Abstract
Bee venom (BV), secreted from the venom gland of the honey bee, contains several biological active compounds. BV has been widely used as a traditional medicine for treating human disease, including cancer. In this study, we have shown the molecular mechanism underlying the therapeutic effect of BV on cancer. Treatment with BV reduced the proliferation of cervical-cancer cells in a dose- and time-dependent manner. Interestingly, the killing effect of BV was specific to HPV-positive cervical-cancer cell lines, such as Caski and HeLa cells, and not to HPV-negative cervical-cancer cells (C33A). BV reduced the expression of HPV E6 and E7 at RNA and protein levels, leading to an increase in the expression of p53 and Rb in Caski and HeLa cells. Further, BV decreased the levels of cell-cycle proteins, such as cyclin A and B, and increased the levels of cell-cycle inhibitors, such as p21 and p27. BV significantly induced apoptosis and inhibited wound healing and migration of cervical-cancer cells. It also upregu-lated the expression of pro-apoptotic BAX and downregulated the expression of anti-apoptotic Bcl-2 and Bcl-XL. Cleavage of caspase-3, caspase-9, and PARP were also induced by BV treatment, whereas the phosphorylation of mitogenic signaling-related proteins, such as AKT, JNK, p38, and ERK, were downregulated. Our results indicate that BV has a therapeutic selectivity for HPV-positive malignant cells, so further clinical studies are needed to assess its clinical application.
Keywords: Bee venom, Cervical cancer, Human papillomavirus
INTRODUCTION
Cervical cancer (CC) is one of the most common malignant cancers in women, with an estimated global mortality rate of 0.25 million deaths per year (1). Cervical cancer is caused by HPV infection, which is related to the metaplastic epithelium of the cervical transformation zones (2). Cervical cancer originates from abnormal cells in the lining of the uterine cervix. There are two primary types of cervical cancer, squamous-cell cancer and adenocarcinoma. Squamous-cell cancer is the most common, and accounts for more than 70 of every 100 cases of cervical cancer. The incidence of adenocarcinoma has been reported to be less than that of squamous-cell cancer, but lately it has become more frequent (3). According to inter-national guidelines, the standard treatment for CC consists of surgery in early-stage tumors, concomitant chemoradiation or neoadjuvant chemotherapy, followed by radical surgery in locally advanced disease, and chemotherapy alone for meta-static or recurrent disease (NCCN guidelines). Although early-stage and locally advanced cancer can be treated with potential curative intent (4), the removal of the cervix and uterus during surgery makes it impossible for the patient to become pregnant. Therefore, new therapeutic targets are needed to prevent problems with pregnancy and to improve the quality of life of women undergoing treatment for CC.
A possible way to inhibit the growth of cancer cells without any side effects is to use oriental medicine, such as bee venom (BV) (5). BV is secreted from the venom gland of the honey bee and contains several biologically active compounds (6). BV therapy is commonly used in Korean medicine as a treatment for various human diseases (7). Currently, BV targeted therapy has been developed for clinical treatment of conditions such as immune-related disorders. Recently several studies have helped explain the anti-cancer effect of BV on lung, liver, renal, prostate, breast and cervical-cancer cells (8). Melittin and phospholipase A2, known BV peptides, can serve as valuable, novel targets in the treatment of some types of cancer. However, further studies are required to investigate the therapeutic effect of BV on cervical cancer.
In this study, we demonstrated the therapeutic mechanism of BV in cervical-cancer cells, and more specifically, we identified the therapeutic effect of BV on HPV-positive and -negative cell lines. We detected induced apoptosis and reduced cell motility after BV treatment. Finally, we also investigated the expression of cell-cycle-regulated proteins and phosphoryl-ation of mitotic signaling-related proteins. Our results show that BV could serve as a potent cervical-cancer therapy agent.
