Abstract
Objective:
Despite the fact that negative mood and executive dysfunction are common following risk-reducing salpingo-oophorectomy (RRSO) – occurring in up to a third of women – little is known about risk factors predicting these negative outcomes. Adverse childhood experiences (ACE) predict poorer health in adulthood and may be a risk factor for negative outcomes following RRSO. Given the complex relationship between early life stress, affective disorders, and cognitive dysfunction, we hypothesized that ACE would be associated with poorer executive function and that mood symptoms would partially mediate this relationship.
Methods:
Women who had undergone RRSO were included in the study (N = 552; age 30–73 years). We measured executive function (continuous performance task, letter n-back task, Brown Attention Deficit Disorder Scale Score), exposure to early life stress (ACE Questionnaire), and mood symptoms (Hospital Anxiety and Depression Scale). Generalized estimating equations were used to evaluate the association between ACE and executive dysfunction and the role of mood symptoms as a mediator in this relationship.
Results:
ACE was associated with greater severity of subjective executive dysfunction (adjusted mean difference (aMD)= 7.1, p=0.0005) and worse performance on both cognitive tasks (CPT: aMD=−0.1, p=0.03; n-back: aMD=−0.17, p=0.007). Mood symptoms partially mediated ACE associations with sustained attention (21.3% mediated; 95% CI: 9.3% - 100%) and subjective report of executive dysfunction (62.8% mediated; 95% CI: 42.3% - 100%).
Conclusions:
The relationship between childhood adversity and executive dysfunction is partially mediated by mood symptoms. These data indicate that assessing history of childhood adversity and current anxiety and depression symptoms may play a role in treating women who report cognitive complaints after RRSO.
Keywords: childhood adversity, executive function, anxiety, depression, menopause, oophorectomy
Introduction
Women with mutations in BRCA1 or BRCA2 (BRCA1/2) susceptibility genes are at increased risk for developing breast and ovarian cancers.1 While risk-reducing salpingo-oophorectomy (RRSO) may lower the risk of breast and ovarian cancer by 37–64% and 69–85%,1,2 respectively, there are several important health consequences of being hypogonadal at such an early age.
Given that ovarian hormones such as estradiol and progesterone have numerous neuromodulatory and neuroprotective effects,3 recent studies have examined RRSO as a potential risk for central nervous system (CNS) impairment and overall decline in quality of life. Large cohort studies have shown women who undergo oophorectomy before natural menopause are at increased risk of dementia4 and cognitive dysfunction during and after the seventh decade of life.4,5 More specifically, the largest declines in cognitive performance following oophorectomy occur in executive functioning domains.6 Previous research has also shown that adverse childhood experiences (ACE) are associated with executive dysfunction in naturally menopausal women in the absence, but not presence, of exogenous estradiol.7,8 Together, these studies suggest that premature, abrupt loss of estradiol with RRSO induces executive function difficulties in many, but not all, hypogonadal women. They also suggest ACE may be one factor contributing to risk versus resilience for executive dysfunction after oophorectomy. However, no studies examining the effect of ACE on executive function after RRSO have been reported.
It is well established that ACE are associated with greater incidence of anxiety and depressive symptoms,9–13 particularly during the menopause transition.14 Previous research has demonstrated that anxiety and depressive symptoms mediate the negative impact of ACE on health-related quality of life,15 smoking status,16 substance dependence,9 and chronic pain disorders.12 Importantly, anxiety and depressive symptoms have been independently associated with executive dysfunction.17 Several studies have shown that mood symptoms negatively impact subjective18,19 and objective20 measures of cognitive function in breast cancer survivors. However, whether ACE is associated with executive dysfunction after RRSO and to what extent ACE effects on executive function in hypogonadal women are mediated by mood symptoms is not clear and has not been reported. That childhood adversity and current mood can be easily assessed with patient questionnaires in the clinical gynecology setting, supports the significance of this line of investigation.
We examined the association between ACE and subjective and objective measures of executive function while rigorously controlling for multiple possible confounding variables in 552 women who underwent RRSO. We hypothesized that high levels of childhood adversity would be associated with greater self-reported symptoms of executive dysfunction and poorer performance on executive function tasks. Furthermore, we hypothesized that mood symptoms would partially mediate the relationship between ACE and executive function.
Method
Participants
Participants were recruited through mailings from the Cancer Risk Evaluation Program at the University of Pennsylvania, local advertising, and an advocacy group for women with genetic risk for breast and ovarian cancer (FORCE; Facing Our Risk of Cancer Empowered). Women considered for inclusion were over the age of 30, had BRCA1 or BRCA2 germline mutations, and had undergone RRSO to reduce the risk of primary or recurrent breast and ovarian cancers given their genetic predisposition. Exclusion criteria included inability to provide informed consent. This study was IRB approved; all participants provided informed consent.
Participants completed online surveys through Qualtrics.21 Surveys assessed mood, cognition, menopausal symptoms, childhood adversity, and medication use. To date, ~55% of participants have repeated these surveys as part of an ongoing, longitudinal study. In this report, we consider the sample of participants from this cohort that completed one or more outcomes of interest at least once. While the impact of ACE on changes in cognitive function over time after RRSO would be of interest, given the short duration of time between testing sessions (~1 year) and the limited portion of the sample that completed both sessions (~55%), we focused on the associations between ACE and cognition rather than the associations between ACE and change in cognition between sessions.
Assessment of subjective executive function
Subjective executive dysfunction was evaluated using a modified Brown Attention Deficit Disorder Scale (BADDS) adapted for online use. The BADDS is a validated22 subjective measure of five domains of executive dysfunction: (1) organization and activating for work, (2) sustaining attention and concentration, (3) alertness, effort, and processing speed, (4) managing affective interference, and (5) working memory and accessing recall. Higher scores indicate greater dysfunction. Total BADDS score was the primary measure of subjective executive function whereas BADDS subscales were secondary outcomes. Given the limited number of items per subscale, participants who skipped a question were excluded from analyses related to that BADDS subscale but not from analyses of other subscales or total score.
Assessment of objective executive function
As part of a comprehensive computerized cognitive battery,23 participants completed two neuropsychological tasks probing executive function domains. In this report, we specifically focused on measures related to executive functions given our hypothesis regarding the association between childhood adversity and executive functioning post-RRSO as well as the converging evidence for the effects of estradiol and early life stress on this domain.3,24 The Penn Continuous Performance Task (CPT) number and letter version25 was used to assess sustained attention. During the task, participants were instructed to respond when the image was a complete letter or number. The primary measure of sustained attention was d’, a signal detection metric that limits the influence of response bias.26
A letter version of the n-back task27 was used to probe working memory function across four conditions: 0-back, 1-back, 2-back, and 3-back. In the 0-back condition, participants responded to a pre-specified target stimulus while participants responded to a stimulus “n” number of stimuli before it in other conditions. Because the 3-back condition places the greatest demands on working memory, 3-back d’ served as the primary measure of working memory.
