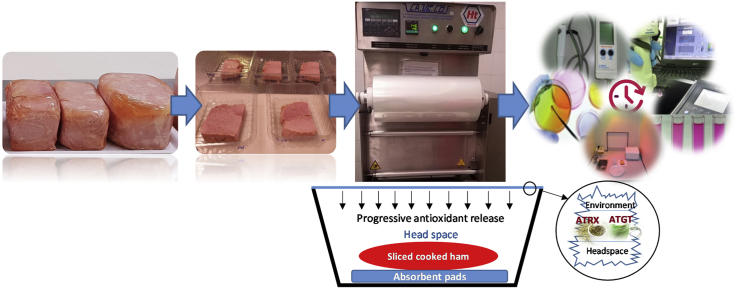
Abstract
The effectiveness of active packaging systems with green tea extract and oregano essential oil was checked for their use in sliced cooked ham. Three packaging systems were evaluated: i) control group without active film, ii) ATGT packed with active film of green tea extract (1%) and iii) ATRX with a mixture of green tea extract and oregano essential oil (1%). The evolution of microbiological, physicochemical (pH, aw, colour and lipid oxidation) and sensory attributes were analysed after 0, 7, 14 and 21 days of refrigerated storage. Microbial populations were below the limits established by the European Regulations (106 UFC/g). The samples packed with ATGT showed the better antimicrobial activity against total viable counts (TVC) and lactic acid bacteria (BAL), while lower counts of Brochothrix thermosphacta was observed in ATRX film (1.48 vs. 1.78 and 2.59 UFC/g for ATRX vs. ATGT and CON, respectively). Regarding colour, low differences were found between the samples packaged with active and control films. Unlike L*, a* and b* parameters showed a progressive diminution throughout the storage in all batches, being the films that contained green tea (ATGT) were the ones that showed the less discolouration at the end of storage (8.86 vs. 8.63 and 7.50 for ATGT vs. CON and ATRX, respectively). The low fat content of this type of product and the use of anaerobic atmosphere for the packaging of cooked ham did not allow to show an antioxidant effect on lipid oxidation (values below 0.15 mg MDA/kg). Finally, the use of ATGT and ATRX did not suppose a modification of the sensorial attributes of the product, being acceptance scores under the acceptance limit during the whole display.
Keywords: Active films, Green tea extract, Oregano essential oil, Shelf life, Meat product
Graphical abstract
Highlights
-
•
ATGT and ATRX could extend shelf life of sliced cured ham.
-
•
ATGT packaging system showed a preservative effect against bacterial spoilage.
-
•
ATGT was the best effective against discoloration.
-
•
Low oxidation values were observed during the whole display.
-
•
Oregano essential oil and green tea extract improved the quality of sliced cooked ham.
1. Introduction
Food trends have their origin in the new needs of consumers related to nutrition and concern for health (Carvalho et al., 2019). Thus, consumers demand natural products, which limits the industry in their use of synthetic additives in foods (Pateiro et al., 2018a, Lorenzo et al., 2018). However, due to meat products are very perishable, it is practically impossible to produce without additives, which leaving manufacturers with few options (Domínguez et al., 2019).
During decades, the use of preservatives and antioxidants in foods were the main strategy to prevent their rapid deterioration and ensure the absence of pathogenic microorganisms. Nowadays, the use of active packaging provides alternatives to limit meat deterioration more efficiently and allows extend its shelf life or improving its sensory properties, while maintaining product quality (Domínguez et al., 2018). Thus, it is a change in the traditional concept of packaging, since it is considered a passive barrier that delays the adverse effect of the environment on the packaged product to make positive the changes that occur during the life of the packaged product.
Active packaging is based on the incorporation of substances that interact with the food and/or the surrounding environment to improve its conservation and extend its shelf life. The active component can be applied to the package formed part of the structure of the packaging material (Domínguez et al., 2018), which has the advantage that the entire surface of the active component comes into contact with the product and that the consumer does not find any foreign element in the product.
According to the active packaging action, they could be subdivide in antimicrobial effect, which are capable of releasing substances that act effectively on microorganisms, limiting their growth by prolonging the latency phase or antioxidant releasers which reduce oxidation processes, improve oxidative stability of lipids and prolong shelf life. Recently there is a growing interest in the use of natural active compounds (as essential oils or polyphenol-rich extracts) in the production of active films, which limit the use of synthetic additives and extending the shelf life through controlled migration of the active substances into the food (Ramos et al., 2012). Several studies have demonstrated the effectiveness in the use of plant extracts and essential oils as part of the packaging material (Lorenzo et al., 2014, Camo et al., 2008, Llana-Ruiz-Cabello et al., 2018). Additionally, it is well known that some natural extracts presented both, antimicrobial and antioxidant activities, which allows double protective function. To this regard, multiple researches found both activities in plant extracts (Al-Rifai et al., 2017, de Araújo et al., 2014, Angiolella et al., 2018) and essential oils (Giweli et al., 2012, Kačániová et al., 2017). Thus, the use of these natural compounds allows reduce synthetic additives and also allows to meet consumer demand (Schumann and Schmid, 2018).
Therefore, the aim of this study was to evaluate the effect of two natural antioxidants (oregano essential oil and green tea extract) incorporated to an active package during shelf life of sliced cooked ham.
