Abstract
OBJECTIVE.
CT colonography (CTC) has been recognized as a complementary approach to evaluating the entire colon after incomplete colonoscopy (IC) in patients with occlusive colorectal cancer (CRC). The objective of this study is to evaluate changes in preoperative surgical planning after CTC is performed for patients with occlusive CRC and IC in an oncologic hospital.
MATERIALS AND METHODS.
This retrospective study included 65 consecutive patients with occlusive CRC who underwent CTC after IC at our institution from February 2000 to April 2016. CTC examinations and radiology reports were reviewed by an abdominal radiologist. Clinical information was obtained from a review of the electronic medical record.
RESULTS.
CTC contributed to a change in the initial surgical plan of the surgeon for 14 of 65 patients (21.5%). In these 14 patients, CTC detected five synchronous proximal colon polyps (35.7%), five synchronous proximal cancers (35.7%), two imprecise CRC locations (14.3%), one case of proximal colon ischemia (7.1%), and one instance of tumor infiltration of the urinary bladder (7.1%). All CTC findings were confirmed at surgery, and all proximal colon polyps were subsequently confirmed to be advanced adenomas.
CONCLUSION.
The preoperative CTC findings optimized the surgical management plan for 21.5% of patients with occlusive CRC and IC.
Keywords: cancer staging, colon cancer, colonoscopy, CT, CT colonography
Colorectal cancer (CRC) is the second most common cause of cancer-related death in the United States [1]. Over the past 2 decades, the decrease in the CRC death rate has been attributed to improvements in early detection and treatment [1]. An important aspect of surgical planning for patients with CRC is preoperative evaluation of the entire colon to rule out potential additional synchronous colorectal neoplasia. Studies in the literature have reported rates of synchronous CRC ranging from 2.2% to 8.1% [2–5] and the presence of adenomatous polyps in up to 28% of cases [4, 5].
Conventional colonoscopy, despite being the reference standard, is not capable of providing full colonic evaluation, mainly because of patient age, poor bowel preparation, prior abdominal surgery, tortuosity of the colon, and history of inflammatory bowel disease [6, 7], as well as occlusive CRC, as was shown by Pagana et al. [5] in 15% of cases. Occlusive CRC is known to occur mainly in the distal colon, increasing the length of the proximal colon not evaluated. As a result, synchronous lesions tend to be more common [8]. Moreover, these synchronous lesions are more difficult to assess during surgery if the proximal colon is distended by feces or gas [9]. This is even more relevant in laparoscopic-assisted and robotic-assisted colorectal surgery because manual palpation is not feasible using these techniques [10]. More proximally located synchronous colon tumors that are not detected before or during surgical resection of an occlusive CRC may potentially result in negative prognostic consequences, including the possibility of increased morbidity related to the need for possible subsequent reoperation and the potential for advanced-stage synchronous colon cancer at the time of diagnosis [11].
CT colonography (CTC), also known as virtual colonoscopy, has been recognized as an alternative approach in evaluating the entire colon after incomplete colonoscopy (IC) [12–19]. The increasing importance of CTC has been highlighted by its inclusion as a current CRC screening test option in screening guidelines issued by several national organizations, including the American Cancer Society, American College of Radiology, and U.S. Multi-Society Task Force [20], the American College of Gastroenterology [21], the European Society of Gastrointestinal Endoscopy and European Society of Gastrointestinal and Abdominal Radiology [19], and, most recently, in a recommendation issued by the U.S. Preventive Services Task Force [22] in 2016.
In this context, the aim of the present study is to evaluate changes in preoperative surgical planning after CTC is performed for patients with occlusive CRC and IC at an oncologic hospital.
Materials and Methods
Study Population
The institutional review board at Memorial Sloan Kettering Cancer Center (MSKCC) approved this HIPAA-compliant single-center retrospective study and waived the requirement for patients’ informed consent.
Consecutive patients at MSKCC who underwent CTC after IC caused by occlusive CRC from February 2000 to April 2016 were included. Patients were excluded if they underwent IC not caused by occlusive CRC or if they did not subsequently undergo surgical resection.
Using a computerized search of the radiology information system at our hospital, we identified 1073 patients who underwent CTC during the selected period, 483 of whom underwent CTC for IC. Of the 483 patients, 73 were preoperative patients with newly diagnosed CRC. Eight of the 73 patients were excluded, including six for whom IC was not caused by an occlusive CRC (one had IC because of a large inguinal hernia containing colon and five had IC because they had colon angulation at least two segments proximal to the tumor) and two who did not subsequently undergo surgical resection because of metastatic disease.
The final study cohort consisted of 65 patients.
