Abstract
Purpose of review:
Hepatopulmonary syndrome (HPS) and portopulmonary hypertension (PoPH) are both pulmonary vascular complications of advanced liver disease; however, these syndromes have distinct pathophysiology, clinical implications, and management.
Recent findings:
While both conditions are associated with portal hypertension, HPS results from diffuse pulmonary capillary vasodilation and PoPH results from vasoconstriction and vascular remodeling of pulmonary arteries. In HPS, no medical therapies clearly improve outcomes; however, patients have excellent post-LT outcomes with near uniform reversal of hypoxemia. In PoPH, several medical therapies used in idiopathic pulmonary hypertension have been shown improve pulmonary hemodynamics, symptoms, and potentially LT outcomes; however, further study is needed to determine best treatment regimens, long-term outcomes on medical therapy, and role of LT.
Summary:
While HPS results in severe hypoxemia that is usually reversible by LT, PoPH patients develop progressive pulmonary hypertension that may improve with medical therapy.
Keywords: hepatopulmonary syndrome, portopulmonary hypertension, liver transplant evaluation, intravascular pulmonary dilation, arterial hypoxemia with elevated A-a gradient, pulmonary hypertension medical therapy
Introduction
Over the last three decades, there has been increased recognition of two important pulmonary vascular complications of liver disease, hepatopulmonary syndrome (HPS) and portopulmonary hypertension (PoPH)(1). While there is a growing literature regarding the impact of these complications on survival and post-liver transplant (LT) outcomes, the pathophysiology and optimal therapeutic strategies are still not well characterized. It is, however, clear that HPS and PoPH are distinct syndromes with differing pathophysiology and clinical implications. This review summarizes our current understanding of these conditions and highlights areas in need of further study.
Hepatopulmonary syndrome
Definition and pathophysiology
HPS is defined as an arterial oxygenation defect secondary to intrapulmonary vascular dilatations (IPVD) in the setting of advanced liver disease. It occurs most commonly in patients with cirrhosis and portal hypertension; however, this syndrome has also been observed in non-cirrhotic portal hypertension, acute liver failure, chronic hepatitis, and congenital portosystemic shunts(1–5). The salient feature on histology is gross dilation of the pulmonary precapillary and capillary vessels up to 100 microns in diameter and an increase in the absolute number of dilated vessels(6,7). Evaluation of explanted livers in HPS patients who underwent LT also revealed increased portal venule thrombosis and extensive vascular proliferation within the liver, suggesting that alterations in the vascular bed of the liver may be a contributing factor(8).
The mechanisms that result in pulmonary vasodilation and vascular remodeling are still under investigation with the current understanding highlighted in Figure 1A. Clear predisposing factors include the presence of hepatic dysfunction and decrease venous blood flow throughout the liver(1,6,8), and while either factor appears to be sufficient as HPS is found in patients with non-cirrhotic portal hypertension and in liver disease without portal hypertension(2–5), the disease course may be more severe in patients with both liver injury and portal hypertension, suggesting at least an additive and potentially synergistic effect(9). Imaging in patients with cirrhosis and HPS demonstrates increased obstruction of intrahepatic portal branches, slowed or hepatofugal portal blood flow, and increased large (> 10mm) abdominal portosystemic shunts compared to patients with cirrhosis and no HPS(8). Animal models and small human studies suggest that four main pulmonary changes are important in the pathogenesis of HPS: (i) decreased vascular tone through increased levels of nitrous oxide (NO) and endothelin-1-induced endothelial dysfunction; (ii) monocyte infiltration of pulmonary vasculature possibly due to increased bacterial translocation from the gut; (iii) intrapulmonary arteriovenous shunt formation through vascular remodeling and angiogenesis; and (iv) defective ventilation and diffusing capacity due to alveolar type II cell dysfunction(10).
Figure 1:
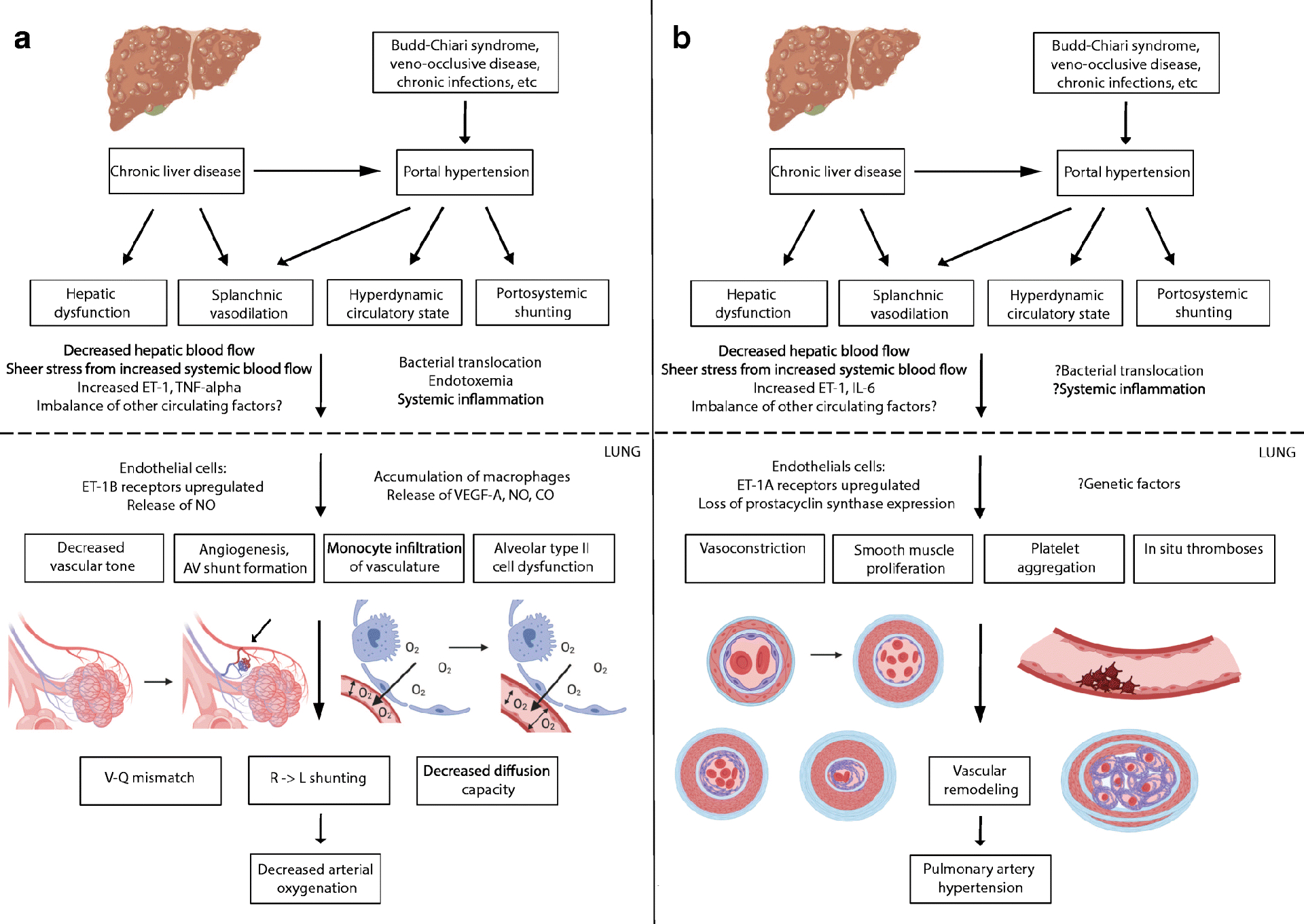
Pathophysiology of Hepatopulmonary Syndrome (A) and Portopulmonary Hypertension (B).
ET: endothelin 1; TNF-alpha: tumor necrosis factor- alpha; IL-6: interleukin-6; NO: nitrous oxide; VEGF: vascular endothelial growth factor; CO: carbon monoxide; AV: arteriovenous; V-Q mismatch: ventilation-perfusion mismatch; R -> L: right to left. Figure made using BioRender graphics.
From a physiologic perspective, abnormal oxygenation in HPS is the result of three distinct mechanisms(6). First and most fundamentally, pulmonary capillary dilation allows for rapid passage of mixed venous blood into the pulmonary veins, leading to a ventilation-perfusion mismatch. Second, pleural and pulmonary arteriovenous connections result in direct right-to-left shunting. Finally, the diffusion capacity of oxygen is decreased due to increased diffusion distance in dilated capillaries and/or thickening of capillary walls in prolonged disease(6,11). While portopulmonary venous anastomoses have also been reported, their effect on arterial oxygenation in HPS seems negligible(12).
Diagnosis
The most common presenting symptom in HPS is dyspnea(13), though a common finding in patients with cirrhosis (ie due to ascites, hepatic hydrothorax, PoPH, anemia, and decreased functional status). Similarly, spider nevi, digital clubbing, and cyanosis on physical examination may suggest the presence of HPS, but are non-specific findings and often only seen in patients with advanced disease(13,14). Platypnea (increased dyspnea when sitting up from supine) and orthodeoxia (decrease in oxygen saturation ≥ 5% from supine to upright positioning) are more sensitive and specific findings for HPS, though not pathognomonic(14,15). Further, oxygen saturation of < 96% by pulse oximetry has a sensitivity of only 28% in diagnosing HPS and 71% in detecting those with a partial pressure of arterial oxygen (PaO2) < 60 mmHg(16,17). As such, HPS should be considered in all patients with chronic liver disease, in patients with unexplained dyspnea, and those undergoing LT evaluation(18,19) (Figure 2).