2. Material and methods
2.1. Manufacture of cooked ham
The pieces of cooked ham were manufactured in the pilot plant of the Meat Technology Center of Galicia (San Cibrao das Viñas, Ourense, Spain). Fresh pieces of pork legs were purchased at local market. The whole pieces were deboned and cleaned of connective tissue in order to facilitate the brine penetration. Then pork legs were injected with 2% brine solution containing sodium chloride (10.8%), dextrose (3%), polyphosphates (1.8%), carrageenan (1.8%), ascorbic acid (0.6%), ham aroma (0.6%), sodium nitrite (0.3%) and colour additive (0.05%). Injection was performed using an injector machine at 2–4 bars and 7 °C. After the injection process a short period of time was necessary in order to obtain an adequate brine homogenization inside the piece. Afterward, a maceration process for 5 h (under vacuum, at 5 °C of temperature and cycles of movement each 20 min) was carried out. Then, ham pieces were packed on vacuum plastic bags and cooked in a cooking kettle until reach an internal temperature of 75 °C. Finally, after the cooking stage, cooked ham was refrigerated until reach an internal temperature of 6 °C for 12 h.
2.2. Storage conditions
Cooked ham pieces were cut in slices (2 cm thickness) and stored in polystyrene tray. Samples were randomly divided into three batches. The first batch (control) was packaged without active film; the second batch was packaged with active film contained 1% of a green tea extract (ATGT) (Baceiredo, Vitoria, Spain), and the third batch was packaged with active film contained a mixture of 1% of green tea extract and of an oregano essential oil (ATRX) (Argolide Química, Barcelona, Spain). The active packaging was prepared by ARTIBAL, S.A. (Sabiñánigo, Spain) under European Patent EP00380302.4. The antioxidant films consisted of a PET/PE/EVOH/PE suitable for use as food packaging with an organic solvent base (ethyl acetate) coating of food-contact approved acrylic base, containing different known concentrations (w/w) of both the natural essential oil (ATRX) and solid extract (ATGT). The grammage of the coating on the film was between 1.5 and 2.0 g/m2. All cooked ham slices were packaged using a packaging machine (CAVECO, Milano, Italy) with a gas mixture of 70% N2/30% CO2 supplied by PRAXAIR (Madrid, Spain).
All packs were stored at 2 ± 1 °C (simulating retail conditions at supermarkets in a refrigeration chamber). This chamber was illuminated by a standard supermarket fluorescent lamp. The samples in the chamber were rotated every 24 h to minimize light intensity differences and possible temperature variations on the surface of the meat. Twelve samples (four of each batch) were removed from the chamber at 0, 7, 14 and 21 days of storage for microbial, sensorial and physico-chemical analysis.
2.3. Microbial analysis of sliced cooked ham
Ten grams of sliced cooked ham were aseptically weighed and homogenized with 90 mL of 0.1% sterile peptone water in a masticator blender (IUL Instruments, Barcelona, Spain) for 2 min at room temperature. Serial decimal dilutions were prepared for each sample in 0.1% peptone solutions (Merck, Darmstadt, Germany), and 1 mL or 0.1 mL of the samples in appropriate dilutions, in duplicate, were poured and spread for total count and selective agar plates, respectively.
Total viable counts (TVC) were enumerated in Plate Count Agar (PCA; Oxoid, Unipath Ltd., Basingstoke, UK) and incubated at 30 °C for 48 h; Lactic acid bacteria (LAB) on the Man Rogosa Sharpe agar (Oxoid, Unipath Ltd., Basingstoke, UK) (pH 5.6), after incubation at 30 °C for 5 days; Enterobacteriaceae on Violet Red Bile Glucose agar (Merck, Darmstadt, Germany) after incubation at 37 °C for 24 h; Brochothrix thermosphacta enumeration, 0.1 ml sample of each dissolution was spread on the surface of STAA Agar Base (Oxoid, Unipath Ltd., Basingstoke, UK) with STAA Selective Supplement (Oxoid, Unipath Ltd., Basingstoke, UK) and incubated at 25 °C for 72 h; Sulphite-reducing clostridia were enumerated on the Sulphite Polymyxin Sulfadiazine Agar (Merck, Darmstadt, Germany) with the plates incubated under anaerobic conditions at 44 °C for 24 h. Staphylococcus aureus were seeded on the surface of Baird Parker and TSA; Listeria monocytogenes and Salmonella was done in depth and the culture medium used was TSA, and they were determined only on the day of packaging. Plates with 30–300 colonies were counted and microbiological data were transformed into logarithms of the number of colony forming units (CFU/g).
2.4. pH, colour parameters and water activity of sliced cooked ham
A portable pH-meter (Hanna Instruments, Eibar, Spain) equipped with a penetration probe was used to measure the pH of the samples. The colour of slices of cooked ham were measured with a portable colorimeter (Konica Minolta CM-600d, Osaka, Japan) at 0° viewing angle geometry and 8 mm aperture size equipped with pulsed xenon arc lamp filtered to illuminant D65 lighting. The colour parameters lightness (L*), redness, (a*) and yellowness (b*) were estimated in three different points of each sample. A white ceramic tile was used to adjust the colorimeter before each series of measurements.
Water activity (aw) was measured using a Decagon Aqualab water activity meter (Decagon Devices Inc., Pullman, WA, USA), previously calibrated with sodium chloride.
2.5. Chemical composition of sliced cooked ham
Moisture (ISO 1442, 1997), protein (ISO 937, 1978) and ash (ISO 936, 1998) were quantified according to the ISO recommended standards, while total fat was extracted according to the AOCS Official Procedure Am 5-04 (Official Proce, 2005).
2.6. Lipid oxidation of sliced cooked ham
Lipid oxidation was evaluated through TBARS index according to the method proposed by Vyncke (1975). Thiobarbituric acid reactive substances (TBARS) values were calculated from a standard curve of malonaldehyde with 1,1,3,3-tetraethoxipropane (TEP) and expressed as mg MDA/kg sample.
