Abstract
The world has witnessed unimaginable damage from the coronavirus disease-19 (COVID-19) pandemic. Because the pandemic is growing rapidly, it is important to consider diverse treatment options to effectively treat people worldwide. Since the immune system is at the hub of the infection, it is essential to regulate the dynamic balance in order to prevent the overexaggerated immune responses that subsequently result in multiorgan damage. The use of stem cells as treatment options has gained tremend-ous momentum in the past decade. The revolutionary mea-sures in science have brought to the world mesenchymal stem cells (MSCs) and MSC-derived exosomes (MSC-Exo) as thera-peutic opportunities for various diseases. The MSCs and MSC-Exos have immunomodulatory functions; they can be used as therapy to strike a balance in the immune cells of patients with COVID-19. In this review, we discuss the basics of the cyto-kine storm in COVID-19, MSCs, and MSC-derived exosomes and the potential and stem-cell-based ongoing clinical trials for COVID-19.
Keywords: COVID-19, Exosome, Immunomodulation, Mesenchymal stem cells (MSCs), Ongoing Clinical trials, Therapy
INTRODUCTION
The world has been facing a dreadful situation due to the spread of the Severe Acute Respiratory Syndrome–Coronavirus-2 (SARS-CoV-2) (1). However, neither confirmed effective antiviral medications nor vaccines are available to deal with this emer-gency (2). Many reports have suggested that it is the cytokine storm in COVID-19 that leads to acute respiratory distress syndrome (ARDS) (3). The cytokine storm in COVID-19 refers to the fact that a variety of cytokines are rapidly produced after viral infections (4). In addition, such a cytokine storm induces hypoxia, and direct viral infection can cause cellular damage. Multiorgan damage and injury have been concomitant with COVID-19, and can be observed more in patients with a more severe form of the disease (5).
Stem cells are specialized cells that can renew themselves by means of cell division and can differentiate into multilineage cells. Mesenchymal stem cell (MSCs) have immunomodulatory features and secrete cytokines and immune receptors that regulate the microenvironment in the host tissue (6). In addition, it has been observed that the crucial role of MSCs in therapy has been mediated by exosomes released by the MSCs. These exo-somes have exhibited immunomodulatory, antiviral, anti-fibrotic, and tissue-repair-related functions in vivo; similar effects have been observed in vitro (6).
COVID-19 AND THE IMMUNE SYSTEM
The dynamic equilibrium maintained by innate and adaptive immunity is essential for impeding the progression of COVID-19 (7). In patients infected with SARS-CoV-2, the plasma levels of IL-1β, IL-1RA, IL-7, IL-8, IL-10, IFN-γ, monocyte chemoattrac-tant peptide (MCP)-1, macrophage inflammatory protein (MIP)-1A, MIP-1B, G-CSF, and TNF-α are significantly higher than in controls. The levels of these factors are also increased in patients who were admitted to ICUs (8). Similarly, reductions in the levels of T cells and NK cells have been observed in COVID-19 patients (9). The loss of such cells can impair the immune system (10). The levels of the helper T cells, cytotoxic suppressive T cells, and regulatory T cells are much lower in patients with COVID-19 than in their healthy and less severe counterparts. The decrease in the regulatory T cells may hamper their ability to inhibit the chronic inflammation (11). Interes-tingly, a remarkable increase is observed in the naïve T cells, where as the memory T cells are reduced in infected patients (10). The reduced expression of memory cells may be a plau-sible explanation for the increased rates of reinfection by SARS-CoV-2.
THE CYTOKINE STORM
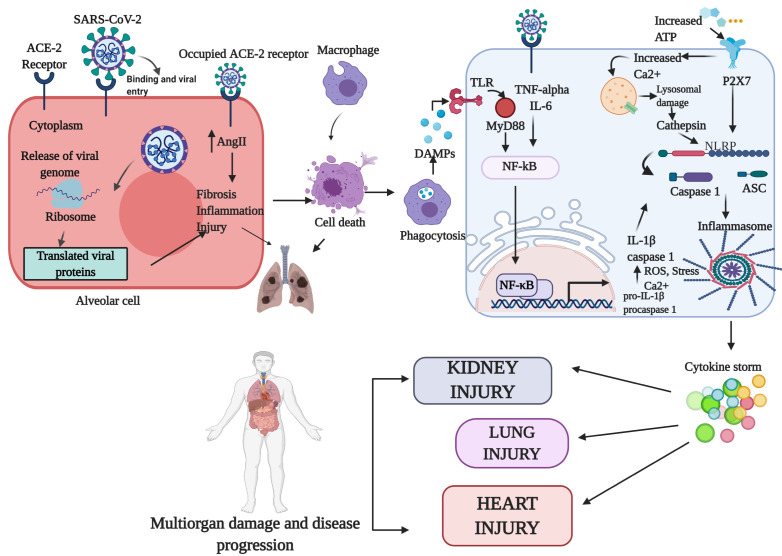
SARS-CoV-2 binds to the Angiotensin-converting enzyme 2 (ACE2) receptor and enters the host cell (1). During infection, the innate and adaptive immune systems work together to inactivate the virus. Since leukocytes and neutrophils are present in higher concentrations in COVID-19 individuals, these immune cells may result in the cytokine storm (10). After viral entry, the virus induces pyroptosis and cell death. The dead cells recruit macrophages to the site of injury that phago-cytose them. The phagocytes then express damage-associated molecular patterns (DAMPs), which bind to the toll-like receptors (TLR) and induce nuclear factor kappa B (NF-κb) signalling by means of the MyD88 pathway. NF-κb enters the nucleus and catalyzes the transcription of pro-IL-1β and pro-caspase-1. When additional signals are detected, the pro-IL-1β and procaspase 1 are cleaved into IL-1β and caspase 1 (12). The activated NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) recruits the apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and pro-cas-pase-1 to form the NLRP3 inflammasome (13). In addition, the phagocytosis releases ATP, which binds to the P2X purino-ceptor 7 (P2RX7) and activates the inflammasome (14). The increased calcium levels caused by the viral proteins results in lysosomal damage, thereby releasing cathepsins that activate the inflammasome (15). Further, the binding of SARS-CoV-2 to the ACE2 reduces the available ACE2 receptors on the cell surface. This increases the levels of Angiotensin II (AngII) in the extracellular space, because ACE2 converts AngI and AngII into Ang 1-9 and Ang1-7, respectively. AngII increases the levels of TNF-α and IL-6 in the cell that upregulates NF-κb, activating the inflammasome (12). The continuous activation of the inflammasome results in a cytokine storm, which recruits more immune cells, necrosis, and cell death. This inflamma-some pathway further causes tissue injury in various organs (Fig. 1).
Fig. 1.
Role of cytokine storm in COVID-19. When SARS-CoV-2 binds the cell, the ACE2 receptors become occupied. This increases AngII which results in lung fibrosis, inflammation, and damage. The infected cell also undergoes cell death as a result of the viral in-fection. Macrophages engulf the dead cells and release DAMPSs, which bind the TLR and activated NF-κb by means of MyD88. Activated NF-κb binding activates the inflammasome. Binding of the virus to the receptor also upregu-lates IL-6 and TNF-αlpha, further activa-ting NF-κb. Increase in ATP binds the-P2X7 receptor, which in turn increases Ca2+, which causes lysosomal damage and further activation of the inflamma-some. Continuous activation of the in-flammasome produces the cytokine storm, resulting in multiorgan damage.
