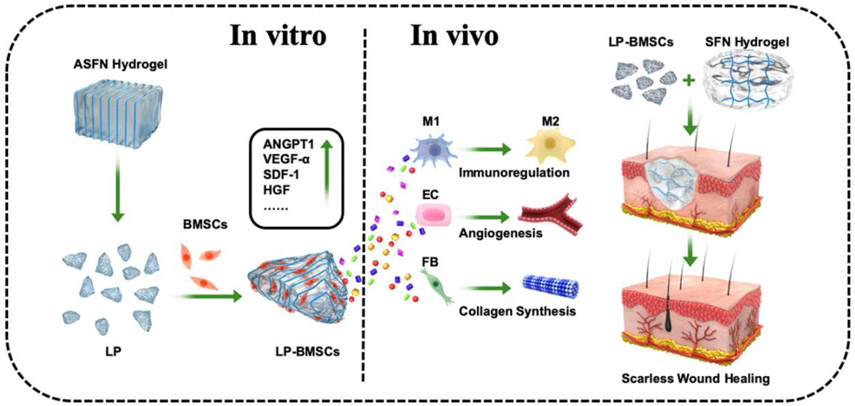
Abstract
Scarless skin regeneration with functional tissue remains a challenge for full-thickness wounds. Here, mesenchymal stem cells (MSC)-laden hydrogels were developed for scarless wound healing with hair follicles. Microgels composed of aligned silk nanofibers were used to load MSCs to actively modulate the paracrine. MSC-laden microgels were dispersed into injectable silk nanofiber hydrogels, forming composites biomaterials containing the cells. The injectable hydrogels protected and stabilized the MSCs in the wounds. The synergistic action of silk-based composite hydrogels and MSCs stimulated angiogenesis and M1-M2 phenotype switching of macrophages, providing a suitable niche for functional recovery of wounds. Compared to skin defects treated with MSC-free hydrogels, the defects treated with the MSC-laden composite hydrogels healed faster and formed scarless tissues with hair follicles. Wound healing could be further improved by adjusting the ratio of silk nanofibers and particles and the loaded MSCs, suggesting tunability of the system. To the best our knowledge, it is the first time scarless skin regeneration with hair follicles based on silk material systems has been reported. The improved wound healing capacity of the systems suggests future in vivo studies to compare to other biomaterial systems related to clinical goals in skin regeneration in the absence of scarring.
Keywords: Injectable hydrogel, Wound healing, MSCs, Silk, Scarless
Graphical Abstract
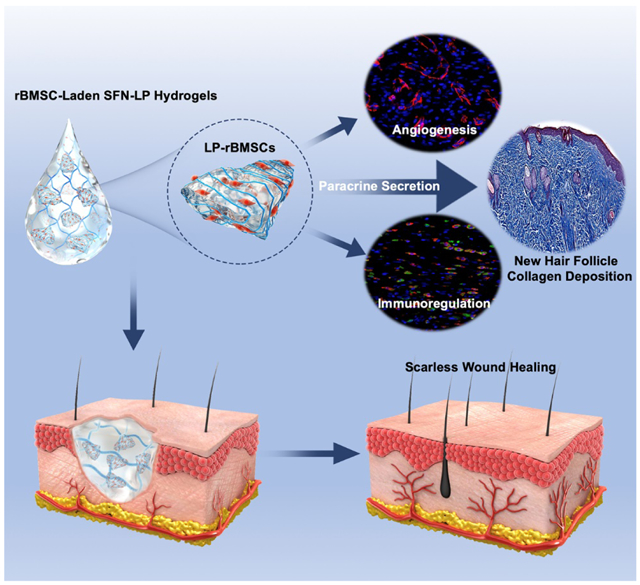
Microgels composed of aligned silk nanofibers were used to load mesenchymal stem cells (MSC) to actively modulate the paracrine. The MSC-laden microgels were blended with injectable silk nanofoiber hydrogels to accelerate the wound healing. The synergistic action of MSC-laden hydrogel systems stimulated angiogenesis and M1-M2 phenotype switching of macrophages, achieving scarless skin regeneration with hair follicles.
1. Introduction
Skin, the largest organ of the homan body, is composed of complex tissues including epidermal, dermal, and hypodermal layers, as well as other functional vasculature/neurons/immune sysems.[1] As a protective barrier to harmful external invasions, skin is easily damaged by trauma, disease and chronic ulcers.[2] Serious skin defects to the depth of skeletal muscle are difficult to heal and usually form nonfunctional scar tissues even when treated with different biomaterial-based wound dressings. Although bioactive biomaterials were developed to improve wound recovery, a challenge remains to regenerate scarless tissues with hair follicles.[3]
Due to the self-renewal and multilineage differentiation potential, as well as their capacity for secreting bioactive factors such as growth factors, cytokines and exosomes, stem cells are promising options for use in skin regeneration.[4] Stem cells play a role in wound healing through tropic and paracrine effects.[5] Therefore, besides the effective loading of stem cells, bioactive cell-laden biomaterial systems have been designed to tune secretory functions, generating a suitable niches for functional skin recovery. Scaffolds, hydrogels and microcapsules with specific microstructure, topography, mechanical and chemical cues were fabricated to guide the behavior of stem cells in the wound healing process.[6] By sensing the microenvironment of these cell-laden biomaterial systems, stem cells can regulate immune responses, vascularization, cell recruitment and tissue remodeling.[5a, 5b, 7] However, a gap exists for stem cell strategies to achieve scarless skin tissue outcomes, thus improved stem cell-laden biomaterial systems are required to strengthen the action of stem cells in skin regeneration.
Microskin is an effective option to solve the shortage of autologous skin grafts for large area skin defects. These autologous grafts are cut into smaller skin particles to increase epithelial surface treatment to achieve larger wound coverage with smaller donor skin samples.[8] Besides as coverings, the microskin could provide suitable physical-chemical cues to recruit and regulate the neighbouring cells, stimulating new skin regeneration. Inspired by microskin grafts, stem cell-laden systems have been developed in which stem cells were loaded onto biomaterial particles to imitate microskin structure.[9] Faster wound healing suggested better efficiency for these cell-laden systems. Unlike microskin grafts, most cell-laden particles lack suitable cues to control the behavior of stem cells. Therefore, further physical/chemical regulation encoded into these cell-laden particle systems would generate improved microenvironments for skin recovery.
Silk fibroin (SF) has been used in skin regeneration due to its biocompatibility and angiogenic capacity.[10] Different stem cells were also loaded on SF matrices and used to accelerate the wounding healing. However, little studies tried to introduce multiple cues to actively control cell behaviors and skin recovery.[11] Recently, SF nanofibers (SFN) were developed to achieve better biocompatibility. Both SFN-based scaffolds and hydrogels were fabricated to stimulate tissue in-growth, resulting in better wound healing.[12] SFN hydrogels with aligned topography were then developed in our group to form better niches with multiple cues .[13] Unlike unaligned SFN hydrogels, the aligned SFN hydrogels could regulate the secretion of different cytokines and growth factors from stem cells, achieving long-term stemness of the stem cells without exogeneous grow factors and fibroblasts (Data not shown). The aligned hydrogels were also induced the anisotropic migration and aggregation of different cells, which was critical for vascularization and granulation ingrowth.[14] However, cells only adhered, migrated and proliferated on the surface, but failed to invade the interior of the aligned hydrogels.[14b] Although the aligned hydrogels could be lyophilized to form anisotropic scaffolds to facilitate the ingrowth of blood vessel and granulation tissue during the wound healing process, the loss of hydrogel state weakened the niches with multiple cues, resulting in the wound healing without hair follicle regeneration.[15] Novel strategies are required to maintained the functions of multiple cues fully, optimzing the control of cell behaviors and skin regeneration. Here, aligned SFN hydrogels were modified to form microparticles without the destroy of aligned microstructures. Stem cells can be loaded in these hydrogel particles, generating microenvironments through the paracrine for potential utility in scarless skin recovery. The objective of the present study was to evaluate the performance of these stem cell-laden particles when blended with injectable SFN hydrogels, as injectable composite biomaterial systems similar to microskin grafts. In vitro cell cultures revealed the desired paracrine control of the stem cells, and in vivo these systems modulated the immune behavior and vascularization in scarless skin recovery with hair follicle regeneration (Scheme 1).
Scheme 1.