Assessment of childhood adversity
The Adverse Childhood Experiences (ACE) Questionnaire28 was used to assess history of childhood abuse, neglect, and household dysfunction. The ACE Questionnaire has been widely used to examine the association between overall early adversity and health-related outcomes in adult life.29 The number of exposures was summed to create the ACE score (range: 0–10). Based on evidence from prior studies indicating increased risk of depressive disorders in later life in those with 2 or more ACEs,14,30 we considered participants with an ACE score ≥ 2 “high ACE” and participants with an ACE score of < 2 “low ACE”.
Assessment of mood symptoms
Depressive and anxiety symptoms were assessed using the 14-item Hospital Anxiety and Depression Scale. This scale contains a 7-item depression subscale that assesses changes in mood, loss of interest, and psychomotor slowing and a 7-item anxiety subscale that assesses mental agitation and psychological distress. In line with prior studies of executive functions that have represented anxiety and depressive symptoms as a composite “mood” score,17 total scores on the depression and anxiety subscales were summed to create a composite measure of mood symptoms.
Statistical analyses
Generalized estimating equations implemented using the geepack package31 in R32 were employed to evaluate associations between ACE and executive function outcomes. This method accommodates multiple survey assessments per woman and adjusts for non-independence of these repeated measures. Age, time since oophorectomy, and age at oophorectomy were assessed as covariates. However, at baseline, age was highly correlated with both age at oophorectomy (r=0.85, p<0.0001) and time since oophorectomy (r=0.49, p<0.0001). To fully account for the effects of age on cognitive outcomes, generalized estimating equations with cognitive measures as the outcomes and age as the predictor were used to obtain age-residualized scores for each outcome: BADDS, CPT, and n-back. Subsequent analyses were performed on these residualized outcomes rather than raw values. Time without hormones (calculated by subtracting the duration of hormone therapy use post-RRSO, if any from the duration of time since oophorectomy), education level, and history of chemotherapy use were included as covariates in all models. Results reported are multivariable adjusted. Bivariable associations, T-tests, Wilcoxon rank sum tests, and Pearson chi-square tests were used as appropriate to assess whether sociodemographic information differed between ACE groups. Two-sided hypotheses were evaluated with statistical significance defined as p<=0.05. Given that all three outcomes are designed to assess the cognitive domain of executive function and adjusting for multiple comparisons may be overly conservative, p-values reported are uncorrected.
Mediation models were used to examine whether ACE associations with executive dysfunction after RRSO were mediated by increased mood symptoms. The three primary outcome measures served as the dependent variable in three separate analyses. Total ACE served as the independent variable while the composite mood score served as the mediator. Similar to models described above, adjusted models are developed to estimate the following components of the mediation model: associations between 1) ACE and executive function (which is the total effect of ACE), 2) ACE and mood, and 3) mood and executive function after accounting for ACE. When these associations were significant, the statistical significance of mood as a mediator was evaluated by estimating the indirect effect of ACE on executive function through mood using the Product of Coefficients approach. Significance testing of the indirect effect estimates were conducted using standard error estimates estimated via a bootstrap resampling approach implemented with the boot package33 using ordinary nonparametric bootstrapping and 2,000 iterations. The percent mediated was computed as the ratio of the indirect effect to the total effect, with 95% CI estimated using bootstrap estimates.
Psychotropic medication use served as an additional covariate in supplemental analyses of primary outcome variables. Supplementary analyses also examined the effects of ACE in the subset of women who underwent oophorectomy prior to age 47.4, 2 standard deviations (1 SD=2.26 years) below the average age (51.9 years) of post-menopause onset.34 To evaluate whether timing of childhood adversity was driving results, we also separately examined the effects of pre-pubertal ACE and post-pubertal ACE on primary outcome measures. Pre-puberty was defined as the period between birth and 2 years prior to menstrual cycle onset. Given that history of cancer could be an important potential confounder, we repeated our primary analyses of ACE associations with executive function with this covariate rather than history of chemotherapy given collinearity.
Results
Participants
Eight hundred participants completed cognitive testing at baseline and were eligible for inclusion in this report. Of these 800 participants, 453 completed a second cognitive testing session, yielding a total of 1,253 sessions that were eligible for inclusion. Sessions included in analyses must have completed at least one outcome of interest (n=1,058). Sessions missing information regarding age at testing (n=16), hormone therapy use post-RRSO (n=165), ACE (n=12), education level (n=8), and mood symptoms (n=30) were excluded. As part of data quality assurance, participants who performed below chance (<50% true positives) on the CPT or 0-back control condition were excluded from analyses of the CPT (n=20) and n-back (n=13), respectively. The final sample included in analyses was 552 participants (Table 1; Figure 1).
Table 1.
Baseline characteristics of participants included in analyses stratified by level of childhood adversity
| p-value | |||||
|---|---|---|---|---|---|
| n | 350 | 202 | |||
| Age (years) | (7.2) | (7.9) | ns | ||
| Age at oophorectomy (years) | (6.0) | (7.3) | ns | ||
| Time since oophorectomy (months) | (46.2) | (48.3) | ns | ||
| Time without hormones (months) | (41.3) | (43.0) | ns | ||
| History of cancer | (44.6) | (50.0) | ns | ||
| Previous hormone therapy use | (42.9) | (39.6) | ns | ||
| Current hormone therapy use | (28.0) | (25.7) | ns | ||
| Previous chemotherapy use | (26.9) | (32.2) | ns | ||
| ns | |||||
| Graduate school | (44.3) | (46.5) | |||
| College | (44.3) | (33.7) | |||
| Some College | (9.7) | (15.8) | |||
| High school | (0.9) | (4.0) | |||
| Some high school | (0.3) | (0.0) | |||
| 8th grade or less | (0.6) | (0.0) | |||
| ns | |||||
| White | (93.1) | (89.6) | |||
| African American | (0.6) | (2.0) | |||
| Asian | (0.9) | (0.0) | |||
| Hispanic | (2.3) | (3.0) | |||
| Other | (1.7) | (1.5) | |||
| Unknown or not disclosed | (2.9) | (4.0) | |||
| HADS total score | (6.7) | (7.1) | 0.0005 | ||
| HADS depression subscale score | (3.8) | (4.0) | 0.001 | ||
| HADS anxiety subscale score | (3.6) | (3.4) | 0.0009 | ||
| Attention deficit disorder | (1.1) | (2.0) | ns | ||
| Depression | (28.0) | (30.7) | 0.004 | ||
| Anxiety | (2.6) | (5.9) | ns | ||
| Neurological conditions | (3.7) | (3.5) | ns | ||
| Narcotics | (0.9) | (1.0) | ns | ||
| SERM antagonist/Aromatase inhibitor | (12.3) | (14.9) | ns | ||
| Tamoxifen/SERM | (9.1) | (7.9) | 0.03 | ||
| <0.0001 | |||||
| Abuse | |||||
| Emotional abuse | (1.7) | (58.9) | <0.0001 | ||
| Physical abuse | (1.1) | (38.6) | <0.0001 | ||
| Sexual abuse | (7.1) | (25.2) | <0.0001 | ||
| Neglect | |||||
| Emotional neglect | (3.1) | (50.5) | <0.0001 | ||
| Physical neglect | (0.3) | (12.4) | <0.0001 | ||
| Household dysfunction | |||||
| Parents divorced/separated | (10.6) | (53.0) | <0.0001 | ||
| Witnessed domestic violence | (0.6) | (12.9) | <0.0001 | ||
| Drug abuse in household | (2.9) | (46.0) | <0.0001 | ||
| Mental illness in household | (2.0) | (46.0) | <0.0001 | ||
| Incarceration of family member | (0.6) | (6.4) | 0.0001 | ||
ACE=Adverse Childhood Experiences; HADS=Hospital Anxiety and Depression Scale; SERM=Selective Estrogen Receptor Modulator; ns=Not Significant (p>0.05)
T-tests, Wilcoxon rank sum tests, and chi-square tests were used to assess whether sociodemographic information differed between ACE groups. Two-sided hypotheses were evaluated with significance defined as p<=0.05.
