Abstract
Purpose:
Patients with triple-negative breast cancer experience high rates of recurrence after radiation, which may be facilitated by the recruitment of circulating tumor cells to proinflammatory microenvironments in the absence of lymphocytes. We hypothesized that patients with lymphopenia and elevated inflammatory hematologic markers after radiation therapy would have an increased risk of locoregional failure.
Methods and Materials:
With approval, we retrospectively studied a cohort of women treated with adjuvant radiation therapy for stage II-III triple-negative breast cancer. We analyzed the relationship between post—radiation therapy neutrophil:lymphocyte ratio (NLR) and locoregional recurrence by using Cox regression.
Results:
One-hundred thirty patients met inclusion criteria, and median follow-up time was 7.6 years. Patients with an NLR ≥3 had a higher rate of locoregional failure (P = .04) and lower overall survival (P = .04). After adjusting for stage (hazard ratio [HR], 5.5; P < .0001) and neoadjuvant chemotherapy (HR, 2.5; P = .0162), NLR was highly predictive of locoregional failure (HR, 1.4; P = .0009). NLR was also highly predictive of overall survival (HR, 1.3; P = .0007) after adjustment for stage and neoadjuvant chemotherapy.
Conclusions:
Innate peripheral inflammation after radiation therapy for triple-negative breast cancer in an immunocompromised setting may be a novel prognostic biomarker for locoregional recurrence, progression, and survival. This finding supports preclinical studies of post—radiation therapy inflammation-mediated tumor progression. Further studies are needed to confirm this finding and develop treatment strategies.
Summary
Preclinical data suggest that triple-negative breast cancer may recur in irradiated tissue as a result of stromal-based attraction of circulating tumor cells into a proinflammatory, lymphocyte-depleted microenvironment. We hypothesized that patients with triple-negative breast cancer are at risk for recurrence based on the inflammatory response to radiation. We studied 130 patients with stage II-III triple-negative breast cancer. We found that elevated neutrophil:lymphocyte ratio after radiation is an independent predictor of locoregional relapse, progression-free survival, and overall survival.
Introduction
Radiation therapy (RT) invokes an immunogenic process by which DNA damage in the tumor cell facilitates the presentation of tumor neoantigens to infiltrating effector lymphocytes for immunorecognition of tumor cells and tumor cell destruction.1 While lymphocytes contribute to the tumor-killing mechanism of RT, lymphocytes are highly radiosensitive.2 Even in the absence of cytotoxic chemotherapy, lymphopenia is common after RT and has been associated with recurrence and mortality in several solid tumors.3 In breast cancer, however, very few studies have examined the relationship between post-RT lymphopenia and outcome. In triple-negative breast cancer (TNBC), lymphocyte-mediated tumor cell death after RT may be even more crucial than in other subtypes with lower mutational burden.4-7 Because mutational burden is thought to correlate with neoantigen load, the probability of TNBC eradication by immune-mediated tumor cell killing may be influenced significantly by lymphocyte activity, viability, and abundance.4,5,8 Thus, in addition to the poor prognosis of TNBC, a substantial impetus exists to correct the dearth of evidence regarding post-RT lymphopenia and TNBC outcomes.9
Recently, a clinical correlation was reported between postdiagnosis lymphopenia and prognosis.10 More recently, a mouse model of lymphopenia was used to show that irradiation of normal breast tissue leads to the recruitment of macrophages through stromal chemokine secretion.11 In this model, these infiltrating macrophages were shown to secrete additional chemokines that attracted circulating TNBC cells, which subsequently invaded the irradiated stroma.11 This finding suggested that TNBC may recur through the creation of irradiated tumor niches by a proinflammatory, lymphocyte-depleted microenvironment.
Although pretreatment inflammation, commonly measured as neutrophil:lymphocyte ratio (NLR), has been correlated with prognosis in breast cancer, very few groups have reported on the prognostic implications of dynamic inflammatory changes after RT.12-16 Furthermore, the relationships between treatment, such as RT, and systemic inflammatory dynamics remain poorly understood; how these changes alter prognosis is also unknown.
To explore the preclinical hypothesis that an irradiated, proinflammatory, and immunocompromised microenvironment leads to TNBC recurrence, we examined the relationship between patient outcomes and the systemic inflammatory response to RT as a surrogate for the microenvironment. Thus, the purpose of our study was to examine the relationship between RT and hematologic markers of inflammation and immunity and to investigate whether time-varying markers of inflammation and immunity after RT were predictive of patient outcomes.
Methods and Materials
Patient population
After obtaining institutional review board approval from each institution, we performed a multi-institutional retrospective cohort study of patients seen between 1999 and 2012 with stage II-III TNBC who were treated with definitive RT. Electronic medical records were compiled by the Vanderbilt Research Derivative and the Stanford Cancer Institute Research Database.17 The Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) criteria were used to guide the design and reporting of this study.