2.7. Sensory evaluation of sliced cooked ham
The sensory acceptance of sliced cooked ham storage in different active packaging system was evaluated by a panel composed by 18 panellists from Meat Technology Center of Galicia (San Cibrao das Viñas, Ourense, Spain), who are usually cooked ham consumers. Sensory evaluation was carried out to analyse the degree of acceptability (liked or disliked) of the cooked ham stored in the different active trays during shelf life. The acceptance test was carried out using a hedonic scale structured in 5-point (1 = excellent and 5 = not acceptable) (Lago et al., 2017). A score of 3 was established as an acceptability limit which would mean a rejection of the product by the consumer (Camo et al., 2011). Colour, surface discoloration and odour were the sensory attributes evaluated by panellists. The sensory sessions were carried out at 0, 7, 14 and 21 days of storage.
2.8. Statistical analysis
A total of 48 samples were used in the present study (4 replicated of sliced cooked ham x 3 batches x 4 sampling points). Normal distribution and variance homogeneity were previously tested (Shapiro–Wilk). Data from microbial analysis, physicochemical parameters (pH, colour, aw) and TBARs were examined using an analysis of variance (ANOVA) with the mixed-model, where these parameters were included in the model as dependent variables, while the type of packaging was included as fixed effect and manufacture process as random effect. The pairwise differences between least squares means were evaluated by Duncan's test. Differences were considered significant at P < 0.05. All statistical analysis were achieved using IBM SPSS statistics for Windows (version 19.0. IBM Corp., New York, NY, USA).
On the other hand, panellist scores (like/dislike) were transformed into numerical values following the hedonic scale (1/5). After that, sensory scores treatment (means and standard deviation) as well as ANOVA test (Tukey) were carried out using XLSTAT for Windows version 2018 (Addinsoft, Paris, France).
3. Results and discussion
3.1. Effect of active packaging on microbial counts during storage time
The low salt content (1.51 g/100 g), pH near 6 (6.57) and high water activity (0.971) which characterized cooked ham make it susceptible to microbial growth, being able to reduce its shelf life (Baños et al., 2012). In the present study, the evolution of total viable counts (TVC) and lactic acid bacteria (LAB) during storage time of sliced cooked ham are shown in Fig. 1. Significant differences (P < 0.05) were observed for the aforementioned counts during storage time and packaging treatment. As expected, microbial counts increased during the 21 days of storage. This in agreement with the results found in other products that used similar packaging materials (Lorenzo et al., 2014).
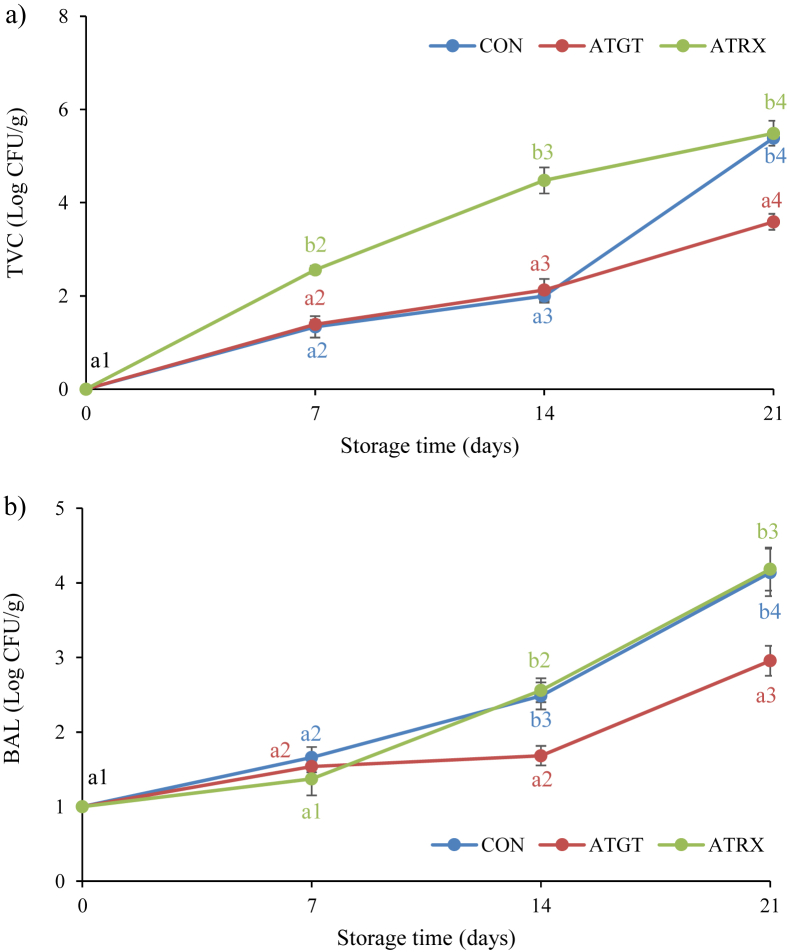
Fig. 1.
Evolution of total viable counts (TVC) (a) and lactic acid bacteria (LAB) (b) during storage time of sliced cooked ham.
TVC is regulated by the European regulation regarding microbiological criteria for foodstuffs (The Commission of the Eur, 2005). This law establishes that the microbial counts for TVC must be less than 106 CFU/g. Final counts reached values between 3.59 and 5.49 CFU/g at 21 days of storage. The values found in CON samples were similar to those found by other authors in the same product (Liu et al., 2012). The lowest values were found in samples packaged with ATGT films, which would confirm the antimicrobial activity associated with compounds of green tea extracts, as reported previously by other authors (An et al., 2004).