Schematic diagram of fabrication process of the MSCs-laden SFN composite hydrogels. Large microparticles with aligned structure were derived from the aligned SFN hydrogels formed in electrical field. The particles were loaded with MSCs and then blended with the injectable SFN hydrogels to form the composite systems. The MSC-laden hydrogels were injected to the wounds to achieve scarless skin regeneration with hair follicles. (ASFN Hydrogel:aligned silk fibroin nanofiber Hydrogel; SFN Hydrogel: silk fibroin nanofiber Hydrogel; LP: large microparticle; BMSCs: Bone marrow mesenchymal stem cells; LP-BMSCs: BMSCs-laden LP; M1: Type 1 macrophage; M2: Type 2 macrophage; EC: endothelial cell; FB: fibroblast)
2. Experimental Section
Preparation of silk nanofiber hydrogels:
Aqueous silk solution was prepared according to our previously published procedures.[16] Bombyx mori silk was degummed in an aqueous solution of sodium carbonate (0.02 M) at 100°C for 20 min. The degummed silks were dissolved in 9.3 M lithium bromide solution at 60°C. After dialysis with distilled water for 72 h, the solution was centrifuged at 9,000 rpm for 20 min to remove the aggregates. Then aqueous silk fibroin (SF) solution with concentration of about 6 wt% was obtained and used to assemble the nanofiber hydrogels via the concentration-dilution-thermal incubation method.[14a] SF nanofibers were assembled when fresh SF solution (6 wt%) was concentrated to 20 wt% at 60°C for at least 24 h. The concentrated SF solutions were diluted to 1 wt% and 2 wt% and stored at 60°C until the hydrogel formation. Following the transition from solution to hydrogel, the particles transformed to nanofibers due to the changes of negative charge repulsion.[17] The SF nanofibers were termed SFN.
Fabrication of aligned SFN microparticles:
Aligned SFN hydrogels were prepared under an electrical field.[13, 14b] The SFN hydrogels (2 wt%) were treated at 50 V DC for 30 min. After the treatment, the aligned hydrogels formed near the positive electrode. The aligned SFN hydrogels were stirred with a magnetic agitator at 200 rpm for 5 min to prepare microparticles. The particles were separated by molecular sieves, obtaining different particles with sizes of 100-300 μm, 300-500 μm, and 500-700 μm, respectively. The microparticles with homogeneous sizes in the different ranges were collected and then used in the following studies. The particles were measured with laser particle size analyzer (Mastersizer 2000, Malvern Panalytical, UK). According to these size ranges, the particles were termed SP (small particles), MP (medium particles) and LP (large particles).
Characterization of aligned SFN microparticles:
After the SFN microparticles were dispersed in distilled water, particle macrostructure was observed with inverted microscopy (Vert.A1, ZEISS, Germany). Scanning electron microscopy (SEM, S-4800, Hitachi, Tokyo, Japan) was also used to measure the micromorphology of the particles. Freeze-dried samples were coated with gold sputter and imaged at 3 kV.[18] The secondary structural conformations of the samples were evaluated with Fourier transform infrared spectroscopy (FTIR, Nicolet FTIR 5700, Thermos Scientific, FL, USA) in the range of 1400-1800 cm−1.
Preparation of SFN-microparticle composite hydrogels (SFN-P):
The aligned particles were directly added to the SFN hydrogels (1 wt%) and stirred at 100 rpm for 5 min to form homogeneous composite hydrogels. The different particles (SP, MP and LP) were blended with the SFN hydrogels and termed SFN-SP, SFN-MP and SFN-LP, respectively. The weight ratios of the particles and the SFN hydrogels were fixed to 40:60.
Dispersion of microparticles in SF hydrogels:
To assess the dispersion of the particles in the hydrogels, the particles were dyed with rhodamine (Sigma, USA) and mixed into the SFN hydrogels (1 wt%). As a control, the particles were also dispersed in ultrapure water (UW). The composite hydrogels were placed at room temperature for 1 min, 1 h, 1 d, 1 w, 2 w and 3 w to observe the homogeneity. Confocal laser scanning microscopy (CLSM, Leica DMIRE2, Leica, Wetzlar, Germany) was used to observe the particle dispersions.
Rheological properties:
The rheological behavior of the SFN-P hydrogels was measured on a rheometer (AR2000, TA Instruments, New Castle, DE) fitted with a 20 mm cone plate (Ti, 20/1°). Frequency sweeps were collected continuously over a frequency range from 100 to 1 rad s−1 at 25°C. The stability of the SFN and SFN-P composite hydrogels was assessed by measuring the rheological behavior under constant frequency over the temperate range of 22 to 40°C.[12a, 19]
Viscosity and Injectability of SFN-P hydrogels:
The viscosities of SFN-P hydrogels were plotted against varying shear rates from 10−1 to 10−2 s−1 to obtain flow curves at room temperature. SFN-P hydrogels were suctioned into 5 mL syringes and pushed through a needle (14 G) at 1.5 N to assess injectability. The inversion study was used to evaluate the stability of the hydrogels. A 3 mL aliquot of the hydrogels (SFN, SFN-SP, SFN-MP, SFN-LP) was placed in cylindrical bottles. After the bottles were inverted for 24 h, the flowing state of the hydrogels was observed over time.
Isolation and identification of Cells:
Six-week-old male Sprague-Dawley (SD) rats were used to isolate bone marrow mesenchymal stem cells (BMSCs). The use of rats was approved by the animal ethics committee of Soochow University (approval number 201906A201). BMSCs were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Grand Island, NY, US) containing 10% fetal bovine serum (FBS) and 100 UmL−1 penicillin-streptomycin (Gibco, Grand Island, NY, US) at a 37°C incubator. After continuous passaging to the third generation, flow cytometric analysis was performed for identification of the purity of BMSCs. Passage 3 BMSCS were labeled with fluorescein isothiocyanate-(FITC) conjugated antibodies against CD90, CD45, CD44, and CD34 (Cyagen Biosciences, China). Other BMSCs cultures were stained with FITC-conjugated mouse IgG1 (Cyagen Biosciences, China) as a negative control.[20] Expression distribution was assessed by single-channel flow cytometry (BD Biosciences, USA).
Cell adhesion on the microparticles:
BMSC suspensions with concentrations of 1×107 cells mL−1 were used to evaluate cell adhesion on the particles. For the studies, 100 μL of microparticles (SP, MP, LP) were added to the wells of 24-well plates, following by the addition of the cell suspension (100 μL). After incubation for 24 h, the microparticles were removed to count the cells adhered on the particles by dissociating the cells using trypsin-ethylenediamine tetraacetic acid (EDTA) solution for 2 min. Cell number was estimated with a Cell Viability Analyzer (Vi-CELL XR, Beckman Coulter, USA). Cell adhesion was calculated using the formula: Cell adhesion rate = Cell adhesion/Cell inoculation volume×100%. [21]
In vitro cytocompatibility of the aligned particles:
When the cells loaded on the microparticles were cultured for 1, 3, 7, and 14 days, cell viability was evaluated with Live/Dead assay (Sigma, USA). For this assessment, 0.5 μL of calcein AM and 2 μL of ethidium homodimer were added to 1 mL PBS to obtain Live/Dead dye solution. The medium was replaced with 300 μL of Live/Dead dye solution and incubated in darkness at 37°C for 15 min to stain the cells. The cells were imaged by CLSM (DMIRE2, Leica, Wetzlar, Germany). Cell viability was determined by calculating the ratio of live cells among all the cells with Image J software.
After culture for 1, 7, 14 days, cells were stained to measure proliferation by CLSM. After fixing in 4% paraformaldehyde solution (PFA, Sigma-Aldrich, St. Louis, MO) for 30 min and permeabilized in 0.1% Triton X-100 for 10 min, the cell actin and nuclei were stained with FITC-phalloidin (Thermo Fisher, Waltham, MA, USA) for 1h, and DAPI solution at a dilution 1:1000 for 10 min, respectively, at 37°C. Cells were imaged with Leica fluorescence microscope. ImageJ software was used to analyze both cell number and morphology. The quantification of cell proliferation was determined by a PicoGreen TM DNA assay (Invitrogen, Carlsbad, CA, USA). At indicated time points (days 1, 3, 7, 14), the samples were immersed in proteinase K solution overnight at 56°C to digest the hydrogel. The digested samples (n = 3) were measured with a spectrofluorometer (BioTeK Synergy 4, Winooski, VT, USA) at an excitation wavelength of 480 nm and emission wavelength of 530 nm. Then the DNA content was calculated based on the standard curve.
Cell viability of the injected BMSC-laden SFN-LP hydrogels:
When BMSC-laden LP particles in medium and SFN hydrogels were injected from a 14 G (inner diameter is 1.54 mm) syringe by hand, cell viability after injection was evaluated with the Live/Dead assay as mentioned above.