RESULTS
Bee venom inhibits the proliferation and expression of HPV E6 and E7 and increases the expression of p53 and Rb proteins in HPV-positive cervical-cancer cells
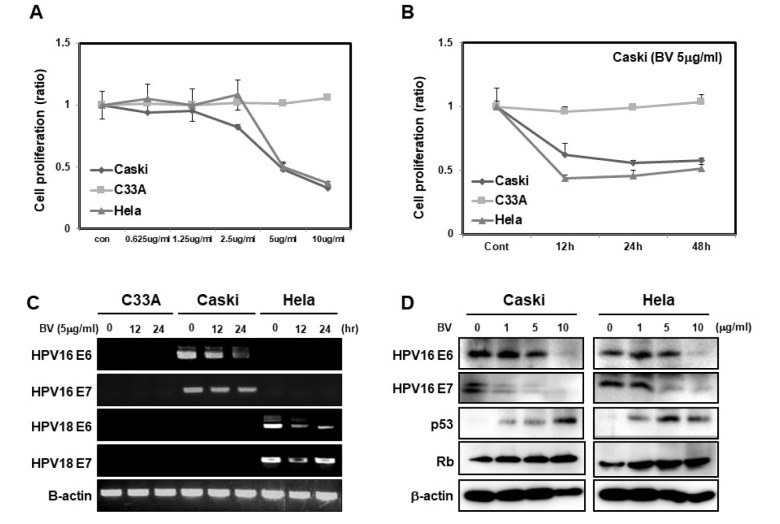
To find out whether BV suppresses cell proliferation, we treated cervical-cancer cell lines Caski, C33A, and HeLa cells with various concentrations of BV (0.625–10 µg/ml). We ob-served a reduction in the proliferation rates of Caski and HeLa cells starting at 2.5 µg/ml, although the same was not true for C33A cells (Fig. 1A). We then treated cells with 5 µg/ml of BV in a time-dependent manner. We found that over time, the proliferation rates of Caski and HeLa cells were reduced by half in response to the treatment; however, C33A cells did not respond to BV (Fig. 1B). Next, we treated the cells with 5 µg/ml of BV and harvested them at various times (0, 12, and 24 h). We showed that mRNA levels of HPV16 E6 and E7, and HPV18 E6 and E7 were decreased in Caski and HeLa cells (Fig. 1C). Since BV treatment of C33A cells did not result in any changes in cell proliferation, we did not quantify the mRNA expression of genes mentioned above. Moreover, we investigated the levels of their respective proteins after treating Caski and HeLa cells with various concentrations of BV. Protein expression of HPV16 E6 and HPV16 E7 was decreased upon increasing the concentrations of BV, whereas the expression of p53 and Rb was increased (Fig. 1D). Overall these results strongly suggest that BV downregulates mRNA expression of E6 and E7, which leads to an increase in p53 and Rb expression, and thus inhibition of cervical-cancer cell proliferation.
Fig. 1.
Bee venom inhibits the prolife-ration and expression of HPV E6 and E7-positive cervical-cancer cells and increases the expression of p53 and Rb proteins. (A) WST cell proliferation assay showing dose-dependent inhibition of C33A, Caski and HeLa cells. C33A, Caski, and HeLa cells were treated with 0, 0.625, 1.25, 2.5, 5, and 10 µg/ml of BV for 24 h (B). At 0, 12, 24, and 48 h of treatment, we detected cell proliferation by MTT assay. (C) RT-PCR-based detection of time-dependent effects of 5 µg/ml of BV on the expression of HPV proteins, HPV16 E6, HPV 16 E7, HPV18 E6, and HPV E7 in C33A, Caski and HeLa cells treated for 0, 12, and 24 h. (D) Protein levels of HPV16 E6, HPV16 E7, p53, and Rb in Caski and HeLa cells treated with 0, 1, 5, and 10 µg/ml BV. β-actin was used as a loading control.
Bee venom significantly induces apoptosis in HPV-positive cervical-cancer cells
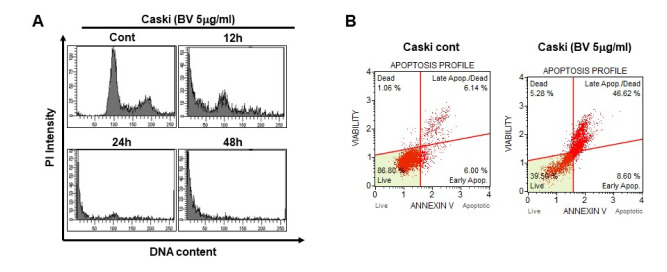
To further investigate whether BV affects cell fate, we carried out PI staining analysis after treating Caski cells with BV. We found that, starting after 12 h treatment, the number of cells entering the sub-G1 phase had increased (Fig. 2A). We then measured the induction of apoptosis in BV-treated Caski cells using annexin V staining followed by FACS analysis, and observed an increase of annexin V positive cells in Caski cells. Less than 10% of BV-untreated cells exhibited background staining with annexin V, whereas after BV treatment, more than 46% of the Caski cells were stained with annexin V, indicating induction of apoptosis and an increased population of dead cells (Fig. 2B). Overall, these data suggest that BV treatment induces apoptosis in HPV-positive cervical-cancer cells.
