j.foodchem.2010.11.027. [DOI] [Google Scholar]
- Li Y., Xiao H., McClements D.J. Encapsulation and delivery of crystalline hydrophobic nutraceuticals using nanoemulsions: factors affecting polymethoxyflavone solubility. Food Biophys. 2012;7(4):341–353. doi: 10.1007/s11483-012-9272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ye A., Je Lee S., Singh H. Influence of gastric digestive reaction on subsequent in vitro intestinal digestion of sodium caseinate-stabilized emulsions. Food Funct. 2012;3(3):320–326. doi: 10.1039/c2fo10242k. [DOI] [PubMed] [Google Scholar]
- Lu X., Zhu J., Pan Y., Huang Q. Assessment of dynamic bioaccessibility of curcumin encapsulated in milled starch particle stabilized Pickering emulsions using TNO's gastrointestinal model. Food Funct. 2019;10(5):2583–2594. doi: 10.1039/c8fo02495b. [DOI] [PubMed] [Google Scholar]
- Lyng E., Havenaar R., Shastri P., Hetsco L., Vick A., Sagart J. Increased bioavailability of celecoxib under fed versus fasted conditions is determined by postprandial bile secretion as demonstrated in a dynamic gastrointestinal model. Drug Dev. Ind. Pharm. 2016;42(8):1334–1339. doi: 10.3109/03639045.2015.1135935. [DOI] [PubMed] [Google Scholar]
- Manthey J.A., Guthrie N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J. Agric. Food Chem. 2002;50:5837–5843. doi: 10.1021/jf020121d. [DOI] [PubMed] [Google Scholar]
- McClements D.J. Enhanced delivery of lipophilic bioactives using emulsions: a review of major factors affecting vitamin, nutraceutical, and lipid bioaccessibility. Food Funct. 2018;9(1):22–41. doi: 10.1039/c7fo01515a. [DOI] [PubMed] [Google Scholar]
- Minekus M., Verhoeckx K., Cotter P., López-Expósito I., Kleiveland C., Lea T., Mackie A., Requena T., Swiatecka D., Wichers H. 2015. The impact of food bioactives on health. in vitro and ex vivo models; pp. 37–46. [DOI] [PubMed] [Google Scholar]
- Mun S., Decker E.A., Park Y., Weiss J., McClements D.J. Influence of interfacial composition on in vitro digestibility of emulsified lipids: potential mechanism for chitosan's ability to inhibit fat digestion. Food Biophys. 2006;1(1):21–29. [Google Scholar]
- Mun S., Kim Y.R., Shin M., McClements D.J. Control of lipid digestion and nutraceutical bioaccessibility using starch-based filled hydrogels: influence of starch and surfactant type. Food Hydrocolloids. 2015;44:380–389. [Google Scholar]
- Naylor T.A., Connolly P.C., Martini L.G., Elder D.P., Minekus M., Havenaar R., Zeijdner E. Use of a gastro-intestinal model and Gastroplus™ for the prediction of in vivo performance. J. Appl. Ther. Res. 2006;6(1):15. [Google Scholar]
- Ozturk B., Argin S., Ozilgen M., McClements D.J. Nanoemulsion delivery systems for oil-soluble vitamins: influence of carrier oil type on lipid digestion and vitamin D3 bioaccessibility. Food Chem. 2015;187:499–506. doi: 10.1016/j.foodchem.2015.04.065. [DOI] [PubMed] [Google Scholar]
- Pilosof A.M.R. Potential impact of interfacial composition of proteins and polysaccharides stabilized emulsions on the modulation of lipolysis. The role of bile salts. Food Hydrocoll. 2017;68:178–185. doi: 10.1016/j.foodhyd.2016.08.030. [DOI] [Google Scholar]