MSCs AND IMMUNOMODULATION
MSCs are predominantly isolated from the bone marrow, adipose tissue, dental pulp, umbilical cord, Wharton’s jelly, placenta, synovial fluid, endometrium, and peripheral blood. These cells exhibit different cell-surface markers and can be used for a variety of treatment options (Table 1). MSCs can undergo in vitro amplification and self-renewal, and have low immunogenicity and immune-modulatory functions; the latter have attracted attention in clinical trials (16). MSCs have been widely used in various cellular therapies, such as pre-clinical studies, as well as in some clinical trials, because of their high safety and efficacy (17, 18). MSCs can exert immune-modu-latory effects in the host cells of both the innate and the adaptive immune system. The direct or indirect interactions of MSCs with the immune cells make the MSCs activate the immunomodulatory responses (19). The immunomodulatory functions of MSCs depend on the environment of the host cells; based on the inflammatory status, the MSCs decide the type of immunoregulatory effect (20). MSCs represent pro-inflammatory immune reactions and anti-inflammatory reactions (21). MSCs regulate the immune system via the transforming growth factor b1 (TGFβ1), which can trigger the proliferation of Tregs, induce IL-6, which prevents the proliferation of neutrophils, and stimulate the prostaglandin E2 (PGE2), which inhibits the antigen presentation by dendritic cells and proliferations of T-effector cells (22, 23). MSCs mediate these kinds of effects by direct contact, where it releases the regulatory cytokines, such as IFN-γ, indoleamine 2,3-dioxygenase, TGFβ, IL-10, and PGE2 (24). Moreover, MSCs can hinder the proliferation and/or func-tions of the CD4+ Th1 and TH17 cells, CD8+ T cells, and the natural killer (NK) cells, mainly by secreting soluble factors, such as TGFβ1 and hepatocyte growth factor (HGF) (16).
Table 1.
Commonly used sources of MSCs
| S. No | Source | Extraction route | Purity level | Proliferation rate | Doubling time | MSCs Marker |
|---|---|---|---|---|---|---|
| 1. | Bone Marrow | Bone Marrow Aspiration | High | Lowest | 40 Hrs | Stro-1, CD271, SSEA-4, CD146 |
| 2. | Adipose Tissue | Liposuction, lipectomy | Medium | Higher | 5 days | CD271, CD146 |
| 3. | Dental pulp | Tooth extraction or root canal | Low | High | 30-40 Hrs | Stro-1, SSEA-4, CD146 |
| 4. | Umbilical Cord | After birth from umbilical cord | High | Medium | 30 Hrs | CD146 |
| 5. | Wharton’s jelly | After birth from umbilical cord | High | High | 30 Hrs | CD73, CD90, CD105 |
| 6. | Placenta | Obtained after delivery | High | High | 36 Hrs | SSEA-4, CD146 |
| 7. | Synovial Fluid | Synovium or synovial fluid | High | High | 10 days | Stro-1, SSEA-4, CD146 |
| 8. | Endometrium | Endometrium biopsies or menstrual blood | High | High | 18-36 Hrs | Stro-1, CD146 |
| 9. | Peripheral Blood | Density Gradient Centrifugation | Low | Low | 95 Hrs | CD133 |
MESENCHYMAL STEM CELLS (MSCs) AND MSC SECRETOME
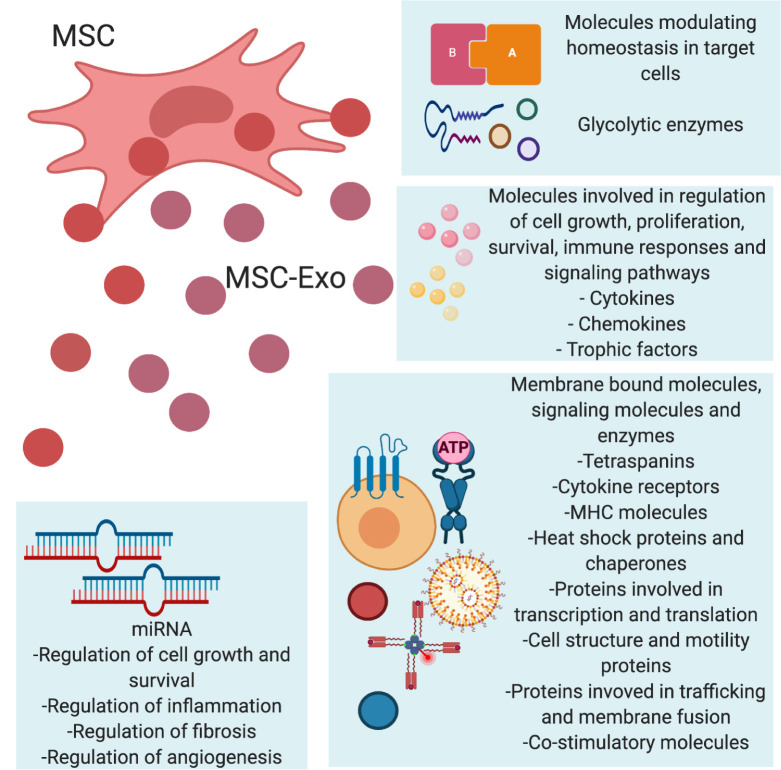
It has currently become apparent that MSCs induce therapeutic characteristics by a paracrine pathway by releasing bioactive substances known as secretomes (25). MSC-secretomes are made of soluble proteins, including cytokines, chemokines, growth factors, and extracellular vesicles (EVs), which include micro-vesicles and exosomes (26). Stem cells release these secretomes by common secretory mechanisms. When the culture medium or secretome are injected into the patients, the neighboring cells assimilate the molecules by paracrine signalling (27). The exosomes themselves contain numerous bioactive molecules, which include microRNAs (miRNA), transfer RNAs (tRNA), long noncoding RNAs (lncRNA), growth factors, proteins, and lipids. The lipid content of the exosomes provide an added advantage by aiding in the infusion of the exosomes with the plasma membrane of the neighboring cells (28). The molecules involved in regulation of cell growth, proliferation, survival, and immune responses are released by exosomes, are elaborately illustrated in Fig. 2. Upon internalization of the mole-cules in the secretome, the neighboring cells modulate various downstream pathways, including immunomodulation, suppression of apoptosis, prevention of fibrosis, and remodelling of the injured tissues (25).
Fig. 2.
Molecules released by MSC-Exos. MSC-Exos affect their targets by means of various molecules that they secrete. The MSC-Exos secrete molecules that maintain the homeostasis in the neighboring cells while also secreting glycolytic enzymes. Other molecules involved in cell growth, proliferation, and modulation of the immune response and signalling pathways are secreted by the MSC-Exos. Some membrane-bound molecules that aid in cell signalling and miRNAs with various functions are also released by MSC-Exos.