Inclusion and exclusion criteria
Female patients who were at least 18 years of age with histologically confirmed, invasive, stage II-III TNBC treated with definitive external beam RT were included. Chemotherapy, RT, and surgery were chosen in a non-randomized manner according to standard of care practice with multidisciplinary tumor board input. TNBC status was defined as <1% immunohistochemical staining for estrogen receptor and progesterone receptor and no amplification or overexpression of human epidermal growth factor receptor 2. Patients with either neoadjuvant or adjuvant chemotherapy, or both, were included. Patients were excluded if they had hematopoietic or bone marrow infiltration disorders. Patients with a history of malignancy were eligible provided there was no evidence of disease at the time of TNBC diagnosis and treatment. Patients were followed for a minimum of 5 years from the end of RT. Patients were required to have at least 1 peripheral complete blood count (CBC) with differential during RT or within 1 year after RT completion based on preclinical evidence of the importance of prolonged inflammation and immunosuppression after RT for TNBC.11 Peripheral blood was obtained, preserved, stored, and analyzed by Clinical Laboratory Improvements Amendments (CLIA) certified laboratories, including at Vanderbilt University Medical Center and Stanford University Medical Center, according to standard protocols using automated flow cytometry. Between 0.5 and 2 mL of whole blood were collected in ethylenediaminetetraacetic acid—containing tubes. All assays were blinded to the study endpoint and obtained outside the context of the study as part of standard of care.
Study predictors
Data were abstracted from the medical record until April 1, 2019. The highest combined histologic grade was recorded. Pretreatment clinical stage was used for patients who underwent neoadjuvant chemotherapy, and the remaining patients were pathologically staged at the time of surgery. Staging was based on the American Joint Committee on Cancer Staging Manual, eightth edition. The presence or absence of lymphovascular invasion (LVI) was recorded at the time of surgery.
Because prior studies suggested prognostic significance of prolonged lymphopenia after RT, every CBC available from the start of RT through 1 year after RT was included in the data to investigate the effects of the pattern of markers over time on prognosis.3,11 From each CBC, platelet count, absolute neutrophil count, absolute lymphocyte count (ALC), and absolute monocyte count were recorded. NLR, platelet: lymphocyte ratio (PLR), and monocyte:lymphocyte ratio (MLR) were then calculated for each CBC. The mean of each hematologic parameter was obtained for each patient.
Outcome measures
The primary study outcome was time to locoregional failure (LRF). Secondary study outcomes were time to progression-free survival (PFS) and time to overall survival (OS). LRF was defined as the time from start of RT until any radiologic or pathologic evidence of disease in the ipsilateral breast/chest wall or ipsilateral regional nodes, with death being considered a competing event for LRF. Distant failure was not considered a competing event for LRF. Time to PFS was defined as time from start of RT until clinically or radiologically suspected local, regional, or distant failure or death, whichever came first. Patients who did not experience any local, regional, or distant failures or death were censored at the date of last follow-up. Time to OS was defined as the time from the start of RT until death from any cause. The sample size was determined by including all patients meeting inclusion and exclusion criteria to maximize statistical power in the multivariable analysis.
Statistical analysis
The time-to-event outcome of LRF was analyzed using competing events analysis, with death as the competing event. The time-to-event outcomes of PFS and OS were analyzed using Kaplan-Meier curves, and medians with 95% confidence interval were calculated using Greenwood’s formula. In these analyses the mean laboratory values per patient were used, and these predictors were dichotomized based on the median. Single-predictor Cox models with time-varying laboratory values were also evaluated. Multivariable Cox models with parsimonious predictor selection were performed to test the hypothesis that time-varying laboratory values contributed meaningful prognostic information for salient clinical predictors in the context of other known prognostic factors. The most optimal models were statistically selected by the score selection method using the branch-and-bound algorithm of Furnival and Wilson; in this approach, the highest likelihood score (χ2) for all possible models was used to build unbiased models so that only variables that contributed significant and unique prognostic information were included.18 For sufficient power and unbiased estimates, each model was required to have at least 10 events per predictor included. Time-to-event outcomes were constrained from the time of first laboratory measurement to the time of event or censor. All tests were 2-sided with an alpha level of 0.05. All analyses were performed using SAS v9.4 (SAS Institute Inc, Cary, NC) and all plots were generated using Prism v8.1 (GraphPad Software, La Jolla, CA).
