Background
Large-scale use and misuse of antibacterial agents for infection prevention and growth promotion in chicken has grown alongside mass production of poultry as a primary protein source in human diet. This has led to concern about promotion of antibiotic resistance among bacteria of clinical significance to human disease. The objectives of this study are to identify which, if any, antimicrobials are commonly found in commercially available broiler chicken and determining the minimum amount of meat with enough microbial inhibitory activity that can be measured in routine culture. Four test organisms, namely ATCC 25922, ATCC 51299, clinical isolate MRSA and clinical isolate E. coli were used. The antimicrobial sensitivity profiles of the test organisms were determined against beta-lactams, tetracyclines, aminoglycosides, sulphonamides, fluoroquinolones and phenicols. 8 mm tissue pieces of liver, muscle and kidney samples were obtained and plated on all four plates of our test organisms. The zones of inhibition, if any, around the tissue samples determined the presence of antimicrobial residues in meat. 270 tissue samples of liver, muscle and kidney were tested for the presence of antimicrobial residues. In total 90 freshly butchered broiler chicken samples were collected, each contributing a liver, kidney and a muscle tissue sample. The samples were collected randomly from butcher shops across geographical bins of the city of Lahore.
The results showed that 73.3% of the samples were positive for antimicrobial activity. Of these 69.6% of the samples were positive for the presence of sulfonamides, 9.3% had flurphenicol, 7.0% had quinolone activity, 6.7% had aminoglycoside activity and 3.7% had tetracyclines in them.
Graphical abstract
Highlights
-
•
Microbiologically significant amounts of antimicrobials are found in nearly 3/4th of commercially available poultry meat.
-
•
Up to one-tenth of such meat may contain more than one antimicrobial agents.
-
•
Sulphonamides were detected in 69.6% of the samples.
-
•
We have attempted to quantify the least amount of poultry meat that contains microbiologically significant concentrations of antibiotics.
-
•
We discovered that nearly 2/3rd of all meat samples containing detectable antibiotic activity, were able to inhibit bacterial growth in culture with 4 g of meat or less.
1. Introduction
In recent times, the consumption of broiler chicken has been rising as a dietary meat source. To keep up with the consumer demands of meat, the producers had to accelerate the production of broilers. This entailed mainly the uninhibited use of antimicrobials in broilers raised on farms (Bulletin of the World Health Organization, 2018). Antimicrobials raise production via growth promotion and disease prevention. Overuse of antibiotics causes a selective pressure that gives rise to resistant microorganisms. These resistant, untreatable microbes cause diseases in birds and contribute towards pathologies in the environment. These microbes are transferable between animals and humans (Agyare et al., 2018). Fig. 1, Fig. 2.
Fig. 1.
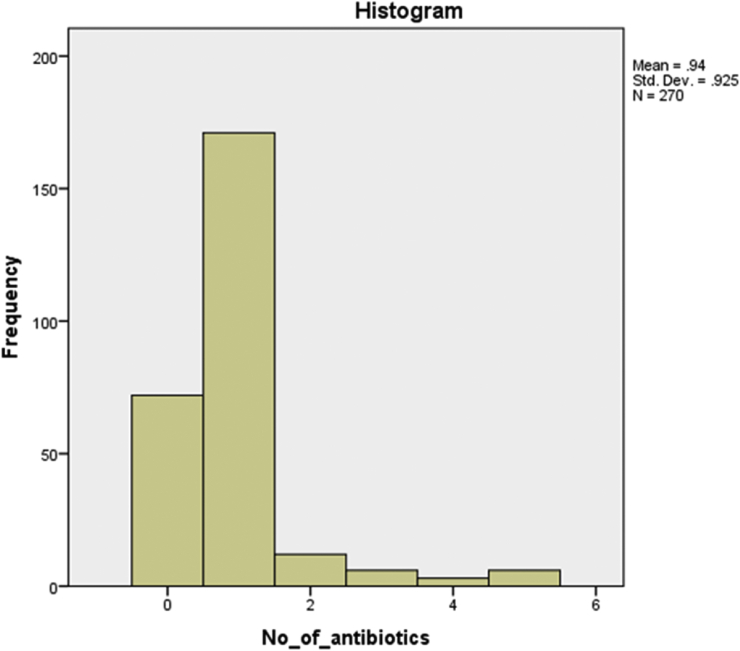
Frequency histogram of number of antibiotic activities.