Fig. 2.
Bee venom significantly induces apoptosis in HPV E6 and E7-positive cervical-cancer cells. (A) Representative data obtained from flow-cytometric analysis of cell-cycle markers after treatment of Caski cells with 5 µg/ml of BV for 0, 12, and 24 h. We fixed cells using 1% PFA and stained DNA with PI as described in Materials and Methods. (B) Caski Cells were treated with BV 5 µg/ml for 24 h and harvested; then DNA was stained with pro-pidium iodide before analysis using FACS.
Bee venom decreases cell motility of HPV-positive cervical-cancer cells
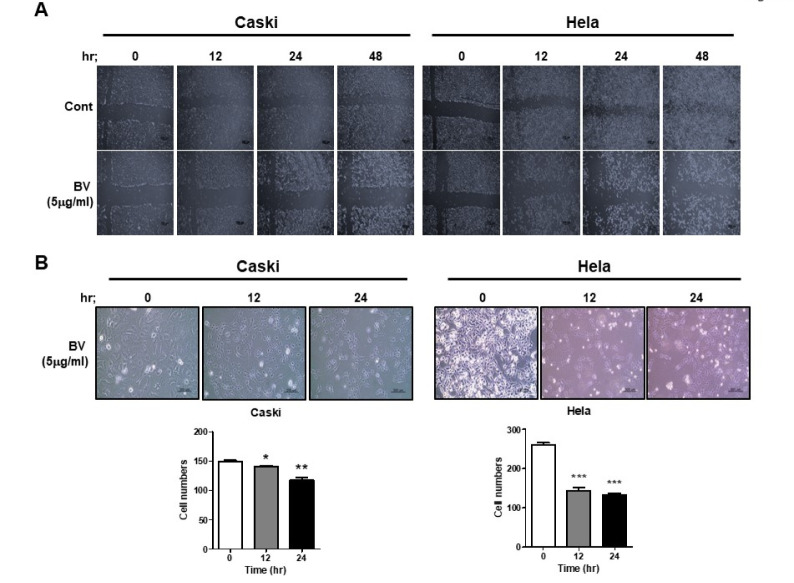
We investigated whether BV-induced morphological changes could be linked to cell migration. In the background of BV treatment (5 µg/ml) in Caski cells and HeLa cells, we did wound healing (Fig. 3A) and migration assays (Fig. 3B). After BV treatment, we investigated cell motility at various times, including 0, 12, 24, and 48 h. We found that BV-treated Caski and HeLa cells exhibited less motility than did untreated cells (Fig. 3A and 3B). These results suggest that BV inhibits cell motility in HPV-positive cervical-cancer cells.
Fig. 3.