IMMUNOMODULATORY POTENTIAL OF MSC-EXOS
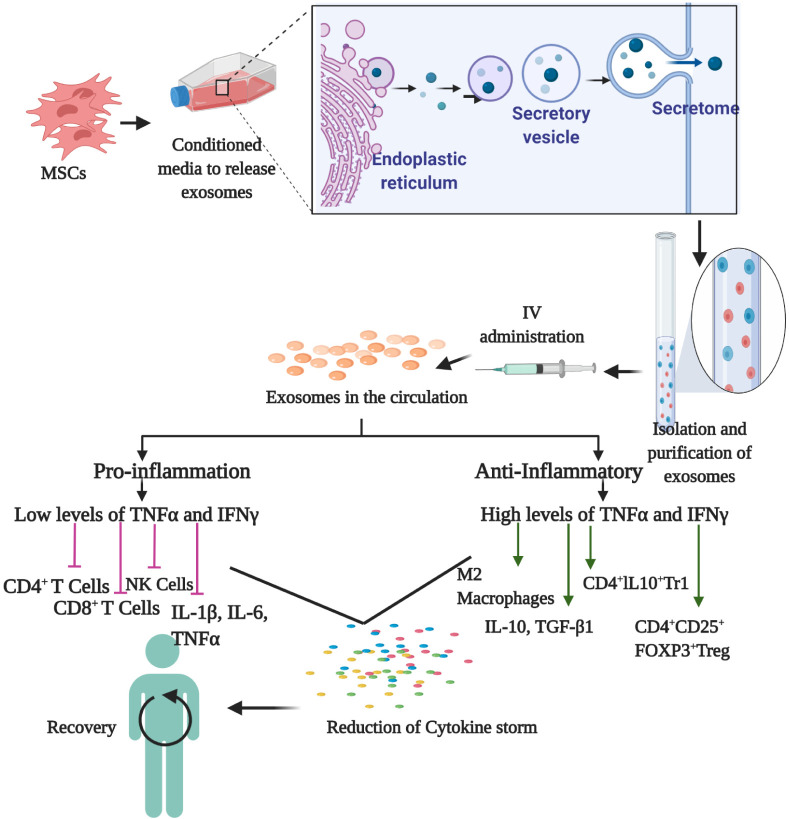
Exosomes are nanoparticles with a diameter of 40-150 nm. To generate and isolate the exosomes, MSCs can be conditioned to increase the release of exosomes by treatment with cyto-kines or by serum starvation or hypoxia (29). The exosomes are then purified and can be subsequently introduced into the body. MSC-Exos can inhibit CD4+ and CD8+ T cells and NK cells (30). They inhibited T cells expressing IL-17 and induced IL-10-expressing regulatory cells that are involved with suppression of inflammation. MSC-Exos also aid in suppressing the differentiation of CD4+ and CD8+ T cells by releasing mole-cules like TGFβ and prevent inflammation in vivo (31). Similarly, treatment with MSC-Exos reduced the proliferation and activa-tion of NK cells (32). MSC-Exos could shift macrophages from the M1 to the M2 phenotype, further suppressing pro-inflam-matory states (33). Moreover, sepsis is an important lethal factor in COVID-19 patients, and treatments with MSC-Exos have increased the rate of survival in mice with sepsis (34). Concomitantly, MSC-Exos also suppressed release of the pro-inflammatory factors TNF-α, IFN-γ, IL-6, IL-17, and IL-1β (35) and promoted release of anti-inflammatory factors, such as IL-4, IL-10 and TGF-β (36). Additionally, MSC-Exos also reduced the number of chemokines in the serum when injected (37). These immunomodulatory effects of MSC-Exos have also been attributed to their anti-inflammatory cargo, such as IDO, HLA-G, PD-L1 and galectin-1 (38, 39). These mechanisms are illustrated in Fig. 3.
Fig. 3.
MSC-Exos therapy for COVID-19. Isolated MSCs are condi-tioned in specialized media that induce release of exosomes. The MSCs identify the external signal and start to pack regulatory factors in secretory vesicles that are released into the culture medium. The exosomes are identified and isolated using specific markers, and are then administered intravenously the i.v. injection. The exosomes inhibit IL-1, IL-6, NK cells, CD4+, and CD8+. This results in suppression of the cytokine storm. Exosomes also activate IL-10, TGF-βeta, M2 macrophages, and T and B regulatory cells to further suppress the immune system. This reduces the proinflammatory cytokines, alleviating symptoms and aiding in recovery of patients.
MSC-EXOS THERAPY FOR COVID-19
In COVID-19, multiorgan damage has been seen in many-infected individuals. MSC-Exos is known to alleviate lung injury in asthmatic models and ARDS (40, 41). MSC-Exos may also be useful in the treatment of cardiovascular (42) and renal pro-blems (43). Hence, they can be used to treat organ damage associated with COVID-19. Similarly, MSC-EVs have also exhi-bited inhibitory activity on the hemagglutination of avian, swine, and human influenza viruses (44). Likewise, MSC-Exos lowered the death rate in H7N9 patients without any toxic effects during follow-up examinations (45). In addition, these exosomes consist of adhesion molecules that accurately guide them to the injured site. The usage of the exosomes may be preferred to the MSCs, since they can easily cross the blood-brain barrier, are inexpensive, and cannot undergo independent self-renewal, hence preventing adverse consequences, such as tumor formation. In this pandemic situation, MSC-Exos may be considered as a good treatment option to alleviate the effect of SARS-CoV-2 infection.
CURRENT CLINICAL TRIALS OF STEM CELL-BASED THERAPY IN COVID-19
Of late, stem-cell-based studies in the treatment of COVID-19 have been gaining momentum. The efficiency and safety of usage of exosomes that had been obtained from BM-MSCs was recently tested on 24 SARS-CoV-2 patients (46). These patients exhibited moderate to severe ARDS. When the exosomes were introduced into the patients, there were no side effects, and patients improved in clinical status and oxygenation (46). In a similar study, patients treated with MSCs showed a remark-able improvement in pulmonary function, higher levels of peripheral lymphocytes, and a reduction in the cells that trigger the cytokine storm. Interestingly, the MSCs did not exhibit ACE2 or TMPRSS2 expression, showing that they may not be infected with COVID-19 (47). Several clinical trials are in the pipeline for usage of stem cells for the treatment of COVID-19 (Table 2). Wharton’s jelly-derived MSCs (WJ-MSCs), which have been used in various studies based on stem-cell therapy and trials, are in progress for their usage for COVID-19 treatment (48). Moreover, adipose tissue-derived AD-MSCs have been used in a few studies in various doses and protocols for COVID-19 therapy (49). Likewise, a novel trial includes inhalation of MSC-Exos for alleviation of symptoms (50). In addition, MSCs from dental pulp (51) and olfactory mucosa (52) were administered in various doses. MSCs in the clinical trials are predominantly administered intravenously; i.v. injection and, in some studies, MSCs have been given as adjuvant therapy in addition to drugs like oseltamivir, hormones, hydroxychloroquine, and azithromycin (53, 54). These trials reveal promising new routes for the battle against COVID-19 (55-94).
Table 2.