Results
Patient characteristics
A total of 130 patients were enrolled (Table 1), including 53 patients treated at Stanford University Medical Center and 77 patients treated at Vanderbilt University Medical Center. Most patients had stage II disease (67%). Almost all patients received chemotherapy (97%), including neoadjuvant chemotherapy in 51% of patients and adjuvant chemotherapy in 72% of patients. After neoadjuvant chemotherapy, 17 (35%) patients had a complete pathologic response at the time of surgery. Most chemotherapy regimens included a combination of anthracyclines, taxanes, and cyclophosphamide. The most common radiation dose to the tumor bed was 60.4 Gy (38%) delivered over 33 fractions, including boost to the surgical scar, and 73% of patients received regional nodal irradiation. Each patient had a median of 4 laboratory measurements in the year after RT (range, 2-40). From the start of RT to 2 months after the conclusion of RT, 112 patients (86%) had laboratory measurements; from 2 months post-RT to 6 months post-RT, 84 patients (64%) had laboratory measurements; and from 6 months post-RT to 12 months post-RT, 101 patients (78%) had laboratory measurements. The overall median ALC was 1.08 K/μL (interquartile range [IQR], 0.79-1.40), the median NLR was 2.99 (IQR, 2.14-4.31), the median PLR was 216 (IQR, 159-286), and the median MLR was 0.40 (IQR, 0.28-0.61). There was no single predictor that correlated with mean laboratory values and clinicopathologic factors, including age, radiation dose, radiation fractions, neoadjuvant chemotherapy, adjuvant chemotherapy, stage, surgery, menopausal status, or nodal irradiation.
Table 1.
Clinicopathologic characteristics
| Parameter | All patients |
|---|---|
| (n = 130) | |
| Age, mean (95% CI), y | 50 (48-52) |
| Race, n (%) | |
| NH white | 82 (63) |
| NH black | 25 (19) |
| NH Asian | 16 (12) |
| Other | 7 (5) |
| Menopausal status, n (%) | |
| Premenopausal | 62 (49) |
| Postmenopausal | 65 (51) |
| Histopathology | |
| Invasive mammary carcinoma, no special type | 120 (94) |
| Combined histologic grade, n (%) | |
| Low | 1 (1) |
| Intermediate | 23 (18) |
| High | 101 (81) |
| Stage, n (%) | |
| II | 87 (67) |
| III | 43 (33) |
| Surgery, n (%) | |
| Lumpectomy | 76 (59) |
| Mastectomy | 53 (41) |
| Lymphovascular invasion at surgery, n (%) | 36 (50) |
| Any form of systemic therapy, n (%) | 126 (97) |
| Neoadjuvant chemotherapy, n (%) | 68 (51) |
| Adjuvant chemotherapy, n (%) | 93 (72) |
| Both neoadjuvant and adjuvant chemotherapy, n (%) | 33 (25) |
| RT target region, n (%) | |
| Whole breast or chest wall | 33 (27) |
| With nodal coverage | 89 (73) |
| RT tumor bed dose, median (IQR), Gy | 60.4 (60.0-60.8) |
Abbreviations: CI = confidence interval; IQR = interquartile range; NH = non-Hispanic; RT = radiation therapy.
At a median follow-up time of 7.6 years (IQR, 0.5-19.3), a total of 34 (26%) deaths were observed during the study period, 31 of which were attributable to TNBC. Relapse occurred in 49 patients (38%). Isolated locoregional recurrence occurred in 9 (7%) patients, and 23 (18%) patients developed both locoregional and distant metastatic disease. The median time to any recurrence was 1.2 years (range, 0.25-10.25) after RT. Mortality occurred at a median time of 2 years after RT (range, 0.6-14). At 5 years after RT, OS was 78%, PFS was 67%, and LRF was 21%.
In single-predictor analysis, the following predictors were significantly correlated with LRF: neoadjuvant chemotherapy (hazard ratio [HR], 2.7; P = .009), adjuvant chemotherapy (HR, 0.2; P < .0001), LVI (HR, 2.4; P = .01), and stage (HR, 5.1; P < .0001) (see Table E1, available online at https://doi.org/10.1016/j.ijrobp.2019.11.398). Neoadjuvant chemotherapy (HR, 2.1; P = .01), adjuvant chemotherapy (HR, 0.37; P = .001), LVI (HR, 2.3; P = .003), stage (HR, 4.6; P < .0001), mastectomy versus lumpectomy (HR, 2.2; P = .006), and pathologic complete response (HR, 0.26; P = .03) were correlated with PFS. OS was correlated with neoadjuvant chemotherapy (HR, 2.5; P = .01), adjuvant chemotherapy (HR, 0.43; P = .02), stage (HR, 4.0; P < .0001), mastectomy versus lumpectomy (HR, 2.6; P = .006), and pathologic complete response (HR, 0.21; P = .03). With the exception of mastectomy versus lumpectomy and pathologic complete response, all of these predictors were significantly correlated with all three time-to-event outcomes. LRF was not significantly correlated with mastectomy versus lumpectomy (HR, 1.6; P = .2) or pathologic complete response (HR, 0.28; P = .09).