Figure 2:
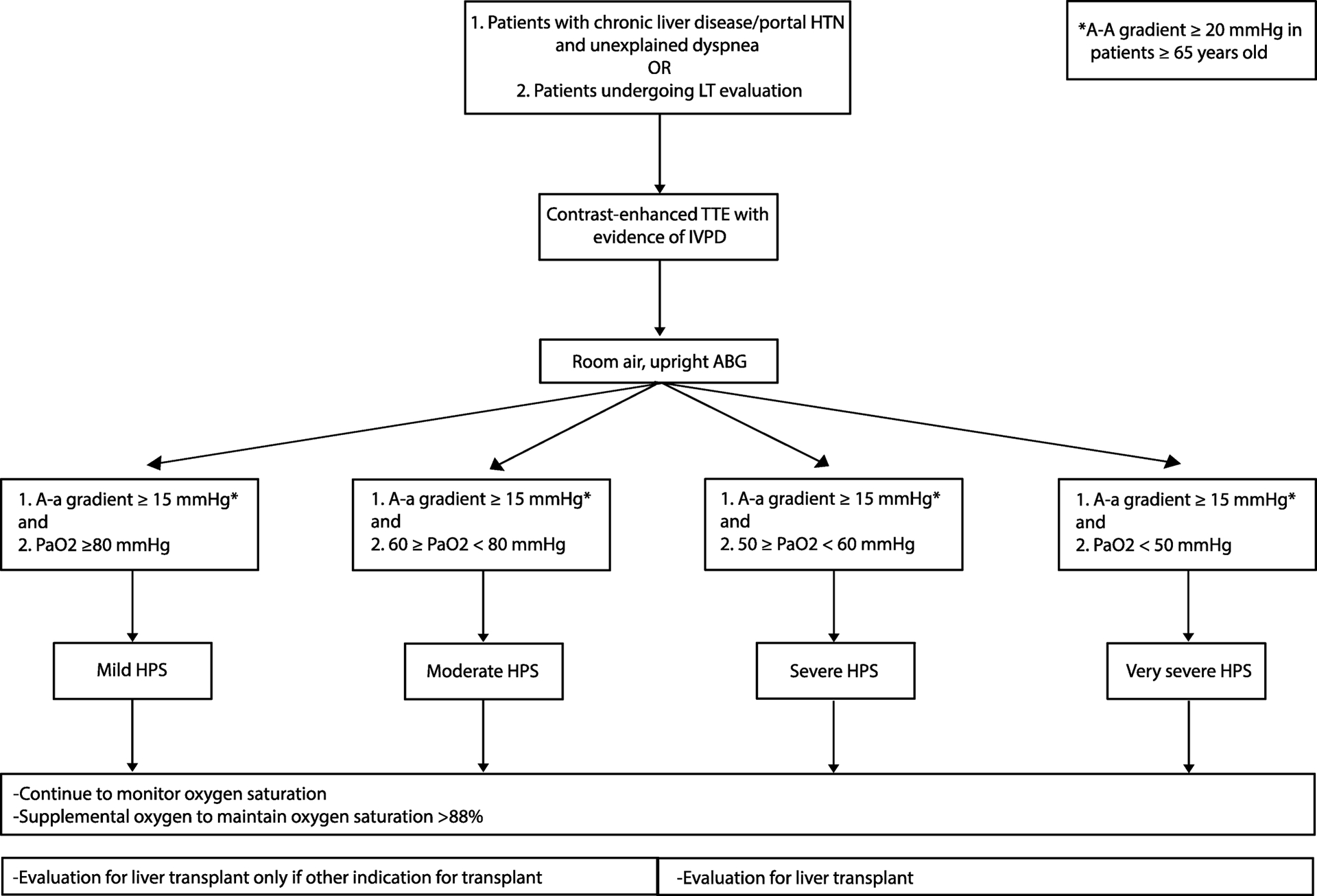
Work-up for Hepatopulmonary Syndrome (HPS).
Portal HTN: portal hypertension; LT: liver transplant; TTE: transthoracic echocardiography; IPVD: intrapulmonary vascular dilation; ABG: arterial blood gas; A-a gradient: alveolar-arterial oxygen gradient; PaO2: partial pressure of arterial oxygen; MELD: model for end-stage liver disease.
The diagnostic criteria for HPS includes (i) chronic liver disease, non-cirrhotic portal hypertension, or other causes of cavopulmonary shunt; (ii) arterial hypoxemia on an upright, room air arterial blood gas defined as an alveolar-arterial oxygen (A-a) gradient ≥ 15 mmHg (≥ 20 mmHg if ≥ 65 years old); and (iii) IPVD as demonstrated by positive contrast-enhanced transthoracic echocardiogram (CE-TTE)(1,18) (Table 1). While there has been debate regarding the appropriate definition of arterial hypoxemia(20), the A-a gradient incorporates the atmospheric pressure, adjusting for changes with altitude, and the partial pressure of arterial carbon dioxide, frequently low in patients with cirrhosis due to hyperventilation(1,6). The severity of HPS is defined by room air PaO2 with ≥ 80 mmHg being mild disease, ≥ 60 to < 80 mmHg moderate disease, ≥ 50 to <60 mmHg severe, and < 50 mmHg very severe(1). Arterial hypoxemia should trigger further diagnostic work-up, including chest x-ray (CXR), pulmonary function tests (PFTs), and chest computed tomography (CT) to evaluate for other causes of hypoxemia. CXR and chest CT may show bibasilar interstitial markings and dilated peripheral pulmonary vessels with increased pulmonary artery to bronchus ratio, respectively(1,21). PFTs may demonstrate decrease diffusion capacity, especially in advanced disease.
Table 1.
Considerations for Liver Transplant Evaluation
| Hepatopulmonary syndrome | Portopulmonary hypertension |
|---|---|
| Screening: | Screening: |
| Diagnostic criteria: | Diagnostic criteria:
|
Indication for liver transplant:
|
Not an indication for liver transplant: |
CE-TTE: contrast-enhanced transesophageal echocardiography; eRVSP: estimated right ventricle systolic pressure; RHC: right heart catherization; A-a gradient : arterial alveolar oxygen gradient; PAH: pulmonary arterial hypertension; PVR: pulmonary vascular resistance; PCWP: pulmonary capillary wedge pressure; IVPD: intrapulmonary vascular dilation; 99mTcMAA: technetium-99m macroaggregated albumin; MELD: model for end stage liver disease; PaO2: partial pressure of arterial oxygen; RV: right ventricle
CE-TTE is the preferred method for detection of IPVD(1,18,22). Hand-agitated saline is administrated peripherally, creating microbubbles of 10–20 microns, which in patients with diffuse capillary dilations or discrete arteriovenous connections will pass through the pulmonary circulation. CE-TTE is considered positive if microbubbles appear in the left atrium within 3 to 6 cardiac cycles after being visualized in the right heart, and, if within 3 cardiac cycles, suggestive of a right-to-left intracardiac shunt(22,23). An alternative method to detect right-to-left shunting is technetium-99m macroaggregated albumin (99mTcMAA) lung perfusion scan, which follows the same principle as CE-TTE given aggregates of 20–90 microns(22,24). The study is considered positive if there is >6% uptake in the brain. Data suggests that CE-TTE is more sensitive for the detection of IPVD in adults, though the opposite may be true in children(22,25). Lung perfusion scans are unable to distinguish between intracardiac and intrapulmonary shunting; however, they are useful in quantifying the degree of hypoxemia caused by HPS, especially in patients with multiple causes of hypoxemia(26,27). Pulmonary angiography is not useful in the routine diagnosis of IPVD(1).
Prevalence and natural history
Epidemiologic data on HPS comes largely from transplant centers with reported prevalence from 4 to 47% in patients with chronic liver disease, averaging approximately 30%(13,14,20,27–31). The large variability in prevalence results from differences in the definition of hypoxemia, diagnostic method employed, and population studied. While more common in adult patients, HPS can also occur in children with chronic liver disease with an estimated prevalence of 3–20%(32). The majority of studies in adult patients report no difference in HPS prevalence based on age, gender, sex, or etiology of liver disease(27–30); however, the largest study of 973 patients with HPS listed for LT reported a higher percentage of woman, non-Hispanic white ethnicity, and hepatitis C and non-alcoholic steatohepatitis cirrhosis compared to patients listed without HPS(33). There is also conflicting data on the association between severity of liver disease or portal hypertension and the presence or severity of HPS(14,20,27–29,31,34,35). It is, however, clear that patients with chronic liver disease and HPS have decreased quality of life and greater than two-fold increase in mortality compared to patients without HPS(13,29,30). Without intervention, HPS patients have a 5-year survival of 20% with cause of death generally due to progressive hepatic dysfunction or other comorbidities rather than secondary to progressive hypoxemia; however, the severity of HPS does appear to be predictive of overall survival(29,30).
