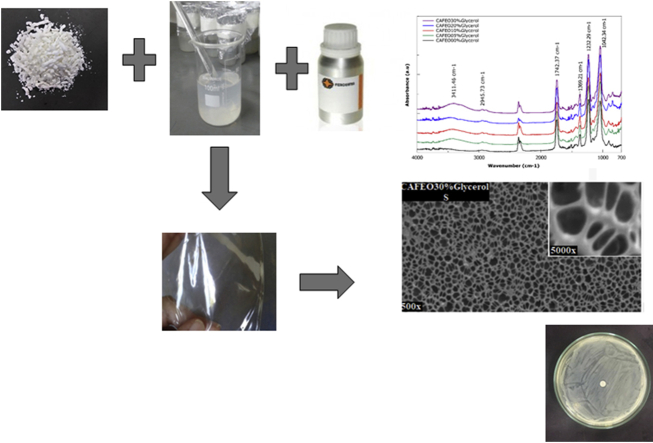
Abstract
Cellulose acetate (CA) films with sweet fennel essential oil (FEO) were evaluated for possible changes caused by the incorporation of 5, 10, 20 and 30% glycerol. The results show that the incorporation of different concentrations of plasticizer caused an increase in thickness, water vapor transmission rate (WVTR), tensile strength (TS), besides altering the optical properties and demonstrating possible chemical interaction with the CA matrix (Fourier transform infrared (FTIR)). Scanning electron microscopy (SEM) images revealed that the addition of glycerol caused morphological changes on the surface and internal region of all films. As for antimicrobial activity, the FEO was effective for Staphylococcus aureus and Escherichia coli. However, all films evaluated did not show activity in inhibiting these microorganisms. Therefore, it is believed that the FEO may have some incompatibility with the CA matrix, being trapped between the polymer chains. Therefore, the results suggest that the incorporation of glycerol caused changes in the functional properties of all films, although it did not result in measurable antimicrobial effects.
Keywords: Natural antimicrobial, Glycerol, Functional properties, Active packaging, Food preservation
Graphical abstract
Highlights
-
•
Cellulose acetate film incorporating sweet fennel essential oil and different concentrations of glycerol showed changes of the functional properties.
-
•
Films with glycerol presented increased tensile strength.
-
•
CA films with essential oil did not exhibit antimicrobial activity.
-
•
Pure FEO was effective for Staphylococcus aureus and Escherichia coli.
1. Introduction
Microbial contamination throughout storage, transport and post-harvest processing has been identified as causing loss of food quality, quantity, nutrients and market value (Prakash et al., 2015). In this sense, active packaging, which interact beneficially with food, can help maintain its quality and extend its commercial validity. The mechanism of action of active packaging consists in the absorption or emission of compounds, such as antimicrobials, responsible for inhibiting and/or reducing microorganisms in food (Khaneghah et al., 2018).
Most food packaging is made from petroleum-derived polymers. Therefore, to reduce the impact generated on the environment, biodegradable natural polymers, such as cellulose acetate (CA), have gained importance as substitutes for synthetic polymers. CA is an abundant biodegradable polymer, obtained through acetylation of cellulose. It is a thermoplastic material with good impact resistance and transparent (Canevaloro, 2006).
Among the active packaging, we highlight the antimicrobial films, which can be made from biopolymers, with the incorporation of antimicrobial agents into their matrix that can be gradually released to the food surface and, in addition, contribute positively to the environment (Galloto et al., 2015, Ribeiro-Santos et al., 2017). An example of antimicrobial additives may be cited as essential oils (OEs), which have been remarkably researched and used for this purpose (Ju et al., 2019).
Essential oils are aromatic liquids composed of a mixture of several components, the type of OEs varies between plants and the part of the plant to which they are extracted (Plant et al., 2019). This antimicrobial additive is well regarded by consumers because it is a natural ingredient and can replace synthetic additives (Santos et al., 2019). An example is sweet fennel EO, with range of applications, including in the cosmetics industry and medicine. The existing studies have shown that fennel essential oil has many antifungal and antibacterial properties, whose major compound is anethole, which in turn has known antimicrobial activity (Sleha et al., 2014, Ribeiro-Santos et al., 2018).
In addition to the added antimicrobial agents for the purpose of imparting activity to films, active antimicrobial packaging is expected to have good physical and mechanical properties, not neglecting one of the main packaging requirements: food protection (Gonçalves et al., 2019). The addition of plasticizers, low volatility molecules, tend to alter the three-dimensional organization of polymers, reducing polymer-polymer interaction and consequently increasing free volume and chain mobility, which improves certain functional properties of films, such as extensibility, dispensability and mechanical properties (Swain et al., 2004, Kokoszka et al., 2010).
Glycerol is a plasticizer widely used in the production of films. Its use tends to desirably alter the tensile strength and elongation properties at break, as well as decrease the moisture barrier of materials (Suderman et al., 2018). Moreover, however small the concentration of additive used, it may modify the properties of materials such as: moisture permeability, mechanical strength, solubility, optical and physical aspects (Pinheiro et al., 2010). Therefore, it is necessary to characterize the materials in order to apply them according to their nature.
It is known that glycerol can produce different effects on packaging depending on its concentration (Liang et al., 2015, Dick et al., 2015, Yun et al., 2016, Paolicelli et al., 2018). However, the plasticizing or antiplasticising effect of glycerol has not been reported when inserted into cellulose acetate-based antimicrobial films incorporated with sweet fennel EO. Thus, this work proposed to evaluate the thickness, morphological structure (SEM), optical properties, chemical structure (FTIR), mechanical, antimicrobial and water vapor transmission rate (TTVA) in view of the incorporation of different concentrations of glycerol in cellulose acetate based films incorporated with sweet fennel OE.