- Qin D., Yang X., Gao S., Yao J., McClements D.J. Influence of hydrocolloids (dietary fibers) on lipid digestion of protein-stabilized emulsions: comparison of neutral, anionic, and cationic polysaccharides. J. Food Sci. 2016;81(7):C1636–C1645. doi: 10.1111/1750-3841.13361. [DOI] [PubMed] [Google Scholar]
- Raman G., Jayaprakasha G.K., Cho M., Brodbelt J., Patil B.S. Rapid adsorptive separation of citrus polymethoxylated flavones in non-aqueous conditions. Separ. Purif. Technol. 2005;45(2):147–152. [Google Scholar]
- Ribnicky D.M., Roopchand D.E., Oren A., Grace M., Poulev A., Lila M.A., Havenaar R., Raskin I. Effects of a high fat meal matrix and protein complexation on the bioaccessibility of blueberry anthocyanins using the TNO gastrointestinal model (TIM-1) Food Chem. 2014;142:349–357. doi: 10.1016/j.foodchem.2013.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah B.N.P., McAinch A.J., Vasiljevic T. Modulation of bovine whey protein digestion in gastrointestinal tract: a comprehensive review. Int. Dairy J. 2016;62:10–18. doi: 10.1016/j.idairyj.2016.07.003. [DOI] [Google Scholar]
- Sarkar A., Ademuyiwa V., Stubley S., Esa N.H., Goycoolea F.M., Qin X., Gonzalez F., Olvera C. Pickering emulsions co-stabilized by composite protein/polysaccharide particle-particle interfaces: impact on in vitro gastric stability. Food Hydrocoll. 2018;84:282–291. [Google Scholar]
- Singh S.P., Tewari D., Patel K., Jain G.K. Permeability determination and pharmacokinetic study of nobiletin in rat plasma and brain by validated high-performance liquid chromatography method. Fitoterapia. 2011;82:1206–1214. doi: 10.1016/j.fitote.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Ting Y., Li C.C., Wang Y., Ho C.T., Huang Q. Influence of processing parameters on morphology of polymethoxyflavone in emulsions. J. Agric. Food Chem. 2015;63(2):652–659. doi: 10.1021/jf504465a. [DOI] [PubMed] [Google Scholar]
- Ting Y., Jiang Y., Lan Y., Xia C., Lin Z., Rogers M.A., Huang Q. Viscoelastic emulsion improved the bioaccessibility and oral bioavailability of crystalline compound: a mechanistic study using in vitro and in vivo models. Mol. Pharm. 2015;12(7):2229–2236. doi: 10.1021/mp5007322. [DOI] [PubMed] [Google Scholar]
- Tokle T., Lesmes U., Decker E.A., McClements D.J. Impact of dietary fiber coatings on behavior of protein-stabilized lipid droplets under simulated gastrointestinal conditions. Food Funct. 2012;3(1):58–66. doi: 10.1039/c1fo10129c. [DOI] [PubMed] [Google Scholar]
- Tung Y.C., Li S., Huang Q., Hung W.L., Ho C.T., Wei G.J., Pan M.H. 5-Demethylnobiletin and 5-acetoxy-6, 7, 8, 3′, 4′-pentamethoxyflavone suppress lipid accumulation by activating the LKB1-AMPK pathway in 3T3-L1 preadipocytes and high fat diet-fed C57BL/6 mice. J. Agric. Food Chem. 2016;64(16):3196–3205. doi: 10.1021/acs.jafc.6b00706. [DOI] [PubMed] [Google Scholar]
- Verwei M., Minekus M., Zeijdner E., Schilderink R., Havenaar R. Evaluation of two dynamic in vitro models simulating fasted and fed state conditions in the upper gastrointestinal tract (TIM-1 and tiny-TIM) for investigating the bioaccessibility of pharmaceutical compounds from oral dosage forms. Int. J. Pharm. 2016;498(1-2):178–186. doi: 10.1016/j.ijpharm.2015.11.048. [DOI] [PubMed] [Google Scholar]