Paracrine secretion of BMSCs loaded on the LP particles in vitro:
Passage 3 BMSCs were seeded alone or loaded on the LP particles with a cell density of 1×106 mL−1 and cultured in the 6-well plates with DMEM containing 10% FBS and 1% penicillin-streptomycin incubated at 37°C in 5% CO2 for 7 days. The culture medium was replaced every two days. At 1, 3 and 7 days, the cells were collected to study cell stemness retention and cytokine secretion. Total RNA was harvested from BMSCs by Lysis Buffer (RZ, RNA simple Total RNA Kit, Tiangen Biotech, China). Gene expression profiles for cell stemness markers included Sex determining region Y-box 2 (Sox2), Octamer-binding transcription factor 4 (Oct4), and Kruppel-like factor 4 (Klf4), as well as cytokine secretion factors including Angiopoietin-1 (ANGPT-1), Vascular endothelial growth factor-α (VEGF-α), the stromal cell-derived factor-1 (SDF-1), Hepatocyte growth factor (HGF), and Basic fibroblast growth factor-2 (FGF-2) were measured using quantitative real time polymerase chain reaction. The sequences of primers are listed in Tables 1 and 2. Extracted RNA underwent reverse transcription and real-time qPCR reaction as reported in our recent studies.[9a] Western blot and Enzyme-linked immunosorbent assays (ELISA) were also used to evaluate the paracrine secretion of BMSCs. For western blot analysis, total proteins of cells were extracted with RIPA lysis buffer. The amount of the protein was measured by a BCA protein assay kit according to our recent study.[10a] Primary antibodies included Anti-Oct4 (1:600, ab19857, abcam), Anti-Klf4 (1:800, ab129473, abcam), Anti-Sox2 (1:400, ab97959, abcam), and Anti-Actin (1:2000, ab34731, abcam) while secondary antibody chose HRP-conjugated secondary antibody(1:2000). For ELISA analysis, culture medium was frozen and stored at −80°C until evaluation with ELISA (R&D Systems, Minneapolis, MN, USA). The cytokine factors in culture medium including ANGPT-1, VEGF-a, SDF-1, HGF, and FGF-2 were measured according to manufacturer instructions (R&D Systems).[5a]
Table 1.
Sequences of primers used for qPCR analysis of BMSC stemness specific gene expression.
| Primer name | Orientation | Sequence (5’-3’) |
|---|---|---|
| Sox2 | Forward | GCCGAGTGGAAACTTTTGTCG |
| Reverse | CGGGAAGCGTGTACTTATCCTT | |
| Oct4 | Forward | GTGTTCAGCCAGACAACCATCT |
| Reverse | GGTCTCCGATTTGCATATCTCCT | |
| Klf4 | Forward | AGGAACTCTCTCACATGAAGCG |
| Reverse | GATCGTTGAACTCCTCGGTC |
Table 2.
Sequences of primers used for qPCR analysis of BMSC secreted key wound healing cytokines specific gene expression.
| Primer name | Orientation | Sequence (5’-3’) |
|---|---|---|
| ANGPT-1 | Forward | AGTCGGAGATGGCCCAGATA |
| Reverse | TGGATTTCAAGACGGGATGTT | |
| VEGF-α | Forward | GCCCTGAGTCAAGAGGACAG |
| Reverse | GAGGAGGAGGAGCCATTACC | |
| SDF-1 | Forward | TGCATCAGTGACGGTAAGCCA |
| Reverse | CTTTTCAGCCTTGCAACAATC | |
| HGF | Forward | AACAAGGGCTTTCCATTCACT |
| Reverse | TGTCCCCTTATAGCTGCCTCC | |
| FGF-2 | Forward | AGCGGCTCTACTGCAAGAAC |
| Reverse | GCCGTCCATCTTCCTTCATA |
In vivo wound healing experiments:
Nine to ten week old Sparague-Dawley (SD) adult male rats were used for the in vivo wound experiments.[12a] All animal experiments were approved by the Animal Care and Experiment Committee of the Soochow university (approval number 201905A182, 201906A340, and 201907A108, Suzhou, China). Thirty SD rats were randomly distributed to six groups: blank, SFN, SFN-LP hydrogels, BMSCs in medium, BMSCs blended with SFN hydrogels (SFN-BMSCs hydrogels) and BMSC-loaded on LP particles and then blended with SFN hydrogels (SFN-LP-BMSCs hydrogels). Since the hydrogel microparticles were used to induce the paracrine of stem cells rather than stimulate the proliferation in vitro, BMSCs-loaded particles were blended with injectable SFN hydrogels and then injected to the wound sites directly without the culture in vitro. Due to the homogenous distribution of the aligned structures, the microparticles could provide sustainable aligned cues even after the degradation of the surface. The injectable SFN hydrogels could facilitate the migration of cells and tissue ingrowth in wound healing process, finally achieving suitable microenvironments for the skin regeneration. A standard excisional wound healing model was used to prevent contraction.[22] Five rats were used for each group. After depilation of the dorsal skin under anesthesia, three full thickness wounds with diameter of 1.5 cm were created on either side of the dorsal midline. Wounds were circumscribed by donut-shaped silicone splints and fixed by 4-0 nylon sutures. For the control group, 250 μL PBS was injected on the wound bed by pipette. For the BMSCs only group, 250 μL of BMSCs-containing PBS (1×106 cells) were injected onto the wound bed. For the SFN and SFN-BMSCs groups, the hydrogels were injected on the wound bed (250 μL, containing 1×106 cells per wound). For SFN-LP hydrogels and SFN-P-BMSCs hydrogel groups, 150 μL hydrogel mixed with 100 μL pure LP microparticles or BMSC-laden LP microparticles (incubated for 24 h, containing 1×106 cells per wound) were injected. After injection, all wounds were coveraged with a transparent film dressing (Tegaderm Film, 3M, USA) and housed individually. Digital images were captured at days 0, 3, 7, 10, 14, 18 and 21, and the wound area was measured using Image J software. The percentage of the original wound area at different time points was assessed by comparing to the initial wound area at the day of surgery.
In vivo bioluminescence imaging:
Luciferase+/GFP+ virus was purchased from GENE company (GENE, China) and transfected into BMSCs by MOI at 50. After culturing with virus for 4 days, puromycin was used to filter the transfected cells for bioluminescence experiments. Luc+/GFP+ BMSCs in PBS (BMSCs, Group A), Luc+/GFP+ BMSCs-loaded SFN hydrogels (SFN-BMSCs, Group B ) and Luc+/GFP+ BMSCs-loaded LP particles in SFN hydrogels (SFN-P-BMSCs, Group C) were injected intraperitoneally with d-luciferin (150 mg kg−1 in PBS, PerkinElmer, USA) to SD rats (6 wounds per group). The cells were imaged using the IVIS Lumina Series III (PerkinElmer, USA). Bioluminescence of the interest region of each wound area was subtracted from background luminescence and quantified as units of total flux using Living Image Software 4.4, PerkinElmer.
Histology analysis and immunofluorescence staining:
The wounds and adjacent skin tissue were harvested and immediately fixed in 4% paraformaldehyde and embedded in paraffin. The blocked samples were then sectioned in 5 μm thick samples for staining with H&E, Masson’s trichrome, and Picrosirius Red. CD31, CD68 and CD206 immunofluorescence staining were performed according to previous studies.[5a, 12b] H&E stained sections were used to evaluate new epidermis formation in each group. Masson’s trichrome and Picrosirius Red stained sections were used to evaluate collagen synthesis and collagen type III/I radio in each group. To evaluate vascularization at 7 days after implantation, tissue sections were reacted with primary antibodies against CD31 (1:200, Abcam, USA), then further treated with the secondary antibodies Alexa Fluor 488 (green)- and CY3 (red)- conjugated (1:1000) for 2 h in darkness at room temperature. Finally, the nuclei were counterstained with DAPI (blue). Fluorescence photos of neovascularization were obtained and analyzed with a confocal microscope (Olympus FV10 inverted microscope, Nagano, Japan). To evaluate the immunoregulation, the samples were collected after 7 days. Primary antibodies of the pan macrophage marker CD68 (mouse anti-rat CD68, ab201340, Abcam), and the M2 macrophage marker CD206 (rabbit anti-rat CD206, ab64693, Abcam) were incubated with specimens at 4°C overnight. After washing with PBS, secondary antibodies (goat anti-mouse Alexa Fluor-594, ab150116, Abcam) and goat anti-rabbit Alexa Fluor-488 were added to the specimen for 1 h at 37°C. The nuclei of the cells were stained by DAPI. Fluorescence mounting medium (AR1109, Boster Bio, USA) for confocal microscopy imaging was applied to cover the slides. The cell number of the different macrophage phenotypes was calculated using NIH ImageJ software.
Modulation of MSC-laden SFN-LP hydrogel systems:
The content of microparticles and the amount of MSCs were regulated to optimize the niches for wound healing. First, different radios of LP microparticles (20%, 40%, 60%) were used to evaluate the efficiency of wound healing. The groups were termed LP20, LP40 and LP60, respectively. For LP20 (20%) group, 50 μL of microparticles were incubated with BMSCs in 24-well plate for 24 h (containing 1×106 cells per wound) and mixed with 200 μL of SFN hydrogel for injection. For the LP40 and LP60 groups, 100 μL and 160 μL of microparticles were mixed with 150 μL and 90 μL of SFN hydrogels, respectively, for the wound healing treatments. Then the ratio of SFN and LP of the hydrogel systems with the best wound healing capacity were fixed to further assess the influence of cell density. Different amounts of cells (3×105, 1×106, 3×106) were laden on the same radio of LP microparticles and blended with same amount of SFN hydrogel to treat the wounds. According to the cell density, the groups were termed L-BMSCs (3×105 cells), M-BMSCs (1×106 cells), and H-BMSCs (1×106 cells). The efficiency of wound healing was observed as described earlier.
Statistical Methods:
Comparisons between multiple groups were evaluated by One-way or Two-way ANOVA. Comparisons between two groups were evaluated by t-test. Values are presented as means ± standard deviations. A p-value of less than 0.05 was considered as significant. All the results were statistically analyzed by SPSS v.16.0 software (IBM SPSS Statistics, US).