2. Experimental
2.1. Materials
Cellulose acetate (AC) (1.48° substitution grain (Sigma-Aldrich, Brazil); sweet fennel essential oil (Foeniculum vulgare dulce) (Ferquima, Brazil); Glycerol PA (Vetec Química, Brazil); acetone PA (Cap-Lab, Brazil). For evaluation of antimicrobial activity, cultures of Escherichia coli (ATCC 11229) and Staphylococcus aureus (ATCC 6538), culture media Mueller-Hinton Agar (Kasvi) and Pepton casein (Himedia, India).
2.2. Film preparation
A 10% w/v AC and acetone gel was prepared, followed by the addition of FEO (50% w/v) and glycerol at different concentrations (5, 10, 20, 30 or 50%) (w/v). By the casting method [20 with modifications], 25 ml of the filmogenic solution was poured and spread on glass, dried at room temperature (25 ± 2 °C) for 10 min and detached. Four samples of each films were preconditioned to 75% relative humidity for 10 days, for analysis.
2.3. Fourier transform infrared attenuated total reflection (FTIR-ATR) spectroscopy
The chemical structure evaluation was followed by FTIR/ATR (FT/IR-4700, Jasco Corporation) according to Moura et al. with modifications (Moura et al., 2012). The spectra were obtained in the wavelength range of 700–4000 cm−1, 4 cm−1 resolution, and 32 scans. Four spectra were collected from each sample, at different points.
2.4. Thickness
Ten measurements of thickness (μm) were made in triplicate with the help of a digital micrometer (Datamed). Measurements were taken at ten points for each film.
2.5. Water vapor transmission rate (WVTR)
The WVTR was performed according to the gravimetric method (ASTM E96-95) with modifications according to the method described by Ghasemlou et al. (2011). CaCl2 (anhydrous calcium chloride) was used inside capsules, which were sealed with the films and packed in a desiccator containing saturated NaCl (sodium chloride) solution to pzromote controlled humidity (75% ± 2) and placed at room temperature (25 °C ± 2 °C). The permeability of the films was determined by linear regression of the constant mass transfer region between weight gain (g) and time (h) which correlated with the exposed area to determine WVTR (Equation (1)).
| WVTR = G/ t. A | 1 |
Where:
WVTR: Water vapor transmission rate expressed in g.m–2.day−1;
G/t: Angular coefficient of the line expressed in g.day−1;
A: Permeation area of the sample expressed in m2.
2.6. Scanning electron microscopy (SEM)
The surface and fracture region of the films were observed with the help of the Scanning Electron Microscope (Carl Zeiss, model EVO MA 10). Samples of 1.5 × 0.7 cm were metallized with gold (Au) (metallizer EMITEC K550X) with current of 25 mA/2 min and subsequently observed in low vacuum SEM, 6000 Kv acceleration voltage, 480 filament current and scans with magnifications of 5000 and 500×.
2.7. Visual aspect of films
Using the colorimeter/spectrophotometer (Minouta CM-5-ID), the films were evaluated for luminosity L∗ (0 = black and 100 = white), red/green (±a∗: -80) chromaticity a 0 = green; 0 to +100 = red), yellow/blue chromaticity (±b∗: -100 to 0 = blue; 0 to +70 = yellow) and total color difference (ΔE∗). The opacity was determined according to the amount of light the films were able to absorb, at 560 nm wavelength, according to ASTM D1746 (American Society for Testing, 2003).
2.8. Mechanical properties
With the help of the TA.XTplus Texturometer (Stable Micro Systems, Surrey, England), the tensile strength (TS) (N), elongation at break (EB) (%) and Young's modulus (YM) (MPa) properties of the films were evaluated. The texturometer was operated according to the standard method ASTM D 882–82, where 10 × 2.5 cm samples were fixed to the claws with an initial length of 25 mm. The claws were separated at a speed of 1 mm/s, with a cell of 30 kg and a force of 0.049 N.
The TS was given by the relation of the maximum force (N) and the sample area (mm), while the YM was calculated from the linear region of the stress versus strain curve, according to the Exponent Texture TEE32 (Stable Micro Systems) program. EB was obtained according to Equation (2):
| 2 |
where:
EB: Elongation at break expressed in %;
L: Distance at moment of breakage expressed in mm;
Ci: Initial sample length in mm.
2.9. In vitro antimicrobial efficiency assessment
The antimicrobial efficiency of FEO and films was determined using the agar diffusion technique according to Moura et al. (2012) with adaptations. For the FEO test, 3 μL of the essential oil was dispersed on filter paper discs (6 mm in diameter). For antimicrobial efficiency of the films, they were sampled in the same dimensions (6 mm in diameter). FEO-soaked filter paper discs and films incorporated with FEO were placed in the center of petri dishes containing solid culture medium (Mueller-Hinton) (15 ml) previously contaminated with 108 CFU/ml Staphylococcus aureus (ATCC 6538) or Escherichia coli (ATCC 11229). The plates were incubated 35 ± 2 °C for 24 h. Antimicrobial efficiency was determined in four replicates, by measuring inhibition halos (considering sample sizes) formed around the films and also by observing microbial growth density.