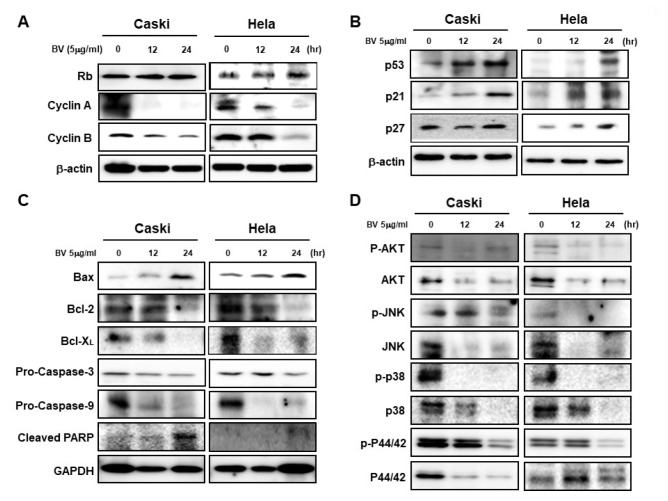
Bee venom regulates the expression of cell-cycle inhibitors, apoptosis-related proteins, and mitogenic signaling-related proteins. (A) Western blot showing the time-dependent effects of BV on Rb, cyclin A, and cyclin B in Caski and HeLa cells treated for 0, 12, and 24 h with BV (5 µg/ml). (B) Expression of p53, p21, and p27 in Caski and HeLa cells treated with 5 µg/ml of BV was measured at 0, 12, and 24 h by Western blot. (C) Western blot of Caski and HeLa cells treated with 5 µg/ml of BV for 0, 12, and 24 h. Immunoblot analysis of Bax, Bcl-2, Bcl-XL, pro-caspase-3, pro-caspase-9, and cleaved PARP. (D) We measured protein levels of p-AKT, AKT, p-JNK, JNK, p-P38, P38, p-P44/42, and P44/42 by Western blot in Caski and HeLa cells treated with 5 µg/ml of BV for 0, 12, and 24 h. β-actin was used as a loading control. Data are presented as mean ± SD (n = 3). Significant differences are indicated with an asterisk (*P < 0.05, *P < 0.01), P values were calculated using the Student’s t test.
Bee venom decreases the expression of cell-cycle-related proteins in HPV-positive cervical-cancer cells
We showed that BV regulates cell-cycle proteins in HPV E6 and E7 oncogene-expressing cervical-cancer cells. Using Western blot analysis, we investigated the expression of cell-cycle proteins in BV-treated (5 µg/ml) Caski and HeLa cells. BV treatment led to a significant increase in the protein levels of Rb and de-creased the levels of cyclin A and cyclin B proteins (Fig. 4A).
Fig. 4.
Bee venom decreases the migration of HPV E6- and E7-positive cervical-cancer cells. (A) We treated Caski and HeLa cells with BV (5 µg /ml) for 0, 12, 24, and 48 h. (B) Transwell migration assay. Caski and HeLa cells were treated with 5 µg/ml of BV for 0, 12, and 24 h, and seeded into the upper chamber of a Transwell plate, before being allowed to vertically migrate towards the lower surface of the membrane. Migrating cells were stained with 0.1% crystal violet.
To further investigate whether BV regulates the expression of tumor suppressors and cyclin-dependent kinase inhibitors, we checked the protein expression of p53, p21Cip1, and p27kip1 after BV treatment at 12 and 24 h. As expected, levels of these proteins were increased in Caski and HeLa cells (Fig. 4B).
Bee venom regulates the expression of apoptosis-related proteins
We also investigated the effect of BV on the expression of apoptosis-related proteins. The levels of pro-apoptotic protein Bax increased after BV treatment, but those of the anti-apop-totic proteins Bcl-2 and Bcl-X decreased (Fig. 4C). Consequent-ly, pro-caspase-3, pro-caspase-9, and PARP were cleaved into their active forms (Fig. 4C).
Bee venom downregulates the mitogenic signaling pathways
We also investigated whether mitogenic signaling pathways are regulated by BV. We measured the expression of proteins related to the AKT, JNK, and mitogen-activated protein kinases (P38, P44/42) signaling pathways in HPV E6- and HPV E7-positive cervical-cancer cells. Compared with BV-untreated cells, the phosphorylation of these proteins was decreased in a time-dependent manner in response to BV treatment (Fig. 4D).
DISCUSSION
HPV (Human Papilloma Virus) is known to be a prerequisite for the development of cervical cancer (9). Increased expression of E6 and E7 is responsible for the carcinogenic effects of HPV (10). Thus, mRNA expression of HPV E6 and E7 is correlated with the progression of cervical cancer. Accordingly, HPV E6 and E7 are used as markers for the diagnosis of cervical cancer (11). Given that the cervix is directly linked to female reproduction, it is crucial to find treatment options without adverse effects. However, surgery, chemotherapy, and radiation therapy are associated with infertility. For addressing this problem, research on alternative treatment approaches is actively under way. Biotoxin-based approaches can also serve as alternative treatments for cancer. One biotoxin in particular, bee venom, is widely known as a treatment for immune-related diseases. Recent studies have shown that the main peptide component of BV, melittin, has an anti-cancer effect (12, 13). Melitin is a non-specific cytolytic peptide that can break down lipid bilayers and is toxic when injected (14). However, via an optimized nanoparticle-based approach, it exhibits anti-cancer effects on several cancer cells, including leukemia, liver cancer, lung cancer, and prostate cancer cells (15).
