Abstract
The near-infrared window of fluorescent heptamethine cyanine dyes greatly facilitates biological imaging because there is deep penetration of the light and negligible background fluorescence. But dye instability, aggregation, and poor pharmacokinetics are current drawbacks that limit performance and the scope of possible applications. All these limitations are simultaneously overcome with a new molecular design strategy that produces a charge balanced and sterically shielded fluorochrome. The key design feature is a meso-Aryl group that simultaneously projects two shielding arms directly over each face of a linear heptamethine polyene. Cell and mouse imaging experiments compared a shielded heptamethine cyanine dye (and several peptide and antibody bioconjugates) to benchmark heptamethine dyes and found that the shielded systems possess an unsurpassed combination of photophysical, physiochemical and biodistribution properties that greatly enhance bioimaging performance.
Keywords: antibodies, cyanines, dyes/pigments, fluorescent probes, imaging agents
Graphical Abstract
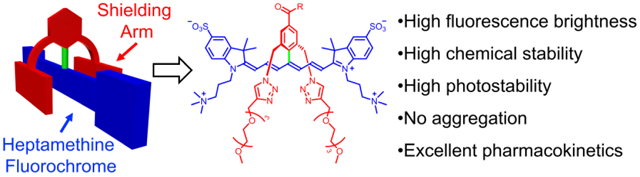
Just like a superhero, an ultrastable shielded heptamethine cyanine dye uses its two strong arms to ward off self-aggregation and non-specific biological interactions. Yet the arms are short enough to allow dye-labeled bioconjugates to selectively target cell receptors for high-contrast and photon-intense microscopy or tumor imaging in living subjects.
Introduction
Fluorescent heptamethine cyanine dyes (known traditionally and commercially as Cy7) have absorption peaks in the near-infrared (NIR) range of 740–840 nm, a favorable wavelength region for in vivo imaging because there is deep penetration of the light through thick biological samples, along with high image contrast due to decreased light scattering and negligible background signal.[1] Heptamethine cyanine dyes are often attached to synthetic or biological molecules to create targeted fluorescent conjugates for diagnostics, microscopy, or in vivo imaging of living subjects, and these frontier technologies are expanding rapidly.[2] The potential value of heptamethine cyanine dyes has increased tremendously in recent years with the realization that the tail of their emission bands extend into the range of 1000–1700 nm which is often called the NIR II region.[3] This is an important discovery with significant practical implications because in vivo imaging in the NIR II region produces brighter and sharper fluorescence images.
By definition, heptamethine cyanine dyes have extended hydrophobic (and polarizable) surface areas and a small polyene HOMO-LUMO band gap, so dye instability, self-aggregation, and poor pharmacokinetics are common technical drawbacks that severely limit the scope of current applications. Shown in Scheme 1 are leading choices of heptamethine cyanine dyes for fabrication of preclinical and clinical fluorescent NIR molecular probes.[2b] The archetype heptamethine dye is Indocyanine Green (ICG), the only NIR dye with absorption/emission > 700 nm that is approved for use in humans. Although used extensively, it is known for its modest stability and mediocre fluorescent properties, and also the absence of a single reactive site for easy bioconjugation.[4] A notable advance in heptamethine cyanine chemistry was the development of conjugatable structures with a central cyclohexyl ring.[5] A benchmark example is polyanionic IRDye CW800® (CW800), a commercially available heptamethine indocyanine dye that has been developed into several fluorescent NIR molecular probes that are currently under clinical evaluation for enhanced intraoperative imaging.[2a,2b] While molecular probes based on CW800 have undoubted value in biomedical imaging, there are three constraining performance limitations. One is undesired, non-specific interaction of the polyanionic fluorochrome (or its conjugate) with off-target proteins, cell membranes, or skin, which often produces moderate background signals and non-optimal pharmacokinetic profiles.[3b,6] A second concern is chemical degradation of CW800 due to nucleophilic displacement of the meso-OAryl group by biological amines or thiols during synthesis, storage, or the course of the imaging experiment.[7] A third concern is susceptibility to photobleaching due to high reactivity of the electron rich heptamethine polyene with electrophilic singlet oxygen.[4b,8]
Scheme 1.
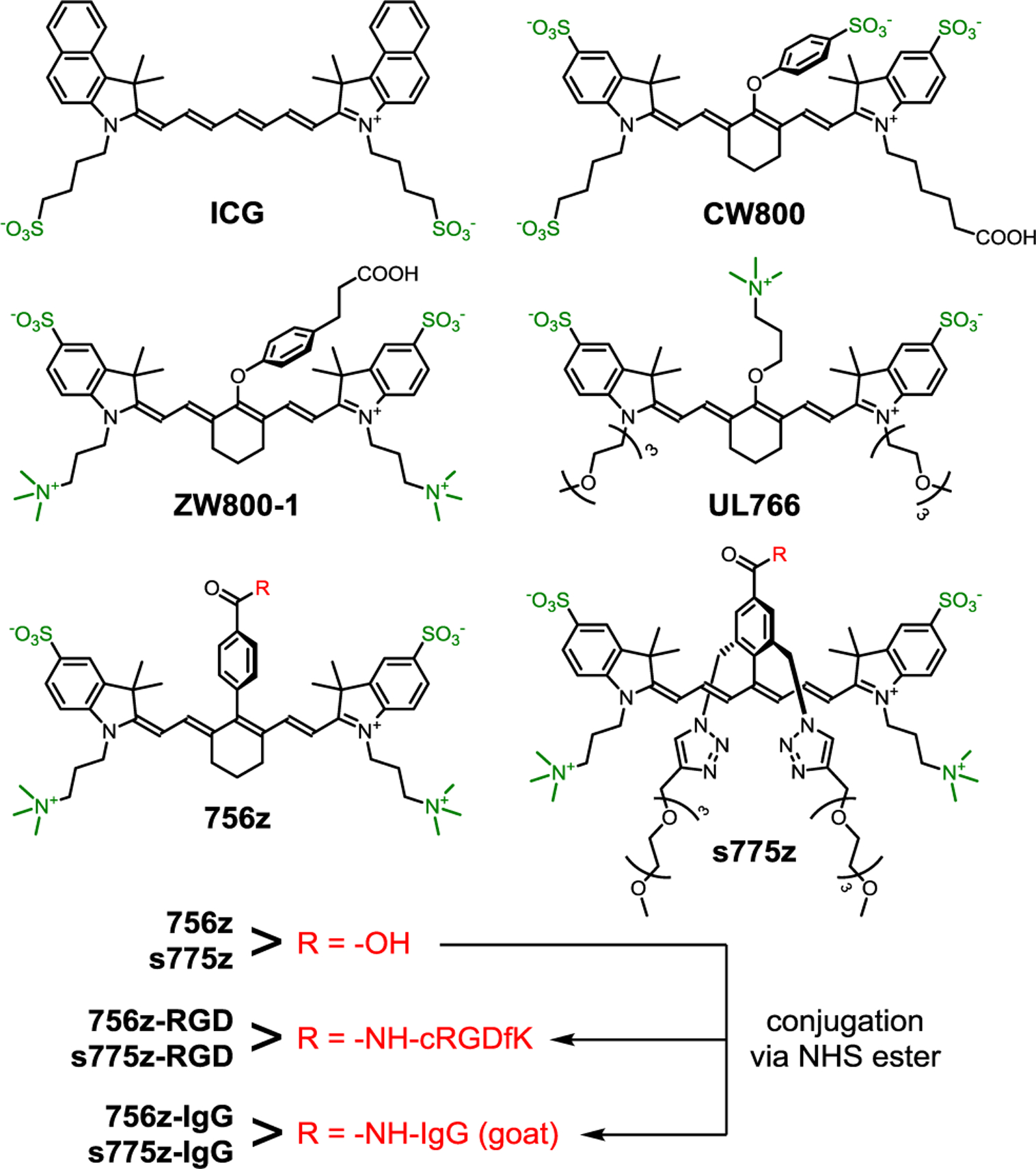
Chemical Structures of Heptamethine Cyanine Dyes.