3. Results and Discussion
3.1. Formation and characterization of injectable SFN-P hydrogel systems
Injectable MSCs-laden hydrogel systems are attractive for wound healing due to the advantage of filling irregular defects, but these systems usually fail to maintain preferable aligned structures to induce more selective cell responses. Inspired by microskin grafts, we developed these injectable composite hydrogel systems composed of SFN and SF particles with aligned cues. As shown in Scheme 1, the BMSCs were loaded on the SF particles with aligned structures and dispersed into the injectable SFN hydrogels where the particles could modulate cell behavior while the nanofiber hydrogel matrices endowed the system with injectability and homogeneous distributions of the MSCs.
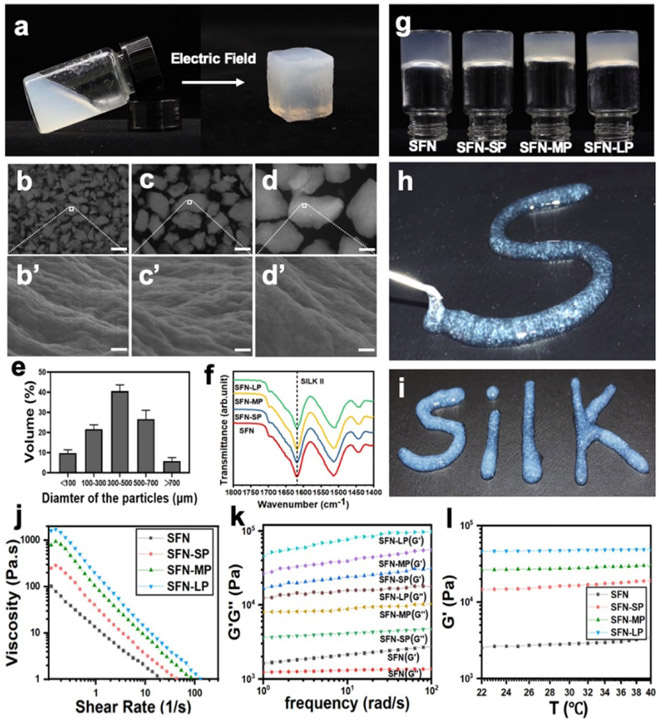
After the separation with molecular sieves, various particle groups with sizes of 100-300 μm, 300-500 μm and 500-700 μm were obtained and termed as SP (100-300 μm), MP (300-500 μm) and LP (500-700 μm), respectively (Figure 1a-d). FTIR spectra showed the same silk II (high beta-sheet) content in all the particles (Figure 1f), suggesting conformational stability during particle preparation. As expected, the particles retained their hierarchically aligned structures derived from bulk ASFN hydrogels (Figure 1b’-d’). The particles were added to the SFN hydrogels with stirring at 100 rpm to form homogeneous composites. The SFN hydrogels with concentration of 1 wt% were used here due to the suitable viscosity. All the SF particles with different sizes were dispersed homogeneously in the hydrogels without further particle disruption. Unlike the particles in ultrapure water that quickly precipitated within 1 hour, all the particles remained in the dispersed state in the viscous SFN hydrogels without any sedimentation after 3 weeks (Figure S1 a,b). These results indicated that the particles with aligned structures could maintain a uniform distribution in the injectable SFN hydrogels, which would facilitate the loading of MSCs. The introduction of the SF particles had no negative influence on the stability and injectability of these SFN hydrogels. All of the composite hydrogels remained in the solid state under gravitational conditions (Figure 1g). Although the viscosity increased when different sizes of SF particles were added in the SFN hydrogels, the composite hydrogels exhibited similar typical shear-thinning behavior, suggesting injectability (Figure 1j). Even the composite hydrogels containing largest particles (700 μm) could be easily pushed through the needles (14 G) by hand, and then maintained the gel state after the removal of force (Figure 1h,i). Similar to other particle-loaded SFN hydrogels, the modulus of the composite hydrogels increased and achieved the highest values for the LP-loaded hydrogels. The mechanical properties of all the hydrogels remained in the range of 103−105 Pa, which was suitable for skin tissue regeneration (Figure 1k). The modulus of the composite hydrogels also remained constant over the temperature range of 22-40°C, indicating stability at body temperature (Figure 1l). Therefore, the composite hydrogels were suitable injectable systems with aligned physical cues for the transplant of MSCs for the study of skin regeneration.
Figure 1.
Formation and characterization of the injectable SFN-P hydrogel systems: (a) Photographs of the hydrogels formed under the electric field, aqueous 2 wt% SFN hydrogel transformed to aligned SFN hydrogel. (b-d) microscopy images of SP, MP, and LP. Scale bars 200 μm; (b’-d’) SEM images of aligned topography in SP, MP, and LP. Scale bars 400 nm. (e) the size distribution of the particles after the blending (n = 6). After separation, SM, MP and LP were obtained. (f) FTIR analysis of SFN, SFN-SP, SFN-MP, and SFN-LP hydrogels. (g) Inversion test of different hydrogels for 24h. (h, i) Injectable ability of SFN-LP hydrogel through 14G syringe. (j) viscosity property of different hydrogels. (k) frequency sweep of different hydrogels. (l) Storage modulus (G’) changes following the increase of temperature of different hydrogels.
3.2. Optimization of BMSC-laden composite hydrogels
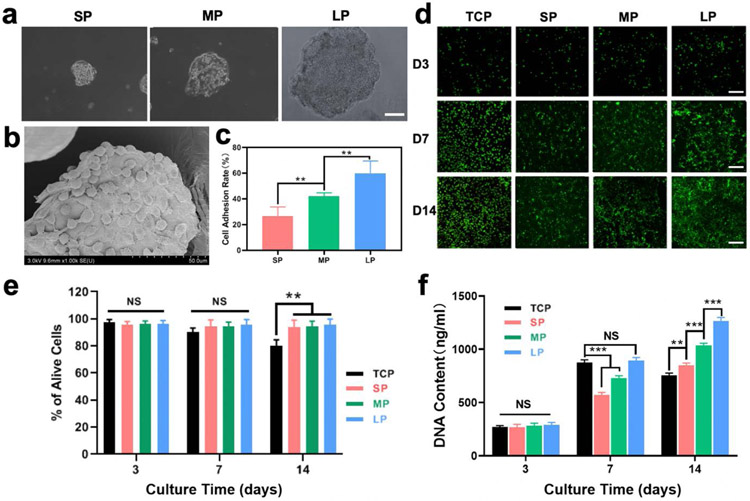
To determine the best MSCs-loaded hydrogel system, BMSCs were cultured on the SF particles (Figure S2a). After incubation on the particles for 24 h, BMSCs adhered uniformly and began to spread on the particles, suggesting strong cell-particle interactions (Figure 2a,b). The size of the particles had a significant impact on the adhesion, as only 30% of the cells adhered on the SP particles while this increased to 60% for the LP particles with sizes of 700 μm (Figure 2c). The cell-loaded particles (cell density 104 cells mL−1) were then cultured for 2 weeks to evaluate cell proliferation. Except for cells cultured on TCP, cells cultured on the different particles showed steady growth up to 14 days without cell death (Figure 2d). Viability remained above 98% for cells cultured on the different particles at days 3, 7 and 14, indicating cytocompatibility (Figure 2e). Although the cells exhibited similar viability on the different particles, better proliferation appeared on the LP particles (Figure 2f, Figure S3). Considering the improved cell adhesion and proliferation on the LP particles, composite hydrogels composed of SFN and LP particles (SFN-LP) were used to load BMSCs to study wound healing.
Figure 2.
In vitro biocompatibility of the BMSCs-laden microparticles: (a) the images of BMSCs-laden SP, MP, and LP particles after incubation for 24h. Scar bars represent 100μm; (b) Specific SEM image of BMSCs-laden particle; (c) Cell adhesion rate on different particles after incubation for 24h (n = 6); (d) CLSM images of BMSCs-laden microparticles when cultured for 3, 7, and 14 days. Live (green) cells are labeled with Calcein AM and dead (red) cells are labeled with ethidium homodimer. Scar bars represent 100μm; (e) Cell viability of different samples when cultured for 3, 7, and 14 days (n = 6); (f) Cell proliferation of different samples when cultured for 3, 7, and 14 days (n = 3). Data presented as mean ± SD, the error bars indicate the SD. P-values are calculated using one-way ANOVA with Sidak’s multiple comparison tests (c) and two-way ANOVA with Turkey’s multiple comparison tests (e,f), statistically significant ** P < 0.01, and *** P < 0.001. NS means no statistic difference.