Management
Currently, the only non-surgical treatment for HPS is supplemental oxygen. There is no proven survival benefit, but, based on experience with other pulmonary conditions and improvement in symptoms, supplemental oxygen is used to maintain oxygen saturation > 88% during rest, exercise, and sleep(18). LT is the only intervention that has been shown to reverse the underlying pathology of HPS and improve survival(27–30,33,36,37). In recent studies, the 5-year post-LT survival rate is 76% for HPS patients, comparable to survival in patients without HPS(33,36,37). Nearly all HPS patients who undergo LT have resolution of hypoxemia and pulmonary vasodilatation, though it may take several months, especially in patients with severe hypoxemia pre-LT(27–30). Recurrence of HPS is extremely rare and the reported cases are in patients who have developed recurrent cirrhosis and portal hypertension(38). POPH developing months to years after the resolution of HPS post-LT has also been reported(39).
There are several important considerations when evaluating patients with HPS for LT, including (i) which HPS patients should be considered; (ii) should these patients receive MELD exception points; and (iii) how can peri-operative risk be reduced in this population. Given the unclear correlation between severity of liver disease and severity of HPS, along with poor outcomes in HPS patients without LT, most major transplant centers and liver societies agree that severe hypoxemia (i.e. room air PaO2 <60 mmHg) from HPS is an indication for LT regardless of degree of hepatic dysfunction(1,18,19); however, there is debate regarding LT for HPS patients with very severe hypoxemia (i.e. room air PaO2 < 50 mmHg). Several multicenter studies, including an analysis of the United Network for Organ Sharing (UNOS) database, have reported increased post-LT morbidity and mortality in HPS patients if PaO2 ≤ 50 mmHg (or ≤ 44 mmHg in one study), raising the question of whether there is a degree of hypoxemia that should be considered too high risk for transplant(27,30,33). On the other hand, 5-year post-LT survival for these patients are still at or above survival for other indications for LT, and these patients have a dismal prognosis without LT. Finally, two more recent single-center studies reported no difference in post-LT mortality based on arterial hypoxemia, suggesting possible center-specific expertise and improved peri-operative care for these patients may play a role(36,37).
Currently, all patients with HPS who have PaO2 < 60 mmHg on room air and no clinically significant pulmonary comorbidities are eligible to receive standard MELD exception points (Table 1). Two analyses of UNOS data under the prior MELD exception system (i.e. 22 points at listing, increased by 3 points every 3 months) showed patients with HPS had lower waitlist mortality than patients without HPS but excellent post-LT outcomes, suggesting an advantage in mild HPS patients while disadvantaging those with severe HPS(33,36). In our current system for standard MELD exception points (listed with median MELD at transplant for center minus 3 points), there remains no difference in MELD exception based on degree of hypoxemia < 60 mmHg.
Regardless, with access to MELD exception points and increased experience caring for these patients, the mortality for HPS patients undergoing LT has decreased over the last few decades. There is, however, still substantial peri-operative morbidity and mortality. Current guidelines suggest the following recommendations to optimize peri-transplant care: (i) continuous intraoperative monitoring of mixed venous oxygen saturation and consideration of extracorporeal membrane oxygenation (ECMO) if < 65%; (ii) early extubation to decrease incidence of ventilator-associated pneumonia; (iii) delivery of 100% inspired oxygen via non-invasive methods to maintain oxygen saturation ≥ 85%; and (iv) goal-directed fluid therapy to avoid fluid overload(18). Risk factors for refractory hypoxemia post-operatively are pre-LT PaO2 ≤ 50 mmHg and intrapulmonary shunt ≥ 20%(40), though as mentioned previously the data are controversial regarding the impact of these risk factors on overall survival. Nayyar et al proposed an algorithm for the management of these patients with refractory hypoxemia, including Trendelenberg positioning, inhaled pulmonary vasodilators, intravenous methylene blue, and, ultimately, consideration of ECMO(41).
Given the morbidity associated with LT and the limited donor organ supply, there is great interest in developing novel interventions and medical therapies for HPS (Table 2). Procedural interventions have possible benefit in select cases, including TIPS(42–44), pulmonary angiography for coil embolization(45,46), and correction of congenital portosystemic shunts in children(5). Pentoxifylline has been shown to reduce hypoxemia in animal models, but human studies have been mixed and no randomized controlled studies exist(47–49). Interestingly, garlic extract has been reported to improve oxygenation and potentially even reverse HPS presumably through altering NO production; however, reports of significant drug-induced liver injury have limited its therapeutic potential(50,51). Further investigation into alternative angiogenesis inhibitors, modulators of endothelin and NO, agents that reduce systemic inflammation and pulmonary monocyte recruitment, and protectors of AT2 cell function are ongoing(10).
Table 2.
Non-surgical Therapies for Hepatopulmonary Syndrome and Portopulmonary Hypertension
| Hepatopulmonary syndrome | Portopulmonary hypertension | ||
|---|---|---|---|
| Supplemental oxygen | Maintain SpO2 >88%, extrapolated from studies of hypoxemia from other etiologies(18) | Supplemental oxygen | Maintain SpO2 >88%, extrapolated from studies of hypoxemia from other etiologies(18) |
| TIPS | Transient improvement in hypoxemia for 1–3 months, unclear long-term benefit. Possible increased benefit using left branch of PV(42–44) | TIPS | Contraindicated in moderate to severe disease(1,83,84) |
| Coil embolization | Most useful in cases of severe hypoxemia and discrete AV connections, possible case of PAH following treatment(45,46,105) | Anti-coagulation | No clear benefit in PAH patients(78,79) |
| Congenital portosystemic shunts | Correction leads to improvement in oxygenation(5) | Calcium channel blockers | No clear benefit in PAH patients, possible harm(80,81) |
| Inhaled L-NAME | Reduced hypoxemia in experimental models, no clear benefit in human studies(106,107) | Beta-blockers | Consider stopping in moderate to severe disease, banding for variceal bleeding prevention(82) |
| Inhaled iloprost | No clear benefit in small study(108) | Prostacyclin analogues | IV Epoprostenol, treprostinil with improved hemodynamics in small studies. Ongoing studies of inhaled iloprost and oral berprost, treprostinil, and selexipag(87–90) |
| Garlic extract | Improved oxygenation in 3 small studies, though reports of DILI(50,51) | Endothelin receptor antagonists | Ambrisentan and bosentan with improved symptoms and hemodynamics in small studies. Currently enrolling RCT of macitentan vs placebo(92–94) |
| Norfloxacin | Reduced hypoxemia in experimental models, no clear benefit in human studies(109) | Phospho- diesterase-5 inhibitors | Sildenafil with increased functional class, exercise tolerance, and hemodynamics in small studies. Ongoing investigation of oral tadalafil(95–97) |
| Pentoxyfylline | Reduced hypoxemia in experimental models, no clear benefit in human studies(47–49) | Riociguat | Improved exercise capacity in RCT of PAH patients, including 13 patients with PoPH(85) |
| Sorafenib | No clear benefit(110) | Combination therapy | Sildenafil or bosentan with prostacyclin showed improved hemodynamics in small studies(91,98) |
SpO2: peripheral oxygen saturation; TIPS: transjugular intrahepatic portosystemic shunt; PV: portal vein; AV: arteriovenous; PAH: pulmonary arterial hypertension; L-NAME: L-N-nitro arginine methyl ester; DILI: drug-induced liver injury; RCT: randomized controlled trial; PoPH portopulmonary hypertension
Portopulmonary hypertension
Definition and pathophysiology
PoPH is defined as pulmonary arterial hypertension (PAH) in the setting of portal hypertension with or without intrinsic liver disease. From a pathologic perspective, the pulmonary vascular remodeling of PoPH is identical to idiopathic PAH on autopsy and lung explant studies(52). As detailed in Figure 1B, findings include pulmonary artery vasoconstriction, medial hypertrophy due to smooth muscle proliferation, and, over time, intimal fibrosis and ultimately plexogenic arteriopathy from transition to fibroblasts. These vascular changes are associated with platelet aggregates and in situ thromboses, leading to pulmonary artery obstruction. The exact mechanisms of this progression are not fully understood, but hepatic injury, splanchnic vasodilation, hyperdynamic state, and portosystemic shunting have all been proposed as mechanisms that increase production and/or decreased clearance of circulating factors that facilitate remodeling(53). Patients with cirrhosis and PoPH have increased levels of endothelin-1 and interleukin-6(54,55) and decreased levels of prostacyclin compare to patients with cirrhosis without PoPH. Several other circulating factors, including thromboxane A2, serotonin, vasoactive intestinal peptide, estrogen, and other interleukins, have also been suggested to play a role, though further study is needed(53).