2.10. Statistical analysis
Statistical analyzes were performed with the help of R software, version 3.2.4 (R Foundation for Computational Statistics, Vienna, Austria). The differences between the samples were obtained with multi comparative test (Tukey) and ANOVA, with 5% significance level. In addition, possible correlations between treatments and/or variables were investigated with the help of Principal Component Analysis (PCA) and Pearson's Correlation. PCA was performed after variables standardized to avoid the influence of different magnitudes and units, in this way to make sure all variables had the same influence on PCA results, and the stronges of Pearson's correlation was evaluated following the rule proposed by (Teles et al., 2019).
3. Results and discussion
3.1. Fourier transform infrared attenuated total reflection (FTIR-ATR) spectroscopy
FTIR spectra (Fig. 1) show typical cellulose band vibrations and increased intensity in the presence of different glycerol concentrations. Bands 3471 cm−1 (free OH groups) and 1742 cm−1 (steric carbonyl elongation) are characteristic in CA spectra (Meireles, 2007). The 3471 cm−1 band, which is in the region representing the free hydroxyl groups (3000–3600 cm−1), had the largest increase compared to 30% glycerol (CAFEO30%Glycerol), which may be a consequence of the presence of glycerol OH groups or possible interaction between glycerol and CA (both are hydrophilic compounds) (Sederavičiūtė et al., 2019). Likewise, slight increases in intensities of other bands, such as 2945 cm−1 and 2882 cm−1 (CH elongation), 1369 cm−1, 1232 cm−1 and 1042 cm−1 (steric carbonyl elongation), before 30% of glycerol, mainly, may be due to chemical interactions between glycerol and polymeric matrix, through hydrogen bonds.
Fig. 1.
FTIR spectra of CA films with FEO (CAFEO0%Glycerol) and CAFEO films with 5, 10, 20 and 30% glycerol (CAFEO5%Glycerol, CAFEO10%Glycerol, CAFEO20%Glycerol and CAFEO30%Glycerol).
3.2. Thickness and water vapor transmission rate (WVTR)
The addition of glycerol caused increasing the thickness of CAFEO films (Table 1), as also shown by Gonçalves et al. (2019). Furthermore, these authors reported that pure CA films presented 41.3 μm thickness, whereas in this work, Table 1 shows 48.16 μm thickness for CA films with 50% FEO (CAFEO0%Glycerol), thus the presence of FEO or glycerol may have caused modification in intra and intermolecular interactions, causing chain distancing and consequent thickness increase. Hasheminya et al. (Mokarram et al., 2019) attributed the increased thickness of kefiran and carboxymethylcellulose films to the increase in solid content following the incorporation of Satureja Khuzestanica EO.
Table 1.
Thickness and water vapor transmission rate (WVTR) of CAFEO films and CAFEO films incorporating with 5, 10, 20 and 30% glycerol.
| Samples | Thickness (μm) | WVTR (g.m-2.day-1) |
|---|---|---|
| CAFEO0%Glycerol | 48.16 ± 2.07c | 155.54 ± 15.97c |
| CAFEO5%Glycerol | 53.3 ± 1.7bc | 177.51 ± 10.27bc |
| CAFEO10%Glycerol | 55.66 ± 1.22b | 172.54 ± 9.29c |
| CAFEO20%Glycerol | 56.1 ± 1.65b | 199.65 ± 11.13ab |
| CAFEO30%Glycerol | 68.96 ± 3.17a | 214.24 ± 4.27ab |
∗Average followed by the same letters do not differ from each other (p > 0.05) by the Tukey test at the 5% level of significance.
The addition of glycerol caused an increase in WVTR (Table 1) for CAFEO films, a result similar to those reported in the literature (Jost et al., 2014, Sanyang et al., 2015), which may occur due to the removal of polymer chains from presence of plasticizer. The type and concentration of plasticizer can cause changes in the barrier properties of polymeric films, including increased gas, water vapor and solute permeability (Barbut and Harper, 2019, Sabbah et al., 2019).
3.3. Scanning electron microscopy (SEM)
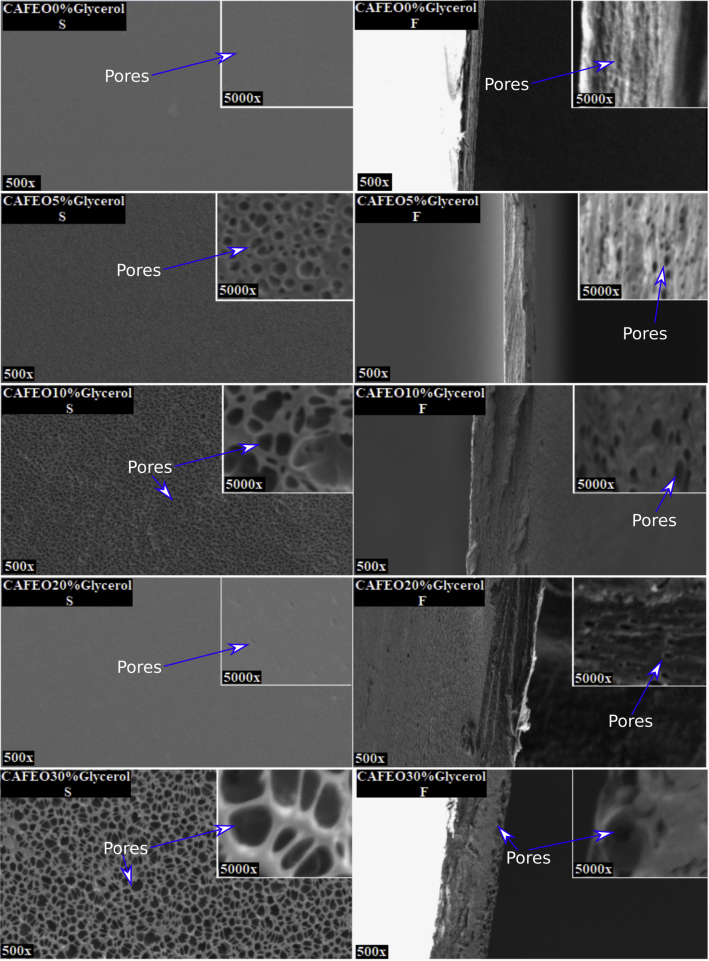
In SEM images (Fig. 2), the control film (CAFEO0%Glycerol S) presented homogeneous surface, while its fracture region (CAFEO0%Glycerol F) shows small rounded structures. Pontes et al. (Pontes, 2013) found similar structures on the surface of EO-embedded methylcellulose films and called them “EO droplets”. With the exception of CAFEO20%Glycerol S film, all other surfaces showed alterations. The surface of the 5% glycerol film (CAFEO5%Glycerol S) had rounded structures, similar to those found by Gonçalves et al. (2019) in CA films with 30 and 50% glycerol. The films with 10 and 30% glycerol (CAFEO10%Glycerol S and CAFEO30%Glycerol S) revealed a net-like surface. For the 20% glycerol film (CAFEO20%Glycerol S), the surface showed small depressions, viewed at 5000× magnification. The internal region (fracture - F) of all films were pores, with a larger amount of pores for the 20% glycerol film (CAFEO20%Glycerol F). The 30% glycerol film (CAFEO30%Glycerol F) presented pores only in the region near the surface.