Regarding BAL, in the first 7 days of storage the counts were very similar to the three batches to differentiate significantly after 14 days of storage until the end of display. As happened with TVC, samples packaged in ATGT showed the lowest counts (2.96 vs. 4.14 and 4.18 CFU/g for ATGT vs. CON and ATRX, respectively). Phenolic compounds present in green tea extract could be the responsible of the antimicrobial effect that showed this active packaging system (Siripatrawan and Noipha, 2012). Although there are many evidences of the synergistic effect associated with many plants and essential oils (Jayasena and Jo, 2013, Pateiro et al., 2018b), in this case the composition of ATRX, a mixture of green tea extract and an oregano essential oil, could in this case have an antagonism effect. This interaction would suppose that the activity of both compounds is less than when they are individually applied (Burt, 2004). Therefore, ATRX would not improve the shelf-life of the product in which they was used.
A significant effect was also found for Brochothrix thermosphacta counts (data not showed). Samples from ATGT and ATRX exhibited lower counts during the whole display than the CON, showing values of 1.48 and 1.77 vs. 2.59 CFU/g for ATRX and ATGT vs. CON at the end of storage. The bioactive compounds present in oregano essential oils as carvacrol, thymol, ρ-cymene, γ-terpinene could be responsible for the antimicrobial activity of ATRX (Pateiro et al., 2018a, Mahmoudzadeh et al., 2017, Ramos et al., 2012), and specifically against Brochothrix thermosphacta (Llana-Ruiz-Cabello et al., 2018, Paparella et al., 2016).
Finally, Enterobacteriaceae, Sulphite-reducing Clostridia, Staphylococcus aureus, Salmonella and Listeria monocytogenes were not detected during the whole display.
3.2. Effect of active packaging on chemical and physico-chemical parameters during storage time
The influence of active films with green tea and oregano has on pH, water activity and colour parameters of sliced cooked ham during their storage in refrigerated conditions is shown in Table 1. At the initial point (day 0), an analysis of proximate composition was conducted. As expected, cooked ham of all batches presented the same chemical composition; they were characterized by 77.6 ± 0.57% of moisture, 2.35 ± 0.29% of fat, 14.14 ± 1.24% of protein and 3.04 ± 0.14% of ashes.
Table 1.
Effect of active packaging on pH, water activity and colour parameter of slice cured ham.
| Day | CON | ATGT | ATRX | SEM | Sig. | |
|---|---|---|---|---|---|---|
| pH | 0 | 6.563 | 6.603 | 6.553 | 0.02 | n.s. |
| 7 | 6.151 | 6.121 | 6.131 | 0.01 | n.s. | |
| 14 | 6.26b2 | 6.26b2 | 6.17a1 | 0.02 | * | |
| 21 | 6.21a12 | 6.26ab2 | 6.33b2 | 0.02 | * | |
| SEM | 0.04 | 0.05 | 0.04 | |||
| Sig. | *** | *** | *** | |||
| aw | 0 | 0.972 | 0.972 | 0.974 | 0.001 | n.s. |
| 7 | 0.971 | 0.971 | 0.973 | 0.001 | n.s. | |
| 14 | 0.970 | 0.974 | 0.977 | 0.001 | n.s. | |
| 21 | 0.973 | 0.973 | 0.976 | 0.001 | n.s. | |
| SEM | 0.001 | 0.001 | 0.001 | |||
| Sig. | n.s. | n.s. | n.s. | |||
| L* | 0 | 64.58b1 | 61.00a1 | 61.19a1 | 0.52 | *** |
| 7 | 65.8712 | 66.942 | 66.632 | 0.39 | n.s. | |
| 14 | 64.81a1 | 65.82b2 | 67.54c2 | 0.38 | *** | |
| 21 | 67.022 | 66.412 | 66.382 | 0.19 | n.s. | |
| SEM | 0.31 | 0.65 | 0.68 | |||
| Sig. | ** | *** | *** | |||
| a* | 0 | 10.01 | 10.773 | 11.003 | 0.32 | n.s. |
| 7 | 9.00 | 8.071 | 8.912 | 0.35 | n.s. | |
| 14 | 9.32b | 7.53a1 | 7.88a12 | 0.26 | *** | |
| 21 | 8.63 | 8.862 | 7.501 | 0.28 | n.s. | |
| SEM | 0.24 | 0.33 | 0.40 | |||
| Sig. | n.s. | *** | *** | |||
| b* | 0 | 10.01ab2 | 9.67a3 | 10.68b3 | 0.17 | * |
| 7 | 8.451 | 8.221 | 8.211 | 0.22 | n.s. | |
| 14 | 8.46a1 | 9.06b2 | 8.40a1 | 0.13 | * | |
| 21 | 8.56ab1 | 7.94a1 | 9.34b2 | 0.24 | * | |
| SEM | 0.25 | 0.19 | 0.28 | |||
| Sig. | * | *** | *** |
a-c Mean values in the same row (different batches on the same storage week) with different letter presented significant differences (P < 0.05; Duncan test); 1−3 Mean values in the same column (same batch in different weeks) with different number presented significant differences (P < 0.05; Duncan test); Sig.: significance: * (P < 0.05), ** (P < 0.01), *** (P < 0.001), ns (not significant); SEM: Standard error of the mean.
Regarding the oxidation stability, insignificant oxidation were observed in all batches and sampling points. In fact, TBARs values were <0.15 mg MDA/kg in all cases. Thus, authors decided describe the lipid oxidation behaviour in the text and not include data of TBARs values, since it does not provide any information because no differences were observed among batches or sampling points. The extremely low TBARs values are related with two factors: the main factor is the low fat content of the cooked ham. Lipids are the substrate for the production of malonaldehyde, which are the major compound detected in TBARs assay (Domínguez et al., 2019). Therefore, the low fat content determines that low amount of substrate are available for the oxidation process. Additionally, the use of anaerobic atmosphere for the packaging of cooked ham, composed by N2 and CO2, prevent the oxidation reactions. It is well known that the presence of O2 is one of the most important factor since it acts as the main component of the propagation stage of lipid oxidation (formation of peroxy radicals) and it is also a source of reactive oxygen species (Domínguez et al., 2019). Obviously, the oxidation values found in this research are well below the threshold for rancidity, set at 2 mg MDA/kg (Campo et al., 2006).