CT Colonography Technique and Bowel Preparation
CTC examinations were performed using a standardized protocol with MDCT scanners after the patient underwent standard bowel preparation with a polyethylene glycol osmotic laxative (MiraLAX, Bayer HealthCare) and without fecal tagging. A CT scout image was obtained with the patient in the decubitus position, to check for the presence of free air in all patients with who underwent IC on the same day that CTC was performed. After confirmation of the absence of free air and placement of a rectal catheter, including inflation of the retention balloon, 1.0 mg of IV or subcutaneous glucagon was administered for temporary interruption of bowel motion. Subsequently, CO2 was carefully insufflated into the colon by use of a CO2 insufflator pump according to each patient’s tolerance (for approximately one-third of the patients, hand pumping of 15–30 pumps of room air was performed before the availability of the CO2 insufflator). Gaseous distention of the colon was evaluated on the CT scout view obtained with the patient in the supine position. Once distention was adequate, CTC was performed, and two sets of images were obtained, one with the patient supine and one with the patient prone. An additional third series of images with the patient in the right lateral decubitus position was obtained when the colon was not completed distended.
The CT parameters used were as follows: detector collimation, 40 mm; tube voltage, 120 kV; tube current, 30–300 mA; and pitch, 0.984 mm. Axial CT images were reconstructed as 1.25-mm and 5-mm slices. CT images were transferred to a remote PC-based workstation using commercially available software. The processed images were viewed on a workstation for diagnostic imaging (TeraRecon, version 4.4.12.138, Aquarius).
Contrast medium was used in 37 CTC examinations because of the request of the referring physician, usually to provide better cancer staging in this preoperative setting. In these cases, 150 mL of iohexol (300 mg I/mL; Omnipaque 300, GE Healthcare) was administered IV by use of a power injector at a rate of 2.5 mL/s.
No patients had CTC-related complications during or after the examination.
Technical Quality of CT Colonography and Colon Visualization
All CTC examinations were reviewed by a board-certified expert abdominal gastrointestinal radiologist (who had 4 years of experience as an attending radiologist) to evaluate the degree of colonic distention and the quality of bowel cleansing, which were subjectively classified as unsatisfactory, suboptimal, or optimal. The large intestine was divided into six segments, including the cecum, ascending colon or hepatic flexure, transverse colon, descending colon or splenic flexure, sigmoid colon, and rectum. Each colorectal segment was analyzed with respect to full visualization (not fully visualized on colonoscopy or CTC, fully visualized on both colonoscopy and CTC, and fully visualized on CTC only).
CT Colonography Evaluation
The structured CTC reports were reviewed by the same radiologist to assess colorectal findings. The visualized CRCs and polyps were evaluated to determine lesion number, location, size, and morphologic findings. If more than one polyp was identified in the same colorectal segment, then the largest lesion was recorded. We included only detected polyps that were larger than 5 mm, as has been recommended by the CT Colonography Reporting and Data System reporting committee [23].
Clinical Data
Patient clinical information was obtained from a detailed review of the electronic medical record. Pathologic, surgical, and colonoscopy reports were evaluated to determine the number, location, size, morphologic features, histologic type, and tumor staging of CRCs and polyps. The plans for surgical management were assessed before and after the patient underwent the CTC examination, on the basis of progress notes acknowledging the CTC results, and changes in treatment resulting from the CTC examination were recorded. Reference standard evaluation of the colon proximal to the occlusive tumor was deemed to be subsequent to postoperative complete colonoscopy (within 2 years of surgical resection), pathologic findings for the completely resected colon, or intraoperative complete colonoscopy successfully able to traverse the occlusive tumor. Colorectal polyps were classified as nonneoplastic (hyperplastic polyps, hamartomas, inflammatory polyps, and lymphoid aggregates) or neoplastic (adenomas, including serrated adenomas) [24]. The neoplastic polyps were divided into two categories: advanced adenomas (i.e., those with a polyp size > 10 mm, a villous growth pattern on histologic analysis, or high-grade dysplasia) and nonadvanced adenomas [25].
Statistical Analysis
The continuous variables were summarized using mean (± SD) values or minimum and maximum values, and the categoric variables were expressed as counts and proportions. The sensitivity and specificity of CTC for the detection of polyps per patient were determined. By-polyp sensitivity was also evaluated. The statistical data were analyzed using statistical software (SPSS software, version 22.0, SPSS-IBM).
Results
Patient Characteristics
The characteristics of the 65 patients with CRC who underwent CTC after IC caused by an occlusive tumor are presented in Table 1.
TABLE 1:
Characteristics of Patients With Occlusive Colorectal Cancer (CRC)
| Characteristic | Value |
|---|---|
| Age (y), mean (range) | 63.2 (27–88) |
| Sex | |
| Male | 35 (54) |
| Female | 30 (46) |
| Previously treated noncolorectal cancer | 10 (15) |
| Location or type of previously treated non-CRC cancer (n) | |
| Breast | 3 |
| Testicular | 2 |
| Head and neck | 2 |
| Bladder | 1 |
| Gallbladder | 1 |
| Leukemia | 1 |
| Colorectal segments not visualized by preoperative colonoscopy because of occlusive CRC, no. (%) of segments | 297 (76) |
| Time interval (d), mean (range) | |
| Between IC and CTC | 23 (0–119) |
| Between CTC and surgery | 1 (1–156) |
| Location of occlusive CRC | |
| Rectum | 11 (17) |
| Sigmoid colon | 36(55) |
| Descending colon or splenic flexure | 14 (22) |
| Transverse colon | 2 (3) |
| Ascending colon or hepatic flexure | 2 (3) |
| TNM category of CRC | |
| T1 | 1 (2) |
| T2 | 4 (6) |
| T3 | 51 (78) |
| T4 | 9 (14) |
| N0 | 26 (40) |
| N1 | 28 (43) |
| N2 | 11 (17) |
| M0 | 49 (75) |
| M1 | 16 (25) |
| CRC stage | |
| I | 4 (6) |
| II | 18 (28) |
| III | 27 (41) |
| IV | 16 (25) |
Note—Unless otherwise indicated, data are number (%) of patients. IC = incomplete colonoscopy, CTC = CT colonography.