- Wang L., Wang J., Fang L., Zheng Z., Zhi D., Wang S., Li S., Ho C.T., Zhao H. Anticancer activities of citrus peel polymethoxyflavones related to angiogenesis and others. BioMed Res. Int. 2014 doi: 10.1155/2014/453972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijaya W., Van der Meeren P., Wijaya C.H., Patel A.R. High internal phase emulsions stabilized solely by whey protein isolate-low methoxyl pectin complexes: effect of pH and polymer concentration. Food Funct. 2017;8(2):584–594. doi: 10.1039/c6fo01027j. [DOI] [PubMed] [Google Scholar]
- Wijaya W., Patel A.R., Setiowati A.D., Van der Meeren P. Functional colloids from proteins and polysaccharides for food applications. Trends Food Sci. Technol. 2017;68:56–69. [Google Scholar]
- Wijaya W., Van der Meeren P., Patel A.R. Cold-set gelation of whey protein isolate and low-methoxyl pectin at low pH. Food Hydrocoll. 2017;65:35–45. [Google Scholar]
- Wijaya W., Van der Meeren P., Patel A.R. Oleogels from emulsion (HIPE) templates stabilized by protein–polysaccharide complexes. In: Patel A.R., editor. Edible Oil Structuring. RSC; Cambridge: 2018. pp. 175–197. [Google Scholar]
- Wijaya W., Van der Meeren P., Dewettinck K., Patel A.R. High internal phase emulsion (HIPE)-templated biopolymeric oleofilms containing an ultra-high concentration of edible liquid oil. Food Funct. 2018;9(4):1993–1997. doi: 10.1039/C7FO01945A. [DOI] [PubMed] [Google Scholar]
- Wijaya W., Qing-Qing S., Vermeir L., Dewettinck K., Patel A.R., Van der Meeren P. pH and protein to polysaccharide ratio control the structural properties and viscoelastic network of HIPE-templated biopolymeric oleogels. Food Struct. 2019;21:100112. [Google Scholar]
- Xia Z., McClements D.J., Xiao H. Influence of physical state of β-carotene (crystallized versus solubilized) on bioaccessibility. J. Agric. Food Chem. 2015;63(3):990–997. doi: 10.1021/jf504673v. [DOI] [PubMed] [Google Scholar]
- Xiao J., Capanoglu E., Jassbi A.R., Miron A. Advance on the flavonoid C-glycosides and health benefits. Crit. Rev. Food Sci. Nutr. 2016;56 doi: 10.1080/10408398.2015.1067595. [DOI] [PubMed] [Google Scholar]
- Xu D., Yuan F., Gao Y., Panya A., McClements D.J., Decker E.A. Influence of whey protein–beet pectin conjugate on the properties and digestibility of β-carotene emulsion during in vitro digestion. Food Chem. 2014;156:374–379. doi: 10.1016/j.foodchem.2014.02.019. [DOI] [PubMed] [Google Scholar]
- Yang Y., Chengying Z., Chen J., Tian G., McClements D.J., Xiao H., Zheng J. Encapsulation of polymethoxyflavones in citrus oil emulsion-based delivery systems. J. Agric. Food Chem. 2017;65(8):1732–1739. doi: 10.1021/acs.jafc.7b00147. [DOI] [PubMed] [Google Scholar]
- Yu H., Shi K., Liu D., Huang Q. Development of a food-grade organogel with high bioaccessibility and loading of curcuminoids. Food Chem. 2012;131(1):48–54. doi: 10.1016/j.foodchem.2011.08.027. [DOI] [Google Scholar]
- Zhang J., Lv Y., Zhao S., Wang B., Tan M., Xie H., Lv G., Ma X. Effect of lipolysis on drug release from self-microemulsifying drug delivery systems (SMEDDS) with different core/shell drug location. AAPS PharmSciTech. 2014;15(3):731–740. doi: 10.1208/s12249-014-0096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.