Fig. 2.
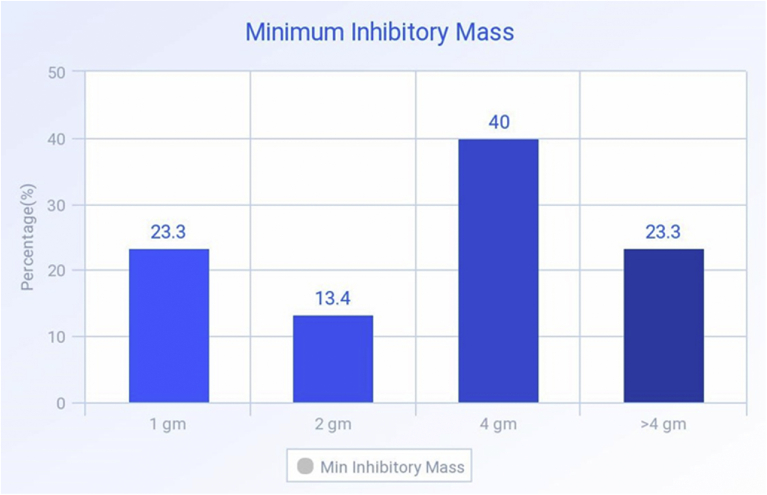
Minimum muscle meat mass causing measurable inhibition in culture.
Another cause of concern is the failure of the poultry farmers to observe antibacterial withdrawal periods in the chicken and slaughtering them for consumption before the drug could be metabolized and excreted by the bird (Khatun et al., 2018). Ongoing consumption of antibiotic residues in food disturbs the local human gut microbiome and facilitates the growth of drug-resistant disease-causing strains (Lammie and Hughes, 2016, Laxminarayan et al., 2013).
Antibiotics belong to a variety of different classes. The mechanism of action of each class is different. Antibiotics in the same class work in a similar way with similar effectiveness and resistance patterns. Most of these have been available over the counter for use in poultry feed (Timmons and Jacobs, 2008). However, due to rising levels of resistance amongst bacteria, recently the availability has been limited and a veterinarian prescription is required to purchase these drugs. The drugs commonly approved for use in poultry feed are the beta-lactams, aminoglycosides, macrolides, quinolones, sulphonamides, phenicols and tetracyclines. While floroquinolones (e.g. enrofloxacin) have, of late, been banned for use in poultry in Pakistan due to the rising floroquinolone resistance in Salmonella and Campylobacter spp., these may occasionally still be used illegally (Biemer, 1973).
In this study, we attempted to determine the frequency with which antimicrobial residues were present in commercially available, unprocessed chicken meat in Lahore city in Pakistan. We further sought a meaningful estimate of the minimum amount of meat with enough antibiotics in it to have a measurable effect on bacterial growth in culture. This is a new and useful finding and helps us determine the scope of the problem. The fact that as little as 4 g of meat could have enough antimicrobials to eliminate an entire minimum infectious dose of an organism is a startling revelation, and one that calls for increased surveillance measures and severe monitoring of antibiotic usage. Hence, the second part of the study focused on a microbial inhibition assay to assess the strength of antimicrobial activity in different masses of muscle tissue.
The main purpose of this study is to motivate the governing authorities and the scientific community to come together and develop a standard for the extraction and analysis of antibiotics in food and to allow an extensive monitoring of residual antibiotics in food, hence ensuring consumer health (Nisha, 2008). We are reporting the presence of residual antibiotics in chicken, while simultaneously introducing simple methods that are applicable in a low cost setting for the a) the determination of residual antibiotics in poultry meat b) detecting minimum inhibitory concentration of such residues (on disease causing germs and human gut flora likewise) that sheds light on the extent of the problem.