Thirty-five (53.8%) of the patients were men and 30 (46.2%) were women. The mean patient age was 63.2 years (range, 49–77 years). Ten patients (15.4%) had undergone prior treatment of a noncolorectal cancer. The mean interval between IC and CTC was 23 days (range, 0–119 days), and six patients underwent CTC on the same day that the IC occurred, mainly because the IC was performed at an outside hospital.
A total of 297 of 390 colorectal segments (76.2%) were not visualized by preoperative colonoscopy because of the occlusive tumor. Forty-two of the 65 patients underwent subsequent complete evaluation of the colorectum after CTC via either postoperative colonoscopy after surgical resection (n = 34), pathologic analysis of the completely resected colorectal surgical specimen (n = 3), or successful total intraoperative colonoscopy that was able to traverse the occlusive tumor (n = 5). The mean interval between surgery and subsequent complete postoperative colonoscopy was 355 days (range, 41–730 days).
Technical Quality of CT Colonography
Colonic distention on CTC was optimal in 60 patients (92.3%), and colonic cleansing was optimal in 51 patients (78.5%). Twelve patients (18.5%) had at least one colorectal segment that was not well visualized on CTC, and all of these patients required an additional series of images obtained with the patient in the lateral decubitus position. CTC was able to visualize 360 of the 390 total colorectal segments (92.3%) and 267 of the 297 colorectal segments (89.9%) not visualized by preoperative colonoscopy. No CTC-related complications were noted.
Polyp Characterization
CTC found a total of 40 synchronous colorectal polyps at least 5 mm in size (mean, 11.7 mm; range, 5–30 mm) proximal to the occlusive tumor in 23 of 65 patients (35.4%). Thirty-four of the polyps (85.0%) were pedunculated, and six (15.0%) were sessile.
Forty-two patients underwent subsequent complete postoperative evaluation of the colorectum after CTC, at which time 19 patients (45.2%) were found to have one or more colorectal polyps (total of 37 polyps) with a mean size of 7.9 mm (range, 5–29 mm). Of the 37 polyps, 25 (67.6%) were tubular adenomas, six (16.2%) were tubulovillous adenomas, four (10.8%) were hyperplastic, one (2.7%) was villous adenoma, and one (2.7%) was serrated adenoma.
On per-person analysis, the sensitivity and specificity of preoperative CTC in detecting colorectal polyps greater than 5 mm were 88.9% (95% CI, 67.2–96.9%) and 83.3% (95% CI, 64.2–93.3%), respectively. On per-polyp analysis, the sensitivity of CTC was 81.1% (95% CI, 65.8–90.0%). When only polyps smaller than 10 mm (n = 9) were considered, the sensitivity was 36.4% (95% CI, 15.2–64.6%), whereas when polyps 10 mm or larger (n = 28) were considered, it was 100% (95% CI, 87.1–100%).
On per-polyp analysis, preoperative CTC did not detect seven polyps, all of which were tubular adenomas (mean size, 7.1 mm; range, 6–9 mm). Furthermore, seven polyps detected by CTC were not confirmed via subsequent reference standard analysis (mean size, 9.6 mm; range, 6–14 mm). Table 2 summarizes the data on the misclassified polyps.
Table 2:
Characteristics of Misclassified Polyps
| Case | CT Colonography | Colonoscopy or Surgery | Pathologic Diagnosis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Distention | Colon Cleansing | IV Contrast Medium | Polyp | Type | Size (mm) | Polyp Present (No. Found) | Type | Size (mm) | ||
| A | Optimal | Optimal | No | Yes | Pedunculated | 6 | No | — | — | — |
| B | Optimal | Insufficient | No | No | — | — | Yes(2) | Sessile | 9 and 7 | Tubular adenoma |
| C | Optimal | Optimal | No | Yes | Pedunculated | 9 | No | — | — | — |
| D | Optimal | Optimal | No | Yes | Pedunculated | 9 | No | — | — | — |
| E | Optimal | Optimal | Yes | Yes | Sessile | 13 | No | — | — | — |
| F | Optimal | Optimal | No | Yes | Pedunculated | 5 | No | — | — | — |
| G | Optimal | Optimal | Yes | Yes | Pedunculated | 14 | No | — | — | — |
| H | Insufficient | Insufficient | No | No | — | — | Yes(2) | Pedunculated | 6 and 6 | Tubular adenoma |
| I | Optimal | Optimal | No | No | — | — | Yes(2) | Pedunculated | 6 and 6 | Tubular adenoma |
| J | Suboptimal | Insufficient | Yes | No | — | — | Yes(2) | Pedunculated | 8 and 7 | Tubular adenoma |
| K | Optimal | Optimal | Yes | Yes | Pedunculated | 8 | No | — | — | — |
Note—Dash denotes absent.