For the last 15 years, international community efforts to solve these three heptamethine cyanine performance problems (non-optimal pharmacokinetics, chemical and photochemical instability) have resulted in two noteworthy structural modifications. In vivo pharmacokinetic profiles have been improved by creating geometrically, charge balanced dye structures (often called zwitterionic) such as ZW800–1 which minimizes binding to serum proteins and membrane surfaces, promotes exclusive renal clearance, and produces an ultralow imaging background and high Tumor-to-Background ratio.[6c,9] The second structural improvement is to replace the dye’s labile meso C-OAryl bond with a more stable covalent linkage. A recent advance developed by Schnermann and coworkers employed a more robust meso C-OAlkyl bond,[6c,8a] and one example of this fluorochrome is UL766 which exhibits excellent chemical stability and very favorable pharmacokinetics due to its charge balanced structure.[10] However, the heptamethine polyene within UL766 (and its close structural analogues) is quite electron rich which means relatively high fluorochrome reactivity with singlet oxygen, and thus susceptibility to photobleaching.[8a] Another way to replace the reactive meso C-OAryl bond in ZW800–1 is to employ a much more stable C-C linkage as exemplified by 756z with its meso-Aryl substituent.[11] However, the rigid hydrophobic core of charge balanced 756z (and its close structural analogues) promotes low water solubility and extensive dye self-aggregation which limits practicality.[11a,11b,12] Self-aggregation of NHS ester versions of 756z is especially problematic during a protein conjugation reaction because it drives attachment of multiple self-aggregated dyes at proximal lysine positions on the protein surface leading to partially quenched (less fluorescent) protein-dye conjugates.[12a,13]
Here we present a new and versatile molecular design strategy that simultaneously overcomes all of the heptamethine performance limitations described above. We have invented a new class of cyanine dyes that we call sterically shielded heptamethine cyanine dyes. The molecular design is based on the underappreciated fact that a cyanine dye with a meso-Aryl substituent adopts a low-energy conformation with the plane of the aryl ring strongly rotated out the plane of the polyene.[14] Adopting this molecular conformation alleviates steric crowding between the meso-Aryl ortho hydrogens and the proximal β hydrogens on the heptamethine chain (Scheme 2). Synthetically, we exploit this structural feature by designing a new three-dimensional architecture that simultaneously projects two shielding arms directly over each face of the polyene. These shielding arms do not greatly increase the molecular weight, but they block undesired biological interactions and enhance photostability. The literature includes a scattering of studies that report self-shielded dyes, but the strategy has not been applied to conjugatable cyanine dyes which are, by far, the most important for NIR fluorescence imaging.[15] To demonstrate the substantial advantages gained by exploiting this approach, we have prepared a new shielded and charge balanced heptamethine cyanine dye called s775z along with two bioconjugates (Scheme 1). We have compared the chemical, photophysical and pharmacokinetic properties of these three fluorescent compounds with an analogous set of compounds that are based on the unshielded analogue 756z and we find major improvements in several different NIR dye properties that lead to broadly enhanced bioimaging performance.
Scheme 2.
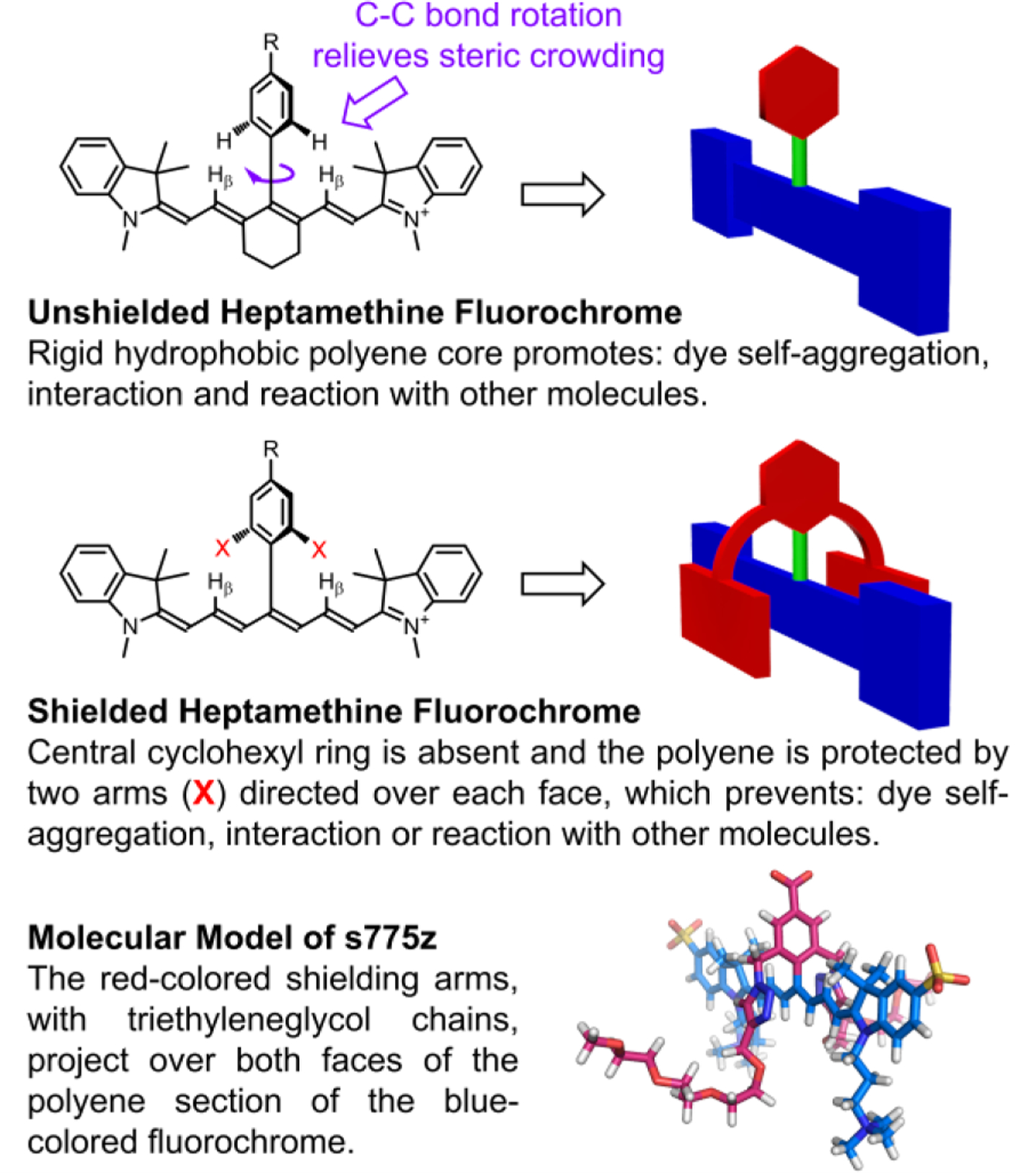
Basic Concept of a Sterically Shielded Heptamethine Cyanine Dye.
Results and Discussion
Design and Synthesis
For comparative studies, we synthesized the benchmark heptamethine dye UL766[10] and purchased ICG. A more transformational synthetic achievement was to prepare the shielded heptamethine s775z and control unshielded analogue 756z, along with two bioconjugates of each dye. The common structural elements in s775z and 756z include a heptamethine indocyanine fluorochrome and a geometric balanced periphery of cationic and anionic residues. There are two crucial structural differences; the presence of the two shielding arms in s775z as discussed in the introduction section, and the presence of the central cyclohexyl ring in 756z. While the central cyclohexyl ring bolsters molecular rigidity, which is often considered a favorable structural attribute for fluorescent dyes,[5a,9c] we reasoned that the rigidity combined with increased hydrophobicity was a factor promoting dye self-aggregation.[11a,11b,12,16] Literature examples of linear heptamethine polyenes that have a meso-positioned substituent but no central cyclohexyl ring are rare and historically hard to make.[17] The synthetic advance that allowed us to prepare linear and meso-functionalized s775z was the newly reported methodology of Štacková and coworkers that involves ring opening of Zincke salts.[18] The significant advantage gained by employing this innovative synthetic strategy is that the C-C link to the center of the heptamethine polyene is formed before the complete polyene is created and thus the C-C coupling reaction does not encounter high steric hindrance. The key synthetic intermediate 1 was prepared in five steps and then converted quantitatively into 2 by conducting a copper catalyzed alkyne azide cycloaddition (CuAAC) reaction that attached two triethyleneglycol chains (Scheme 3). The next step was a Zincke reaction; a two-step process that first formed a pyridinium salt, 3, and then reacted it with two molar equivalents of charge balanced indolenium 4[9e] to give the t-butyl protected heptamethine dye which was converted into shielded s775z.