Single-predictor biomarker prognostic analysis
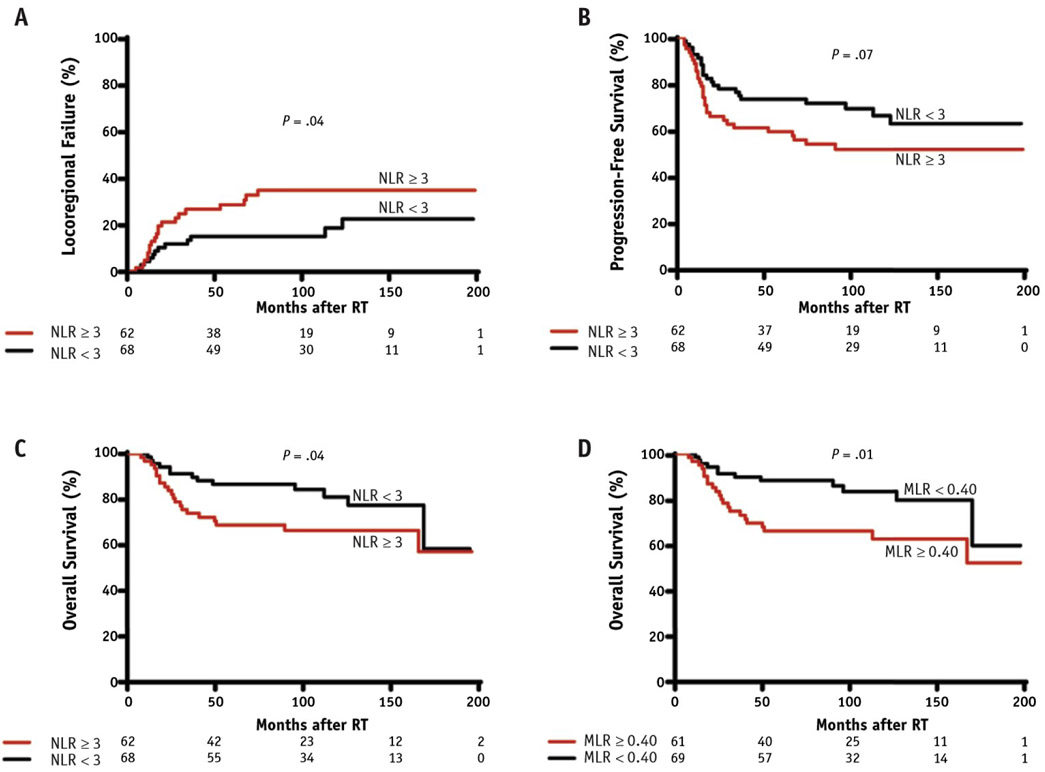
The relationships between postradiation systemic markers of inflammation and immunity and LRF, PFS, and OS are reported in Table 2. Elevated NLR as a continuous variable after RT was predictive of LRF (HR, 1.37; P = .0029), PFS (HR, 1.32; P = .0012), and OS (HR, 1.21; P = .0054). These results were consistent with the Kaplan-Meier analysis, which showed that a mean NLR ≥3 after RT was predictive of a higher cumulative incidence of LRF (P = .04) and lower OS (P = .04) (Fig. 1A-C). In the single-predictor Cox model, continuous MLR was significantly correlated with OS (HR, 3.8; P = .0196) but not with LRF (HR, 2.8; P = .0872) or PFS (HR, 2.1; P = .4130). In the Kaplan-Meier analysis, a mean MLR value ≥0.40 was correlated with lower survival (P = .01) (Fig. 1D). However, this mean MLR threshold was not significant for LRF (P = .3589) or PFS (P = .0913). Patients with higher continuous lymphocyte counts after RT had a lower cumulative incidence of LRF (HR, 0.40; P = .0189) and a higher rate of PFS (HR, 0.52; P = .0280). However, ALC was not associated with OS (HR, 0.53; P = .0734). The Kaplan-Meier analysis did not show any mean ALC threshold predictive of any study outcome.
Table 2.
Single-predictor time-dependent competing risk analysis for locoregional failure and time-dependent Cox proportional hazards regression for progression-free survival and overall survival
| Predictor | LRF |
PFS |
OS |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| ALC | 0.40* | 0.18-0.86 | .02 | 0.52* | 0.29-0.93 | .03 | 0.53 | 0.26-1.1 | .07 |
| PLR | 1.0 | 1.0-1.0 | .1 | 1.0 | 0.99-1.0 | .4 | 1.0 | 0.99-1.0 | .4 |
| MLR | 2.8 | 0.86-9.1 | .09 | 2.1 | 0.75-5.9 | .2 | 3.8* | 1.2-11 | .02 |
| NLR | 1.4* | 1.1-1.7 | .003 | 1.3* | 1.1-1.6 | .001 | 1.2* | 1.1-1.4 | .005 |
Abbreviations: ALC = absolute lymphocyte count; CI = confidence interval; HR = hazard ratio; LRF = locoregional failure; MLR = monocyte: lymphocyte ratio; NLR = neutrophil:lymphocyte ratio; OS = overall survival; PFS = progression-free survival; PLR = platelet:lymphocyte ratio.
All time-dependent predictors were evaluated as continuous variables.
Statistically significant hazard ratios.
Fig. 1.
High neutrophil:lymphocyte ratio (NLR) after radiation predicts poor outcome in triple-negative breast cancer. At-risk subjects are indicated along the x-axis. (A) Competing risk analysis of locoregional failure (LRF) comparing patients with NLR <3 and NLR ≥3. Kaplan-Meier curves for the outcome of (B) progression-free survival (PFS) comparing patients with NLR <3 and NLR ≥3, (C) overall survival (OS) comparing patients with NLR <3 and NLR ≥3, and (D) OS comparing patients with monocyte:lymphocyte ratio (MLR) <0.40 and MLR ≥0.40.