In contrast to our results, TBARs values showed a significant increase in foal meat samples packed with these active films (2% of oregano and 1% of green tea) (Lorenzo et al., 2014). Although foal meat also presented low fat value, the MAP atmosphere was composed by high oxygen content (80% O2 + 20% CO2), which confirm our hypothesis that O2 content is the most important factor in the lipid oxidation process. In that study, TBARs values of foal steaks increased from <0.1 mg MDA/kg at the initial point to around 2 mg MDA/kg at day 14 of storage. It is also important to note that oregano film showed a potent antioxidant activity, while green tea film did not present antioxidant effect and the TBARs values in this case was the same that those observed in control samples (Lorenzo et al., 2014).
At the beginning of storage no significant effect of active packaging was observed for pH values, which ranged between 6.55 and 6.60. However, a significant decrease was observed in pH values along storage time for all the analysed batches, which could be related with the growth of LAB during the storage. In similar way, other authors also reported the relation between the microbial growth and the pH diminution (Liu et al., 2012). Another possible explanation to the diminution of pH values could be the dissolution of CO2 from the modified atmosphere in the aqueous phase of the meat product (Liu et al., 2012, Leygonie et al., 2011). Thus, our results agree with those obtained by other authors, who reported similar pH values at the initial point in cooked ham and also described a progressive diminution of pH value as increase the storage time (Liu et al., 2012).
On the other hand, active packaging only affect the pH value at 14 and 21 days. To this regard, at 14 days, samples from ATRX showed significant lower values than the other two batches, while at 21 days CON samples displayed significantly (P < 0.05) lower value (6.21) than samples from ATRX batch (6.33) and ATGT which presented intermediate values (6.26). Beside these significant differences, the pH values did not follow a clear trend and they were so similar among bathes.
Throughout the storage and among the three different batches studied no significant differences were observed in the water activity values, which agree with the results reported by other researchers, who also did not found variations in aw values during storage of cooked ham during 90 days (Liu et al., 2012). In our case, the values of aw ranged in all cases between 0.970 and 0.977.
On the other hand, the colour parameters were affected by sampling point, while among batches, although had differences, they did not follow a clear trend. Colour is the most important parameter that influenced consumer acceptability due it is considered as quality indicator (Pateiro et al., 2018a). Regard to luminosity values (L*), control samples had the highest values at the initial point, while at the day 14 this value was higher in active packaging samples (65.82 and 67.54 in ATGT and ATRX respectively, vs. 64.81 in CON samples). These suggest that L* differences among batches could related with the differences inherent to the samples, and not to the effect of packaging. In contrast, a clear and significant increase of L* values was observed during storage in all batches. The initial values increased to final values between 66.38 and 67.02 at day 21. This increase could be due to the water exuded during storage. The storage stage affect the proteins, which decreased water retention in the product. This fact results in a greater dispersion of light, thus L* values increased (Pateiro et al., 2018a). In this study the Pseudomonas were not analysed, therefore we cannot affirm that the L* values increase was due to the growth Pseudomonas in the cooked ham surface. To this regard, other authors observed in fresh foal meat that the increase of Pseudomonas resulted in an increase of L* values (Lorenzo et al., 2014). These authors, who tested the same films that we used in the present research, also observed a progressive increment of L* values throughout the refrigeration storage in both, control and active packaged samples. In contrast, other authors reported that the L* values decreased during storage of cooked ham vacuum packaged (Liu et al., 2012) and in pork sausages packaged with chitosan + green tea active film (Siripatrawan and Noipha, 2012). However, pork sausages packaged with control and chitosan active film suffered a significant increment of L*, as occurs in the present research (Siripatrawan and Noipha, 2012).
In contrast to the behaviour of L*, the values of redness (a*) and yellowness (b*) decreased during storage. The a* values showed a progressive diminution throughout the storage in all batches, although this decrease was not significant in control samples. In all batches, the values decreased from around 10.5 at initial point to ≈8 at day 21. Statistical analysis only show significant differences among batches at day 14; in this case, control samples had higher a* values than the samples packed with active films (9.32 in CON vs. ≈7.7 in the other batches). Several researches reported a significant redness decreased during meat and meat products storage (Lorenzo et al., 2014, Camo et al., 2008, Liu et al., 2012, Siripatrawan and Noipha, 2012). To this regard, some studies conducted in fresh meat showed that oregano films prevent discoloration more effectively than rosemary (Camo et al., 2008) or green tea films (Lorenzo et al., 2014). In these researches, although a decrease of a* values were also reported in oregano film samples during storage, redness were much higher than both, control and active packaging with other natural extracts. In pork sausages, the use of chitosan or a mixture chitosan-green tea films also prevent the discolouration during storage (Siripatrawan and Noipha, 2012). The protective effect of films that contain natural extracts and essential oils against discolouration can be related with they had polyphenolic compounds with high antioxidant activity (Pateiro et al., 2018a). However, in the present study the use of a cooked meat product resulted in a more stable product against discolouration process than raw meat, which explain the low differences found between the samples packaged with active and control films.