Diagnosis
The most common presenting symptom in PoPH is exertional dyspnea(1). Other non-specific symptoms can include dyspnea at rest, chest pain, palpitations, pre-syncope, and syncope. Physical examination signs include those of pulmonary hypertension (accentuated and split S2, right ventricular heave) and right heart failure (right-sided S3 gallop, jugular venous distension, ascites, and lower extremity edema); however, in mild POPH, patients may be asymptomatic with a normal exam(57). Mild hypoxemia is a common finding, but it is rare to have clubbing, cyanosis, and severe hypoxemia as seen in HPS. CXR may show cardiomegaly and enlargement of central pulmonary arteries with rightward axis and right bundle branch block on electrocardiogram but these are late findings. As such, all patients with unexplained dyspnea and/or hypoxemia and all patients being evaluated for LT should undergo TTE for further evaluation(18,58,59) (Figure 3). Elevated estimated right ventricular systolic pressure (RVSP) requires further hemodynamic assessment by right heart catheterization (RHC) to confirm mean pulmonary arterial pressure (mPAP) and rule out other causes of pulmonary hypertension, There is debate over the appropriate cutoff for RSVP requiring further evaluation, and some have argued that RSVP is too inaccurate be used for screening, especially in this population, as 20% of patients with cirrhosis and portal hypertension have a mildly elevated mPAP due to their high-flow state(58–60). Other TTE parameters have been proposed to increased specificity for PAH(61,62). Currently, the AASLD recommends RHC in patients with RVSP ≥ 45 mmHg and/or other evidence of elevated mPAP on TTE, while the ERS Task Force and Mayo Clinic use RVSP ≥ 50 mmHg(1,18,19,59).
Figure 3:
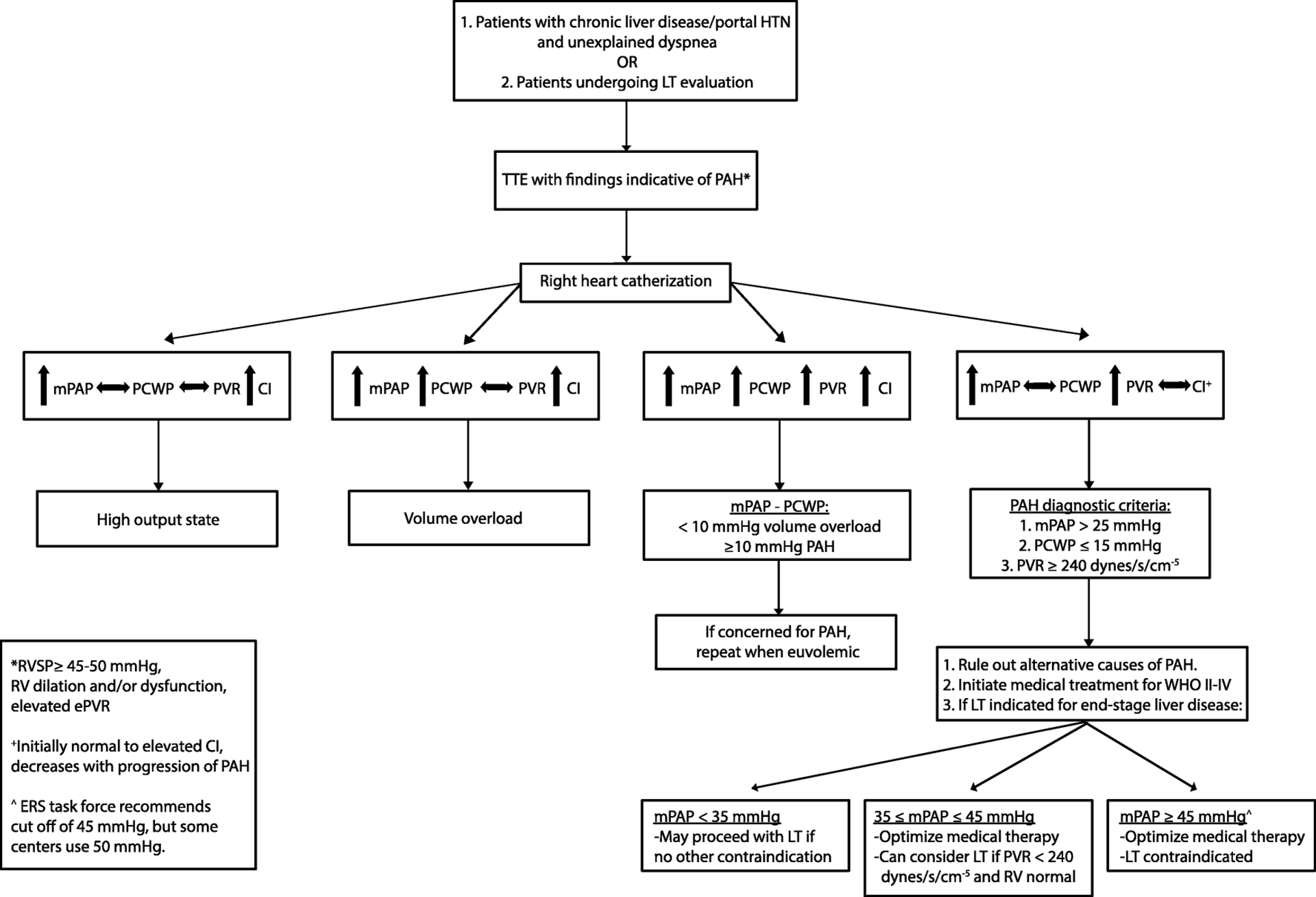
Work-up for Portopulmonary Hypertension (PoPH).
Portal HTN: portal hypertension; LT: liver transplant; TTE: transthoracic echocardiography; PAH: pulmonary arterial hypertension; mPAP: mean pulmonary artery pressure; PCWP: pulmonary capillary wedge pressure; PVR: pulmonary vascular resistance; CI: cardiac index; WHO II-IV: World Health Organization functional class II-IV; RVSP: right ventricular systolic pressure; RV: right ventricle; ePVR: estimated pulmonary vascular resistance; ERS: European Respiratory Society
Diagnostic criteria for PoPH include a clinical diagnosis of portal hypertension and evidence of PAH as defined by (i) mPAP > 25 mmHg on RHC; (ii) peripheral vascular resistance (PVR) > 3 Woods units (240 dynes/s per cm−5); and (iii) pulmonary capillary wedge pressure (PCWP) < 15 mm Hg (Table 1). The severity of PoPH is based on RHC and graded as mild (25 ≤ mPAP < 35 mmHg), moderate (35 ≤ mPAP < 45 mmHg), and severe (mPAP ≥ 45 mmHg(1). Notably, not all patients with an elevated mPAP have PAH, as the high-flow state observed in many cirrhotic patients can lead to high mPAP but normal PVR. Left-sided volume overload can also lead to an elevated mPAP, but normal PVR and increased PCWP. Similarly, patients with PoPH can also have left-sided dysfunction, which, especially in the setting of concurrent renal dysfunction, can worsen overall volume overload and lead to an elevated PCWP. In these cases, a transpulmonary gradient (i.e. mPAP – PCWP) greater than 10 mmHg is suggestive of increased pulmonary resistance and should be re-evaluated once volume status is optimized(1). Work-up also requires excluding other causes of PAH with PFTs, sleep study, ventilation-perfusion scan, antinuclear antibody, screening for human immunodeficiency virus, and any other testing indicated by the clinical presentation(57). Compared with idiopathic PAH, patients with PoPH generally have higher cardiac output (CO) and less severe PAH by mPAP and PVR(63).
Prevalence and Natural History
PoPH, more rare than HPS, is present in approximately 0.7% of patients with cirrhosis, 2% of patients with portal hypertension, and 5–9% of patients undergoing LT evaluation(59,64–67), and accounts for 5–15% of PAH cases(68,69). While uncommon, it can also occur in children(70). Female sex, autoimmune etiologies of liver disease, elevated estradiol levels, large spontaneous or surgical portosystemic shunts, portocaval shunts, hepatofugal blood flow, and splenectomy have been reported as possible risk factors for PoPH(71–74); however, the severity of liver disease determined by MELD or CTP scores and the degree of portal hypertension assessed by hepatic venous pressure gradient (HVPG) do not correlate with the presence or severity of PoPH(65,71). Without treatment, patients have a 1- and 5-year survival between 35–46% and 4–14%, respectively(75,76), and cause of death is split between right heart failure and complications of liver disease. As demonstrated in the REVEAL registry, PoPH patients have worse survival than idiopathic PAH despite higher CO and lower PVR (40% vs 60% survival at 5 years, respectively)(63). Notably, given the baseline high output state in patients with cirrhosis, the finding of “normal CO” in a patient with PoPH is actually a sign of significant RV dysfunction, which may partially explain this discrepancy. Additionally, the investigators noted a delay in initiating PAH-specific therapy in the PoPH group, likely due to the exclusion of PoPH patients from randomized controlled trials of PAH treatments.
Management
Several studies suggest improved survival and quality of life after initiation of medical therapy in PoPH patients regardless of LT; however, these were retrospective cohort studies, often with inclusion of historical controls, and small uncontrolled prospective studies(77). The true impact of medical therapy on survival and optimal PAH-directed regimens are still unknown and are extrapolated from data in other etiologies of PAH (Table 2). As there is uncertain benefit from anticoagulation in PAH and increased bleeding risk in patients with portal hypertension, anticoagulation is not recommended(78,79). Calcium channel blockers should be avoided due to lack of established benefit and potential to worsen portal hypertension(80,81). In moderate-to-severe PoPH, withdrawal of beta-blockade should be considered along with an alternative method for prevention of variceal bleeding (i.e. band ligation)(82). Severe PoPH and heart failure are absolute contraindications to TIPS, and moderate PoPH is a relative contraindication and TIPS should be avoided in these patients if possible(83,84).