Multivariable Cox analysis
The following predictors were submitted for evaluation for inclusion in each multivariable model: each time-varying laboratory measurement, age, body mass index, clinical T stage, clinical N stage, overall stage, receipt of neoadjuvant chemotherapy, pathologic complete response, histologic grade, mastectomy versus lumpectomy, lymphovascular invasion, and menopausal status. For each study outcome, the following predictors were consistently retained by multivariable optimized predictor selection: NLR, stage, adjuvant chemotherapy, neoadjuvant chemotherapy, and mastectomy versus lumpectomy. Only the PFS model had sufficient statistical power to include 5 predictors in a single model. In this 5-predictor model, stage (HR, 3.8; P < .0001), NLR (HR, 1.3; P = .004), and adjuvant chemotherapy (HR, 0.41; P = .01) retained statistical significance, but mastectomy versus lumpectomy (HR, 1.6; P = .1) and complete pathologic response (HR, 0.27; P = .08) did not (Table E2, available online at https://doi.org/10.1016/j.ijrobp.2019.11.398). Therefore, the most optimal PFS models included 3 predictors only; owing to power, the LRF and OS models also included 3 predictors.
For each study outcome, there were 3 consistent models. All models included NLR, stage, and 1 of the following predictors: mastectomy versus lumpectomy, adjuvant chemotherapy, or neoadjuvant chemotherapy (Table 3). For LRF, mastectomy versus lumpectomy was not significant (HR, 1.32; P = .4621), and for OS, adjuvant chemotherapy was not significant (HR, 0.52; P = .0836). Thus, the final model for LRF, PFS, and OS consisted of NLR, stage, and neoadjuvant chemotherapy. Compared with stage II disease, stage III disease was associated with a greater hazard of LRF (HR, 5.53; P < .0001), PFS (HR, 4.81; P < .0001), and dying (HR, 4.46; P < .0001). A 1 unit increase in the value of NLR (ie, a change from an NLR of 3 to 4) was associated with a greater hazard of LRF (HR, 1.40; P = .0009), PFS (HR, 1.35; P = .0004), and dying (HR, 1.27; P = .0007). Receiving neoadjuvant chemotherapy was associated with a greater hazard of LRF (HR, 2.53; P = .0162), PFS (HR, 2.02; P = .0241), and dying (HR, 2.32; P = .0271).
Table 3.
Multivariable time-dependent competing risk analysis for locoregional failure and multivariable time-dependent Cox proportional hazards regression analysis for progression-free survival and overall survival
| Predictor | LRF |
PFS |
OS |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Stage II vs III | 5.5* | 2.6-11 | <.0001 | 4.6* | 2.6-8.3 | <.0001 | 3.6* | 1.8-7.1 | .0003 |
| NLR | 1.4* | 1.1-1.7 | .002 | 1.3* | 1.1-1.5 | .002 | 1.2* | 1.1-1.4 | .006 |
| Mastectomy vs lumpectomy | 1.3 | 0.63-2.8 | .5 | 1.9* | 1.1-3.5 | .03 | 2.4* | 1.2-4.7 | .02 |
| Stage II vs III | 4.7* | 2.2-9.9 | <.0001 | 4.5* | 2.5-8.2 | <.0001 | 4.3* | 2.1-8.9 | <.0001 |
| NLR | 1.3* | 1.1-1.6 | .005 | 1.3* | 1.1-1.6 | .001 | 1.246* | 1.1-1.4 | .002 |
| Adjuvant chemotherapy | 0.36* | 0.17-0.79 | .01 | 0.52* | 0.27-0.98 | .04 | 0.52 | 0.25-1.1 | .08 |
| Stage II vs III | 5.5* | 2.7-11 | <.0001 | 4.8* | 2.7-8.6 | <.0001 | 4.5* | 2.2-9.1 | <.0001 |
| NLR | 1.4* | 1.1-1.7 | .0009 | 1.3* | 1.1-1.6 | .0004 | 1.3* | 1.1-1.4 | .0007 |
| Neoadjuvant chemotherapy | 2.5* | 1.2-5.4 | .02 | 2.018* | 1.1-3.7 | .02 | 2.316* | 1.1-4.9 | .03 |
Abbreviations: CI = confidence interval; HR = hazard ratio; LRF = locoregional failure; NLR = neutrophil:lymphocyte ratio; OS = overall survival; PFS = progression-free survival.
NLR was evaluated as a continuous variable.
Statistically significant hazard ratios.