Similarly, the b* values also suffered a significant reduction during storage in all samples. The initial values were between 9.67 and 10.68, while at day 21 they decreased to 7.94–9.34. The active packaging also influenced the b* values, since at initial and final sampling point ATRX samples presented the highest values, while at day 14 samples from ATGT batch had higher values than the other batches. As commented above, besides these differences were significant, samples did not showed a clear trend. Thus, the differences could be due to the samples themselves and not to the effect of packaging. In contrast to our data, b* values from other studies made with fresh foal meat (Lorenzo et al., 2014) and cooked ham (Liu et al., 2012) showed a significant increment during refrigeration storage. Other authors who tested the effect of active films in pork sausages observed differences between control samples, in which b* values increased and active packed samples in which b* values suffered a significant decreased (Siripatrawan and Noipha, 2012).
3.3. Sensory
The effect of active packaging on sensory attributes (red colour, surface discoloration and off-odour) of sliced cooked ham is showed in Fig. 2, Fig. 3. The evolution of acceptance scores of the sensory attributes was to increase over the shelf life (Fig. 2). For the three studied sensory attributes, all scores were obtained between 1 and 3 on the hedonic scale used, ranging between “excellent” and “acceptable”, respectively. The cooked ham packaged in ATRX obtained the best acceptance scores for the three studied sensorial attributes.
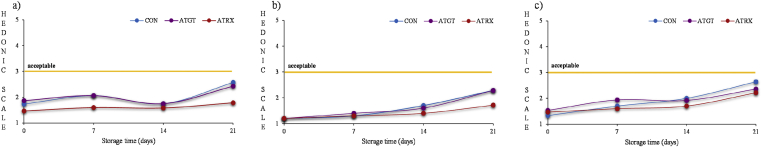
Fig. 2.
Sensory scores attributed by the panelist for colour (a), surface discolouration (b) and odour (c) during storage time of sliced cooked ham. Hedonic scale used: 1 = excellent, 2 = good, 3 = acceptable, 4 = hardly acceptable, 5 = not acceptable.
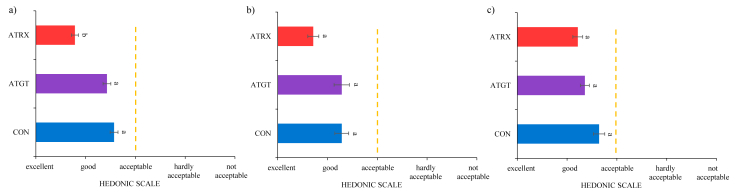
Fig. 3.
Acceptance scores for colour (a), surface discolouration (b) and odour (c) of sliced cooked hams in the last day of storage time (21 days).
Specifically, in terms of colour and surface discoloration, although the score values increased along the shelf life of sliced cooked ham, the values were below the score considered as the limit of acceptability and that would mean an obvious discoloration of the product (Djenane et al., 2003). This fact was linked with the fact that colour parameters, especially a*, decreased along the time. In the present study, as commented above, the use of a cooked meat product resulted in a more stable product against discolouration process than raw meat, which would explain the low differences found between the samples packaged with active and control films. It should be noted that the highest acceptance scores were displayed by ATRX, which could be suggest a higher antioxidant capacity of compounds present in essential oregano which would protect better the colour loss in the product than ATGT. These results are in agreement with previous results found during the shelf life of fresh meat packaged in similar antioxidant active packaging (Lorenzo et al., 2014, Camo et al., 2008).
Accordingly with other authors, TBARs values was related with oxidized lipid flavour, since values higher than 0.6 mg MDA/kg would be necessary to appreciate oxidized flavours (Martínez et al., 2006, Greene and Cumuze, 1982). This relationship between TBARs and sensory odour was also found by other authors in other meat products, where the sensory scores were higher the acceptability limit (score higher than 3) when TBARs values exceed 2.0 mg MDA/kg (de Carvalho et al., 2019). As commented before, the batches evaluated in the present study showed in all cases TBARs values lower than 0.15 mg MDA/kg. These values were far below those necessary for the detection of off-odours by the consumer, showing in all cases values below 3.
Focusing on the results obtained in the last day of the shelf life, significant (P < 0.01) differences were found among the studied batches for colour attribute (Fig. 3). The colour and odour acceptance scores observed in CON batch were close to the acceptance limit. Similar results were obtained in beef steaks packaged with films with similar added amounts of oregano extract (Camo et al., 2011). Therefore, the use of ATGT and ATRX did not suppose a modification of the sensorial attributes of the product or the consumer acceptance.
4. Conclusions
Active packaging is a good strategy which allows to extend the shelf life of meat products. In this sense, the use of ATGT or ATRX could delay the spoilage and oxidation of sliced cooked ham. These packaging systems combined with appropriate storage conditions (anaerobic conditions) allowed to preserve the quality of these cooked meat products, since decrease the microbial growth of TVC, BAL and Brochothrix thermosphacta. ATGT active packaging showed an effective antimicrobial activity against these microorganism. These lowest microbial counts also affected the colour changes that occur during the shelf life. In this way, ATGT showed the lowest discolouration during storage probably associated with the protective effect of the polyphenolic compounds that contain green tea extract. During storage, TBARs values were lower than 0.15 mg MDA/kg in all the batches, therefore they not contribute to rancid flavour. From the sensory point of view, packaging composed of mixture of oregano essential oil and green tea presented greater ability to maintain the colour, avoiding the surface discolouration and improving the odour acceptance of cooked ham than those obtained in active packaging system that contained only green tea extract.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
Authors are grateful to RTA2017-00021-00-00 (INIA-MINECO) and GAIN (Axencia Galega de Innovación, grant number IN607A2019/01) for the financial support. Jose M. Lorenzo is member of the HealthyMeat network, funded by CYTED (ref. 119RT0568). Paulo E. S. Munekata acknowledges postdoctoral fellowship support from Ministry of Economy and Competitiveness (MINECO, Spain) “Juan de la Cierva” program (FJCI-2016-29486).