Colorectal Cancer Characterization
CTC detected all 65 occlusive CRCs previously characterized on incomplete preoperative colonoscopy and four additional proximal synchronous colon cancers in different patients, including one cancer in the cecum, two in the ascending colon, and one in the rectum. All four of the additional synchronous colon cancers were subsequently confirmed as adenocarcinomas on pathologic analysis. In addition, CTC also detected a synchronous appendiceal tumor more proximally, which was subsequently confirmed on pathologic analysis as a grade 1 neuroendocrine tumor of the appendix.
Surgical Treatment Changes
The preoperative clinical information provided by CTC contributed to a change in the surgeon’s initial surgical management plan in 14 of 65 patients (21.5%). One patient underwent laparoscopic surgery, and the remaining patients underwent open surgery. The change was caused by proximal synchronous colon polyps in five patients (35.7%), proximal synchronous tumors in five (35.7%; four colon tumors and one appendiceal tumor), imprecise tumor location determined at colonoscopy in two (14.3%), proximal colon ischemia in one (7.1%), and detection of tumor infiltration of the urinary bladder in one (7.1%). Table 3 summarizes the changes in surgical planning resulting from preoperative CTC findings.
TABLE 3:
Changes in Surgical Planning Resulting From CT Colonography (CTC) Findings
| Case | Surgical Planning Before CTC | Surgical Planning After CTC | Type of Change | Reason for Change | Pathologic Analysis of Unknown Lesion (Polyp Size)a |
|---|---|---|---|---|---|
| 1 | Left hemicolectomy | Left hemicolectomy and appendectomy | Added appendectomy | Incidental lesion in appendix | Neuroendocrine tumor |
| 2 | Sigmoid resection | Sigmoid resection and right hemicolectomy | Added right hemicolectomy | Proximal synchronous lesion | Adenocarcinoma |
| 3 | Left hemicolectomy | Total colectomy | Changed left hemicolectomy to total colectomy | Polyps in the proximal colon | Tubulovillous and tubular adenoma (10–20 mm) |
| 4 | Sigmoid resection | Left hemicolectomy | Changed sigmoid resection to left hemicolectomy | Modification in tumor location | Adenocarcinoma |
| 5 | Left hemicolectomy | Subtotal colectomy | Changed left hemicolectomy to subtotal colectomy | Proximal synchronous lesion | Adenocarcinoma |
| 6 | Total mesorectal excision | Sigmoid resection | Changed rectal surgery to sigmoid surgery | Modification in tumor location | Adenocarcinoma |
| 7 | Sigmoid resection | Sigmoid resection and extended left hemicolectomy | Added extended left hemicolectomy | Proximal synchronous lesion | Adenocarcinoma |
| 8 | Left hemicolectomy | Subtotal colectomy | Changed left hemicolectomy to subtotal colectomy | Proximal synchronous lesion | Adenocarcinoma |
| 9 | Total mesorectal excision | Total proctocolectomy | Added total colectomy | Numerous polyps in the proximal colon | Tubular and tubulovillous adenomas with high-grade dysplasia (1–12 mm) |
| 10 | Left hemicolectomy | Extended left hemicolectomy | Changed left hemicolectomy to extended left hemicolectomy | Proximal polyp | Tubulovillous adenoma (12 mm) |
| 11 | Left hemicolectomy | Extended left hemicolectomy | Changed left hemicolectomy to extended left hemicolectomy | Proximal polyp | Tubular adenomas (6–15 mm) |
| 12 | Low anterior resection | Low anterior resection and cystectomy | Added cystectomy | T4 tumor | Adenocarcinoma |
| 13 | Sigmoid resection | Sigmoid resection and right colectomy | Added right colectomy | Proximal polyp | Tubular adenomas with high-grade dysplasia (24–43 mm) |
| 14 | Left hemicolectomy | Extended left hemicolectomy | Changed left hemicolectomy to extended left hemicolectomy | Proximal colonic ischemia | Ischemic changes |
If more than one polyp was present, the size range is provided.
All findings on CTC that were proximal to the occlusive CRC and resulted in changes in preoperative surgical management planning were subsequently confirmed at surgery. Furthermore, all proximal synchronous colon polyps were proven to be advanced adenomas (one patient had polyps ≥ 10 mm; two patients had polyps ≥ 10 mm with a villous component; one patient had polyps ≥ 10 mm and high-grade dysplasia; and one patient had polyps ≥ 10 mm with a villous component and also had high-grade dysplasia).
Two examples illustrate changes in surgical management based on CTC findings. Case 1 was an appendiceal neuroendocrine tumor detected by preoperative CTC in a 67-year-old man with an occlusive descending colon cancer. On the basis of these findings, an appendectomy was added to the initially planned surgical left hemicolectomy (Fig. 1). Case 2 was a 52-year-old woman with an occlusive sigmoid cancer and IC who underwent an anterior sigmoid resection and right hemicolectomy because of a synchronous lesion in the ascending colon (detected on CTC), which was found to be an adenocarcinoma (Fig. 2).