Scheme 3.
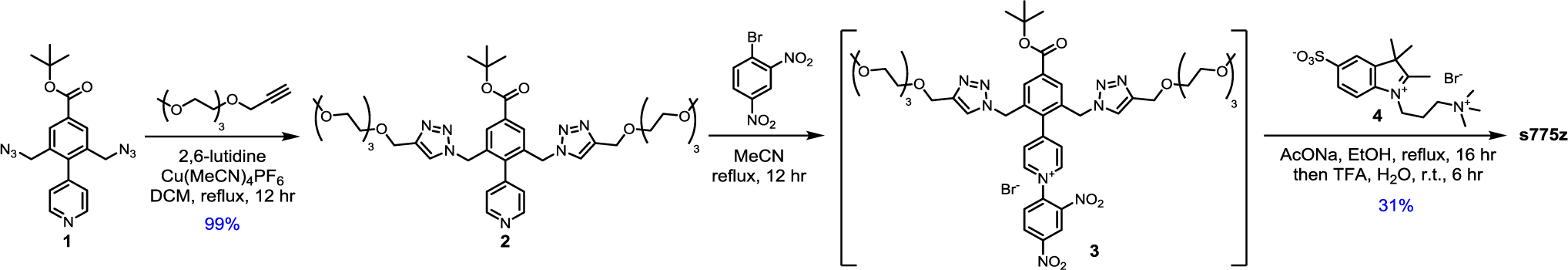
Synthesis of s775z.
Molecular Structure of s775z
The energy minimized molecular model of s775z in Scheme 2 shows how the two shielding arms, with triethyleneglycol chains, project over both faces of the heptamethine polyene that is an all-trans conformation.[4a] The model is consistent with literature X-ray crystal structures showing the meso-Aryl ring strongly rotated out of the plane of the polyene.[14,19] Close inspection of the 1H NMR spectra for s775z (Figure S3) in water reveals the heptamethine proton coupling constants (3JHH) to all be 13.5 Hz indicating a polyene chain with an all-trans conformation.[20] In addition, 1H-1H NOE experiments (Figure S4) identified cross relaxation between indolenine gem-dimethyl protons and polyene protons, as well as shielding chain protons, all consistent with an all-trans polyene.[21] Finally, the chemical shifts for the heptamethine β-protons and indolenine gem-dimethyl groups in 756z and s775z are substantially upfield of the analogous peaks in related heptamethine structures that do not have a meso-Aryl substituent (Figure S2), reflecting strong magnetic shielding of these diagnostic protons by the face of the rotated meso-Aryl ring.
Spectral Properties and Stability
As shown in Tables 1, S1 and S2, the fluorescence brightness of shielded s775z and benchmark UL766 were listed within experimental error. Importantly, the excitation/emission wavelengths of s775z (ex: 775 nm, em: 794, in PBS) closely match the typical default settings of commercial closed box and open field imaging stations, which means minimal refinement of machine configuration is needed for future utilization of molecular probes that are based on s775z.[22]
Table 1.
Spectral and Reactivity Properties of Dyes in PBS (pH 7.4).[a]
| 756z | s775z | UL766[g] | ZW800–1[g] | CW800[g] | |
|---|---|---|---|---|---|
| λabsmax (nm) | 681 (a)[f] 756 (m)[f] | 775 | 766 | 770 | 775 |
| λemmax (nm) | 773 | 794 | 789 | 788 | 796 |
| ε (M−1cm−1) (R2)[b] | 99,000 (0.942)[b] | 201,000 (0.999) | 229,000 | 246,000 | 242,000 |
| QY[c] | 0.097 | 0.090 | 0.095 | 0.135 | 0.090 |
| Brightness[d] | 9,600 | 18,000 | 22,000 | 33,000 | 22,000 |
| Stable to nucleophiles[e] | Yes | Yes | Yes | No | No |
Concentration range of dyes is 0 – 5 μM. All measurements were made at room temperature.
Molar absorptivity of monomer band, nonlinear relationship with concentration due to dye self-aggregation.
Quantum yield relative to UL766, error is ±10%.
ε x QY, error is ±15%.
a = aggregate; m = monomer.
Aqueous samples of s775z can be stored indefinitely at 4°C, and samples of s775z in 100% fetal bovine serum (FBS) do not change at 37 °C over 24 hours (Figure S13a) which is in contrast to the known degradation of CW800 and ZW800–1 under very similar conditions.[10,12]
High photostability is also a highly desired, but an elusive heptamethine cyanine dye property.[4a,24] Photobleaching of a heptamethine cyanine dye is primarily caused by a bimolecular reaction of the heptamethine polyene with photogenerated singlet oxygen.[4b,8a–c] The predominant reaction pathway forms a strained dioxetane intermediate followed by a fragmentation cascade. A possible second minor pathway is electron transfer from the polyene to singlet oxygen leading to a dimerized dye structure.[20]
Shown in Figure 1 are the results of two separate photostability studies. The first study irradiated four different cuvettes, each containing a solution of dye in PBS, with a Xenon lamp (filtered to allow wavelengths > 620 nm) and monitored for a decrease in the dye’s absorption maxima band (Figure 1a, see Figures S15–S18 for the entire set of spectral plots and Table S3 for quantification). The order of photostabilities was observed to be s775z > 756z > UL766 > ICG. An additional competitive experiment irradiated a single solution containing a mixture of s775z and UL766 which ensured that both dyes were exposed to the same number of photons and photogenerated singlet oxygen. Analysis of the solution mixture after irradiation revealed slight decomposition of the s775z but complete loss of all UL766 (Figure S19).
Figure 1.
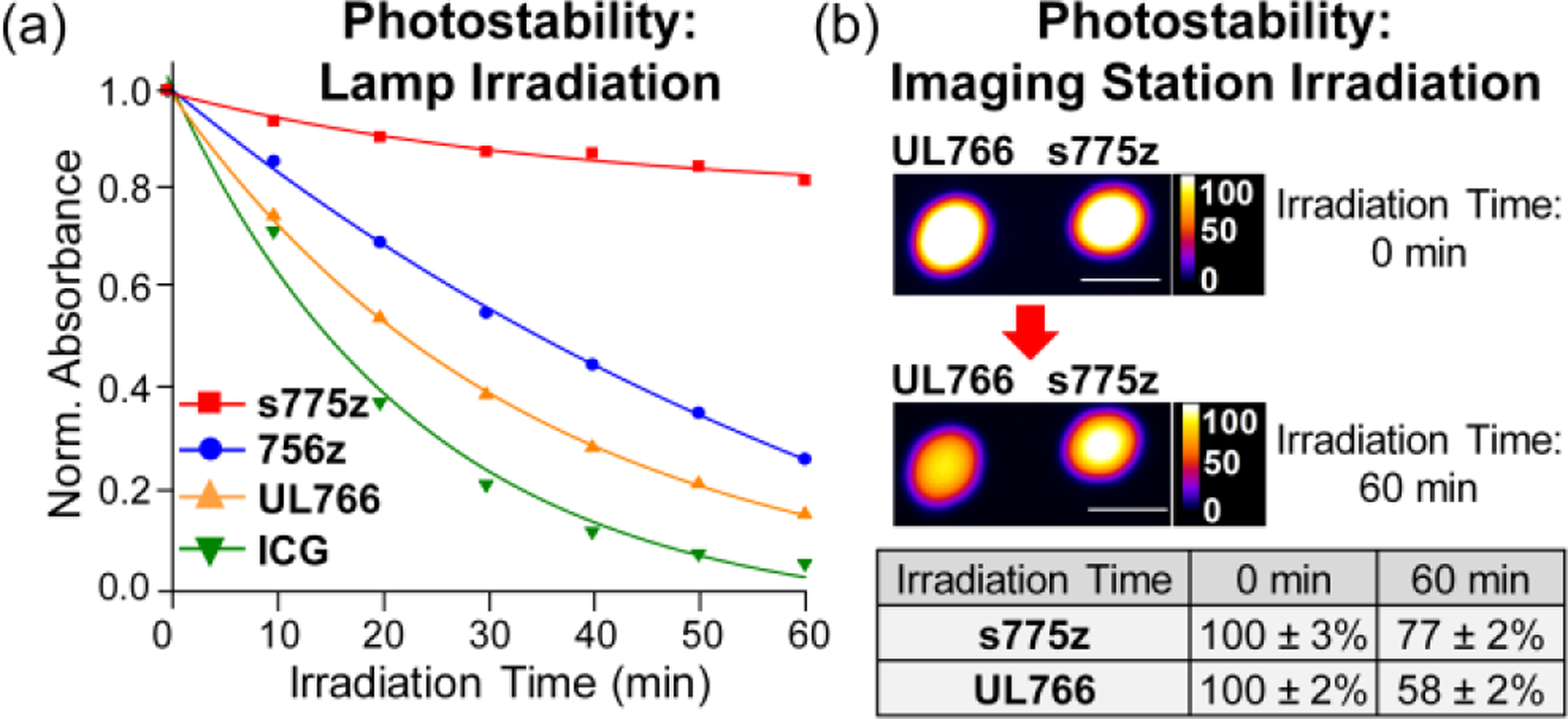
Two separate photostability studies. (a) Lamp irradiation: Four separate cuvettes, each containing 1 μM dye in PBS buffer, pH 7.4, were irradiated by a 150 W Xenon lamp with a 620 nm long-pass filter. The plot of normalized dye absorbance versus time was fit to a one-phase exponential decay. (b) Imaging station irradiation: Imaging phantoms (immobilized 100 μL drops containing s775z or UL766, 10 μM in PBS buffer, pH 7.4) were irradiated with an in vivo imaging station’s 745 nm LED for a total period of 60 min. The mean pixel intensity (MPI) values for the fluorescence images (ex: 745 nm, em: 850 nm) are listed (N=3 for each phantom). The length scale bar on each NIR fluorescence image is 1 cm.