Ongoing stem cell based clinical trials in COVID-19
| Study title | Intervention | Study size | Description | Status | Country | Reference |
|---|---|---|---|---|---|---|
| Treatment of COVID-19 patients using Wharton’s Jelly-Mesenchymal Stem Cells | WJ-MSCs | 5 | Dose: 3 IV doses of 1×10e6/kg | Phase 1 | Jordan | 55 |
| Time: 3 days apart | ||||||
| Safety and Efficacy study of Allogenic Human Dental Pulp Mesenchymal Stem Cells to Treat Severe COVID-19 Patients | Allogenic Human Dental Pulp MSCs | 20 | Dose: IV of 3.0x10e7 human dental pulp stem cell solution (30 ml) on day 1, day 4 and day 7 | Phase 2 | China | 56 |
| Placebo: Intravenous Saline | IV of 3 ml of 0.9% saline at the same interval | |||||
| NestCell Mesenchymal Stem Cells to Treat Patients with Severe COVID-19 Pneumonia | NestCell | 66 | Dose: 2×107 cells (20 million cells) | Phase 1 | Brazil | 57 |
| Time: days 1, 3 and 5 in addition to standard care. | ||||||
| On day 7, cells will only be administered if necessary | ||||||
| A Randomized, Double-Blind, Placebo-Controlled Clinical Trial to Determine the Safety and Efficacy of Hope Biosciences Allogeneic Mesenchymal Stem Cell Therapy (HB-adMSCs) to Provide Protection Against COVID-19 | HB-adMSCs | 100 | 3 groups of patients, will receive five IVs at 200, 100 and 50 million cells/dose | Phase 2 | USA | 58 |
| Infusions will occur at week 0, 2, 6, 10 and 14. Placebo is saline | ||||||
| Clinical Trial to Assess the Safety and Efficacy of Intravenous Administration of Allogeneic Adult Mesenchymal Stem Cells of Expanded Adipose Tissue in Patients With Severe Pneumonia Due to COVID-19 | Allogenic expanded adMSCs | 26 | Two doses of 80 million adipose-tissue derived mesenchymal stem cells | Phase 2 | Spain | 59 |
| A Clinical Trial to Determine the Safety and Efficacy of Hope Biosciences Autologous Mesenchymal Stem Cell Therapy (HB-adMSCs) to Provide Protection Against COVID-19 | HB-adMSCs | 56 | Dose: five IV infusions | Phase 2 | USA | 60 |
| Time: follow-up inflammatory data will be obtained at 6, 14, 26 weeks; and PHQ-9 Questionnaires at weeks 2, 6, 10, 14, 18, 22, 26 | ||||||
| Novel Coronavirus Induced Severe Pneumonia Treated by Dental Pulp Mesenchymal Stem cells | Dental pulp MSCs | 24 | Dose: 1.0×106 cells/kg | Early Phase 1 | China | 51 |
| The injection of dental mesenchymal stem cells will be increased on day 1, 3 and 7 | ||||||
| Mesenchymal Stem Cell Treatment for Pneumonia Patients Infected With COVID-19 | MSCs | 20 | China | 61 | ||
| Treatment With Mesenchymal Stem Cells for Severe Corona Virus Disease 2019 (COVID-19) | MSCs | 90 | 3 times of MSCs (3.0*10E7 MSCs intravenously at Day 0, Day 3, Day 6) | Phase 1 | China | 62 |
| Saline containing 1% Human serum albumin (solution of MSC) | ||||||
| Bone Marrow-Derived Mesenchymal Stem Cell Treatment for Severe Patients With Coronavirus Disease 2019 (COVID-19) | BM-MSCs | 20 | Participants will receive conventional treatment plus BM-MSCs (1*10E6/kg body weight intravenously at Day 1) | Phase 2 | China | 63 |
| Study of Human Umbilical Cord Mesenchymal Stem Cells in the Treatment of Severe COVID-19 | UC-MSCs | 48 | 4 times of UC-MSCs (0.5*10E6 UC-MSCs/kg body weight intravenously at Day 1, Day 3, Day 5, Day 7) | Not yet recrui-ting | China | 64 |
| Safety and Effectiveness of Mesenchymal Stem Cells in the Treatment of Pneumonia of Coronavirus Disease 2019 | Drug: Oseltamivir and hormones MSCs | 60 | Umbilical cord mesenchymal stem cells were given at 106/Kg body weight/time, once every 4 days for a total of 4 times Peripheral intravenous infusion was given within 3 days of first admission | Early Phase 1 | China | 53 |
| Clinical Research of Human Mesenchymal Stem Cells in the Treatment of COVID-19 Pneumonia | UC-MSCs | 30 | 1*10E6 UC-MSCs/kg suspended in 100 ml saline | Phase 2 | China | 65 |
| Mesenchymal Stem Cell Therapy for SARS-CoV-2-related Acute Respiratory Distress Syndrome | Cell therapy | 60 | Protocol 1 (n=20). Two doses of MSCs 100×10e6 (± 10%) at Day 0 and Day 2 plus Conventional treatment | Phase 3 | Iran | 66 |
| Protocol 2: Two doses of MSCs 100×10e6 (± 10%)at Day 0 and Day 2, intravenously plus two doses of EVs at Day 4 and Day 6 plus conventional treatment | ||||||
| Role of Immune and Inflammatory Response in Recipients of Allogeneic Haematopoietic Stem Cell Transplantation (SCT) Affected by Severe COVID19 | No intervention | 40 | Comparison of biomarkers | Active, not re-cruiting | United King-dom | 67 |
| Use of UC-MSCs for COVID-19 Patients | UC-MSCs | 24 | UC-MSC will be administered at 100×106 cells/infusion administered intravenously in addition to the standard of care treatment | Phase 2 | USA | 68 |
| Stem Cell Educator Therapy Treat the Viral Inflammation in COVID-19 | Stem Cell Educator-Treated Mononuclear Cells Apheresis | 20 | SCE therapy circulates a patient’s blood through a blood cell separator, briefly cocultures the patient’s immune cells with adherent CB-SC in vitro, and returns the autologous immune cells to the patient’s circulation | Phase 2 | USA | 69 |
| Efficacy and Safety Study of Allogeneic HB-adMSCs for the Treatment of COVID-19 | Drug: HB-and MSC | 110 | Dose: 4 IV of HB-adMSCs at 100 million cells/dose + hydroxychloroquine and azithromycin | Phase 2 | USA | 54 |
| Drug: Placebo | HB-adMSC infusions will occur at day 0, 3, 7, and 10 | |||||
| Drug: HC | Placebo: similar intervals without the HB-adMSCs | |||||
| Drug: AZ | ||||||
| Therapy for Pneumonia Patients Infected by 2019 Novel Coronavirus | Biological: UC-MSCs | N.A | 0.5*10E6 UC-MSCs/kg body weight suspended in 100 ml saline containing 1% human albumin intravenously at Day 1, Day 3, Day 5, Day 7 | With-drawn | China | 70 |
| Battle Against COVID-19 Using Mesenchymal Stromal Cells | Allogeneic and expanded adipose tissue-derived MSCs | 100 | Two serial doses of 1.5 million adipose-tissue derived mesenchymal stem cells/kg | Phase 2 | Madrid | 71 |
| Safety and Efficacy of CAStem for Severe COVID-19 Associated With/Without ARDS | CAStem | 9 | A dose-escalation with 3 cohorts with 3 patients/cohort who receive doses of 3, 5 or 10 million cells/kg | Phase 2 | China | 72 |
| ASC Therapy for Patients With Severe Respiratory COVID-19 | Stem Cell Product | 40 | 100 million allogeneic adipose-derived mesenchymal stromal cells diluted in 100 ml saline | Phase 2 | Denmark | 73 |
| Mesenchymal Stem Cells (MSCs) in Inflammation-Resolution Programs of Coronavirus Disease 2019 (COVID-19) Induced Acute Respiratory Distress Syndrome (ARDS) | MSC | 40 | Infusion of allogeneic bone marrow-derived human mesenchymal stem (stromal) cells | Phase 2 | Germany | 74 |
| Umbilical Cord(UC)-Derived Mesenchymal Stem Cells(MSCs) Treatment for the 2019-novel Coronavirus (nCOV) Pneumonia | UC-MSCs | 10 | UC-MSCs infusion intravenously on day 1, day 3, day 5, and day 7 | Phase 2 | China | 75 |
| A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia | MSCs-derived exosomes | 30 | 5 times aerosol inhalation of MSCs-derived exosomes (2.0*10E8 nano vesicles/3 ml at Day 1, Day 2, Day 3, Day 4, Day 5) | Phase 1 | China | 50 |