2. Materials and methods
A total of 270 freshly slaughtered broiler chicken (Gallus gallus domesticus) samples (90 each of muscle, liver and kidney tissue) were collected from butcher shops across Lahore city. The points of collection were selected by dividing the city into 10 bins and selecting three points of fresh poultry meat sale from each bin by a random draw. Three specimens each, of muscle (breast, commonly consumed), liver and kidney meat were collected from each sale point. Each sample was placed in a separate plastic zipper bag, labelled and transferred to the microbiology lab over ice and assessed immediately or stored at −20 °C until tested.
2.1. Qualitative detection of antibiotics in chicken tissues
2.1.1. Sample preparation
The four test organisms ATCC 25922, ATCC 51299, Clinical isolate E.coli and Clinical isolate MRSA were subjected to antimicrobial susceptibility testing and profiling as per the modified Kirby Bauer method (Biemer, 1973) and described as per CLSI 2018 criteria (CLSI, 2018). These were tested on Nutrient agar against the antibiotic classes mentioned previously. Interpretation was done according to CLSI criteria 2018. The ATCC 25922 was found susceptible to beta-lactams, aminoglycosides and quinolones. The ATCC 51299 showed growth inhibition in response to tetracyclines. The E.coli isolate was found susceptible to phenicols alone and MRSA was determined to be susceptible to sulphonamides and aminoglycosides.
From each broiler chicken sample 3 tissue types, namely, its muscle, liver and kidney were taken. Approximately 10 g of each organ was taken. Samples of each broiler chicken were added to a separate polythene bag and labelled. From each shop at least 2 broiler chickens were sampled. The samples were transported to the Microbiology Lab at University of Health Sciences, Lahore, on an ice pack and tested right away.
From bag 1 the broiler chicken muscle sample (SI) was extruded. The surface was cleaned with povidone-iodine solution and the sterilized surface was removed using sterile blade. A sterile surgical blade was used to cut out a piece of tissue measuring 8 mm into 2 mm was. Similarly, 4 pieces of tissue were obtained from the muscle tissuee from broiler chicken sample (SI). The liver and kidney samples from SI were also cut out as above. Again, cutting four pieces out of each tissue.
The four MH (Mueller Hinton) agar plates labelled 1, 2, 3 and 4 were taken out of the incubator. Onto each plate 1 muscle, 1 liver and 1 kidney sample from SI were applied. This procedure was followed with all four plates. The plates were incubated at 38C for 24 h.
The principle was to identify that if the tissue samples contained any antimicrobial drugs then these tissue pieces would act as an antibiotic disc as in the Kirby Bauer disc diffusion method and a zone of inhibition of the plated bacteria will be noticed around the discs of tissue. Positive samples were indicated by a complete inhibition of growth in an annular zone not less than 2 mm around the piece of meat. While less than 2 mm of inhibitory zones were indicated as negative result (Sajid et al., 2016).
For each specimen of poultry, three tissues were assayed. The total number of samples analyzed is thus 270. While more muscle specimens appear to show antibiotic activity, there is no statistically significant difference in the distribution of the number of antibiotics used when analyzed against the tissue type (p-value for Fisher's exact test = 0.884). (Table 1).
Table 1.
Distribution of number of antibiotic activities by tissue type.
| No_of_antibiotics |
Total | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| Tissue | |||||||
| Muscle | 24 | 60 | 4 | 1 | 1 | 0 | 90 |
| Liver | 22 | 57 | 4 | 2 | 1 | 4 | 90 |
| Kidney | 26 | 54 | 4 | 3 | 1 | 2 | 90 |
| Total | 72 | 171 | 12 | 6 | 3 | 6 | 270 |
2.1.2. Microbial inhibition assay
4-5 bacterial colonies of test organism Staphylococcus aureus were (ATCC 25923) obtained using a sterile wire loop. The colonies were not older than 24 h. The reason behind the use of ATCC 25923 in part of my research is that this particular bacterial strain is susceptible to most antibiotics and hence is a good indicator of presence of antimicrobials in muscle tissue samples. These were added to a rich media broth and a suspension was prepared to match 0.5 McFarland standard. The final inoculum hence prepared contains 107–108 CFU/ml.