Fig. 2.
SEM of surface (S) and fracture region (F) of CA films with FEO (CAFEO0%Glycerol) and CAFEO films with 5, 10, 20 and 30% glycerol (CAFEO5% Glycerol, CAFEO10% Glycerol, CAFEO20% Glycerol and CAFEO30% Glycerol).
3.4. Visual aspect of films
Table 2 shows that the presence of different concentrations of glycerol caused a decrease in luminosity (L∗) of CAFEO films. For red/green chromaticity (a∗), it is observed that the film with FEO showed slightly greenish (-a∗) (CAFEO0%Glycerol), while all films with glycerol were red (+a∗), with the highest values for the films with 10 and 20% glycerol (CAFEO10%Glycerol and CAFEO20%Glycerol). For yellow/blue (b∗) chromaticity, the presence of glycerol caused an increase in the yellow color (+b∗) of the films, especially CAFEO10%Glycerol and CAFEO20%Glycerol films, which had the highest values. As for the total difference (ΔE∗), CAFEO10%Glycerol and CAFEO20%Glycerol films presented the largest total color difference, which, in turn, is directly related to the highest values observed for +a∗ and +b∗. These results corroborate the findings of Dick et al. (2015) who observed reduction of luminosity, increase of +a∗, +b∗ and ΔE∗ of chia seed mucilage films with 75% glycerol.
Table 2.
Visual properties of CAFEO films and CAFEO films incorporating with 5, 10, 20 and 30% glycerol.
| Samples | L∗ | a∗ | b∗ | ΔE∗ | Opacity |
|---|---|---|---|---|---|
| CAFEO0%Glycerol | 97.15 ± 0.08a | −0.01 ± 0d | 0.17 ± 0.03d | 3.22 ± 0.1b | 92.848 ± 0.21a |
| CAFEO5%Glycerol | 96.77 ± 0.86a | 0.38 ± 0.06c | 0.85 ± 0.21c | 2.32 ± 1.77b | 91.97 ± 2.09ab |
| CAFEO10%Glycerol | 94.83 ± 2.58b | 1.06 ± 0.09a | 2.6 ± 0.9b | 14.49 ± 18.3b | 87.54 ± 5.92b |
| CAFEO20%Glycerol | 90.77 ± 1.53c | 1.02 ± 0.06a | 3.44 ± 0.19a | 48.875 ± 18.2a | 79.79 ± 3.67c |
| CAFEO30%Glycerol | 96.71 ± 0.48ab | 0.59 ± 0.04b | 1.1 ± 0.09c | 1.581 ± 0.68b | 91.82 ± 1.16ab |
∗Average followed by the same letters do not differ from each other (p > 0.05) by the Tukey test at the 5% level of significance.
Opacity is given according to the amount of light absorbed by the film, so the greater the amount of light absorbed, the greater the opacity. Therefore, it is noted that glycerol caused a reduction in the opacity of all films, with lower values for CAFEO10%Glycerol and CAFEO20%Glycerol films. According to SEM images (Fig. 2), the different concentrations of glycerol caused changes in the surface and the region of fracture of the films, which, in turn, were characterized by pore-like structures. Thus, glycerol may have caused the chains to move away and, consequently, provided greater light passage through the material, showing more transparent films. Moreover, the different opacities observed in Table 2 may be justified by the presence of non-miscible material, since the hydrophobicity of the FEO and its components may have hindered their interaction with the CA matrix and glycerol, which, in turn, are notably hydrophilic. Thus, the heterogeneity of films may have favored different refractions for each phase (Diao et al., 2014, Villalobos et al., 2005).
3.5. Mechanical properties
With the exception of 5% glycerol film (CAFEO5%Glycerol), all other concentrations caused increased tensile strength (TS) (Table 3). The action of the plasticizer basically consists in causing changes in the polymer network, with consequent increase of flexibility and reduction of the TS of the films. However, polymeric plasticization depends on the type and especially on the amount of plasticizer used. Besides the interaction between glycerol, FEO and the polymeric matrix, we can have antiplastifying effect, with consequent increase of TS, as reported by Gonçalves et al. (2019) and Reis et al. (2015).