References
- Al-Rifai A., Aqel A., Al-Warhi T., Wabaidur S.M., Al-Othman Z.A., Badjah-Hadj-Ahmed A.Y. Antibacterial, antioxidant activity of ethanolic plant extracts of some convolvulus species and their DART-ToF-MS profiling. Evid. Based Complement Altern. Med. 2017;2017:5694305. doi: 10.1155/2017/5694305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An B.J., Kwak J.H., Son J.H., Park J.M., Lee J.Y., Jo C., Byun M.W. Biological and anti-microbial activity of irradiated green tea polyphenols. Food Chem. 2004;88:549–555. doi: 10.1016/j.foodchem.2004.01.070. [DOI] [Google Scholar]
- Angiolella L., Sacchetti G., Efferth T. Antimicrobial and antioxidant activities of natural compounds. Evid. Based Complement Altern. Med. 2018;2018:1945179. doi: 10.1155/2018/1945179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOCS, AOCS Official Procedure Am5-04 . American Oil Chemists Society; Urbana, IL, USA: 2005. Rapid Determination of Oil/fat Utilizing High Temperature Solvent Extraction. [Google Scholar]
- Baños A., Ananou S., Martínez-Bueno M., Gálvez A., Maqueda M., Valdivia E. Prevention of spoilage by enterocin AS-48 combined with chemical preservatives, under vacuum, or modified atmosphere in a cooked ham model. Food Control. 2012;24:15–22. doi: 10.1016/J.FOODCONT.2011.08.001. [DOI] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/J.IJFOODMICRO.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Camo J., Beltrán J.A., Roncalés P. Extension of the display life of lamb with an antioxidant active packaging. Meat Sci. 2008;80:1086–1091. doi: 10.1016/j.meatsci.2008.04.031. [DOI] [PubMed] [Google Scholar]
- Camo J., Lorés A., Djenane D., Beltrán J.A., Roncalés P. Display life of beef packaged with an antioxidant active film as a function of the concentration of oregano extract. Meat Sci. 2011;88:174–178. doi: 10.1016/J.MEATSCI.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Campo M.M., Nute G.R., Hughes S.I., Enser M., Wood J.D., Richardson R.I. Flavour perception of oxidation in beef. Meat Sci. 2006;72:303–311. doi: 10.1016/j.meatsci.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Carvalho F.A.L., Pateiro M., Domínguez R., Barba-Orellana S., Mattar J., Rimac Brnčić S., Barba F.J., Lorenzo J.M. Replacement of meat by spinach on physicochemical and nutritional properties of chicken burgers. J. Food Process. Preserv. 2019;43(e13935):1–8. doi: 10.1111/jfpp.13935. [DOI] [Google Scholar]
- de Araújo K., de Lima A., Silva J., Rodrigues L., Amorim A., Quelemes P., dos Santos R., Rocha J., de Andrades É., Leite J., Mancini-Filho J., da Trindade R. Identification of phenolic compounds and evaluation of antioxidant and antimicrobial properties of Euphorbia tirucalli L. Antioxidants. 2014;3:159–175. doi: 10.3390/antiox3010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho F.A.L., Lorenzo J.M., Pateiro M., Bermúdez R., Purriños L., Trindade M.A. Effect of guarana (Paullinia cupana) seed and pitanga (Eugenia uniflora L.) leaf extracts on lamb burgers with fat replacement by chia oil emulsion during shelf life storage at 2 °C. Food Res. Int. 2019;125:108554. doi: 10.1016/J.FOODRES.2019.108554. [DOI] [PubMed] [Google Scholar]
- Djenane D., Sánchez-Escalante A., Beltrán J.A., Roncalés P. Extension of the shelf life of beef steaks packaged in a modified atmosphere by treatment with rosemary and displayed under UV-free lighting. Meat Sci. 2003;64:417–426. doi: 10.1016/S0309-1740(02)00210-3. [DOI] [PubMed] [Google Scholar]
- Domínguez R., Barba F.J., Gómez B., Putnik P., Bursać Kovačević D., Pateiro M., Santos E.M., Lorenzo J.M. Active packaging films with natural antioxidants to be used in meat industry: a review. Food Res. Int. 2018;113:93–101. doi: 10.1016/j.foodres.2018.06.073. [DOI] [PubMed] [Google Scholar]
- Domínguez R., Pateiro M., Gagaoua M., Barba F.J., Zhang W., Lorenzo J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants. 2019;8:429. doi: 10.3390/antiox8100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giweli A., Džamić A.M., Soković M., Ristić M.S., Marin P.D. Antimicrobial and antioxidant activities of essential oils of satureja thymbra growing wild in Libya. Molecules. 2012;17:4836–4850. doi: 10.3390/molecules17054836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene B.E., Cumuze T.H. Relationship between TBA numbers and inexperienced panelists' assessments of oxidized flavor in cooked beef. J. Food Sci. 1982;47:52–54. doi: 10.1111/j.1365-2621.1982.tb11025.x. [DOI] [Google Scholar]
- ISO 1442 . International Organization for Standarization; Geneva, Switzerland: 1997. International Standards Meat and Meat Products - Determination of Moisture Content. [Google Scholar]
- ISO 936 . International Organization for Standarization; Geneva, Switzerland: 1998. International Standards Meat Andmeat Products - Determination of Ash Content. [Google Scholar]
- ISO 937 . International Organization for Standarization; Geneva, Switzerland: 1978. International Standardsmeat Andmeat Products - Determination of Nitrogen Content. [Google Scholar]