In addition to diuretic therapy, PAH-directed therapy should be considered for PoPH patients with mPAP ≥ 35 mmHg (Table 2)(1,59). The only randomized controlled trial that included PoPH patients evaluated riociguat, a soluble guanylate cyclase stimulator, and included 13 PoPH patients of which 11 received riociguat. As such, no conclusions could be made about efficacy in PoPH, though overall patients with PAH had improved exercise capacity(85). Continuous infusion of prostacyclin analogues (esoprostenol, treprostinil) have been used to improve hemodynamics as a bridge to LT with success, though there are technical challenges to continuous infusion in patients prone to hepatic encephalopathy(86–89). Investigation of inhaled iloprost, oral beraprost (not available in the US), and several other oral prostacyclin anaglogues (oral treprostinil, selexipag) are ongoing(90,91). Oral endothelin receptor antagonists (ambrisentan, bosentan) have shown promising results in small studies of PoPH patients, including improved hemodynamics, decreased symptoms, and, for bosentan, improved survival compared to those treated with inhaled iloprost(92–94). Notably, approximately 10% of PAH patients have had liver toxicity with bosentan, though this has not been reported with ambrisentan to date. Similarly, phosphodiesterase-5 inhibitors (sildenafil, possibly tadalafil) may increase functional class, exercise tolerance, and improve hemodynamics in PoPH patients(95–97). Sildenafil has also been used in combination with prostacylins and bosentan with improved hemodynamics noted in 11 and 7 patients, respectively(91,98).
Most published studies on LT outcomes in PoPH patients are small, retrospective, and uncontrolled, using variable hemodynamic cutoffs and medical therapy. Overall, post-LT outcomes are highly variable, though there is increasing literature to support the use of medical therapy as a bridge to LT and normalization of pulmonary hemodynamics following LT in some patients.(28,67,76,86,98,99). Studies prior to availability of medical therapy for PoPH reported no increase in perioperative mortality if mPAP was < 35 mmHg, but significantly increased mortality if mPAP ≥ 35 mmHg (100% mortality if mPAP ≥ 50 mmHg in one study)(28,100,101). As such, PoPH patients are generally considered for LT if their baseline mPAP is < 35 mmHg, though the presence of mild PoPH itself is not an indication for transplant (Table 1)(1,18,59). Patients with PoPH and mPAP ≥ 35 mmHg are recommended for medical optimization first with consideration for listing and allocation of MELD exception points if on treatment mPAP < 35 mmHg and PVR < 5 Woods units (400 dynes/s per cm−5). Patients can also be considered for LT if mPAP ≥ 35 on treatment but PVR < 240 dynes/s per cm−5 with normal RV function. Per the ERS Task force, mPAP ≥ 45 mmHg is considered an absolute contraindication to transplant, though some transplant centers use mPAP ≥ 50 mmHg(18,59). With these general guidelines, 5-year post-LT survival for PoPH patients is 63–67% based on available data(76,86,102,103). Analysis of the UNOS database from 2006 to 2012 reported increased waitlist and post-LT mortality in patients who met PoPH criteria and received MELD exception points compared to patients without PoPH (5-year survival 64% vs 70%, respectively)(103). Notably, in this study and others, mortality is high within the first year- some studies reporting over 50% of deaths within 30-days of LT and up to 36% in-hospital mortality(28,103).
There are several important considerations to optimization of post-LT outcomes for PoPH patients. All patients should have a pulmonary artery catheter placed before the abdominal incision is made(1,18), and, if mPAP ≥ 35 mmHg, a full hemodynamic assessment with CO, PCWP, and PVR repeated, with case cancellation if mPAP ≥ 45–50 mmHg or acute therapy is unable to lower mPAP to < 40 mmHg with normal PVR. PAH-targeted therapy should be continued during the transplant surgery with transesophageal echocardiography (TEE) available if needed for RV monitoring. Reperfusion of the transplanted liver is an especially critical time during the procedure, as the acute rise in PA pressures may precipitate RV failure(104). Various interventions have been utilized including inhaled NO, intravenous prostacyclin, milrinone, and ECMO. RV assist devices should not be used due to potential for PA rupture. Immediately following transplant, major changes in PAH-directed therapy should be avoided and patients should be monitored with serial TTE every 4 to 6 months and consideration for weaning of medical therapy in patients with normalized hemodynamics. There is no clear method for the execution of this process and studies report variable outcomes with 33–82% of patients weaned off therapy over months to years post-LT(67,76,86,98,99).
Conclusions
While often discussed together, HPS and PoPH are distinct pulmonary vascular sequelae of liver disease with unique pathophysiology and clinical implications. HPS is characterized by arterial hypoxemia secondary to IPVD, and, while there are no effective medical therapies, LT usually facilitate complete resolution of hypoxemia and outcomes are excellent. On the other hand, patients with PoPH rarely have significant hypoxemia and instead develop progressive right heart failure from PAH. PoPH patients have highly variable outcomes with LT and high peri-operative mortality; however, several promising medical therapies are available to help minimize these risks in well selected LT candidates. Further study is needed in both HPS and PoPH to determine the best allocation strategy to minimize waitlist mortality and maximize post-LT survival.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Disclosures:
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Rodríguez-Roisin R, Krowka MJ, Hervé P, Fallon MB, Barberá JA, Cáneva JO, et al. Pulmonary-hepatic vascular disorders (PHD). Eur Respir J. 2004. November;24(5):861–80. [DOI] [PubMed] [Google Scholar]
- 2.De BK, Sen S, Biswas PK, Mandal SK, Das D, Das U, et al. Occurrence of hepatopulmonary syndrome in Budd-Chiari syndrome and the role of venous decompression. Gastroenterology. 2002;122(4):897–903. [DOI] [PubMed] [Google Scholar]
- 3.Regev A, Yeshurun M, Rodriguez M, Sagie A, Neff GW, Molina EG, et al. Transient hepatopulmonary syndrome in a patient with acute hepatitis A. J Viral Hepat. 2001;8(1):83–6. [DOI] [PubMed] [Google Scholar]
- 4.Teuber G, Teupe C, Dietrich CF, Caspary WF, Buhl R, Zeuzem S. Pulmonary dysfunction in non-cirrhotic patients with chronic viral hepatitis. Eur J Intern Med. 2002;13(5):311–8. [DOI] [PubMed] [Google Scholar]
- 5.Baiges A, Turon F, Simón-Talero M, Tasayco S, Bueno J, Zekrini K, et al. Congenital Extrahepatic Portosystemic Shunts (Abernethy Malformation): An International Observational Study. Hepatology. 2020;71(2):658–69. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary Syndrome-A Liver-Induced Lung Vascular Disorder. N Engl J Med. 2008;458(22):2378–87. [DOI] [PubMed] [Google Scholar]
- 7.Berthelot P, Walker JG, Sherlock S, Reid L. Arterial Changes in the Lungs in Cirrhosis of the Liver — Lung Spider Nevi. N Engl J Med. 1966;274(6):291–8. [DOI] [PubMed] [Google Scholar]
- 8. *.Lejealle C, Paradis V, Bruno O, de Raucourt E, Francoz C, Soubrane O, et al. Evidence for an Association Between Intrahepatic Vascular Changes and the Development of Hepatopulmonary Syndrome. Chest. 2019. January 1;155(1):123–36. [DOI] [PubMed] [Google Scholar]; -New evidence that there are vascular changes within the liver itself as well as pulmonary and splanchnic vascular changes in HPS.
- 9.Borkar VV, Poddar U, Kapoor A, Ns S, Srivastava A, Yachha SK Hepatopulmonary Syndrome in children: A comparative study of non-cirrhotic vs. cirrhotic portal hypertension. Liver Int. 2015;35(6):1665–72. [DOI] [PubMed] [Google Scholar]
- 10. *.Raevens S, Fallon MB. Potential Clinical Targets in Hepatopulmonary Syndrome: Lessons From Experimental Models. Hepatology. 2018;68(5):2016–28. [DOI] [PMC free article] [PubMed] [Google Scholar]; -Excellent review of experimental models of HPS and potential clinical targets for treatments.