Table 3.
Mechanical properties of CAFEO films and CAFEO films added with 5, 10, 20 and 30% glycerol.
| Samples | Tensile strength (TS) | Young's Modulus (YM) | Elongation at break (EB) |
|---|---|---|---|
| CAFEO0%Glycerol | 29.92 ± 0.39b | 4.37 ± 0.27ab | 20.34 ± 5.26b |
| CAFEO5%Glycerol | 29.46 ± 2.41b | 3.67 ± 0.29bc | 28.64 ± 3.35a |
| CAFEO10%Glycerol | 35.29 ± 1.5a | 3.19 ± 0.26c | 12.54 ± 5.78c |
| CAFEO20%Glycerol | 37.05 ± 0.93a | 4.04 ± 0.51ab | 20.52 ± 1.52b |
| CAFEO30%Glycerol | 37.53 ± 0.58a | 4.41 ± 0.59ab | 10.11 ± 0.73c |
∗Average followed by the same letters do not differ from each other (p > 0.05) by the Tukey test at the 5% level of significance.
For Young's modulus, the only films that differed from the control film (CAFEO0%Glycerol) were 5 and 10% glycerol films (CAFEO5%Glycerol and CAFEO10%Glycerol) (Table 3), which were less rigid. It is expected that the higher the TS, the higher the YM, however, we can observe that, although CAFEO10%Glycerol film had one of the highest values for TS, it showed lower stiffness, ie, lower value for YM. According to SEM images (Fig. 2), the incorporation of glycerol caused alterations in the whole structure of the films, therefore, this may have allowed different behaviors due to mechanical stress. Thus, it can be predicted that the incorporation of 5 and 10% glycerol may have reduced the linear region of the stress versus strain curve, revealing materials with less plastic deformation (Canevaloro, 2006).
Data for elongation at rupture (EB) also reveal the effects of glycerol incorporation (Table 3). The highest elongations were presented by CAFEO5%Glycerol and CAFEO20%Glycerol films, while CAFEO10%Glycerol and CAFEO30%Glycerol films showed lower EB. CAFEO10%Glycerol film presented lower YM, but also had lower value for EB. Less rigid materials are expected to have greater elongation (Canevaloro, 2006), so it is believed that the presence of glycerol may have caused a reduction in both elastic and plastic deformation, revealing a film with rupture soon after application of mechanical stress.
Gonçalves et al. (2019) reported that CA film incorporated with 50% glycerol presented TS equal to 39.04 N, while Table 3 shows that CAFEO0%Glycerol film had TS equal to 29.92 N. Therefore, the addition of only 50% of FEO had superior effect when compared to 50% glycerol on CA film for TS. However, as mentioned, the hydrophobic characteristic of FEO (Reis et al., 2015) may have precluded its chemical interaction with the CA polymer matrix. Thus, it is believed that the FEO was trapped between the CA chains, pulling them apart and, consequently, reducing the TS. The SEM image shows that the inner region of the film with FEO (Fig. 2 CAFEO0%Glycerol F) had rounded structures, which, in turn, may be the trapped FEO droplets (Pontes, 2013). Thus, the possible non-miscible material characteristics may explain all the mechanical behavior variations of the films, which, in turn, were shown to be highly heterogeneous.
3.6. In vitro antimicrobial efficiency assessment
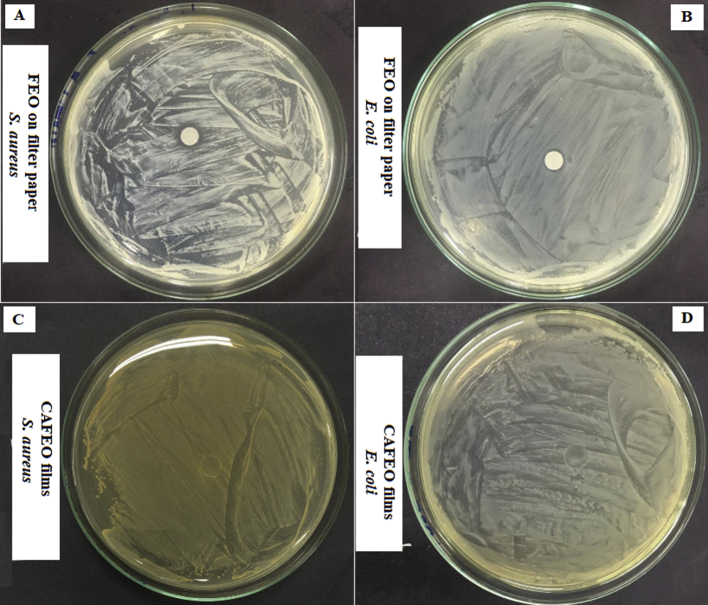
The results of the antimicrobial efficiency of FEO are shown in Fig. 3. A higher efficiency is observed for Staphylococcus aureus (Fig. 3A) than for Escherichia coli (Fig. 3B), with inhibition halos of 8.76 mm and 7.18 mm, respectively. However, no antimicrobial efficiency was observed for all films analyzed (Fig. 3C and D). The possible entrapment of the oil in the CA matrix may have prevented the FEO migration, or it may have migrated in insufficient quantity to inhibit the microbial load present. Thus, even with the addition of glycerol and consequent morphological changes visualized in the SEM images, such events were not sufficient to promote the release of FEO from the polymeric matrix to the culture medium.