A second, independent photostability study confirmed the difference between s775z and UL766 under milder irradiation conditions that more closely resembled an in vivo imaging experiment or clinical intraoperative imaging procedure. Imaging phantoms were created by immobilizing stable drops of s775z or UL766 (100 μL, 10 μM in PBS buffer, pH 7.4) on a black non-reflective sheet. The phantoms were placed inside a commercial in vivo imaging station and continuously exposed to the station’s 745 nm LED. The data in Figure 1b shows the change in mean pixel intensity (MPI) for the phantom images. After 60 min of constant irradiation, the images of phantoms containing UL766 had decreased to 58 ± 2 % of initial intensity; whereas, the images of phantoms containing s775z had only decreased to 77 ± 2 % of initial intensity.
These heptamethine photostability trends suggest that the meso-Aryl group in s775z with its two shielding arms induces three synergistic effects that inhibit bimolecular reaction of its heptamethine polyene with electrophilic singlet oxygen: (a) the meso-Aryl group within s775z electronically deactivates polyene reactivity (lowers the HOMO energy) compared to UL766 which has an electron donating meso-OAlkyl group, (b) the steric bulk of the meso-Aryl group in s775z destabilizes any putative dioxetane intermediate formed by oxygen/polyene cycloaddition, and (c) the two shielding arms in s775z sterically inhibit singlet oxygen attack at the polyene, compared to unshielded 756z, providing more opportunity for the short-lived singlet oxygen to relax by another physical pathway.[15e]
Aggregation of Dyes and Bioconjugates
The solubility of s775z in water is remarkably high at >100 mM and a 1 mM stock solution of s775z in water was found to be unchanged after one month storage at 4 °C. In contrast, a freshly prepared 1 mM stock solution of unshielded 756z in water forms a precipitate after 24 hr, and the insoluble material cannot be redissolved after sonication (Figure S1). The difference in water solubility between s775z and 756z correlates with the propensities to form self-aggregates. Self-aggregation of heptamethine cyanine dyes is readily indicated by conversion of monomer absorption bands into aggregate bands, in this case blue-shifted H-aggregates.[13b,13c] As shown by the absorption spectra in Figures 2a and S7–S10, the control dye 756z exists largely as non-fluorescent H-aggregates (see excitation spectra in Figure S11), whereas the shielded dye s775z is in a fluorescent monomeric state. A series of dye/protein association studies (Figures S10 and S14) found that charge balanced 756z and s775z have similar weak affinities for bovine serum albumin (BSA) with Ka values of 1.6 × 104 M−1 and 1.3 × 104 M−1, respectively, which is about 40 times lower than the Ka for BSA association with ICG.[13b,25]
Figure 2.
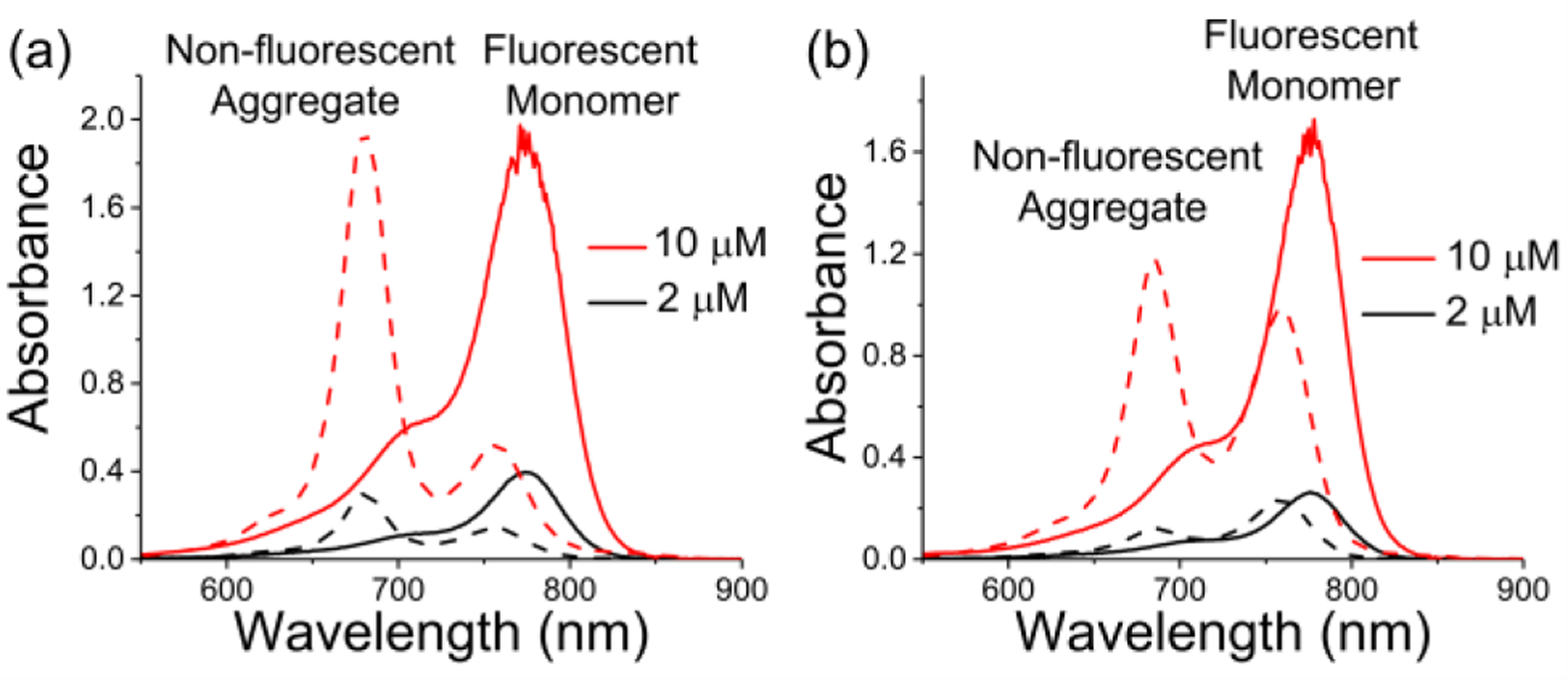
Absorption spectra. (a) s775z (solid line) and 756z (dashed line), (b) s775z-RGD (solid line) and 756z-RGD (dashed line), in water at different concentrations.
Standard amide bond conjugation chemistry was used to react the NHS ester of 756z or s775z with a free amine on the cyclic peptide targeting unit, cRGDfK, and create the homologous fluorescent peptide probes 756z-RGD and s775z-RGD, respectively (Scheme 1). The absorption spectra in Figure 2b and Figure S12 show that the unshielded probe 756z-RGD exists as a concentration-dependent mixture of fluorescent monomer and non-fluorescent H-aggregate (see excitation spectrum in Figure S11), whereas the shielded probe s775z-RGD is a fluorescent monomer in water even at the highest concentration tested (10 μM).