| MultiStem Administration for COVID-19 Induced ARDS (MACoVIA) | MultiStem | 400 | IV infusion of MultiStem | Phase 3 | USA | 76 |
| Cell Therapy Using Umbilical Cord-derived Mesenchymal Stromal Cells in SARS-CoV-2-related ARDS | UC Wharton’s jelly-derived human | 60 | Dose: 1 million/kg through an intravenous route | Phase 2 | France | 48 |
| Placebo: NaCl 0.9% | ||||||
| Treatment of Severe COVID-19 Pneumonia With Allogeneic Mesenchymal Stromal Cells (COVID_MSV) | Mesenchymal Stromal Cells | 24 | IV injection of 1 million MSV cells/Kg diluted in 100 ml saline | Phase 2 | Spain | 77 |
| Mesenchymal Stromal Cells for the Treatment of SARS-CoV-2 Induced Acute Respiratory Failure (COVID-19 Disease) | Mesenchymal Stromal Cells | 30 | Dose:1 × 108 MSCs through IV | Early Phase 1 | USA | 78 |
| Repair of Acute Respiratory Distress Syndrome by Stromal Cell Administration (REALIST) (COVID-19) | Remestemcel-L | 300 | Administered twice during the first week, with the second infusion at 4 days following the first injection (± 1 day) | Phase 3 | USA | 79 |
| Treatment of Covid-19 Associated Pneumonia With Allogenic Pooled Olfactory Mucosa-derived Mesenchymal Stem Cells | Allogenic pooled olfactory mucosa-derived MSCs | 40 | IV injection | Phase 2 | Minsk | 52 |
| Autologous Adipose-derived Stem Cells (AdMSCs) for COVID-19 | Autologous adMSCs | 200 | 3 doses of 200 million cells through IV every 3 days | Phase 2 | USA | 49 |
| Mesenchymal Stem Cell Infusion for COVID-19 Infection | MSCs | 20 | Dose: 2 × 106 cells/kg, administered on day 1, 7 in addition to supportive care | Phase 2 | Pakistan | 80 |
| Safety and Efficacy of Mesenchymal Stem Cells in the Management of Severe COVID-19 Pneumonia (CELMA) | UC-MSCs | 30 | Dose: 1*106 cells/Kg | Phase 2 | USA | 81 |
| Mesenchymal Stem Cell for Acute Respiratory Distress Syndrome Due for COVID-19 (COVID-19) | MSC | 10 | Dose: 1 million/Kg | Phase 2 | Mexico | 82 |
| NestaCellⓇ Mesenchymal Stem Cell to Treat Patients With Severe COVID-19 Pneumonia (HOPE) | NestaCellⓇ | 90 | Dose : 2×107 cells on days 1, 3, 5 and 7 | Phase 2 | Brazil | 83 |
| Treatment With Human Umbilical Cord-derived Mesenchymal Stem Cells for Severe Corona Virus Disease 2019 (COVID-19) | UC-MSCs | 100 | Dose: 3 of 4.0*10E7 cells at Day 0, Day 3, Day 6 | Phase 2 | China | 84 |
| Efficacy of Intravenous Infusions of Stem Cells in the Treatment of COVID-19 Patients | MSCs | 20 | IV injection of Cultured stem cells at days 1, 3 and 5 | Phase 2 | Turkey | 85 |
| Clinical Use of Stem Cells for the Treatment of Covid-19 | MSCs | 30 | Dose: 3 million cells/kg on days 0, 3 and 6 | Phase 2 | Turkey | 86 |
| Safety and Efficacy of Intravenous Wharton’s Jelly Derived Mesenchymal Stem Cells in Acute Respiratory Distress Syndrome Due to COVID 19 | WJ-MSCs | 40 | 2 doses | Phase 2 | Colombia | 87 |
| MSCs in COVID-19 ARDS | Remestemcel-L | 300 | Twice in the first week with a gap of 4 days between the injections | Phase 3 | USA | 88 |
| Efficacy and Safety Evaluation of Mesenchymal Stem Cells for the Treatment of Patients With Respiratory Distress Due to COVID-19 (COVIDMES) | WJ-MSCs | 30 | Administration along with standard care | Phase 2 | Spain | 89 |
| Cellular Immuno-Therapy for COVID-19 Acute Respiratory Distress Syndrome - Vanguard (CIRCA-19) | MSCs | 9 | IV administration | Phase 1 | Canada | 90 |
| ACT-20 in Patients With Severe COVID-19 Pneumonia | Allogenic UC-MSCs | 70 | 1 million cells/kg body weight in 100 ml in conditioned media | Phase 2 | 91 | |
| Study of the Safety of Therapeutic Tx With Immunomodulatory MSC in Adults With COVID-19 Infection Requiring Mechanical Ventilation | Allogenic BM-MSC | 45 | IV administration | Phase 1 | USA | 92 |
| Double-Blind, Multicenter, Study to Evaluate the Efficacy of PLX PAD for the Treatment of COVID-19 | MSCs | 140 | 15 IM injections (1 ml each). Twice with an interval of 1 week | Phase 2 | USA | 93 |
| A Study of Cell Therapy in COVID-19 Subjects With Acute Kidney Injury Who Are Receiving Renal Replacement Therapy | MSCs and a plasmapheresis device | 24 | Administered through integration into a Continuous Renal Replacement Therapy circuit | Phase 2 | 94 |
WJ: Wharton’s Jelly; MSC: Mesenchymal stem cells; adMSCs: adipose derived MSCs; UC: Umbilical cord; IV: Intravenous; BM: Bone marrow; HC: hydroxychloroquine; AZ: azithromycin.
FUTURE DIRECTIONS
Stem cells have been studied extensively for their ability to regenerate and for the treatment of various diseases. Recently, we devised an improved protocol for the isolation of urine-derived stem cells and their further differentiation into immune cells (95). Moreover, our research group promoted the hematopoietic differentiation of hiPSCs using a novel small molecule (96). At the advent of COVID-19, it has become mandatory to discover therapeutic strategies that are easily reproducible and cost effective. Drugs currently available for the treatment of COVID-19 include ones that target viral replication. These drugs include camo-stat mesylate, which is involved in the inhibition of viral fusion to the cell membrane, and favipiravir and remdesivir, which are anti-viral drugs. However, because the cytokine storm is found predominantly in COVID-19 patients, it is essential to consider drugs that inhibit viral replication while treating the cytokine storm. Hence, MSC-Exos may be appropriate therapeutic agents for COVID-19 (97). MSCs can be more advantageous than other anti-inflammatory agents, because they can provide immunomodulatory effects based on the host cells. In addition to these effects, MSCs can prevent fibrosis of tissues, enable reversal of lung dysfunction, and aid in the regeneration of damaged tissue, which can be significantly beneficial for COVID-19-associated organ damage (98, 99). Because the healing properties of the MSCs can be primarily attributed to the secretomes or exosomes, using them may be more effective than using MSCs themselves. Exosomes can be mass-produced, administered systematically with minimaltoxicity, and be able to reach the cell targets more efficiently. In addition to their inherent immunomodulatory potential, the MSC-Exos can also be used as a drug-delivery system (100). MSC-Exos can be modified in vivo to release exosomes that have a higher immunomodulatory potential (101) and can be cultured using various cytokines to exhibit an anti-inflammatory state (102). Although MSC-Exos appear to be promising thera-peutic agents for COVID-19, more experimental research is necessary for them to be used clinically. Moreover, it is es-sential to optimize the protocols for storage and isolation of MSC-Exos for the treatment of COVID-19. It is also imperative to do experiments to understand the underlying mechanisms of COVID-19 in order to optimize MSC-Exo therapy for treatment (97). Further, it is also essential to find the optimum dosage, route of administration, and treatment schedule for MSC-Exos. Hence, since MSCs are more widely studied in these aspects than are MSC-Exos, they are predominantly preferred in clinical trials for COVID-19 (103).