Fig. 1—
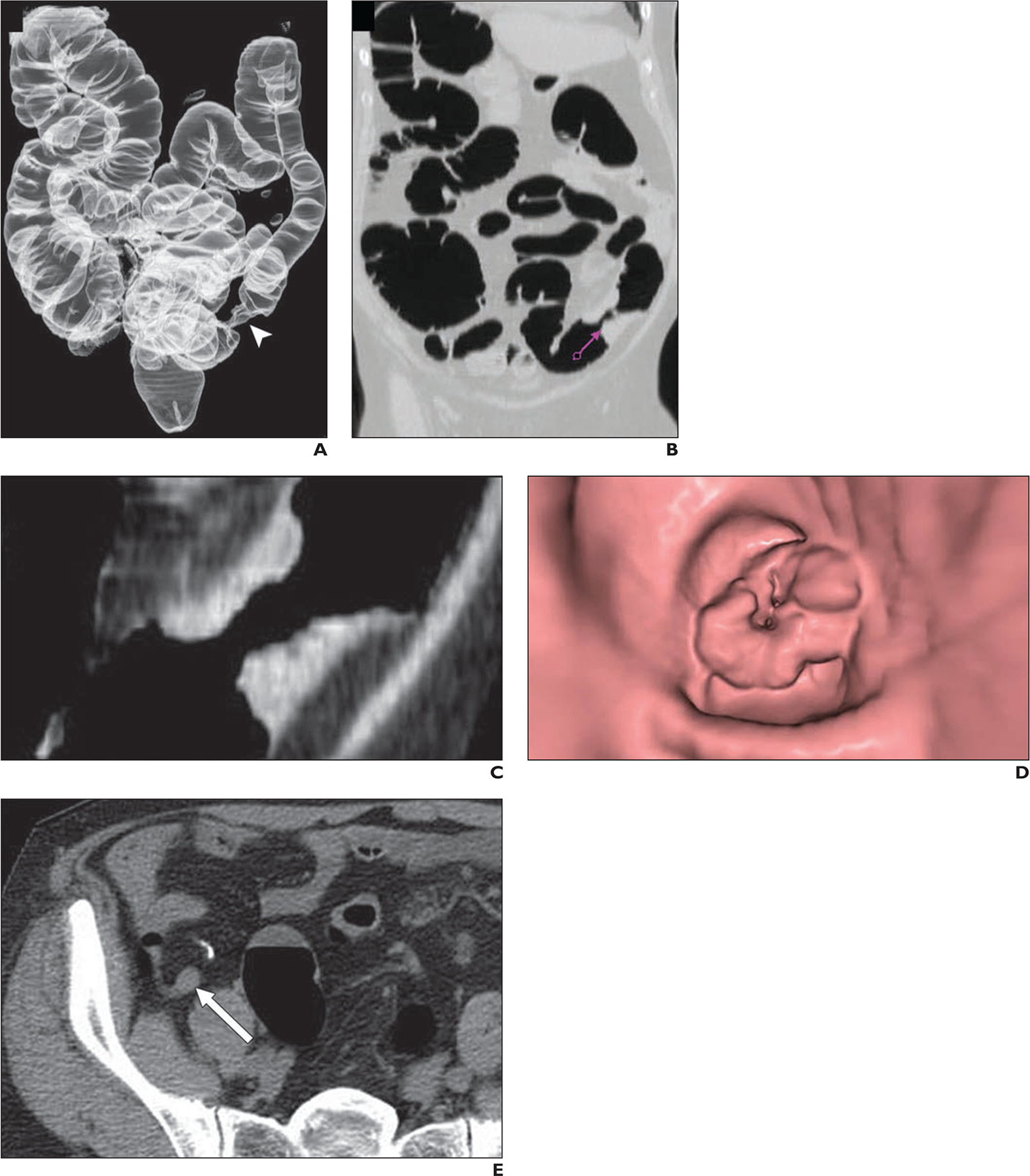
67-year-old man with newly diagnosed occlusive cancer in descending colon. Synchronous appendiceal lesion was noted in setting of occlusive descending colon cancer.
A, Three-dimensional edge-enhanced CT image shows known descending lesion (arrowhead) in colon, which presented as typical apple-core wall deformity.
B and C, Lesion was characterized as focal wall thickening on CT images. Purple arrow (B) represents 3D endoluminal view.
D, Three-dimensional endoluminal view from CT colonography (D) corresponding to CT views in B and C characterizes lesion as luminal narrowing.
E, CT image shows additional lesion detected in tip of appendix (arrow).