Amide bond formation was also used to attach multiple copies of either 756z or s775z to an antibody. Two sets of antibody conjugates were each prepared by reacting goat Immunoglobulin G (IgG) with dye NHS ester followed by size exclusion purification to remove any unreacted dye (see Figures S20–S22 for gel electrophoresis proof-of-purity). Purified samples of 756z-IgG (degree of labeling (DOL) = 2.1) and s775z-IgG (DOL = 2.3) were found to be stable over 7 days when stored at 4 °C in PBS buffer (Figure S25), unlike antibody conjugates of ZW800–1 which have been reported to partially degrade over 24 hours.[12b] The absorption spectrum of control antibody conjugate 756z-IgG (Figure 3a) shows a blue-shifted H-aggregate peak at 680 nm corresponding to close stacking of the appended fluorochromes because they are attached to the antibody at proximal positions (see Scheme S1 for a schematic picture).[13] A patch of stacked appended fluorescent dyes on an antibody surface is problematic for several reasons, including: (a) the stacked dyes can disrupt antibody folding or structural dynamics and thus antibody function; (b) the H-aggregate peak is non-fluorescent which weakens utility of the antibody conjugate for high sensitivity fluorescence imaging or diagnostics; (c) a patch of stacked appended dyes can become a hydrophobic hot spot on the antibody surface and promote undesired antibody aggregation or association with biological interfaces. This latter point became apparent when we prepared versions of control 756z-IgG with DOL > 2.1; absorption spectra for these samples indicated extensive light scattering (Figure S24) due to intermolecular aggregation of the antibody conjugate. In stark contrast, the absorption spectrum of an analogous antibody conjugate, s775z-IgG, did not exhibit a stacked fluorochrome peak (Figure 3a). Shown in Figure 3b is a plot of relative fluorescence intensity for different polyacrylamide gel bands comprised of s775z-IgG with increasing DOL. The plot reveals an inverse exponential dependence of relative fluorescence on DOL, up to the highest DOL tested which was 10.7. Even at this unusually high DOL, there was no stacked fluorochrome peak in the conjugate’s absorption spectrum (Figure S23), indicating that the 10.7 (on average) copies of s775z covalently appended to the surface of the IgG were not spatially close enough for strong Coulombic coupling of dye excitons.[26] The fact that fluorescence intensity for s775z-IgG continually increases with DOL, without reaching a maximum value, is unusual for a protein labeled with a cyanine dye, especially a heptamethine cyanine.[12a,27] This finding has important practical implications because it suggests that bright, densely labeled s775z-antibodies can be used at very low doses for diagnostics or imaging applications. This is crucial in the field of fluorescence guided surgery where the procedural and practical benefits of conducting clinical trials under microdosing regimes are well recognized,[28] but to date very few microdose trials have been attempted with fluorescent antibodies because they are not sufficiently bright.[2a,29]
Figure 3.
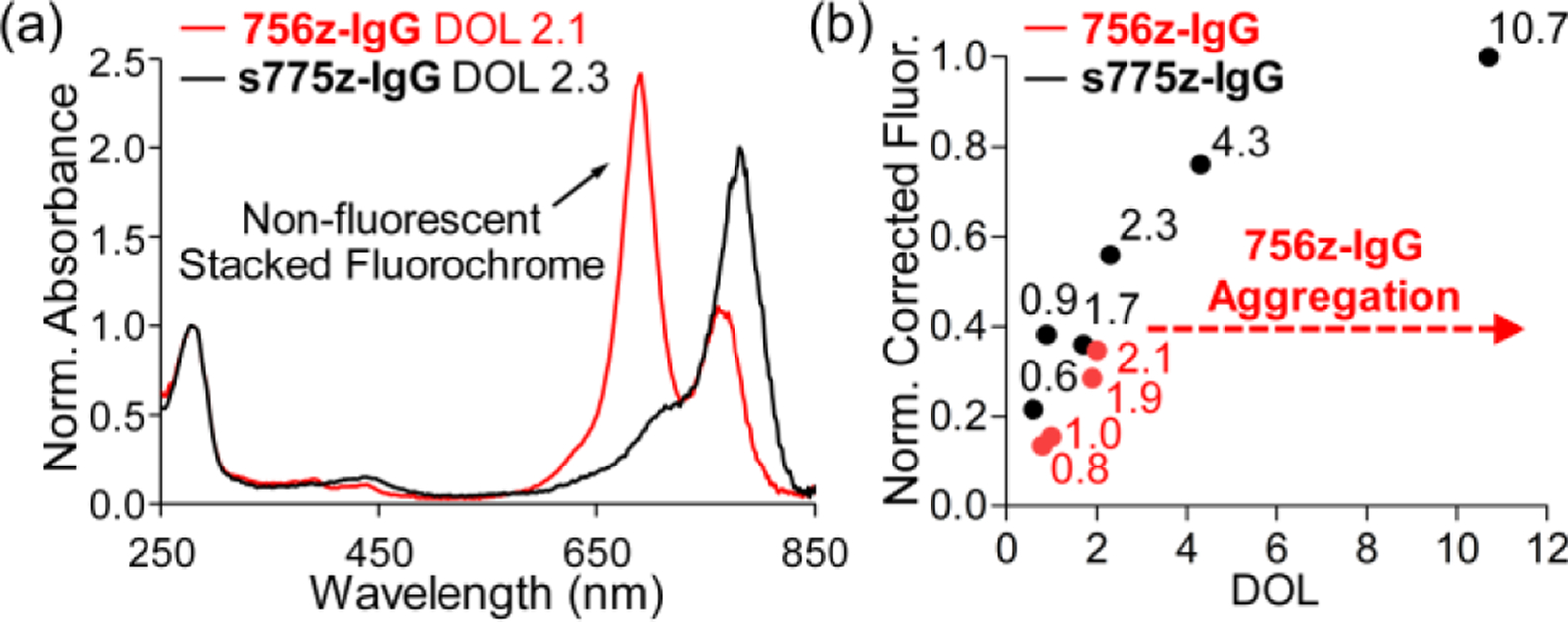
(a) Absorbance spectra (normalized to the absorbance at 280 nm) for samples of 756z-IgG or s775z-IgG with very similar DOL. Only the 756z-IgG spectrum exhibits a blue-shifted peak corresponding to non-fluorescent stacked fluorochrome. (b) Plot of DOL for 756z-IgG or s775z-IgG versus fluorescence intensity (corrected for protein concentration and normalized relative to s775z-IgG DOL 10.7) for different bands of pure 756z-IgG or s775z-IgG on a polyacrylamide gel.
Biological Imaging Studies
The overall goal of the biological imaging studies was to determine if the heptamethine steric shielding effect promoted high performance NIR fluorescence imaging. More specifically, we needed to prove that the length of the shielding triethyleneglycol chains in s775z was long enough to block non-specific interactions with membrane surfaces, serum proteins, and the extracellular matrix. Yet the shielding arms had to be short enough to permit strong association of dye-labeled bioconjugates with specific cell receptors and also allow rapid renal excretion of any unbound probe.[30]
The hypothesis of low non-specific binding was first tested by measuring the cell uptake, cell toxicity, and mouse biodistribution of s775z. Cell microscopy experiments showed negligible cell uptake of s775z, and there was no significant drop in cell viability after 24 hours of dye incubation at the low micromolar concentrations commonly used for biological imaging (Figure S26). Mouse biodistribution studies injected two separate cohorts of normal mice with a 10 nmol dose of ICG or s775z, followed by whole body imaging over time (all mouse experiments used protocols that were approved by the university’s Institutional Animal Care and Use Committee). After 2 hours the mice were sacrificed and the abdominal cavity of each animal was exposed and imaged. The live mouse images (Figure S28a) showed that both dyes were quickly cleared from the mouse bloodstream. But as revealed by the representative NIR images of exposed abdomen in Figure 4a and the associated biodistribution graph (Figure S28b), the blood clearance pathways were very different. As expected, virtually all of the ICG remained within the animals, where it accumulated in the intestines and liver. In contrast, most of the s775z had underwent near-exclusive renal clearance after 2 hours, with only weak NIR fluorescence remaining in the urine-containing bladder and kidneys.