CONCLUDING REMARKS
COVID-19 has invoked frenzy in individuals worldwide. The unceasing increase of infection and death has halted the lives of the citizens of countries everywhere. Hence, it is important to discover novel therapeutic platforms and productive measures without further delay (104). The therapies produced must be easily reproducible and available in large quantities so that enough bioactive molecules will be available for all indivi-duals who have succumbed to COVID-19. MSCs and MSC-Exos can be used for their immunomodulatory effects in indi-viduals with COVID-19.
ACKNOWLEDGEMENTS
The author Dr. VB would like to thank Bharathiar University for providing the necessary infrastructure facility and Project funded and supported by MHRD-RUSA 2.0 – BEICH to carry out this manuscript of diagnostic and therapeutic approaches (Ref No. BU/RUSA/BEICH/2019/65).
Dr. SMD would like to thank the Science and Engineering Research Board (SERB) (ECR/2018/000718), Government of India, New Delhi, for providing necessary help in carrying out this review process. The study was supported by a grant from the National Research Foundation (NRF) funded by the Korean government (Grant no: 2019M3A9H1030682).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interest.
References
- 1.Vellingiri B, Jayaramayya K, Iyer M, et al. COVID-19: A promising cure for the global pani. Sci Total Environ. 2020;725:138277. doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iyer M, Jayaramayya K, Subramaniam MD, et al. COVID-19: an update on diagnostic and therapeutic approache. BMB Rep. 2020;53:191–205. doi: 10.5483/BMBRep.2020.53.4.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Ital. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun X, Wang T, Cai D, et al. Cytokine storm intervention in the early stages of COVID-19 pneumoni. Cytokine Growth Factor Rev. 2020 doi: 10.1016/j.cytogfr.2020.04.002. doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balachandar V, Mahalaxmi I, Devi SM, et al. Follow-up studies in COVID-19 recovered patients-is it mandatory. Sci Total Environ. 2020;729:139021. doi: 10.1016/j.scitotenv.2020.139021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taghavi-farahabadi M, Mahmoudi M, Soudi S, Hashemi SM. Hypothesis for the management and treatment of the COVID-19-induced acute respiratory distress syndrome and lung injury using mesenchymal stem cell-derived exosome. Med Hypotheses. 2020;144:109865. doi: 10.1016/j.mehy.2020.109865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune response. J Med Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, Chin. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, He J, Wu S. The definition and risks of cytokine release syndrome-like in 11 COVID-19-infected pneumonia critically ill patients: disease characteristics and retrospective analysi. 2020 doi: 10.1101/2020.02.26.20026989. doi: 10.1101/2020.02.26.20026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tufan A, Güler AA, Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drug. Turk J Med Sci. 2020;50:620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune syste. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 12.Zbinden‐Foncea H, Francaux M, Deldicque L, Hawley JA. Does high cardiorespiratory fitness confer some protection against pro‐inflammatory responses after infection by SARS‐CoV‐2. Obesity. 2020 doi: 10.1002/oby.22849. doi: 10.1002/oby.22849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shneider A, Kudriavtsev A, Vakhrusheva A. Can melatonin reduce the severity of COVID-19 pandemic. Int Rev Immunol. 2020;39:153–162. doi: 10.1080/08830185.2020.1756284. [DOI] [PubMed] [Google Scholar]
- 14.Guo J, Huang Z, Lin L, Lv J. Coronavirus Disease 2019 (COVID‐19) and Cardiovascular Disease: A viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infectio. J Am Heart Assoc. 2020;9:e01. doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conti P, Ronconi G, Caraffa A, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategie. J Biol Regul Homeost Agents. 2020;34:1. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Q, Ren H, Han Z. Mesenchymal stem cells: Immunomodulatory capability and clinical potential in immune disease. J Cell Immunother. 2016;2:3–20. doi: 10.1016/j.jocit.2014.12.001. [DOI] [Google Scholar]
- 17.Prockop DJ. The exciting prospects of new therapies with mesenchymal stromal cells. Cytotherapy. 2017;19:1–8. doi: 10.1016/j.jcyt.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson JG, Liu KD, Zhuo H, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical tria. Lancet Respir Med. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and diseas. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 20.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxid. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotyp. PLoS One. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, Rameshwar P. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-β. J Immunol. 2010;184:5885–5894. doi: 10.4049/jimmunol.0903143. [DOI] [PubMed] [Google Scholar]
- 23.Raffaghello L, Bianchi G, Bertolotto M, et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow nich. Stem Cells. 2008;26:151–162. doi: 10.1634/stemcells.2007-0416. [DOI] [PubMed] [Google Scholar]
- 24.Thanunchai M, Hongeng S, Thitithanyanont A. Mesenchymal stromal cells and viral infectio. Stem Cells Int. 2015;2015:860950. doi: 10.1155/2015/860950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bari E, Ferrarotti I, Saracino L, Perteghella S, Torre ML, Corsico AG. Mesenchymal stromal cell secretome for severe COVID-19 infections: Premises for the therapeutic us. Cells. 2020;9:924. doi: 10.3390/cells9040924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crivelli B, Chlapanidas T, Perteghella S, et al. Mesenchymal stem/stromal cell extracellular vesicles: From active principle to next generation drug delivery syste. J Controlled Release. 2017;262:104–117. doi: 10.1016/j.jconrel.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Deffune E, Prudenciatti A, Moroz A. Mesenchymal stem cell (MSc) secretome: a possible therapeutic strategy for intensive-care COVID-19 patient. Med Hypotheses. 2020;142:109769. doi: 10.1016/j.mehy.2020.109769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicin. Stem Cell Res Ther. 2018;9:63. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver disease. Exp Mol Med. 2017;49:e346–e346. doi: 10.1038/emm.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai P, Chen X, Guo L, et al. A potent immunomodulatory role of exosomes derived from mesenchymal stromal cells in preventing cGVH. J Hematol Oncol. 2018;11:135. doi: 10.1186/s13045-018-0680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Álvarez V, Sánchez-Margallo FM, Macías-García B, et al. The immunomodulatory activity of extracellular vesicles derived from endometrial mesenchymal stem cells on CD4+ T cells is partially mediated by TGFβet. J Tissue Eng Regen Med. 2018;12:2088–2098. doi: 10.1002/term.2743. [DOI] [PubMed] [Google Scholar]
- 32.Fan Y, Herr F, Vernochet A, Mennesson B, Oberlin E, Durrbach A. Human fetal liver mesenchymal stem cell-derived exosome impair natural killer cell functio. Stem Cells Dev. 2019;28:44–55. doi: 10.1089/scd.2018.0015. [DOI] [PubMed] [Google Scholar]
- 33.Domenis R, Cifù A, Quaglia S, et al. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosome. Sci Rep. 2018;8:13325. doi: 10.1038/s41598-018-31707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song Y, Dou H, Li X, et al. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1b-primed mesenchymal stem cells against sepsi. Stem Cells. 2017;35:1208–1221. doi: 10.1002/stem.2564. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Huang Y, Han J, et al. Immunomodu-latory effects of mesenchymal stromal cells-derived exosom. Immunol Res. 2016;64:831–840. doi: 10.1007/s12026-016-8798-6. [DOI] [PubMed] [Google Scholar]