5 ml of the solution of the prepared broth culture was added to 3 test tubes each, autoclaved at 121 for 15 min 1 g of minced muscle tissue from the first chicken muscle sample in the study was added to a test tube. To two other test tubes, 2 and 4 g of minced muscle tissue from the same broiler chicken were added respectively. A micropipette was used to remove 0.1 μl of the culture solution from the test tube and dropped onto a Nutrient agar plate. This was done with the culture solution in all three test tubes. Using a sterile loop, a homogenous bacterial lawn was prepared. The petri dishes were incubated at 30 °C for 24 h. This was repeated on the muscle tissues from all the broiler chicken samples.
The petri dishes were removed from the incubator. The colonies were counted using a colony counter. The minimum weight of meat that prevented the visible growth of the organisms was assumed to be the minimum weight of meat that contained enough antibiotic residues to affect food contaminants in the digestive tract or gut flora.
3. Results
While more muscle specimens appear to show antibiotic activity, there was no statistically significant difference in the distribution of the number of antibiotics used when analyzed against the tissue type (p-value for Fisher's exact test = 0.884).
3.1. Distribution of number of antibiotic activities across samples
The average poultry meat specimen had one antibiotic activity detected in culture (mean = 0.94, SD = 0.925). The most commonly used antibacterial group appears to be sulphonamides, isolated in 69.6% of all specimens tested. The percentage frequency of all antibiotic activities isolated are depicted in Table 2.
Table 2.
Frequency of antibacterial agents detected across all meat specimens.
| Antibiotic | Percentage Frequency (%) |
|---|---|
| Sulphonamides | 69.6 |
| Flurphenicol | 9.3 |
| Quinolone | 7.0 |
| Aminoglycoside | 6.7 |
| Tetracycline | 3.7 |
| None | 26.7 |
Over 73% of samples were found to contain at least one antibiotic activity. Of these, up to 63.3% of specimens contained at least one antibiotic, with as many as 2.2% showing as high as 5 different antibiotic activities (Table 3).
Table 3.
Number of antibiotics in each specimen tested.
| Number of Antibiotics | Frequency | Percent (%) |
|---|---|---|
| 0 | 72 | 26.7 |
| 1 | 171 | 63.3 |
| 2 | 12 | 4.4 |
| 3 | 6 | 2.2 |
| 4 | 3 | 1.1 |
| 5 | 6 | 2.2 |
3.2. Frequency of each antibiotic activity found in tissue specimens
We then attempted to estimate the minimum mass (in grams) that would elicit meaningful and measurable inhibition of microbial growth in culture plates. For this purpose, we worked with muscle tissue only, as that is the most commonly consumed part of poultry meat. We plated discs of 1 g, 2 g and 4 g meat in a culture of multidrug sensitive ATCC isolate. Out of a total of 90 muscle tissue specimens, 40 (44.4%) showed inhibition with 4 g or less of muscle tissue. The frequency of cultures inhibited by various values of tissue mass are listed in Table 4.
Table 4.
Minimum muscle meat mass causing measurable inhibition in culture.
| Min. Tissue Mass (g) Causing inhibition in Culture | Frequency | Percentage (%) |
|---|---|---|
| 1 | 14 | 21.2 |
| 2 | 8 | 12.1 |
| 4 | 18 | 27.3 |
| >4 (not measured) | 26 | 39.4 |
4. Discussion
In this research we have used a relatively cheap and cost-effective method to indirectly detect and quantify antimicrobial activity in poultry meat and offal (Sajid et al., 2016a). We used bacterial strains with well-defined and overlapping antibacterial resistance profile to determine if they were inhibited by test-specimens of poultry meat. Our findings indicate that up to 3/4th of all specimens tested had at least 1 antibiotic at concentrations detectable by routine microbiological methods. Furthermore, our findings suggest that withdrawal periods, ranging from a few days to weeks, defined as the time required for 99% of birds in a flock to be free of the drug residues above the tolerance level specific to each drug (Engberg et al., 2001), are not observed.