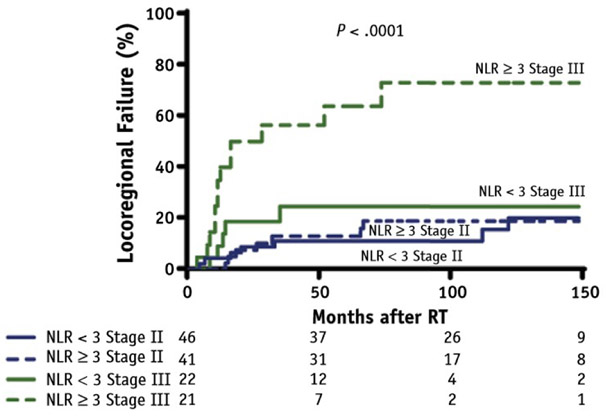
A cumulative incidence model was fit with LRF as the outcome and death as a competing risk, demonstrating a difference (P < .0001) among 4 cohorts stratified by stage and mean postradiation NLR: NLR <3 and stage II, NLR ≥3 and stage II, NLR <3 and stage III, and NLR ≥3 and stage III (Fig. 2). As suggested by the magnitude of the HR of the Cox multivariable analysis, stage is the strongest predictor of LRF, and the 2 groups with stage II have lower rates of LRF than the 2 groups with stage III. Within each stage, patients who have NLR <3 have a lower rate of LRF compared with patients with NLR ≥3.
Fig. 2.
Competing risks analysis of locoregional failure (LRF) with stage II versus III and neutrophil:lymphocyte ratio (NLR) <3 versus NLR ≥3.
Discussion
In this retrospective study of TNBC, systemic inflammation and immunocompromised status after RT were independently predictive of LRF, PFS, and OS. These clinical findings support the preclinical hypothesis that TNBC recurrence is mediated in part by a proinflammatory, lymphocyte-poor microenvironment within irradiated stroma. This study also suggests that systemic inflammatory measurements, such as NLR, PLR, and MLR, may be valuable prognostic biomarkers after RT. In addition to prognostic information, these biomarkers may provide an avenue to clinically model the characteristics of the irradiated microenvironment in real time.
The host adaptive immune response is thought to recognize RT-induced tumor neoantigen presentation and subsequently enhance RT-related tumor killing.19 This notion has been supported by the relationship between intratumoral lymphocytes and prognosis.20 Although RT promotes antitumor immunostimulation, normal tissue damage also activates the innate inflammatory response.21,22 Although RT damage leading to neutrophil and macrophage infiltration signals for cleanup of necrotic debris within the microenvironment, the relationship between tumor cells and the innate immune system is controversial.23-25 In fact, there is some evidence that the inflammatory response to RT may inadvertently promote TNBC progression locally and even distantly, and this hypothesis has been postulated in other cancers as well.16,26-28
In support of the hypothesis that RT promotes systemic inflammation and immunosuppression, resulting in a microenvironment conducive to TNBC recurrence, we find that an elevated NLR after RT strongly and independently predicts LRF, PFS, and OS. The prognostic significance demonstrated by this finding advances the concept of protumor neutrophil phenotypes induced by RT in the tumor microenvironment. In the irradiated microenvironment, such protumor neutrophils may directly promote immunosuppression by regulating infiltrating effector lymphocytes through mediators such as reactive oxygen species, nitric oxide, and arginase.29-31 Radiation-driven neutrophil-mediated immunosuppression, in addition to radiation-induced lymphopenia, may therefore facilitate tumor escape via immunodetection avoidance.11
Interestingly, MLR did not provide direct prognostic information on LRF or PFS but instead on OS. The utility of MLR for predicting local failure may be challenged by the dichotomy of pro- and antitumor macrophage polarization states, differences between macrophages derived from circulating monocytes and resident tissue macrophages, and microenvironmental dynamics between tumor-associated macrophages, tumor, and infiltrating monocytes, with nuances that are poorly evaluated by peripheral monocyte count.11-32-34
This work highlights several critical areas for further study. Determining the influence of host genetics, environmental factors, and tumor composition, among other factors, on patient response to RT may allow for more precise prediction of outcome before treatment. Such precision medicine techniques may even offer a clinical platform for tailoring TNBC therapies based on the predicted inflammatory and immunologic response to RT. In the era of checkpoint inhibitors, the significance of immunologically based predictive biomarkers may be even more relevant.35 Although immunotherapy is not currently the standard of care for localized TNBC, adjuvant locoregional TNBC treatment paradigms may shift to include immunotherapy concurrently or in sequence with RT based on positive results from the metastatic literature. For example, the phase 3 IMpassion 130 trial recently reported that programmed death ligand 1 inhibition plus chemotherapy in untreated metastatic TNBC provided a PFS benefit enriched in programmed death ligand 1—positive tumors.36 In light of such promising data, an increased understanding of the underlying mechanisms behind the clinical findings reported in our study may provide a basis for personalized strategies to minimize the protumor inflammatory milieu of the irradiated microenvironment. The prognostic biomarkers reported here may also be a potential indication for intensification of treatment. For instance, patients who achieve a complete pathologic response after neoadjuvant chemotherapy but demonstrate high-risk inflammatory biomarkers after adjuvant RT may be candidates for adjuvant chemotherapy or chemoimmunotherapy strategies.