Fig. 2—
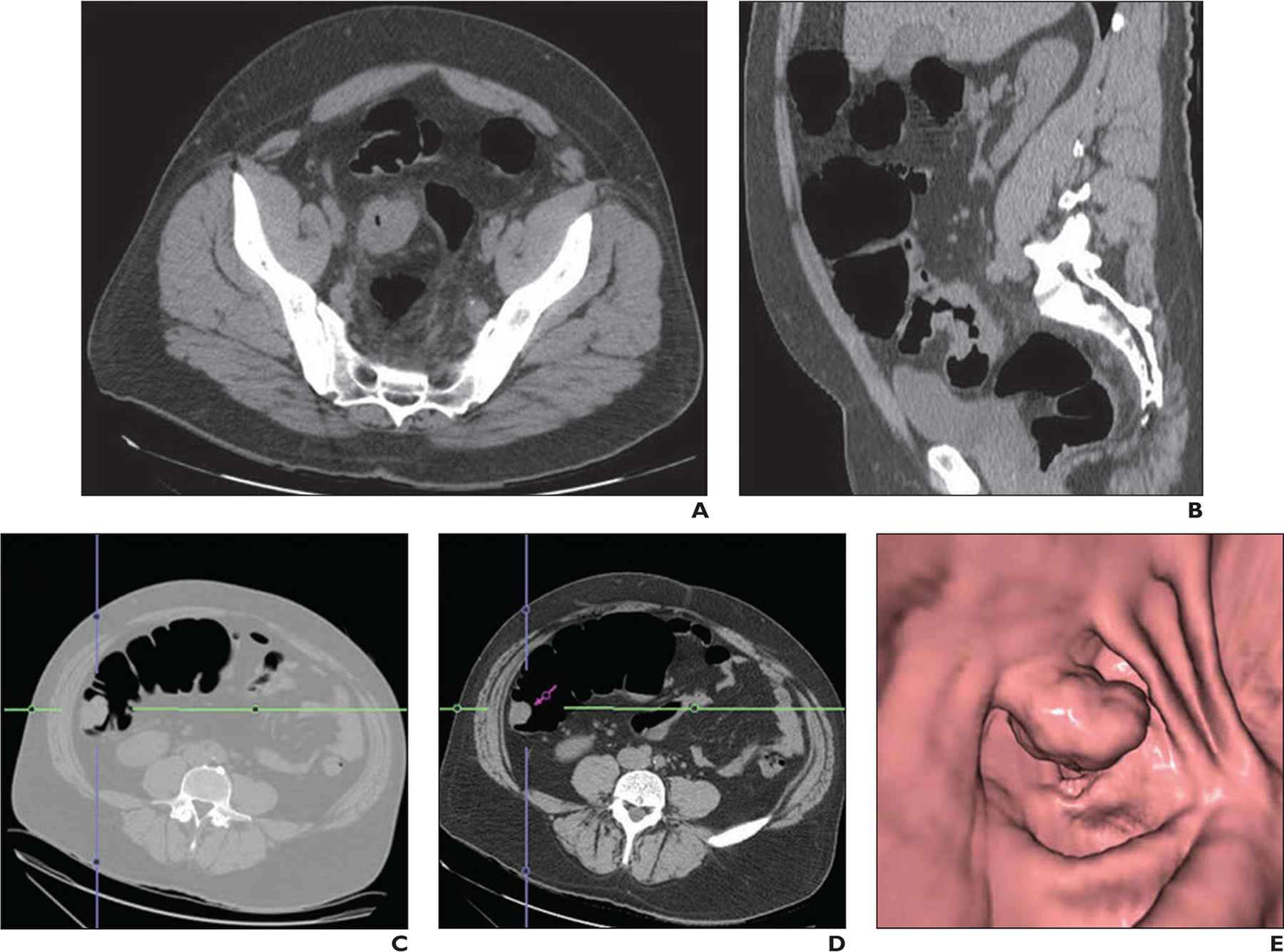
52-year-old woman with recently diagnosed occlusive sigmoid adenocarcinoma who had synchronous ascending colon lesion. Virtual colonoscopy was performed to evaluate proximal colon.
A and B, Unenhanced axial (A) and sagittal (B) CT images show occlusive sigmoid colon cancer.
C–E, CT images (C and D) and 3D endoluminal view from CT colonography (E) show synchronous polypoid lesion in ascending colon. Right hemicolectomy was included in surgical planning of patient because of incidental finding in right colon. Pathologic analysis revealed two adenocarcinomas, T3 lesion in sigmoid colon and T1 lesion in ascending colon. Blue and green lines (C and D) are reference lines for 3D view and purple arrow (D) represents 3D endoluminal view.
Discussion
In our study cohort of 65 consecutive patients with occlusive CRC and preoperative IC followed by CTC, the rectosigmoid segments were the most frequent location of the colorectal tumors (72.3% [47/65]), which left 76.1% (297/390) of colonic segments inaccessible to preoperative conventional colonoscopy. CTC safely completed the preoperative evaluation of 267 of the 297 inaccessible segments (89.9%), which left only 30 segments (10.1%) not fully evaluated by both examinations. Our study shows that preoperative CTC findings allowed the surgeon to more accurately design the surgical management plan for 14 of 65 patients (21.5%). Of importance, the data provided by CTC findings allowed safe resection of these additional colonic segments in one setting. These data also indicate the potential to avoid the costs and potential morbidity that would have been associated with a second operation for these patients if these additional neoplasms had not been identified.