Figure 4.
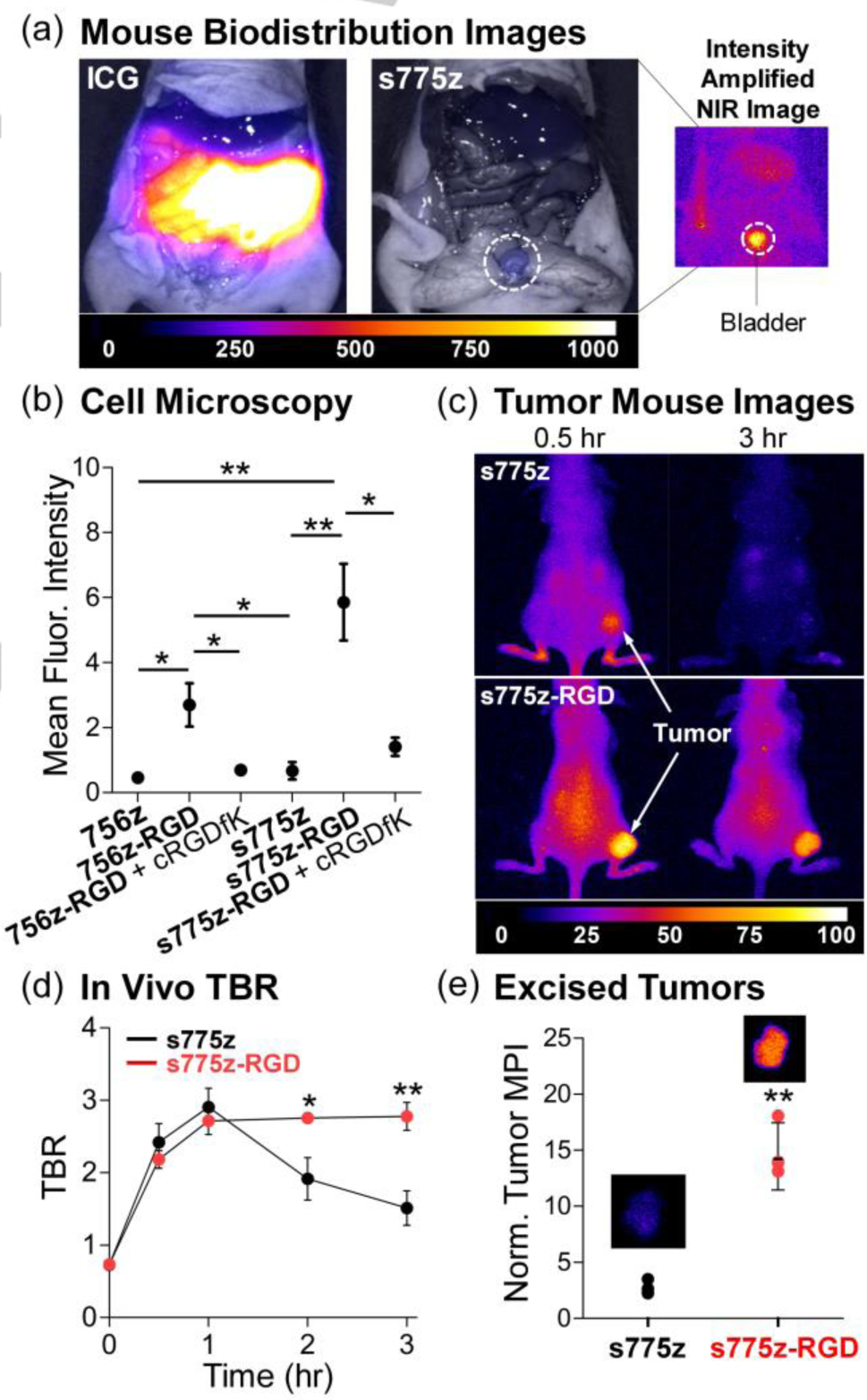
(a) Representative overlaid brightfield and fluorescence images of exposed abdomen of normal mice (no tumor) sacrificed 2 hr after retro-orbital injection of either ICG or s775z (10 nmol). The fluorescence intensity scale, in arbitrary units, is the same for both overlaid images, whereas the intensity of smaller NIR fluorescence image is amplified. (b) Plot of intracellular mean fluorescence intensities as a measure of NIR dye cell uptake. Integrin positive A549 cells were treated for 1 hr with 10 μM of NIR probe. The blocking experiments added 100 μM of free cRGDfK prior to the incubation with RGD probes. (c) Representative whole-body NIR fluorescence images of living mice bearing a subcutaneous A549 tumor at 0.5 and 3 hr after retro-orbital injection of either s775z or s775z-RGD (10 nmol). (d) Plot of Tumor-to-Background Ratio (TBR) in living mice at different post-injection time points. (e) Plot of MPI for excised tumors normalized to thigh muscle from the same mouse sacrificed at 3 hr post-injection. Average for each cohort (N=4) is indicated by a black line, with error bars indicating ± SEM. Representative NIR fluorescence image of an excised tumor is shown above each cohort. * indicates p<0.05, and ** p<0.01.
The next step was to prove that the two shielding triethyleneglycol chains in s775z did not prevent a targeted version of the dye from binding to cancer cell-surface receptors. This was done by first studying the cell targeting properties of the peptide conjugates, s775z-RGD and 756z-RGD. These two conjugates include the cyclic peptide sequence cRGDfK that is well-known to have nanomolar affinity for cell-surface integrin receptors, more specifically the receptor sub-types αvβ3 and αvβ5.[31] The ubiquity of RGD-based molecular probes makes cRGDfK a sensible choice of targeting unit for comparative studies of biological imaging performance.[2a,2b] We focused on A549 cancer cells (human lung adenocarcinoma) which is a cell line that overexpresses integrin αvβ5 receptors and selectively internalizes fluorescent cRGDfK conjugates.[31,32] A comparative set of fluorescence microscopy experiments incubated separate samples of A549 cells with each fluorescent compound (s775z-RGD, 756z-RGD, s775z, or 756z) and observed much higher cell uptake of two cRGDfK targeted probes compared to their untargeted counterparts (Figure S27). Moreover, cell uptake of the shielded s775z-RGD was higher than cell uptake of the unshielded and self-aggregated 756z-RGD. In both cases, the cell uptake of targeted probe was successfully blocked by pre-incubating the cells with an excess amount of the optically transparent targeting peptide cRGDfK (Figure 4b), strongly indicating that cell uptake was caused by integrin-selective binding and subsequent endocytosis.
The high level of A549 cell uptake by cancer targeted s775z-RGD prompted us to conduct in vivo imaging studies using a subcutaneous mouse tumor model. Nude mice (N=8) bearing a subcutaneous tumor (A549 cells) in the right rear flank were randomly divided into two cohorts and given a retro-orbital injection of either s775z or s775z-RGD (10 nmol).[33] Each mouse was imaged periodically over 3 hours (Figure 4c and S29) and the change in tumor fluorescence MPI and Tumor-to-Background ratio was plotted (Figure 4d and S30). The live animal images were consistent with the standard pharmacokinetic model for tumor partitioning of small untargeted and targeted probes.[25,34] The mice dosed with s775z showed transient uptake into the subcutaneous tumor followed by washout of the untargeted dye. In contrast, the images of mice dosed with the targeted s775z-RGD showed much slower washout from the tumor leading to a significantly higher Tumor-to-Background ratio at the 2 hour and 3 hour time points (Figures 4d and S29–S30). This difference in tumor imaging capability reflects the high affinity of the targeted s775z-RGD probe for the overexpressed integrin receptors on the surface of the cancer cells and endothelial cells that line the tumor vasculature.[32] After the 3 hour time point, the mice were sacrificed, and a mock surgery was performed on the mouse cohort dosed with s775z-RGD (Figure S31). Subsequently, all tumors and major organs were removed and the amount of dye in the different tissues was quantified by measuring the fluorescence MPI. Shown in Figure 4e is a plot of MPI for excised tumors, normalized to thigh muscle, and also a pair of representative NIR fluorescence images of the excised tumors. The complete set of tumor NIR fluorescence images is provided in Figure S32 and a plot of normalized MPI for all excised tissues is shown in Figure S33. The normalized tumor MPI for mice dosed with cancer targeted s775z-RGD (14.4 ± 3.0) was much higher than the value for mice dosed with untargeted s775z (2.6 ± 0.5), and reflects a combination of high affinity for the overexpressed integrin receptors in the tumor tissue and very low affinity for background muscle tissue.[9d,33] From the perspective of fluorescence guided cancer surgery, s775z-RGD achieved the highly desirable combination of rapid, near-exclusive renal clearance from the bloodstream, very high Tumor-to-Background ratio, and ultralow retention in background tissue.[2a,2b] Thus, s775z-RGD has high potential for passage towards clinical translation.