- 11.Stanley NN, Williams AJ, Dewar CA, Blendis LM, Reid L. Hypoxia and hydrothoraces in a case of liver cirrhosis: Correlation of physiological, radiographic, scintigraphic, and pathological findings. Thorax 1977. August 1;32(4):457–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigue Roisin, Agusti R The hepatopulmonary syndrome: new name, old complexities. Thorax. 1992;47:897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fallon MB, Krowka MJ, Brown RS, Trotter JF, Zacks S, Roberts KE, et al. Impact of Hepatopulmonary Syndrome on Quality of Life and Survival in Liver Transplant Candidates. Gastroenterology. 2008;135(4):1168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Younis I, Sarwar S, Butt Z, Tanveer S, Qaadir A, Jadoon NA. Clinical characteristics, predictors, and survival among patients with hepatopulmonary syndrome. Ann Hepatol. 2015. May 1;14(3):354–60. [PubMed] [Google Scholar]
- 15.Krowka MJ, Dickson ER, Cortese DA. Hepatopulmonary syndrome: Clinical observations and lack of therapeutic response to somatostatin analogue. Chest. 1993;104(2):515–21. [DOI] [PubMed] [Google Scholar]
- 16. *.Forde KA, Fallon MB, Krowka MJ, Sprys M, Goldberg DS, Krok KL, et al. Pulse Oximetry Is Insensitive for Detection of Hepatopulmonary Syndrome in Patients Evaluated for Liver Transplantation. Hepatology. 2019. January;69(1):270–81. [DOI] [PMC free article] [PubMed] [Google Scholar]; -Prior guidelines (institutions, national, international) have suggested pulse oximetry for HPS screening in adult patients undergoing liver transplantation evaluation, but this study suggests this is inadequate and all patients need contrast-enhanced echocardiography.
- 17.Hoerning A, Raub S, Neudorf U, Müntjes C, Kathemann S, Lainka E, et al. Pulse oximetry is insufficient for timely diagnosis of hepatopulmonary syndrome in children with liver cirrhosis. J Pediatr. 2014. March 1;164(3):546–552.e2. [DOI] [PubMed] [Google Scholar]
- 18. **.Krowka MJ, Fallon MB, Kawut SM, Fuhrmann V, Heimbach JK, Ramsay MAE, et al. International liver transplant society practice guidelines: Diagnosis and management of hepatopulmonary syndrome and portopulmonary hypertension. Transplantation. 2016;100(7):1440–52. [DOI] [PubMed] [Google Scholar]; -Thorough guidelines on many aspects of HPS and PoPH.
- 19.Martin Paul, Andrea DiMartini Sandy Feng, Robert Brown Jr MF. Evaluation for Liver Transplantation in Adults: 2013 Practice Guideline by the AASLD and the American Society of Transplantation. Hepatology. 2014;1144–465. [DOI] [PubMed] [Google Scholar]
- 20.Schenk P, Fuhrmann V, Madl C, Funk G, Lehr S, Kandel O, et al. Hepatopulmonary syndrome: Prevalence and predictive value of various cut offs for arterial oxygenation and their clinical consequences. Gut. 2002. December 1;51(6):853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folador L, Torres FS, Zampieri JF, Machado BC, Knorst MM, Gazzana MB. Hepatopulmonary syndrome has low prevalence of pulmonary vascular abnormalities on chest computed tomography. PLoS One. 2019;14(10):e0223805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abrams GA, Jaffe CC, Hoffer PB, Binder HJ, Fallon MB. Diagnostic utility of contrast echocardiography and lung perfusion scan in patients with hepatopulmonary syndrome. Gastroenterology. 1995;109(4):1283–8. [DOI] [PubMed] [Google Scholar]
- 23.Tonelli AR, Naal T, Dakkak W, Park MM, Dweik RA, Stoller JK. Assessing the kinetics of microbubble appearance in cirrhotic patients using transthoracic saline contrast-enhanced echocardiography. Echocardiography. 2017;34(10):1439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fragaki M, Sifaki-Pistolla D, Samonakis DN, Koulentaki M, Koukouraki S, Stathaki M, et al. Screening for Hepatopulmonary Syndrome in Cirrhotic Patients Using Technetium 99m-macroaggregated Albumin Perfusion Lung Scan (Tc-MAA): Diagnostic Approach and Clinical Correlations. J Clin Gastroenterol. 2018;52(9):828–34. [DOI] [PubMed] [Google Scholar]
- 25.El-Shabrawi MH, Omran S, Wageeh S, Isa M, Okasha S, Mohsen NA, et al. 99mTechnetium-macroaggregated albumin perfusion lung scan versus contrast enhanced echocardiography in the diagnosis of the hepatopulmonary syndrome in children with chronic liver disease. Eur J Gastroenterol Hepatol. 2010;22(8):1006–12. [DOI] [PubMed] [Google Scholar]
- 26.Martinez G, Barberà JA, Navasa M, Roca J, Visa J, Rodriguez-Roisin R. Hepatopulmonary syndrome associated with cardiorespiratory disease. J Hepatol. 1999. May 1;30(5):882–9. [DOI] [PubMed] [Google Scholar]
- 27.Arguedas MR, Abrams GA, Krowka MJ, Fallon MB. Prospective evaluation of outcomes and predictors of mortality in patients with hepatopulmonary syndrome undergoing liver transplantation. Hepatology. 2003. January 1;37(1):192–7. [DOI] [PubMed] [Google Scholar]
- 28.Krowka MJ, Mandell MS, Ramsay MAE, Kawut SM, Fallon MB, Manzarbeitia C, et al. Hepatopulmonary syndrome and portopulmonary hypertension: A report of multicenter liver transplant database. Liver Transplant. 2004. February;10(2):174–82. [DOI] [PubMed] [Google Scholar]
- 29.Schenk P, Schöniger-Hekele M, Fuhrmann V, Madl C, Silberhumer G, Müller C. Prognostic significance of the hepatopulmonary syndrome in patients with cirrhosis. Gastroenterology. 2003. October 1;125(4):1042–52. [DOI] [PubMed] [Google Scholar]
- 30.Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: Impact of liver transplantation. Hepatology. 2005;41(5):1122–9. [DOI] [PubMed] [Google Scholar]
- 31.Al-Harbi A, Abdullah K, Al-Abdulkareem A, Alghamdi A, Al-Jahdali H. Prevalence, severity, and prognostic effect of hepatopulmonary syndrome in liver transplant candidates. Ann Transplant. 2016;21:180–4. [DOI] [PubMed] [Google Scholar]
- 32.Warner S, McKiernan PJ, Hartley J, Ong E, van Mourik ID, Gupte G, et al. Hepatopulmonary Syndrome in Children: A 20-Year Review of Presenting Symptoms, Clinical Progression, and Transplant Outcome. Liver Transplant. 2018;24(9):1271–9. [DOI] [PubMed] [Google Scholar]
- 33.Goldberg DS, Krok K, Batra S, Trotter JF, Kawut SM, Fallon MB. Impact of the hepatopulmonary syndrome MELD exception policy on outcomes of patients after liver transplantation: An analysis of the UNOS database. Gastroenterology. 2014. May 1;146(5):1256–1265.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HY, Choi MS, Lee SC, Park SW, Lee JH, Koh KC, et al. Outcomes in patients with hepatopulmonary syndrome undergoing liver transplantation. Transplant Proc. 2004. November;36(9):2762–3. [DOI] [PubMed] [Google Scholar]
- 35.Pascasio JM, Grilo I, Lõpez-Pardo FJ, Ortega-Ruiz F, Tirado JL, Sousa JM, et al. Prevalence and severity of hepatopulmonary syndrome and its influence on survival in cirrhotic patients evaluated for liver transplantation. Am J Transplant. 2014;14(6):1391–9. [DOI] [PubMed] [Google Scholar]
- 36.Iyer VN, Swanson KL, Cartin-Ceba R, Dierkhising RA, Rosen CB, Heimbach JK, et al. Hepatopulmonary syndrome: Favorable outcomes in the MELD exception era. Hepatology. 2013. June;57(6):2427–35. [DOI] [PubMed] [Google Scholar]
- 37.Gupta S, Castel H, Rao RV., Picard M, Lilly L, Faughnan ME, et al. Improved survival after liver transplantation in patients with hepatopulmonary syndrome. Am J Transplant. 2010. February;10(2):354–63. [DOI] [PubMed] [Google Scholar]
- 38.Casey S, Schelleman A, Angus P. Recurrence of hepatopulmonary syndrome post-orthotopic liver transplantation in a patient with noncirrhotic portal hypertension. Hepatology. 2013;58(6):2205–6. [DOI] [PubMed] [Google Scholar]
- 39.Aucejo F, Miller C, Vogt D, Eghtesad B, Nakagawa S, Stoller JK. Pulmonary hypertension after liver transplantation in patients with antecedent hepatopulmonary syndrome: a report of 2 cases and review of the literature. Liver Transpl. 2006;12(8):1278–82. [DOI] [PubMed] [Google Scholar]
- 40.Nayyar D, Jeffrey Man HS, Granton J, Gupta S. Defining and characterizing severe hypoxemia after liver transplantation in hepatopulmonary syndrome. Liver Transplant. 2014;20(2):182–90. [DOI] [PubMed] [Google Scholar]
- 41.Nayyar D, Man HSJ, Granton J, Lilly LB, Gupta S. Proposed management algorithm for severe hypoxemia after liver transplantation in the hepatopulmonary syndrome. Vol. 15, American Journal of Transplantation. 2015. p. 903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsauo Jiaywei, Weng Ningna, Ma Huaiyuan, Jiang Mingshan, Zhao He and XY. Role of Transjugular Intrahepatic Portosystemic Shunts in the Management of Hepatopulmonary Syndrome: A Systemic Literature Review. J Vasc Interv Radiol. 2015;26(37):1266–71. [DOI] [PubMed] [Google Scholar]
- 43. *.Tsauo J, Zhao H, Zhang X, Ma H, Jiang M, Weng N, et al. Effect of Transjugular Intrahepatic Portosystemic Shunt Creation on Pulmonary Gas Exchange in Patients with Hepatopulmonary Syndrome: A Prospective Study. J Vasc Interv Radiol. 2019;30(2):170–7. [DOI] [PubMed] [Google Scholar]; -Prior review (42) suggested TIPS improved oxygenation in patients with HPS; however, in this prospective study of 23 patients with HPS, oxygenation only improved transiently but returned to baseline by 3 months.