We found that the most common reason for changing the surgical management plan was the detection of proximal synchronous neoplasia, including colon polyps (n = 5) or cancer (colon cancer [n = 4] and appendiceal neuroendocrine tumor [n = 1]), accounting for 71.4% of the total number of cases with a revised surgical management plan. All of these preoperative CTC findings proximal to the occlusive tumor were subsequently confirmed and justified the change in surgical management plans. However, preoperative CTC did not detect seven proximal synchronous colon polyps, none of which were subsequently found to be advanced adenomas on pathologic analysis. On the basis of per-person and per-polyp analysis, we found overall sensitivities of 88.9% and 81.1%, respectively.
Our observations suggest that CTC plays an important role in the preoperative evaluation of the proximal colon in patients with occlusive CRC, by accessing approximately 90% of the colon segments, which were not evaluable by conventional colonoscopy. Our study shows the utility of CTC to help identify lesions requiring expeditious resection in the setting of occlusive disease not amenable to complete colonoscopy. Specifically, we found that most of the proximal findings detected by CTC were neoplasms (adenocarcinoma and neuroendocrine tumor) and advanced adenomas, and none of the seven uncharacterized polyps were advanced adenomas on pathologic analysis.
Most occlusive CRCs in our cohort (93.8% [61/65]) were located in the left colon (sigmoid colon > rectum > descending colon or splenic flexure), a slightly higher prevalence than has been observed in some previous studies [13, 18]. This distal preponderance of tumor obstruction is important to note when considering the length of the more proximal colon segment that is rendered inaccessible at conventional colonoscopy, which may contribute to the increased the number of proximal colon lesions in our study.
Flor et al. [18] evaluated changes in surgical management planning resulting from preoperative CTC colonic findings in a study of laparoscopic procedures performed on 69 patients, and they showed that the information provided by CTC changed the surgical strategy for 10 patients (14%). In five patients, the change was caused by synchronous cancers (in three patients, CTC clarified the exact location of the lesion, and in two patients, planning was changed as a result of the detection of proximal polyps larger than 6 mm) [18]. Other authors have also reported that CTC detected additional relevant findings in 16–23% of the patients with occlusive CRC, and most of the findings were synchronous proximal colon neoplastic lesions and change in primary tumor localization [12, 13, 15, 17]. However, they did not specifically explore the changes in surgical planning, and advanced adenomas were not recorded.
Preoperative evaluation of the entire colon is important given the high frequency of synchronous lesions [2, 3]. Occlusive cancer is more frequent in the distal colon, leaving many segments of the colon inaccessible to colonoscopy. Patients with CRC who undergo complete evaluation of the colon preoperatively may have less local recurrence, less chance of developing distant metastases, and a longer disease-free survival compared with patients who do not [26, 27]. Furthermore, precise localization of CRC, which may be achieved using CTC, is critical for the surgeon performing open, laparoscopic, and robotic-assisted surgery, to allow more precise planning of the operation, including determining trocar placement in minimally invasive settings, assessing the efficiency of the operation, and obtaining an adequate oncologic resection [28].
Our study has limitations. These include its retrospective nature, the fact that patients were retrieved over a long period during which changes in CT technology occurred, and the lack of fecal tagging (which some radiology experts routinely use to improve the accuracy of CTC) during the CTC examinations performed [29, 30]. Furthermore, only 42 of 65 patients underwent a subsequent evaluation of the colon after preoperative CTC, 34 via surveillance colonoscopy performed up to 2 years after surgery
Nonetheless, our study has a number of strengths, including its evaluation of the specific changes in surgical planning on the basis of a detailed review of the electronic medical records of an oncologic population. We also have adhered to the nomenclature for advanced adenomas versus nonadvanced adenomas among the neoplastic polyps, to better assess the changes in surgical planning owing to presence of proximal polyps.
Conclusion
In conclusion, CTC findings in our population optimized the surgical management plan for 21.5% of patients with occlusive CRC and IC assessment of the proximal colon.