In this study, we confirmed that BV can effectively treat HPV E6 and E7-infected cervical-cancer cells. This could help us in specifically targeting cervical-cancer cells. We confirmed that BV downregulated the proliferation in HPV E6 and HPV E7-positive cervical-cancer cells; p53 and Rb, both tumor-sup-pressive proteins, are associated with HPV E6 and E7 proteins, suggesting a mechanism for viral proteins to induce cervical cancer (16). E6 protein promotes the degradation of the tumor suppressor p53 by forming a trimeric complex consisting of E6, p53, and a cellular ubiquitin ligase, E6-AP, thereby promoting the progression of cancer cells (17). A decrease in the levels of E6 proteins inhibits P53 function, thereby deregu-lating the cell cycle and stimulating the growth of cancer cells (18). Here, we observed an increased expression of p53 and Rb proteins in cervical-cancer cells after treatment with BV. In addition, several reports have shown that BV induces caspase-dependent apoptosis (19). Our results revealed that the expression of Bcl2 family proteins was greatly altered, a pheno-menon that seems to be a direct result of p53 activity.
In addition, we investigated whether BV affects mitogenic signaling pathways. The AKT signaling pathway plays an important role in various biological processes and in cancer progression and survival (20). Akt is activated through phos-phorylation at the serine 473 residue (21). Activated Akt can phosphorylate downstream proteins, such as mTOR, BAD, and FOXO1, to regulate cancer-cell proliferation and viability. The JNK pathway is one of the pathways downstream to AKT and plays a crucial role in apoptosis and cancer-cell fate determi-nation (22). Two upstream MAPKKs promote JNK activation (23). The MAPK signaling pathway plays a crucial role in tumor growth and apoptosis (24), and p38, a major MAP kinase is activated in cells in response to apoptosis and inflammatory cytokines. MAP/ERK kinase 1 and 2 phosphoryl-ate p42 and p44 (ERK) MAP kinases. MAP3Ks trigger p28 MAPK activation, which promotes the activation of the JNK pathway (25), and p38 MAPK is an important factor in determining cancer-cell fate (26). We observed a significant decrease in AKT, JNK, p38, and p44/42 signals following BV treatment. Additionally, BV also inhibited the phosphorylation of these proteins in HPV E6 and E7 positive cervical-cancer cells.
Taken together, this study demonstrates that BV can play a role in inhibiting cervical-cancer tumorigenesis. Therefore, we posit that BV is an ideal approach for treating cervical cancer.
MATERIALS AND METHODS
Cell line preparation and Bee venom treatment
We obtained human cervical-cancer cell lines, C33A, Caski, and HeLa, from ATCC (Manassas, VA, USA). Cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% antibiotics (Invitrogen, Carlsbad, CA, USA), at 37°C in 5% CO2 atmos-phere, as previously described (27, 28). Cervical-cancer cells were seeded in 6-well plates and subsequently treated with BV 0-10 µg/ml for 24 h. Bee Venom (Apis mellifera caucasia), Dried Bee Venom Powder was purchased by MyBiosouece (San Diego, USA).
Analysis of cell proliferation
We seeded cervical-cancer cells, C33A, Caski, and HeLa in 96-well culture plates (3 × 103 cells/well). After 24 h, we treated C33A, Caski and HeLa cells with BV (0-10 µg/ml) for 24 h. Following this treatment, highly sensitive water-soluble tetra-zolium salt (WST) solution, purchased from Daeil (Seoul, Korea), was added to each well. After 1-3 h of incubation, we measured absorbance using an ELISA reader at a test wavelength of 450 nm (29, 30).