The timing and frequency of biomarker measurements were nonprotocolized and not uniform within our study. Although we allowed each biomarker to vary over time in the regression models, we did not find an optimized time interval for biomarker measurement. Based on this multi-institutional experience, we recommend CBC with differential before RT, at the conclusion of RT, 1 month after RT, and every 3 months after RT for at least 1 year posttreatment.
Other limitations of this study include its retrospective nature and sample size. Although our study represents the patient population of 2 tertiary care centers, our results may not be extrapolatable to all practice settings. Our cohort was nonrandomized and included both stage II and stage III TNBC. Although stage and other predictors were included in multivariable analysis, our sample was underpowered, and we could not fit a model that included all relevant predictors. Given selection bias and nonrandomization, patients who received mastectomy or neoadjuvant chemotherapy were likely at greater baseline risk for recurrence and mortality. Therefore, it is unlikely that the receipt of neoadjuvant chemotherapy or mastectomy causally worsened survival, which would conflict with evidence from large randomized trials, but rather that the correlation observed with these 2 variables relates to the imbalance between groups owing to selection bias.37,38 Although this imbalance is a notable limitation, our multivariable analysis affirms that NLR provides independent, unique, and significant prognostic information irrespective of whether patients received neoadjuvant chemotherapy or mastectomy.
A further limitation of our study is the correlational, rather than causational, relationships of biomarkers and outcome. Preradiation markers were not analyzed in light of the significant variability in time of collection in relation to RT, surgery, and chemotherapy, as well as a large number of patients with missing pre-RT markers, although the prognostic meaning of a marker measurement collected at only 1 time point before RT is debatable. This is because the pattern of markers over time is more likely to inform the underlying disease pathophysiology and probability of recurrence and is why we chose to incorporate time-varying markers in our multivariable analysis. Furthermore, because most of our patients were treated with conventionally fractionated RT, we could not establish a dose-response relationship between radiation dose and biomarker quantification. The dose-response relationship between RT and inflammatory biomarkers should be examined in conventional and hypofractionated regimens as well as dosimetric studies of nodal, cardiopulmonary, and marrow irradiation. An additional limitation to this study is the heterogeneity of chemotherapy regimens and variance of chemotherapy sequence with RT. Our study did not find any relationship between neoadjuvant or adjuvant chemotherapy sequence and inflammatory biomarkers. However, future studies should confirm this finding with a well-powered prospective study.
Conclusions
Radiation-induced elevations in NLR may be an independent prognostic biomarker for LRF, PFS, and OS in patients with stage II-III TNBC. Preclinical hypotheses purporting mechanisms of tumor recurrence through increases in neutrophil and macrophage activity with concurrent immunosuppression in an irradiated microenvironment appear to be supported by the clinical findings reported here. The inflammatory biomarkers used in this study are routinely obtained, cost-effective, easily accessible, and readily calculated from conventional blood tests. Future clinical trials may benefit from incorporating systemic inflammatory biomarkers in studying TNBC outcome and evaluating methods of attenuating systemic inflammation in patients with poor prognostic inflammatory biomarkers after radiation.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health grant #R00CA201304 (M. Rafat) and grant #P30CA068485 (A. B. Chakravarthy) from the National Cancer Institute and CTSA award #UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Institutes of Health.
Footnotes
Disclosures: I.M. reports research grant funding from Novartis and Pfizer and an advisory role at Novartis, AstraZeneca, Genentech, Lilly, GlaxoSmithKline, Immunomedics, Microgenics, and Seattle Genetics.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2019.11.398.