Acknowledgments
Supported by core grant P30CA008748 from the National Institutes of Health.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66:7–30 [DOI] [PubMed] [Google Scholar]
- 2.Cunliffe WJ, Hasleton PS, Tweedle DE, Schofield PF. Incidence of synchronous and metachronous colorectal carcinoma. Br J Surg 1984; 71:941–943 [DOI] [PubMed] [Google Scholar]
- 3.Huang CS, Yang SH, Lin CC, et al. Synchronous and metachronous colorectal cancers: distinct disease entities or different disease courses? Hepatogastroenterology 2015; 62:286–290 [PubMed] [Google Scholar]
- 4.Arenas RB, Fichera A, Mhoon D, Michelassi F. Incidence and therapeutic implications of synchronous colonic pathology in colorectal adenocarcinoma. Surgery 1997; 122:706–709; discussion, 709–710 [DOI] [PubMed] [Google Scholar]
- 5.Pagana TJ, Ledesma EJ, Mittelman A, Nava HR. The use of colonoscopy in the study of synchronous colorectal neoplasms. Cancer 1984; 53:356–359 [DOI] [PubMed] [Google Scholar]
- 6.Koido S, Ohkusa T, Nakae K, et al. Factors associated with incomplete colonoscopy at a Japanese academic hospital. World J Gastroenterol 2014; 20:6961–6967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah HA, Paszat LF, Saskin R, Stukel TA, Rabeneck L. Factors associated with incomplete colonoscopy: a population-based study. Gastroenterology 2007; 132:2297–2303 [DOI] [PubMed] [Google Scholar]
- 8.Bat L, Neumann G, Shemesh E. The association of synchronous neoplasms with occluding colorectal cancer. Dis Colon Rectum 1985; 28:149–151 [DOI] [PubMed] [Google Scholar]
- 9.Heald RJBH. Clinical experiences at St Mark’s Hospital with multiple synchronous cancers of the colon and rectum. Dis Colon Rectum 1975; 18:6–10 [DOI] [PubMed] [Google Scholar]
- 10.Ahad S, Figueredo EJ. Laparoscopic colectomy. MedGenMed 2007; 9:37. [PMC free article] [PubMed] [Google Scholar]
- 11.Achiam MP, Burgdorf SK, Wilhelmsen M, Alamili M, Rosenberg J. Inadequate preoperative colonic evaluation for synchronous colorectal cancer. Scand J Surg 2009; 98:62–67 [DOI] [PubMed] [Google Scholar]
- 12.Fenlon HM, McAneny DB, Nunes DP, Clarke PD, Ferrucci JT. Occlusive colon carcinoma: virtual colonoscopy in the preoperative evaluation of the proximal colon. Radiology 1999; 210:423–428 [DOI] [PubMed] [Google Scholar]
- 13.Neri E, Giusti P, Battolla L, et al. Colorectal cancer: role of CT colonography in preoperative evaluation after incomplete colonoscopy. Radiology 2002; 223:615–619 [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Kim WH, Kim TI, et al. Incomplete colonoscopy in patients with occlusive colorectal cancer: usefulness of CT colonography according to tumor location. Yonsei Med J 2007; 48:934–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagata K, Ota Y, Okawa T, Endo S, Kudo SE. PET/CT colonography for the preoperative evaluation of the colon proximal to the obstructive colorectal cancer. Dis Colon Rectum 2008; 51:882–890 [DOI] [PubMed] [Google Scholar]
- 16.Maras-Simunic M, Druzijanic N, Simunic M, Roglic J, Tomic S, Perko Z. Use of modified multidetector CT colonography for the evaluation of acute and subacute colon obstruction caused by colorectal cancer: a feasibility study. Dis Colon Rectum 2009; 52:489–495 [DOI] [PubMed] [Google Scholar]
- 17.da Fonte AC, Chojniak R, de Oliveira Ferreira F, Pinto PN, dos Santos Neto PJ, Bitencourt AG. Inclusion of computed tomographic colonography on pre-operative CT for patients with colorectal cancer. Eur J Radiol 2012; 81:e298–e303 [DOI] [PubMed] [Google Scholar]
- 18.Flor N, Ceretti AP, Mezzanzanica M, et al. Impact of contrast-enhanced computed tomography colonography on laparoscopic surgical planning of colorectal cancer. Abdom Imaging 2013; 38:1024–1032 [DOI] [PubMed] [Google Scholar]
- 19.Spada C, Stoker J, Alarcon O, et al. Clinical indications for computed tomographic colonography: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Guideline. Eur Radiol 2015; 25:331–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008; 134:1570–1595 [DOI] [PubMed] [Google Scholar]
- 21.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol 2009; 104:739–750 [Erratum in Am J Gastroenterol 2009; 104:613] [DOI] [PubMed] [Google Scholar]
- 22.US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA 2016; 315:2564–2575 [DOI] [PubMed] [Google Scholar]
- 23.Pooler BD, Kim DH, Lam VP, Burnside ES, Pickhardt PJ. CT Colonography Reporting and Data System (C-RADS): benchmark values from a clinical screening program. AJR 2014; 202:1232–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colucci PM, Yale SH, Rall CJ. Colorectal polyps. Clin Med Res 2003; 1:261–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winawer SJ, Zauber AG. The advanced adenoma as the primary target of screening. Gastrointest Endosc Clin N Am 2002; 12:1–9 [v] [DOI] [PubMed] [Google Scholar]
- 26.Howard ML, Greene FL. The effect of preoperative endoscopy on recurrence and survival following surgery for colorectal carcinoma. Am Surg 1990; 56:124–127 [PubMed] [Google Scholar]
- 27.Isler JT, Brown PC, Lewis FG, Billingham RP. The role of preoperative colonoscopy in colorectal cancer. Dis Colon Rectum 1987; 30:435–439 [DOI] [PubMed] [Google Scholar]
- 28.Wexner SD, Cohen SM, Ulrich A, Reissman P. Laparoscopic colorectal surgery: are we being honest with our patients? Dis Colon Rectum 1995; 38:723–727 [DOI] [PubMed] [Google Scholar]
- 29.Gryspeerdt S, Lefere P, Herman M, et al. CT colonography with fecal tagging after incomplete colonoscopy. Eur Radiol 2005; 15:1192–1202 [DOI] [PubMed] [Google Scholar]
- 30.Buccicardi D, Grosso M, Caviglia I, et al. CT colonography: patient tolerance of laxative free fecal tagging regimen versus traditional cathartic cleansing. Abdom Imaging 2011; 36:532–537 [DOI] [PubMed] [Google Scholar]