3.3. Characterization of BMSC-loaded SFN-LP composite hydrogel systems
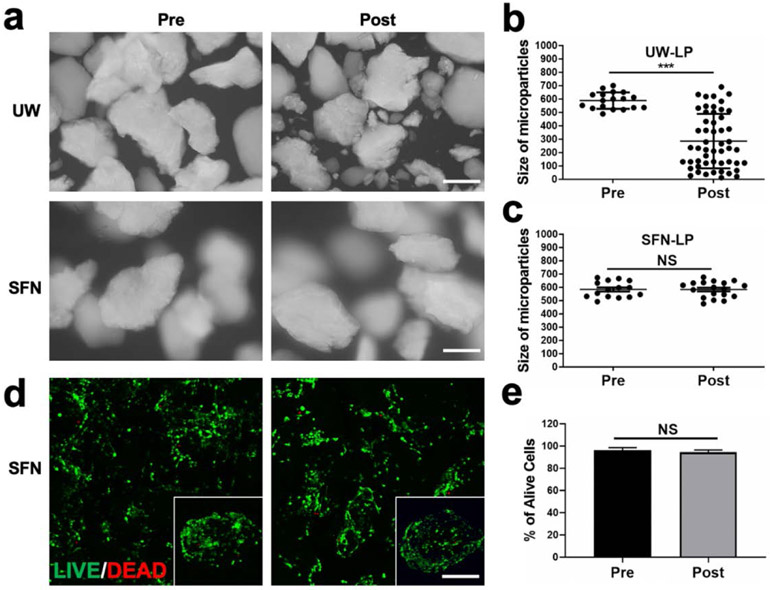
BMSCs have been injected directly into wound sites in clinical applications.[4b, 23] The shear force can often damages the cells.[24] Previous studies revealed that hydrogels with shear thinning properties can protect the cells from the damage during injection, improving the viability of MSCs at wound sites.[6,25] To evaluate the protective effect of the SFN hydrogels, the cell-free SFN-LP hydrogels were injected (14 G needles) and the size of the LPs were measured after injection. The LP particles, when dispersed in ultrapure water, changed to smaller particles after injection due to the shear force, while the LP particles in the composite hydrogels maintained the original size after the injection, indicating the protection of the SFN hydrogels for the particles (Figure 3a-c). Then the BMSC-loaded composite hydrogels were injected from the needles (14 G), and compared to the cells before the injection, few dead cells were found. This confirmed that the BMSC-loaded composite system avoided the negative influence of the injection (Figure 3d,e).
Figure 3.
Effect of SFN-LP hydrogel on cell viability after injection: (a) Microscopy images of LP particles in water and SFN hydrogels before and after injection; (b, c) Quantification of LP particle size distribution in water and SFN hydrogels before and after injection; (d) Live/Dead assay of BMSCs-laden LP particles in SFN hydrogels before and after injection; (e) Quantification analysis of cell viability from Live/Dead assay. Data presented as mean ± SD, n = 6, the error bars indicate the SD, P-values are calculated using t-test, statistically significant *** P < 0.001. NS means no statistic difference. All scale bars represent 100 μm.
Specific physical and chemical cues can modulate secretory functions of BMSCs and accelerate functional recovery of wounds.[4a, 5a, 15, 26] To evaluate the function of aligned physical cues of the LP particles, BMSCs were cultured on the LP particles for 7 days to measure the secretion of different cytokines, including stemness genes (Sox2, Oct4, and Klf4) and specific wound healing cytokines (ANGPT-1, VEGF-α, SDF-1, HGF, bFGF) where ANGPT-1, VEGF-α, SDF-1, HGF and bFGF were critical regulators in vascularization, cell recruitment, tissue ingrowth and immunomodulation, respectively. The upregulation of these cytokines suggested possible promotion of vascularization, immunomodulation and granulation ingrowth, which would accelerate wound healing. Since the unaligned SFN particles with similar size failed to be prepared through different methods, the cells cultured on TCP were used as control. Both Weston blot, ELISA and PCR results revealed that the LP particles effectively regulated the secretion of different cytokines. Compared to the cells cultured on TCP, the cells on the LP particles showed slightly higher expression of Sox2 and similar expression of Oct4 and Klf4. The results indicated that the LP particles had no negative influence on the stemness of the MSCs (Figure 4a,b). After culture for 7 days, although the secretion of bFGF was similar for the cells on TCP and LP particles, significantly higher secretion of ANGPT-1, VEGF-α, SDF-1 and HGF was achieved for the cells on the LP particles. For example, the secreted amount of ANGPT-1 on the LP particles was eight times higher than that on the TCP while the secretion of HGF increased five-fold (Figure 4c,d). Since ANGPT-1, VEGF-α, SDF-1 and HGF play roles in vascularization, cell recruitment, tissue ingrowth and immunomodulation,[4b, 23] the higher expression level of different cytokines suggested that the aligned SF particles could modulate the MSCs to generate niches for wound healing.
Figure 4.
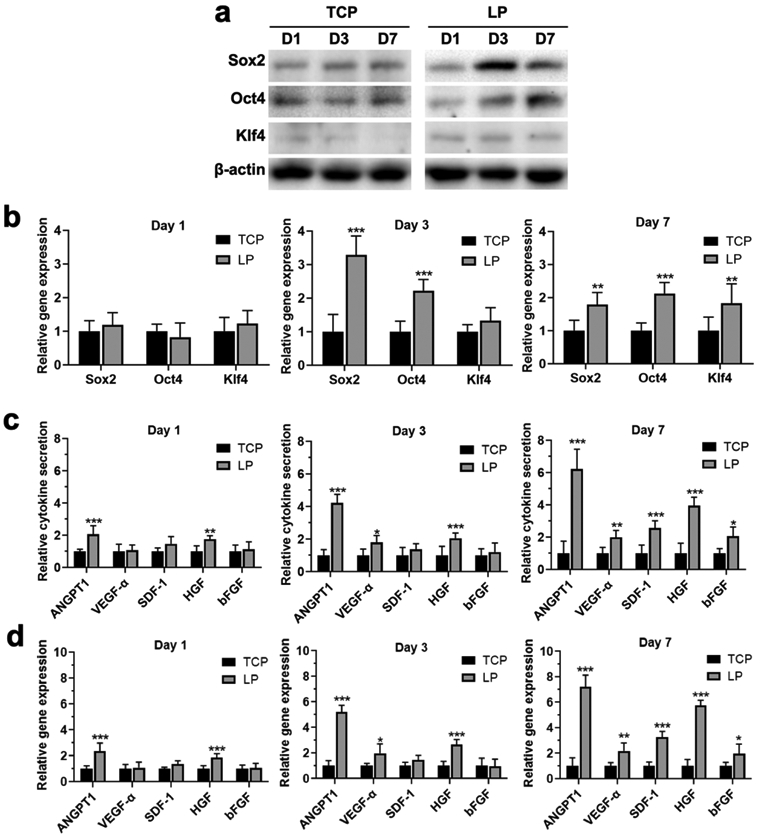
Stemness retention and cytokine secretion form BMSCs cultured on TCP and LP particles: (a) Western blotting analysis of stemness markers of Sox2, Oct4, and Klf4 from BMSCs when incubation on TCP and LP particles for 1, 3, and 7 days; (b) qPCR analysis of Sox2, Oct4, and Klf4 gene expression from BMSCs when incubation on TCP and LP particles for 1, 3, and 7 days; (c) ELISA analysis of the secretion of key cytokines from BMSCs when incubation on TCP and LP particles for 1, 3, and 7 days; (d) qPCR analysis of the secretion of key cytokine gene expression from BMSCs when incubation on TCP and LP particles for 1, 3, and 7 days. Data presented as mean ± SD, n = 3. Statistically significant *P < 0.05, ** P < 0.01, and *** P < 0.001.
Due to the undesirable cell loss caused by injection, rapid cell migration and apoptosis at wound sites, the therapeutic effects of stem cell therapy are usually lower than expected.[3a] To investigate whether the hydrogel systems in the present work would protect the implanted cells from death and migration in vivo, the BMSCs were transfected with Luc+/GFP+ (Figure S2b, c), loaded on the microparticles and then dispersed in SFN hydrogels. The cell loaded hydrogels were injected into the wounds and cell viability based on the bioluminescence was measured (Figure 5). As a control, BMSCs were dispersed in cell medium and in the injectable SFN hydrogels, respectively, to evaluate the function of SFN and LP particles. After the injections, more than 70% of the BMSCs in the medium disappeared at the wound site after 3 days and no BMSCs were found after 7 days. In contrast, the injectable SFN hydrogels protected and stabilized the BMSCs at the wound sites. After 5 days, more than 50% of the viable BMSCs remained in the wound area for the SFN and SFN-NP groups. Viable BMSCs remained visible after 10 days, suggesting the superiority of the injectable SFN hydrogels as MSC-laden systems. Although no statistical difference was found for the cells loaded in the SFN hydrogels or the SFN-LP hydrogels, a few viable cells remained after 2 weeks for the SFN-LP composite hydrogels, suggesting better protection than the SFN hydrogels. Considering the multiple advantages of the SFN-LP composite hydrogels, such as protection/stabilization of the BMSCs, and regulation of paracrine behavior at the wounds, the BMSC-laden composite hydrogels were the most suitable matrices to study scarless skin regeneration.