Detection of cell cycle and apoptosis
Caski cells were seeded in culture plates and treated with 5 µg/ml of BV. Cells were harvested and then fixed with 70% EtOH (5 ml) overnight at −20°C. After fixation, cells were resuspended in propidium iodide (PI solution; RNaseA 50 µg/ml, PI 50 µg/ml in PBS) and transferred to FACS filter tubes. We evaluated cell-cycle distribution by PI staining using fluorescence-activated cell sorting (FACS). Caski cells were seeded in culture plates and treated with 5 µg/ml of BV. After 24 h, cells were harvested and resuspended (1 × 106 cells/ml) in Annexin V. Then, we transferred the solution (100 µl) to a 1-ml culture tube, and added 5 ml of FITC Annexin V and 7-AAD to each sample. Cells were incubated for 15 min at RT in the dark. Then, we added 400 µl of 1x Annexin Binding Buffer to each tube, after which the solution was transferred to FACS filter tubes. We measured apoptosis distribution by Annexin V staining using FACS (31).
Wound-healing assay
Caski and HeLa cells were seeded in 12-well plates and treated with 5 µg/ml of BV. A straight wound was created by scratching the cell surface with a 200-µl pipette tip. Cells, maintained in serum-free DMEM, migrated to the wound area; these were then imaged using wide-field microscopy at 0, 12, 24, and 48 h (32).
Transwell migration assay
We treated Caski and HeLa cells with 5 µg/ml of BV. After 24-h of treatment, cells (2 × 104/well) were isolated and added into the upper chamber of a Transwell plate (Corning, New York, USA). Chambers coated with 0.5 mg/ml collagen type I were purchased from BD bioscience (Seoul, Korea) for the migration assay. We added DMEM supplemented with 10% FBS and 1% antibiotics to the lower chamber. Cells were then incubated for a further 24 h, and those that migrated to the lower chamber were quantified after H&E staining. For quantification, cells were counted in 5 randomly selected areas in each well using wide-field microscopy. Data were expressed as mean ± SEM from three independent experiments.
Western blot analysis
Cells were lysed in RIPA buffer (Biosesang, Seoul, Korea) containing an Xpert protease-inhibitor cocktail (GenDEPOT, Barker, TX, USA) and a phosphatase inhibitor (NaF, Na3VO4). The cell lysate was centrifuged at 13,200 rpm for 20 min at 4°C, after which the supernatant was collected. Proteins (20 µg) were separated by SDS-PAGE and transferred onto PVDF membranes (Merck Millipore, Darmstadt, Germany). After transfer, membranes were blocked with 5% skim milk (Becton Dickinson, Sparks, MD, USA) and incubated with primary antibody prepared in 5% BSA (Bovagen Biologicals, Victoria, Australia) overnight at 4°C. We then probed membranes with HRP-conjugated anti-mouse or anti-rabbit secondary antibody (Bethyl Laboratories, Montgomery, TX, USA) for 1 h. Protein bands were detected by Clarity western ECL substrate (Bio-Rad, Hercules, CA, USA) using LAS 3000 (29). The following antibodies were used: anti-β-actin, anti-Rb, anti-Cyclin A, anti-Cyclin B, anti-P53, anti-P21, anti-P27, anti-BAX, anti-BCl 2, anti-BCl-XL, anti-P-Akt, anti-Akt, anti-p-JNK, anti-JNK, anti-p-P38 anti-P38, anti-p-P44/42, and anti-P44/42 (Santa Cruz, Dallas, TX, USA). We also used anti-pro-caspase3 and anti-pro-caspase9 antibodies that were purchased from Cell Signaling Technology (Beverly, MA, USA).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
We extracted total RNA from cells using Isol-RNA Lysis Reagent, which was purchased from 5prime (Gaithersburg, MD, USA) and used according to the manufacturer’s protocol (33). We synthesized cDNAs using 1 µg of total RNA by using ReverTra Ace® qPCR RT Master Mix (TOYOBO, Tokyo, Japan). We did PCR using EX-taq DNA Polymerase, purchased from TaKaRa (Kyoto, Japan). The primer sequences were as follows: β-actin, 5'-AGCCTCGCCTTTGCCGA-3' (sense) and 5'-CTG GTGCCTGGGGCG-3' (anti-sense); HPV16 E6, 5'-GAA CAGCAATACAACACAAACCG-3' (sense) and 5'-CCACCGAC CCCTTATATTATG-3' (anti-sense) HPV16 E7, 5'-CAGCTCAGA GGAGGAGGATG-3' (sense) and 5'-CACAACCGAAGCGTAGA GTC-3' (anti-sense); HPV18 E6, 5'-ATCCAACACGGCGACCC TACAA-3' (sense) and 5'-CTGGATTCTATGTCACGAGCAAT-3' (anti-sense); HPV18 E7, 5'-ACCTTCTATGTCACGAGCAAT-3' (sense) and 5'-CGGACACACAAAGGACAGGGT-3' (anti-sense); P53, 5'-GGCCCACTTCACCGTACTAA-3' (sense) and 5'-GTGG TTTCAAGGCCAGATGT-3' (anti-sense); and Rb, 5'-TGTATCG GCTAGCCTATCTC-3' (sense) and 5'-AATTAACAAGGTGTGG TGG-3' (anti-sense).