Conclusion
For about thirty years, chemical research on heptamethine cyanine dyes has focused on flat molecules with a polar periphery. This study validates a new three-dimensional structural strategy that simultaneously projects two shielding arms directly over each face of the polyene. Compared to the benchmark heptamethine cyanine dyes listed in Scheme 1, shielded s775z and its bioconjugates exhibit an unsurpassed combination of photophysical, physiochemical and biodistribution properties that greatly enhance bioimaging performance. Shielded s775z has a C-Aryl group at the meso position of a heptamethine polyene which makes the fluorochrome chemically more stable than the popular heptamethine cyanines CW800 or ZW800–1 which each have a more labile meso C-OAryl linkage.[12] A large set of comparative NIR fluorescence studies compared s775z to unshielded control dye 756z and found that shielding prevents dye self-aggregation and non-specific biological interactions. Importantly, the shielding arms do not prevent high affinity targeting of bioconjugates to cell surface receptors, or renal clearance from the blood stream. Notably, the integrin targeted probe s775z-RGD permitted high contrast cancer cell microscopy and mouse tumor imaging, with the latter producing a very high Tumor-to-Background ratio and ultralow retention in background tissue. Additional bioconjugation studies showed that multiple copies of shielded s775z can be attached to an antibody to produce a densely labeled conjugate without any stacking of appended fluorochromes. Next generation versions of densely labeled s775z-antibodies can likely be used as very bright, fluorescent probes for deployment at microdoses in various diagnostics or clinical imaging procedures. Furthermore, shielded s775z exhibits much better photostability than the benchmark heptamethine cyanines CW800, ZW800–1, or UL766 whose polyenes are electronically activated to react with photogenerated singlet oxygen. The remarkably high photostability of s775z makes it very attractive for incorporation into modern photon-intensive microscopy experiments such as single molecule tracking or super resolution imaging, as well as emerging clinical procedures, such as fluorescence guided surgery, which require long periods of sustained light exposure.[4a] The synthetic modularity that underlies the structure of s775z enables easy customization of bioimaging performance by modifying the two shielding arms to rationally fine-tune pharmacokinetics,[30,35] or the polyene structure to enhance photophysical properties.[36]
Supplementary Material
Acknowledgements
We are grateful for funding support from the US NIH (R01GM059078, R35GM136212 and T32GM075762) and a Berry Family Foundation fellowship from the University of Notre Dame.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].a) Sun W, Guo S, Hu C, Fan J, Peng X, Chem. Rev 2016, 116, 7768–7817; [DOI] [PubMed] [Google Scholar]; b) Shi C, Wu JB, Pan D, J. Biomed. Opt 2016, 21, 050901. [DOI] [PubMed] [Google Scholar]
- [2].a) Hernot S, Manen LV, Debie P, Sven J, Mieog D, Vahrmeijer AL, Lancet Oncol. 2019, 20, e354–e367; [DOI] [PubMed] [Google Scholar]; b) Debie P, Hernot S, Front. Pharmacol 2019, 10, 510; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Levitus M, Ranjit S, Q. Rev. Biophys 2011, 44, 123–151. [DOI] [PubMed] [Google Scholar]
- [3].a) Zhu S, Yung BC, Chandra S, Niu G, Antaris AL, Chen X, Theranostics 2018, 8, 4141–4151; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhu S, Hu Z, Tian R, Yung BC, Yang Q, Zhao S, Kiesewetter DO, Niu G, Sun H, Antaris AL, Chen X, Adv. Mater 2018, 30, 1802546. [DOI] [PubMed] [Google Scholar]
- [4].a) Gorka AP, Nani RR, Schnermann MJ, Acc. Chem. Res 2018, 51, 3226–3235; [DOI] [PubMed] [Google Scholar]; b) Gorka AP, Nani RR, Schnermann MJ, Org. Biomol. Chem 2015, 13, 7584–7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Ma X, Laramie M, Henary M, Bioorg. Med. Chem. Lett 2018, 28, 509–514; [DOI] [PubMed] [Google Scholar]; b) Strekowski L, Lipowska M, Patonay G, J. Org. Chem 1992, 57, 4578–4580. [Google Scholar]
- [6].a) Debie P, van Quathem J, Hansen I, Bala G, Massa S, Devoogdt N, Xavier C, Hernot S, Mol. Pharm 2017, 14, 1145–1153; [DOI] [PubMed] [Google Scholar]; b) Cilliers C, Nessler I, Christodolu N, Thurber GM, Mol. Pharm 2017, 14, 1623–1633; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Sato K, Gorka AP, Nagaya T, Michie MS, Nani RR, Nakamura Y, Coble VL, Vasalatiy OV, Swenson RE, Choyke PL, Schnermann MJ, Kobayashi H, Bioconjug. Chem 2016, 27, 404–413; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Owens EA, Henary M, Fakhri G. El, Choi HS, Acc. Chem. Res 2016, 49, 1731–1740; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Bunschoten A, Welling MM, Bioconjug. Chem 2016, 27, 1253–1258; [DOI] [PubMed] [Google Scholar]; f) Yu M, Liu J, Ning X, Zheng J, Angew. Chem. Int. Ed 2015, 54, 15434–15438; Angew. Chem. 2015, 127, 15654–15658; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Sato K, Paik CH, Schnermann MJ, Gorka AP, Nagaya T, Nakamura Y, Harada T, Shaum JB, Kim I, Kobayashi H, Nani RR, Choyke PL, Mol. Pharm 2015, 12, 3303–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].a) Lim SY, Hong KH, Kim DI, Kwon H, Kim HJ, J. Am. Chem. Soc 2014, 136, 7018–7025; [DOI] [PubMed] [Google Scholar]; b) Zaheer A, Wheat TE, Frangioni JV, Mol. Imaging 2002, 1, 354–364. [DOI] [PubMed] [Google Scholar]
- [8].a) Luciano MP, Crooke SN, Nourian S, Dingle I, Nani RR, Kline G, Patel NL, Robinson CM, Difilippantonio S, Kalen JD, Finn MG, Schnermann MJ, ACS Chem. Biol 2019, 14, 934–940; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nani RR, Kelley JA, Ivanic J, Schnermann MJ, Chem. Sci 2015, 6, 6556–6563; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Samanta A, Vendrell M, Das R, Chang Y-T, Chem. Commun 2010, 46, 7406–7408; [DOI] [PubMed] [Google Scholar]; d) Mellanby RJ, Scott JI, Mair I, Fernandez A, Saul L, Arlt J, Moral M, Vendrell M, Chem. Sci 2018, 9, 7261–7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) Yang Z, Usama SM, Li F, Burgess K, Li Z, Med. Chem. Comm 2018, 31, 1754–1760; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hyun H, Henary M, Gao T, Narayana L, Owens EA, Lee JH, Park G, Wada H, Ashitate Y, Frangioni JV, Choi HS, Mol. Imaging Biol 2016, 52–61; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Choi HS, Narayana L, Henary M, Njiojob CN, Owens EA, Hyun, J. Med. Chem 2015, 58, 2845–2854; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Choi HS, Gibbs SL, Lee JH, Kim SH, Ashitate Y, Liu F, Hyun H, Park G, Xie Y, Bae S, Henary M, Frangioni JV, Nat. Biotechnol 2013, 31, 148–153; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Choi HS, Nasr K, Alyabyev S, Feith D, Lee JH, Kim SH, Ashitate Y, Hyun H, Patonay G, Strekowski L, Henary M, Frangioni JV, Angew. Chem. Int. Ed 2011, 50, 6258–6263; Angew. Chem. 2011, 123, 6382–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cha J, Nani RR, Luciano MP, Kline G, Broch A, Kim K, Namgoong J.-m., Kulkarni RA, Meier JL, Kim P, Schnermann MJ, Bioorg. Med. Chem. Lett 2018, 28, 2741–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].a) Su D, Teoh CL, Samanta A, Kang NY, Park SJ, Chang YT, Chem. Commun 2015, 51, 3989–3992; [DOI] [PubMed] [Google Scholar]; b) Wu Z, Shao P, Zhang S, Bai M, J. Biomed. Opt 2014, 19, 36006; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lee H, Mason JC, Achilefu S, J. Org. Chem 2006, 71, 7862–7865. [DOI] [PubMed] [Google Scholar]