Figure 1. Consort diagram.
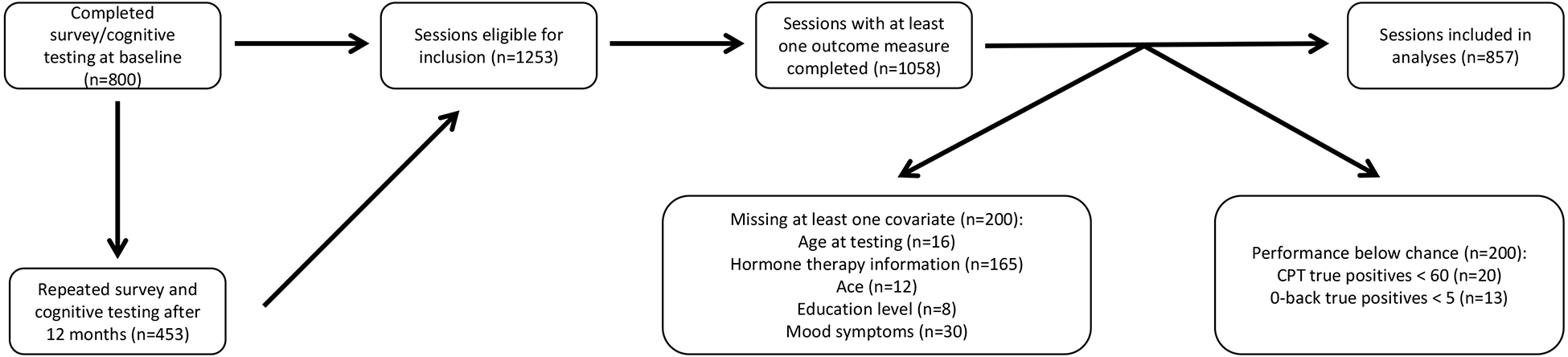
Eight hundred participants completed cognitive testing at baseline and 453 participants repeated cognitive testing after 1 year, yielding a total of 1,253 sessions that were eligible for inclusion. Sessions included in analyses must have completed at least one outcome of interest (n=1,058). Sessions missing information regarding age at testing (n=16), hormone therapy use (n=165), ACE (n=12), education level (n=8), and mood symptoms (n=30) were excluded. Participants who performed below chance (<50% true positives) on the CPT were excluded from analyses of the CPT (n=20). Similarly, participants who performed below chance (<5 true positive responses) on the 0-back control condition were excluded from analyses of the n-back (n=13). Complete data for at least one outcome measure and all primary covariates were obtained on 494 participants at baseline and 363 participants at the 1-year follow-up session, yielding a total of 552 participants and 857 sessions included in analyses. ACE=adverse childhood experiences.
ACE associations with executive function
ACE was associated with higher age-residualized total BADDS score (n=493, adjusted mean difference (aMD)= 7.1, p=0.0005) (Figure 2a) as well as each BADDS subscale (organization/activation for work, aMD=1.7, p= 0.002; attention/concentration, aMD=1.8, p=0.001; alertness/effort/processing speed, aMD=1.4, p=0.004; managing affective interference, aMD=1.3, p=0.0007; working memory/accessing recall, aMD=0.8 p=0.03) (Figure 2b). The high ACE group also performed worse on both the n-back (n=479, aMD=−0.17, p=0.007) (Figure 3a) and CPT (n=500, aMD=−0.1, p=0.03) (Figure 3b) in comparison to the low ACE group. Chemotherapy use was negatively associated with sustained attention (aMD=−0.17, p=0.006) (Figure 4) but was not associated with subjective symptoms or working memory.
Figure 2. ACE associations with subjective symptoms of executive dysfunction.