ACKNOWLEDGEMENTS
This study was supported by the Bio & Medical Technology Development Program of the National Research Foundation of Korea (NRF) funded by the Korean government, MSIP (NRF-2015M3A9B6073835, NRF-2015M3A9B6073833), and the NRF grant awarded by the Korean government (NRF-2019R1A 2C2089237) to K.H.C.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Hwang LY, Ma Y, Shiboski SC, Farhat S, Jonte J, Moscicki AB. Active squamous metaplasia of the cervical epithelium is associated with subsequent acquisi-tion of human papillomavirus 16 infection among healthy young women. J Infect Dis. 2012;206:504–511. doi: 10.1093/infdis/jis398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marth C, Landoni F, Mahner S, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv72–iv83. doi: 10.1093/annonc/mdx220. [DOI] [PubMed] [Google Scholar]
- 4.Tomao F, Santangelo G, Musacchio L, et al. Targeting cervical cancer: Is there a role for poly (ADP-ribose) polymerase inhibition? J Cell Physiol. 2020;6:5050–5058. doi: 10.1002/jcp.29440. [DOI] [PubMed] [Google Scholar]
- 5.Chaisakul J, Hodgson WC, Kuruppu S, Prasongsook N. Effects of animal venoms and toxins on hallmarks of cancer. J Cancer. 2016;7:1571–1578. doi: 10.7150/jca.15309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hossen MS, Shapla UM, Gan SH, Khalil MI. Impact of bee venom enzymes on diseases and immune responses. Molecules. 2017;22:25. doi: 10.3390/molecules22010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YS, Jun H, Chae Y, et al. The practice of Korean medicine: An overview of clinical trials in acupuncture. Evid Based Complement Alternat Med. 2005;2:325–352. doi: 10.1093/ecam/neh102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YW, Chaturvedi PK, Chun SN, Lee YG, Ahn WS. Honeybee venom possesses anticancer and antiviral effects by differential inhibition of HPV E6 and E7 expression on cervical cancer cell line. Oncol Rep. 2015;33:1675–1682. doi: 10.3892/or.2015.3760. [DOI] [PubMed] [Google Scholar]
- 9.Walboomers JMM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 10.Cyprian FS, Al-Farsi HF, Vranic S, Akhtar S, Al Moustafa AE. Epstein-Barr virus and human papillomaviruses interactions and their roles in the initiation of epithelial-mesenchymal transition and cancer progression. Front Oncol. 2018;8:111. doi: 10.3389/fonc.2018.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao YL, Tian QF, Cheng B, Cheng YF, Ye J, Lu WG. Human papillomavirus (HPV) E6/E7 mRNA detection in cervical exfoliated cells: a potential triage for HPV-positive women. J Zhejiang Univ Sci B. 2017;18:256–262. doi: 10.1631/jzus.B1600288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orsolic N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012;31:173–194. doi: 10.1007/s10555-011-9339-3. [DOI] [PubMed] [Google Scholar]
- 13.Rady I, Siddiqui IA, Rady M, Mukhtar H. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett. 2017;402:16–31. doi: 10.1016/j.canlet.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong JJ, Lu XM, Deng ZX, Xiao SF, Yuan B, Yang K. How melittin inserts into cell membrane: confor-mational changes, Inter-Peptide Cooperation, and Distur-bance on the Membrane. Molecules. 2019;24:1775. doi: 10.3390/molecules24091775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu SJ, Yu M, He Y, et al. Melittin prevents liver cancer cell metastasis through inhibition of the Rac1-dependent pathway. Hepatology. 2008;47:1964–1973. doi: 10.1002/hep.22240. [DOI] [PubMed] [Google Scholar]