References
- 1.Lhuillier C, Rudqvist N, Elemento O, Formenti S, Demaria S. Radiation therapy and antitumor immunity: Exposing immunogenic mutations to the immune system. Genome Med 2019;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakamura N, Kusunoki Y, Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res 1990;123:224–227. [PubMed] [Google Scholar]
- 3.Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol 2018;123:42–51. [DOI] [PubMed] [Google Scholar]
- 4.Chan TA, Yarchoan M, Jaffee E, et al. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann Oncol 2019;30:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turajlic S, Litchfield K, Xu H, et al. Insertion-and-deletion-derived tumor-specific neoantigens and the immunogenic phenotype: A pancancer analysis. Lancet Oncol 2017;18:1009–1021. [DOI] [PubMed] [Google Scholar]
- 7.Silwal-Pandit L, Vollan HKM, Chin SF, et al. TP53 mutation spectrum in breast cancer is subtype specific and has distinct prognostic relevance. Clin Cancer Res 2014;20:3569–3580. [DOI] [PubMed] [Google Scholar]
- 8.Thomas A, Routh ED, Pullikuth A, et al. Tumor mutational burden is a determinant of immune-mediated survival in breast cancer. Oncoimmunology 2018;7:e1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Yang J, Peng L, et al. Triple-negative breast cancer has worse overall survival and cause-specific survival than nontriple-negative breast cancer. Breast Cancer Res Treat 2017;161:279–287. [DOI] [PubMed] [Google Scholar]
- 10.Afghahi A, Purington N, Han SS, et al. Higher absolute lymphocyte counts predict lower mortality from early-stage triple-negative breast cancer. Clin Cancer Res 2018;24:2851–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rafat M, Aguilera TA, Vilalta M, et al. Macrophages promote circulating tumor cell-mediated local recurrence following radiotherapy in immunosuppressed patients. Cancer Res 2018;78:4241–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh CH, Bhoo-Pathy N, Ng KL, et al. Utility of pretreatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer 2015;113:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei B, Yao M, Xing C, et al. The neutrophil lymphocyte ratio is associated with breast cancer prognosis: An updated systematic review and meta-analysis. Onco Targets Ther 2016;9:5567–5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Qu JK, Zhang J, et al. Prognostic role of pretreatment neutrophil to lymphocyte ratio in breast cancer patients. Med (United States) 2017;96:e8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mei Z, Shi L, Wang B, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: A systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev 2017;58:1–13. [DOI] [PubMed] [Google Scholar]
- 16.Mason M, Maurice C, McNamara MG, et al. Neutrophil—lymphocyte ratio dynamics during concurrent chemo-radiotherapy for glioblastoma is an independent predictor for overall survival. J Neurooncol 2017;132:463–471. [DOI] [PubMed] [Google Scholar]
- 17.Danciu I, Cowan JD, Basford M, et al. Secondary use of clinical data: The Vanderbilt approach. J Biomed Inform 2014;52:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furnival GM, Wilson RW. Regressions by leaps and bounds. Technometrics 1974;16:499–511. [Google Scholar]
- 19.Rodríguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. Immunologic mechanisms responsible for radiation-induced abscopal effect. Trends Immunol 2018;39:644–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dieci MV, Mathieu MC, Guarneri V, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol 2015;26:1698–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schernberg A, Blanchard P, Chargari C, Deutsch E. Neutrophils, a candidate biomarker and target for radiation therapy? Acta Oncol (Madr) 2017;56:1522–1530. [DOI] [PubMed] [Google Scholar]
- 22.Wilkins AC, Patin EC, Harrington KJ, Melcher AA. The immunological consequences of radiation-induced DNA damage. J Pathol 2019;247:606–614. [DOI] [PubMed] [Google Scholar]
- 23.Derer A, Deloch L, Rubner Y, Fietkau R, Frey B, Gaipl US. Radioimmunotherapy-induced immunogenic cancer cells as basis for induction of systemic antitumor immune responses - preclinical evidence and ongoing clinical applications. Front Immunol 2015;6:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coffelt SB, Wellenstein MD, De Visser KE. Neutrophils in cancer: Neutral no more. Nat Rev Cancer 2016;16:431–446. [DOI] [PubMed] [Google Scholar]
- 25.Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 2015;528:413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee IH, Hwang S, Lee SJ, et al. Systemic inflammatory response after preoperative chemoradiotherapy can affect oncologic outcomes in locally advanced rectal cancer. Anticancer Res 2017;37:1459–1465. [DOI] [PubMed] [Google Scholar]
- 27.Gao C, Kozlowska A, Nechaev S, et al. TLR9 signaling in the tumor microenvironment initiates cancer recurrence after radiotherapy. Cancer Res 2013;73:7211–7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Rayes T, Catena R, Lee S, et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc Natl Acad Sci USA 2015;112:16000–16005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez PC, Ernstoff MS, Hernandez C, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a sub-population of activated granulocytes. Cancer Res 2009;69:1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller I, Munder M, Kropf P, Hänsch GM. Polymorphonuclear neutrophils and T lymphocytes: Strange bedfellows or brothers in arms? Trends Immunol 2009;30:522–530. [DOI] [PubMed] [Google Scholar]
- 31.Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 2009;16:183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 1996;56:4625–4629. [PubMed] [Google Scholar]
- 33.Mantovani A, Sica A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr Opin Immunol 2010;22:231–237. [DOI] [PubMed] [Google Scholar]
- 34.Lahmar Q, Keirsse J, Laoui D, Movahedi K, Van Overmeire E, Van Ginderachter JA. Tissue-resident versus monocyte-derived macrophages in the tumor microenvironment. Biochim Biophys Acta 2016;1865:23–34. [DOI] [PubMed] [Google Scholar]
- 35.Pike LRG, Bang A, Mahal BA, et al. The impact of radiation therapy on lymphocyte count and survival in metastatic cancer patients receiving PD-1 immune checkpoint inhibitors, Int J Radiat Oncol 2019;103:141–151. [DOI] [PubMed] [Google Scholar]
- 36.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108–2121. [DOI] [PubMed] [Google Scholar]
- 37.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227–1232. [DOI] [PubMed] [Google Scholar]
- 38.Van der Hage JA, Van de Velde CJH, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: Results from the European Organization for Research and Treatment of Cancer Trial 10902. J Clin Oncol 2001; 19:4224–4237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.