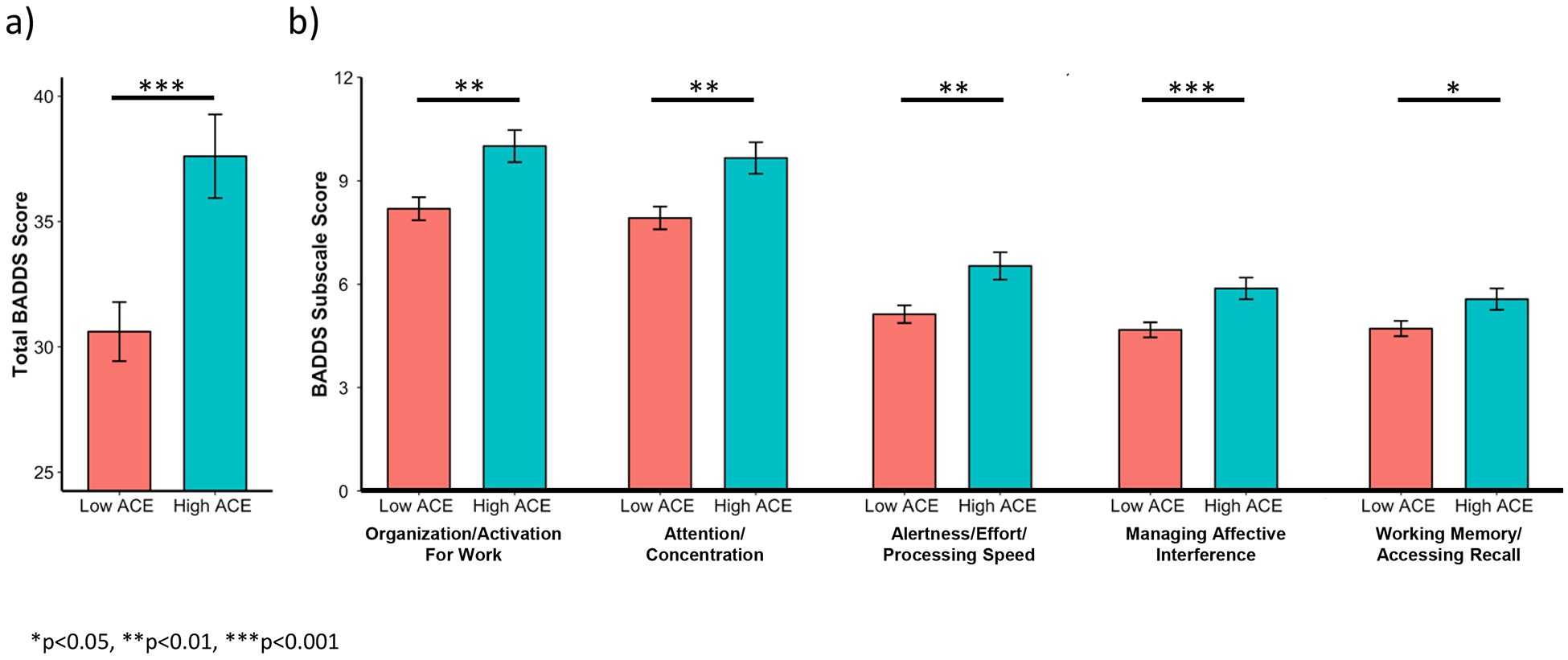
ACE was associated with higher total BADDS score (a) as well as higher scores on each BADDS subscale (b). Bars represent means and error bars represent standard error. BADDS=Brown Attention Deficit Disorder Scale. ACE=adverse childhood experiences
Figure 3. ACE associations with sustained attention and working memory.
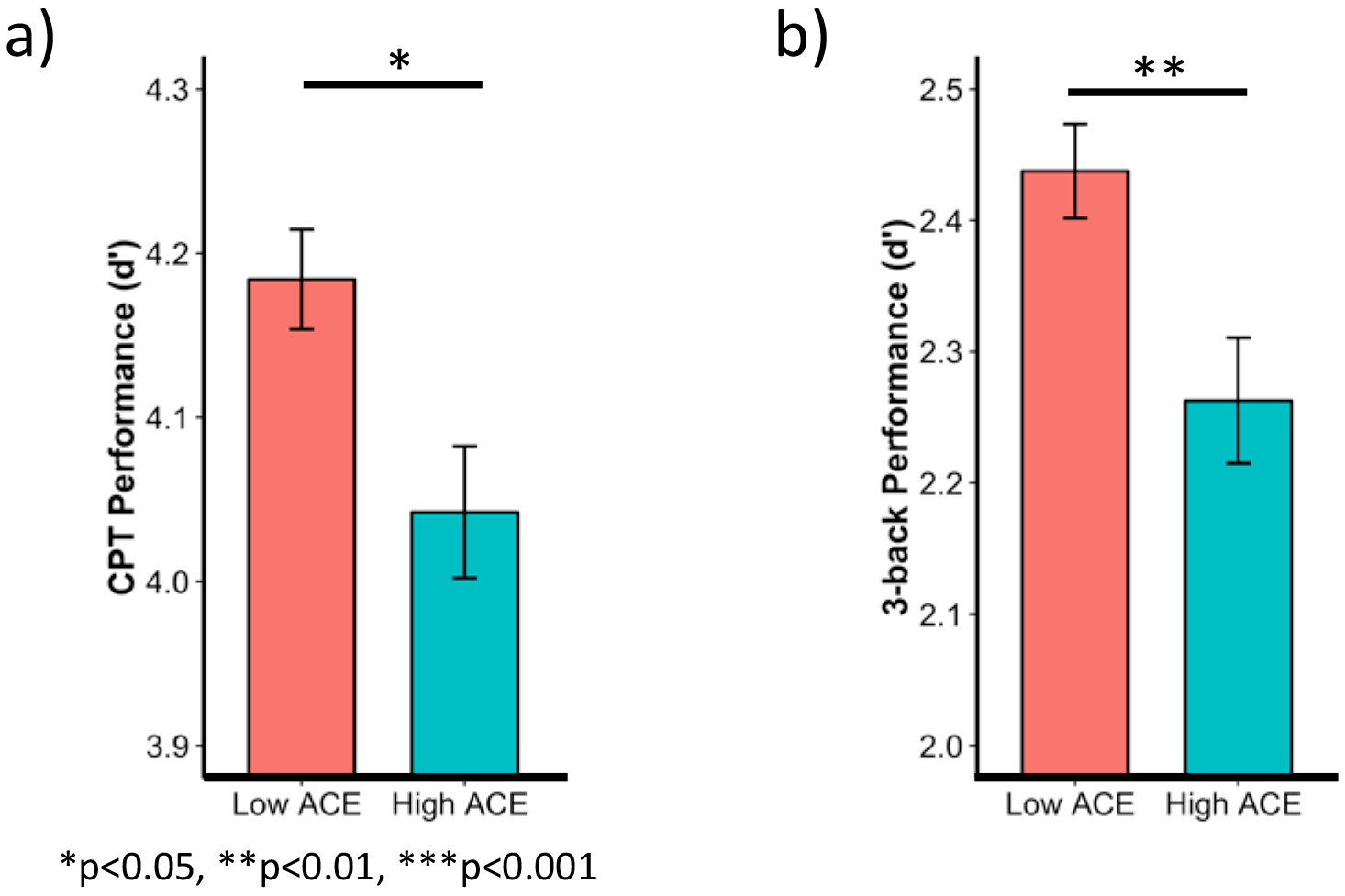
When controlling for age, time without hormones, chemotherapy use, and education level, ACE was associated with worse performance worse on both the CPT (a) and n-back (b). Bars represent means and error bars represent standard error. CPT=continuous performance task. ACE=adverse childhood experiences.
Figure 4. Chemotherapy associations with sustained attention.