- 16.Yim EK, Park JS. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res Treat. 2005;37:319–324. doi: 10.4143/crt.2005.37.6.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipari F, McGibbon GA, Wardrop E, Cordingley MG. Purification and biophysical characterization of a minimal functional domain and of an N-terminal Zn2+-binding fragment from the human papillomavirus type 16 E6 protein. Biochemistry. 2001;40:1196–1204. doi: 10.1021/bi001837+. [DOI] [PubMed] [Google Scholar]
- 18.Crook T, Tidy JA, Vousden KH. Degradation of P53 can be targeted by Hpv E6 sequences distinct from those required for P53 binding and transactivation. Cell. 1991;67:547–556. doi: 10.1016/0092-8674(91)90529-8. [DOI] [PubMed] [Google Scholar]
- 19.Naseri MH, Mahdavi M, Davoodi J, Tackallou SH, Goudarzvand M, Neishabouri SH. Up regu-lation of Bax and down regulation of Bcl2 during 3-NC mediated apoptosis in human cancer cells. Cancer Cell Int. 2015;15:55. doi: 10.1186/s12935-015-0204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 21.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Weston CR, Davis RJ. The JNK signal trans-duction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Tournier C, Dong C, Turner TK, Jones SN, Flavell RA, Davis RJ. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflam-matory cytokines. Genes Dev. 2001;15:1419–1426. doi: 10.1101/gad.888501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koul HK, Pal M, Koul S. Role of p38 MAP kinase signal transduction in solid tumors. Genes Cancer. 2013;4:342–359. doi: 10.1177/1947601913507951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochemical J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 26.Koul HK, Khandrika L. Signal transduction targets in prostate cancer. Curr Signal Transduct Ther. 2008;3:112–128. doi: 10.2174/157436208784223143. [DOI] [Google Scholar]
- 27.Lee HW, Jang KS, Choi HJ, Jo A, Cheong JH, Chun KH. Celastrol inhibits gastric cancer growth by induc-tion of apoptosis and autophagy. BMB Rep. 2014;47:697–702. doi: 10.5483/BMBRep.2014.47.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang HG, Kim WJ, Kang HG, Chun KH, Kim SJ. Galectin-3 Interacts with C/EBPbeta and Upregulates Hyaluronan-Mediated Motility Receptor Expression in Gastric Cancer. Mol Cancer Res. 2019;18:403–413. doi: 10.1158/1541-7786.MCR-19-0811. [DOI] [PubMed] [Google Scholar]
- 29.Cho Y, Kang HG, Kim SJ, et al. Post-translational modification of OCT4 in breast cancer tumorigenesis. Cell Death Differ. 2018;25:1781–1795. doi: 10.1038/s41418-018-0079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baek JH, Kim NJ, Song JK, Chun KH. Kahweol inhibits lipid accumulation and induces Glucose-uptake through activation of AMP-activated protein kinase (AMPK) BMB Rep. 2017;50:566–571. doi: 10.5483/BMBRep.2017.50.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SJ, Lee HW, Baek JH, et al. Activation of nuclear PTEN by inhibition of Notch signaling induces G2/M cell cycle arrest in gastric cancer. Oncogene. 2016;35:251–260. doi: 10.1038/onc.2015.80. [DOI] [PubMed] [Google Scholar]
- 32.Kim SJ, Choi IJ, Cheong TC, et al. Galectin-3 increases gastric cancer cell motility by up-regulating fascin-1 expression. Gastroenterology. 2010;138:1035–1045. doi: 10.1053/j.gastro.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 33.Kim NJ, Baek JH, Lee J, Kim H, Song JK, Chun KH. A PDE1 inhibitor reduces adipogenesis in mice via regulation of lipolysis and adipogenic cell signaling. Exp Mol Med. 2019;51:5. doi: 10.1038/s12276-018-0198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]