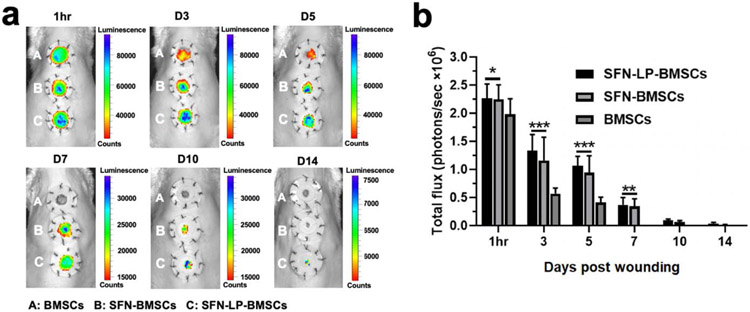
Figure 5.
In vivo cell retention of transplanted BMSCs-laden SFN-LP hydrogel system: (a) In vivo bioluminescence imaging of the Luc+/GFP+ BMSCs at wound area when free cells (A), cell-laden SFN hydrogels (B) and cell-laden SFN-LP hydrogels (C) were injected for different times.The inner diameter of silicone splints is 1.5 cm; (b) Quantification of total flux in the different groups. Hydrogels significantly improved the retention of BMSCs at wound sites. Data presented as mean ± SD, n = 6, the error bars indicate the SD. P-values are calculated using two-way ANOVA with Turkey’s multiple comparison tests, statistically significant *P < 0.05, *** P < 0.001. NS means no statistic difference.
3.4. Scarless wound healing with hair follicles
Cell-free SFN hydrogels as matrices have been used to accelerate wound healing.[12a] To reveal the synergistic action of SFN, LP and BMSCs, cell-free SFN and SFN-LP hydrogels, BMSCs in medium, BMSCs-laden SFN hydrogels and BMSCs-laden SFN-LP hydrogels were used to fill full-thickness wounds. Since all the wounds healed completely within 21 days (Figure 6a,b), the rats were reared for 21 days to evaluate the wound healing quality. Compared to the blank group, gradually faster healing was achieved following the introduction of the SFN hydrogels and BMSCs, where the healing days decreased from 21 to 18 days for the SFN, SFN-LP, and BMSCs groups, and then further decreased to 16 and 14 days for BMSC-laden SFN and BMSCs-laden SFN-LP groups, respectively (Figure 6c). Similar to previous studies[5c, 10], SFN hydrogels and BMSCs accelerated wound healing. The synergistic influence of SFN-based hydrogels and BMSCs provided better microenvironments, resulting in faster tissue regeneration. Although both BMSCs-laden SFN and SFN-LP hydrogels established cooperative cell-biomaterial niches for the wounds, and the best healing capacity was achieved in the BMSC-laden SFN-LP hydrogel group in which the wounds closed by 90% at day 10. The results make sense that the LPs with aligned structures stimulated the paracrine secretion of BMSCs and further regulated the microenvironment at wound sites. Since HE images of wounds are not reliable to compare the wound sizes in healing process, the migration distance of new epidermis was used to confirm different wound healing behavior. The best re-epithelialization was also achieved in the wounds treated with the BMSC-laden SFN-LP hydrogels in which new epidermis totally covered the wounds after 14 days (Figure 6d,e). The results confirmed that the cell-laden SFN-LP hydrogels provided the most wound healing friendly outcomes in the study.
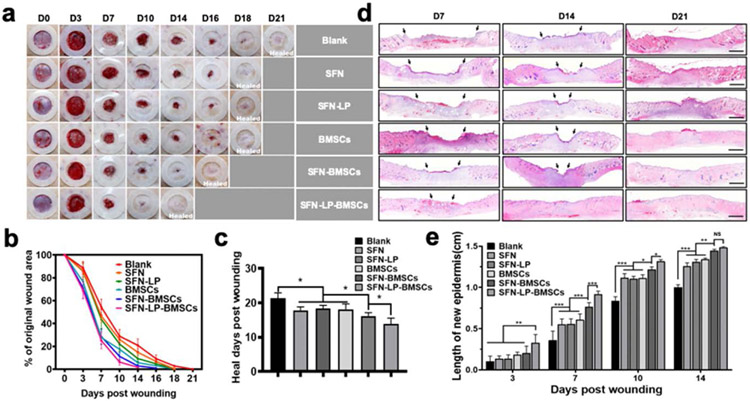
Figure 6.
Wound healing behaviors when treated with different groups: (a) Representative photo micrographs of wounds when treated with different groups for various days. The inner diameter of silicone splints is 1.5 cm; (b) Wound closure curves when treated with different groups; (c) Average healed time of the wounds treated with different groups; (d) Hematoxylin and Eosin (H&E) images of wounds when treated with different groups for 7, 14, and 21 days. Scale bars represent 2 mm; (e) The length of new epidermis of the wounds treated with different groups for 3, 7, 10, and 14 days. Data presented as mean ± SD, n = 6, the error bars indicate the SD, P-values are calculated using one-way ANOVA with Sidak’s multiple comparison tests (c) and two-way ANOVA with Turkey’s multiple comparison tests (e), statistically significant *P < 0.05, ** P < 0.01, and *** P < 0.001. NS means no statistic difference.
Bioactive biomaterials with physical/chemical cues are known to modulate paracrine secretion of BMSCs and regulate vascularization, immunoregulation and tissue remodeling.[25] Since the in vitro results revealed that LP particles could stimulate the secretion of multiple cytokines from the MSCs, we anticipated preferred cell behavior on the wounds treated with the BMSC-laden SFN-LP hydrogels, resulting in faster and better healing. Vascularization and immunoregulation are critical factors in regulating scarless tissue formation.[3a] After implantation of the hydrogels for 14 days, the neovascularization at the wound sites was analyzed with immunohistochemistry (Figure 7a,c,d). Neovessels appeared in the blank wounds due to inferior angiogenesis (Figure 7a a’, a’’). Both the introduction of SFN hydrogels and BMSCs facilitated more vessel formation since the SF nanofibers could induce the migration of endothelial cells while BMSCs could recruit the cells through paracrine effects (Figure 7a b’-d’, b’’-d’’).[7, 16b] Further improvement of angiogenesis was achieved in the wounds treated with the BMSC-laden SFN hydrogels due to the cooperative effect of SFN hydrogels and BMCs (Figure 7a e’, e’’). Finally, the LP particles with aligned structures actively regulated the secretion behavior of the BMCs, resulting in the most extensive vascularization in the wounds treated with the BMSC-laden SFN-LP hydrogels (Figure 7a f’, f’’). The phenotype switching of macrophages from M1 (proinflammatory state) to M2 (anti-inflammatory state) can regulate the differentiation of fibroblasts and reduce scar formation (Figure 7b,e,f).[27] Similar to the angiogenesis results, the highest ratio of M2/M1 macrophages was achieved in the wounds treated with the BMSC-laden SFN-LP hydrogels. Several recent studies indicated that biomaterials with suitable cues could control the paracrine of stem cells and then stimulate the secretion of different cytokines and growth factors, finally inducing the phenotype switching of macrophages.[5a, 6] Our recent results also revealed the upregulation of multiple cytokines in the medium cultured with embryonic stem cells, which resulted in more M2 phenotype when the macrophages were cultured in the medium in vitro (Data not shown). Therefore, it is reasonable to deduce that the paracrine effects of BMSCs on the aligned hydrogel microparticles promote the M1-M2 phenotype switching in vivo. As expected, the SFN-LP composite hydrogels provided preferable regulators to control paracrine signaling of the loaded MSCs, and further tuned the angiogenesis and immune behavior in the wounds, resulting in faster wound healing.
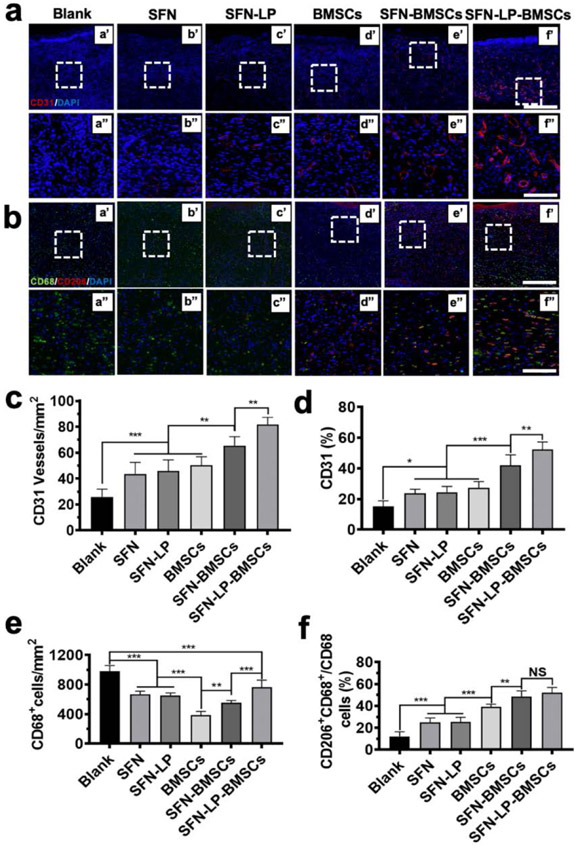
Figure 7.