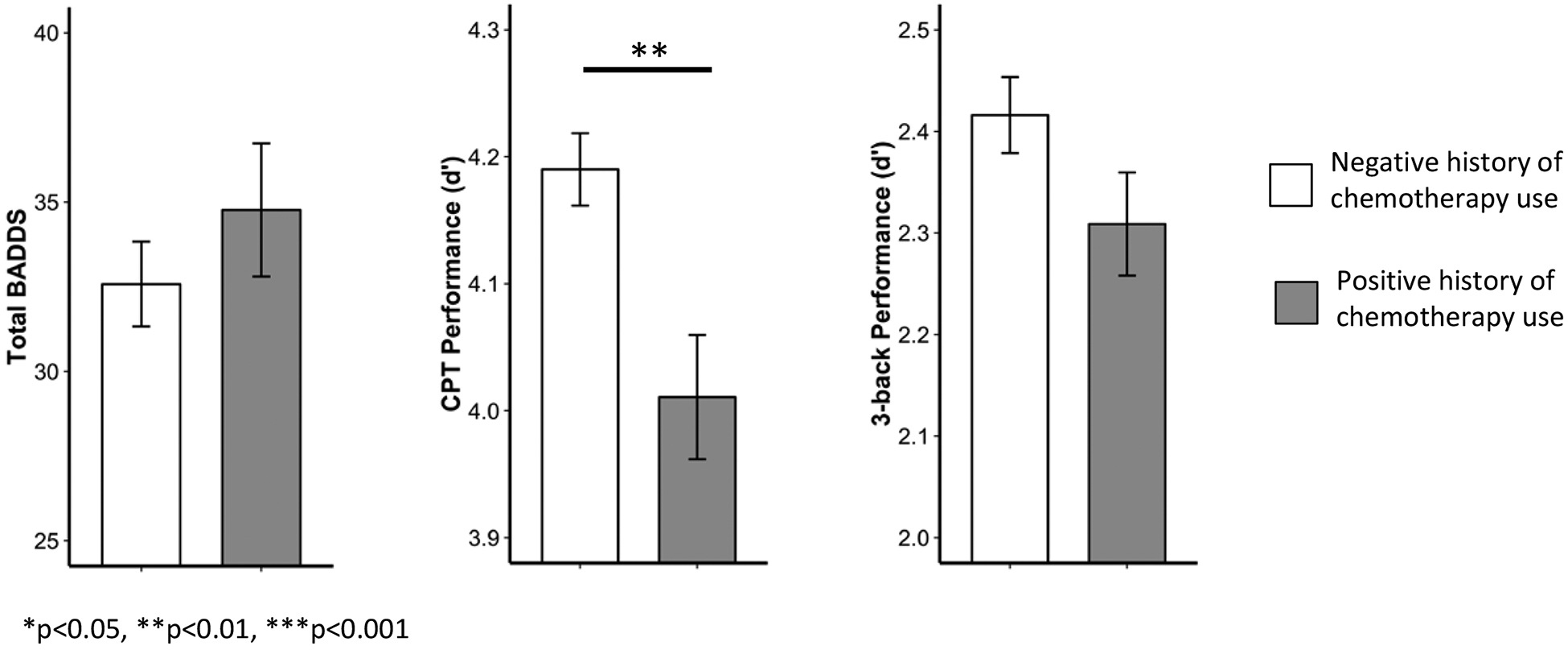
When controlling for ACE group, age, time without hormones, and education level, history (previous or current) of chemotherapy use was associated with worse sustained attention. There was no effect of history of chemotherapy on subjective symptoms of executive dysfunction or working memory performance. Bars represent means and error bars represent standard error. BADDS=Brown Attention Deficit Disorder Scale. CPT=continuous performance task.
Mood symptoms mediate ACE associations with executive function
We next examined whether mood symptoms mediated ACE associations with executive function (Figure 5a). ACE was significantly associated with greater mood symptoms (aMD=2.1, p=0.0003) and mood symptoms were significantly associated with age-residualized total BADDS scores (coefficient=2.1, p<0.0001). After adjusting for mood symptoms, the direct effect of ACE on total BADDS scores was lower (aMD=2.8, p=0.08). The estimated indirect effect of ACE on total BADDS through mood was also statistically significant (aMD=4.48, p=0.0004), an indication that mood symptoms partially mediate the association between ACE and total BADDS score. (62.8% mediated; 95% CI: 42.3% - 100%).
Figure 5. Mood symptoms mediate ACE associations with executive function.
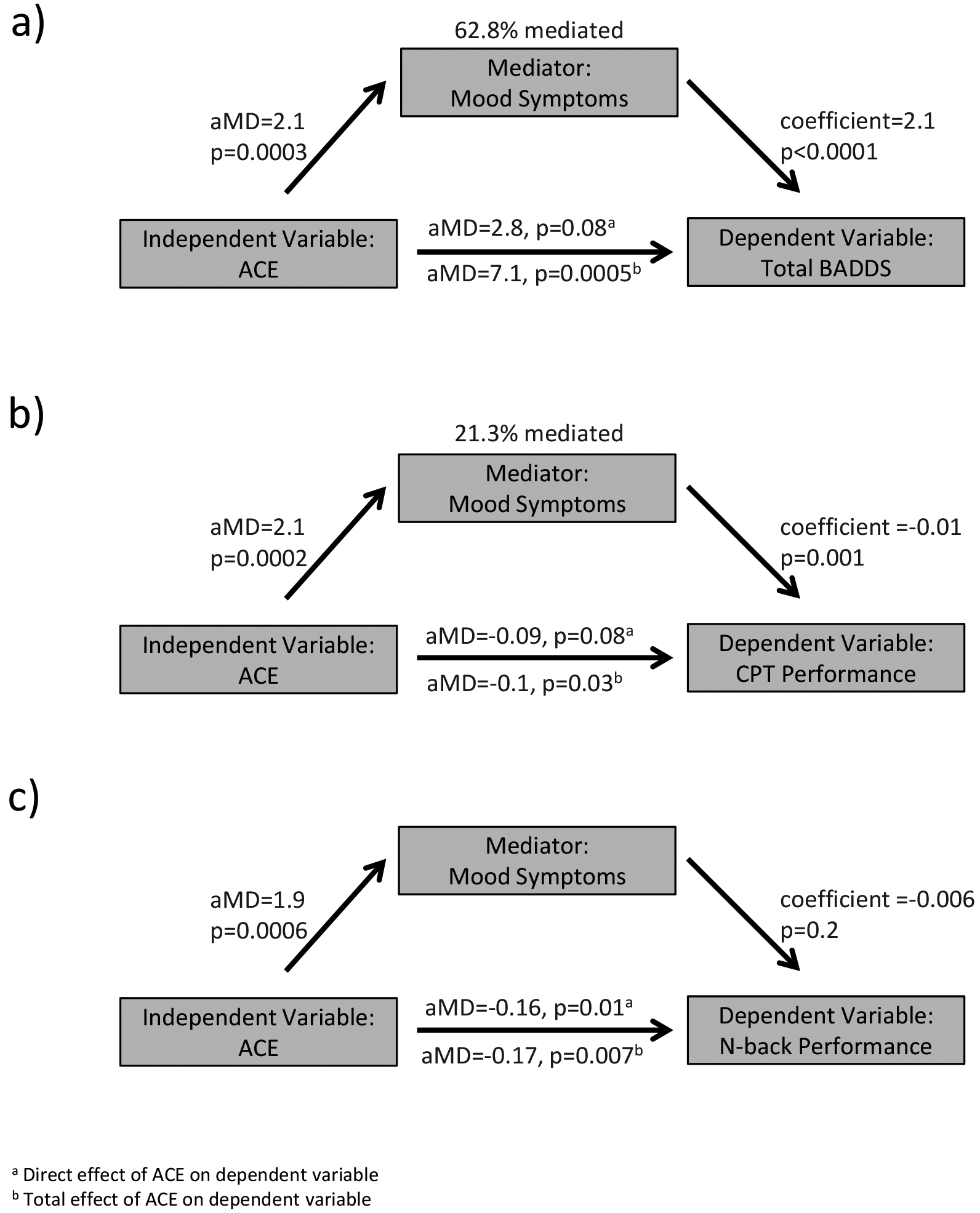
Depressive and anxiety symptoms were assessed using the 14-item Hospital Anxiety and Depression Scale. Total scores on the depression and anxiety subscales were summed to create a composite measure of mood symptoms. This composite score was assessed as a mediator of the relationship between ACE and executive outcomes. a) Mood symptoms partially mediated the relationship between ACE and total BADDS score. b) Mood symptoms partially mediated the relationship between ACE and sustained attention. c) Mood symptoms did not mediate a significant proportion of the relationship between ACE and working memory performance. BADDS=Brown Attention Deficit Disorder Scale. CPT=continuous performance task. ACE=adverse childhood experiences. aMD= adjusted mean difference. Coefficient= regression coefficient in a continuous-by-continuous regression.