Fig. 3.
Antimicrobial efficiency of FEO on filter paper for Staphylococcus aureus (A) and Escherichia coli (B); Antimicrobial efficiency of CAFEO films for Staphylococcus aureus (C) and Escherichia coli (D).
3.7. Principal component analysis (PCA) and Pearson's correlation
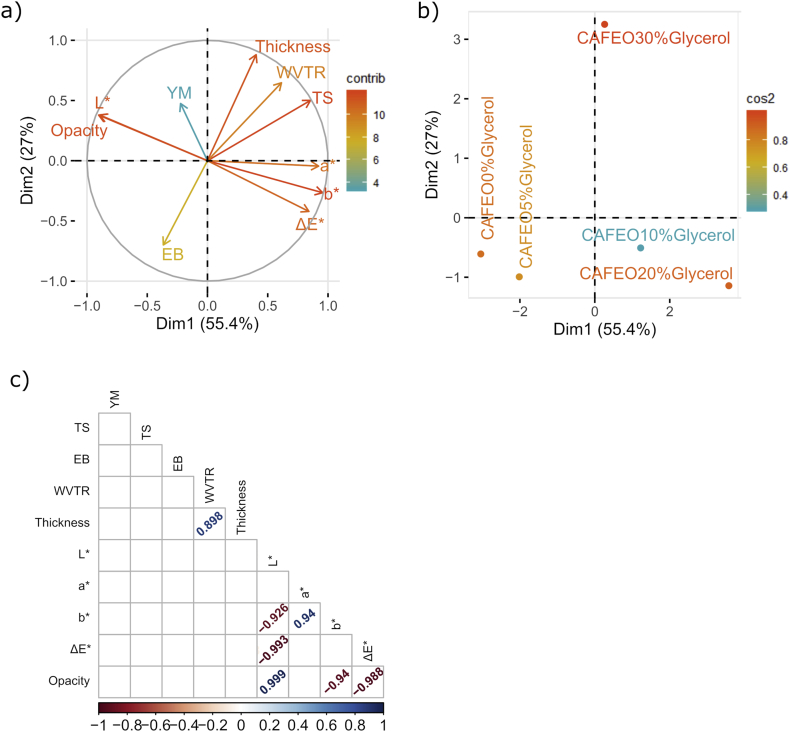
Fig. 4 (a and b) shows that PC1 (55.4%) and PC2 (27%) accounted for approximately 82.4% of the total variability of the data obtained in the study. Thickness, L∗, b∗, Opacity and TS (Fig. 4a) had the greatest influence on the differentiation of variables (greater influence on the most distant variables from the center (0)), while a∗, ΔE∗, WVTR and EB (Fig. 4a) showed lower influences (closer to the center (0)). It is noted that YM (Fig. 4a) had the lowest influence, which can be justified by the analysis of Table 3, where the values for YM were slightly changed in view of different glycerol concentrations. Observing Fig. 4a, it is noted that the thickness, WVTR and TS occupy the upper right quadrant, which is equivalent to CAFEO30%Glycerol film (Fig. 4b), which can be justified with Table 1, Table 3, where the films incorporated with 30% of glycerol presented the highest values for such variables. ΔE∗, a∗ and b∗ are located in the lower right quadrant (Fig. 4a), belonging to CAFEO10%Glycerol and CAFEO20%Glycerol films (Fig. 4b). L∗, opacity and YM occupy the upper left quadrant (Fig. 4a) between CAFEO0%Glycerol, CAFEO5%Glycerol and CAFEO30%Glycerol films (Fig. 4b), which corroborates the data presented in Table 2, Table 3. EB occupies, lower left quadrant (Fig. 4a), belonging to CAFEO5%Glycerol and CAFEO0%Glycerol films (Fig. 4b). However, according to the position at 4a and 4b, EB is closer to CAFEO5%Glycerol, which can be justified by Table 3, where the highest value for EB was found for the 5% glycerol film.
Fig. 4.
Principal component analyses (PCA), (a) PCA response variables and (b) PCA for treatments. CAFEO0%Glycerol is cellulose acetate film with fennel essential oil (CAFEO); CAFEO incorporated with glycerol (CAFEO5%Glycerol, CAFEO10%Glycerol, CAFEO20%Glycerol and CAFEO30%Glycerol). Pearson's correlation (c) for dependent variables for CAFEO and CAFEO with glycerol.
Fig. 4c shows strong or very strong positive correlations (Teles et al., 2019) shown in blue (thickness with WVTR; L∗ with opacity; a∗ with b∗). Variables with positive correlation are those that showed similar behavior in relation to different glycerol concentrations, that is, the same glycerol concentration was able to increase or decrease the a∗ and b∗, which is in accordance with the provisions of Table 2. Contrary situation, that is, strong or very strong negative correlations (negative values in red), were observed (L∗ with b∗; L∗ with ΔE∗; b∗ with opacity; ΔE∗ with opacity). For these variables, the same glycerol concentration was able to cause opposite responses, as shown in Table 2, where 20% glycerol (CAFEO20%Glycerol) caused a reduction in L∗ and an increase in ΔE∗ of the film. Therefore, it is concluded that the different glycerol concentrations caused changes in CA films for the studied variables.
4. Conclusion
The results indicate that the addition of different concentrations of glycerol in CAFEO films caused significant changes in their functional properties, detected in all evaluated parameters and confirmed by principal component analysis (PCA) and Pearson's correlation.