- 44. *.Zhao H, Liu F, Yue Z, Wang L, Fan Z, He F. Clinical efficacy of transjugular intrahepatic portosystemic shunt in the treatment of hepatopulmonary syndrome. Med (United States). 2017;96(49):e9080. [DOI] [PMC free article] [PubMed] [Google Scholar]; -Retrospective study that seperated HPS patients who had TIPS by location of shunt (main, right, or left portal vein) and reported that left portal vein TIPS was associated with improved oxygenation compared to right or main portal vein TIPS, which could potentially explain the discrepancy in patient responses in prior studies.
- 45.Grady K, Gowda S, Kingah P, Soubani AO. Coil embolization of pulmonary arteries as a palliative treatment of diffuse type I hepatopulmonary syndrome. Respir Care. 2015;60(2):e20–5. [DOI] [PubMed] [Google Scholar]
- 46.Ikubo Y, Kasai H, Sugiura T, Saito T, Shoji H, Sakao S, et al. Pulmonary hypertension that developed during treatment for hepatopulmonary syndrome and pulmonary arteriovenous malformation. Intern Med. 2019;58(12):1765–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Luo B, Tang L, Wang Y, Stockard CR, Kadish I, et al. Pulmonary Angiogenesis in a Rat Model of Hepatopulmonary Syndrome. Gastroenterology. 2009;136(3):1070–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanikella R, Philips GM, Faulk DK, Kawut SM, Fallon MB. Pilot study of pentoxifylline in hepatopulmonary syndrome. Liver Transplant. 2008. August;14(8):1199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kianifar HR. Pentoxifylline in hepatopulmonary syndrome. World J Gastroenterol. 2012;18(35):4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De BK, Pal SK, Mbbs G, Das S, Mbbs B, Mbbs AP. The role of garlic in hepatopulmonary syndrome: A randomized controlled trial. Can J Gastroenterol. 2010;24(3):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaikh SA, Tischer S, Choi EK, Fontana RJ. Good for the lung but bad for the liver? Garlic-induced hepatotoxicity following liver transplantation. J Clin Pharm Ther 2017;42(5):646–8. [DOI] [PubMed] [Google Scholar]
- 52.Krowka MJ, Edwards WD. A spectrum of pulmonary vascular pathology in portopulmonary hypertension. Liver Transplant. 2000. March;6(2):241–2. [DOI] [PubMed] [Google Scholar]
- 53.Cartin-Ceba R, Krowka MJ. Portopulmonary hypertension. Clin Liver Dis. 2014;18(2):421–38. [DOI] [PubMed] [Google Scholar]
- 54.Benjaminov FS, Prentice M, Sniderman KW, Siu S, Liu P, Wong F. Portopulmonary hypertension in decompensated cirrhosis with refractory ascites. Gut. 2003;52(9):1355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pellicelli AM, Barbaro G, Puoti C, Guarascio P, Lusi EA, Bellis L, et al. Plasma Cytokines and Portopulmonary Hypertension in Patients With Cirrhosis Waiting for Orthotopic Liver Transplantation. Angiology. 2010;61(8):802–6. [DOI] [PubMed] [Google Scholar]
- 56.Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, et al. Prostacyclin Synthase Expression Is Decreased in Lungs from Patients with Severe Pulmonary Hypertension. Am J Respir Crit Care Med. 1999;159(6):1925–32. [DOI] [PubMed] [Google Scholar]
- 57.Gaine S Pulmonary hypertension. JAMA. 2000;284(24):3160–8. [DOI] [PubMed] [Google Scholar]
- 58.Kim WR, Krowka MJ, Plevak DJ, Lee J, Rettke SR, Frantz RP, et al. Accuracy of doppler echocardiography in the assessment of pulmonary hypertension in liver transplant candidates. Liver Transplant. 2000;6(4):453–8. [DOI] [PubMed] [Google Scholar]
- 59.Krowka MJ, Swanson KL, Frantz RP, McGoon MD, Wiesner RH. Portopulmonary hypertension: Results from a 10-year screening algorithm. Hepatology. 2006;44(6):1502–10. [DOI] [PubMed] [Google Scholar]
- 60.Cotton CL, Gandhi S, Vaitkus PT, Massad MG, Benedetti E, Mrtek RG, et al. Role of echocardiography in detecting portopulmonary hypertension in liver transplant candidates. Liver Transplant. 2002. November 1;8(11):1051–4. [DOI] [PubMed] [Google Scholar]
- 61. *.DesJardin JT, Manicardi M, Svetlichnaya Y, Kolaitis NA, Papolos AI, Selby VN, et al. Noninvasive estimation of pulmonary vascular resistance improves portopulmonary hypertension screening in liver transplant candidates. Clin Transplant. 2019;33(7). [DOI] [PubMed] [Google Scholar]; -There is debate over what is the appropriate estimated RVSP (eRVSP) on doppler echocardiography to necessitate right RHC when screening patients for PoPH because eRVSP is especially inaccurate in patients with cirrhosis due to high flow state. This paper shows that using estimated PVR in patients with eRVSP 35–45 mmHg may help improve sensitvity and specficity of this screening tool.
- 62.Farzaneh-Far R, McKeown BH, Dang D, Roberts J, Schiller NB, Foster E. Accuracy of Doppler-estimated pulmonary vascular resistance in patients before liver transplantation. Am J Cardiol. 2008;101(2):259–62. [DOI] [PubMed] [Google Scholar]
- 63.Krowka MJ, Miller DP, Barst RJ, Taichman D, Dweik RA, Badesch DB, et al. Portopulmonary hypertension: A report from the US-based REVEAL registry. Chest. 2012;141(4):906–15. [DOI] [PubMed] [Google Scholar]
- 64.McDonnell PJ, Toye PA, Hutchins GM. Primary pulmonary hypertension and cirrhosis: Are they related? Am Rev Respir Dis. 1983;127(4):437–41. [DOI] [PubMed] [Google Scholar]
- 65.Hadengue A, Benhayoun MK, Lebrec D, Benhamou JP. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology. 1991;100(2):520–8. [DOI] [PubMed] [Google Scholar]
- 66.Colle I, Moreau R, Godhino E, Belghiti J, Ettori F, Cohan-Solal A, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: A prospective study. Hepatology. 2003;37(2):401–9. [DOI] [PubMed] [Google Scholar]
- 67.Ramsay MAE, Simpson BR, Nguyen AT, Ramsay KJ, East C, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transplant Surg. 1997;3(5):494–500. [DOI] [PubMed] [Google Scholar]
- 68.Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987. August 1;107(2):216–23. [DOI] [PubMed] [Google Scholar]
- 69.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: Results from a national registry. Am J Respir Crit Care Med. 2006;173(9):1023–30. [DOI] [PubMed] [Google Scholar]
- 70.Condino AA, Ivy DD, O’Connor JA, Narkewicz MR, Mengshol S, Whitworth JR, et al. Portopulmonary hypertension in pediatric patients. J Pediatr. 2005;147(1):20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawut SM, Krowka MJ, Trotter JF, Roberts KE, Benza RL, Badesch DB, et al. Clinical risk factors for portopulmonary hypertension. Hepatology. 2008. July;48(1):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Talwalkar JA, Swanson KL, Krowka MJ, Andrews JC, Kamath PS. Prevalence of spontaneous portosystemic shunts in patients with portopulmonary hypertension and effect on treatment. Gastroenterology. 2011;141(5):1673–9. [DOI] [PubMed] [Google Scholar]
- 73.Senior RM, Britton RC, Turino GM, Wood JA, Langer GA, Fishman AP. Pulmonary hypertension associated with cirrhosis of the liver and with portacaval shunts. Circulation. 1968;37(1):88–96. [DOI] [PubMed] [Google Scholar]
- 74.Peacock AJ. Pulmonary hypertension after splenectomy: A consequence of loss of the splenic filter or is there something more? Thorax. 2005;60(12):983–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robalino BD, Moodie DS. Association between primary pulmonary hypertension and portal hypertension: Analysis of its pathophysiology and clinical, laboratory and hemodynamic manifestations. J Am Coll Cardiol. 1991;17(2):492–8. [DOI] [PubMed] [Google Scholar]
- 76.Swanson KL, Wiesner RH, Nyberg SL, Rosen CB, Krowka MJ. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant. 2008. November;8(11):2445–53. [DOI] [PubMed] [Google Scholar]
- 77. *.AbuHalimeh B, Krowka MJ, Tonelli AR. Treatment Barriers in Portopulmonary Hypertension. Hepatology. 2019;69(1):431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]; -Excellent review of the current challenges and knowledge gaps in the medical management of PoPH.