- [12].a) van der Wal S, Kuil J, Valentijn ARPM, van Leeuwen FWB, Dyes Pigm. 2016, 132, 7–19; [Google Scholar]; b) Hyun H, Owens EA, Narayana L, Wada H, Gravier J, Bao K, Frangioni JV, Choi HS, Henary M, RSC Adv. 2014, 4, 58762–58768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].a) Ji Y, Wang Z, Bao K, Park GK, Kang H, Hu S, McDonald E, Kim MS, Kashiwagi S, Choi HS, Quant. Imaging Med. Surg 2019, 9, 1548–1555; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Spa SJ, Hensbergen AW, van der Wal S, Kuil J, van Leeuwen FWB, Dyes Pigm. 2018, 152, 19–28; [Google Scholar]; c) Gruber HJ, Hahn CD, Kada G, Riener CK, Harms GS, Ahrer W, Dax TG, Knaus H-GG, Bioconjug. Chem 2000, 11, 696–704. [DOI] [PubMed] [Google Scholar]
- [14].a) Al-Karmi S, Albu SA, Vito A, Janzen N, Czorny S, Banevicius L, Nanao M, Zubieta J, Capretta A, Valliant JF, Chem. Eur. J 2017, 23, 254–258; [DOI] [PubMed] [Google Scholar]; b) He L, Lin W, Xu Q, Ren M, Wei H, Wang J-Y, Chem. Sci 2015, 6, 4530–4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].a) Ji C, Cheng W, Yuan Q, Mullen K, Yin M, Acc. Chem. Res 2019, 52, 2266–2277; [DOI] [PubMed] [Google Scholar]; b) Kühne C, Haag R, Heek T, Urner LH, Pagel K, Huth K, Achazi K, Dernedde J, Chem. Eur. J 2017, 23, 4849–4862; [DOI] [PubMed] [Google Scholar]; c) Zhu S, Yang Q, Antaris AL, Yue J, Ma Z, Wang H, Huang W, Wan H, Wang J, Diao S, Zhang B, Li X, Zhong Y, Yu K, Hong G, Luo J, Liang Y, Dai H, Proc. Natl. Acad. Sci. U.S.A 2017, 114, 962–967; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Wycisk V, Pauli J, Welker P, Justies A, Resch-Genger U, Haag R, Licha K, Bioconjug. Chem 2015, 26, 773–781; [DOI] [PubMed] [Google Scholar]; e) Yau CMS, Pascu SI, Odom SA, Warren JE, Klotz EJF, Frampton MJ, Williams CC, Coropceanu V, Kuimova MK, Phillips D, Barlow S, Brédas JL, Marder SR, Millar V, Anderson HL, Chem. Commun 2008, 2897–2899. [DOI] [PubMed] [Google Scholar]
- [16].Thavornpradit S, Usama SM, Park GK, Shrestha JP, Nomura S, Baek Y, Choi HS, Burgess K, Theranostics 2019, 9, 2856–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Arjona-Esteban A, Stolte M, Würthner F, Angew. Chem. Int. Ed 2016, 55, 2470–2473; Angew. Chem. 2016, 128, 2516–2519. [DOI] [PubMed] [Google Scholar]
- [18].Štacková L, Štacko P, Klán P, J. Am. Chem. Soc 2019, 141, 7155–7162. [DOI] [PubMed] [Google Scholar]
- [19].The Cambridge Crystallographic Data Centre contains relevant X-ray structures, including: 1404767, 1404768 and 1404769. [Google Scholar]
- [20].Rüttger F, Mindt S, Golz C, Alcarazo M, John M, Eur. J. Org. Chem 2019, 2019, 4791–4796. [Google Scholar]
- [21].At present, we cannot rule out the possibility that a small fraction of s775z adopts a polyene conformation with one of its methine-methine bonds in a cis orientation (this putative conformational exchange would be rapid on the NMR time scale, see reference 20 for further discussion). This minor structural point does not change any conclusions concerning the practical value of s775z.
- [22].DSouza AV, Lin H, Henderson ER, Samkoe KS, Pogue BW, J. Biomed. Opt 2016, 21, 080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].a) Hyun H, Bordo MW, Nasr K, Feith D, Lee JH, Kim SH, Ashitate Y, Moffitt LA, Rosenberg M, Henary M, Choi HS, Frangioni JV, Contrast Media Mol. Imaging 2012, 7, 516–524; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ohnishi S, Lomnes SJ, Laurence RG, Gogbashian A, Mariani G, Frangioni JV, Mol. Imaging 2005, 4, 172–181. [DOI] [PubMed] [Google Scholar]
- [24].König SG, Krämer R, Chem. Eur. J 2017, 23, 9306–9312. [DOI] [PubMed] [Google Scholar]
- [25].Berezin MY, Guo K, Akers W, Livingston J, Solomon M, Lee H, Liang K, Agee A, Achilefu S, Biochemistry 2011, 50, 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Szabó Á, Szendi-Szatmári T, Ujlaky-Nagy L, Rádi I, Vereb G, Szöllősi J, Nagy P, Biophys. J 2018, 114, 688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Berlier JE, Rothe A, Buller G, Bradford J, Gray DR, Filanoski BJ, Telford WG, Yue S, Liu J, Cheung CY, Chang W, Hirsch JD, Beechem JM, Haugland RP, Haugland RP, J. Histochem. Cytochem 2003, 51, 1699–1712. [DOI] [PubMed] [Google Scholar]
- [28].Kleinjan GH, Bunschoten A, Berg NSVD, Eur. J. Nuc. Med. Mol. Imaging 2016, 1857–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lamberts LE, Koch M, Jong JSD, Adams ALL, Kranendonk EG, Scheltinga AGTTV, Jorritsma-Smit A, Linssen MD, Boer ED, Vegt BVD, Nagengast WB, Elias SG, Oliveira S, Witkamp AJ, Wall EVD, Diest PJV, Vries EGED, Clin. Cancer Res 2017, 23, 2730–2742.28119364 [Google Scholar]
- [30].Du B, Jiang X, Huang Y, Li S, Lin JC, Yu M, Zheng J, Bioconjug. Chem 2019, 31, 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shaw SK, Liu W, Gómez-Durán CFA, Schreiber CL, Betancourt-Mendiola M. de L., Zhai C, Roland FM, Padanilam SJ, Smith BD, Chem. Eur. J 2018, 24, 13821–13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schreiber CL, Zhai C, Dempsey JM, Mcgarraugh HH, Matthews BP, Christmann CR, Smith BD, Bioconjug. Chem 2020, 31, 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Handgraaf HJM, Boonstra MC, Prevoo HAJM, Kuil J, Bordo MW, Boogerd LSF, Sibinga Mulder BG, Sier CFM, Vinkenburg-van Slooten ML, Valentijn ARPM, Burggraaf J, van de Velde CJH, Frangioni JV, Vahrmeijer AL, Oncotarget 2017, 8, 21054–21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tichauer KM, Samkoe KS, Sexton KJ, Hextrum SK, Yang HH, Klubben WS, Gunn JR, Hasan T, Pogue BW, Mol. Imaging Biol 2012, 14, 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yazaki PJ, Lwin TM, Minnix M, Li L, Sherman A, Molnar J, Miller A, Frankel P, Chea J, Poku E, Bowles N, Hoffman RM, Shively JE, Bouvet M, J. Biomed. Opt 2019, 24, 066012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].a) Michie MS, Götz R, Franke C, Bowler M, Kumari N, Magidson V, Levitus M, Loncarek, Sauer M, Schnermann MJ, J. Am. Chem. Soc 2017, 139, 12406–12409; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Li B, Lu L, Zhao M, Lei Z, Zhang F, Angew. Chem. Int. Ed 2018, 57, 7483–7487; Angew. Chem. 2018, 130, 7605–7609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