Angiogenesis and immunoregulation at wound sites when treated with different groups for 14 days: (a) Representative fluorescent images of vascular endothelial cells at wounds treated with different groups. Endothelial cells were marked with CD31 (red) and DAPI (nuclei; blue) for difference treatment groups. Scale bars in a' -f' represent 400 μm, scale bars in a"-f" represent 100 μm; (b) Representative fluorescent images of macrophages at wounds treated with different groups. Macrophages were marked with pan-maker CD68 (green), M2 phenotype were marked with marker CD 206. The nuclei was marked with DAPI (blue). Scale bars in a' -f' represent 400 μm, scale bars in a"-f" represent 100 μm; (c) Blood vessel numbers per mm2 at wound sites treated with different group; (d) The percentage of CD31 vessel volume in fluorescent images; (e) CD68+ macrophages number per mm2 at wound sites treated with different group; (f) The percentage of M2 phenotype macrophages over total CD68+ macrophages at wound sites treated with difference treatment groups. Data presented as mean ± SD, n = 6, the error bars indicate the SD, P-values are calculated using one-way ANOVA with Sidak’s multiple comparison tests, statistically significant *P < 0.05, ** P < 0.01, and *** P < 0.001. NS means no statistic difference.
Phenotype switching of macrophages from M1 to M2 is a typical regulatory factor in determining scarless tissue formation.[5a] The ratio of type III versus type I collagen, a key characteristic of scarless wound healing, was used to evaluate the quality of the regenerated tissue.[28] Consistent with the M1-M2 phenotype switching behaviors, the introduction of MSCs significantly reduced scar tissue formation, superior to the cell-free SFN hydrogels (Figure 8a,b). Less scar tissue was formed in the wounds treated with the MSC-laden SFN hydrogels due to the synergistic effects of BMSC and SFN hydrogels. The LP particles inside the cell-laden hydrogels further modulated the BMSCs, resulting in scarless tissues without significant differences with normal dermis. To the best our knowledge, it is the first time scarless wound healing similar to normal dermis has been achieved using SF-based biomaterials. The paracrine secretion of BMSCs can also stimulate the regeneration of hair follicles, an outcome difficult for cell-free biomaterial systems to attain.[29] Although the cell-free SNF hydrogels and BMSCs in the medium exhibited similar promotion for wound healing, few hair follicles were regenerated in the wounds treated with the BMSCs or the cell-free hydrogels (Figure 8c, d). BMSCs loaded in the SFN hydrogels exhibited better stimulation, leading to more hair follicles in the healed wounds. Similarly, the SFN-LP composite hydrogels provided more active cues to modulate the BMSCs. The most extensive hair follicle regeneration was achieved in the BMSC-laden SFN-LP hydrogel groups. Therefore, using the microskin strategy, BMSC-laden injectable hydrogels with suitable physical cues to tune paracrine secretion and to modulate angiogenesis and immune reactions in the wounds, established a suitable niche to induce scarless tissue recovery with hair follicles.
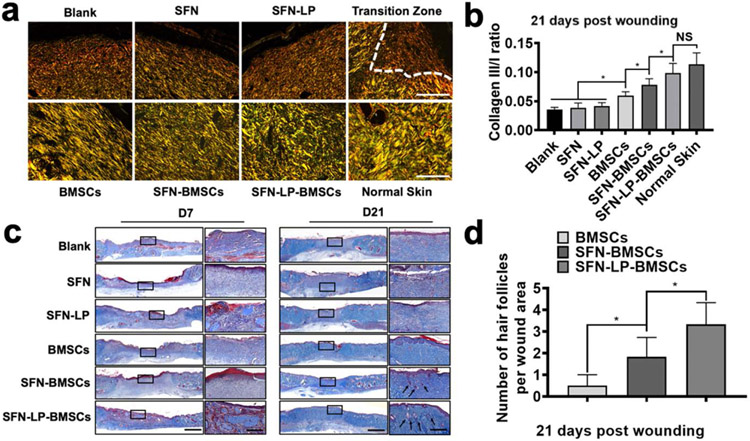
Figure 8.
Collagen deposition and hair follicle regeneration at wound sites treated with different groups for 21 days: (a) Picrosirius red staining of the wounds treated with difference groups The dotted line from left to right showed the transition of wounded skin from scar healing to normal tissue. Scale bars represent 100 μm; (b) The ratios of collagen III/I at wound sites treated with different groups for 21 days; (c) Masson’s trichrome staining of wound sites treated with different group for 7 and 21 days. Squares refered to the close-up areas. Original scale bars represent 2000 μm, and close-up scale bars represent 200 μm; (d) The number of hair follicles in the wound area on day 21. Data presented as mean ± SD, n = 6, the error bars indicate the SD, P-values are calculated using one-way ANOVA with Sidak’s multiple comparison tests, statistically significant *P < 0.05. NS means no statistic difference.
3.5. Modulation of BMSC-laden SFN-LP hydrogel systems
Unlike previous injectable BMSCs-laden systems,[7, 30] these new hydrogels were composed of two flexible SFN units, endowing the hydrogel system with tunability. The content of LP particles and the amount of BMSCs can be regulated to optimize the niche for wound healing. Different ratios of LP particles (20%, 40%, and 60%) were used to fabricate the cell-laden composite hydrogels. Although same quantity of BMSCs was loaded in the different composite hydrogels, different healing behavior appeared in the wounds, where the fastest healing was achieved in the wounds treated with the hydrogels with 40% of LP particles (Figure S4 a,b). Tissue staining also confirmed the most epidermis coverage and scarless tissue formation in the groups with 40% of LP particles (Figure S4 c-e). Different quantities of BMSCs (0.3×106, 1×106, and 3×106) were also loaded in the composite hydrogels with 40% LP particles. Similar healing rates and healing quality were obtained for the hydrogels with different numbers of BMSCs. The results suggested that wound healing was not determined by the loaded MSCs (Figure S5). As reported in previous studies, BMSC-modulated wound healing is mainly determined with paracrine signaling.[30-31] Further study is necessary to reveal whether similar paracrine secretion occurred for the different amounts of BMSCs loaded in the systems, or whether thresholds of bioactivity were reached. Although optimal BMSC-laden SFN-LP composite hydrogels need to be explored in future studies, the present results suggest a tunable platform to establish better niches for scarless, functional recovery of skin. The high tunability of the injectable systems also suggests the versatility. Through altering stem cell types, the concentration of SFN hydrogels as well as the ratio of stem cells, microparticles and SFN hydrogels, various injectable cell-laden systems would be developed to provide better niches for soft tissues such as nerve and muscles.
4. Conclusions
An injectable BMSC-laden silk nanofiber hydrogel system was developed to generate suitable microenvironments for wound healing. Aligned SFN particles modulated paracrine secretion of the stem cells while the SFN nanofiber hydrogels endowed the system with injectability and cell protection. Scarless healing with hair follicle recovery was achieved with the BMSC-laden hydrogels where the modulation of the BMSCs with the aligned SFN particles provided preferable angiogenesis and immune environments to facilitate skin regeneration. The hydrogel system also showed tunability to further improve the skin recovery quality. The in vitro and in vivo results suggested promising directions to pursue for these BMSCs-laden hydrogels in terms of potential clinical translation.
Supplementary Material
Acknowledgements
X. Zheng, Z. Ding and W. Cheng have the same contribution to the work. The authors thank the National Key R&D Program of China (2016YFE0204400), National Nature Science Foundation of China (81171712) and the NIH (R01NS094218, R01AR070975). We also thank the Social Development Program of Jiangsu Province (BE2018626, BE2019662), the Fundamental Research Funds for the Central Universities (JUSRP52014B) and The Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2020RC144) for support of this work.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Xin Zheng, Department of Orthopedics, The Second Affiliated Hospital of Soochow University, Suzhou 215000, P. R. China; Department of Orthopedics, Taizhou Municipal Hospital, Taizhou 318000, P. R. China.
Zhaozhao Ding, Department of Burns and Plastic Surgery, The Affiliated Hospital of Jiangnan University, Wuxi 214041, P. R. China; Engineering Research Center of the Ministry of Education for Wound Repair Technology, Jiangnan University, Wuxi 214041, P. R. China.
Weinan Cheng, Department of Orthopedics, The First Affiliated Hospital of Xiamen University, Xiamen 361000, P. R. China.
Qiang Lu, Department of Orthopedics, The Second Affiliated Hospital of Soochow University, Suzhou 215000, P. R. China; Department of Burns and Plastic Surgery, The Affiliated Hospital of Jiangnan University, Wuxi 214041, P. R. China; Engineering Research Center of the Ministry of Education for Wound Repair Technology, Jiangnan University, Wuxi 214041, P. R. China; National Engineering Laboratory for Modern Silk & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou 215123, P. R. China
Xiangdong Kong, Zhejiang-Mauritius Joint Research Center for Biomaterials and Tissue Engineering, School of Materials Science and Engineering, Zhejiang Sci-Tech University, Hangzhou 310018, P. R. China.