Similarly, we evaluated the extent that mood symptoms mediated the association between ACE and sustained attention (Figure 5b). ACE was significantly associated with greater mood symptoms (aMD=2.1, p=0.0002) and mood symptoms were significantly negatively associated with age-residualized CPT d’ (coefficient=−0.01, p=0.001). After adjusting for mood symptoms, the direct effect of ACE on CPT d’ was diminished (aMD=−0.09, p=0.08). The estimated indirect effect of ACE on sustained attention through mood was also statistically significant (aMD= −0.024, p=0.03), an indication that mood symptoms partially mediated ACE associations with sustained attention (21.3% mediated; 95% CI: 9.3% - 100%).
In contrast, mood symptoms did not mediate a significant proportion of the relationship between ACE and working memory performance (6.5% mediated, 95% CI: −1.4% - 50%; Figure 5c), given that mood symptoms were not significantly associated with n-back d’ (coefficient =−0.006, p=0.20) after accounting for mood symptoms, the direct effect of ACE on n-back d’ was practically unchanged (aMD=−0.16, p=0.01).
Supplementary analyses
ACE associations with subjective executive function (aMD=6.1, p=0.002), sustained attention (aMD=−0.11, p=0.03), and working memory (aMD=−0.16, p=0.01) were similar when controlling for use of psychoactive medications. Results were also similar in the subset of women who underwent oophorectomy before age 47.4 (total BADDS, n=353, aMD =8.4, p=0.0008; CPT d’, n=350, aMD = −0.12, p=0.06; n-back d’, n=382, aMD = −0.16, p=0.06). While pre-pubertal ACE was associated with significantly more subjective executive function difficulties (aMD=5.6, p=0.03), post-pubertal ACE was not. In contrast, post-pubertal ACE was associated with worse sustained attention (aMD=−0.24, p=0.006) while pre-pubertal ACE was not. Pre-pubertal ACE (aMD=−0.13, p=0.08) and post-pubertal ACE (aMD=−0.22, p=0.07) were both associated with poorer working memory. Given that history of cancer could be an important potential confounder, we repeated our primary analyses of ACE associations with executive function with this covariate rather than history of chemotherapy given collinearity. ACE association with subjective executive function (aMD=8.4, p=0.01) and working memory (aMD=−0.18, p=0.02) were similar, but ACE association with sustained attention was no longer significant (aMD=−0.08, p=0.18).
Discussion
In this large study of cognitive function in BRCA1/2 mutation carriers who underwent RRSO, we examined the association between childhood adversity and executive function as well as the mediating role of mood. Women with higher levels of childhood adversity reported more symptoms of dysfunction across executive function domains. High ACE women also performed worse on executive function tasks probing sustained attention and working memory. Although mood symptoms partially mediated ACE associations with sustained attention and subjective report of executive dysfunction, the negative associations between ACE and these measures remained present after accounting for the effects of anxiety and depression symptoms. Taken together, these data emphasize that childhood adversity is associated with increased risk of executive dysfunction after RRSO.
Evidence for ACE associations with executive function
As predicted, we found that ACE was associated with greater subjective report of executive dysfunction and poorer performance on neuropsychological tasks of executive function. Childhood adversity has been shown to adversely impact cognitive performance in a general population.35–38 Several studies have demonstrated that early life adversity is associated with poorer executive function during childhood and adolescence39–41 and that this negative effect persists into adulthood.38 However, ACE associations with subjective and objective measures of executive function have not been studied in women who have undergone RRSO, a specific surgical procedure that leads to hypogonadism and is associated with a number of psychological issues related to concerns about cancer risk or recurrence. We have recently shown that ACE has negative impacts on executive function in naturally menopausal women7,8 and that the negative impact of ACE is heightened in the absence of exogenous estradiol.7 This literature suggests childhood adversity likely alters the developmental trajectory of the executive system24 and that abrupt loss of estradiol regulation of executive system function after RRSO is a potential mechanism underlying the observed differences in executive function between high and low ACE groups.3
Evidence for mood as a mediator of ACE associations with executive function
ACE associations with subjective report of executive dysfunction and sustained attention after RRSO were partially mediated by anxiety and depressive symptoms. Our prior studies focusing on naturally menopausal women have shown that healthy women experience onset of executive function difficulties during menopause concurrent with loss of estradiol.7,8,42–44 There is also an increased risk for affective disturbances, including major depressive disorder with the transition to menopause45 and among high ACE women in particular.14 Further, loss of estradiol has been shown to impact brain neurochemistry, structure and function.3 Our more recent studies have provided preliminary evidence that childhood adversity may be a risk factor for both executive function difficulties3 as well as mood changes14 during periods of waning estradiol. Mood symptoms are also associated with executive system dysfunction in both youths17 and adults46, though this has not specifically been studied in post-menopause. Further, our previous work has suggested that ACE-associated vulnerability for executive function difficulties with loss of estradiol during menopause involves alterations in serotonergic neurotransmission,7 highlighting the importance of jointly considering the role of early adversity and mood changes on executive functions after RRSO as we do in this report. Notably, the mediating role of mood and timing of pre- versus post-pubertal adversity varied across domains, suggesting future studies of ACE effects on executive function would benefit from further dissociation between sustained attention, working memory, and subjective executive dysfunction symptoms as well as timing of adversity onset in relation to puberty. Our result demonstrating a significant impact of mood on CPT but not n-back performance is consistent with a recent study examining correlations between depressive symptoms and performance on several executive functioning tasks in a mixed sample of older male and female adults. Similar to our findings, depressive symptoms in that study were associated with poorer scores on an attention task but not with a working memory task.47 That negative affect did not mediate the ACE effect on working memory is particularly important given a growing neuroimaging literature showing that ACEs impacts neural7 and network8 outcomes during working memory tasks in postmenopausal women.