- Jayasena D.D., Jo C. Essential oils as potential antimicrobial agents in meat and meat products: a review. Trends Food Sci. Technol. 2013;34:96–108. doi: 10.1016/J.TIFS.2013.09.002. [DOI] [Google Scholar]
- Kačániová M., Terentjeva M., Vukovic N., Puchalski C., Roychoudhury S., Kunová S., Klūga A., Tokár M., Kluz M., Ivanišová E. The antioxidant and antimicrobial activity of essential oils against Pseudomonas spp. isolated from fish. Saudi Pharm. J. 2017;25:1108–1116. doi: 10.1016/J.JSPS.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago A.M.T., Vidal A.C.C., Schiassi M.C.E.V., Reis T., Pimenta C., Pimenta M.E.S.G. Influence of the addition of minced fish on the preparation of fish sausage: effects on sensory properties. J. Food Sci. 2017;82:492–499. doi: 10.1111/1750-3841.13586. [DOI] [PubMed] [Google Scholar]
- Leygonie C., Britz T.J., Hoffman L.C. Protein and lipid oxidative stability of fresh ostrich M. Iliofibularis packaged under different modified atmospheric packaging conditions. Food Chem. 2011;127:1659–1667. doi: 10.1016/j.foodchem.2011.02.033. [DOI] [Google Scholar]
- Liu G., Wang Y., Gui M., Zheng H., Dai R., Li P. Combined effect of high hydrostatic pressure and enterocin LM-2 on the refrigerated shelf life of ready-to-eat sliced vacuum-packed cooked ham. Food Control. 2012;24:64–71. doi: 10.1016/j.foodcont.2011.09.004. [DOI] [Google Scholar]
- Llana-Ruiz-Cabello M., Pichardo S., Bermudez J.M., Baños A., Ariza J.J., Guillamón E., Aucejo S., Cameán A.M. Characterisation and antimicrobial activity of active polypropylene films containing oregano essential oil and Allium extract to be used in packaging for meat products. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018;35:782–791. doi: 10.1080/19440049.2017.1422282. [DOI] [PubMed] [Google Scholar]
- Lorenzo J.M., Batlle R., Gómez M. Extension of the shelf-life of foal meat with two antioxidant active packaging systems. LWT - Food Sci Technol. 2014;59:181–188. doi: 10.1016/J.LWT.2014.04.061. [DOI] [Google Scholar]
- Lorenzo J.M., Pateiro M., Domínguez R., Barba F.J., Putnik P., Kovačević D.B., Shpigelman A., Granato D., Franco D. Berries extracts as natural antioxidants in meat products: a review. Food Res. Int. 2018;106:1095–1104. doi: 10.1016/j.foodres.2017.12.005. [DOI] [PubMed] [Google Scholar]
- Mahmoudzadeh M., Hosseini H., Shahraz F., Akhondzadeh-Basti A., Khaneghah A.M., Azizkhani M., Sant’ana A.D.S., Haghshenas M., Mahmoudzadeh L. Essential oil composition and antioxidant capacity of Carum copticum and its antibacterial effect on Staphylococcus aureus, Enterococcus faecalis and Escherichia coli O157:H7. J. Food Process. Preserv. 2017;41:e12938. doi: 10.1111/jfpp.12938. [DOI] [Google Scholar]
- Martínez L., Cilla I., Beltrán J.A., Roncalés P. Antioxidant effect of rosemary, borage, green tea, pu-erh tea and ascorbic acid on fresh pork sausages packaged in a modified atmosphere: influence of the presence of sodium chloride. J. Sci. Food Agric. 2006;86:1298–1307. doi: 10.1002/jsfa.2492. [DOI] [Google Scholar]
- Paparella A., Mazzarrino G., Chaves-López C., Rossi C., Sacchetti G., Guerrieri O., Serio A. Chitosan boosts the antimicrobial activity of Origanum vulgare essential oil in modified atmosphere packaged pork. Food Microbiol. 2016;59:23–31. doi: 10.1016/J.FM.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Pateiro M., Vargas F.C., Chincha A.A.I.A., Sant'Ana A.S., Strozzi I., Rocchetti G., Barba F.J., Domínguez R., Lucini L., do Amaral Sobral P.J., Lorenzo J.M. Guarana seed extracts as a useful strategy to extend the shelf life of pork patties: UHPLC-ESI/QTOF phenolic profile and impact on microbial inactivation, lipid and protein oxidation and antioxidant capacity. Food Res. Int. 2018;114:55–63. doi: 10.1016/J.FOODRES.2018.07.047. [DOI] [PubMed] [Google Scholar]
- Pateiro M., Barba F.J.F.J., Domínguez R., Sant'Ana A.S.A.S., Mousavi Khaneghah A., Gavahian M., Gómez B., Lorenzo J.M.J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: a review. Food Res. Int. 2018;113:156–166. doi: 10.1016/J.FOODRES.2018.07.014. [DOI] [PubMed] [Google Scholar]
- Ramos M., Jiménez A., Peltzer M., Garrigós M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012;109:513–519. doi: 10.1016/J.JFOODENG.2011.10.031. [DOI] [Google Scholar]
- Schumann B., Schmid M. Packaging concepts for fresh and processed meat – recent progresses. Innov. Food Sci. Emerg. Technol. 2018;47:88–100. doi: 10.1016/j.ifset.2018.02.005. [DOI] [Google Scholar]
- Siripatrawan U., Noipha S. Active film from chitosan incorporating green tea extract for shelf life extension of pork sausages. Food Hydrocolloids. 2012;27:102–108. doi: 10.1016/J.FOODHYD.2011.08.011. [DOI] [Google Scholar]
- The Commission of the European Communities Commission Regulation (EC) No 2073/2005 of 15th November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union. 2005;L338:1–26. [Google Scholar]
- Vyncke W. Evaluation of the direct thiobarbituric acid extraction method for determining oxidative rancidity in mackerel. Fette Seifen Anstrichm. 1975;77:239–240. doi: 10.1002/lipi.19750770610. [DOI] [Google Scholar]