- 36.Ji L, Bao L, Gu Z, et al. Comparison of immuno-modulatory properties of exosomes derived from bone marrow mesenchymal stem cells and dental pulp stem cell. Immunol Res. 2019;67:432–442. doi: 10.1007/s12026-019-09088-6. [DOI] [PubMed] [Google Scholar]
- 37.He Ping. AB0291E the effect of human umbilical cord mesenchymal stem cells-derived exosomes on chemokines in collagen-induced arthritis rat. BMJ. 2019;1606 doi: 10.1136/annrheumdis-2019-eular.7165. [DOI] [Google Scholar]
- 38.Mardpour S, Hamidieh AA, Taleahmad S, Sharifzad F, Taghikhani A, Baharvand H. Interaction be-tween mesenchymal stromal cell-derived extracellular vesicles and immune cells by distinct protein conten. J Cellular Physiol. 2019;234:8249–8258. doi: 10.1002/jcp.27669. [DOI] [PubMed] [Google Scholar]
- 39.Baharlooi H, Azimi M, Salehi Z, Izad M. Mesenchymal stem cell-derived exosomes: A Promising therapeutic ace card to address autoimmune disease. Int J Stem Cells. 2020;13:13–23. doi: 10.15283/ijsc19108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du Y, Zhuansun Y, Chen R, Lin L, Lin Y, Li J. Mesenchymal stem cell exosomes promote immunosup-pression of regulatory T cells in asthm. Exp Cell Res. 2018;363:114–120. doi: 10.1016/j.yexcr.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 41.Fujita Y, Kosaka N, Araya J, Kuwano K, Ochiya T. Extracellular vesicles in lung microenvironment and pathogenesi. Trends Mol Med. 2015;21:533–542. doi: 10.1016/j.molmed.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Barani B, Rajasingh S, Rajasingh J. Exosomes: Outlook for future cell-free cardiovascular disease therap. Adv Exp Med Biol. 2017;2017:285–307. doi: 10.1007/978-981-10-4397-0_19. [DOI] [PubMed] [Google Scholar]
- 43.Tsuji K, Kitamura S, Wada J. Secretomes from mesenchymal stem cells against acute kidney injury: possible heterogeneit. Stem Cells Int. 2018;2018:8693137. doi: 10.1155/2018/8693137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khatri M, Richardson LA, Meulia T. Mesen-chymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig mode. Stem Cell Res Ther. 2018;9:17. doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J, Hu C, Chen L, et al. Clinical study of mesenchymal stem cell treating acute respiratory distress syndrome induced by epidemic Influenza A (H7N9) infection, a hint for COVID-19 treatmen. Engineering. 2020 doi: 10.1016/j.eng.2020.02.006. doi: 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vikram S, Sascha S, Angel L, et al. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-1. Stem Cells Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumoni. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cell Therapy Using Umbilical Cord-derived Mesenchymal Stromal Cells in SARS-CoV-2-related ARDS (STROMA-CoV2). ClinicalTrials.gov Identifier: NCT04333368. [Accessed on 26th June 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT043.
- 49.Autologous Adipose-derived Stem Cells (AdMSCs) for COVID-19.. ClinicalTrials.gov Identifier: NCT04428801. [Accessed on 26th June 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT0442.
- 50.A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia. ClinicalTrials.gov Identifier: NCT 04276987. [Accessed on 26th June 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04276987.
- 51.Novel Coronavirus Induced Severe Pneumonia Treated by Dental Pulp Mesenchymal Stem Cells. ClinicalTrials.gov Identifier: NCT04302519. [Accessed on 26th June 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04302519.
- 52.Treatment of Covid-19 Associated Pneumonia with Allogenic Pooled Olfactory Mucosa-derived Mesenchymal Stem Cells. ClinicalTrials.gov Identifier: NCT04382547. [Accessed on 26th June 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT043.
- 53.Safety and Effectiveness of Mesenchymal Stem Cells in the Treatment of Pneumonia of Corona-virus Disease 2019. ClinicalTrials.gov Identifier: NCT04 371601. [Accessed on 26th June 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04371601.
- 54.Efficacy and Safety Study of Allogeneic HB-adMSCs for the Treatment of COVID-19. ClinicalTrials.gov Identifier: NCT04362189. [Accessed on 26th June 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/
- 55.Treatment of COVID-19 patients using Wharton's Jelly-Mesenchymal Stem Cells. ClinicalTrials.gov Identifier: NCT04313322. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/
- 56.Safety and Efficacy study of Allogenic Human Dental Pulp Mesenchymal Stem Cells to Treat Severe COVID-19 Patients. ClinicalTrials.gov Identifier: NCT04336254. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04.
- 57.NestCell Mesenchymal Stem Cells to Treat Patients with Severe COVID-19 Pneumonia. ClinicalTrials.gov Identifier: NCT04315987. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04315987.
- 58.A Randomized, Double-Blind, Placebo-Controlled Clinical Trial to Determine the Safety and Efficacy of Hope Biosciences Allogeneic Mesenchymal Stem Cell Therapy (HB-adMSCs) to Provide Protection Against COVID-19. ClinicalTrials.gov Identifier: NCT04 348435. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04348435.
- 59.Clinical Trial to Assess the Safety and Efficacy of Intravenous Administration of Allogeneic Adult Mesenchymal Stem Cells of Expanded Adipose Tissue in Patients With Severe Pneumonia Due to COVID-19. ClinicalTrials.gov Identifier: NCT04366323. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04366323.
- 60.A Clinical Trial to Determine the Safety and Efficacy of Hope Biosciences Autologous Mesenchymal Stem Cell Therapy (HB-adMSCs) to Provide Protection Against COVID-19. ClinicalTrials.gov Identifier: NCT04 349631. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04349631.
- 61.Mesenchymal Stem Cell Treatment for Pneumonia Patients Infected With COVID-19.ClinicalTrials.gov Identifier: NCT04252118. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04252118.
- 62.Clinicaltrials.gov
- 63.Bone Marrow-Derived Mesenchymal Stem Cell Treatment for Severe Patients With Coronavirus Disease 2019 (COVID-19). ClinicalTrials.gov Identifier: NCT04346368. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04.
- 64.Study of Human Umbilical Cord Mesenchymal Stem Cells in the Treatment of Severe COVID-19. ClinicalTrials.gov Identifier: NCT04273646. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04273646.
- 65.Clinical Research of Human Mesen-chymal Stem Cells in the Treatment of COVID-19 Pneumonia. ClinicalTrials.gov Identifier: NCT04339660. [Accessed on 16th July 2020];Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT04339660.
- 66.Mesenchymal Stem Cell Therapy for SARS-CoV-2-related Acute Respiratory Distress Syndrome. ClinicalTrials.gov Identifier: NCT04366063. [Accessed on 16th July 2020];Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT04366063.
- 67.Role of Immune and Inflammatory Response in Recipients of Allogeneic Haematopoietic Stem Cell Transplantation (SCT) Affected by Severe COVID19. ClinicalTrials.gov Identifier: NCT04349540. [Accessed on 16th July 2020];Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT04349540.
- 68.Use of UC-MSCs for COVID-19 Patients. ClinicalTrials.gov Identifier: NCT04355728. [Accessed on 16th July 2020];Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT04355728.
- 69.Stem Cell Educator Therapy Treat the Viral Inflammation in COVID-19. ClinicalTrials.gov Identifier: NCT0429915. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04.
- 70.Therapy for Pneumonia Patients Infected by 2019 Novel Coronavirus. ClinicalTrials.gov Identifier: NCT04293692. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04.