No studies to our knowledge have pulled ACE and mood into one study to address the complexity of their contribution to executive dysfunction after RRSO with or without history of chemotherapy. By doing so in a large sample of well-characterized women post RRSO, we were able to determine the relative impact of ACE and ACE related negative affect on our outcomes of interest. Our results highlight that addressing concurrent mood changes is a critical step in treating surgical menopause-induced executive function difficulties. Importantly, we found that ACE associations were mediated by anxiety and depressive symptoms, which may not translate to a full diagnosis of generalized anxiety disorder, major depressive disorder, or other mood disorder. The mediating role of mood was significant even though the average anxiety and depression subscale scores in this sample (Table 1) were below those corresponding to full categorical diagnoses of anxiety (8+) or depressive disorders (8+),48 further emphasizing the importance of screening for sub-threshold mood symptoms in this population.
Limitations
While this study had a large sample size and controlled for multiple possible confounds, certain limitations should be acknowledged. First, participants completed cognitive testing at home rather than in a controlled environment under supervision of research staff, potentially reducing validity of these neuropsychological tests due to lack of consistency across testing settings. However, excluding participants who performed below chance on control conditions allowed us to limit this effect. Additionally, such a study design enabled us to examine the effects of early adversity on cognition post-RRSO at a scale that would have been much less feasible with in-office visits. Although we detected statistically and clinically significant differences in subjective symptoms, the absolute difference in cognitive task performance between groups was small and may not be clinically significant. Second, all participants in this study were surveyed after undergoing RRSO, so it is not possible to ascertain whether ACE has similar associations prior to RRSO. However, we have previously shown that treatment with estradiol attenuates the negative impact of ACE on executive function in naturally menopausal women,7 suggesting that ACE associations, whether present prior to RRSO or not, may be magnified by the abrupt loss of estradiol. The relative risks of hormone therapy use in the current study population present an inherent limitation to examining the moderating impact of estradiol, so longitudinal studies that evaluate participants before and after RRSO would be necessary to support or refute this hypothesis.
Conclusions
In summary, these data provide novel evidence regarding the impact of childhood adversity on executive function after RRSO. These results emphasize that mood symptoms, in part, mediate the negative associations between early adversity and executive function, underscoring the importance of assessing anxiety and depressive symptoms in women who have undergone RRSO.
Pending future longitudinal studies, questionnaires assessing childhood adversity and mood symptoms may play a role in identifying at-risk women, providing greater context for the discussion of cognitive effects of premature menopause when counseling women who would benefit from RRSO. The largest study (n=846) on quality of life in women who underwent RRSO to date noted that a portion of women in their sample who underwent RRSO experienced anxiety that affected their mood (~30%) and everyday functioning (~10%).49 Given that executive dysfunction is also associated with poorer quality of life,50,51 assessment of childhood adversity may help identify women who are more likely to experience executive function difficulties and mood symptoms after surgery, with the goal of treating these difficulties before symptoms negatively impact quality of life. Our results also suggest that current anxiety and depression symptoms should be evaluated in women who report cognitive complaints after RRSO. Assessment of mood symptoms would also be beneficial in selecting a non-hormonal treatment for women who experience vasomotor symptoms, a significant complaint affecting quality of life in women who have undergone RRSO. Selective serotonin and serotonin-norepinephrine reuptake inhibitors are effective treatments for both conditions.52
Supplementary Material
Acknowledgements:
We thank Korrina Duffy, Ph.D., for her help with editing and submitting the manuscript.
Conflicts of Interest/Sources of Funding: Dr. Epperson reports that during the course of conducting this research, she received funding from Shire Pharmaceuticals for investigator-initiated research and was the site investigator for a multi-site, randomized clinical trial funded by Sage Therapeutics. Dr. Epperson also consulted to Shire Pharmaceuticals and continues to conduct research with Sage Therapeutics. Dr. Epperson also discloses personal investments in the following companies during conduct of this research, but has now divested: Pfizer, Johnson and Johnson, Merck, Abbott, and Abbvie. Dr. Brown reports consultation for Ironshore Pharmaceuticals and publication royalties from American Psychiatric Association Publishing, Jossey-Bass/Wiley, Pearson, Routledge, and Yale University Press. Dr. Gur has no disclosures to report. Dr. Domchek has received honoraria from AstraZeneca, Clovis, and BMS and Penn has received funding for clinical trials from AZ, Clovis, and Pharmamar.
Sources of funding: This research was supported by R01 CA215587 (Epperson), K24 DA030301 (Epperson), P50 MH099910 (Epperson, Sammel), R01 AG048839 (Epperson, Sammel), K12 HD0585848 (Epperson, Sammel), F30 AG055256 (Shanmugan), R25MH119043, the Basser Research Center in the Abramson Cancer Center, and the Susan G. Komen for the Cure Foundation.
Footnotes
Previously presented at the 2018 Annual Meeting of the Society for Biological Psychiatry in New York, New York.