- 78.Olsson KM, Delcroix M, Ghofrani HA, Tiede H, Huscher D, Speich R, et al. Anticoagulation and survival in pulmonary arterial hypertension: Results from the comparative, prospective registry of newly initiated therapies for pulmonary hypertension (COMPERA). Circulation. 2014;129(1):57–65. [DOI] [PubMed] [Google Scholar]
- 79.Preston Ioana R; Roberts Kari E; Miller Dave P; Sen Ginny P; Selej Mona; Benton Wade W; Hill Nicholas S; Farber HW. Effect of Warfarin Treatment on Survival of Patients With Pulmonary Arterial Hypertension (PAH) in the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL). Circulation. 2015;132(25):2403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fan Z, Chen Y, Liu H. Calcium channel blockers for pulmonary arterial hypertension. Cochrane Database Syst Rev. 2015;2015(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ota K, Shijo H, Kokawa H, Kubara K, Kim T, Akiyoshi N, et al. Effects of nifedipine on hepatic venous pressure gradient and portal vein blood flow in patients with cirrhosis. J Gastroenterol Hepatol. 1995;10(2):198–204. [DOI] [PubMed] [Google Scholar]
- 82.Provencher S, Herve P, Jais X, Lebrec D, Humbert M, Simonneau G, et al. Deleterious effects of β-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. Gastroenterology. 2006;130(1):120–6. [DOI] [PubMed] [Google Scholar]
- 83.Boyer TD, Haskal ZJ. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: Update 2009. Hepatology. 2010;51(1):306. [DOI] [PubMed] [Google Scholar]
- 84.Van der Linden P Pulmonary hypertension after transjugular intrahepatic portosystemic shunt: Effects on right ventricular function. Hepatology. 1996;23(5):982–7. [DOI] [PubMed] [Google Scholar]
- 85. *.Cartin-Ceba R, Halank M, Ghofrani H-A, Humbert M, Mattson J, Fritsch A, et al. Riociguat treatment for portopulmonary hypertension: a subgroup analysis from the PATENT-1/−2 studies. Pulm Circ. 2018;8(2):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]; -Only RCT for pulmonary hypertension medications that included patients with PoPH-unfortunately still to small sample size to compare within PoPH alone; however, in all PAH patients, riociguat improved exercising capacity.
- 86.Ashfaq M, Chinnakotla S, Rogers L, Ausloos K, Saadeh S, Klintmalm GB, et al. The impact of treatment of portopulmonary hypertension on survival following liver transplantation. Am J Transplant. 2007;7(5):1258–64. [DOI] [PubMed] [Google Scholar]
- 87.Fix OK, Bass NM, De Morco T, Merriman RB. Long-term follow-up of portopulmonary hypertension: Effect of treatment with epoprostenol. Liver Transplant. 2007. June;13(6):875–85. [DOI] [PubMed] [Google Scholar]
- 88.Sakai T, Planinsic RM, Mathier MA, De Vera ME, Venkataramanan R. Initial experience using continuous intravenous treprostinil to manage pulmonary arterial hypertension in patients with end-stage liver disease. Transpl Int. 2009;22(5):554–61. [DOI] [PubMed] [Google Scholar]
- 89.Awdish RLA, Cajigas HR. Early initiation of prostacyclin in portopulmonary hypertension: 10 years of a transplant center’s experience. Lung. 2013;191(6):593–600. [DOI] [PubMed] [Google Scholar]
- 90.Melgosa MT, Ricci GL, García-Pagan JC, Blanco I, Escribano P, Abraldes JG, et al. Acute and long-term effects of inhaled iloprost in portopulmonary hypertension. Liver Transpl. 2010;16(3):348–56. [DOI] [PubMed] [Google Scholar]
- 91.Hoeper MM, Seyfarth HJ, Hoeffken G, Wirtz H, Spiekerkoetter E, Pletz MW, et al. Experience with inhaled iloprost and bosentan in portopulmonary hypertension. Eur Respir J. 2007;30(6):1096–102. [DOI] [PubMed] [Google Scholar]
- 92.Hoeper MM, Halank M, Marx C, Hoeffken G, Seyfarth HJ, Schauer J, et al. Bosentan therapy for portopulmonary hypertension. Eur Respir J. 2005;25(3):502–8. [DOI] [PubMed] [Google Scholar]
- 93.Savale L, Magnier R, Le Pavec J, Jaïs X, Montani D, O’Callaghan DS, et al. Efficacy, safety and pharmacokinetics of bosentan in portopulmonary hypertension. Eur Respir J. 2013;41(1):96–103. [DOI] [PubMed] [Google Scholar]
- 94.Cartin-Ceba R, Swanson K, Iyer V, Wiesner RH, Krowka MJ. Safety and efficacy of ambrisentan for the treatment of portopulmonary hypertension. Chest. 2011;139(1):109–14. [DOI] [PubMed] [Google Scholar]
- 95.Hemnes AR, Robbins IM. Sildenafil monotherapy in portopulmonary hypertension can facilitate liver transplantation. Liver Transplant. 2009;15(1):15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reichenberger F, Voswinckel R, Steveling E, Enke B, Kreckel A, Olschewski H, et al. Sildenafil treatment for portopulmonary hypertension. Eur Respir J. 2006;28(3):563–7. [DOI] [PubMed] [Google Scholar]
- 97.Gough MS, White RJ. Sildenafil therapy is associated with improved hemodynamics in liver transplantation candidates with pulmonary arterial hypertension. Liver Transpl. 2009;15(1):30–6. [DOI] [PubMed] [Google Scholar]
- 98.Hollatz TJ, Musat A, Westphal S, Decker C, D’Alessandro AM, Keevil J, et al. Treatment with sildenafil and treprostinil allows successful liver transplantation of patients with moderate to severe portopulmonary hypertension. Liver Transplant. 2012. June;18(6):686–95. [DOI] [PubMed] [Google Scholar]
- 99.Khaderi S, Khan R, Safdar Z, Stribling R, Vierling JM, Goss JA, et al. Long-term follow-up of portopulmonary hypertension patients after liver transplantation. Liver Transplant. 2014;20(6):724–7. [DOI] [PubMed] [Google Scholar]
- 100.Mangus RS, Kinsella SB, Marshall GR, Fridell JA, Wilkes KR, Tector AJ. Mild to moderate pulmonary hypertension in liver transplantation. J Surg Res. 2013;184(2):1150–6. [DOI] [PubMed] [Google Scholar]
- 101.Krowka MJ, Plevak DJ, Findlay JY, Rosen CB, Wiesner RH, Krom RAF. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transplant. 2000;6(4):443–50. [DOI] [PubMed] [Google Scholar]
- 102.Le Pavec J, Souza R, Herve P, Lebrec D, Savale L, Tcherakian C, et al. Portopulmonary hypertension: Survival and prognostic factors. Am J Respir Crit Care Med. 2008;178(6):637–43. [DOI] [PubMed] [Google Scholar]
- 103.Goldberg DS, Batra S, Sahay S, Kawut SM, Fallon MB. MELD exceptions for portopulmonary hypertension: Current policy and future implementation. Am J Transplant. 2014;14(9):2081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramsay M Portopulmonary hypertension and right heart failure in patients with cirrhosis. Curr Opin Anaesthesiol. 2010;23(2):145–50. [DOI] [PubMed] [Google Scholar]
- 105.Saad NEA, Lee DE, Waldman DL, Saad WEA. Pulmonary Arterial Coil Embolization for the Management of Persistent Type I Hepatopulmonary Syndrome after Liver Transplantation. J Vasc Interv Radiol. 2007;18(12):1576–80. [DOI] [PubMed] [Google Scholar]
- 106.Gómez FP, Barberá JA, Roca J, Burgos F, Gistau C, Rodríguez-Roisin R. Effects of nebulized NG-nitro-L-arginine methyl ester in patients with hepatopulmonary syndrome. Hepatology. 2006;43(5):1084–91. [DOI] [PubMed] [Google Scholar]
- 107.Brussino L, Bucca C, Morello M, Scappaticci E, Mauro M, Rolla G. Effect on dyspnoea and hypoxaemia of inhaled N(G)-nitro-L-arginine methyl ester in hepatopulmonary syndrome. Lancet (London, England). 2003;362(9377):43–4. [DOI] [PubMed] [Google Scholar]
- 108.Krug S, Seyfarth HJ, Hagendorff A, Wirtz H. Inhaled iloprost for hepatopulmonary syndrome: Improvement of hypoxemia. Eur J Gastroenterol Hepatol. 2007;19(12):1140–3. [DOI] [PubMed] [Google Scholar]
- 109.Gupta S, Faughnan ME, Lilly L, Hutchison S, Fowler R, Bayoumi AM. Norfloxacin therapy for hepatopulmonary syndrome: A pilot randomized controlled trial. Clin Gastroenterol Hepatol. 2010;8(12):1095–8. [DOI] [PubMed] [Google Scholar]
- 110. *.Kawut SM, Ellenberg SS, Krowka MJ, Goldberg D, Vargas H, Koch D, et al. Sorafenib in Hepatopulmonary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Liver Transplant. 2019;25(8):1155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]; -Despite angiogenesis being implicated in pathogenesis of HPS in experimental models, there was no benefit in this RCT.