Our method is similar, though not identical, to that of Hakem et al. (2013). Our findings are consistent with studies from across the developing world over the last decade or so, citing antimicrobial detection rates in poultry meat ranging from 93% (Hakem et al., 2013), through 62% (Rahmatallah et al., 2018) to 52% (Prajapati et al., 2018). Some of these differences may be attributable to the methodology used for detection of antibiotic residues. As an example, a recent study (Sarker et al., 2018a) from Mardan, Pakistan, reports a very low rate of antibiotic residue detection of only 8% in poultry liver specimens tested against clinical E. coli and S. aureus isolates. However, they do not consider the possibility of antibiotic resistance in these clinical isolates. This is an important point that we have meticulously tried to study using carefully standardized indicator species with differing, partially overlapping, susceptibility profiles. In agreement with existing literature from Pakistan (Sajid et al., 2016b), sulphonamides were the most commonly detected active antibiotic in commercially available poultry meat.
A number of factors determine whether these antibiotic residues are of concern for humans who consume them as part of their diet. Aminoglycosides are concentrated in kidneys and have poor absorption through the gut. These are therefore likely to be of less concern as dietary contaminants. While the concentration of tetracycline significantly declines during cooking and food processing, sulphonamides and fluoroquinolones are likely to retain significant activity through routine cooking and processing (Moreno and Lanusse, 2017).
While some studies seem to suggest higher concentrations of antibiotic residues in liver or kidney tissue as compared to muscle (Muaz et al., 2018), others (Sarker et al., 2018b), as in our case, did not find such differences. Such findings may be attributable to the type of antibiotic considered (e.g. aminoglycosides are heavily concentrated in the kidneys and certain third generation cephalosporins are excreted in bile), or to the duration of drug withdrawal before tissue concentrations were measured. A few authors have also reported higher concentrations of certain antibiotics in breast meat while certain others seem to be detected in higher amounts in thigh meet (Rahmatallah et al., 2018, Prajapati et al., 2018). In this study we did not take any measures to resolve these differences and used meat from a variety of muscle tissues.
We have attempted to quantify the least amount of poultry meat that contains microbiologically significant concentrations of antibiotics. We achieved this by plating varying masses of muscle tissue samples in multi-drug sensitive culture plates. We discovered that nearly 2/3rd of all meat samples containing detectable antibiotic activity, were able to inhibit bacterial growth in culture with 4 g of meat or less. This is a significant, and to the best of our knowledge, a novel discovery as it defines a replicable measure to determine the burden of antibiotic consumed in poultry meat.
The development of resistance to multiple antimicrobials in gut flora and pathogenic organisms is threatening to human health. Furthermore, residuals concentrations of some antibiotics, such as sulphamethazine and oxytetracycline are known to cause carcinogenicity. Chloramphenicol is known to be nephrotoxic and hepatotoxic (Allen and Stanton, 2014). There has been an alarming increase in the burden of infections due to multi-drug resistant organisms in Pakistan. The Global Action Plan to fight Antibiotic Resistance was recommended and approved in a meeting of the World Health Assembly in 2015 by Pakistan and many other counrtries. The Global Antibiotic Resistance Partnership funded by the Bill and Melinda Gates Foundation facilitates the development of policy proposals on antibiotic resistance. The systems policies fail due to a lack of AMR data in Pakistan, a lack of awareness, limited lab facilities and the rising trend in the widespread acceptance of antibiotics as the magic bullet. This study is a step towards dealing with such problems.
Conclusion
Based on the results of our analysis, we conclude that microbiologically significant amounts of antimicrobials are found in nearly 3/4th of commercially available poultry meat. Up to one-tenth of such meat may contain more than one antimicrobial agents. As the concentration of these agents detected in commercially available poultry meat have detectable inhibitory effects on clinically relevant bacteria even at a small fraction of the mass consumed by an average human, these findings raise the specter that this common dietary component of the average person's diet may be significantly altering the human microbiome, potentially leading to a host of pathological outcomes.
Funding
This study was supported by internal funding from the University of Health Sciences Lahore.
Transparency declarations
None to Declare.
CRediT authorship contribution statement
Rabia Ali: Methodology, Software, Data curation, Writing - original draft, Formal analysis, Investigation, Writing - review & editing, Project administration, Validation. Sidrah Saleem: Conceptualization, Supervision, Resources, Funding acquisition.
Declaration of Competing Interest
None to declare.
Contributor Information
Rabia Ali, Email: rabyaskhan@gmail.com.
Sidrah Saleem, Email: microbiology@uhs.edu.pk.