Xiaozhong Zhou, Department of Orthopedics, The Second Affiliated Hospital of Soochow University, Suzhou 215000, P. R. China
Guozhong Lu, Department of Burns and Plastic Surgery, The Affiliated Hospital of Jiangnan University, Wuxi 214041, P. R. China; Engineering Research Center of the Ministry of Education for Wound Repair Technology, Jiangnan University, Wuxi 214041, P. R. China
David L Kaplan, Department of Biomedical Engineering, Tufts University, Medford, Massachusetts 02155, United States
References
- [1].Kim BS, Kwon YW, Kong JS, Park GT, Gao G, Han W, Kim MB, Lee H, Kim JH, Cho DW, Biomaterials 2018, 168, 38. [DOI] [PubMed] [Google Scholar]
- [2].Rodrigues M, Kosaric N, Bonham CA, Gurtner GC, Physiol. Rev. 2019, 99, 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].a) Chouhan D, Dey N, Bhardwaj N, Mandal BB, Biomaterials 2019, 216, 119267; [DOI] [PubMed] [Google Scholar]; b) Salamone JC, Salamone AB, Swindle-Reilly K, Leung KX, McMahon RE, Regen. Biomater 2016, 3, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Gur-Cohen S, Yang H, Baksh SC, Miao Y, Levorse J, Kataru RP, Liu X, de la Cruz-Racelis J, Mehrara BJ, Fuchs E, Science 2019, 366, 1218; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dehkordi A. Nourian, Babaheydari F. Mirahmadi, Chehelgerdi M, Dehkordi S. Raeisi, Stem Cell. Res. Ther 2019, 10, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Li T, Ma H, Ma H, Ma Z, Qiang L, Yang Z, Yang X, Zhou X, Dai K, Wang J, ACS Appl. Mater. Interfaces 2019, 11, 17134; [DOI] [PubMed] [Google Scholar]; b) Huang R, Wang J, Chen H, Shi X, Wang X, Zhu Y, Tan Z, Biomater. Sci 2019, 24, 4248; [DOI] [PubMed] [Google Scholar]; c) Hu MS, Borrelli MR, Lorenz HP, Longaker MT, Wan DC, Stem Cells. Int 2018, 2018, 6901983; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Henriques-Antunes H, Cardoso RMS, Zonari A, Correia J, Leal EC, Jiménez-Balsa A, Lino MM, Barradas A, Kostic I, Gomes C, Karp JM, Carvalho E, Ferreira L, ACS Nano 2019, 13, 8694. [DOI] [PubMed] [Google Scholar]
- [6].Mitrousis N, Fokina A, Shoichet MS, Nature Rev. Mat 2018, 3, 441. [Google Scholar]
- [7].Yang Z, He C, He J, Chu J, Liu H, Deng X, Stem Cell. Res. Ther 2018, 9, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yuru S, Dawei L, Chuanan S, Kai Y, Li M, Longzhu L, Dongxu Z, Wenfeng C, J. Burn. Care. Res 2017, 38, 348. [DOI] [PubMed] [Google Scholar]
- [9].a) Zhao X, Liu S, Yildirimer L, Zhao H, Ding R, Wang H, Cui W, Weitz D, Adv. Funct. Mater 2016, 26, 2809; [Google Scholar]; b) Wang Z, Wu D, Zou J, Zhou Q, Liu W, Zhang W, Zhou G, Wang X, Pei G, Cao Y, Zhang ZY, J. Mat. Chem. B 2017, 5, 62. [DOI] [PubMed] [Google Scholar]
- [10].Farokhi M, Mottaghitalab F, Fatahi Y, Khademhosseini A, Kaplan DL, Trends Biotechnol. 2018, 36, 907. [DOI] [PubMed] [Google Scholar]
- [11].a) Wang J, Chen Y, Zhou G, Chen Y, Mao C, Yang MY, ACS Appl. Mater. Interfaces 2019, 11, 34736; [DOI] [PubMed] [Google Scholar]; b) Millan-Rivero JE, Martinez CM, Romecin PA, Aznar-Cervantes SD, Carpes-Ruiz M, Cenis JL, Moraleda JM, Atucha NM, Garcia-Bernal D, Stem Cell Res. Ther 2019, 10, 126; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Huang T, Wang G, Tseng C, Su W, Mater. Sci. Eng. C 2019, 104, 109986. [DOI] [PubMed] [Google Scholar]
- [12].a) Ding Z, Zhou M, Zhou Z, Zhang W, Jiang X, Lu X, Zuo B, Lu Q, Kaplan DL, ACS Biomater. Sci. Eng 2019, 5, 4077; [DOI] [PubMed] [Google Scholar]; b) Lu G, Ding Z, Wei Y, Lu X, Lu Q, Kaplan DL, ACS Appl. Mater. Interfaces 2018, 10, 44314. [DOI] [PubMed] [Google Scholar]
- [13].Lu Q, Bai S, Ding Z, Guo H, Shao Z, Zhu H, Kaplan DL, Adv. Mater. Interfaces 2016, 31500687. [Google Scholar]
- [14].a) Sleep E, Cosgrove BD, McClendon MT, Preslar AT, Chen CH, Sangji MH, Perez CMR, Meade TJ, Blau HM, Stupp SI, Proc. Natl. Acad. Sci. U. S. A 2017, 114, e7919; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang LL, Lu GZ, Lu Q, Kaplan DL, ACS Biomater. Sci. Eng 2018, 4, 933. [DOI] [PubMed] [Google Scholar]
- [15].Lu G, Ding Z, Wei Y, Lu X, Lu Q, Kaplan DL, ACS Appl. Mater. Interfaces 2018, 10, 44314. [DOI] [PubMed] [Google Scholar]
- [16].a) Ding Z, Han H, Fan Z, Lu H, Sang Y, Yao Y, Cheng Q, Lu Q, Kaplan DL, ACS Appl. Mater. Interfaces 2017, 9, 16913; [DOI] [PubMed] [Google Scholar]; b) Lu X, Ding Z, Xu F, Lu Q, Kaplan DL, ACS Appl. Bio. Mater 2019, 2, 3108. [DOI] [PubMed] [Google Scholar]
- [17].Lu Q, Zhu H, Zhang C, Zhang F, Zhang B, Kaplan DL, Biomacromolecules 2012, 13, 826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dong X, Zhao Q, Xiao L, Lu Q, Kaplan DL, Biomacromolecules 2016, 17, 3000. [DOI] [PubMed] [Google Scholar]
- [19].Arun Kumar R, Sivashanmugam A, Deepthi S, Iseki S, Chennazhi KP, Nair SV, Jayakumar R, ACS Appl. Mater. Interfaces 2015, 7, 9399. [DOI] [PubMed] [Google Scholar]
- [20].Liang Y, Li M, Lu T, Peng W, Wu JH, Biomed. Res. Int 2017, 2017, 4210867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kankala RK, Zhao J, Liu CG, Song XJ, Yang DY, Zhu K, Wang SB, Zhang YS, Chen AZ, Small 2019, 15, e1901397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dong Y, Rodrigues M, Kwon SH, Li X, S. A, Brett EA, Elvassore N, Wang W, Gurtner GC, Adv. Healthc. Mater 2018, 7, e1800432. [DOI] [PubMed] [Google Scholar]
- [23].Liu L, Chiu PW, Lam PK, Poon CC, Lam CC, Ng EK, Lai PB, Br. J. Surg 2015, 102, e158. [DOI] [PubMed] [Google Scholar]
- [24].Dong Y, S. A, Rodrigues M, Li X, Kwon SH, Kosaric N, Khong S, Gao Y, Wang W, Gurtner GC, Adv. Funct. Mater 2017, 27, 1606619. [Google Scholar]
- [25].Amer MH, White LJ, Shakesheff KM, J. Pharm. Pharmacol 2015, 67, 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Su N, Gao PL, Wang K, Wang JY, Zhong Y, Luo Y, Biomaterials 2017, 141, 74. [DOI] [PubMed] [Google Scholar]
- [27].Chu SY, Chou CH, Huang HD, Yen MH, Hong HC, Chao PH, Wang YH, Chen PY, Nian SX, Chen YR, Liou LY, Liu YC, Chen HM, Lin FM, Chang YT, Chen CC, Lee OK, Nat. Commun 2019, 10, 1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lim CH, Sun Q, Ratti K, Lee SH, Zheng Y, Takeo M, Lee W, Rabbani P, Plikus MV, Cain JE, Wang DH, Watkins DN, Millar S, Taketo MM, Myung P, Cotsarelis G, Ito M, Nat. Commun 2018, 9, 4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen S, Shi J, Zhang M, Chen Y, Wang X, Zhang L, Tian Z, Yan Y, Li Q, Zhong W, Xing M, Zhang L, Zhang L, Sci. Rep 2015, 5, 18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lei Z, Singh G, Min Z, Shixuan C, Xu K, Pengcheng X, Xueer W, Yinghua C, Lu Z, Lin Z, Mater Sci. Eng. C. Mater. Biol. Appl 2018, 90, 159. [DOI] [PubMed] [Google Scholar]
- [31].Chew JRJ, Chuah SJ, Teo KYW, Zhang S, Lai RC, Fu JH, Lim LP, Lim SK, Toh WS, Acta Biomater 2019, 89, 252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.