- 71.Battle Against COVID-19 Using Mesen-chymal Stromal Cells. ClinicalTrials.gov Identifier: NCT 04348461. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04348461.
- 72.Safety and Efficacy of CAStem for Severe COVID-19 Associated With/Without ARDS ClinicalTrials.gov Identifier: NCT04331613. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04331613.
- 73.ASC Therapy for Patients With Severe Respiratory COVID-19. ClinicalTrials.gov Identifier: NCT 04341610. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04341610.
- 74.Mesenchymal Stem Cells (MSCs) in Inflammation-Resolution Programs of Coronavirus Disease 2019 (COVID-19) Induced Acute Respiratory Distress Syndrome (ARDS). ClinicalTrials.gov Identifier: NCT043 77334. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04377334.
- 75.Umbilical Cord(UC)-Derived Mesen-chymal Stem Cells(MSCs) Treatment for the 2019-novel Coronavirus (nCOV) Pneumonia. ClinicalTrials.gov Identifier: NCT04269525. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT.
- 76.MultiStem Administration for COVID-19 Induced ARDS (MACoVIA). ClinicalTrials.gov Identifier: NCT04367077. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04.
- 77.Treatment of Severe COVID-19 Pneu-monia with Allogeneic Mesenchymal Stromal Cells (COVID_MSV) Clinical Trials.gov Identifier: NCT04361 942. Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04361942.
- 78.Mesenchymal Stromal Cells for the Treatment of SARS-CoV-2 Induced Acute Respiratory Failure (COVID-19 Disease). ClinicalTrials.gov Identifier: NCT04345601. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04.
- 79.Repair of Acute Respiratory Distress Syndrome by Stromal Cell Administration (REALIST) (COVID-19)ClinicalTrials.gov Identifier: NCT03042143. [Accessed on 16th July 2020];Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT03042143.
- 80.Mesenchymal Stem Cell Infusion for COVID-19 Infection. ClinicalTrials.gov Identifier: NCT 04444271. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT044442.
- 81.Safety and Efficacy of Mesenchymal Stem Cells in the Management of Severe COVID-19 Pneumonia (CELMA). ClinicalTrials.gov Identifier: NCT 04429763. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT044297.
- 82.Mesenchymal Stem Cell for Acute Respiratory Distress Syndrome Due for COVID-19 (COVID-19). ClinicalTrials.gov Identifier: NCT04416139. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04416139.
- 83.NestaCell® Mesenchymal Stem Cell to Treat Patients With Severe COVID-19 Pneumonia (HOPE). ClinicalTrials.gov Identifier: NCT04315987. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04315987.
- 84.Treatment With Human Umbilical Cord-derived Mesenchymal Stem Cells for Severe Corona Virus Disease 2019 (COVID-19). ClinicalTrials.gov Identifier: NCT04288102. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT.
- 85.Efficacy of Intravenous Infusions of Stem Cells in the Treatment of COVID-19 Patients. ClinicalTrials.gov Identifier: NCT04437823. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04437823.
- 86.Clinical Use of Stem Cells for the Treatment of Covid-19. ClinicalTrials.gov Identifier: NCT 04392778. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04392.
- 87.Safety and Efficacy of Intravenous Wharton's Jelly Derived Mesenchymal Stem Cells in Acute Respiratory Distress Syndrome Due to COVID 19. ClinicalTrials.gov Identifier: NCT04390152. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04390152.
- 88.MSCs in COVID-19 ARDS.Clinical Trials.gov Identifier: NCT0437139. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04371393.
- 89.Efficacy and Safety Evaluation of Mesenchymal Stem Cells for the Treatment of Patients With Respiratory Distress Due to COVID-19 (COVIDMES). ClinicalTrials.gov Identifier: NCT04390139. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04390139.
- 90.Cellular Immuno-Therapy for COVID-19 Acute Respiratory Distress Syndrome - Vanguard (CIRCA-19). ClinicalTrials.gov Identifier: NCT04400032. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04400032.
- 91.ACT-20 in Patients With Severe COVID-19 Pneumonia. ClinicalTrials.gov Identifier: NCT04398303. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04398303.
- 92.Study of the Safety of Therapeutic Tx With Immunomodulatory MSC in Adults With COVID-19 Infection Requiring Mechanical Ventilation ClinicalTrials.gov Identifier: NCT04397796. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04397796.
- 93.Double-Blind, Multicenter, Study to Evaluate the Efficacy of PLX PAD for the Treatment of COVID-1. ClinicalTrials.gov Identifier: NCT04389450. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04389450.
- 94.A Study of Cell Therapy in COVID-19 Subjects With Acute Kidney Injury Who Are Receiving Renal Replacement Therapy. ClinicalTrials.gov Identifier: NCT0444522. [Accessed on 16th July 2020];Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04.
- 95.Kim K, Gil M, Dayem AA, et al. Improved isolation and culture of urine-derived stem cells (USCs) and enhanced production of immune cells from the USC-derived induced pluripotent stem cell. J Clin Med. 2020;9:827. doi: 10.3390/jcm9030827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim K, Abdal Dayem A, Gil M, et al. 3,2'-Dihydroxyflavone Improves the proliferation and survival of human pluripotent stem cells and their differen-tiation into hematopoietic progenitor cell. J Clin Med. 2020;9:669. doi: 10.3390/jcm9030669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsuchiya A, Takeuchi S, Iwasawa T, et al. Thera-peutic potential of mesenchymal stem cells and their exosomes in severe novel coronavirus disease 2019 (COVID-19) case. Inflammation and Regeneration. 2020;40:1–6. doi: 10.1186/s41232-020-00121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Akkoc T. COVID-19 and mesenchymal stem cell treatment; mystery or no. Adv Exp Med Biol. 2020:1–10. doi: 10.1007/5584_2020_557. doi: 10.1007/5584_2020_557. [DOI] [PubMed] [Google Scholar]
- 99.Pinky, Gupta S, Krishnakumar V, et al. Mesen-chymal stem cell derived exosomes: a nano platform for therapeutics and drug delivery in combating COVID-1. Stem Cell Rev Rep 1-11 doi. 2020:1–11. doi: 10.1007/s12015-020-10002-z. doi: 10.1007/s12015-020-10002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muraca M, Pessina A, Pozzobon M, et al. Mesen-chymal stromal cells and their secreted extracellular vesicles as therapeutic tools for COVID-19 pneumonia. J Control Release. 2020:135–140. doi: 10.1016/j.jconrel.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O'Driscoll L. Extracellular vesicles from mesen-chymal stem cells as a Covid-19 treatmen. Drug Dis-covery Today. 2020:S1359-6446(20)30170-7. doi: 10.1016/j.drudis.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sleem A, Saleh F. Mesenchymal stem cells in the fight against viruses: Face to face with the invisible enem. Curr Res Transl Med. 2020:S2452-3186(20)30031-3. doi: 10.1016/j.retram.2020.04.003. doi: 10.1016/j.retram.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harrell CR, Jovicic N, Djonov V, et al. Therapeutic Use of Mesenchymal Stem Cell-Derived Exosomes: From Basic Science to Clinic. Pharmaceutics. 2020;12:474. doi: 10.3390/pharmaceutics12050474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Balachandar V, Mahalaxmi I, Kaavya J, et al. COVID-19: emerging protective measure. Eur Rev Med Pharmacol Sci. 2020;24:3422–3425. doi: 10.26355/eurrev_202003_20713. [DOI] [PubMed] [Google Scholar]