References
- Agyare C., Boamah V.E., Zumbi C.N. 2018. Antibiotic Use in Poultry Production and its Effects on Bacterial Resistance. [Google Scholar]
- Allen H.K., Stanton T.B. Altered egos: antibiotic effects on food animal microbiomes. Annu. Rev. Microbiol. 2014;68:297–315. doi: 10.1146/annurev-micro-091213-113052. [DOI] [PubMed] [Google Scholar]
- Biemer J.J. Antimicrobial susceptibility testing by the Kirby-Bauer disc diffusion method. Ann. Clin. Lab. Sci. 1973;3(2):135–140. [PubMed] [Google Scholar]
- Bulletin of the World Health Organization. 2018;96(2):77–144. doi: 10.2471/BLT.17.195834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg J., Aarestrup F.M., Taylor D.E. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 2001;7(1):24. doi: 10.3201/eid0701.010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakem A., Titouche Y., Houali K., Yabrir B., Malki O., Chenouf N., Yahiaoui S., Labiad M., Ghenim H., Kechin-Bounar S., Chirila F. Screening of antibiotics residues in poultry meat by microbiological methods. Bulletin of university of agricultural Sciences and veterinary medicine Cluj-napoca. Vet. Med. 2013;70(1):77–82. [Google Scholar]
- Khatun R., Howlader A.J., Ahmed S. Validation of the declared withdrawal periods of antibiotics. Univ. J. Public Health. 2018;6:14–22. [Google Scholar]
- Lammie S.L., Hughes J.M. Antimicrobial resistance, food safety, and one health: the need for convergence. Annual Rev. Food Sci. Tech. 2016;7:287–312. doi: 10.1146/annurev-food-041715-033251. [DOI] [PubMed] [Google Scholar]
- Laxminarayan R., Duse A., Wattal C. Antibiotic resistance—the need for global solutions. Lancet Infect. Dis. 2013;13(12):1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- Moreno L., Lanusse C. New aspects of meat quality. Woodhead Publishing; 2017. Specific veterinary drug residues of concern in meat production; pp. 605–627. [Google Scholar]
- Muaz K., Riaz M., Akhtar S. Antibiotic residues in chicken meat: global prevalence, threats, and decontamination strategies: a review. J. Food Protect. 2018;81(4):619–627. doi: 10.4315/0362-028X.JFP-17-086. [DOI] [PubMed] [Google Scholar]
- Nisha A.R. Antibiotic residues-a global health hazard. Vet. World. 2008;1(12):375. [Google Scholar]
- Prajapati M., Ranjit E., Shrestha R. Status of antibiotic residues in poultry meat of Nepal. Np. Veterinary J. 2018;35:55–62. [Google Scholar]
- Rahmatallah N., El Rhaffouli H., Lahlou Amine I. Consumption of antibacterial molecules in broiler production in Morocco. Veterinary Med. Sci. 2018;4(2):80–90. doi: 10.1002/vms3.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajid A., Kashif N., Kifayat N., Ahmad S. Detection of antibiotic residues in poultry meat. Pak. J. Pharm. Sci. 2016;29(5):1691–1694. [PubMed] [Google Scholar]
- Sajid A., Kashif N., Kifayat N. Detection of antibiotic residues in poultry meat. Pak. J. Pharm. Sci. 2016;29(5):1691–1694. [PubMed] [Google Scholar]
- Sarker Y.A., Hasan M.M., Paul T.K. Screening of antibiotic residues in chicken meat in Bangladesh by thin layer chromatography. J. Adv. Veterinary Animal Res. 2018;5(2):140–145. [Google Scholar]
- Sarker Y.A., Hasan M.M., Paul T.K. Screening of antibiotic residues in chicken meat in Bangladesh by thin layer chromatography. J. Adv. Veterinary Animal Res. 2018;5(2):140–145. [Google Scholar]
- Timmons J., Jacobs J.E., Reynnells R.D. Combined proceedings 2008 and 2007 national extension workshops: Poultry Science Association Annual Meeting Niagra Falls, Ontario, Canada July 21, 2008 and Joint Animal, Dairy, and Poultry Science Association Annual Meeting San Antonio, TX July 10, 2007. United States. Cooperative State Research, Education, and Extension Service. Plant and Animal Systems; 2008. [Google Scholar]