References
- 1.Domchek SM, Friebel TM, Singer CF, et al. : Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. Jama 304:967–75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finch AP, Lubinski J, Moller P, et al. : Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol 32:1547–53, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanmugan S, Epperson CN: Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp 35:847–65, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocca WA, Bower JH, Maraganore DM, et al. : Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 69:1074–83, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Ryan J, Scali J, Carriere I, et al. : Impact of a premature menopause on cognitive function in later life. Bjog 121:1729–39, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Kurita K, Henderson VW, Gatz M, et al. : Association of bilateral oophorectomy with cognitive function in healthy, postmenopausal women. Fertil Steril 106:749–756.e2, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanmugan S, Loughead J, Cao W, et al. : Impact of Tryptophan Depletion on Executive System Function during Menopause is Moderated by Childhood Adversity. Neuropsychopharmacology, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shanmugan S, Satterthwaite TD, Sammel MD, et al. : Impact of early life adversity and tryptophan depletion on functional connectivity in menopausal women: A double-blind, placebo-controlled crossover study. Psychoneuroendocrinology 84:197–205, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas KR, Chan G, Gelernter J, et al. : Adverse childhood events as risk factors for substance dependence: partial mediation by mood and anxiety disorders. Addict Behav 35:7–13, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giovanelli A, Reynolds AJ, Mondi CF, et al. : Adverse Childhood Experiences and Adult Well-Being in a Low-income, Urban Cohort. Pediatrics 137, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mersky JP, Topitzes J, Reynolds AJ : Impacts of adverse childhood experiences on health, mental health, and substance use in early adulthood: a cohort study of an urban, minority sample in the U.S. Child Abuse Negl 37:917–25, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sachs-Ericsson NJ, Sheffler JL, Stanley IH, et al. : When Emotional Pain Becomes Physical: Adverse Childhood Experiences, Pain, and the Role of Mood and Anxiety Disorders. J Clin Psychol, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schilling EA, Aseltine RH Jr., Gore S: Adverse childhood experiences and mental health in young adults: a longitudinal survey. BMC Public Health 7:30, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epperson CN, Sammel MD, Bale TL, et al. : Adverse Childhood Experiences and Risk for First-Episode Major Depression During the Menopause Transition. J Clin Psychiatry 78:e298–e307, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salinas-Miranda AA, Salemi JL, King LM, et al. : Adverse childhood experiences and health-related quality of life in adulthood: revelations from a community needs assessment. Health Qual Life Outcomes 13:123, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh EG, Cawthon SW: The mediating role of depressive symptoms in the relationship between adverse childhood experiences and smoking. Addict Behav 39:1471–6, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Shanmugan S, Wolf DH, Calkins ME, et al. : Common and Dissociable Mechanisms of Executive System Dysfunction Across Psychiatric Disorders in Youth. Am J Psychiatry 173:517–26, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castellon SA, Ganz PA, Bower JE, et al. : Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol 26:955–69, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Van Dyk K, Hunter AM, Ercoli L, et al. : Evaluating cognitive complaints in breast cancer survivors with the FACT-Cog and quantitative electroencephalography. Breast Cancer Res Treat, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Ganz PA, Kwan L, Castellon SA, et al. : Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J Natl Cancer Inst 105:791–801, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qualtrics. Provo, Utah, 2017 [Google Scholar]
- 22.Sandra Kooij JJ, Marije Boonstra A, Swinkels SH, et al. : Reliability, validity, and utility of instruments for self-report and informant report concerning symptoms of ADHD in adult patients. J Atten Disord 11:445–58, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Gur RC, Richard J, Hughett P, et al. : A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods 187:254–62, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shanmugan S, Satterthwaite TD: Neural Markers of the Development of Executive Function: Relevance for Education. Curr Opin Behav Sci 10:7–13, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurtz MM, Ragland JD, Bilker W, et al. : Comparison of the continuous performance test with and without working memory demands in healthy controls and patients with schizophrenia. Schizophr Res 48:307–16, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Snodgrass JG, Corwin J: Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen 117:34–50, 1988 [DOI] [PubMed] [Google Scholar]
- 27.Ragland JD, Turetsky BI, Gur RC, et al. : Working memory for complex figures: an fMRI comparison of letter and fractal n-back tasks. Neuropsychology 16:370–9, 2002 [PMC free article] [PubMed] [Google Scholar]
- 28.Felitti VJ, Anda RF, Nordenberg D, et al. : Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14:245–58, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Division of Violence Prevention: About the CDC-Kaiser ACE Study, 2014
- 30.Chapman DP, Whitfield CL, Felitti VJ, et al. : Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord 82:217–25, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Højsgaard S, Halekoh U, J Y: The R Package geepack for Generalized Estimating Equations. Journal of Statistical Software 15/2:1––11, 2006 [Google Scholar]
- 32.Team RC: R: A Language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing, 2015 [Google Scholar]
- 33.Canty A, D. RB: boot: Bootstrap R (S-Plus) Functions, 2016
- 34.Freeman EW, Sammel MD, Lin H, et al. : Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab 97:1673–80, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korten NC, Penninx BW, Pot AM, et al. : Adverse Childhood and Recent Negative Life Events: Contrasting Associations With Cognitive Decline in Older Persons. J Geriatr Psychiatry Neurol 27:128–38, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Radford K, Delbaere K, Draper B, et al. : Childhood Stress and Adversity is Associated with Late-Life Dementia in Aboriginal Australians. Am J Geriatr Psychiatry 25:1097–1106, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Almanza-Sepulveda ML, Chico E, Gonzalez A, et al. : Executive function in teen and adult women: Association with maternal status and early adversity. Dev Psychobiol 60:849–861, 2018 [DOI] [PubMed] [Google Scholar]
- 38.Philip NS, Sweet LH, Tyrka AR, et al. : Exposure to childhood trauma is associated with altered n-back activation and performance in healthy adults: implications for a commonly used working memory task. Brain Imaging Behav 10:124–35, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hostinar CE, Stellern SA, Schaefer C, et al. : Associations between early life adversity and executive function in children adopted internationally from orphanages. Proc Natl Acad Sci U S A 109 Suppl 2:17208–12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loman MM, Johnson AE, Westerlund A, et al. : The effect of early deprivation on executive attention in middle childhood. J Child Psychol Psychiatry 54:37–45, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heyman M, Hauser-Cram P: Negative life events predict performance on an executive function task in young adults with developmental disabilities. J Intellect Disabil Res 59:746–54, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Epperson CN, Pittman B, Czarkowski KA, et al. : Impact of atomoxetine on subjective attention and memory difficulties in perimenopausal and postmenopausal women. Menopause 18:542–8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Epperson CN, Shanmugan S, Kim DR, et al. : New onset executive function difficulties at menopause: a possible role for lisdexamfetamine. Psychopharmacology (Berl) 232:3091–100, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shanmugan S, Loughead J, Nanga RP, et al. : Lisdexamfetamine Effects on Executive Activation and Neurochemistry in Menopausal Women with Executive Function Difficulties. Neuropsychopharmacology 42:437–445, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freeman EW, Sammel MD, Boorman DW, et al. : Longitudinal pattern of depressive symptoms around natural menopause. JAMA Psychiatry 71:36–43, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janiri D, Moser DA, Doucet GE, et al. : Shared Neural Phenotypes for Mood and Anxiety Disorders: A Meta-analysis of 226 Task-Related Functional Imaging Studies. JAMA Psychiatry:1–8, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klojcnik M, Kavcic V, Bakracevic Vukman K: Relationship of Depression With Executive Functions and Visuospatial Memory in Elderly. Int J Aging Hum Dev:91415017712186, 2017. [DOI] [PubMed] [Google Scholar]
- 48.Bjelland I, Dahl AA, Haug TT, et al. : The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 52:69–77, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Madalinska JB, Hollenstein J, Bleiker E, et al. : Quality-of-life effects of prophylactic salpingo-oophorectomy versus gynecologic screening among women at increased risk of hereditary ovarian cancer. J Clin Oncol 23:6890–8, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Tolman AW, Kurtz MM: Neurocognitive predictors of objective and subjective quality of life in individuals with schizophrenia: a meta-analytic investigation. Schizophr Bull 38:304–15, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wehmeier PM, Schacht A, Barkley RA: Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J Adolesc Health 46:209–17, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Nelson HD, Vesco KK, Haney E, et al. : Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. Jama 295:2057–71, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