CA Films incorporating with glycerol proved to be thicker, reddish, yellowish, less opaque and luminous, and more to water vapor permeable. SEM images revealed surface alteration and presence of porosity in the internal region of all films with glycerol. In addition, the presence of glycerol caused increased tensile strength of the films. However, for the other mechanical parameters (Young's modulus and elongation at break), the films showed discrepant results, which reflect their heterogeneity.
In addition, some parameters (opacity and SEM) revealed that chemical incompatibility may have occurred between FEO and the CA matrix, which, in turn, negatively influenced the antimicrobial efficiency of the films for Staphylococcus aureus and Escherichia coli, since the antimicrobial effectiveness for pure FEO was positive. Therefore, there may not have been sufficient migration of FEO from the polymer matrix. Thus, given the exposed results, it is concluded that, most films are able to pack food, as long as the purpose is not the active antimicrobial conservation, since the films did not present adequate.
CRediT authorship contribution statement
Sheyla Moreira Gonçalves: Investigation, Writing - original draft, Validation. Joyce Fagundes Gomes Motta: Investigation, Writing - original draft. Regiane Ribeiro-Santos: Writing - original draft. Davy William Hidalgo Chávez: Formal analysis. Nathália Ramos de Melo: Resources, Funding acquisition, Supervision.
Declaration of Competing Interest
Authors declare no conflicts of interest.
Acknowledgements
This research was funded by the Coordination for the Improvement of Higher Education Personnel - Brazil - Financial Code 001, Brazilian Agricultural Research Corporation - Brazil (Embrapa), and Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro - Brazil (FAPERJ). In addition, the authors would also like to thank the Dr. Carlos Wanderlei Piler de Carvalho (Embrapa-Rio de Janeiro), Federal Rural University of Rio de Janeiro (UFRRJ) and the Federal University of Fluminense (UFF) for their support in the research.
References
- American Society for Testing and Materials – ASTM . Annual Book of ASTM Standards; Philadelphia: 2003. Designation: D1746: Standard Test Method for Transparency of Plastic Sheeting; p. 352. [Google Scholar]
- Barbut S., Harper B.A. Dried Ca-alginate films: effects of glycerol, relative humidity, soy fibers, and carrageenan. LWT. 2019;103:260–265. [Google Scholar]
- Canevaloro S.V., Jr. São Paulo: São Paulo; Artliber Editora: 2006. Ciência dos polímeros: um texto básico para tecnólogos e engenheiros, 2a edição. [Google Scholar]
- Diao Wen-Rui, Hu Qing-Ping, Zhang H., Xu Jian-Guo. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.) Food Contr. 2014;35:109–116. [Google Scholar]
- Dick M., Costa T.M.H., Gomaa A., Subirade M., Flôres S.H. Edible film production from chia seed mucilage: effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr. Polym. 2015;130:198–205. doi: 10.1016/j.carbpol.2015.05.040. [DOI] [PubMed] [Google Scholar]
- Galloto M.J., Guarda A., Dicastillo C.L.D. vol. 10. United States of America: Scrivener Publishing; 2015. Antimicrobial active polymers in food packaging. (Functional Polymers in Food Science: from Technology to Biology). [Google Scholar]
- Ghasemlou M., Khodaiyan F., Oromiehie A., Yarmand M.S. Characterization of edible emulsified films with low affinity to water based on kefiran and oleic acid. Int. J. Biol. Macromol. 2011;49:378–884. doi: 10.1016/j.ijbiomac.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Gonçalves S.M., Santos D.C., Motta J.F.G., Ribeiro-Santos R., Chávez D.W.H., Melo N.R. Structure and functional properties of cellulose acetate films incorporated with glycerol. Carbohydr. Polym. 2019;209:190–197. doi: 10.1016/j.carbpol.2019.01.031. [DOI] [PubMed] [Google Scholar]
- Jost V., Kobsik K., Schmid M., Noller K. Influence of plasticiser on the barrier, mechanical and greaseresistance properties of alginate cast films. Carbohydr. Polym. 2014;110:309–319. doi: 10.1016/j.carbpol.2014.03.096. [DOI] [PubMed] [Google Scholar]
- Ju J., Chen X., Xie Y., Yu H., Guo Y., Cheng Y., Quian H., Yao W. Application of essential oil as a sustained release preparation in food packaging. Trends Food Sci. Technol. 2019;92:22–32. [Google Scholar]
- Khaneghah A.M., Hashemi S.M.B., Limbo S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: an overview of approaches and interactions. Food Bioprod. Process. 2018;111:1–19. [Google Scholar]
- Kokoszka S., Debeaufort F., Hambleton A., Lenart A., Voilley A. Protein and glycerol contents affect physico-chemical properties of soy protein isolate-based edible films. Innovat. Food Sci. Emerg. Technol. 2010;11:503–510. [Google Scholar]
- Liang J., Xia Q., Wang S., Li J., Huang Q., Ludescher R.D. Influence of glycerol on the molecular mobility, oxygen permeability and microstructure of amorphous zein films. Food Hidrocolloids. 2015;44:94–100. [Google Scholar]
- Meireles C.S. Universidade Federal de Uberlândia; 2007. Desenvolvimento de nanoemulsões de óleos essenciais incorporadas em filme de metilcelulose para uso em alimentos, Dissertação (mestrado em Química) Uberlândia. [Google Scholar]
- Hasheminya Seyedeh-Maryam, Mokarram R.R., Ghanbarzadeh B., Hamishekar H., Kafil H.S., Dehghannya J. Development and characterization of biocomposite films made from kefiran, carboxymethyl cellulose and Satureja Khuzestanica essential oil. Food Chem. 2019;289:443–452. doi: 10.1016/j.foodchem.2019.03.076. [DOI] [PubMed] [Google Scholar]
- Moura M.R., Mattoso L.H., Zucolotto V. Development of cellulose-based bactericidal monocomposites containing silver nanoparticles and their use as active food packaging. J. Food Eng. 2012;109:520–524. [Google Scholar]
- Paolicelli P., Petralito S., Varani G., Nardoni M., Pacelli S., Di Muzio L., Tirillò J., Bartuli C., Cesa S., Casadei M.A., Adrover A. Effect of glycerol on the physical and mechanical properties of thin gellan gum films for oral drug delivery. Int. J. Pharm. 2018;547:226–234. doi: 10.1016/j.ijpharm.2018.05.046. [DOI] [PubMed] [Google Scholar]
- Pinheiro A.C., Cerqueira M.A., Souza B.W.S., Martins J.T., Teixeira J.A., Vicente A.A. Utilização de revestimentos/filmes edíveis para aplicações alimentares. Boletim de Biotecnologia. 2010;85:18–28. [Google Scholar]
- Plant R.M., Dinh L., Argo S., Shah M. The essentials of essential oils. Adv. Pediatr. 2019;66:111–112. doi: 10.1016/j.yapd.2019.03.005. [DOI] [PubMed] [Google Scholar]
- Pontes S.F.O. 2013. Desenvolvimento de nanoemulsões de óleos essenciais incorporadas em filme de metilcelulose para uso em alimentos, Tese (doutorado em Ciência e Tecnologia de Alimentos) - Universidade Federal de Viçosa – Viçosa. [Google Scholar]
- Prakash B., Kedia A., Mishra P.K., Dubey N.K. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities – potentials and challenges. Food Contr. 2015;47:381–391. [Google Scholar]
- Reis L.C.B., Souza C.O., Silva J.B.A., Martins A.C., Nunes I.L., Druzian J.I. Active biocomposites of cassava starch: the effect of yerba mate extract and mango pulp as antioxidant additives on the properties and the stability of a packaged product. Food Bioprod. Process. 2015;94:382–391. [Google Scholar]
- Ribeiro-Santos R., Andrade M., Sanches-Silva A. Application of encapsulated essential oils as antimicrobial agents in food packaging. Cur. Opin. Food Sci. 2017;14:78–84. [Google Scholar]
- Ribeiro-Santos R., Ventura L.A.F., Santos D.C., Melo N.R., Costa B.S. Effects of oregano, cinnamon, and sweet fennel essential oils and their blends on foodborne microorganisms. Int. Food Res. J. 2018;25:540–544. [Google Scholar]
- Sabbah M., Di Pierro P., Cammarota M., Dell'Olmo E., Arciello A., Porta R. Development and properties of new chitosan-based films plasticized with spermidine and/or glycerol. Food Hydrocolloids. 2019;87:245–252. [Google Scholar]
- Santos J., Alfaro M.C., Trujillo-Cayado L.A., Calero N., Muñoz J. Encapsulation of β-carotene in emulgels-based delivery systems formulated with sweet fennel oil. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2019;100:189–195. [Google Scholar]
- Sanyang M.L., Sapuan S.M., Jawaid M., Ishak M.R., Sahari J. Effect of plasticizer type and concentration on tensile, thermal and barrier properties of biodegradable films based on sugar palm (Arenga pinnata) starch. Polymers. 2015;7:1106–1124. doi: 10.1007/s13197-015-2009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederavičiūtė F., Bekampienė P., Domskienė J. Effect of pretreatment procedure on properties of Kombucha fermented bacterial cellulose membrane. Polym. Test. 2019;(78) [Google Scholar]
- Sleha R., Mosio P., Vydrzalova M., Jantovska A., Bostikova V., Mazurova J. In vitro antimicrobial activities of cinnamon bark oil, anethole, carvacrol, eugenol and guaiazulene against Mycoplasma hominis clinical isolates. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2014;158:208–211. doi: 10.5507/bp.2012.083. [DOI] [PubMed] [Google Scholar]
- Suderman N., Isa M.I.N., Sarbon N.M. The effect of plasticizers on the functional properties of biodegradable gelatin-based film: a review. Food Biosci. 2018;24:111–119. [Google Scholar]
- Swain S.N., Biswal S.M., Nanda P.K., Nayak P.L. Biodegradable soy-based plastics: opportunities and challenges. Journal of Polymer Environment. 2004;12:35–42. [Google Scholar]
- Teles A.S.C., Chávez D.W.H., Oliveira R.A., Bon E.P.S. Use of grape pomace for the production of hydrolytic enzymes by solid-state fermentation and recovery of its bioactive compounds. Food Res. Int. 2019;120:441–448. doi: 10.1016/j.foodres.2018.10.083. [DOI] [PubMed] [Google Scholar]
- Villalobos R., Chanona J., Hernández P., Gutiérrez G., Chiralt A. Gloss and transparency of hydroxypropyl methylcellulose films containing surfactants as affected by their microstructure. Food Hydrocolloids. 2005;19:53–61. [Google Scholar]
- Yun H., Kim M.K., Kwak H.W., Lee J.Y., Kim M.H., Lee K.H. The role of glycerol and water in flexible silk sericin film. Int. J. Biol. Macromol. 2016;82:945–951. doi: 10.1016/j.ijbiomac.2015.11.016. [DOI] [PubMed] [Google Scholar]